SIRT3 Modulates Endothelial Mitochondrial Redox State during Insulin Resistance
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cell Culture and Treatments
2.2. Cell Viability Detection
2.3. SIRT3 Overexpressing
2.4. Glucose Uptake Analysis
2.5. Lactate Dehydrogenase (LDH) Assay
2.6. Lipid Peroxidation Detection
2.7. Cytokine Levels Determination
2.8. Evaluation of ROS
2.9. Programmed Cell Death Mechanisms
2.10. Cell Lysis and Immunoblotting Analysis
2.11. RNA Isolation and Quantitative RT-PCR
- −
- SIRT2 (gene ID: 22933): F-5′-GCCCTTTACCAACATGGCTG-3′, R-5′-TTCGTACAACACCCAGAGCG-3′;
- −
- SIRT2 SIRT3 (gene ID: 23410): F-5′-AGAAGAGATGCGGGACCTTG-3′, R-5′-GGTCCATCAAGCCTAGAGCAG-3′;
- −
- SIRT2 SIRT4 (gene ID: 23409): F-5′-GGCAGGAATCTCCACCGAAT-3′, R-5′-GCACTCCGGACAAAATCACC-3′;
- −
- SIRT2 SIRT5 (gene ID: 23408): F-5′-GGTGTTCCGACCTTCAGAGG-3′, R-5′-GTGGTAGAACTCCCACACCC-3′;
- −
- SIRT2 GAPDH (gene ID: 2597): F-5′-GAAGGTGAAGGTCGGAGTC-3′, R-5′-GAAGATGGTGATGGGATTTC-3′.
2.12. Confocal Laser Scanning Microscopy
2.13. Statistical Analysis
3. Results
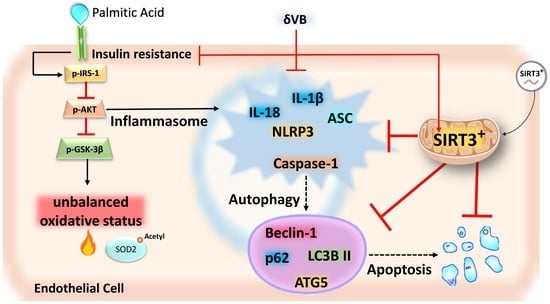
3.1. Effects of PA on IR, Mitochondria Oxidative Status, and Sirtuins
3.2. SIRT3+ Decreased the PA-Induced Cytotoxicity
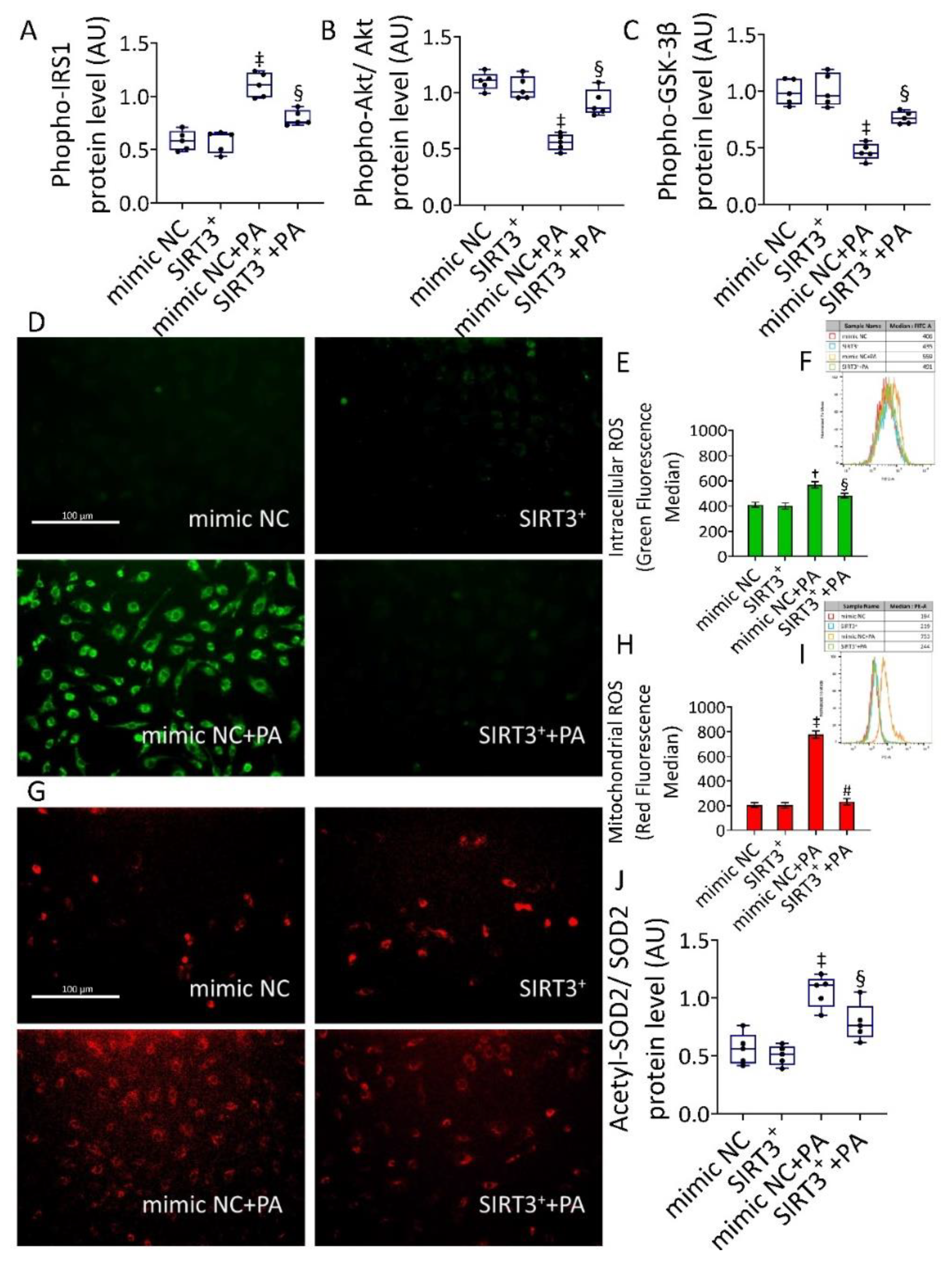
3.3. SIRT3+ Reverted the PA-Induced IR State and Oxidative Stress
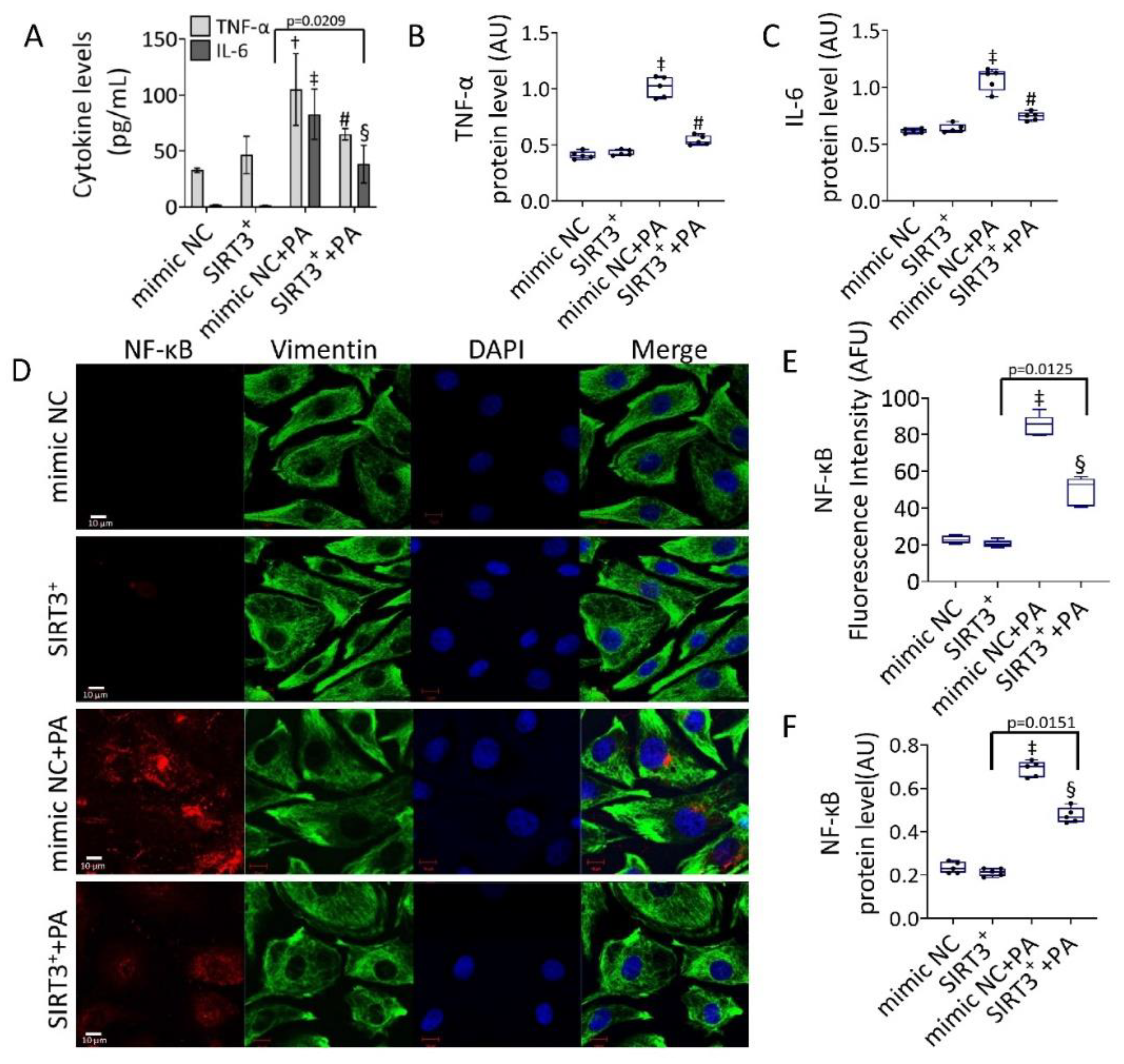
3.4. Effects of SIRT3+ on PA-Induced Inflammation
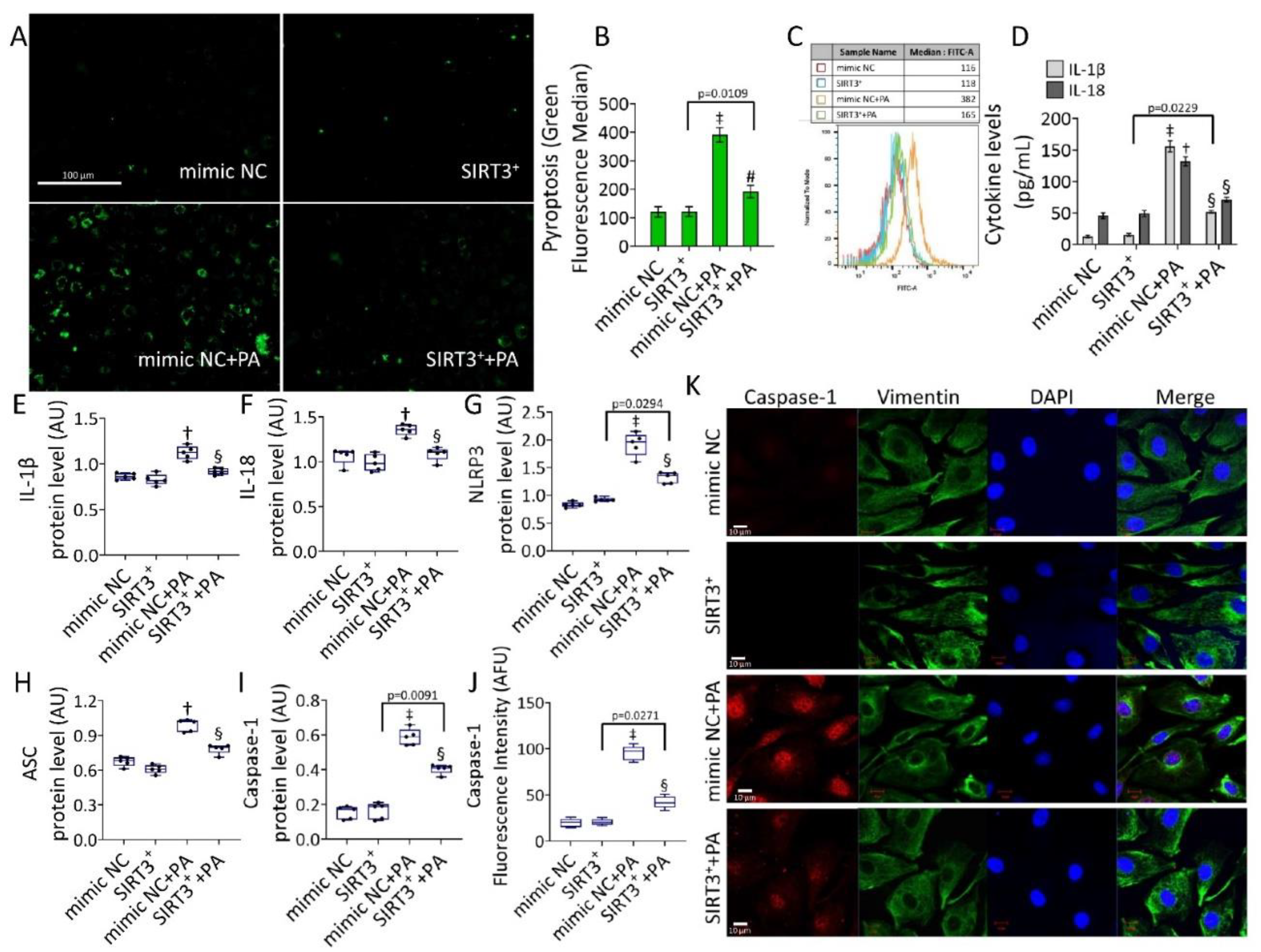
3.5. SIRT3+ Reduced the PA-Induced Pyroptosis
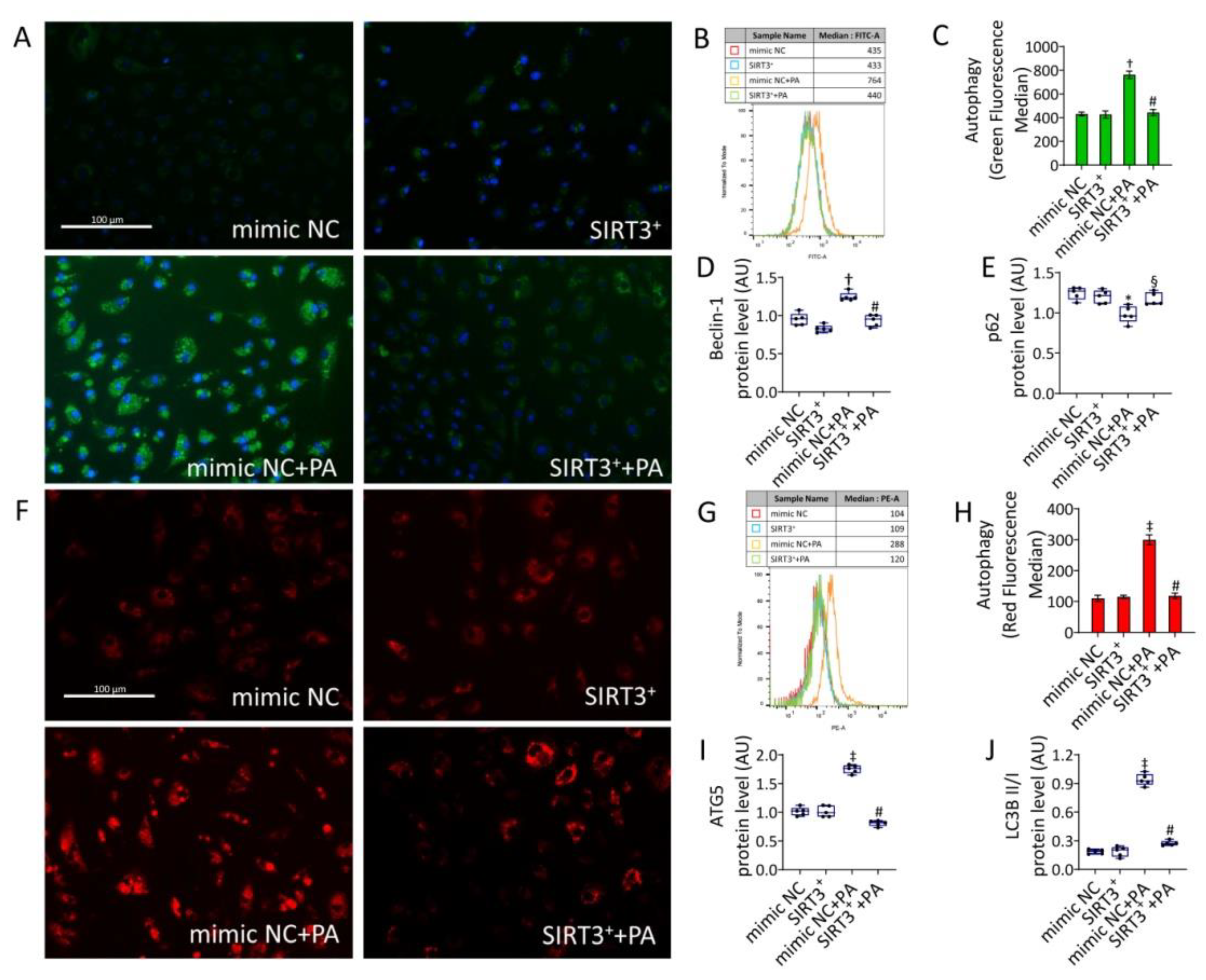
3.6. SIRT3+ Reduced the Autophagy Induced by PA
3.7. Effects of δVB on Mitochondrial ROS and Sirtuin Modulation
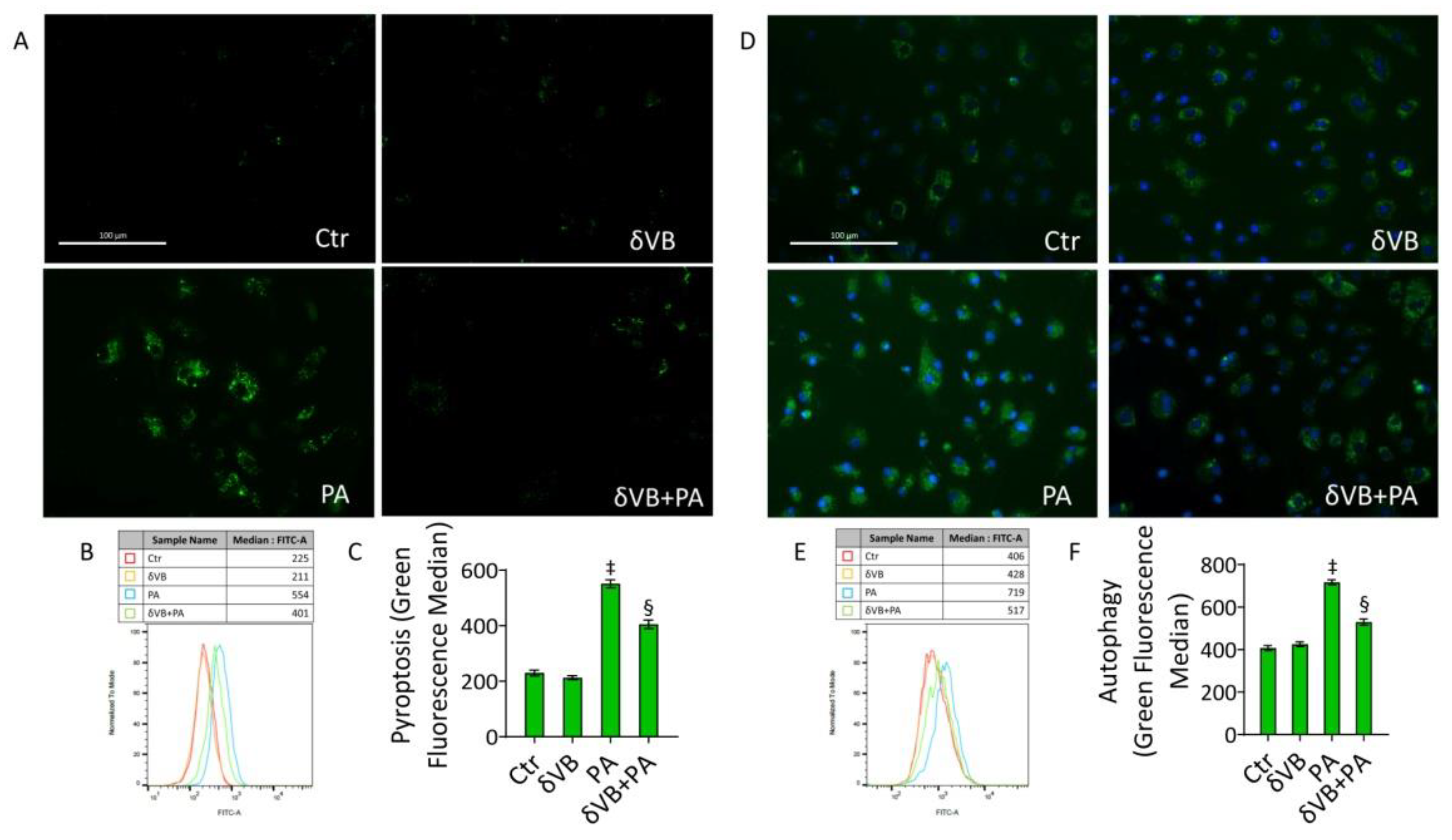
3.8. δVB as a Modulator of PA-Induced Pyroptosis and Autophagy
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- He, X.; Zeng, H.; Chen, J.X. Emerging role of SIRT3 in endothelial metabolism, angiogenesis, and cardiovascular disease. J. Cell. Physiol. 2019, 234, 2252–2265. [Google Scholar] [CrossRef] [PubMed]
- Kane, A.E.; Sinclair, D.A. Sirtuins and NAD+ in the development and treatment of metabolic and cardiovascular diseases. Circ. Res. 2018, 123, 868–885. [Google Scholar] [CrossRef] [PubMed]
- Colloca, A.; Balestrieri, A.; Anastasio, C.; Balestrieri, M.L.; D’Onofrio, N. Mitochondrial sirtuins in chronic degenerative diseases: New metabolic targets in colorectal cancer. Int. J. Mol. Sci. 2022, 23, 3212. [Google Scholar] [CrossRef] [PubMed]
- D’Onofrio, N.; Vitiello, M.; Casale, R.; Servillo, L.; Giovane, A.; Balestrieri, M.L. Sirtuins in vascular diseases: Emerging roles and therapeutic potential. Biochim. Biophys. Acta 2015, 1852, 1311–1322. [Google Scholar] [CrossRef]
- D’Onofrio, N.; Servillo, L.; Balestrieri, M.L. SIRT1 and SIRT6 Signaling pathways in cardiovascular disease protection. Antioxid. Redox Signal 2018, 28, 711–732. [Google Scholar] [CrossRef]
- Riehle, C.; Abel, E.D. Insulin signaling and heart failure. Circ. Res. 2016, 118, 1151–1169. [Google Scholar] [CrossRef]
- De Meyts, P. The Insulin Receptor and Its Signal Transduction Network; Feingold, K.R., Anawalt, B., Boyce, A., Chrousos, G., de Herder, W.W., Dhatariya, K., Dungan, K., Hershman, J.M., Hofland, J., Kalra, S., et al., Eds.; Endotext: South Dartmouth, MA, USA, 2016. [Google Scholar]
- Zhang, Q.J.; McMillin, S.L.; Tanner, J.M.; Palionyte, M.; Abel, E.D.; Symons, J.D. Endothelial nitric oxide synthase phosphorylation in treadmill-running mice: Role of vascular signalling kinases. J. Physiol. 2009, 587 Pt 15, 3911–3920. [Google Scholar] [CrossRef]
- Gélinas, D.S.; Bernatchez, P.N.; Rollin, S.; Bazan, N.G.; Sirois, M.G. Immediate and delayed VEGF-mediated NO synthesis in endothelial cells: Role of PI3K, PKC and PLC pathways. Br. J. Pharmacol. 2002, 137, 1021–1030. [Google Scholar] [CrossRef]
- Masaki, N.; Ido, Y.; Yamada, T.; Yamashita, Y.; Toya, T.; Takase, B.; Hamburg, N.M.; Adachi, T. Endothelial insulin resistance of freshly isolated arterial endothelial cells from radial sheaths in patients with suspected coronary artery disease. J. Am. Heart Assoc. 2019, 8, e010816. [Google Scholar] [CrossRef]
- Sharma, D.; Kanneganti, T.D. The cell biology of inflammasomes: Mechanisms of inflammasome activation and regulation. J. Cell Biol. 2016, 213, 617–629. [Google Scholar] [CrossRef]
- Zheng, D.; Shi, Z.; Yang, M.; Liang, B.; Zhou, X.; Jing, L.; Sun, Z. NLRP3 inflammasome-mediated endothelial cells pyroptosis is involved in decabromodiphenyl ethane-induced vascular endothelial injury. Chemosphere 2021, 267, 128867. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Tang, D.; Zhou, X.; Yang, X.; Chen, A.F. PhospholipaseCγ1/calcium dependent membranous localization of Gsdmd-N drives endothelial pyroptosis, contributing to lipopolysaccharide-induced fatal outcome. Am. J. Physiol. Heart Circ. Physiol. 2020, 319, H1482–H1495. [Google Scholar] [CrossRef] [PubMed]
- Li, H.B.; Jin, C.; Chen, Y.; Flavell, R.A. Inflammasome activation and metabolic disease progression. Cytokine Growth Factor Rev. 2014, 25, 699–706. [Google Scholar] [CrossRef] [PubMed]
- Jo, E.K.; Kim, J.K.; Shin, D.M.; Sasakawa, C. Molecular mechanisms regulating NLRP3 inflammasome activation. Cell. Mol. Immunol. 2016, 13, 148–159. [Google Scholar] [CrossRef]
- Kärkkäinen, O.; Lankinen, M.A.; Vitale, M.; Jokkala, J.; Leppänen, J.; Koistinen, V.; Lehtonen, M.; Giacco, R.; Rosa-Sibakov, N.; Micard, V.; et al. Diets rich in whole grains increase levels of betainized compounds associated with glucose metabolism. Am. J. Clin. Nutr. 2018, 108, 971–979. [Google Scholar] [CrossRef]
- Kärkkäinen, O.; Tuomainen, T.; Koistinen, V.; Tuomainen, M.; Leppänen, J.; Laitinen, T.; Lehtonen, M.; Rysä, J.; Auriola, S.; Poso, A.; et al. Whole grain intake associated molecule 5- aminovaleric acid betaine decreases β-oxidation of fatty acids in mouse cardiomyocytes. Sci. Rep. 2018, 8, 13036. [Google Scholar] [CrossRef] [PubMed]
- Tuomainen, M.; Kärkkäinen, O.; Leppänen, J.; Auriola, S.; Lehtonen, M.; Savolainen, M.J.; Hermansen, K.; Risérus, U.; Åkesson, B.; Thorsdottir, I.; et al. Quantitative assessment of betainized compounds and associations with dietary and metabolic biomarkers in the randomized study of the healthy Nordic diet (SYSDIET). Am. J. Clin. Nutr. 2019, 110, 1108–1118. [Google Scholar] [CrossRef] [PubMed]
- D’Onofrio, N.; Balestrieri, A.; Neglia, G.; Monaco, A.; Tatullo, M.; Casale, R.; Limone, A.; Balestrieri, M.L.; Campanile, G. Antioxidant and anti-inflammatory activities of buffalo milk δ-Valerobetaine. J. Agric. Food Chem. 2019, 67, 1702–1710. [Google Scholar] [CrossRef] [PubMed]
- D’Onofrio, N.; Cacciola, N.A.; Martino, E.; Borrelli, F.; Fiorino, F.; Lombardi, A.; Neglia, G.; Balestrieri, M.L.; Campanile, G. ROS-mediated apoptotic cell death of human colon cancer LoVo cells by milk δ-valerobetaine. Sci. Rep. 2020, 10, 8978. [Google Scholar] [CrossRef]
- D’Onofrio, N.; Martino, E.; Mele, L.; Colloca, A.; Maione, M.; Cautela, D.; Castaldo, D.; Balestrieri, M.L. Colorectal cancer apoptosis induced by dietary δ-valerobetaine involves PINK1/Parkin dependent-mitophagy and SIRT3. Int. J. Mol. Sci. 2021, 22, 8117. [Google Scholar] [CrossRef]
- D’Onofrio, N.; Mele, L.; Martino, E.; Salzano, A.; Restucci, B.; Cautela, D.; Tatullo, M.; Balestrieri, M.L.; Campanile, G. Synergistic effect of dietary betaines on SIRT1-mediated apoptosis in human oral squamous cell carcinoma Cal 27. Cancers 2020, 12, 2468. [Google Scholar] [CrossRef] [PubMed]
- Tatullo, M.; Marrelli, B.; Benincasa, C.; Aiello, E.; Amantea, M.; Gentile, S.; Leonardi, N.; Balestrieri, M.L.; Campanile, G. Potential impact of functional biomolecules-enriched foods on human health: A randomized controlled clinical trial. Int. J. Med. Sci. 2022, 19, 563–571. [Google Scholar] [CrossRef]
- Servillo, L.; Giovane, A.; Cautela, D.; Castaldo, D.; Balestrieri, M.L. Where does N(ε)-trimethyllysine for the carnitine biosynthesis in mammals come from? PLoS ONE 2014, 9, e84589. [Google Scholar] [CrossRef] [PubMed]
- Servillo, L.; D’Onofrio, N.; Giovane, A.; Casale, R.; Cautela, D.; Castaldo, D.; Iannaccone, F.; Neglia, G.; Campanile, G.; Balestrieri, M.L. Ruminant meat and milk contain δ-valerobetaine, another precursor of trimethylamine N-oxide (TMAO) like γ-butyrobetaine. Food Chem. 2018, 260, 193–199. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Fu, W.; Xue, Y.; Lu, Z.; Li, Y.; Yu, P.; Yu, X.; Xu, H.; Sui, D. Ginsenoside Rc ameliorates endothelial insulin resistance via upregulation of angiotensin-converting enzyme 2. Front. Pharmacol. 2021, 12, 620524. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, C.S.S.; Cerqueira, N.M.F.S.A.; Gomes, P.; Sousa, S.F. A molecular perspective on sirtuin activity. Int. J. Mol. Sci. 2020, 21, 8609. [Google Scholar] [CrossRef]
- Shahgaldi, S.; Kahmini, F.R. A comprehensive review of Sirtuins: With a major focus on redox homeostasis and metabolism. Life Sci. 2021, 282, 119803. [Google Scholar] [CrossRef]
- Liu, G.; Park, S.H.; Imbesi, M.; Nathan, W.J.; Zou, X.; Zhu, Y.; Jiang, H.; Parisiadou, L.; Gius, D. Loss of NAD-dependent protein deacetylase sirtuin-2 alters mitochondrial protein acetylation and dysregulates mitophagy. Antioxid. Redox Signal 2017, 26, 849–863. [Google Scholar] [CrossRef]
- Lee, A.Y.; Christensen, S.M.; Duong, N.; Tran, Q.A.; Xiong, H.M.; Huang, J.; James, S.; Vallabh, D.; Talbott, G.; Rose, M.; et al. Sirt3 pharmacologically promotes insulin sensitivity through PI3/AKT/mTOR and their downstream pathway in adipocytes. Int. J. Mol. Sci. 2022, 23, 3740. [Google Scholar] [CrossRef]
- Yang, L.; Zhang, J.; Xing, W.; Zhang, X.; Xu, J.; Zhang, H.; Chen, L.; Ning, X.; Ji, G.; Li, J.; et al. SIRT3 deficiency induces endothelial insulin resistance and blunts endothelial-dependent vasorelaxation in mice and human with obesity. Sci. Rep. 2016, 6, 23366. [Google Scholar] [CrossRef]
- He, X.; Zeng, H.; Chen, S.T.; Roman, R.J.; Aschner, J.L.; Didion, S.; Chen, J.X. Endothelial specific SIRT3 deletion impairs glycolysis and angiogenesis and causes diastolic dysfunction. J. Mol. Cell Cardiol. 2017, 112, 104–113. [Google Scholar] [CrossRef] [PubMed]
- Vandanmagsar, B.; Youm, Y.H.; Ravussin, A.; Galgani, J.E.; Stadler, K.; Mynatt, R.L.; Ravussin, E.; Stephens, J.M.; Dixit, V.D. The NLRP3 inflammasome instigates obesity-induced inflammation and insulin resistance. Nat. Med. 2011, 17, 179–188. [Google Scholar] [CrossRef]
- Luo, H.; Mu, W.C.; Karki, R.; Chiang, H.H.; Mohrin, M.; Shin, J.J.; Ohkubo, R.; Ito, K.; Kanneganti, T.D.; Chen, D. Mitochondrial stress-initiated aberrant activation of the NLRP3 inflammasome regulates the functional deterioration of hematopoietic stem cell aging. Cell Rep. 2019, 26, 945–954.e4. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Zhang, L.; Hua, F.; Zhang, C.; Zhang, C.; Mi, X.; Qin, N.; Wang, J.; Zhu, A.; Qin, Z.; et al. FOXM1-activated SIRT4 inhibits NF-κB signaling and NLRP3 inflammasome to alleviate kidney injury and podocyte pyroptosis in diabetic nephropathy. Exp. Cell Res. 2021, 408, 112863. [Google Scholar] [CrossRef] [PubMed]
- Guan, C.; Huang, X.; Yue, J.; Xiang, H.; Shaheen, S.; Jiang, Z.; Tao, Y.; Tu, J.; Liu, Z.; Yao, Y.; et al. SIRT3-mediated deacetylation of NLRC4 promotes inflammasome activation. Theranostics 2021, 11, 3981–3995. [Google Scholar] [CrossRef] [PubMed]
- Tabit, C.E.; Shenouda, S.M.; Holbrook, M.; Fetterman, J.L.; Kiani, S.; Frame, A.A.; Kluge, M.A.; Held, A.; Dohadwala, M.M.; Gokce, N.; et al. Protein kinase C-β contributes to impaired endothelial insulin signaling in humans with diabetes mellitus. Circulation 2013, 127, 86–95. [Google Scholar] [CrossRef]
- Bretòn-Romero, R.; Feng, B.; Holbrook, M.; Farb, M.G.; Fetterman, J.L.; Linder, E.A.; Berk, B.D.; Masaki, N.; Weisbrod, R.M.; Inagaki, E.; et al. Endothelial dysfunction in human diabetes is mediated by Wnt5a-JNK signaling. Arterioscler. Thromb. Vasc. Biol. 2016, 36, 561–569. [Google Scholar] [CrossRef]
- Straub, L.G.; Efthymiou, V.; Grandl, G.; Balaz, M.; Challa, T.D.; Truscello, L.; Horvath, C.; Moser, C.; Rachamin, Y.; Arnold, M.; et al. Antioxidants protect against diabetes by improving glucose homeostasis in mouse models of inducible insulin resistance and obesity. Diabetologia 2019, 62, 2094–2105. [Google Scholar] [CrossRef]
- Gong, L.; Guo, S.; Zou, Z. Resveratrol ameliorates metabolic disorders and insulin resistance in high-fat diet-fed mice. Life Sci. 2020, 242, 117212. [Google Scholar] [CrossRef]
- Alam, M.A.; Subhan, N.; Rahman, M.M.; Uddin, S.J.; Reza, H.M.; Sarker, S.D. Effect of citrus flavonoids, naringin and naringenin, on metabolic syndrome and their mechanisms of action. Adv. Nutr. 2014, 5, 404–417. [Google Scholar] [CrossRef]
- MacDonald-Ramos, K.; Michán, L.; Martínez-Ibarra, A.; Cerbón, M. Silymarin is an ally against insulin resistance: A review. Ann. Hepatol. 2021, 23, 100255. [Google Scholar] [CrossRef] [PubMed]
- Gurau, F.; Baldoni, S.; Prattichizzo, F.; Espinosa, E.; Amenta, F.; Procopio, A.D.; Albertini, M.C.; Bonafè, M.; Olivieri, F. Antisenescence compounds: A potential nutraceutical approach to healthy aging. Ageing Res. Rev. 2018, 46, 14–31. [Google Scholar] [CrossRef] [PubMed]
- Haikonen, R.; Kärkkäinen, O.; Koistinen, V.; Hanhineva, K. Diet- and microbiota-related metabolite, 5-aminovaleric acid betaine (5-AVAB), in health and disease. Trends Endocrinol. Metab. 2022, 33, 463–480. [Google Scholar] [CrossRef] [PubMed]
- Adam, J.; Brandmaier, S.; Leonhardt, J.; Scheerer, M.F.; Mohney, R.P.; Xu, T.; Bi, J.; Rotter, M.; Troll, M.; Chi, S.; et al. Metformin effect on nontargeted metabolite profiles in patients with type 2 diabetes and in multiple murine tissues. Diabetes 2016, 65, 3776–3785. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martino, E.; Balestrieri, A.; Anastasio, C.; Maione, M.; Mele, L.; Cautela, D.; Campanile, G.; Balestrieri, M.L.; D’Onofrio, N. SIRT3 Modulates Endothelial Mitochondrial Redox State during Insulin Resistance. Antioxidants 2022, 11, 1611. https://doi.org/10.3390/antiox11081611
Martino E, Balestrieri A, Anastasio C, Maione M, Mele L, Cautela D, Campanile G, Balestrieri ML, D’Onofrio N. SIRT3 Modulates Endothelial Mitochondrial Redox State during Insulin Resistance. Antioxidants. 2022; 11(8):1611. https://doi.org/10.3390/antiox11081611
Chicago/Turabian StyleMartino, Elisa, Anna Balestrieri, Camilla Anastasio, Martina Maione, Luigi Mele, Domenico Cautela, Giuseppe Campanile, Maria Luisa Balestrieri, and Nunzia D’Onofrio. 2022. "SIRT3 Modulates Endothelial Mitochondrial Redox State during Insulin Resistance" Antioxidants 11, no. 8: 1611. https://doi.org/10.3390/antiox11081611
APA StyleMartino, E., Balestrieri, A., Anastasio, C., Maione, M., Mele, L., Cautela, D., Campanile, G., Balestrieri, M. L., & D’Onofrio, N. (2022). SIRT3 Modulates Endothelial Mitochondrial Redox State during Insulin Resistance. Antioxidants, 11(8), 1611. https://doi.org/10.3390/antiox11081611