The Impact of Follicular Fluid Oxidative Stress Levels on the Outcomes of Assisted Reproductive Therapy
Abstract
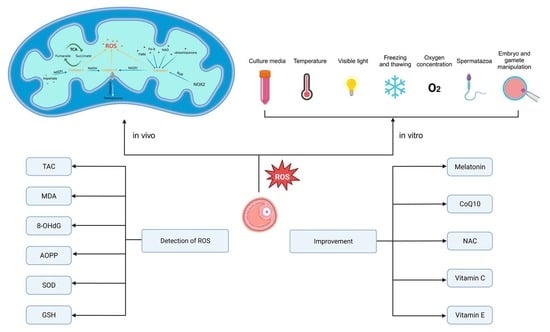
:1. Introduction
2. Generation of Reactive Oxygen Species in Human Follicular Fluid and Its Clinical Significance
3. Oxidative Stress Biomarkers in Follicular Fluid
3.1. Total Antioxidant Capacity (TAC)
3.2. Malondialdehyde (MDA)
3.3. 8-Oxodeoxyguanosine (8-OHdG)
3.4. Advanced Oxidation Protein Products (AOPP)
3.5. Superoxide Dismutase (SOD)
3.6. Glutathione (GSH)
4. Measures to Improve Follicular Fluid Oxidative Stress Status
4.1. Melatonin
4.2. Coenzyme Q10 (CoQ10)
4.3. Resveratrol
| Species | Protocol | Treatment Outcome |
|---|---|---|
| Mouse | Addition of 1.0 μM resveratrol in IVM culture medium | Improved oocyte maturation rates and oocyte quality, significantly reducing the proportion of abnormal oocytes [66]. |
| Addition of 25 μM resveratrol in vitrification medium (ES, VS), warming medium (TS, DS, and HM), and post-warming medium (IVF medium) | Reduced oxidative stress in vitrified oocytes and alleviated abnormal mitochondrial distribution after vitrification [72]. | |
| Pig | Addition of 2 μM resveratrol in IVM growth medium | Enhanced mitochondrial function by activating SIRT1, increasing ATP levels, and improving oocyte quality [69]. |
| Resveratrol at 2 μmol/L for 68 h in maturation medium | Significantly improved the quality of aged oocytes, including the assembly of meiotic apparatus, redistribution of cortical granules, and mitochondria in pig oocytes [71]. | |
| Cow | Addition of different concentrations of resveratrol (0, 0.1, 1.0, or 10.0 μM) in maturation medium | Reduce ROS levels, improving subsequent development after in vitro fertilization or parthenogenetic activation [65]. |
| Cultured in medium containing 0 or 0.5 µM resveratrol for 1 or 5 days | Enhanced mitochondrial functions via SIRT1 expression, reduced lipid content via beta-oxidation, improved the rate of embryonic development to the blastocyst stage, and improved blastocyst cryotolerance [67]. | |
| Cultured in TCM-199 medium supplemented with 10% FCS and 0 or 20 µM resveratrol | Improved the quality of oocytes by improving mitochondrial quantity and quality, enhanced SIRT1 protein expression in oocytes, and improved fertilization [70]. |
4.4. N-Acetylcysteine (NAC)
5. Conclusions and Prospects
Funding
Conflicts of Interest
References
- Wang, L.; Zhu, Y.; Wang, T.; Xu, X.; Tang, Q.; Li, J.; Wang, Y.; Hu, W.; Wu, W. Feasibility analysis of incorporating infertility into medical insurance in China. Front. Endocrinol. 2022, 13, 967739. [Google Scholar] [CrossRef]
- Sang, Q.; Ray, P.F.; Wang, L. Understanding the genetics of human infertility. Science 2023, 380, 158–163. [Google Scholar] [CrossRef]
- Ishak, G.M.; Feugang, J.M.; Pechanova, O.; Pechan, T.; Peterson, D.G.; Willard, S.T.; Ryan, P.L.; Gastal, E.L. Follicular-fluid proteomics during equine follicle development. Mol. Reprod. Dev. 2022, 89, 298–311. [Google Scholar] [CrossRef] [PubMed]
- Da Broi, M.G.; Giorgi, V.S.I.; Wang, F.; Keefe, D.L.; Albertini, D.; Navarro, P.A. Influence of follicular fluid and cumulus cells on oocyte quality: Clinical implications. J. Assist. Reprod. Genet. 2018, 35, 735–751. [Google Scholar] [CrossRef] [PubMed]
- Gode, F.; Gulekli, B.; Dogan, E.; Korhan, P.; Dogan, S.; Bige, O.; Cimrin, D.; Atabey, N. Influence of follicular fluid GDF9 and BMP15 on embryo quality. Fertil. Steril. 2011, 95, 2274–2278. [Google Scholar] [CrossRef] [PubMed]
- Liu, N.; Ma, Y.; Li, R.; Jin, H.; Li, M.; Huang, X.; Feng, H.L.; Qiao, J. Comparison of follicular fluid amphiregulin and EGF concentrations in patients undergoing IVF with different stimulation protocols. Endocrine 2012, 42, 708–716. [Google Scholar] [CrossRef]
- Yang, W.J.; Liu, F.C.; Hsieh, J.S.; Chen, C.H.; Hsiao, S.Y.; Lin, C.S. Matrix metalloproteinase 2 level in human follicular fluid is a reliable marker of human oocyte maturation in in vitro fertilization and intracytoplasmic sperm injection cycles. Reprod. Biol. Endocrinol. 2015, 13, 102. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, A.; Aponte-Mellado, A.; Premkumar, B.J.; Shaman, A.; Gupta, S. The effects of oxidative stress on female reproduction: A review. Reprod. Biol. Endocrinol. 2012, 10, 49. [Google Scholar] [CrossRef]
- Liu, Y.; Yu, Z.; Zhao, S.; Cheng, L.; Man, Y.; Gao, X.; Zhao, H. Oxidative stress markers in the follicular fluid of patients with polycystic ovary syndrome correlate with a decrease in embryo quality. J. Assist. Reprod. Genet. 2021, 38, 471–477. [Google Scholar] [CrossRef]
- Artini, P.G.; Scarfò, G.; Marzi, I.; Fusi, J.; Obino, M.E.; Franzoni, F.; Zappelli, E.; Chelucci, E.; Martini, C.; Cela, V.; et al. Oxidative Stress-Related Signaling Pathways Predict Oocytes’ Fertilization In Vitro and Embryo Quality. Int. J. Mol. Sci. 2022, 23, 13442. [Google Scholar] [CrossRef]
- Reiter, R.J.; Sharma, R.; Romero, A.; Manucha, W.; Tan, D.X.; Zuccari, D.; Chuffa, L.G.A. Aging-Related Ovarian Failure and Infertility: Melatonin to the Rescue. Antioxidants 2023, 12, 695. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Wang, Z.; Cao, J.; Chen, Y.; Dong, Y. A novel and compact review on the role of oxidative stress in female reproduction. Reprod. Biol. Endocrinol. 2018, 16, 80. [Google Scholar] [CrossRef] [PubMed]
- Tamura, H.; Jozaki, M.; Tanabe, M.; Shirafuta, Y.; Mihara, Y.; Shinagawa, M.; Tamura, I.; Maekawa, R.; Sato, S.; Taketani, T.; et al. Importance of Melatonin in Assisted Reproductive Technology and Ovarian Aging. Int. J. Mol. Sci. 2020, 21, 1135. [Google Scholar] [CrossRef] [PubMed]
- Prasad, S.; Tiwari, M.; Pandey, A.N.; Shrivastav, T.G.; Chaube, S.K. Impact of stress on oocyte quality and reproductive outcome. J. Biomed. Sci. 2016, 23, 36. [Google Scholar] [CrossRef] [PubMed]
- An, Q.; Peng, W.; Cheng, Y.; Lu, Z.; Zhou, C.; Zhang, Y.; Su, J. Melatonin supplementation during in vitro maturation of oocyte enhances subsequent development of bovine cloned embryos. J. Cell. Physiol. 2019, 234, 17370–17381. [Google Scholar] [CrossRef] [PubMed]
- Tamura, H.; Takasaki, A.; Miwa, I.; Taniguchi, K.; Maekawa, R.; Asada, H.; Taketani, T.; Matsuoka, A.; Yamagata, Y.; Shimamura, K.; et al. Oxidative stress impairs oocyte quality and melatonin protects oocytes from free radical damage and improves fertilization rate. J. Pineal Res. 2008, 44, 280–287. [Google Scholar] [CrossRef] [PubMed]
- Nasiri, N.; Moini, A.; Eftekhari-Yazdi, P.; Karimian, L.; Salman-Yazdi, R.; Arabipoor, A. Oxidative Stress Statues in Serum and Follicular Fluid of Women with Endometriosis. Cell J. 2017, 18, 582–587. [Google Scholar]
- Scutiero, G.; Iannone, P.; Bernardi, G.; Bonaccorsi, G.; Spadaro, S.; Volta, C.A.; Greco, P.; Nappi, L. Oxidative Stress and Endometriosis: A Systematic Review of the Literature. Oxidative Med. Cell. Longev. 2017, 2017, 7265238. [Google Scholar] [CrossRef]
- Bedaiwy, M.A.; Elnashar, S.A.; Goldberg, J.M.; Sharma, R.; Mascha, E.J.; Arrigain, S.; Agarwal, A.; Falcone, T. Effect of follicular fluid oxidative stress parameters on intracytoplasmic sperm injection outcome. Gynecol. Endocrinol. 2012, 28, 51–55. [Google Scholar] [CrossRef]
- Palini, S.; Benedetti, S.; Tagliamonte, M.C.; De Stefani, S.; Primiterra, M.; Polli, V.; Rocchi, P.; Catalani, S.; Battistelli, S.; Canestrari, F.; et al. Influence of ovarian stimulation for IVF/ICSI on the antioxidant defence system and relationship to outcome. Reprod. Biomed. Online 2014, 29, 65–71. [Google Scholar] [CrossRef]
- Drejza, M.A.; Rylewicz, K.; Majcherek, E.; Gross-Tyrkin, K.; Mizgier, M.; Plagens-Rotman, K.; Wójcik, M.; Panecka-Mysza, K.; Pisarska-Krawczyk, M.; Kędzia, W.; et al. Markers of Oxidative Stress in Obstetrics and Gynaecology-A Systematic Literature Review. Antioxidants 2022, 11, 1477. [Google Scholar] [CrossRef]
- Al-Saleh, I.; Coskun, S.; Al-Rouqi, R.; Al-Rajudi, T.; Eltabache, C.; Abduljabbar, M.; Al-Hassan, S. Oxidative stress and DNA damage status in couples undergoing in vitro fertilization treatment. Reprod. Fertil. 2021, 2, 117–139. [Google Scholar] [CrossRef] [PubMed]
- Oyawoye, O.; Abdel Gadir, A.; Garner, A.; Constantinovici, N.; Perrett, C.; Hardiman, P. Antioxidants and reactive oxygen species in follicular fluid of women undergoing IVF: Relationship to outcome. Hum. Reprod. 2003, 18, 2270–2274. [Google Scholar] [CrossRef] [PubMed]
- Appasamy, M.; Jauniaux, E.; Serhal, P.; Al-Qahtani, A.; Groome, N.P.; Muttukrishna, S. Evaluation of the relationship between follicular fluid oxidative stress, ovarian hormones, and response to gonadotropin stimulation. Fertil. Steril. 2008, 89, 912–921. [Google Scholar] [CrossRef]
- Pasqualotto, E.B.; Agarwal, A.; Sharma, R.K.; Izzo, V.M.; Pinotti, J.A.; Joshi, N.J.; Rose, B.I. Effect of oxidative stress in follicular fluid on the outcome of assisted reproductive procedures. Fertil. Steril. 2004, 81, 973–976. [Google Scholar] [CrossRef] [PubMed]
- Kazemi, A.; Ramezanzadeh, F.; Nasr-Esfahani, M.H.; Saboor Yaraghi, A.A.; Ahmadi, M. Does dietary fat intake influence oocyte competence and embryo quality by inducing oxidative stress in follicular fluid? Iran. J. Reprod. Med. 2013, 11, 1005–1012. [Google Scholar] [PubMed]
- Debbarh, H.; Louanjli, N.; Aboulmaouahib, S.; Jamil, M.; Ahbbas, L.; Kaarouch, I.; Sefrioui, O.; Cadi, R. Antioxidant activities and lipid peroxidation status in human follicular fluid: Age-dependent change. Zygote 2021, 29, 490–494. [Google Scholar] [CrossRef] [PubMed]
- Seino, T.; Saito, H.; Kaneko, T.; Takahashi, T.; Kawachiya, S.; Kurachi, H. Eight-hydroxy-2′-deoxyguanosine in granulosa cells is correlated with the quality of oocytes and embryos in an in vitro fertilization-embryo transfer program. Fertil. Steril. 2002, 77, 1184–1190. [Google Scholar] [CrossRef]
- Péntek, S.; Várnagy, Á.; Farkas, B.; Mauchart, P.; Gödöny, K.; Varjas, T.; Kőszegi, T.; Kaltenecker, P.; Jakabfi-Csepregi, R.; Kovács, K.; et al. Telomere Length and Telomerase Activity of Granulosa Cells and Follicular Fluid in Women Undergoing In Vitro Fertilization. Antioxidants 2023, 12, 419. [Google Scholar] [CrossRef]
- Song, Y.L.; Quan, S.; Tian, J.W.; Li, H.; Chen, S.M.; Xing, F.Q. [Relationship between protein oxidation levels in the follicular fluid and the outcome parameters of in vitro fertilization-embryo transplantation]. Nan Fang Yi Ke Da Xue Xue Bao J. South. Med. Univ. 2009, 29, 160–163. [Google Scholar]
- Kokot, I.; Piwowar, A.; Jędryka, M.; Kratz, E.M. Is There a Balance in Oxidative-Antioxidant Status in Blood Serum of Patients with Advanced Endometriosis? Antioxidants 2021, 10, 1097. [Google Scholar] [CrossRef]
- Santulli, P.; Chouzenoux, S.; Fiorese, M.; Marcellin, L.; Lemarechal, H.; Millischer, A.E.; Batteux, F.; Borderie, D.; Chapron, C. Protein oxidative stress markers in peritoneal fluids of women with deep infiltrating endometriosis are increased. Hum. Reprod. 2015, 30, 49–60. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Liu, J.; Qiu, Z.; Chen, D.; Luo, C.; Liu, X.; Hua, R.; Zhu, X.; Lin, Y.; Li, L.; et al. Advanced oxidation protein products from the follicular microenvironment and their role in infertile women with endometriosis. Exp. Ther. Med. 2018, 15, 479–486. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, J.C.; Braga, P.C.; Martins, A.D.; Silva, B.M.; Alves, M.G.; Oliveira, P.F. Antioxidants Present in Reproductive Tract Fluids and Their Relevance for Fertility. Antioxidants 2021, 10, 1441. [Google Scholar] [CrossRef] [PubMed]
- Combelles, C.M.; Holick, E.A.; Paolella, L.J.; Walker, D.C.; Wu, Q. Profiling of superoxide dismutase isoenzymes in compartments of the developing bovine antral follicles. Reproduction 2010, 139, 871–881. [Google Scholar] [CrossRef] [PubMed]
- Matzuk, M.M.; Dionne, L.; Guo, Q.; Kumar, T.R.; Lebovitz, R.M. Ovarian function in superoxide dismutase 1 and 2 knockout mice. Endocrinology 1998, 139, 4008–4011. [Google Scholar] [CrossRef] [PubMed]
- Yalçınkaya, E.; Cakıroğlu, Y.; Doğer, E.; Budak, O.; Cekmen, M.; Calışkan, E. Effect of follicular fluid NO, MDA and GSH levels on in vitro fertilization outcomes. J. Turk. Ger. Gynecol. Assoc. 2013, 14, 136–141. [Google Scholar] [CrossRef] [PubMed]
- Liang, S.; Guo, J.; Choi, J.W.; Kim, N.H.; Cui, X.S. Effect and possible mechanisms of melatonin treatment on the quality and developmental potential of aged bovine oocytes. Reprod. Fertil. Dev. 2017, 29, 1821–1831. [Google Scholar] [CrossRef]
- Ezzati, M.; Roshangar, L.; Soleimani Rad, J.; Karimian, N. Evaluating The Effect of Melatonin on HAS2, and PGR expression, as well as Cumulus Expansion, and Fertility Potential in Mice. Cell J. 2018, 20, 108–112. [Google Scholar]
- Tian, X.; Wang, F.; Zhang, L.; Ji, P.; Wang, J.; Lv, D.; Li, G.; Chai, M.; Lian, Z.; Liu, G. Melatonin Promotes the In Vitro Development of Microinjected Pronuclear Mouse Embryos via Its Anti-Oxidative and Anti-Apoptotic Effects. Int. J. Mol. Sci. 2017, 18, 988. [Google Scholar] [CrossRef]
- Kang, J.T.; Koo, O.J.; Kwon, D.K.; Park, H.J.; Jang, G.; Kang, S.K.; Lee, B.C. Effects of melatonin on in vitro maturation of porcine oocyte and expression of melatonin receptor RNA in cumulus and granulosa cells. J. Pineal Res. 2009, 46, 22–28. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Jin, J.X.; Taweechaipaisankul, A.; Kim, G.A.; Lee, B.C. Stimulatory Effects of Melatonin on Porcine In Vitro Maturation Are Mediated by MT2 Receptor. Int. J. Mol. Sci. 2018, 19, 1581. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Shan, X.; Jiang, H.; Guo, Z. Exogenous Melatonin Directly and Indirectly Influences Sheep Oocytes. Front. Vet. Sci. 2022, 9, 903195. [Google Scholar] [CrossRef] [PubMed]
- Tian, X.; Wang, F.; Zhang, L.; He, C.; Ji, P.; Wang, J.; Zhang, Z.; Lv, D.; Abulizi, W.; Wang, X.; et al. Beneficial Effects of Melatonin on the In Vitro Maturation of Sheep Oocytes and Its Relation to Melatonin Receptors. Int. J. Mol. Sci. 2017, 18, 834. [Google Scholar] [CrossRef] [PubMed]
- Park, H.J.; Park, S.Y.; Kim, J.W.; Yang, S.G.; Kim, M.J.; Jegal, H.G.; Kim, I.S.; Choo, Y.K.; Koo, D.B. Melatonin Improves Oocyte Maturation and Mitochondrial Functions by Reducing Bisphenol A-Derived Superoxide in Porcine Oocytes In Vitro. Int. J. Mol. Sci. 2018, 19, 3422. [Google Scholar] [CrossRef] [PubMed]
- Cheng, L.; Qin, Y.; Hu, X.; Ren, L.; Zhang, C.; Wang, X.; Wang, W.; Zhang, Z.; Hao, J.; Guo, M.; et al. Melatonin protects in vitro matured porcine oocytes from toxicity of Aflatoxin B1. J. Pineal Res. 2019, 66, e12543. [Google Scholar] [CrossRef] [PubMed]
- Jia, Y.; Liu, W.; Bai, D.; Zhang, Y.; Li, Y.; Liu, Y.; Yin, J.; Chen, Q.; Ye, M.; Zhao, Y.; et al. Melatonin supplementation in the culture medium rescues impaired glucose metabolism in IVF mice offspring. J. Pineal Res. 2022, 72, e12778. [Google Scholar] [CrossRef]
- Zou, H.; Chen, B.; Ding, D.; Gao, M.; Chen, D.; Liu, Y.; Hao, Y.; Zou, W.; Ji, D.; Zhou, P.; et al. Melatonin promotes the development of immature oocytes from the COH cycle into healthy offspring by protecting mitochondrial function. J. Pineal Res. 2020, 68, e12621. [Google Scholar] [CrossRef]
- Zhang, Z.; Mu, Y.; Ding, D.; Zou, W.; Li, X.; Chen, B.; Leung, P.C.; Chang, H.M.; Zhu, Q.; Wang, K.; et al. Melatonin improves the effect of cryopreservation on human oocytes by suppressing oxidative stress and maintaining the permeability of the oolemma. J. Pineal Res. 2021, 70, e12707. [Google Scholar] [CrossRef]
- Song, C.; Peng, W.; Yin, S.; Zhao, J.; Fu, B.; Zhang, J.; Mao, T.; Wu, H.; Zhang, Y. Melatonin improves age-induced fertility decline and attenuates ovarian mitochondrial oxidative stress in mice. Sci. Rep. 2016, 6, 35165. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, Z.; Wang, J.; Lv, D.; Zhu, T.; Wang, F.; Tian, X.; Yao, Y.; Ji, P.; Liu, G. Melatonin regulates the activities of ovary and delays the fertility decline in female animals via MT1/AMPK pathway. J. Pineal Res. 2019, 66, e12550. [Google Scholar] [CrossRef]
- Dong, L.; Teh, D.B.L.; Kennedy, B.K.; Huang, Z. Unraveling female reproductive senescence to enhance healthy longevity. Cell Res. 2023, 33, 11–29. [Google Scholar] [CrossRef]
- Fernando, S.; Wallace, E.M.; Vollenhoven, B.; Lolatgis, N.; Hope, N.; Wong, M.; Lawrence, M.; Lawrence, A.; Russell, C.; Leong, K.; et al. Melatonin in Assisted Reproductive Technology: A Pilot Double-Blind Randomized Placebo-Controlled Clinical Trial. Front. Endocrinol. 2018, 9, 545. [Google Scholar] [CrossRef]
- Keshavarzi, S.; Salehi, M.; Farifteh-Nobijari, F.; Hosseini, T.; Hosseini, S.; Ghazifard, A.; Ghaffari Novin, M.; Fallah-Omrani, V.; Nourozian, M.; Hosseini, A. Melatonin Modifies Histone Acetylation During In Vitro Maturation of Mouse Oocytes. Cell J. 2018, 20, 244–249. [Google Scholar]
- Martinez, C.A.; Cuello, C.; Parrilla, I.; Maside, C.; Ramis, G.; Cambra, J.M.; Vazquez, J.M.; Rodriguez-Martinez, H.; Gil, M.A.; Martinez, E.A. Exogenous Melatonin in the Culture Medium Does Not Affect the Development of In Vivo-Derived Pig Embryos but Substantially Improves the Quality of In Vitro-Produced Embryos. Antioxidants 2022, 11, 1177. [Google Scholar] [CrossRef]
- Marques, T.C.; da Silva Santos, E.C.; Diesel, T.O.; Leme, L.O.; Martins, C.F.; Dode, M.; Alves, B.G.; Costa, F.; de Oliveira, E.B.; Gambarini, M.L. Melatonin reduces apoptotic cells, SOD2 and HSPB1 and improves the in vitro production and quality of bovine blastocysts. Reprod. Domest. Anim. 2018, 53, 226–236. [Google Scholar] [CrossRef]
- Tian, X.; Wang, F.; He, C.; Zhang, L.; Tan, D.; Reiter, R.J.; Xu, J.; Ji, P.; Liu, G. Beneficial effects of melatonin on bovine oocytes maturation: A mechanistic approach. J. Pineal Res. 2014, 57, 239–247. [Google Scholar] [CrossRef]
- Zhao, X.M.; Wang, N.; Hao, H.S.; Li, C.Y.; Zhao, Y.H.; Yan, C.L.; Wang, H.Y.; Du, W.H.; Wang, D.; Liu, Y.; et al. Melatonin improves the fertilization capacity and developmental ability of bovine oocytes by regulating cytoplasmic maturation events. J. Pineal Res. 2017, 64, e12445. [Google Scholar] [CrossRef] [PubMed]
- Mancini, A.; Festa, R.; Raimondo, S.; Pontecorvi, A.; Littarru, G.P. Hormonal influence on coenzyme Q(10) levels in blood plasma. Int. J. Mol. Sci. 2011, 12, 9216–9225. [Google Scholar] [CrossRef] [PubMed]
- Miles, M.V.; Horn, P.S.; Tang, P.H.; Morrison, J.A.; Miles, L.; DeGrauw, T.; Pesce, A.J. Age-related changes in plasma coenzyme Q10 concentrations and redox state in apparently healthy children and adults. Clin. Chim. Acta; Int. J. Clin. Chem. 2004, 347, 139–144. [Google Scholar] [CrossRef] [PubMed]
- Ben-Meir, A.; Kim, K.; McQuaid, R.; Esfandiari, N.; Bentov, Y.; Casper, R.F.; Jurisicova, A. Co-Enzyme Q10 Supplementation Rescues Cumulus Cells Dysfunction in a Maternal Aging Model. Antioxidants 2019, 8, 58. [Google Scholar] [CrossRef]
- Florou, P.; Anagnostis, P.; Theocharis, P.; Chourdakis, M.; Goulis, D.G. Does coenzyme Q(10) supplementation improve fertility outcomes in women undergoing assisted reproductive technology procedures? A systematic review and meta-analysis of randomized-controlled trials. J. Assist. Reprod. Genet. 2020, 37, 2377–2387. [Google Scholar] [CrossRef]
- Xu, Y.; Nisenblat, V.; Lu, C.; Li, R.; Qiao, J.; Zhen, X.; Wang, S. Pretreatment with coenzyme Q10 improves ovarian response and embryo quality in low-prognosis young women with decreased ovarian reserve: A randomized controlled trial. Reprod. Biol. Endocrinol. 2018, 16, 29. [Google Scholar] [CrossRef]
- Ma, L.; Cai, L.; Hu, M.; Wang, J.; Xie, J.; Xing, Y.; Shen, J.; Cui, Y.; Liu, X.J.; Liu, J. Coenzyme Q10 supplementation of human oocyte in vitro maturation reduces postmeiotic aneuploidies. Fertil. Steril. 2020, 114, 331–337. [Google Scholar] [CrossRef]
- Wang, F.; Tian, X.; Zhang, L.; He, C.; Ji, P.; Li, Y.; Tan, D.; Liu, G. Beneficial effect of resveratrol on bovine oocyte maturation and subsequent embryonic development after in vitro fertilization. Fertil. Steril. 2014, 101, 577–586. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.J.; Sun, A.G.; Zhao, S.G.; Liu, H.; Ma, S.Y.; Li, M.; Huai, Y.X.; Zhao, H.; Liu, H.B. Resveratrol improves in vitro maturation of oocytes in aged mice and humans. Fertil. Steril. 2018, 109, 900–907. [Google Scholar] [CrossRef] [PubMed]
- Abe, T.; Kawahara-Miki, R.; Hara, T.; Noguchi, T.; Hayashi, T.; Shirasuna, K.; Kuwayama, T.; Iwata, H. Modification of mitochondrial function, cytoplasmic lipid content and cryosensitivity of bovine embryos by resveratrol. J. Reprod. Dev. 2017, 63, 455–461. [Google Scholar] [CrossRef] [PubMed]
- Bahramrezaie, M.; Amidi, F.; Aleyasin, A.; Saremi, A.; Aghahoseini, M.; Brenjian, S.; Khodarahmian, M.; Pooladi, A. Effects of resveratrol on VEGF & HIF1 genes expression in granulosa cells in the angiogenesis pathway and laboratory parameters of polycystic ovary syndrome: A triple-blind randomized clinical trial. J. Assist. Reprod. Genet. 2019, 36, 1701–1712. [Google Scholar] [PubMed]
- Itami, N.; Shirasuna, K.; Kuwayama, T.; Iwata, H. Resveratrol improves the quality of pig oocytes derived from early antral follicles through sirtuin 1 activation. Theriogenology 2015, 83, 1360–1367. [Google Scholar] [CrossRef] [PubMed]
- Takeo, S.; Sato, D.; Kimura, K.; Monji, Y.; Kuwayama, T.; Kawahara-Miki, R.; Iwata, H. Resveratrol improves the mitochondrial function and fertilization outcome of bovine oocytes. J. Reprod. Dev. 2014, 60, 92–99. [Google Scholar] [CrossRef] [PubMed]
- Ma, R.; Zhang, Y.; Zhang, L.; Han, J.; Rui, R. Sirt1 protects pig oocyte against in vitro aging. Anim. Sci. J. 2015, 86, 826–832. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, M.; Chen, Z.J.; Du, Y. Resveratrol promotes the embryonic development of vitrified mouse oocytes after in vitro fertilization. Vitr. Cell. Dev. Biol. Anim. 2018, 54, 430–438. [Google Scholar] [CrossRef]
- Issels, R.D.; Nagele, A.; Eckert, K.G.; Wilmanns, W. Promotion of cystine uptake and its utilization for glutathione biosynthesis induced by cysteamine and N-acetylcysteine. Biochem. Pharmacol. 1988, 37, 881–888. [Google Scholar] [CrossRef]
- Mao, X.; Wang, T.; Liu, Y.; Irwin, M.G.; Ou, J.S.; Liao, X.L.; Gao, X.; Xu, Y.; Ng, K.F.; Vanhoutte, P.M.; et al. N-acetylcysteine and allopurinol confer synergy in attenuating myocardial ischemia injury via restoring HIF-1α/HO-1 signaling in diabetic rats. PLoS ONE 2013, 8, e68949. [Google Scholar] [CrossRef] [PubMed]
- Fiordaliso, F.; Bianchi, R.; Staszewsky, L.; Cuccovillo, I.; Doni, M.; Laragione, T.; Salio, M.; Savino, C.; Melucci, S.; Santangelo, F.; et al. Antioxidant treatment attenuates hyperglycemia-induced cardiomyocyte death in rats. J. Mol. Cell. Cardiol. 2004, 37, 959–968. [Google Scholar] [CrossRef] [PubMed]
- Sun, W.S.; Jang, H.; Park, M.R.; Oh, K.B.; Lee, H.; Hwang, S.; Xu, L.J.; Hwang, I.S.; Lee, J.W. N-acetyl-L-cysteine Improves the Developmental Competence of Bovine Oocytes and Embryos Cultured In Vitro by Attenuating Oxidative Damage and Apoptosis. Antioxidants 2021, 10, 860. [Google Scholar] [CrossRef] [PubMed]
- Whitaker, B.D.; Casey, S.J.; Taupier, R. The effects of N-acetyl-L-cysteine supplementation on in vitro porcine oocyte maturation and subsequent fertilisation and embryonic development. Reprod. Fertil. Dev. 2012, 24, 1048–1054. [Google Scholar] [CrossRef] [PubMed]
- Whitaker, B.D.; Knight, J.W. Mechanisms of oxidative stress in porcine oocytes and the role of anti-oxidants. Reprod. Fertil. Dev. 2008, 20, 694–702. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Li, L.; Fan, L.H.; Jing, Y.; Li, J.; Ouyang, Y.C.; Wang, Z.B.; Hou, Y.; Sun, Q.Y. N-acetyl-L-cysteine (NAC) delays post-ovulatory oocyte aging in mouse. Aging 2019, 11, 2020–2030. [Google Scholar] [CrossRef] [PubMed]
- Truong, T.; Gardner, D.K. Antioxidants improve IVF outcome and subsequent embryo development in the mouse. Hum. Reprod. 2017, 32, 2404–2413. [Google Scholar] [CrossRef]
- Matilla, E.; Martín-Cano, F.E.; González-Fernández, L.; Sánchez-Margallo, F.M.; Álvarez, I.S.; Macías-García, B. N-acetylcysteine addition after vitrification improves oocyte mitochondrial polarization status and the quality of embryos derived from vitrified murine oocytes. BMC Vet. Res. 2019, 15, 31. [Google Scholar] [CrossRef]
- Li, X.; Wang, Z.; Wang, H.; Xu, H.; Sheng, Y.; Lian, F. Role of N-acetylcysteine treatment in women with advanced age undergoing IVF/ICSI cycles: A prospective study. Front. Med. 2022, 9, 917146. [Google Scholar] [CrossRef] [PubMed]
- Thakker, D.; Raval, A.; Patel, I.; Walia, R. N-acetylcysteine for polycystic ovary syndrome: A systematic review and meta-analysis of randomized controlled clinical trials. Obstet. Gynecol. Int. 2015, 2015, 817849. [Google Scholar] [CrossRef]
- Whitaker, B.D.; Knight, J.W. Effects of N-acetyl-cysteine and N-acetyl-cysteine-amide supplementation on in vitro matured porcine oocytes. Reprod. Domest. Anim. 2010, 45, 755–759. [Google Scholar]
- Nishihara, T.; Hashimoto, S.; Ito, K.; Nakaoka, Y.; Matsumoto, K.; Hosoi, Y.; Morimoto, Y. Oral melatonin supplementation improves oocyte and embryo quality in women undergoing in vitro fertilization-embryo transfer. Gynecol. Endocrinol. 2014, 30, 359–362. [Google Scholar] [CrossRef] [PubMed]
- Espino, J.; Macedo, M.; Lozano, G.; Ortiz, Á.; Rodríguez, C.; Rodríguez, A.B.; Bejarano, I. Impact of Melatonin Supplementation in Women with Unexplained Infertility Undergoing Fertility Treatment. Antioxidants 2019, 8, 338. [Google Scholar] [CrossRef] [PubMed]
- Ochiai, A.; Kuroda, K.; Ikemoto, Y.; Ozaki, R.; Nakagawa, K.; Nojiri, S.; Takeda, S.; Sugiyama, R. Influence of resveratrol supplementation on IVF-embryo transfer cycle outcomes. Reprod. Biomed. Online 2019, 39, 205–210. [Google Scholar] [CrossRef] [PubMed]
- Crha, I.; Hrubá, D.; Ventruba, P.; Fiala, J.; Totusek, J.; Visnová, H. Ascorbic acid and infertility treatment. Cent. Eur. J. Public Health 2003, 11, 63–67. [Google Scholar]
- Fatemi, F.; Mohammadzadeh, A.; Sadeghi, M.R.; Akhondi, M.M.; Mohammadmoradi, S.; Kamali, K.; Lackpour, N.; Jouhari, S.; Zafadoust, S.; Mokhtar, S.; et al. Role of vitamin E and D(3) supplementation in Intra-Cytoplasmic Sperm Injection outcomes of women with polycystic ovarian syndrome: A double blinded randomized placebo-controlled trial. Clin. Nutr. 2017, 18, 23–30. [Google Scholar] [CrossRef]
- Rizzo, P.; Raffone, E.; Benedetto, V. Effect of the treatment with myo-inositol plus folic acid plus melatonin in comparison with a treatment with myo-inositol plus folic acid on oocyte quality and pregnancy outcome in IVF cycles. A prospective, clinical trial. Eur. Rev. Med. Pharmacol. Sci. 2010, 14, 555–561. [Google Scholar]
- Unfer, V.; Raffone, E.; Rizzo, P.; Buffo, S. Effect of a supplementation with myo-inositol plus melatonin on oocyte quality in women who failed to conceive in previous in vitro fertilization cycles for poor oocyte quality: A prospective, longitudinal, cohort study. Gynecol. Endocrinol. 2011, 27, 857–861. [Google Scholar] [CrossRef]
- Rao, M.; Zhou, F.; Tang, L.; Zeng, Z.; Hu, S.; Wang, Y.; Ke, D.; Cheng, G.; Xia, W.; Zhang, L.; et al. Follicular fluid humanin concentration is related to ovarian reserve markers and clinical pregnancy after IVF-ICSI: A pilot study. Reprod. Biomed. Online 2019, 38, 108–117. [Google Scholar] [CrossRef]
- Wang, Y.; Li, N.; Zeng, Z.; Tang, L.; Zhao, S.; Zhou, F.; Zhou, L.; Xia, W.; Zhu, C.; Rao, M. Humanin regulates oxidative stress in the ovaries of polycystic ovary syndrome patients via the Keap1/Nrf2 pathway. Mol. Hum. Reprod. 2021, 27, gaaa081. [Google Scholar] [CrossRef]
- Terashita, K.; Hashimoto, Y.; Niikura, T.; Tajima, H.; Yamagishi, Y.; Ishizaka, M.; Kawasumi, M.; Chiba, T.; Kanekura, K.; Yamada, M.; et al. Two serine residues distinctly regulate the rescue function of Humanin, an inhibiting factor of Alzheimer’s disease-related neurotoxicity: Functional potentiation by isomerization and dimerization. J. Neurochem. 2003, 85, 1521–1538. [Google Scholar] [CrossRef]
- Huang, J.; Feng, Q.; Zou, L.; Liu, Y.; Bao, M.; Xia, W.; Zhu, C. [Gly14]-humanin exerts a protective effect against D-galactose-induced primary ovarian insufficiency in mice. Reprod. Biomed. Online 2023, 103330. [Google Scholar] [CrossRef]
- Yang, Y.; Shi, L.; Fu, X.; Ma, G.; Yang, Z.; Li, Y.; Zhou, Y.; Yuan, L.; Xia, Y.; Zhong, X.; et al. Metabolic and epigenetic dysfunctions underlie the arrest of in vitro fertilized human embryos in a senescent-like state. PLoS Biol. 2022, 20, e3001682. [Google Scholar] [CrossRef]
- Wang, L.; Chen, Y.; Wei, J.; Guo, F.; Li, L.; Han, Z.; Wang, Z.; Zhu, H.; Zhang, X.; Li, Z.; et al. Administration of nicotinamide mononucleotide improves oocyte quality of obese mice. Cell Prolif. 2022, 55, e13303. [Google Scholar] [CrossRef]
- Miao, Y.; Cui, Z.; Gao, Q.; Rui, R.; Xiong, B. Nicotinamide Mononucleotide Supplementation Reverses the Declining Quality of Maternally Aged Oocytes. Cell Rep. 2020, 32, 107987. [Google Scholar] [CrossRef]
- Bertoldo, M.J.; Listijono, D.R.; Ho, W.J.; Riepsamen, A.H.; Goss, D.M.; Richani, D.; Jin, X.L.; Mahbub, S.; Campbell, J.M.; Habibalahi, A.; et al. NAD(+) Repletion Rescues Female Fertility during Reproductive Aging. Cell Rep. 2020, 30, 1670–1681.e1677. [Google Scholar] [CrossRef]
- Huang, P.; Zhou, Y.; Tang, W.; Ren, C.; Jiang, A.; Wang, X.; Qian, X.; Zhou, Z.; Gong, A. Long-term treatment of Nicotinamide mononucleotide improved age-related diminished ovary reserve through enhancing the mitophagy level of granulosa cells in mice. J. Nutr. Biochem. 2022, 101, 108911. [Google Scholar] [CrossRef]
- Tarín, J.J.; Pérez-Albalá, S.; Pertusa, J.F.; Cano, A. Oral administration of pharmacological doses of vitamins C and E reduces reproductive fitness and impairs the ovarian and uterine functions of female mice. Theriogenology 2002, 57, 1539–1550. [Google Scholar] [CrossRef]
- Bjelakovic, G.; Nikolova, D.; Gluud, L.L.; Simonetti, R.G.; Gluud, C. Antioxidant supplements for prevention of mortality in healthy participants and patients with various diseases. Cochrane Database Syst. Rev. 2012, 2012, Cd007176. [Google Scholar] [CrossRef]
| Species | Protocol | Treatment Outcome |
|---|---|---|
| Mouse | Addition of melatonin at a concentration of 10−6 M in IVM medium | Enhanced cumulus–oocyte complex expansion, increased oocyte maturation rates, and decreased oocyte histone acetylation levels [54]. |
| Microinjection of 10−7 M melatonin | Markedly improved blastocyst rates, increased blastocyst cell numbers, reduced blastocyst apoptosis rates, and enhanced embryo implantation efficiency and neonatal birth rates [40]. | |
| Melatonin at 10 μM for 6 h in culture medium | Increased HAS2 and PGR expression, indicating cumulus expansion and increased fertilization rates in IVF [39]. | |
| Addition of 10−6 M melatonin in IVM medium | Significant improvement in mitochondrial function and blastocyst formation rates. Rescued fetal growth restriction and glucose intolerance, and enhanced energy expenditure in IVF mice [47]. | |
| Pig | Addition of melatonin at 4.3 × 10−8 M in IVM medium | Marked increase in oocyte maturation rates, and a significantly higher proportion of parthenogenetic activation of oocytes developing into blastocysts [41]. |
| Addition of melatonin at 10−9 M in maturation, fertilization, and embryo culture media | While melatonin added to maturation and fertilization media did not increase fertilization rates and blastocyst formation rates of porcine in vitro-fertilized oocytes, its addition to embryo culture medium increased glutathione (GSH) levels, accelerated embryo development, and improved the outcomes and quality of in vitro-produced (IVP) embryos [55]. | |
| Addition of melatonin at 10−9 M in IVM medium | Significant increases in parthenogenetic blastocyst formation rates and total blastocyst cell numbers, increased cumulus expansion, possibly mediated by MT2 receptors [42]. | |
| Sheep | Addition of melatonin at 10−7 M in culture medium | Increased cumulus–oocyte complex expansion, higher cleavage rates, and blastocyst rates in parthenogenetically activated embryos, likely mediated by MT1 receptors [44]. |
| Cow | Addition of melatonin at 10−9 M in IVM medium | Enhanced blastocyst formation rates and embryo quality, with reduced apoptotic cell counts [56]. |
| Addition of melatonin at 10−9 to 10−7 M in GV-stage oocyte IVM medium | Increased oocyte maturation rates, improved embryo development, and significantly increased average cell numbers produced after in vitro fertilization. The involvement of melatonin receptors in mediating this phenomenon has been demonstrated [57]. | |
| Addition of melatonin at 10−7, 10−9, and 10−11 M in IVM medium | Enhanced oocyte fertilization and development capabilities, possibly regulated through improvements in organelle distribution, increased GSH and ATP levels, and enhanced expression of antioxidant genes, thereby promoting cytoplasmic maturation [58]. |
| Antioxidant | Protocol | Experimental Design | Experimental Results |
|---|---|---|---|
| Melatonin | Oral melatonin (2 mg/day) administered for at least 3 weeks before hCG trigger dose | In the no supplementation group, 78 were inseminated by ICSI and 19 by conventional IVF (cIVF). In the melatonin supplementation group, 83 cycles were inseminated by ICSI, and 14 by cIVF. | Increased fertilization rate and blastocyst quality after melatonin treatment [85]. |
| Daily oral administration of 3 mg or 6 mg melatonin from the start of controlled ovarian stimulation to the day of follicle puncture | 40 patients were divided into 4 groups: Group 1 (control n = 10), group 2 (n = 10), women who did not take melatonin; group 3 (n = 10), women who took a daily dose of 3 mg of melatonin; and group 4 (n = 10), women who took a daily dose of 6 mg of melatonin. | Both melatonin doses improved oxidative balance in infertile patients’ follicles and oocyte quality, resulting in increased pregnancy/live birth rates [86]. | |
| 115 patients with low fertilization rates in the previous IVF-ET cycle treated with melatonin (600 mg/day) | 115 patients were divided into two groups: 56 patients with melatonin treatment (3 mg/day) and 59 patients without melatonin treatment. | Significantly increased fertilization rates, with no statistical difference in pregnancy rates compared to the control group [16]. | |
| 150IVF/ICSI patients randomized into placebo, 2 mg/day melatonin, 4 mg/day melatonin, and 8 mg/day melatonin groups | 150 were randomized to receive placebo (n = 36), melatonin 2 mg (n = 38), melatonin 4 mg (n = 36), or melatonin 8 mg (n = 40) | Although melatonin concentrations in follicular fluid significantly increased in the melatonin treatment group, there were no differences in oocyte maturation rates, fertilization rates, embryo quality, or clinical pregnancy rates [53]. | |
| 193 immature oocytes collected from hyperstimulated ovarian cycles, randomly divided into a 10 μmol/L melatonin-treated group and control group | 193 immature oocytes were divided into two groups: 10 μmol/L MT (n = 105, M group) and no MT (n = 88, NM group) | Higher blastocyst formation rate, significantly reduced aneuploidy rate, and successful delivery of three healthy babies in the melatonin group [48]. | |
| Coenzyme Q10 | Randomized pre-treatment with CoQ10 (200 mg, three times daily for 60 days) or no pre-treatment for 10 days before IVF-ICSI cycles in PCOS patients | 76 participants treated with CoQ10 and 93 control participants without any additional treatment. | Coenzyme Q10 pre-treatment group had increased E2 peak levels, significantly higher retrieval of oocytes, fertilization rates, and high-quality embryos, leading to higher clinical pregnancy and live birth rates, although without statistical differences [63]. |
| Addition of 50 mmol/L CoQ10 to IVM culture medium for 24 h | 166 immature human oocytes were obtained (GV stage) from 63 women (45 patients aged ≥ 38 years and 18 patients aged ≤ 30 years) | In older women, CoQ10 treatment significantly improved oocyte maturation rates and reduced oocyte and chromosomal aneuploidy rates. In younger women, there were no significant differences in these parameters [64]. | |
| Resveratrol | Immature oocytes from ICSI patients treated with 1.0 μm resveratrol for 24 and 36 h | A total of 75 GV oocytes from 64 patients >38 years of age were randomly divided into two groups: 1.0 μm resveratrol and DMSO supplemented IVM media group. | Improved oocyte maturation and oocyte quality, with a significant reduction in abnormal oocyte proportions [66]. |
| 800 PCOS patients undergoing ICSI randomized into treatment with daily oral resveratrol (40 mg) from the start of the menstrual cycle until oocyte retrieval | 62 patients were randomly assigned to two groups: took resveratrol 800 mg/day, or placebo for 40 days | Higher rates of high-quality oocytes and high-quality embryos. Possible reductions in serum total testosterone and LH levels, and increased TSH and FSH levels [68]. | |
| Comparison of transplant outcomes between resveratrol-treated group (200 mg/day) and control group in IVF-ET patients | The RES group (204 cycles, 102 women) receiving resveratrol supplementation (200 mg/day) continuously was compared with the control group (7073 cycles, 2958 women) | Reduced clinical pregnancy rates and increased miscarriage risk in the resveratrol treatment group [87]. | |
| Vitamin C | Daily intake of vitamin C (500 mg/day) in women undergoing ART procedures | 76 women (38 of them smokers, 38 non-smokers) were studied. Half the women (19 smokers and 19 non-smokers) were administered vitamin C. The control group consisted of the same number of smokers and non-smokers. | Significantly higher pregnancy rates in women who consumed vitamin C compared to the control group [88]. |
| Vitamin E and Vitamin D3 | Randomized allocation of infertile women planning ICSI into vitamin treatment group (vitamin E, 400 mg/day, vitamin D3, 50,000 IU/one in two weeks) and placebo group | 105 PCOS infertile women scheduled for ICSI were enrolled to treatment group(n = 52) or placebo group (n = 53) for 8 weeks. | Increased implantation and clinical pregnancy rates in the vitamin treatment group [89]. |
| Melatonin + Inositol + Folate | Starting from the day of GnRH administration, one group received 3 g melatonin + 4 g inositol + 200 mg folate, while the other received inositol with folate | 65 women undergoing IVF cycles were randomized into two groups to receive myo-inositol plus folic acid plus melatonin (32 women, group A), and myo-inositol plus folic acid (33 women, group B) | The melatonin combination treatment group showed significantly increased oocyte maturation rates, higher clinical pregnancy rates, and implantation rates, though without statistical significance [90]. |
| Three months of treatment with 3 mg melatonin + 4 g inositol + 4 g folate in women who failed to conceive in IVF cycles due to poor oocyte quality | All 46 women were treated with myo-inositol and melatonin for 3 months. Results were compared to the previous IVF cycle. | Significant increases in oocyte maturation rates, fertilization rates, total number of transferred embryos, and embryo quality [91]. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Y.; Yang, J.; Zhang, L. The Impact of Follicular Fluid Oxidative Stress Levels on the Outcomes of Assisted Reproductive Therapy. Antioxidants 2023, 12, 2117. https://doi.org/10.3390/antiox12122117
Chen Y, Yang J, Zhang L. The Impact of Follicular Fluid Oxidative Stress Levels on the Outcomes of Assisted Reproductive Therapy. Antioxidants. 2023; 12(12):2117. https://doi.org/10.3390/antiox12122117
Chicago/Turabian StyleChen, Yu, Jiahao Yang, and Ling Zhang. 2023. "The Impact of Follicular Fluid Oxidative Stress Levels on the Outcomes of Assisted Reproductive Therapy" Antioxidants 12, no. 12: 2117. https://doi.org/10.3390/antiox12122117
APA StyleChen, Y., Yang, J., & Zhang, L. (2023). The Impact of Follicular Fluid Oxidative Stress Levels on the Outcomes of Assisted Reproductive Therapy. Antioxidants, 12(12), 2117. https://doi.org/10.3390/antiox12122117