Effects of Low-Fish-Meal Diet Supplemented with Coenzyme Q10 on Growth Performance, Antioxidant Capacity, Intestinal Morphology, Immunity and Hypoxic Resistance of Litopenaeus vannamei
Abstract
:1. Introduction
2. Material and Methods
2.1. Ethics Approval
2.2. Diets Design and Preparation
2.3. Rearing Conditions and Sampling
2.4. Acute Hypoxic Stress Experiment
2.5. Analysis of the Whole Body Proximate Composition
2.6. Intestinal Morphology Analysis
2.7. Antioxidant Capacity Examination
2.8. Quantitative Real-Time PCR Analysis
2.9. Calculations and Statistical Analysis
3. Results
3.1. Growth Performance and Feed Utilization
3.2. Whole-Body Proximate Composition
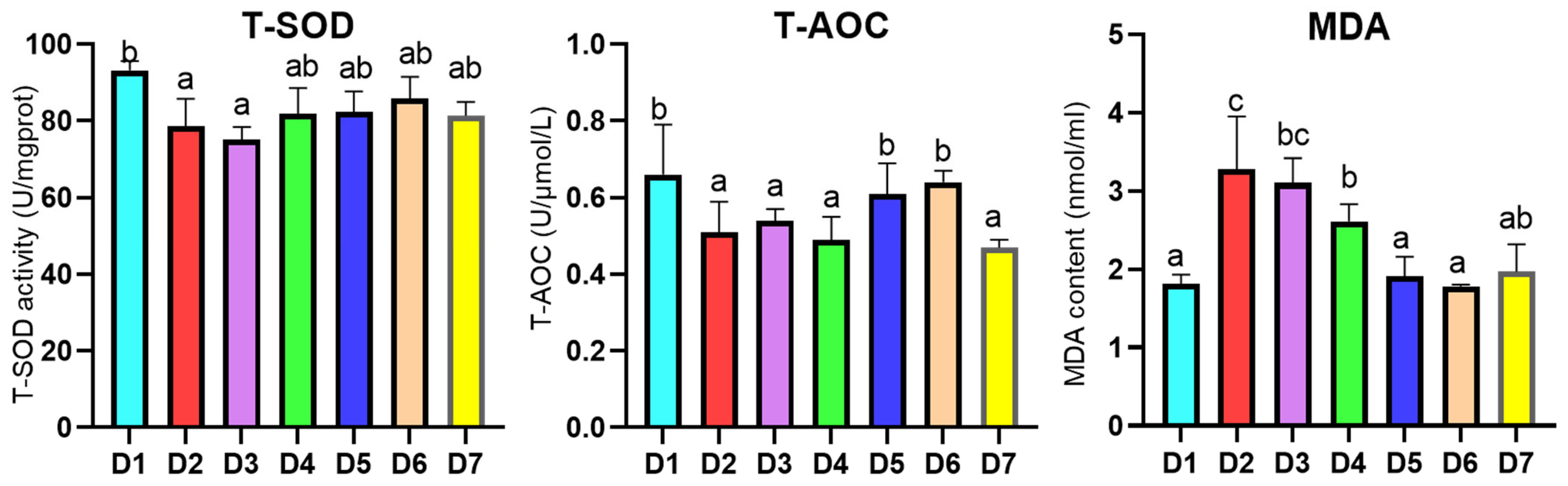
3.3. Hepatopancreatic Antioxidant Enzyme Analysis
3.4. Relative Expression of Antioxidant and Immune Genes in Hepatopancreas
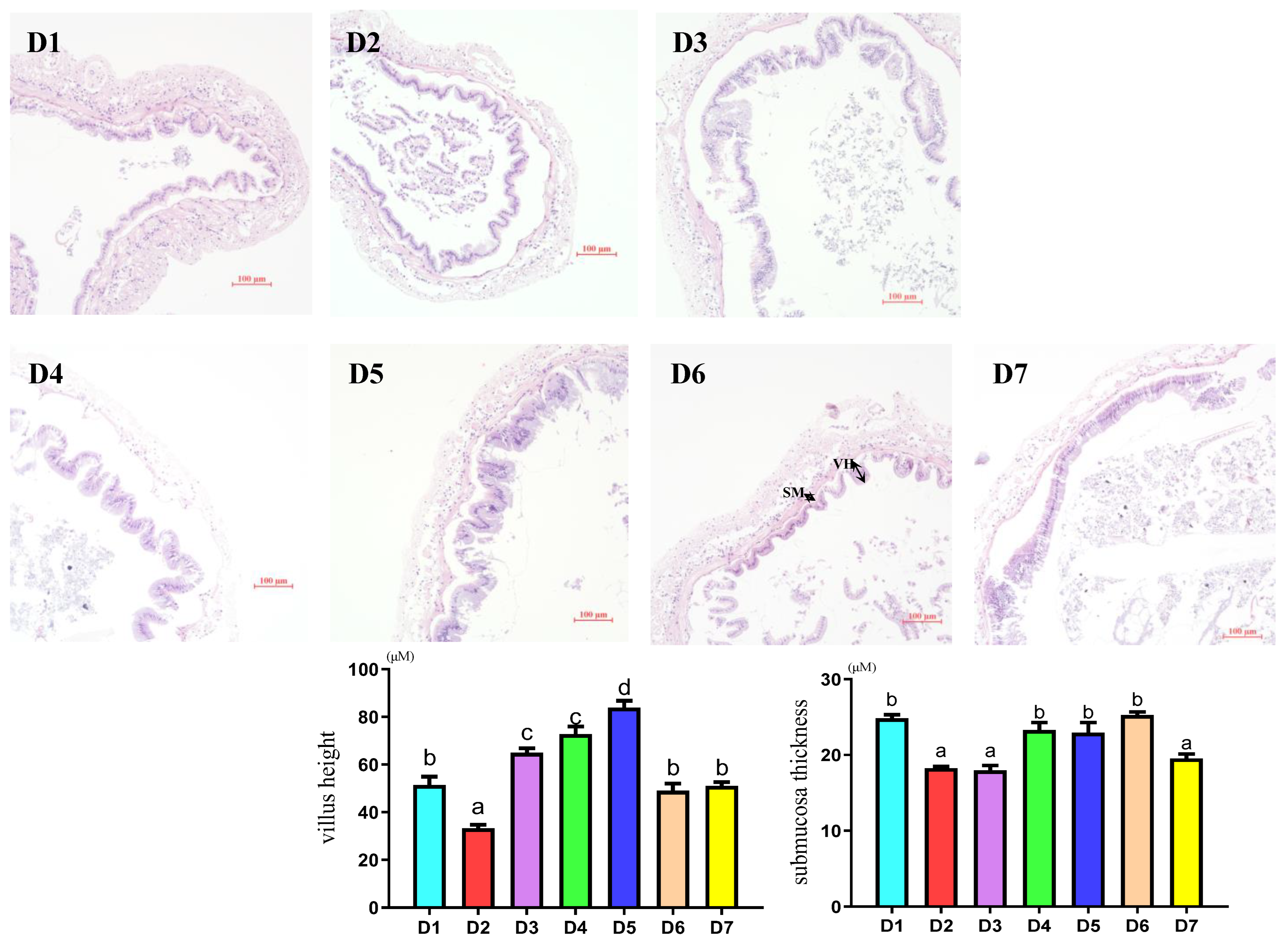
3.5. Intestinal Morphology Measurement
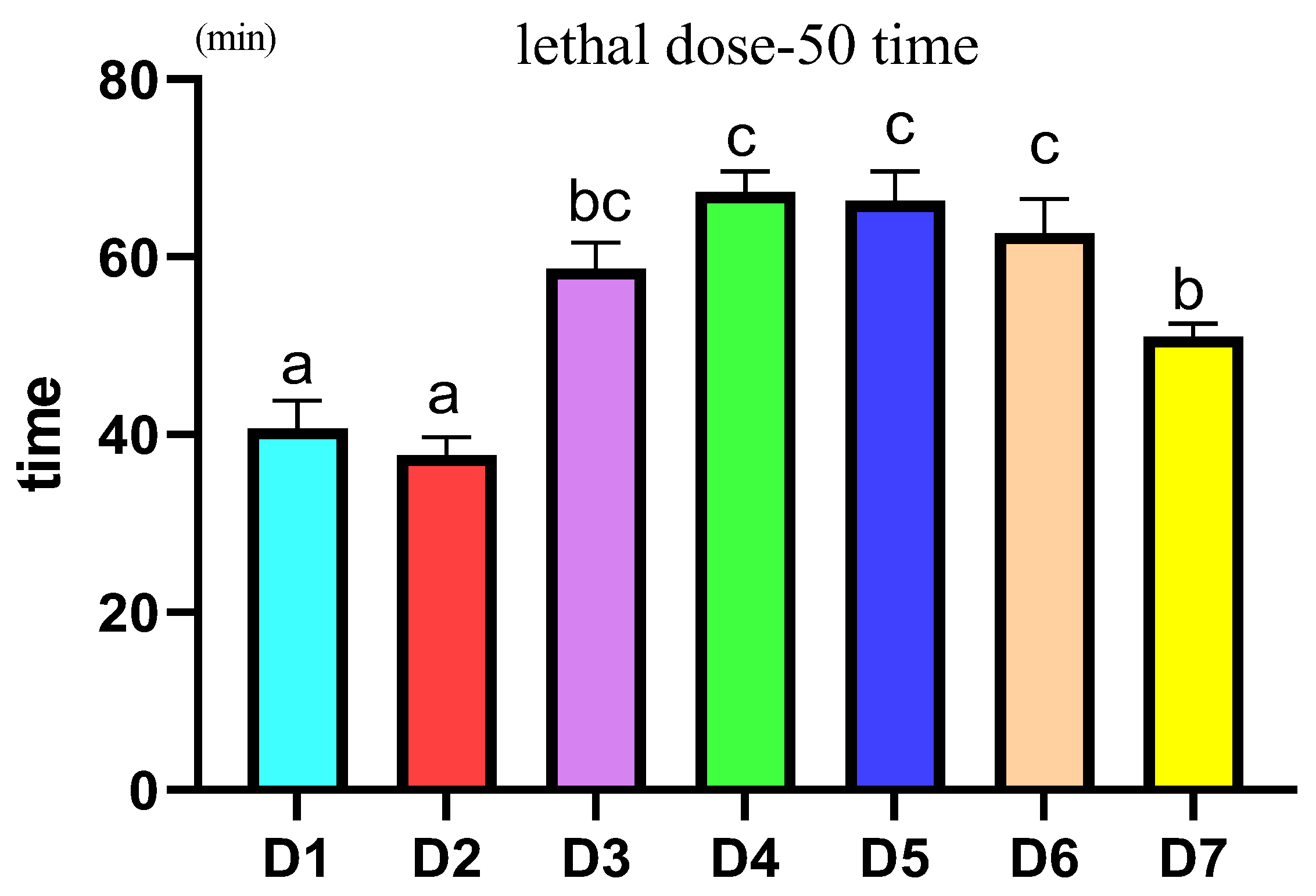
3.6. Acute Hypoxia Stress
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fisheries Administration Bureau. 2022 China Fishery Statistical Yearbook; Fisheries Administration Bureau: Beijing, China, 2022. [Google Scholar]
- FAO. The State of World Fisheries and Aquaculture 2020. Sustainability in Action; FAO: Rome, Italy, 2020. [Google Scholar] [CrossRef]
- Tacon, A.G.J.; Metian, M. Global Overview on the Use of Fish Meal and Fish Oil in Industrially Compounded Aquafeeds: Trends and Future Prospects. Aquaculture 2008, 285, 146–158. [Google Scholar] [CrossRef]
- Olsen, R.L.; Hasan, M.R. A Limited Supply of Fishmeal: Impact on Future Increases in Global Aquaculture Production. Trends Food Sci. Technol. 2012, 27, 120–128. [Google Scholar] [CrossRef]
- Zhang, C.; Rahimnejad, S.; Wang, Y.R.; Lu, K.; Song, K.; Wang, L.; Mai, K. Substituting Fish Meal with Soybean Meal in Diets for Japanese Seabass (Lateolabrax japonicus): Effects on Growth, Digestive Enzymes Activity, Gut Histology, and Expression of Gut Inflammatory and Transporter Genes. Aquaculture 2018, 483, 173–182. [Google Scholar] [CrossRef]
- Suárez, J.A.; Gaxiola, G.; Mendoza, R.; Cadavid, S.; Garcia, G.; Alanis, G.; Suárez, A.; Faillace, J.; Cuzon, G. Substitution of Fish Meal with Plant Protein Sources and Energy Budget for White Shrimp Litopenaeus vannamei (Boone, 1931). Aquaculture 2009, 289, 118–123. [Google Scholar] [CrossRef]
- Erisman, B.E.; Bolser, D.G.; Ilich, A.; Frasier, K.E.; Glaspie, C.N.; Moreno, P.T.; Dell’Apa, A.; de Mutsert, K.; Yassin, M.S.; Nepal, S.; et al. A Meta-Analytical Review of the Effects of Environmental and Ecological Drivers on the Abundance of Red Snapper (Lutjanus Campechanus) in the U.S. Gulf of Mexico. Rev. Fish Biol. Fish. 2020, 30, 437–462. [Google Scholar] [CrossRef]
- Tacon, A.G.J.; Metian, M.; Hasan, M.R. Feed Ingredients and Fertilizers for Farmed Aquatic Animals: Sources and Composition; FAO: Rome, Italy, 2009; Volume 540. [Google Scholar]
- Luthada-Raswiswi, R.; Mukaratirwa, S.; O’brien, G. Animal Protein Sources as a Substitute for Fishmeal in Aquaculture Diets: A Systematic Review and Meta-Analysis. Appl. Sci. 2021, 11, 3854. [Google Scholar] [CrossRef]
- Molina-Poveda, C.; Lucas, M.; Jover, M. Evaluation of the Potential of Andean Lupin Meal (Lupinus mutabilis Sweet) as an Alternative to Fish Meal in Juvenile Litopenaeus vannamei Diets. Aquaculture 2013, 410–411, 148–156. [Google Scholar] [CrossRef]
- Oujifard, A.; Seyfabadi, J.; Abedian Kenari, A.; Rezaei, M. Growth and Apparent Digestibility of Nutrients, Fatty Acids and Amino Acids in Pacific White Shrimp, Litopenaeus vannamei, Fed Diets with Rice Protein Concentrate as Total and Partial Replacement of Fish Meal. Aquaculture 2012, 342–343, 56–61. [Google Scholar] [CrossRef]
- Yang, Q.; Tan, B.; Dong, X.; Chi, S.; Liu, H. Effect of Replacing Fish Meal with Extruded Soybean Meal on Growth, Feed Utilization and Apparent Nutrient Digestibility of Juvenile White Shrimp (Litopenaeus vannamei). J. Ocean. Univ. China 2015, 14, 865–872. [Google Scholar] [CrossRef]
- Aya, F.A.; Cuvin-Aralar, M.L.; Coloso, R.M. Potential of Cowpea (Vigna Unguiculata L.) Meal as an Alternative Protein Source in Diets for Giant Freshwater Prawn (Macrobrachium Rosenbergii, de Man 1879). In Proceedings of the International Workshop on Resource Enhancement and Sustainable Aquaculture Practices in Southeast Asia 2014, Iloilo City, Philippines, 5–7 March 2014. [Google Scholar]
- Xie, S.; Niu, J.; Zhou, W.; Liu, Y.; Tian, L. Developing a Low Fishmeal Diet for Juvenile Pacific White Shrimp, Litopenaeus vannamei, Using the Nutritional Value of FM as the Reference Profile. Aquac. Nutr. 2018, 24, 1184–1197. [Google Scholar] [CrossRef]
- Habte-Tsion, H.-M.; Kolimadu, G.D.; Rossi, W.; Filer, K.; Kumar, V. Effects of Schizochytrium and Micro-Minerals on Immune, Antioxidant, Inflammatory and Lipid-Metabolism Status of Micropterus salmoides Fed High- and Low-Fishmeal Diets. Sci. Rep. 2020, 10, 7457. [Google Scholar] [CrossRef] [PubMed]
- Bu, X.; Wang, Y.; Chen, F.; Tang, B.; Luo, C.; Wang, Y.; Ge, X.; Yang, Y. An Evaluation of Replacing Fishmeal with Rapeseed Meal in the Diet of Pseudobagrus ussuriensis: Growth, Feed Utilization, Nonspecific Immunity, and Growth-related Gene Expression. J. World Aquac. Soc. 2018, 49, 1068–1080. [Google Scholar] [CrossRef]
- Yigit, M.; Erdem, M.; Koshio, S.; Ergun, S.; Turker, A.; Karaali, B. Substituting Fish Meal with Poultry by-Product Meal in Diets for Black Sea Turbot Psetta maeotica. Aquac. Nutr. 2006, 12, 340–347. [Google Scholar] [CrossRef]
- Bureau, D.; Harris, A.; Bevan, D.; Simmons, L.; Azevedo, P.; Cho, C. Feather Meals and Meat and Bone Meals from Different Origins as Protein Sources in Rainbow Trout (Oncorhynchus mykiss) Diets. Aquaculture 2000, 181, 281–291. [Google Scholar] [CrossRef]
- Tacon, A.G.J. Utilisation of Conventional and Unconventional Protein Sources in Practical Fish Feeds. In Nutrition and Feeding in Fish; Academic Press: Cambridge, MA, USA, 1985. [Google Scholar]
- Galkanda-Arachchige, H.S.; Wilson, A.E.; Davis, D.A. Success of Fishmeal Replacement through Poultry by-Product Meal in Aquaculture Feed Formulations: A Meta-Analysis. Rev. Aquac. 2020, 12, 1624–1636. [Google Scholar] [CrossRef]
- Novriadi, R. A Meta-Analysis Approach toward Fish Meal Replacement with Fermented Soybean Meal: Effects on Fish Growth Performance and Feed Conversion Ratio. Asian Fish. Sci. 2017, 30, 227–244. [Google Scholar] [CrossRef]
- Mugwanya, M.; Dawood, M.A.O.; Kimera, F.; Sewilam, H. Replacement of Fish Meal with Fermented Plant Proteins in the Aquafeed Industry: A Systematic Review and Meta-Analysis. Rev. Aquac. 2023, 15, 62–88. [Google Scholar] [CrossRef]
- Bautista-Teruel, M.N.; Fermin, A.C.; Koshio, S.S. Diet Development and Evaluation for Juvenile abalone, Haliotis Asinina: Animal and Plant Protein Sources. Aquaculture 2003, 219, 645–653. [Google Scholar] [CrossRef]
- Dawood, M.A.O.; Koshio, S. Application of Fermentation Strategy in Aquafeed for Sustainable Aquaculture. Rev. Aquac. 2020, 12, 987–1002. [Google Scholar] [CrossRef]
- McGoogan, B.B.; Gatlin, D.M. Effects of Replacing Fish Meal with Soybean Meal in Diets for Red Drum Sciaenops ocellatus and Potential for Palatability Enhancement. J. World Aquac. Soc. 1997, 28, 374–385. [Google Scholar] [CrossRef]
- Dias, J.; Gomes, E.F.; Kaushik, S.J. Improvement of Feed Intake through Supplementation with an Attractant Mix in European Seabass Fed Plant-Protein Rich Diets. Aquat. Living Resour. 1997, 10, 385–389. [Google Scholar] [CrossRef]
- Kader, M.A.; Koshio, S.; Ishikawa, M.; Yokoyama, S.; Bulbul, M. Supplemental Effects of Some Crude Ingredients in Improving Nutritive Values of Low Fishmeal Diets for Red Sea Bream, Pagrus major. Aquaculture 2010, 308, 136–144. [Google Scholar] [CrossRef]
- Bae, J.; Hamidoghli, A.; Won, S.; Choi, W.; Lim, S.-G.; Kim, K.-W.; Lee, B.-J.; Hur, S.-W.; Bai, S.C. Evaluation of Seven Different Functional Feed Additives in a Low Fish Meal Diet for Olive Flounder, Paralichthys olivaceus. Aquaculture 2020, 525, 735333. [Google Scholar] [CrossRef]
- Yang, X.; He, Y.; Chi, S.; Tan, B.; Lin, S.; Dong, X.; Yang, Q.; Liu, H.; Zhang, S. Supplementation with Saccharomyces cerevisiae Hydrolysate in a Complex Plant Protein, Low-Fishmeal Diet Improves Intestinal Morphology, Immune Function and Vibrio harveyi Disease Resistance in Epinephelus coioides. Aquaculture 2020, 529, 735655. [Google Scholar] [CrossRef]
- Xie, S.-W.; Li, Y.T.; Zhou, W.-W.; Tian, L.-X.; Li, Y.-M.; Zeng, S.-L.; Liu, Y.-J. Effect of γ-Aminobutyric Acid Supplementation on Growth Performance, Endocrine Hormone and Stress Tolerance of Juvenile Pacific white Shrimp, Litopenaeus vannamei, Fed Low Fishmeal Diet. Aquac. Nutr. 2017, 23, 54–62. [Google Scholar] [CrossRef]
- Niu, K.-M.; Khosravi, S.; Kothari, D.; Lee, W.-D.; Lim, J.-M.; Lee, B.-J.; Kim, K.-W.; Lim, S.-G.; Lee, S.-M.; Kim, S.-K. Effects of Dietary Multi-Strain Probiotics Supplementation in a Low Fishmeal Diet on Growth Performance, Nutrient Utilization, Proximate Composition, Immune Parameters, and Gut Microbiota of Juvenile Olive Flounder (Paralichthys olivaceus). Fish Shellfish. Immunol. 2019, 93, 258–268. [Google Scholar] [CrossRef]
- Nepal, P.R.; Han, H.-K.; Choi, H.-K. Enhancement of Solubility and Dissolution of Coenzyme Q10 Using Solid Dispersion Formulation. Int. J. Pharm. 2010, 383, 147–153. [Google Scholar] [CrossRef]
- Greenberg, S.; Frishman, W.H. Co-Enzyme Q10: A New Drug for Cardiovascular Disease. J. Clin. Pharmacol. 1990, 30, 596–608. [Google Scholar] [CrossRef]
- Tran, M.T.; Mitchell, T.M.; Kennedy, D.T.; Giles, J.T. Role of Coenzyme Q10 in Chronic Heart Failure, Angina, and Hypertension. Pharmacotherapy 2001, 21, 797–806. [Google Scholar] [CrossRef]
- Montano, S.J.; Grünler, J.; Nair, D.; Tekle, M.; Fernandes, A.P.; Hua, X.; Holmgren, A.; Brismar, K.; Ungerstedt, J.S. Glutaredoxin Mediated Redox Effects of Coenzyme Q10 Treatment In Type 1 and Type 2 Diabetes Patients. BBA Clin. 2015, 4, 14–20. [Google Scholar] [CrossRef]
- Sohmiya, M.; Tanaka, M.; Tak, N.W.; Yanagisawa, M.; Tanino, Y.; Suzuki, Y.; Okamoto, K.; Yamamoto, Y. Redox Status of Plasma Coenzyme Q10 Indicates Elevated Systemic Oxidative Stress in Parkinson’s Disease. J. Neurol. Sci. 2004, 223, 161–166. [Google Scholar] [CrossRef] [PubMed]
- Sugiyama, S.; Kitazawa, M.; Ozawa, T.; Suzuki, K.; Izawa, Y. Anti-Oxidative Effect of Coenzyme Q10. Experientia 1980, 36, 1002–1003. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Park, H.-H.; Choi, H.; Kim, Y.S.; Yu, H.-J.; Lee, K.-Y.; Lee, Y.J.; Kim, S.H.; Koh, S.-H. Coenzyme Q10 Protects Neural Stem Cells against Hypoxia by Enhancing Survival Signals. Brain Res. 2012, 1478, 64–73. [Google Scholar] [CrossRef] [PubMed]
- Amin, M.M.; Asaad, G.F.; Salam, R.M.A.; El-Abhar, H.S.; Arbid, M.S. Novel CoQ10 Antidiabetic Mechanisms Underlie Its Positive Effect: Modulation of Insulin and Adiponectine Receptors, Tyrosine Kinase, PI3K, Glucose Transporters, sRAGE and Visfatin in Insulin Resistant/Diabetic Rats. PLoS ONE 2014, 9, e89169. [Google Scholar] [CrossRef]
- Honda, K.; Kamisoyama, H.; Motoori, T.; Saneyasu, T.; Hasegawa, S. Effect of Dietary Coenzyme Q10 on Cholesterol Metabolism in Growing Chickens. J. Poult. Sci. 2010, 47, 41–47. [Google Scholar] [CrossRef]
- Varela-López, A.; Giampieri, F.; Battino, M.; Quiles, J.L. Coenzyme Q and Its Role in the Dietary Therapy against Aging. Molecules 2016, 21, 373. [Google Scholar] [CrossRef]
- Henriksen, J.E.; Andersen, C.B.; Hother-Nielsen, O.; Vaag, A.; Mortensen, S.A.; Beck-Nielsen, H. Impact of Ubiquinone (Coenzyme Q10) Treatment on Glycaemic Control, Insulin Requirement and Well-Being in Patients with Type 1 Diabetes Mellitus. Diabet. Med. 1999, 16, 312–318. [Google Scholar] [CrossRef]
- Suksomboon, N.; Poolsup, N.; Juanak, N. Effects of Coenzyme Q10 Supplementation on Metabolic Profile in Diabetes: A Systematic Review and Meta-Analysis. J. Clin. Pharm. Ther. 2015, 40, 413–418. [Google Scholar] [CrossRef]
- Johansson, P.; Dahlström, Ö.; Dahlström, U.; Alehagen, U. Improved Health-Related Quality of Life, and More Days Out of Hospital with Supplementation with Selenium and Coenzyme Q10 Combined. Results from a Double Blind, Placebo-Controlled Prospective Study. J. Nutr. Health Aging 2015, 19, 870–877. [Google Scholar] [CrossRef]
- Vetvicka, V.; Vetvickova, J. Combination Therapy with Glucan and Coenzyme Q10in Murine Experimental Autoimmune Disease and Cancer. Anticancer. Res. 2018, 38, 3291–3297. [Google Scholar] [CrossRef]
- Todorovic, K.; Jovanovic, G.; Todorovic, A.; Mitic, A.; Stojiljkovic, N.; Ilic, S.; Stojanovic, N.; Stojnev, S. Effects of Coenzyme Q10 Encapsulated in Nanoliposomes on Wound Healing Processes after Tooth Extraction. J. Dent. Sci. 2018, 13, 103–108. [Google Scholar] [CrossRef] [PubMed]
- Kessler, R.C.; Sonnega, A.; Bromet, E.; Hughes, M.; Nelson, C.B. Posttraumatic Stress Disorder in the National Comorbidity Survey. Arch. Gen. Psychiatry 1995, 52, 1048–1060. [Google Scholar] [CrossRef] [PubMed]
- Shults, C.W.; Oakes, D.; Kieburtz, K.; Beal, M.F.; Haas, R.; Plumb, S.; Juncos, J.L.; Nutt, J.; Shoulson, I.; Carter, J.; et al. Effects of Coenzyme Q10 in Early Parkinson Disease: Evidence of Slowing of the Functional Decline. Arch. Neurol. 2002, 59, 1541–1550. [Google Scholar] [CrossRef] [PubMed]
- Fan, L.; Feng, Y.; Chen, G.-C.; Qin, L.-Q.; Fu, C.-L.; Chen, L.-H. Effects of Coenzyme Q10 Supplementation on Inflammatory Markers: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Pharmacol. Res. 2017, 119, 128–136. [Google Scholar] [CrossRef] [PubMed]
- De Barcelos, I.P.; Haas, R.H. CoQ10 and Aging. Biology 2019, 8, 28. [Google Scholar] [CrossRef]
- Liao, Z.; Gong, Y.; Zhao, W.; He, X.; Wei, D.; Niu, J. Comparison Effect of Rhodobacter sphaeroides Protein Replace Fishmeal on Growth Performance, Intestinal Morphology, Hepatic Antioxidant Capacity and Immune Gene Expression of Litopenaeus vannamei under Low Salt Stress. Aquaculture 2022, 547, 737488. [Google Scholar] [CrossRef]
- Sánchez-Muros, M.-J.; Barroso, F.G.; Manzano-Agugliaro, F. Insect Meal as Renewable Source of Food for Animal Feeding: A Review. J. Clean. Prod. 2014, 65, 16–27. [Google Scholar] [CrossRef]
- Hardy, R.W. Utilization of Plant Proteins in Fish Diets: Effects of Global Demand and Supplies of Fishmeal. Aquac. Res. 2010, 41, 770–776. [Google Scholar] [CrossRef]
- Shiu, Y.-L.; Wong, S.-L.; Guei, W.-C.; Shin, Y.-C.; Liu, C.-H. Increase in the Plant Protein Ratio in the Diet of White Shrimp, Litopenaeus vannamei (Boone), Using Bacillus subtilis E20-Fermented Soybean Meal as a Replacement. Aquac. Res. 2015, 46, 382–394. [Google Scholar] [CrossRef]
- Liu, X.-H.; Ye, J.-D.; Wang, K.; Kong, J.-H.; Yang, W.; Zhou, L. Partial Replacement of Fish Meal with Peanut Meal in Practical Diets for the Pacific White Shrimp, Litopenaeus vannamei. Aquac. Res. 2012, 43, 745–755. [Google Scholar] [CrossRef]
- Molina-Poveda, C.; Cárdenas, R.; Jover, M. Evaluation of Amaranth (Amaranthus caudatus L.) and Quinoa (Chenopodium quinoa) Protein Sources as Partial Substitutes for Fish Meal in Litopenaeus vannamei Grow-Out Diets. Aquac. Res. 2017, 48, 822–835. [Google Scholar] [CrossRef]
- Wan, M.; Yin, P.; Fang, W.; Xie, S.; Chen, S.J.; Tian, L.X.; Niu, J. The Effect of Replacement of Fishmeal by Concentrated Dephenolization Cottonseed Protein on the Growth, Body Composition, Haemolymph Indexes and Haematological Enzyme Activities of the Pacific White Shrimp (Litopenaeus vannamei). Aquac. Nutr. 2018, 24, 1845–1854. [Google Scholar] [CrossRef]
- Hernández, C.; Lizárraga-Velázquez, C.E.; Contreras-Rojas, D.; Sánchez-Gutiérrez, E.Y.; Martínez-Montaño, E.; Ibarra-Castro, L.; Peña-Marín, E.S. Fish Meal Replacement by Corn Gluten in Feeds for Juvenile Spotted Rose Snapper (Lutjanus guttatus): Effect on Growth Performance, Feed Efficiency, Hematological Parameters, Protease Activity, Body Composition, and Nutrient Digestibility. Aquaculture 2021, 531, 735896. [Google Scholar] [CrossRef]
- Pratoomyot, J.; Bendiksen, E.; Bell, J.; Tocher, D. Effects of Increasing Replacement of Dietary Fishmeal with Plant Protein Sources on Growth Performance and Body Lipid Composition of Atlantic Salmon (Salmo salar L.). Aquaculture 2010, 305, 124–132. [Google Scholar] [CrossRef]
- Regost, C.; Arzel, J.; Kaushik, S. Partial or Total Replacement of Fish Meal by Corn Gluten Meal in Diet for Turbot (Psetta maxima). Aquaculture 1999, 180, 99–117. [Google Scholar] [CrossRef]
- Kumar, V.; Lee, S.; Cleveland, B.M.; Romano, N.; Lalgudi, R.S.; Benito, M.R.; McGraw, B.; Hardy, R.W. Comparative Evaluation of Processed Soybean Meal (EnzoMealTM) vs. Regular Soybean Meal as a Fishmeal Replacement in Diets of Rainbow Trout (Oncorhynchus mykiss): Effects on Growth Performance and Growth-Related Genes. Aquaculture 2020, 516, 734652. [Google Scholar] [CrossRef]
- Younis, E.-S.M.; Al-Quffail, A.S.; Al-Asgah, N.A.; Abdel-Warith, A.-W.A.; Al-Hafedh, Y.S. Effect of Dietary Fish Meal Replacement by Red Algae, Gracilaria arcuata, on Growth Performance and Body Composition of Nile Tilapia Oreochromis niloticus. Saudi J. Biol. Sci. 2018, 25, 198–203. [Google Scholar] [CrossRef]
- Sitjà-Bobadilla, A.; Peña-Llopis, S.; Gómez-Requeni, P.; Médale, F.; Kaushik, S.; Pérez-Sánchez, J. Effect of Fish Meal Replacement by Plant Protein Sources on Non-Specific Defence Mechanisms and Oxidative Stress in Gilthead Sea Bream (Sparus aurata). Aquaculture 2005, 249, 387–400. [Google Scholar] [CrossRef]
- Xi, L.; Lu, Q.; Liu, Y.; Su, J.; Chen, W.; Gong, Y.; Han, D.; Yang, Y.; Zhang, Z.; Jin, J.; et al. Effects of Fish Meal Replacement with Chlorella Meal on Growth Performance, Pigmentation, and Liver Health of Largemouth Bass (Micropterus salmoides). Anim. Nutr. 2022, 10, 26–40. [Google Scholar] [CrossRef]
- Richard, L.; Surget, A.; Rigolet, V.; Kaushik, S.J.; Geurden, I. Availability of Essential Amino Acids, Nutrient Utilisation and Growth in Juvenile Black Tiger Shrimp, Penaeus monodon, Following Fishmeal Replacement by Plant Protein. Aquaculture 2011, 322–323, 109–116. [Google Scholar] [CrossRef]
- Ye, J.; Liu, X.; Wang, Z.; Wang, K. Effect of Partial Fish Meal Replacement by Soybean Meal on the Growth Performance and Biochemical Indices of Juvenile Japanese Flounder Paralichthys olivaceus. Aquac. Int. 2011, 19, 143–153. [Google Scholar] [CrossRef]
- Feher, J.; Nemeth, E.; Nagy, V.; Lengyel, G.; Feher, J. The Preventive Role of Coenzyme Q10 and Other Antioxidants in Injuries Caused by Oxidative Stress. Arch. Med. Sci. 2007, 3, 305–314. [Google Scholar]
- El Basuini, M.F.; Shahin, S.A.; Teiba, I.I.; Zaki, M.A.; El-Hais, A.M.; Sewilam, H.; Almeer, R.; Abdelkhalek, N.; Dawood, M.A. The Influence of Dietary Coenzyme Q10 and Vitamin C on the Growth Rate, Immunity, Oxidative-Related Genes, and the Resistance against Streptococcus agalactiae of Nile Tilapia (Oreochromis niloticus). Aquaculture 2021, 531, 735862. [Google Scholar] [CrossRef]
- El Basuini, M.F.; Teiba, I.I.; Zaki, M.A.; Alabssawy, A.N.; El-Hais, A.M.; Gabr, A.A.; Dawood, M.A.; Zaineldin, A.I.; Mzengereza, K.; Shadrack, R.S.; et al. Assessing the Effectiveness of CoQ10 Dietary Supplementation on Growth Performance, Digestive Enzymes, Blood Health, Immune Response, and Oxidative-Related Genes Expression of Nile Tilapia (Oreochromis niloticus). Fish Shellfish. Immunol. 2020, 98, 420–428. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.C.; Ślesak, I.; Jordá, L.; Sotnikov, A.; Melzer, M.; Miszalski, Z.; Mullineaux, P.M.; Parker, J.E.; Karpińska, B.; Karpiński, S. Arabidopsis Chloroplastic Glutathione Peroxidases Play a Role in Cross Talk between Photooxidative Stress and Immune Responses. Plant Physiol. 2009, 150, 670–683. [Google Scholar] [CrossRef] [PubMed]
- Mahmoud, H.; Reda, F.; Alagawany, M.; Farag, M. The Stress of Abamectin Toxicity Reduced Water Quality, Growth Performance, Immunity and Antioxidant Capacity of Oreochromis niloticus Fish: Modulatory Role of Simmondsia chinensis Extract as a Dietary Supplement. Aquaculture 2021, 534, 736247. [Google Scholar] [CrossRef]
- Zhao, W.; Yao, R.; He, X.-S.; Liao, Z.-H.; Liu, Y.-T.; Gao, B.-Y.; Zhang, C.-W.; Niu, J. Beneficial Contribution of the Microalga Odontella aurita to the Growth, Immune Response, Antioxidant Capacity, and Hepatic Health of Juvenile Golden Pompano (Trachinotus ovatus). Aquaculture 2022, 555, 738206. [Google Scholar] [CrossRef]
- Wang, Y.; Li, Z.; Li, J.; Duan, Y.-F.; Niu, J.; Wang, J.; Huang, Z.; Lin, H.-Z. Effects of Dietary Chlorogenic Acid on Growth Performance, Antioxidant Capacity of White Shrimp Litopenaeus vannamei under Normal Condition and Combined Stress of Low-Salinity and Nitrite. Fish Shellfish. Immunol. 2015, 43, 337–345. [Google Scholar] [CrossRef]
- Pipe, R.; Porte, C.; Livingstone, D. Antioxidant Enzymes Associated with the Blood Cells and Haemolymph of the Mussel Mytilus edulis. Fish Shellfish. Immunol. 1993, 3, 221–233. [Google Scholar] [CrossRef]
- Garcia, M.U.; Foote, C.; van Es, S.; Devreotes, P.N.; Alexander, S.; Alexander, H. Differential Developmental Expression and Cell Type Specificity of Dictyostelium catalases and Their Response to Oxidative Stress and UV-Light. Biochim. Biophys. Acta (BBA) Gene Struct. Expr. 2000, 1492, 295–310. [Google Scholar] [CrossRef]
- Margis, R.; Dunand, C.; Teixeira, F.K.; Margis-Pinheiro, M. Glutathione Peroxidase Family—An Evolutionary Overview. FEBS J. 2008, 275, 3959–3970. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.-L.; Xi, Q.-Y.; Yang, L.; Li, H.-Y.; Jiang, Q.-Y.; Shu, G.; Wang, S.-B.; Gao, P.; Zhu, X.-T.; Zhang, Y.-L. The Effect of Dietary Panax ginseng Polysaccharide Extract on the Immune Responses in White Shrimp, Litopenaeus vannamei. Fish Shellfish. Immunol. 2011, 30, 495–500. [Google Scholar] [CrossRef] [PubMed]
- Zheng, C.; Zhao, Q.; Li, E.; Zhao, D.; Sun, S. Role of hypoxia in the Behaviour, Physiology, Immunity and Response Mechanisms of Crustaceans: A Review. Rev. Aquac. 2022, 14, 676–687. [Google Scholar] [CrossRef]
- Liao, Z.; Gong, Y.; Wang, Z.; Wang, Y.; Yao, R.; Chen, M.; Wei, D.; Zhao, W.; He, X.; Niu, J. Effects of Dietary Rhodobacter sphaeroides Protein Substitution of Fishmeal and Coenzyme Q10 Supplementation on Growth Performance, Intestinal Microbiota and Stress Tolerance of Litopenaeus vannamei in Acute Low Salinity. Front. Mar. Sci. 2022, 9, 851649. [Google Scholar] [CrossRef]
- Bonakdar, R.A.; Guarneri, E. Coenzyme Q10. Am. Fam. Physician 2005, 72, 1065–1070. [Google Scholar]
- Mantle, D.; Heaton, R.A.; Hargreaves, I.P. Coenzyme Q10 and Immune Function: An Overview. Antioxidants 2021, 10, 759. [Google Scholar] [CrossRef]
- Amparyup, P.; Charoensapsri, W.; Tassanakajon, A. Prophenoloxidase System and Its Role in Shrimp Immune Responses against Major Pathogens. Fish Shellfish. Immunol. 2013, 34, 990–1001. [Google Scholar] [CrossRef]
- Paulsen, S.M.; Engstad, R.E.; Robertsen, B. Enhanced Lysozyme Production in Atlantic Salmon (Salmo salar L.) Macrophages Treated with Yeast β-Glucan and Bacterial Lipopolysaccharide. Fish Shellfish. Immunol. 2001, 11, 23–37. [Google Scholar] [CrossRef]
- Littarru, G.P. Biomedical and Clinical Aspects of Coenzyme Q. Clin. Investig. 1993, 71, 587–588. [Google Scholar] [CrossRef]
- Crane, F.L.; Sun, I.L.; Sun, E.E. The Essential Functions of Coenzyme Q. Clin. Investig. 1993, 71, S55–S59. [Google Scholar] [CrossRef]
- Yang, M.; Sun, S.; Fu, H.; Qiao, H.; Zhang, W.; Gong, Y.; Jiang, S.; Xiong, Y.; Xu, L.; Zhao, C.; et al. Hypoxia and Reoxygenation on Antioxidant Enzyme Activities and Histological Structure of Macrobrachium nipponense. J. Fish. Sci. China 2019, 26, 493. [Google Scholar] [CrossRef]
- Sun, S.; Xuan, F.; Fu, H.; Zhu, J.; Ge, X.; Gu, Z. Transciptomic and Histological Analysis of Hepatopancreas, Muscle and Gill Tissues of Oriental River Prawn (Macrobrachium nipponense) in Response to Chronic Hypoxia. BMC Genom. 2015, 16, 491. [Google Scholar] [CrossRef] [PubMed]
- Duan, Y.; Zhang, J.; Dong, H.; Wang, Y.; Liu, Q.; Li, H. Effect of Desiccation and Resubmersion on the Oxidative Stress Response of the Kuruma Shrimp Marsupenaeus japonicus. Fish Shellfish. Immunol. 2016, 49, 91–99. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.; Xuan, F.; Ge, X.; Fu, H.; Zhu, J.; Zhang, S. Identification of Differentially Expressed Genes in Hepatopancreas of Oriental River Prawn, Macrobrachium nipponense Exposed to Environmental Hypoxia. Gene 2014, 534, 298–306. [Google Scholar] [CrossRef]
- Zenteno-Savín, T.; Saldierna, R.; Ahuejote-Sandoval, M. Superoxide Radical Production in Response to Environmental Hypoxia in Cultured Shrimp. Comp. Biochem. Physiol. Part. C Toxicol. Pharmacol. 2006, 142, 301–308. [Google Scholar] [CrossRef]
- Yang, S.; Fan, B.; Chen, X.; Meng, Z. Supplementation of the Freezing Medium with Coenzyme Q10 Attenuates Oxidative Stress and Improves Function of Frozen-Thawed Giant Grouper (Epinephelus lanceolatus) Spermatozoa. Theriogenology 2021, 175, 77–82. [Google Scholar] [CrossRef]
- Chenga, W.; Liub, C.H.; Hsuc, J.P.; Chend, J.C. Effect of Hypoxia on the Immune Response of Giant Freshwater Prawn Macrobrachium rosenbergii and Its Susceptibility to Pathogen Enterococcus. Fish Shellfish. Immunol. 2002, 13, 351–365. [Google Scholar] [CrossRef]
- Le Moullac, G.; Soyez, C.; Saulnier, D.; Ansquer, D.; Avarre, J.C.; Levy, P. Effect of Hypoxic Stress on the Immune Response and the Resistance to Vibriosis of the Shrimp Penaeus stylirostris. Fish Shellfish. Immunol. 1998, 8, 621–629. [Google Scholar] [CrossRef]
- Li, C.; Wang, S.; He, J. The Two NF-ΚB Pathways Regulating Bacterial and WSSV Infection of Shrimp. Front. Immunol. 2019, 10, 1785. [Google Scholar] [CrossRef]
- Valanne, S.; Wang, J.-H.; Rämet, M. The Drosophila Toll Signaling Pathway. J. Immunol. 2011, 186, 649–656. [Google Scholar] [CrossRef]
- Huang, X.-D.; Yin, Z.-X.; Jia, X.-T.; Liang, J.-P.; Ai, H.-S.; Yang, L.-S.; Liu, X.; Wang, P.-H.; Li, S.-D.; Weng, S.-P.; et al. Identification and Functional Study of a Shrimp Dorsal homologue. Dev. Comp. Immunol. 2010, 34, 107–113. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Wang, W.; Ren, Q. Dorsal Transcription Factor Is Involved in Regulating Expression of Crustin Genes during White Spot Syndrome Virus Infection. Dev. Comp. Immunol. 2016, 63, 18–26. [Google Scholar] [CrossRef] [PubMed]



| Ingredients (%) | D1 | D2 | D3 | D4 | D5 | D6 | D7 |
|---|---|---|---|---|---|---|---|
| Fish meal | 20 | 15 | 15 | 15 | 15 | 15 | 15 |
| Soybean meal | 28 | 28 | 28 | 28 | 28 | 28 | 28 |
| Peanut meal | 12 | 12 | 12 | 12 | 12 | 12 | 12 |
| Corn gluten meal | 0 | 5 | 5 | 5 | 5 | 5 | 5 |
| Wheat flour | 23.85 | 23.25 | 23.248 | 23.246 | 23.244 | 23.242 | 23.24 |
| Krill meal | 2 | 2 | 2 | 2 | 2 | 2 | 2 |
| Beer yeast | 4 | 4 | 4 | 4 | 4 | 4 | 4 |
| Fish oil | 1.5 | 1.8 | 1.8 | 1.8 | 1.8 | 1.8 | 1.8 |
| Soybean lecithin | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| Soy oil | 1.5 | 1.5 | 1.5 | 1.5 | 1.5 | 1.5 | 1.5 |
| Vitamin premix a | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| Mineral premix b | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| Choline | 0.5 | 0.5 | 0.5 | 0.5 | 0.5 | 0.5 | 0.5 |
| Ca(H2PO4)2 | 1.7 | 1.7 | 1.7 | 1.7 | 1.7 | 1.7 | 1.7 |
| Lysine | 0.29 | 0.53 | 0.53 | 0.53 | 0.53 | 0.53 | 0.53 |
| Vitamin C | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 |
| Methionine | 0.24 | 0.25 | 0.25 | 0.25 | 0.25 | 0.25 | 0.25 |
| Threonine | 0.32 | 0.37 | 0.37 | 0.37 | 0.37 | 0.37 | 0.37 |
| Sodium alginate | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| Coenzyme Q10 c | 0 | 0 | 0.002 | 0.004 | 0.006 | 0.008 | 0.01 |
| Total | 100 | 100 | 100 | 100 | 100 | 100 | 100 |
| Proximate composition (%) | |||||||
| Moisture | 9.38 | 9.66 | 9.27 | 9.68 | 9.63 | 9.55 | 9.81 |
| Crude protein | 37.73 | 38.20 | 37.86 | 38.02 | 38.12 | 38.21 | 37.92 |
| Crude lipid | 6.96 | 7.24 | 7.02 | 7.12 | 7.32 | 7.18 | 7.07 |
| Gene | Primer Sequence (5′ to 3′) | Genbank No. | Products Length |
|---|---|---|---|
| β-actin F | CTTGTGTGCGACAATGGCTC | XM_027364954.1 | 194 |
| β-actin R | TCGATGGGGTACTTGAGGGT | ||
| MnSOD F | TGTTGCACAAGCCATTGACG | XM_027381242.1 | 157 |
| MnSOD R | ATCCTGGTTCTGGCAAGTGG | ||
| CAT F | GCGACCAGAAACAACACACC | XM_027383071.1 | 166 |
| CAT R | CTTGATGCCTTGGTCCGTCT | ||
| GPX F | GGCACCAGGAGAACACTAC | XM_027368745.1 | 102 |
| GPX R | CGACTTTGCCGAACATAAC | ||
| ProPO F | TCCCGGACCGAGAAGATAGT | AY723296.1 | 105 |
| ProPO R | TGTGGTATCATTCCCTGCGAG | ||
| LZM F | CGATGATATCACGGAGGCCC | XM_027352857.1 | 111 |
| LZM R | TTGCTGTTGTAAGCCACCCA | ||
| Toll F | TTTCGGAGGATTGGAGTGCC | DQ923424.1 | 112 |
| Toll R | GGTTTGTGAGGGAGTCCAGG | ||
| Myd88 F | CCTCAGCCCAGCTTCTAAACA | JX073566.1 | 110 |
| Myd88 R | CAGCCTGTTCTGCCAATCCT | ||
| TRAF6 F | GGAGGTTGCAGGACACATGA | HM581680.1 | 132 |
| TRAF6 R | TGTAGCTGCGTGGCTTGTAA | ||
| Pelle F | CGTCAGTATTACGTCACGGC | KC346864 | 195 |
| Pelle R | TGACTTCCAAGATGTGCGCT | ||
| Dorsal F | CACGACCCATCAGAGTAGCC | XM_027382194.1 | 153 |
| Dorsal R | AAACTGGAGGCTTCACAGCA |
| Parameters | D1 | D2 | D3 | D4 | D5 | D6 | D7 |
|---|---|---|---|---|---|---|---|
| IBW (g) | 0.66 ± 0.01 | 0.67 ± 0.01 | 0.68 ± 0.00 | 0.66 ± 0.01 | 0.66 ± 0.00 | 0.68 ± 0.00 | 0.66 ± 0.01 |
| FBW (g) | 26.94 ± 0.78 | 24.71 ± 0.49 | 25.28 ± 0.47 | 25.71 ± 0.21 | 25.08 ± 1.17 | 26.08 ± 0.26 | 26.19 ± 0.77 |
| WG (%) | 3967.76 ± 120.48 b | 3606.94 ± 62.50 a | 3613.86 ± 70.97 a | 3790.40 ± 25.70 ab | 3708.63 ± 172.00 ab | 3749.86 ± 42.72 ab | 3899.68 ± 128.75 ab |
| SGR (%, day−1) | 5.88 ± 0.05 b | 5.73 ± 0.03 a | 5.74 ± 0.03 a | 5.81 ± 0.01 ab | 5.77 ± 0.07 ab | 5.79 ± 0.02 ab | 5.85 ± 0.05 ab |
| FCR | 1.46 ± 0.03 | 1.50 ± 0.03 | 1.48 ± 0.04 | 1.53 ± 0.09 | 1.56 ± 0.06 | 1.44 ± 0.03 | 1.51 ± 0.03 |
| CF (g cm−3) | 1.38 ± 0.03 b | 1.28 ± 0.04 a | 1.24 ± 0.03 a | 1.23 ± 0.02 a | 1.21 ± 0.02 a | 1.22 ± 0.02 a | 1.26 ± 0.01 a |
| Survival (%) | 89.17 ± 5.51 | 89.17 ± 4.38 | 86.67 ± 4.91 | 88.33 ± 5.18 | 88.33 ± 3.97 | 90.00 ± 1.36 | 87.50 ± 1.60 |
| Parameter | D1 | D2 | D3 | D4 | D5 | D6 | D7 |
|---|---|---|---|---|---|---|---|
| Moisture | 72.77 ± 0.14 b | 73.36 ± 0.37 bc | 74.43 ± 0.53 c | 71.06 ± 0.41 a | 72.77 ± 0.67 b | 74.06 ± 0.30 bc | 73.00 ± 0.75 bc |
| Crude protein | 19.23 ± 0.08 | 18.42 ± 0.08 | 18.46 ± 0.13 | 19.18 ± 0.42 | 18.05 ± 0.30 | 18.58 ± 0.30 | 19.10 ± 0.28 |
| Crude lipid | 1.75 ± 0.18 | 1.97 ± 0.05 | 1.90 ± 0.15 | 2.18 ± 0.12 | 2.02 ± 0.06 | 1.86 ± 0.11 | 2.01 ± 0.20 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Y.; Zhuang, Z.; Liao, Z.; Yao, R.; Chen, M.; Wei, H.; Zhao, W.; Niu, J. Effects of Low-Fish-Meal Diet Supplemented with Coenzyme Q10 on Growth Performance, Antioxidant Capacity, Intestinal Morphology, Immunity and Hypoxic Resistance of Litopenaeus vannamei. Antioxidants 2023, 12, 2042. https://doi.org/10.3390/antiox12122042
Liu Y, Zhuang Z, Liao Z, Yao R, Chen M, Wei H, Zhao W, Niu J. Effects of Low-Fish-Meal Diet Supplemented with Coenzyme Q10 on Growth Performance, Antioxidant Capacity, Intestinal Morphology, Immunity and Hypoxic Resistance of Litopenaeus vannamei. Antioxidants. 2023; 12(12):2042. https://doi.org/10.3390/antiox12122042
Chicago/Turabian StyleLiu, Yantao, Zhenxiao Zhuang, Zhihong Liao, Rong Yao, Mengdie Chen, Hanlin Wei, Wei Zhao, and Jin Niu. 2023. "Effects of Low-Fish-Meal Diet Supplemented with Coenzyme Q10 on Growth Performance, Antioxidant Capacity, Intestinal Morphology, Immunity and Hypoxic Resistance of Litopenaeus vannamei" Antioxidants 12, no. 12: 2042. https://doi.org/10.3390/antiox12122042
APA StyleLiu, Y., Zhuang, Z., Liao, Z., Yao, R., Chen, M., Wei, H., Zhao, W., & Niu, J. (2023). Effects of Low-Fish-Meal Diet Supplemented with Coenzyme Q10 on Growth Performance, Antioxidant Capacity, Intestinal Morphology, Immunity and Hypoxic Resistance of Litopenaeus vannamei. Antioxidants, 12(12), 2042. https://doi.org/10.3390/antiox12122042







