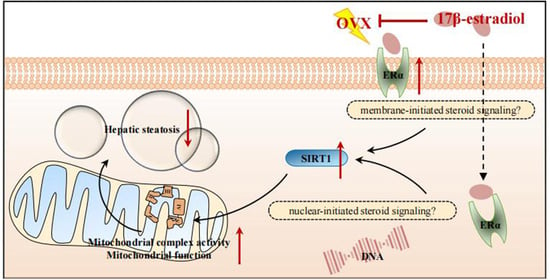
17β-Estradiol (E2) Upregulates the ERα/SIRT1/PGC-1α Signaling Pathway and Protects Mitochondrial Function to Prevent Bilateral Oophorectomy (OVX)-Induced Nonalcoholic Fatty Liver Disease (NAFLD)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animal and Study Design
2.2. Cytological Examination of Vaginal Exfoliation
2.3. Histopathological Analysis
2.4. Immunohistochemistry (Paraffin) (IHC-P)
2.5. Blood Biochemistry
2.6. Transmission Electron Microscopy (TEM)
2.7. Transcriptome Analysis
2.8. Untargeted Metabolomics
2.9. Western Blot (WB)
2.10. Cell Lines and Treatment
2.11. Measurement of Mitochondrial Complex Activity
2.12. Lipid Peroxidation Assay
2.13. Statistical Analysis
3. Results
3.1. E2 Supplementation Alleviated OVX-Induced NAFLD in Female Rats
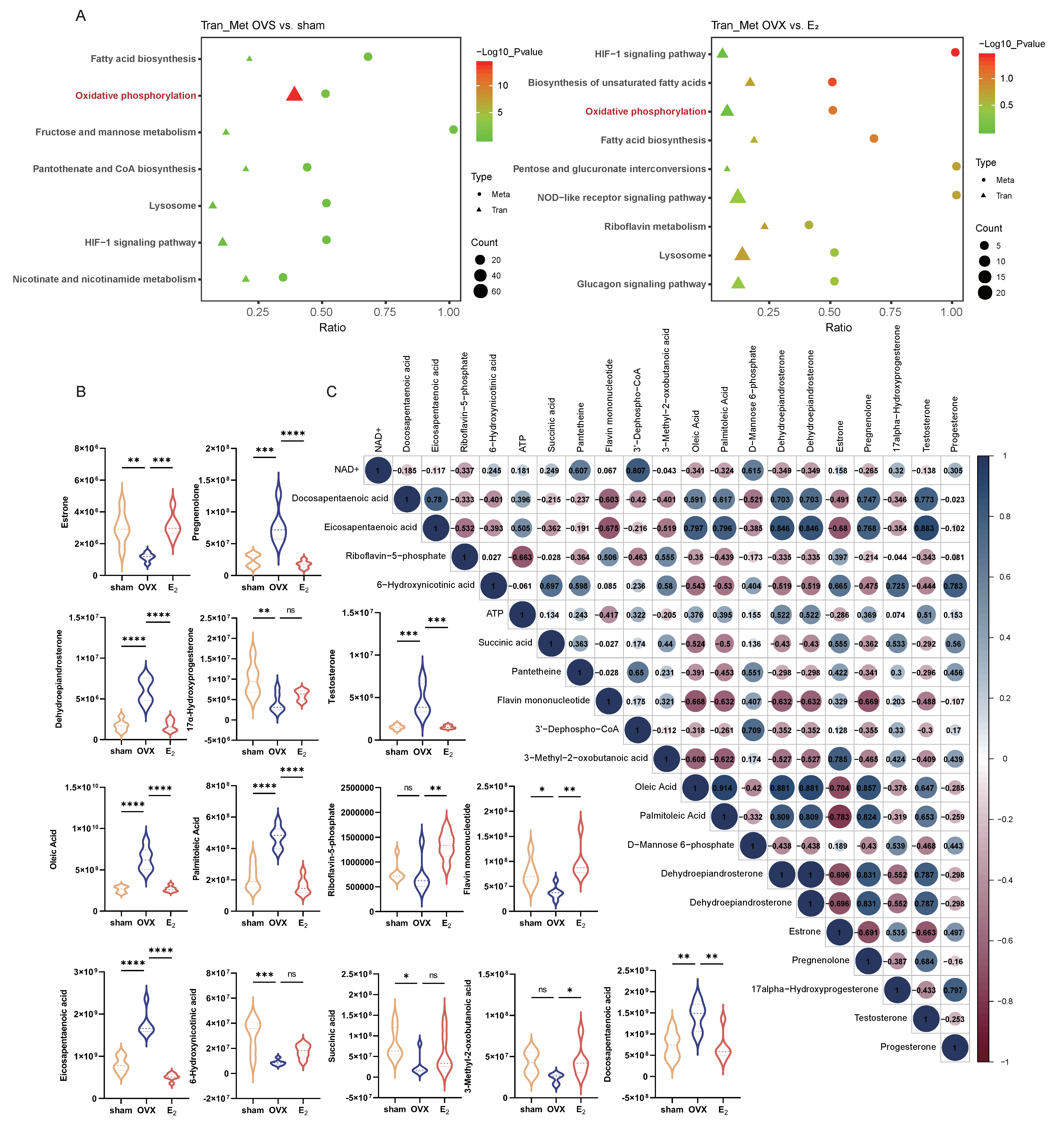
3.2. E2 Supplementation Maintained Some of the Pathways in Liver Tissue That Were Altered by OVX
3.3. The Combination of Transcriptomic and Metabolomic Analyses Revealed Enrichment of Oxidative Phosphorylation as a Key Pathway That Was Altered in the OVX-Induced NAFLD and E2 Supplementation Groups
3.4. E2 Supplementation Upregulated the ERα/SIRT1 Pathway and Alleviated OVX-Induced NAFLD
3.5. E2 Treatment Alleviated LA-Induced Hepatic Steatosis by Upregulating the ERα/SIRT1 Pathway
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wai-Sun Wong, V.; Ekstedt, M.; Wong, G.L.-H.; Hagström, H. Changing epidemiology, global trends and implications for outcomes of NAFLD. J. Hepatol. 2023, 79, 842–852. [Google Scholar] [CrossRef]
- Xu, X.; Poulsen, K.L.; Wu, L.; Liu, S.; Miyata, T.; Song, Q.; Wei, Q.; Zhao, C.; Lin, C.; Yang, J. Targeted therapeutics and novel signaling pathways in non-alcohol-associated fatty liver/steatohepatitis (NAFL/NASH). Signal Transduct. Target. Ther. 2022, 7, 287. [Google Scholar] [CrossRef]
- Buzzetti, E.; Pinzani, M.; Tsochatzis, E.A. The multiple-hit pathogenesis of non-alcoholic fatty liver disease (NAFLD). Metabolism 2016, 65, 1038–1048. [Google Scholar] [CrossRef] [PubMed]
- Koliaki, C.; Szendroedi, J.; Kaul, K.; Jelenik, T.; Nowotny, P.; Jankowiak, F.; Herder, C.; Carstensen, M.; Krausch, M.; Knoefel, W.T.; et al. Adaptation of hepatic mitochondrial function in humans with non-alcoholic fatty liver is lost in steatohepatitis. Cell Metab. 2015, 21, 739–746. [Google Scholar] [CrossRef] [PubMed]
- Berson, A.; De Beco, V.; Lettéron, P.; Robin, M.A.; Moreau, C.; El Kahwaji, J.; Verthier, N.; Feldmann, G.; Fromenty, B.; Pessayre, D. Steatohepatitis-inducing drugs cause mitochondrial dysfunction and lipid peroxidation in rat hepatocytes. Gastroenterology 1998, 114, 764–774. [Google Scholar] [CrossRef] [PubMed]
- Nassir, F.; Ibdah, J.A. Role of mitochondria in nonalcoholic fatty liver disease. Int. J. Mol. Sci. 2014, 15, 8713–8742. [Google Scholar] [CrossRef] [PubMed]
- Klinge, C.M. Estrogenic control of mitochondrial function. Redox Biol. 2020, 31, 101435. [Google Scholar] [CrossRef] [PubMed]
- Pafili, K.; Paschou, S.A.; Armeni, E.; Polyzos, S.A.; Goulis, D.G.; Lambrinoudaki, I. Non-alcoholic fatty liver disease through the female lifespan: The role of sex hormones. J. Endocrinol. Investig. 2022, 45, 1609–1623. [Google Scholar] [CrossRef]
- Ryu, S.; Chang, Y.; Choi, Y.; Kwon, M.-J.; Kim, C.-W.; Yun, K.E.; Jung, H.-S.; Kim, B.-K.; Kim, Y.J.; Ahn, J.; et al. Age at menarche and non-alcoholic fatty liver disease. J. Hepatol. 2015, 62, 1164–1170. [Google Scholar] [CrossRef]
- Lu, J.; Zhang, J.; Du, R.; Wang, T.; Xu, M.; Xu, Y.; Wang, W.; Bi, Y.; Li, D.; Chen, Y.; et al. Age at menarche is associated with the prevalence of non-alcoholic fatty liver disease later in life. J. Diabetes 2017, 9, 53–60. [Google Scholar] [CrossRef]
- Wang, J.; Wu, A.H.; Stanczyk, F.Z.; Porcel, J.; Noureddin, M.; Terrault, N.A.; Wilkens, L.R.; Setiawan, V.W. Associations Between Reproductive and Hormone-Related Factors and Risk of Nonalcoholic Fatty Liver Disease in a Multiethnic Population. Clin. Gastroenterol. Hepatol. 2021, 19, 1258–1266.e1. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.D.; Abdelmalek, M.F.; Pang, H.; Guy, C.D.; Smith, A.D.; Diehl, A.M.; Suzuki, A. Gender and menopause impact severity of fibrosis among patients with nonalcoholic steatohepatitis. Hepatology 2014, 59, 1406–1414. [Google Scholar] [CrossRef] [PubMed]
- Klair, J.S.; Yang, J.D.; Abdelmalek, M.F.; Guy, C.D.; Gill, R.M.; Yates, K.; Unalp-Arida, A.; Lavine, J.E.; Clark, J.M.; Diehl, A.M.; et al. A longer duration of estrogen deficiency increases fibrosis risk among postmenopausal women with nonalcoholic fatty liver disease. Hepatology 2016, 64, 85–91. [Google Scholar] [CrossRef] [PubMed]
- Mahboobifard, F.; Pourgholami, M.H.; Jorjani, M.; Dargahi, L.; Amiri, M.; Sadeghi, S.; Tehrani, F.R. Estrogen as a key regulator of energy homeostasis and metabolic health. Biomed. Pharmacother. 2022, 156, 113808. [Google Scholar] [CrossRef] [PubMed]
- Borrás, C.; Sastre, J.; García-Sala, D.; Lloret, A.; Pallardó, F.V.; Viña, J. Mitochondria from females exhibit higher antioxidant gene expression and lower oxidative damage than males. Free Radic. Biol. Med. 2003, 34, 546–552. [Google Scholar] [CrossRef] [PubMed]
- Borrás, C.; Ferrando, M.; Inglés, M.; Gambini, J.; Lopez-Grueso, R.; Edo, R.; Mas-Bargues, C.; Pellicer, A.; Viña, J. Estrogen Replacement Therapy Induces Antioxidant and Longevity-Related Genes in Women after Medically Induced Menopause. Oxid. Med. Cell. Longev. 2021, 2021, 8101615. [Google Scholar] [CrossRef]
- Beikoghli Kalkhoran, S.; Kararigas, G. Oestrogenic Regulation of Mitochondrial Dynamics. Int. J. Mol. Sci. 2022, 23, 1118. [Google Scholar] [CrossRef]
- Austad, S.N.; Ballinger, S.; Buford, T.W.; Carter, C.S.; Smith, D.L.; Darley-Usmar, V.; Zhang, J. Targeting whole body metabolism and mitochondrial bioenergetics in the drug development for Alzheimer’s disease. Acta Pharm. Sin. B 2022, 12, 511–531. [Google Scholar] [CrossRef]
- Yoh, K.; Ikeda, K.; Horie, K.; Inoue, S. Roles of Estrogen, Estrogen Receptors, and Estrogen-Related Receptors in Skeletal Muscle: Regulation of Mitochondrial Function. Int. J. Mol. Sci. 2023, 24, 1853. [Google Scholar] [CrossRef]
- Ling, W.; Krager, K.; Richardson, K.K.; Warren, A.D.; Ponte, F.; Aykin-Burns, N.; Manolagas, S.C.; Almeida, M.; Kim, H.-N. Mitochondrial Sirt3 contributes to the bone loss caused by aging or estrogen deficiency. JCI Insight 2021, 6, e146728. [Google Scholar] [CrossRef]
- McCoin, C.S.; Von Schulze, A.; Allen, J.; Fuller, K.N.Z.; Xia, Q.; Koestler, D.C.; Houchen, C.J.; Maurer, A.; Dorn, G.W.; Shankar, K.; et al. Sex modulates hepatic mitochondrial adaptations to high-fat diet and physical activity. Am. J. Physiol. Endocrinol. Metab. 2019, 317, E298–E311. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.; Ding, Y.; Li, X.; Pu, R.; Liu, W.; Li, P.; Yin, J. Association of ESR1 gene polymorphisms with the susceptibility to Hepatitis B virus infection and the clinical outcomes. J. Med. Virol. 2023, 95, e28510. [Google Scholar] [CrossRef] [PubMed]
- Jeon, Y.; Yoo, J.E.; Rhee, H.; Kim, Y.-J.; Kim, G.I.; Chung, T.; Yoon, S.; Shin, B.; Woo, H.G.; Park, Y.N. YAP inactivation in estrogen receptor alpha-positive hepatocellular carcinoma with less aggressive behavior. Exp. Mol. Med. 2021, 53, 1055–1067. [Google Scholar] [CrossRef] [PubMed]
- Della Torre, S.; Benedusi, V.; Pepe, G.; Meda, C.; Rizzi, N.; Uhlenhaut, N.H.; Maggi, A. Dietary essential amino acids restore liver metabolism in ovariectomized mice via hepatic estrogen receptor α. Nat. Commun. 2021, 12, 6883. [Google Scholar] [CrossRef]
- Pedram, A.; Razandi, M.; O’mahony, F.; Harvey, H.; Harvey, B.J.; Levin, E.R. Estrogen reduces lipid content in the liver exclusively from membrane receptor signaling. Sci. Signal. 2013, 6, ra36. [Google Scholar] [CrossRef] [PubMed]
- Meda, C.; Barone, M.; Mitro, N.; Lolli, F.; Pedretti, S.; Caruso, D.; Maggi, A.; Della Torre, S. Hepatic ERα accounts for sex differences in the ability to cope with an excess of dietary lipids. Mol. Metab. 2020, 32, 97–108. [Google Scholar] [CrossRef] [PubMed]
- Strom, J.O.; Theodorsson, A.; Ingberg, E.; Isaksson, I.-M.; Theodorsson, E. Ovariectomy and 17beta-estradiol replacement in rats and mice: A visual demonstration. J. Vis. Exp. 2012, 64, e4013. [Google Scholar] [CrossRef]
- Tian, Y.; Xie, Y.; Guo, Z.; Feng, P.; You, Y.; Yu, Q. 17β-oestradiol inhibits ferroptosis in the hippocampus by upregulating DHODH and further improves memory decline after ovariectomy. Redox Biol. 2023, 62, 102708. [Google Scholar] [CrossRef]
- Zhang, T.; Wang, Y.; Wang, L. Comparison of two staining methods for rat vaginal smears. Chin. J. Comp. Med. 2018, 28, 98–101. [Google Scholar] [CrossRef]
- Nogueiras, R.; Habegger, K.M.; Chaudhary, N.; Finan, B.; Banks, A.S.; Dietrich, M.O.; Horvath, T.L.; Sinclair, D.A.; Pfluger, P.T.; Tschöop, M.H. Sirtuin 1 and sirtuin 3: Physiological modulators of metabolism. Physiol. Rev. 2012, 92, 1479–1514. [Google Scholar] [CrossRef]
- Zheng, Q.; Liu, H.; Zhang, H.; Han, Y.; Yuan, J.; Wang, T.; Gao, Y.; Li, Z. Ameliorating Mitochondrial Dysfunction of Neurons by Biomimetic Targeting Nanoparticles Mediated Mitochondrial Biogenesis to Boost the Therapy of Parkinson’s Disease. Adv. Sci. 2023, 10, e2300758. [Google Scholar] [CrossRef]
- Ye, F.; Wu, L.; Li, H.; Peng, X.; Xu, Y.; Li, W.; Wei, Y.; Chen, F.; Zhang, J.; Liu, Q. SIRT1/PGC-1α is involved in arsenic-induced male reproductive damage through mitochondrial dysfunction, which is blocked by the antioxidative effect of zinc. Environ. Pollut. 2023, 320, 121084. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Ling, L.; Lu, J.; Jiang, F.; Sun, J.; Zhang, Z.; Huang, Y.; Liu, X.; Zhu, Y.; Fu, X.; et al. Reactive oxygen species-responsive mitochondria-targeted liposomal quercetin attenuates retinal ischemia-reperfusion injury via regulating SIRT1/FOXO3A and p38 MAPK signaling pathways. Bioeng. Transl. Med. 2023, 8, e10460. [Google Scholar] [CrossRef]
- McGarry, J.D.; Mannaerts, G.P.; Foster, D.W. A possible role for malonyl-CoA in the regulation of hepatic fatty acid oxidation and ketogenesis. J. Clin. Investig. 1977, 60, 265–270. [Google Scholar] [CrossRef]
- Lambert, J.E.; Ramos-Roman, M.A.; Browning, J.D.; Parks, E.J. Increased de novo lipogenesis is a distinct characteristic of individuals with nonalcoholic fatty liver disease. Gastroenterology 2014, 146, 726–735. [Google Scholar] [CrossRef]
- Lobo, R.A.; Gompel, A. Management of menopause: A view towards prevention. Lancet Diabetes Endocrinol. 2022, 10, 457–470. [Google Scholar] [CrossRef]
- Robeva, R.; Mladenović, D.; Vesković, M.; Hrnčić, D.; Bjekić-Macut, J.; Stanojlović, O.; Livadas, S.; Yildiz, B.O.; Macut, D. The interplay between metabolic dysregulations and non-alcoholic fatty liver disease in women after menopause. Maturitas 2021, 151, 22–30. [Google Scholar] [CrossRef]
- Galmés-Pascual, B.M.; Martínez-Cignoni, M.R.; Morán-Costoya, A.; Bauza-Thorbrügge, M.; Sbert-Roig, M.; Valle, A.; Proenza, A.M.; Lladó, I.; Gianotti, M. 17β-estradiol ameliorates lipotoxicity-induced hepatic mitochondrial oxidative stress and insulin resistance. Free Radic. Biol. Med. 2020, 150, 148–160. [Google Scholar] [CrossRef]
- Galmés-Pascual, B.M.; Nadal-Casellas, A.; Bauza-Thorbrügge, M.; Sbert-Roig, M.; García-Palmer, F.J.; Proenza, A.M.; Gianotti, M.; Lladó, I. 17β-estradiol improves hepatic mitochondrial biogenesis and function through PGC1B. J. Endocrinol. 2017, 232, 297–308. [Google Scholar] [CrossRef]
- Besse-Patin, A.; Léveillé, M.; Oropeza, D.; Nguyen, B.N.; Prat, A.; Estall, J.L. Estrogen Signals Through Peroxisome Proliferator-Activated Receptor-γ Coactivator 1α to Reduce Oxidative Damage Associated with Diet-Induced Fatty Liver Disease. Gastroenterology 2017, 152, 243–256. [Google Scholar] [CrossRef]
- Zuo, Q.; Chen, K.L.; Eve, A.A.; Liu, Y.-J.; Kim, S.H.; Katzenellenbogen, B.S.; Katzenellenbogen, J.A.; Madak-Erdogan, Z. Pathway Preferential Estrogens Prevent Hepatosteatosis Due to Ovariectomy and High-Fat Diets. Nutrients 2021, 13, 3334. [Google Scholar] [CrossRef]
- Chen, C.Y.; Li, Y.; Zeng, N.; He, L.; Zhang, X.; Tu, T.; Tang, Q.; Alba, M.; Mir, S.; Stiles, E.X.; et al. Inhibition of Estrogen-Related Receptor alpha Blocks Liver Steatosis and Steatohepatitis and Attenuates Triglyceride Biosynthesis. Am. J. Pathol. 2021, 191, 1240–1254. [Google Scholar] [CrossRef]
- Yang, M.; Liu, Q.; Huang, T.; Tan, W.; Qu, L.; Chen, T.; Pan, H.; Chen, L.; Liu, J.; Wong, C.-W.; et al. Dysfunction of estrogen-related receptor alpha-dependent hepatic VLDL secretion contributes to sex disparity in NAFLD/NASH development. Theranostics 2020, 10, 10874–10891. [Google Scholar] [CrossRef]
- Hart-Unger, S.; Arao, Y.; Hamilton, K.J.; Lierz, S.; Malarkey, D.E.; Hewitt, S.; Freemark, M.; Korach, K.S. Hormone signaling and fatty liver in females: Analysis of estrogen receptor α mutant mice. Int. J. Obes. 2017, 41, 945–954. [Google Scholar] [CrossRef]
- Zeng, C.; Chen, M. Progress in Nonalcoholic Fatty Liver Disease: SIRT Family Regulates Mitochondrial Biogenesis. Biomolecules 2022, 12, 1079. [Google Scholar] [CrossRef]
- Long, J.K.; Dai, W.; Zheng, Y.-W.; Zhao, S.-P. miR-122 promotes hepatic lipogenesis via inhibiting the LKB1/AMPK pathway by targeting Sirt1 in non-alcoholic fatty liver disease. Mol. Med. 2019, 25, 26. [Google Scholar] [CrossRef]
- Ren, H.; Ren, H.; Hu, F.; Hu, F.; Wang, D.; Wang, D.; Kang, X.; Kang, X.; Feng, X.; Feng, X.; et al. Sirtuin 2 Prevents Liver Steatosis and Metabolic Disorders by Deacetylation of Hepatocyte Nuclear Factor 4α. Hepatology 2021, 74, 723–740. [Google Scholar] [CrossRef]
- Li, R.; Xin, T.; Li, D.; Wang, C.; Zhu, H.; Zhou, H. Therapeutic effect of Sirtuin 3 on ameliorating nonalcoholic fatty liver disease: The role of the ERK-CREB pathway and Bnip3-mediated mitophagy. Redox Biol. 2018, 18, 229–243. [Google Scholar] [CrossRef]
- Luo, P.; Qin, C.; Zhu, L.; Fang, C.; Zhang, Y.; Zhang, H.; Pei, F.; Tian, S.; Zhu, X.; Gong, J.; et al. Ubiquitin-Specific Peptidase 10 (USP10) Inhibits Hepatic Steatosis, Insulin Resistance, and Inflammation through Sirt6. Hepatology 2018, 68, 1786–1803. [Google Scholar] [CrossRef]
- Ryu, J.; Kim, E.; Kang, M.; Song, D.; Shin, E.; Lee, H.; Jung, J.W.; Nam, S.H.; Kim, J.E.; Kim, H.; et al. Differential TM4SF5-mediated SIRT1 modulation and metabolic signaling in nonalcoholic steatohepatitis progression. J. Pathol. 2021, 253, 55–67. [Google Scholar] [CrossRef]
- Liou, C.J.; Wu, S.-J.; Shen, S.-C.; Chen, L.-C.; Chen, Y.-L.; Huang, W.-C. Phloretin ameliorates hepatic steatosis through regulation of lipogenesis and Sirt1/AMPK signaling in obese mice. Cell Biosci. 2020, 10, 114. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Li, J.; Zhang, M.; Yang, W.; Qin, W.; Zheng, Q.; Chu, Y.; Wu, Y.; Wu, D.; Yuan, X. 11β-HSD1 Inhibitor Alleviates Non-Alcoholic Fatty Liver Disease by Activating the AMPK/SIRT1 Signaling Pathway. Nutrients 2022, 14, 2358. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Anand, S.K.; Singh, N.; Dwarkanath, A.; Dwivedi, U.N.; Kakkar, P. Berbamine induced activation of the SIRT1/LKB1/AMPK signaling axis attenuates the development of hepatic steatosis in high-fat diet-induced NAFLD rats. Food Funct. 2021, 12, 892–909. [Google Scholar] [CrossRef] [PubMed]
- Tsai, H.Y.; Shih, Y.-Y.; Yeh, Y.-T.; Huang, C.-H.; Liao, C.-A.; Hu, C.-Y.; Nagabhushanam, K.; Ho, C.-T.; Chen, Y.-K. Pterostilbene and Its Derivative 3′-Hydroxypterostilbene Ameliorated Nonalcoholic Fatty Liver Disease Through Synergistic Modulation of the Gut Microbiota and SIRT1/AMPK Signaling Pathway. J. Agric. Food Chem. 2022, 70, 4966–4980. [Google Scholar] [CrossRef] [PubMed]
- Gui, W.; Zhu, Y.; Sun, S.; Zhu, W.; Tan, B.; Zhao, H.; Shang, C.; Zheng, F.; Lin, X.; Li, H. Knockdown of insulin-like growth factor 2 gene disrupts mitochondrial functions in the liver. J. Mol. Cell Biol. 2021, 13, 543–555. [Google Scholar] [CrossRef] [PubMed]
- Armeni, E.; Paschou, S.A.; Goulis, D.G.; Lambrinoudaki, I. Hormone therapy regimens for managing the menopause and premature ovarian insufficiency. Best Pract. Res. Clin. Endocrinol. Metab. 2021, 35, 101561. [Google Scholar] [CrossRef]
- López-Grueso, R.; Gambini, J.; Abdelaziz, K.M.; Monleón, D.; Díaz, A.; El Alami, M.; Bonet-Costa, V.; Borrás, C.; Viña, J. Early, but not late onset estrogen replacement therapy prevents oxidative stress and metabolic alterations caused by ovariectomy. Antioxid. Redox Signal. 2014, 20, 236–246. [Google Scholar] [CrossRef]
- North American Menopause Society. The 2017 hormone therapy position statement of The North American Menopause Society. Menopause 2018, 25, 1362–1387. [Google Scholar] [CrossRef]
- Jin, K.; Shi, Y.; Zhang, H.; Zhangyuan, G.; Wang, F.; Li, S.; Chen, C.; Zhang, J.; Wang, H.; Zhang, W.; et al. A TNFα/Miz1-positive feedback loop inhibits mitophagy in hepatocytes and propagates nonalcoholic steatohepatitis. J. Hepatol. 2023, 79, 403–416. [Google Scholar] [CrossRef]
- Fan, L.; Gokaltun, A.; Maggipinto, S.; Kitagawa, Y.; Martyn, J.; Yeh, H.; Uygun, B.E.; Yarmush, M.L.; Usta, O.B. Alterations in Cytoskeleton and Mitochondria in the Development and Reversal of Steatosis in Human Hepatocytes. Cell. Mol. Gastroenterol. Hepatol. 2023, 16, 243–261. [Google Scholar] [CrossRef]
- Lonardo, A.; Nascimbeni, F.; Ballestri, S.; Fairweather, D.; Win, S.; Than, T.A.; Abdelmalek, M.F.; Suzuki, A. Sex Differences in Nonalcoholic Fatty Liver Disease: State of the Art and Identification of Research Gaps. Hepatology 2019, 70, 1457–1469. [Google Scholar] [CrossRef] [PubMed]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tian, Y.; Hong, X.; Xie, Y.; Guo, Z.; Yu, Q. 17β-Estradiol (E2) Upregulates the ERα/SIRT1/PGC-1α Signaling Pathway and Protects Mitochondrial Function to Prevent Bilateral Oophorectomy (OVX)-Induced Nonalcoholic Fatty Liver Disease (NAFLD). Antioxidants 2023, 12, 2100. https://doi.org/10.3390/antiox12122100
Tian Y, Hong X, Xie Y, Guo Z, Yu Q. 17β-Estradiol (E2) Upregulates the ERα/SIRT1/PGC-1α Signaling Pathway and Protects Mitochondrial Function to Prevent Bilateral Oophorectomy (OVX)-Induced Nonalcoholic Fatty Liver Disease (NAFLD). Antioxidants. 2023; 12(12):2100. https://doi.org/10.3390/antiox12122100
Chicago/Turabian StyleTian, Ying, Xinyu Hong, Yuan Xie, Zaixin Guo, and Qi Yu. 2023. "17β-Estradiol (E2) Upregulates the ERα/SIRT1/PGC-1α Signaling Pathway and Protects Mitochondrial Function to Prevent Bilateral Oophorectomy (OVX)-Induced Nonalcoholic Fatty Liver Disease (NAFLD)" Antioxidants 12, no. 12: 2100. https://doi.org/10.3390/antiox12122100
APA StyleTian, Y., Hong, X., Xie, Y., Guo, Z., & Yu, Q. (2023). 17β-Estradiol (E2) Upregulates the ERα/SIRT1/PGC-1α Signaling Pathway and Protects Mitochondrial Function to Prevent Bilateral Oophorectomy (OVX)-Induced Nonalcoholic Fatty Liver Disease (NAFLD). Antioxidants, 12(12), 2100. https://doi.org/10.3390/antiox12122100