MARCKS Is an Essential Regulator of Reactive Oxygen Species Production in the Monocytic Cell Type
Abstract
1. Introduction
2. Materials and Methods
2.1. CRISPR/Cas9-Mediated Generation of WT, Intermediate (IM), and KO Cells
2.1.1. CRISPR/Cas9 Plasmids
2.1.2. Transfection of THP-1 Cells with CRISPR/Cas9 Plasmids
2.1.3. Generation of MARCKS WT, IM, and KO Clones
2.1.4. Generation of PKCβ KO Clones
2.1.5. Western Blot
2.1.6. Flow Cytometry
2.1.7. Cycle Sequencing
2.2. Isolation of Primary Human Monocytes and Cell Culture Conditions
2.2.1. Isolation of Primary Monocytes
2.2.2. Cell Culture
2.2.3. Inhibitors and Activators
2.2.4. Differentiation of THP-1 and THP-1-Derived Cells
2.3. Detection of Total and Intracellular ROS Production
2.3.1. ROS-Inducing Stimuli
2.3.2. Luminometer-Based Analysis of ROS
2.3.3. Flow Cytometry-Based Analysis of ROS
2.4. MARCKS Reconstitution in MARCKS KO Cells
2.4.1. MARCKS Expression Plasmid
2.4.2. Transfection of MARCKS KO Cells
2.5. Proliferation Assay
2.6. Determination of Cytokine Expression
2.7. Phagocytosis Assay
2.7.1. Labelling of Bacteria
2.7.2. Phagocytosis Assay
3. Results
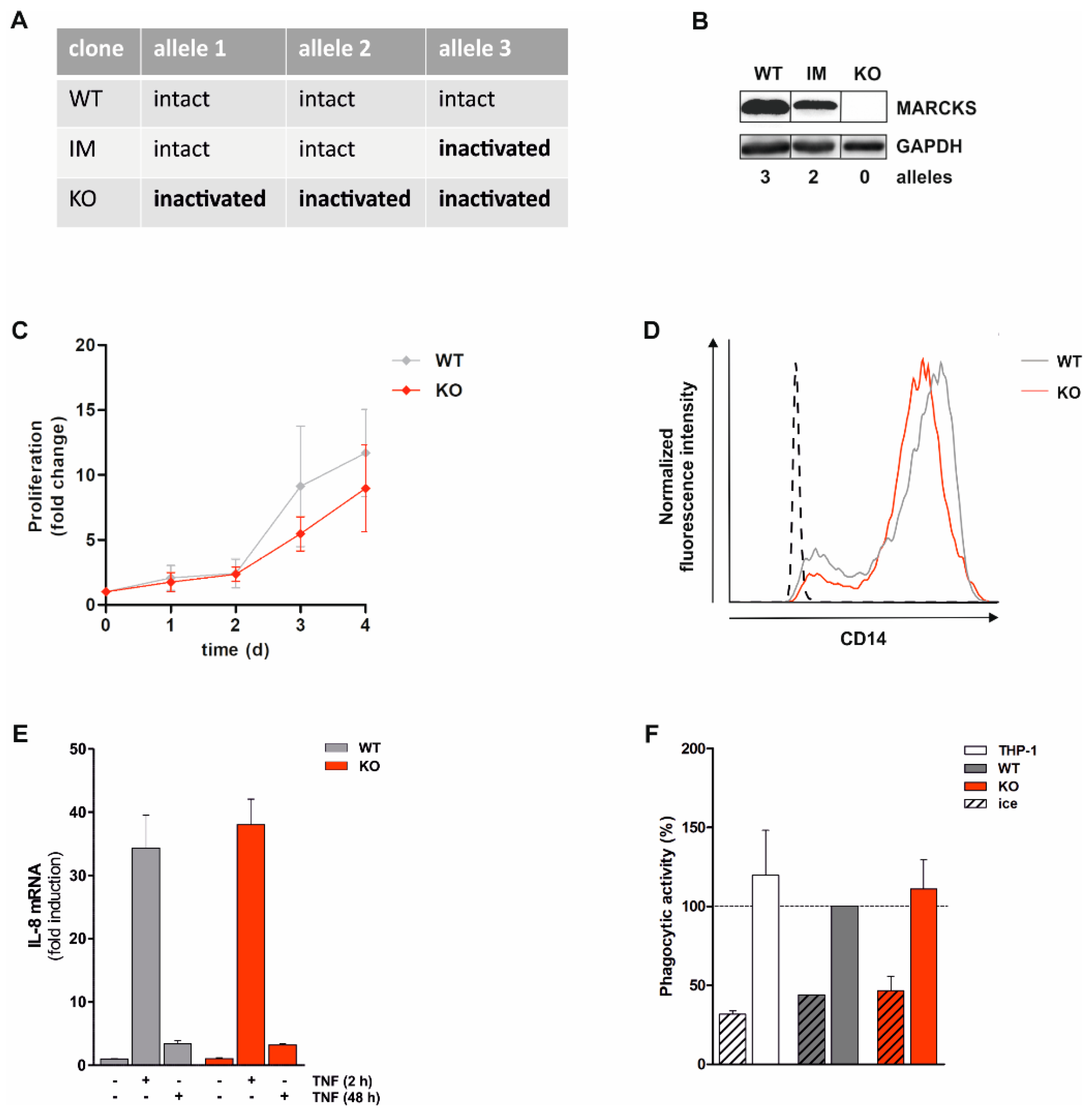
3.1. Generation and Characterization of Monocytic MARCKS KO Cell Lines
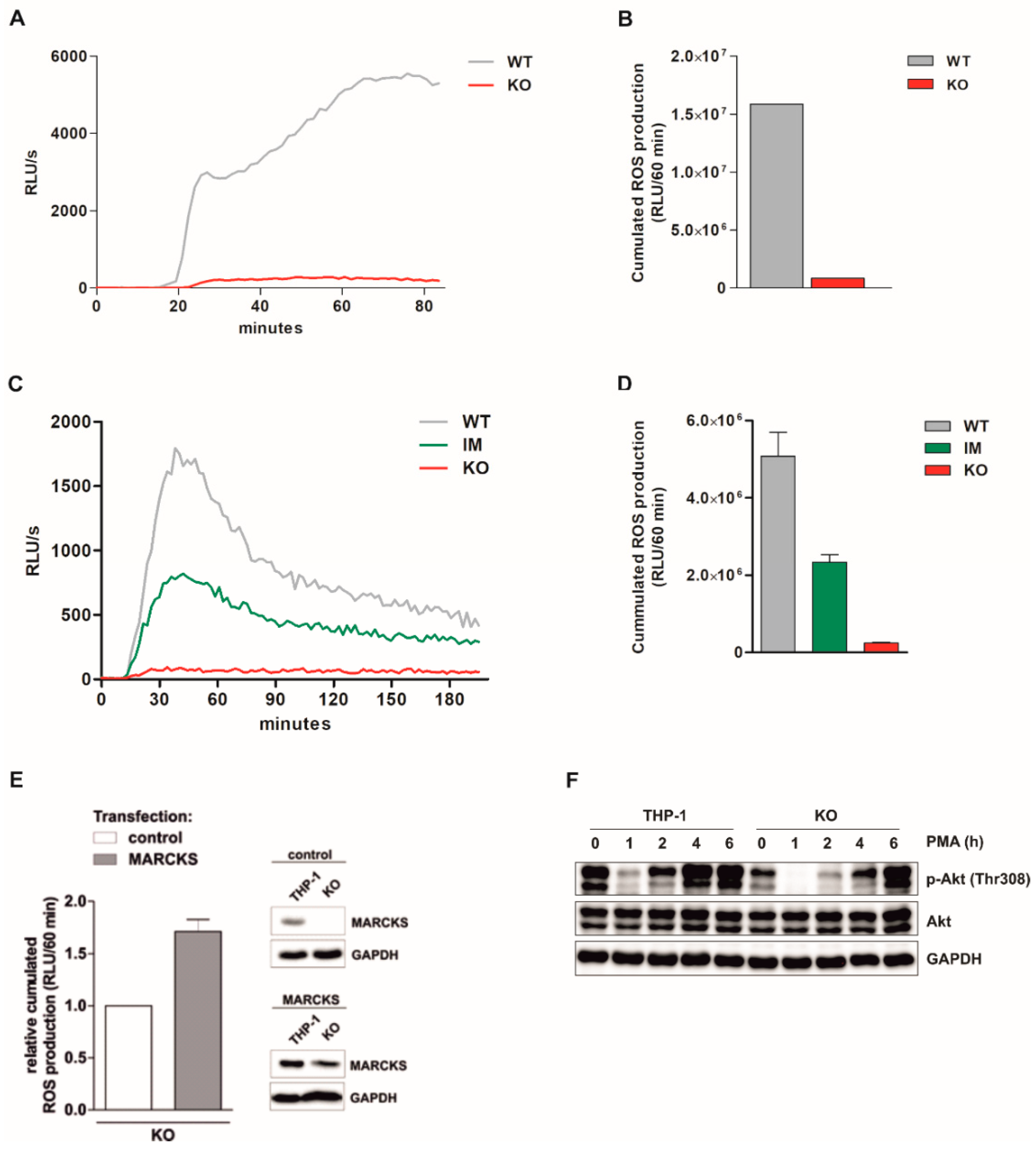
3.2. PMA-Induced Total ROS Production Is Completely Abolished in Monocytic MARCKS KO Cells
3.3. PMA-Induced Intracellular ROS Production Is Strongly Inhibited in MARCKS KO Clones and Reduced in MARCKS IM Cells
3.4. Reconstitution of MARCKS in KO Cells Restores PMA-Induced ROS Production
3.5. Basal Akt Levels Are Reduced and Akt Re-Phosphorylation Is Delayed in MARCKS KO Cells
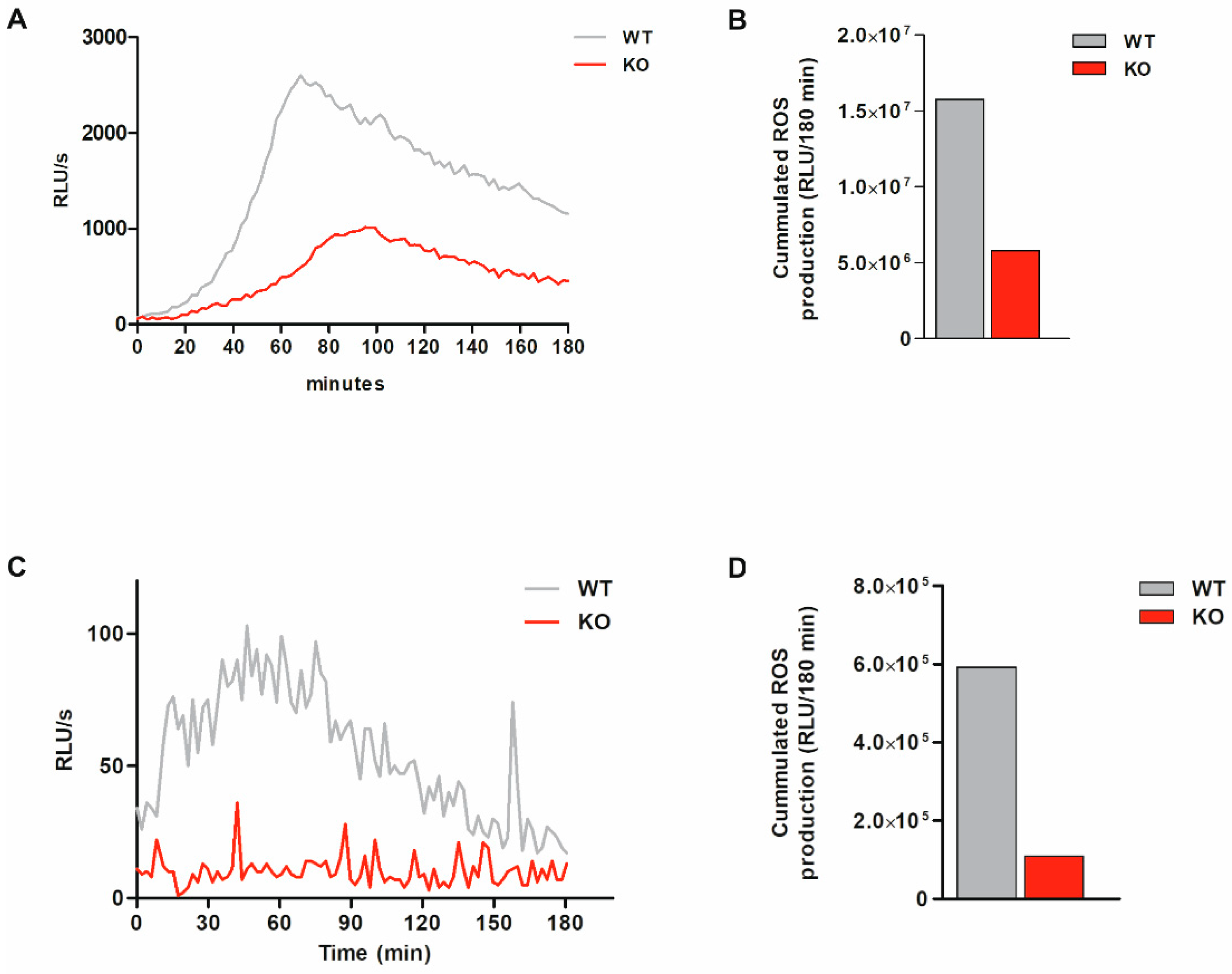
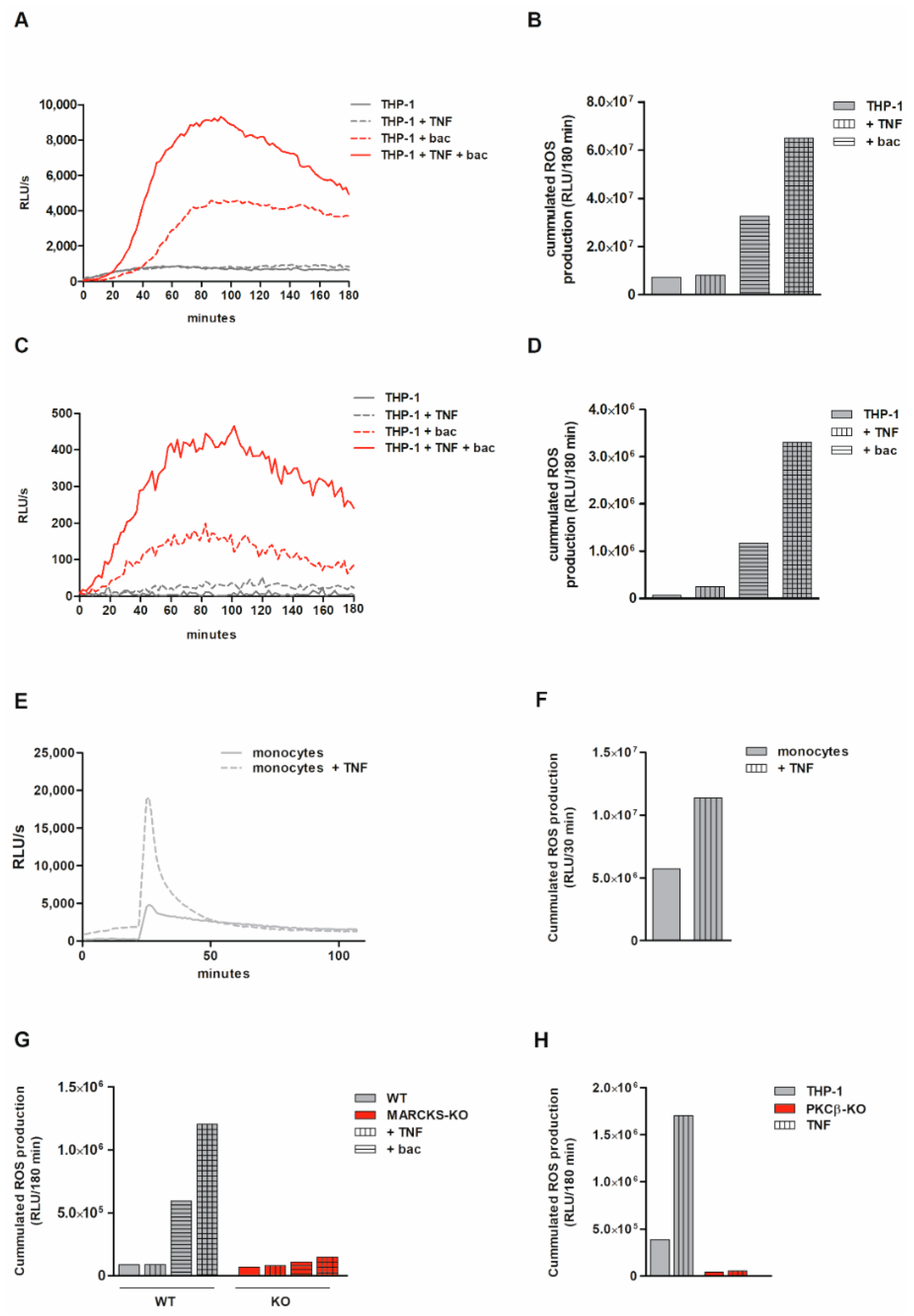
3.6. Total ROS Production Is Markedly Reduced and Intracellular ROS Production Is Abolished in Bacteria-Exposed MARCKS KO Cells
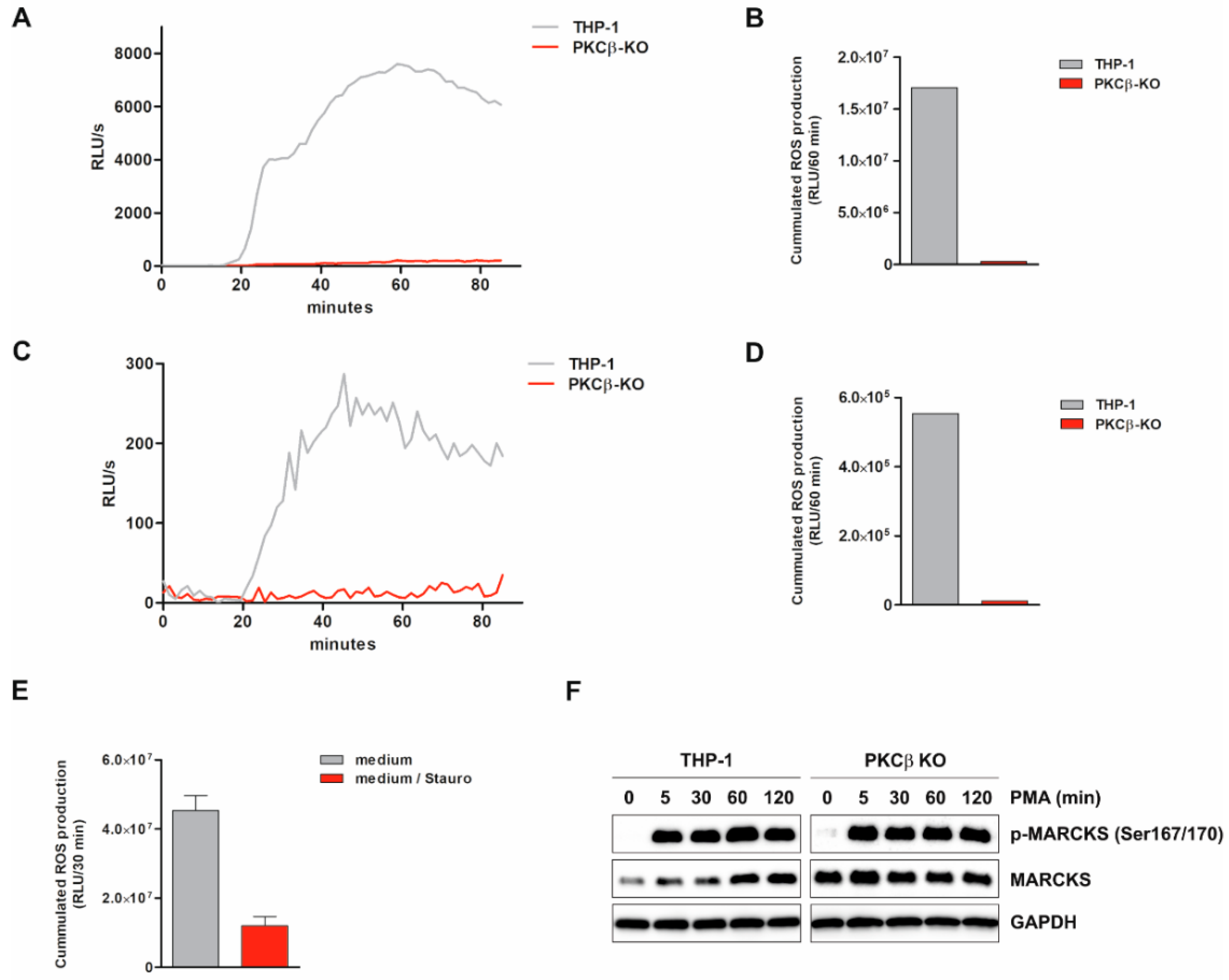
3.7. ROS Production Is Suppressed by PKCβ KO and PKC Inhibitor Staurosporine
3.8. Monocytic ROS Production Is Increased by TNF Tong-Term Preincubation Which Is Dependent on MARCKS and PKCβ
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fong, L.W.R.; Yang, D.C.; Chen, C.H. Myristoylated alanine-rich C kinase substrate (MARCKS): A multirole signaling protein in cancers. Cancer Metastasis Rev. 2017, 36, 737–747. [Google Scholar] [CrossRef] [PubMed]
- El Amri, M.; Fitzgerald, U.; Schlosser, G. MARCKS and MARCKS-like proteins in development and regeneration. J. Biomed. Sci. 2018, 25, 43. [Google Scholar] [CrossRef] [PubMed]
- Thelen, M.; Rosen, A.; Nairn, A.C.; Aderem, A. Regulation by phosphorylation of reversible association of a myristoylated protein kinase C substrate with the plasma membrane. Nature 1991, 351, 320–322. [Google Scholar] [CrossRef] [PubMed]
- Swierczynski, S.L.; Blackshear, P.J. Membrane association of the myristoylated alanine-rich C kinase substrate (MARCKS) protein. Mutational analysis provides evidence for complex interactions. J. Biol. Chem. 1995, 270, 13436–13445. [Google Scholar] [CrossRef]
- Hartwig, J.H.; Thelen, M.; Rosen, A.; Janmey, P.A.; Nairn, A.C.; Aderem, A. MARCKS is an actin filament crosslinking protein regulated by protein kinase C and calcium-calmodulin. Nature 1992, 356, 618–622. [Google Scholar] [CrossRef]
- Glaser, M.; Wanaski, S.; Buser, C.A.; Boguslavsky, V.; Rashidzada, W.; Morris, A.; Rebecchi, M.; Scarlata, S.F.; Runnels, L.W.; Prestwich, G.D.; et al. Myristoylated alanine-rich C kinase substrate (MARCKS) produces reversible inhibition of phospholipase C by sequestering phosphatidylinositol 4,5-bisphosphate in lateral domains. J. Biol. Chem. 1996, 271, 26187–26193. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.H.; Deng, C.Y.; Liu, Y.; He, M.; Peng, J.; Wang, T.; Yuan, L.; Zheng, Z.S.; Blackshear, P.J.; Luo, Z.G. MARCKS regulates membrane targeting of Rab10 vesicles to promote axon development. Cell Res. 2014, 24, 576–594. [Google Scholar] [CrossRef] [PubMed]
- Nagumo, H.; Ikenoya, M.; Sakurada, K.; Furuya, K.; Ikuhara, T.; Hiraoka, H.; Sasaki, Y. Rho-associated kinase phosphorylates MARCKS in human neuronal cells. Biochem. Biophys. Res. Commun. 2001, 280, 605–609. [Google Scholar] [CrossRef]
- Ziemba, B.P.; Burke, J.E.; Masson, G.; Williams, R.L.; Falke, J.J. Regulation of PI3K by PKC and MARCKS: Single-Molecule Analysis of a Reconstituted Signaling Pathway. Biophys. J. 2016, 110, 1811–1825. [Google Scholar] [CrossRef]
- Iyer, D.N.; Faruq, O.; Zhang, L.; Rastgoo, N.; Liu, A.; Chang, H. Pathophysiological roles of myristoylated alanine-rich C-kinase substrate (MARCKS) in hematological malignancies. Biomark. Res. 2021, 9, 34. [Google Scholar] [CrossRef]
- Chen, Z.; Zhang, W.; Selmi, C.; Ridgway, W.M.; Leung, P.S.C.; Zhang, F.; Gershwin, M.E. The myristoylated alanine-rich C-kinase substrates (MARCKS): A membrane-anchored mediator of the cell function. Autoimmun. Rev. 2021, 20, 102942. [Google Scholar] [CrossRef] [PubMed]
- Singer, M.; Martin, L.D.; Vargaftig, B.B.; Park, J.; Gruber, A.D.; Li, Y.; Adler, K.B. A MARCKS-related peptide blocks mucus hypersecretion in a mouse model of asthma. Nat. Med. 2004, 10, 193–196. [Google Scholar] [CrossRef] [PubMed]
- Brudvig, J.J.; Weimer, J.M. X MARCKS the spot: Myristoylated alanine-rich C kinase substrate in neuronal function and disease. Front. Cell Neurosci. 2015, 9, 407. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.H.; Thai, P.; Yoneda, K.; Adler, K.B.; Yang, P.C.; Wu, R. A peptide that inhibits function of Myristoylated Alanine-Rich C Kinase Substrate (MARCKS) reduces lung cancer metastasis. Oncogene 2014, 33, 3696–3706. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.C.; Li, J.M.; Xu, J.; Oldham, J.; Phan, S.H.; Last, J.A.; Wu, R.; Chen, C.H. Tackling MARCKS-PIP3 circuit attenuates fibroblast activation and fibrosis progression. FASEB J. 2019, 33, 14354–14369. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Fang, Y.; Yang, Z.; Jing, Y.; Zhang, Y.; Liu, C.; Liu, W. MARCKS regulates tonic and chronic active B cell receptor signaling. Leukemia 2019, 33, 710–729. [Google Scholar] [CrossRef]
- Green, T.D.; Park, J.; Yin, Q.; Fang, S.; Crews, A.L.; Jones, S.L.; Adler, K.B. Directed migration of mouse macrophages in vitro involves myristoylated alanine-rich C-kinase substrate (MARCKS) protein. J. Leukoc. Biol. 2012, 92, 633–639. [Google Scholar] [CrossRef]
- Ziemba, B.P.; Falke, J.J. A PKC-MARCKS-PI3K regulatory module links Ca2+ and PIP3 signals at the leading edge of polarized macrophages. PLoS ONE 2018, 13, e0196678. [Google Scholar] [CrossRef]
- Eckert, R.E.; Neuder, L.E.; Park, J.; Adler, K.B.; Jones, S.L. Myristoylated alanine-rich C-kinase substrate (MARCKS) protein regulation of human neutrophil migration. Am. J. Respir. Cell Mol. Biol. 2010, 42, 586–594. [Google Scholar] [CrossRef]
- Sheats, M.K.; Sung, E.J.; Adler, K.B.; Jones, S.L. In Vitro Neutrophil Migration Requires Protein Kinase C-Delta (delta-PKC)-Mediated Myristoylated Alanine-Rich C-Kinase Substrate (MARCKS) Phosphorylation. Inflammation 2015, 38, 1126–1141. [Google Scholar] [CrossRef]
- Allen, L.H.; Aderem, A. A role for MARCKS, the alpha isozyme of protein kinase C and myosin I in zymosan phagocytosis by macrophages. J. Exp. Med. 1995, 182, 829–840. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; D’Annibale-Tolhurst, M.A.; Adler, K.B.; Fang, S.; Yin, Q.; Birkenheuer, A.J.; Levy, M.G.; Jones, S.L.; Sung, E.J.; Hawkins, E.C.; et al. A myristoylated alanine-rich C kinase substrate-related peptide suppresses cytokine mRNA and protein expression in LPS-activated canine neutrophils. Am. J. Respir. Cell Mol. Biol. 2013, 48, 314–321. [Google Scholar] [CrossRef] [PubMed]
- Takashi, S.; Park, J.; Fang, S.; Koyama, S.; Parikh, I.; Adler, K.B. A peptide against the N-terminus of myristoylated alanine-rich C kinase substrate inhibits degranulation of human leukocytes in vitro. Am. J. Respir. Cell Mol. Biol. 2006, 34, 647–652. [Google Scholar] [CrossRef] [PubMed]
- Karunakaran, D.; Kockx, M.; Owen, D.M.; Burnett, J.R.; Jessup, W.; Kritharides, L. Protein kinase C controls vesicular transport and secretion of apolipoprotein E from primary human macrophages. J. Biol. Chem. 2013, 288, 5186–5197. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.M.; Suk, K.; Lee, W.H. Myristoylated alanine-rich C kinase substrate (MARCKS) regulates the expression of proinflammatory cytokines in macrophages through activation of p38/JNK MAPK and NF-kappaB. Cell Immunol. 2015, 296, 115–121. [Google Scholar] [CrossRef] [PubMed]
- Welz, B.; Bikker, R.; Junemann, J.; Christmann, M.; Neumann, K.; Weber, M.; Hoffmeister, L.; Preuss, K.; Pich, A.; Huber, R.; et al. Proteome and Phosphoproteome Analysis in TNF Long Term-Exposed Primary Human Monocytes. Int. J. Mol. Sci. 2019, 20, 1241. [Google Scholar] [CrossRef]
- Doudna, J.A.; Charpentier, E. Genome editing. The new frontier of genome engineering with CRISPR-Cas9. Science 2014, 346, 1258096. [Google Scholar] [CrossRef]
- Paijo, J.; Doring, M.; Spanier, J.; Grabski, E.; Nooruzzaman, M.; Schmidt, T.; Witte, G.; Messerle, M.; Hornung, V.; Kaever, V.; et al. cGAS Senses Human Cytomegalovirus and Induces Type I Interferon Responses in Human Monocyte-Derived Cells. PLoS Pathog. 2016, 12, e1005546. [Google Scholar] [CrossRef]
- Ran, F.A.; Hsu, P.D.; Wright, J.; Agarwala, V.; Scott, D.A.; Zhang, F. Genome engineering using the CRISPR-Cas9 system. Nat. Protoc. 2013, 8, 2281–2308. [Google Scholar] [CrossRef]
- Tsuchiya, S.; Yamabe, M.; Yamaguchi, Y.; Kobayashi, Y.; Konno, T.; Tada, K. Establishment and characterization of a human acute monocytic leukemia cell line (THP-1). Int. J. Cancer 1980, 26, 171–176. [Google Scholar] [CrossRef]
- Odero, M.D.; Zeleznik-Le, N.J.; Chinwalla, V.; Rowley, J.D. Cytogenetic and molecular analysis of the acute monocytic leukemia cell line THP-1 with an MLL-AF9 translocation. Genes Chromosomes Cancer 2000, 29, 333–338. [Google Scholar] [CrossRef]
- Doench, J.G.; Fusi, N.; Sullender, M.; Hegde, M.; Vaimberg, E.W.; Donovan, K.F.; Smith, I.; Tothova, Z.; Wilen, C.; Orchard, R.; et al. Optimized sgRNA design to maximize activity and minimize off-target effects of CRISPR-Cas9. Nat. Biotechnol. 2016, 34, 184–191. [Google Scholar] [CrossRef]
- Gunther, J.; Vogt, N.; Hampel, K.; Bikker, R.; Page, S.; Muller, B.; Kandemir, J.; Kracht, M.; Dittrich-Breiholz, O.; Huber, R.; et al. Identification of two forms of TNF tolerance in human monocytes: Differential inhibition of NF-kappaB/AP-1- and PP1-associated signaling. J. Immunol. 2014, 192, 3143–3155. [Google Scholar] [CrossRef] [PubMed]
- Welz, B.; Bikker, R.; Hoffmeister, L.; Diekmann, M.; Christmann, M.; Brand, K.; Huber, R. Activation of GSK3 Prevents Termination of TNF-Induced Signaling. J. Inflamm. Res. 2021, 14, 1717–1730. [Google Scholar] [CrossRef] [PubMed]
- Huber, R.; Panterodt, T.; Welz, B.; Christmann, M.; Friesenhagen, J.; Westphal, A.; Pietsch, D.; Brand, K. C/EBPbeta-LAP*/LAP Expression Is Mediated by RSK/eIF4B-Dependent Signalling and Boosted by Increased Protein Stability in Models of Monocytic Differentiation. PLoS ONE 2015, 10, e0144338. [Google Scholar] [CrossRef]
- Haas, S.C.; Huber, R.; Gutsch, R.; Kandemir, J.D.; Cappello, C.; Krauter, J.; Duyster, J.; Ganser, A.; Brand, K. ITD- and FL-induced FLT3 signal transduction leads to increased C/EBPbeta-LIP expression and LIP/LAP ratio by different signalling modules. Br. J. Haematol. 2010, 148, 777–790. [Google Scholar] [CrossRef]
- Arendt, L.M.; McCready, J.; Keller, P.J.; Baker, D.D.; Naber, S.P.; Seewaldt, V.; Kuperwasser, C. Obesity promotes breast cancer by CCL2-mediated macrophage recruitment and angiogenesis. Cancer Res. 2013, 73, 6080–6093. [Google Scholar] [CrossRef]
- Hoxhaj, G.; Manning, B.D. The PI3K-AKT network at the interface of oncogenic signalling and cancer metabolism. Nat. Rev. Cancer 2020, 20, 74–88. [Google Scholar] [CrossRef]
- Elzagallaai, A.; Rose, S.D.; Trifaro, J.M. Platelet secretion induced by phorbol esters stimulation is mediated through phosphorylation of MARCKS: A MARCKS-derived peptide blocks MARCKS phosphorylation and serotonin release without affecting pleckstrin phosphorylation. Blood 2000, 95, 894–902. [Google Scholar] [CrossRef]
- Volpe, C.M.O.; Villar-Delfino, P.H.; Dos Anjos, P.M.F.; Nogueira-Machado, J.A. Cellular death, reactive oxygen species (ROS) and diabetic complications. Cell Death Dis. 2018, 9, 119. [Google Scholar] [CrossRef]
- Bikker, R.; Christmann, M.; Preuss, K.; Welz, B.; Friesenhagen, J.; Dittrich-Breiholz, O.; Huber, R.; Brand, K. TNF phase III signalling in tolerant cells is tightly controlled by A20 and CYLD. Cell Signal. 2017, 37, 123–135. [Google Scholar] [CrossRef] [PubMed]
- Huber, R.; Bikker, R.; Welz, B.; Christmann, M.; Brand, K. TNF Tolerance in Monocytes and Macrophages: Characteristics and Molecular Mechanisms. J. Immunol. Res. 2017, 2017, 9570129. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Ongil, S.; Torrecillas, G.; Perez-Sala, D.; Gonzalez-Santiago, L.; Rodriguez-Puyol, M.; Rodriguez-Puyol, D. Mechanisms involved in the contraction of endothelial cells by hydrogen peroxide. Free Radic. Biol. Med. 1999, 26, 501–510. [Google Scholar] [CrossRef]
- Reddy, M.M.; Fernandes, M.S.; Salgia, R.; Levine, R.L.; Griffin, J.D.; Sattler, M. NADPH oxidases regulate cell growth and migration in myeloid cells transformed by oncogenic tyrosine kinases. Leukemia 2011, 25, 281–289. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kalwa, H.; Sartoretto, J.L.; Martinelli, R.; Romero, N.; Steinhorn, B.S.; Tao, M.; Ozaki, C.K.; Carman, C.V.; Michel, T. Central role for hydrogen peroxide in P2Y1 ADP receptor-mediated cellular responses in vascular endothelium. Proc. Natl. Acad. Sci. USA 2014, 111, 3383–3388. [Google Scholar] [CrossRef] [PubMed]
- Kalwa, H.; Sartoretto, J.L.; Sartoretto, S.M.; Michel, T. Angiotensin-II and MARCKS: A hydrogen peroxide- and RAC1-dependent signaling pathway in vascular endothelium. J. Biol. Chem. 2012, 287, 29147–29158. [Google Scholar] [CrossRef]
- Jin, B.Y.; Lin, A.J.; Golan, D.E.; Michel, T. MARCKS protein mediates hydrogen peroxide regulation of endothelial permeability. Proc. Natl. Acad. Sci. USA 2012, 109, 14864–14869. [Google Scholar] [CrossRef]
- Jakubzick, C.V.; Randolph, G.J.; Henson, P.M. Monocyte differentiation and antigen-presenting functions. Nat. Rev. Immunol. 2017, 17, 349–362. [Google Scholar] [CrossRef]
- Carballo, E.; Pitterle, D.M.; Stumpo, D.J.; Sperling, R.T.; Blackshear, P.J. Phagocytic and macropinocytic activity in MARCKS-deficient macrophages and fibroblasts. Am. J. Physiol. 1999, 277, C163–C173. [Google Scholar] [CrossRef]
- Kinchen, J.M.; Ravichandran, K.S. Phagosome maturation: Going through the acid test. Nat. Rev. Mol. Cell Biol. 2008, 9, 781–795. [Google Scholar] [CrossRef]
- Li, J.; Aderem, A. MacMARCKS, a novel member of the MARCKS family of protein kinase C substrates. Cell 1992, 70, 791–801. [Google Scholar] [CrossRef]
- Mochly-Rosen, D.; Das, K.; Grimes, K.V. Protein kinase C, an elusive therapeutic target? Nat. Rev. Drug. Discov. 2012, 11, 937–957. [Google Scholar] [CrossRef]
- Deng, B.; Xie, S.; Wang, J.; Xia, Z.; Nie, R. Inhibition of protein kinase C beta(2) prevents tumor necrosis factor-alpha-induced apoptosis and oxidative stress in endothelial cells: The role of NADPH oxidase subunits. J. Vasc. Res. 2012, 49, 144–159. [Google Scholar] [CrossRef] [PubMed]
- Haller, M.; Khalid, S.; Kremser, L.; Fresser, F.; Furlan, T.; Hermann, M.; Guenther, J.; Drasche, A.; Leitges, M.; Giorgio, M.; et al. Novel Insights into the PKCbeta-dependent Regulation of the Oxidoreductase p66Shc. J. Biol. Chem. 2016, 291, 23557–23568. [Google Scholar] [CrossRef] [PubMed]
- Bey, E.A.; Xu, B.; Bhattacharjee, A.; Oldfield, C.M.; Zhao, X.; Li, Q.; Subbulakshmi, V.; Feldman, G.M.; Wientjes, F.B.; Cathcart, M.K. Protein kinase C delta is required for p47phox phosphorylation and translocation in activated human monocytes. J. Immunol. 2004, 173, 5730–5738. [Google Scholar] [CrossRef] [PubMed]
- Lu, D.; Yang, H.; Lenox, R.H.; Raizada, M.K. Regulation of angiotensin II-induced neuromodulation by MARCKS in brain neurons. J. Cell Biol. 1998, 142, 217–227. [Google Scholar] [CrossRef]
- Inoguchi, T.; Sonta, T.; Tsubouchi, H.; Etoh, T.; Kakimoto, M.; Sonoda, N.; Sato, N.; Sekiguchi, N.; Kobayashi, K.; Sumimoto, H.; et al. Protein kinase C-dependent increase in reactive oxygen species (ROS) production in vascular tissues of diabetes: Role of vascular NAD(P)H oxidase. J. Am. Soc. Nephrol. 2003, 14, S227–S232. [Google Scholar] [CrossRef]
- Dekker, L.V.; Leitges, M.; Altschuler, G.; Mistry, N.; McDermott, A.; Roes, J.; Segal, A.W. Protein kinase C-beta contributes to NADPH oxidase activation in neutrophils. Biochem. J. 2000, 347 Pt 1, 285–289. [Google Scholar] [CrossRef]
- Blaser, H.; Dostert, C.; Mak, T.W.; Brenner, D. TNF and ROS Crosstalk in Inflammation. Trends Cell Biol. 2016, 26, 249–261. [Google Scholar] [CrossRef]
- Buckley, C.D.; Gilroy, D.W.; Serhan, C.N.; Stockinger, B.; Tak, P.P. The resolution of inflammation. Nat. Rev. Immunol. 2013, 13, 59–66. [Google Scholar] [CrossRef]
- Fullerton, J.N.; Gilroy, D.W. Resolution of inflammation: A new therapeutic frontier. Nat. Rev. Drug. Discov. 2016, 15, 551–567. [Google Scholar] [CrossRef] [PubMed]
- Nathan, C.; Cunningham-Bussel, A. Beyond oxidative stress: An immunologist’s guide to reactive oxygen species. Nat. Rev. Immunol. 2013, 13, 349–361. [Google Scholar] [CrossRef] [PubMed]
- Ezraty, B.; Gennaris, A.; Barras, F.; Collet, J.F. Oxidative stress, protein damage and repair in bacteria. Nat. Rev. Microbiol. 2017, 15, 385–396. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huber, R.; Diekmann, M.; Hoffmeister, L.; Kühl, F.; Welz, B.; Brand, K. MARCKS Is an Essential Regulator of Reactive Oxygen Species Production in the Monocytic Cell Type. Antioxidants 2022, 11, 1600. https://doi.org/10.3390/antiox11081600
Huber R, Diekmann M, Hoffmeister L, Kühl F, Welz B, Brand K. MARCKS Is an Essential Regulator of Reactive Oxygen Species Production in the Monocytic Cell Type. Antioxidants. 2022; 11(8):1600. https://doi.org/10.3390/antiox11081600
Chicago/Turabian StyleHuber, René, Mareike Diekmann, Leonie Hoffmeister, Friederike Kühl, Bastian Welz, and Korbinian Brand. 2022. "MARCKS Is an Essential Regulator of Reactive Oxygen Species Production in the Monocytic Cell Type" Antioxidants 11, no. 8: 1600. https://doi.org/10.3390/antiox11081600
APA StyleHuber, R., Diekmann, M., Hoffmeister, L., Kühl, F., Welz, B., & Brand, K. (2022). MARCKS Is an Essential Regulator of Reactive Oxygen Species Production in the Monocytic Cell Type. Antioxidants, 11(8), 1600. https://doi.org/10.3390/antiox11081600





