Genetic and Pharmacological Inhibition of GCN2 Ameliorates Hyperglycemia and Insulin Resistance in Type 2 Diabetic Mice
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents and Antibodies
2.2. Experimental Animals
2.3. Histopathology Staining
2.4. Western Blot Analysis and Quantitative Real-Time Polymerase Chain Reaction (qPCR) Analysis
2.5. Data and Statistical Analysis
3. Results
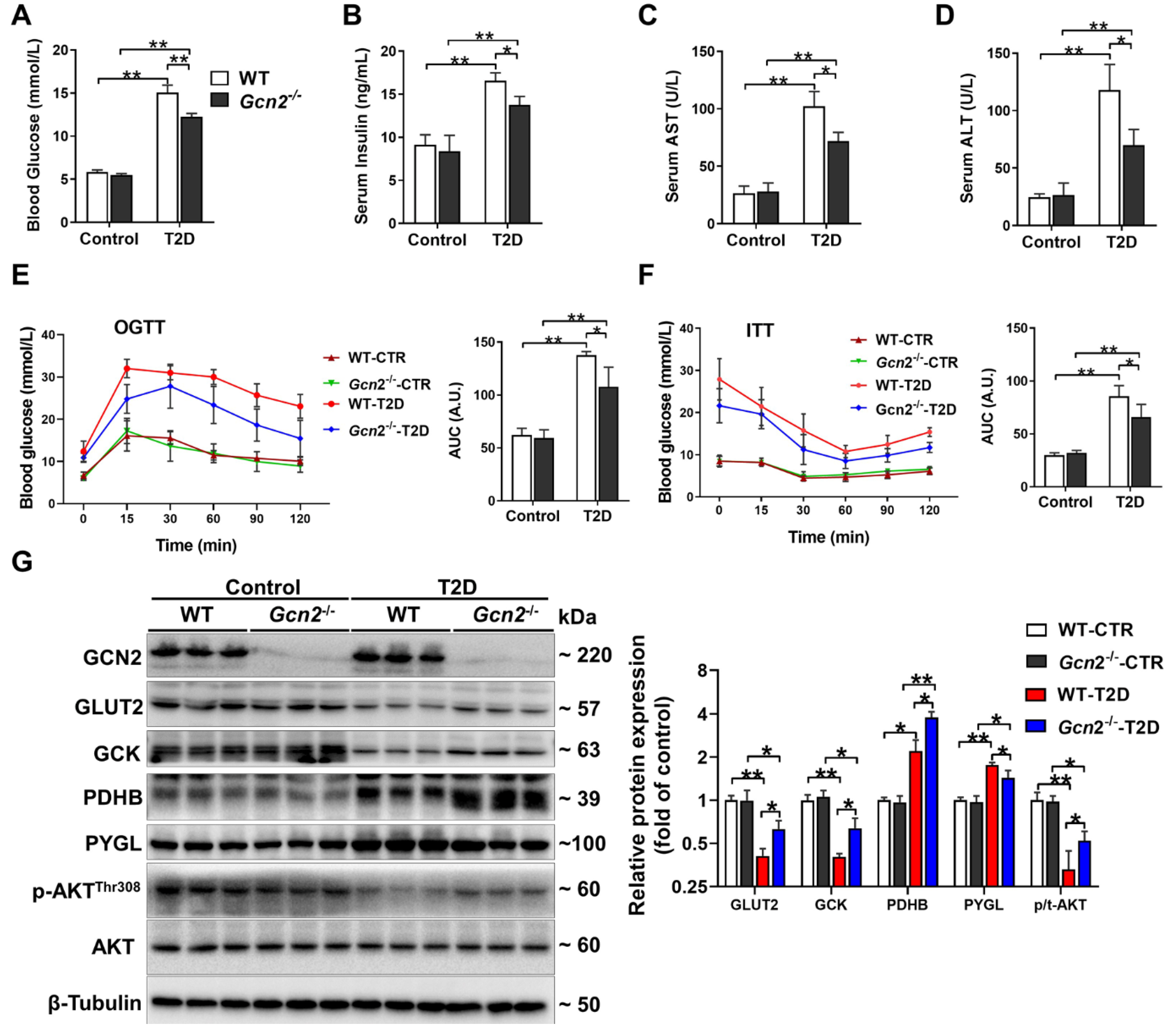
3.1. GCN2 Deficiency Ameliorates Hyperglycemia, Liver Dysfunction, and Insulin Resistance in HFD/STZ-Induced T2D Mice
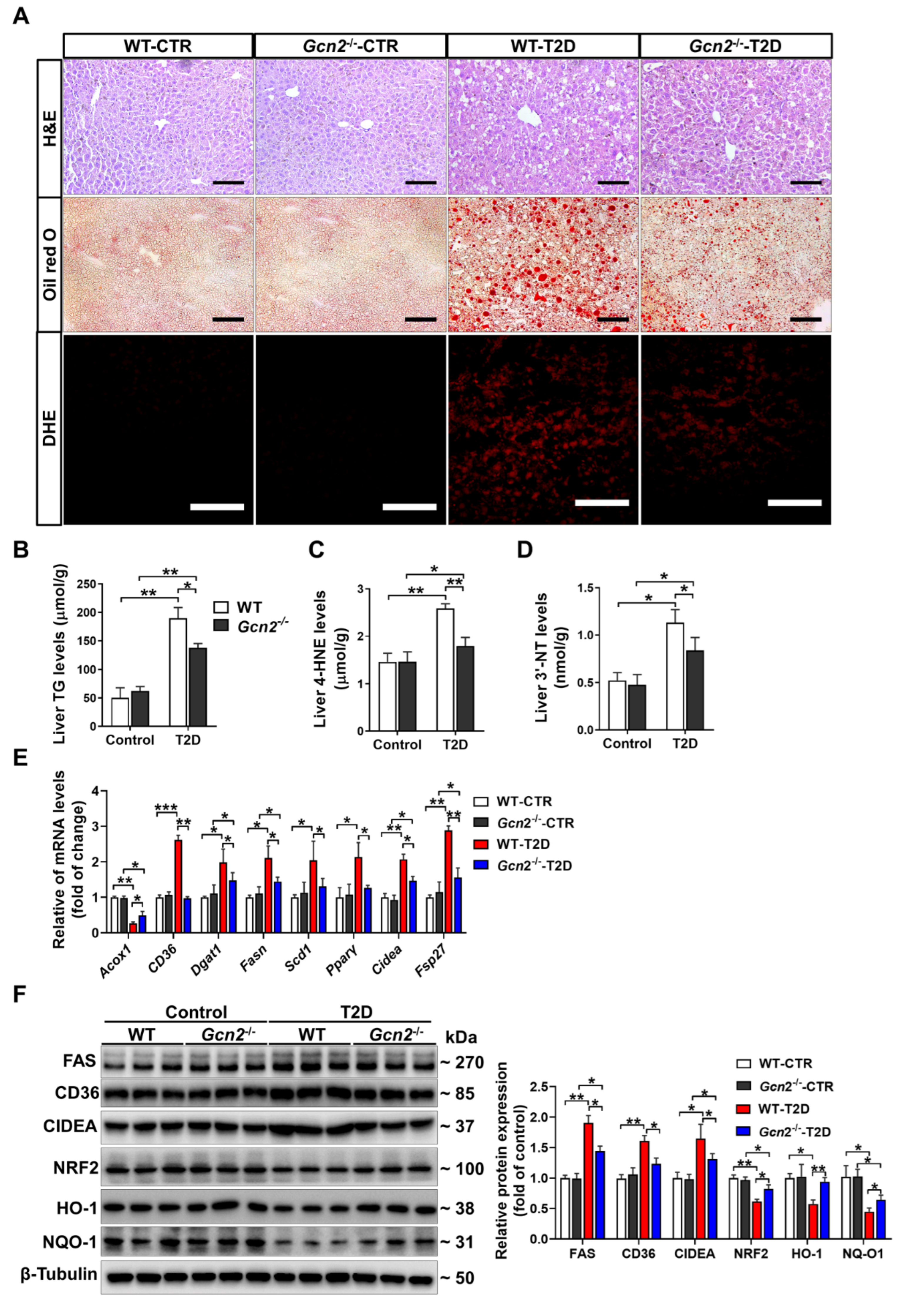
3.2. GCN2 Deficiency Alleviates Hepatic Steatosis and Oxidative Stress in T2D Mice
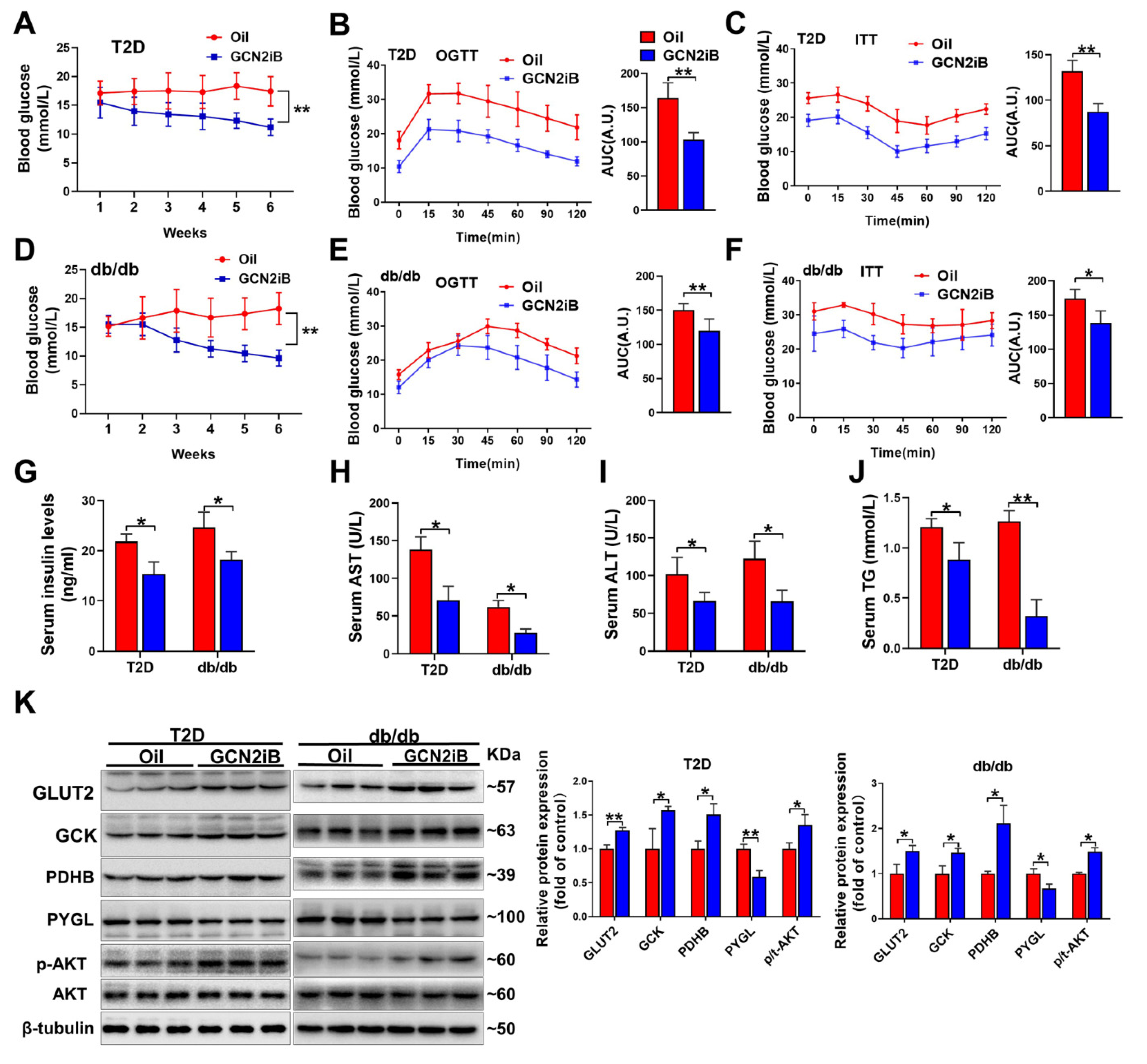
3.3. Inhibition of GCN2 Improves Insulin Sensitivity and Alleviates Hepatic Steatosis and Oxidative Stress in T2D Mice
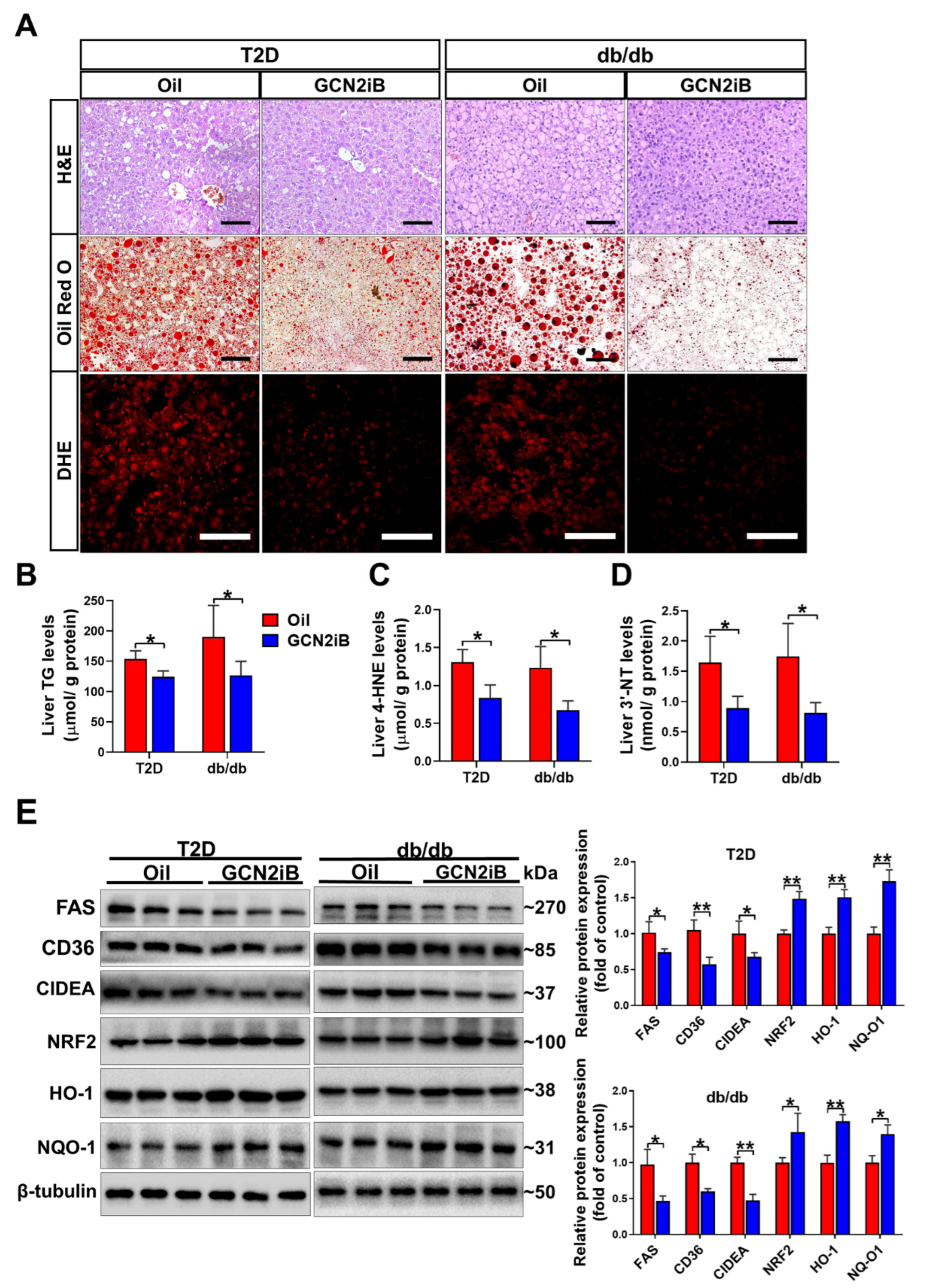
3.4. GCN2iB Ameliorates Hepatic Steatosis and Oxidative Stress in T2D Mice
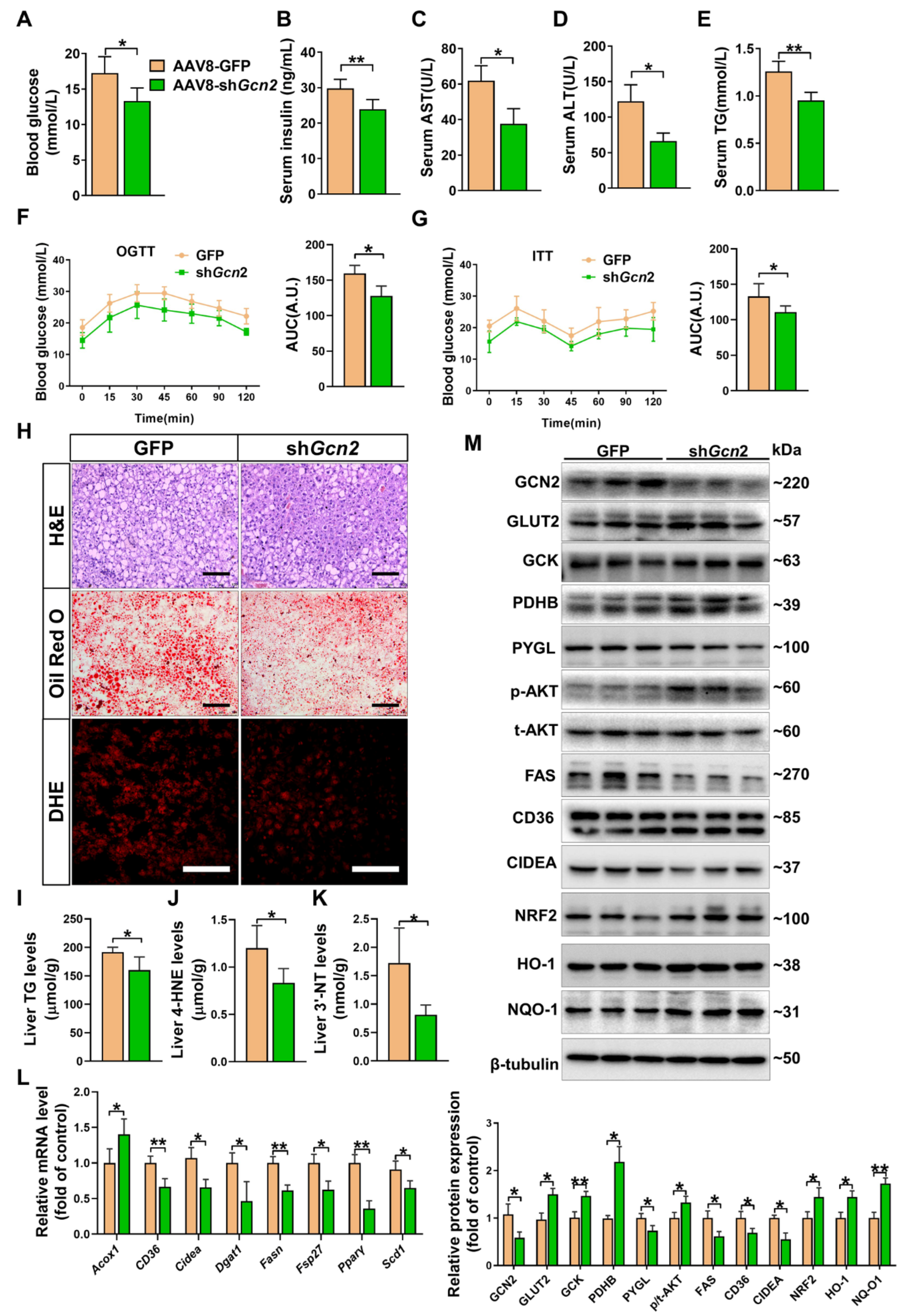
3.5. Depletion of Hepatic Gcn2 Ameliorates Insulin Resistance, Hepatic Steatosis, and Oxidative Stress in db/db Mice
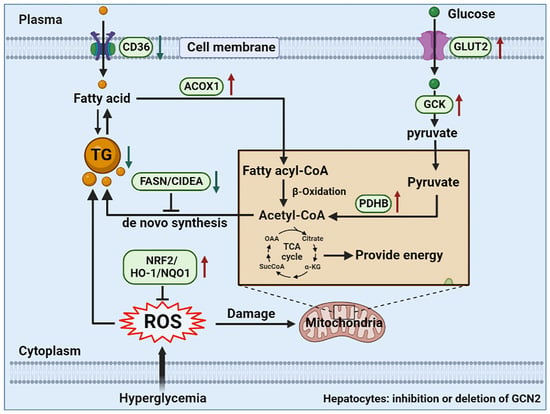
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Goedeke, L.; Perry, R.J.; Shulman, G.I. Emerging Pharmacological Targets for the Treatment of Nonalcoholic Fatty Liver Disease, Insulin Resistance, and Type 2 Diabetes. Annu. Rev. Pharmacol. Toxicol. 2019, 59, 65–87. [Google Scholar] [CrossRef]
- Hunter, R.W.; Hughey, C.C.; Lantier, L.; Sundelin, E.I.; Peggie, M.; Zeqiraj, E.; Sicheri, F.; Jessen, N.; Wasserman, D.H.; Sakamoto, K. Metformin reduces liver glucose production by inhibition of fructose-1-6-bisphosphatase. Nat. Med. 2018, 24, 1395–1406. [Google Scholar] [CrossRef]
- Madiraju, A.K.; Qiu, Y.; Perry, R.J.; Rahimi, Y.; Zhang, X.M.; Zhang, D.; Camporez, J.G.; Cline, G.W.; Butrico, G.M.; Kemp, B.E.; et al. Metformin inhibits gluconeogenesis via a redox-dependent mechanism in vivo. Nat. Med. 2018, 24, 1384–1394. [Google Scholar] [CrossRef]
- Cao, J.; Zheng, R.; Chang, X.; Zhao, Y.; Zhang, D.; Gao, M.; Yin, Z.; Jiang, C.; Zhang, J. Cyclocarya paliurus triterpenoids suppress hepatic gluconeogenesis via AMPK-mediated cAMP/PKA/CREB pathway. Phytomed. Int. J. Phytother. Phytopharm. 2022, 102, 154175. [Google Scholar] [CrossRef]
- Hu, M.; Phan, F.; Bourron, O.; Ferré, P.; Foufelle, F. Steatosis and NASH in type 2 diabetes. Biochimie 2017, 143, 37–41. [Google Scholar] [CrossRef]
- Perry, R.J.; Samuel, V.T.; Petersen, K.F.; Shulman, G.I. The role of hepatic lipids in hepatic insulin resistance and type 2 diabetes. Nature 2014, 510, 84–91. [Google Scholar] [CrossRef]
- Dong, J.; Qiu, H.; Garcia-Barrio, M.; Anderson, J.; Hinnebusch, A.G. Uncharged tRNA activates GCN2 by displacing the protein kinase moiety from a bipartite tRNA-binding domain. Mol. Cell 2000, 6, 269–279. [Google Scholar] [CrossRef]
- Sood, R.; Porter, A.C.; Olsen, D.A.; Cavener, D.R.; Wek, R.C. A mammalian homologue of GCN2 protein kinase important for translational control by phosphorylation of eukaryotic initiation factor-2alpha. Genetics 2000, 154, 787–801. [Google Scholar] [CrossRef]
- Xiao, F.; Huang, Z.; Li, H.; Yu, J.; Wang, C.; Chen, S.; Meng, Q.; Cheng, Y.; Gao, X.; Li, J.; et al. Leucine deprivation increases hepatic insulin sensitivity via GCN2/mTOR/S6K1 and AMPK pathways. Diabetes 2011, 60, 746–756. [Google Scholar] [CrossRef]
- Hanrui, Y.; Feixiang, Y.; Fuxin, J.; Yuguo, N.; Xiaoxue, J.; Jiali, D.; Yajie, G.; Shanghai, C.; Qiwei, Z.; Cheng, H.; et al. Intermittent Leucine Deprivation Produces Long-Lasting Improvement in Insulin Sensitivity by Increasing Hepatic Gcn2 Expression. Diabetes 2021, 10, 124–135. [Google Scholar] [CrossRef]
- Xu, X.; Hu, J.; McGrath, B.C.; Cavener, D.R. GCN2 regulates the CCAAT enhancer binding protein beta and hepatic gluconeogenesis. American journal of physiology. Endocrinol. Metab. 2013, 305, E1007–E1017. [Google Scholar] [CrossRef][Green Version]
- Liu, S.; Yuan, J.; Yue, W.; Bi, Y.; Shen, X.; Gao, J.; Xu, X.; Lu, Z. GCN2 deficiency protects against high fat diet induced hepatic steatosis and insulin resistance in mice. Biochimica et biophysica acta. Mol. Basis Dis. 2018, 1864, 3257–3267. [Google Scholar] [CrossRef]
- Feng, W.; Lei, T.; Wang, Y.; Feng, R.; Yuan, J.; Shen, X.; Wu, Y.; Gao, J.; Ding, W.; Lu, Z. GCN2 deficiency ameliorates cardiac dysfunction in diabetic mice by reducing lipotoxicity and oxidative stress. Free. Radic. Biol. Med. 2019, 130, 128–139. [Google Scholar] [CrossRef]
- Yuan, J.; Yu, Z.; Gao, J.; Luo, K.; Shen, X.; Cui, B.; Lu, Z. Inhibition of GCN2 alleviates hepatic steatosis and oxidative stress in obese mice: Involvement of NRF2 regulation. Redox Biol. 2022, 49, 102224. [Google Scholar] [CrossRef]
- Shi, L.; Zhao, C.; Wang, H.; Lei, T.; Liu, S.; Cao, J.; Lu, Z. Dimethylarginine Dimethylaminohydrolase 1 Deficiency Induces the Epithelial to Mesenchymal Transition in Renal Proximal Tubular Epithelial Cells and Exacerbates Kidney Damage in Aged and Diabetic Mice. Antioxid. Redox Signal. 2017, 27, 1347–1360. [Google Scholar] [CrossRef]
- Li, T.; Feng, R.; Zhao, C.; Wang, Y.; Wang, J.; Liu, S.; Cao, J.; Wang, H.; Wang, T.; Guo, Y.; et al. Dimethylarginine Dimethylaminohydrolase 1 Protects Against High-Fat Diet-Induced Hepatic Steatosis and Insulin Resistance in Mice. Antioxid. Redox Signal. 2017, 26, 598–609. [Google Scholar] [CrossRef]
- Puigserver, P.; Rhee, J.; Donovan, J.; Walkey, C.J.; Yoon, J.C.; Oriente, F.; Kitamura, Y.; Altomonte, J.; Dong, H.; Accili, D.; et al. Insulin-regulated hepatic gluconeogenesis through FOXO1-PGC-1alpha interaction. Nature 2003, 423, 550–555. [Google Scholar] [CrossRef]
- Thiel, G.; Guethlein, L.A.; Rössler, O.G. Insulin-Responsive Transcription Factors. Biomolecules 2021, 11, 1886. [Google Scholar] [CrossRef]
- Jung, J.G.; Yi, S.A.; Choi, S.E.; Kang, Y.; Kim, T.H.; Jeon, J.Y.; Bae, M.A.; Ahn, J.H.; Jeong, H.; Hwang, E.S.; et al. TM-25659-Induced Activation of FGF21 Level Decreases Insulin Resistance and Inflammation in Skeletal Muscle via GCN2 Pathways. Mol. Cells 2015, 38, 1037–1043. [Google Scholar] [CrossRef]
- Bröer, S. Amino acid transporters as modulators of glucose homeostasis. Trends Endocrinol. Metab. TEM 2022, 33, 120–135. [Google Scholar] [CrossRef]
- Kanno, A.; Asahara, S.I.; Furubayashi, A.; Masuda, K.; Yoshitomi, R.; Suzuki, E.; Takai, T.; Kimura-Koyanagi, M.; Matsuda, T.; Bartolome, A.; et al. GCN2 regulates pancreatic β cell mass by sensing intracellular amino acid levels. JCI Insight 2020, 5, e128820. [Google Scholar] [CrossRef]
- Seyer, P.; Vallois, D.; Poitry-Yamate, C.; Schütz, F.; Metref, S.; Tarussio, D.; Maechler, P.; Staels, B.; Lanz, B.; Grueter, R.; et al. Hepatic glucose sensing is required to preserve β cell glucose competence. J. Clin. Investig. 2013, 123, 1662–1676. [Google Scholar] [CrossRef]
- Laukkanen, O.; Lindström, J.; Eriksson, J.; Valle, T.T.; Hämäläinen, H.; Ilanne-Parikka, P.; Keinänen-Kiukaanniemi, S.; Tuomilehto, J.; Uusitupa, M.; Laakso, M. Polymorphisms in the SLC2A2 (GLUT2) gene are associated with the conversion from impaired glucose tolerance to type 2 diabetes: The Finnish Diabetes Prevention Study. Diabetes 2005, 54, 2256–2260. [Google Scholar] [CrossRef]
- Liu, P.; Jiang, L.; Kong, W.; Xie, Q.; Li, P.; Liu, X.; Zhang, J.; Liu, M.; Wang, Z.; Zhu, L.; et al. PXR activation impairs hepatic glucose metabolism partly via inhibiting the HNF4α-GLUT2 pathway. Acta Pharm. Sin. B 2022, 12, 2391–2405. [Google Scholar] [CrossRef]
- de Souza Cordeiro, L.M.; Bainbridge, L.; Devisetty, N.; McDougal, D.H.; Peters, D.J.M.; Chhabra, K.H. Loss of function of renal Glut2 reverses hyperglycaemia and normalises body weight in mouse models of diabetes and obesity. Diabetologia 2022, 65, 1032–1047. [Google Scholar] [CrossRef]
- Lu, G.; Teng, X.; Zheng, Z.; Zhang, R.; Peng, L.; Zheng, F.; Liu, J.; Huang, H.; Xiong, H. Overexpression of a glucokinase point mutant in the treatment of diabetes mellitus. Gene Ther. 2016, 23, 323–329. [Google Scholar] [CrossRef]
- Caro, J.F.; Triester, S.; Patel, V.K.; Tapscott, E.B.; Frazier, N.L.; Dohm, G.L. Liver glucokinase: Decreased activity in patients with type II diabetes. Horm. Metab. Res. 1995, 27, 19–22. [Google Scholar] [CrossRef]
- Haeusler, R.A.; Camastra, S.; Astiarraga, B.; Nannipieri, M.; Anselmino, M.; Ferrannini, E. Decreased expression of hepatic glucokinase in type 2 diabetes. Mol. Metab. 2015, 4, 222–226. [Google Scholar] [CrossRef]
- Thilagavathi, R.; Hosseini-Zare, M.S.; Malini, M.; Selvam, C. A comprehensive review on glucokinase activators: Promising agents for the treatment of Type 2 diabetes. Chem. Biol. Drug Des. 2022, 99, 247–263. [Google Scholar] [CrossRef]
- Zhang, W.; Patil, S.; Chauhan, B.; Guo, S.; Powell, D.R.; Le, J.; Klotsas, A.; Matika, R.; Xiao, X.; Franks, R.; et al. FoxO1 regulates multiple metabolic pathways in the liver: Effects on gluconeogenic, glycolytic, and lipogenic gene expression. J. Biol. Chem. 2006, 281, 10105–10117. [Google Scholar] [CrossRef]
- Kotronen, A.; Juurinen, L.; Hakkarainen, A.; Westerbacka, J.; Cornér, A.; Bergholm, R.; Yki-Järvinen, H. Liver fat is increased in type 2 diabetic patients and underestimated by serum alanine aminotransferase compared with equally obese non-diabetic subjects. Diabetes Care 2008, 31, 165–169. [Google Scholar] [CrossRef]
- Matsuzaka, T.; Shimano, H. Molecular mechanisms involved in hepatic steatosis and insulin resistance. J. Diabetes Investig. 2011, 2, 170–175. [Google Scholar] [CrossRef]
- Vezza, T.; de Marañón, A.M.; Canet, F.; Díaz-Pozo, P.; Marti, M.; D’Ocon, P.; Apostolova, N.; Rocha, M.; Víctor, V.M. MicroRNAs and Oxidative Stress: An Intriguing Crosstalk to Be Exploited in the Management of Type 2 Diabetes. Antioxidants 2021, 10, 802. [Google Scholar] [CrossRef]
- Yaribeygi, H.; Sathyapalan, T.; Atkin, S.L.; Sahebkar, A. Molecular Mechanisms Linking Oxidative Stress and Diabetes Mellitus. Oxidative Med. Cell. Longev. 2020, 2020, 8609213. [Google Scholar] [CrossRef]
- Lee, J.; Noh, S.; Lim, S.; Kim, B. Plant Extracts for Type 2 Diabetes: From Traditional Medicine to Modern Drug Discovery. Antioxidants 2021, 10, 81. [Google Scholar] [CrossRef]
- Tian, S.; Li, X.; Wang, Y.; Lu, Y. The protective effect of sulforaphane on type II diabetes induced by high-fat diet and low-dosage streptozotocin. Food Sci. Nutr. 2021, 9, 747–756. [Google Scholar] [CrossRef]
- Zhang, Y.; Wu, Q.; Liu, J.; Zhang, Z.; Ma, X.; Zhang, Y.; Zhu, J.; Thring, R.W.; Wu, M.; Gao, Y.; et al. Sulforaphane alleviates high fat diet-induced insulin resistance via AMPK/Nrf2/GPx4 axis. Biomed. Pharmacother. 2022, 152, 113273. [Google Scholar] [CrossRef]
- Zhang, T.; He, Q.; Liu, Y.; Chen, Z.; Hu, H. Efficacy and Safety of Curcumin Supplement on Improvement of Insulin Resistance in People with Type 2 Diabetes Mellitus: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Evid. Based Complementary Altern. Med. Ecam 2021, 2021, 4471944. [Google Scholar] [CrossRef]
- Shamsi-Goushki, A.; Mortazavi, Z.; Mirshekar, M.A.; Mohammadi, M.; Moradi-Kor, N.; Jafari-Maskouni, S.; Shahraki, M. Comparative Effects of Curcumin versus Nano-Curcumin on Insulin Resistance, Serum Levels of Apelin and Lipid Profile in Type 2 Diabetic Rats. Diabetes Metab. Syndr. Obes. Targets Ther. 2020, 13, 2337–2346. [Google Scholar] [CrossRef]
- Su, M.; Zhao, W.; Xu, S.; Weng, J. Resveratrol in Treating Diabetes and Its Cardiovascular Complications: A Review of Its Mechanisms of Action. Antioxidants 2022, 11, 1085. [Google Scholar] [CrossRef]
- Sharma, R.S.; Harrison, D.J.; Kisielewski, D.; Cassidy, D.M.; McNeilly, A.D.; Gallagher, J.R.; Walsh, S.V.; Honda, T.; McCrimmon, R.J.; Dinkova-Kostova, A.T.; et al. Experimental Nonalcoholic Steatohepatitis and Liver Fibrosis Are Ameliorated by Pharmacologic Activation of Nrf2 (NF-E2 p45-Related Factor 2). Cell. Mol. Gastroenterol. Hepatol. 2018, 5, 367–398. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yuan, J.; Li, F.; Shen, X.; Gao, J.; Yu, Z.; Luo, K.; Cui, B.; Lu, Z. Genetic and Pharmacological Inhibition of GCN2 Ameliorates Hyperglycemia and Insulin Resistance in Type 2 Diabetic Mice. Antioxidants 2022, 11, 1584. https://doi.org/10.3390/antiox11081584
Yuan J, Li F, Shen X, Gao J, Yu Z, Luo K, Cui B, Lu Z. Genetic and Pharmacological Inhibition of GCN2 Ameliorates Hyperglycemia and Insulin Resistance in Type 2 Diabetic Mice. Antioxidants. 2022; 11(8):1584. https://doi.org/10.3390/antiox11081584
Chicago/Turabian StyleYuan, Juntao, Fang Li, Xiyue Shen, Junling Gao, Zhuoran Yu, Kai Luo, Bingqing Cui, and Zhongbing Lu. 2022. "Genetic and Pharmacological Inhibition of GCN2 Ameliorates Hyperglycemia and Insulin Resistance in Type 2 Diabetic Mice" Antioxidants 11, no. 8: 1584. https://doi.org/10.3390/antiox11081584
APA StyleYuan, J., Li, F., Shen, X., Gao, J., Yu, Z., Luo, K., Cui, B., & Lu, Z. (2022). Genetic and Pharmacological Inhibition of GCN2 Ameliorates Hyperglycemia and Insulin Resistance in Type 2 Diabetic Mice. Antioxidants, 11(8), 1584. https://doi.org/10.3390/antiox11081584