Abstract
The present study examined the efficacy of dietary selenium-enriched spirulina (SeE-SP) on growth performance, antioxidant response, liver and intestinal health, immunity and disease resistance of Asian seabass, Lates calcarifer. A total of 480 seabass juveniles with an initial weight of 9.22 ± 0.09 g/fish were randomly assigned to four dietary groups. The fish were fed a fishmeal protein replacement diets with SeE-SP at 5%, 10%, and 20%, namely SeE-SP5, SeE-SP10, and SeE-SP20, and a fishmeal-based diet as control for 8 weeks. The results indicated that seabass juveniles fed SeE-SP5 and SeE-SP10 diets grew at the same rate as the fish fed a fishmeal-based control diet after 8 weeks of feeding, while SeE-SP20 grew at a significantly lower rate than the control (p < 0.05). Although most of the measured biochemical parameters were not influenced by the Se-SP diets, serum antioxidant-enzyme glutathione peroxidase (GPx) and immunological indices, such as lysozyme activity and immunoglobulin-M, were found significantly higher in the SeE-SP5 and SeE-SP10 diets compared to control. In addition, the fish fed the SeE-SP5 diet showed significantly lower mortalities after the 14-day of bacterial challenge with V. harveyi. These outcomes indicated that up to 10% inclusion of SeE-SP in the diet of juvenile Asian seabass does not compromise growth, while SeE-SP5 enhanced disease resistance in juvenile seabass.
1. Introduction
Improved and diversified production techniques along, with the intrinsic superior production features of fish, have fueled the aquaculture industry’s rapid rise. As a result, by 2050, fish will be the primary source of food for 9 billion people [1]. However, there are various challenges associated with the production of fish in aquaculture systems, one of which is the vulnerability to disease outbreaks. In this case, the addition of feed additives in the feed formulation of fish, such as probiotic, micronutrients and microalgae, are seen as a promising field in the aquaculture sector [2,3,4].
Selenium (Se) is a key micronutrient that enhances fish health by acting as a potent antioxidant when included in the diet. The antioxidative defenses formed by Se protect cells from oxidation [5]. Furthermore, Se is a component of at least 25 selenoproteins and an important co-factor in the antioxidant enzyme system. Se consumption has an impact on nutrition utilization, productivity, antioxidative mechanisms, reproductive function, hormone metabolism, and immune system responses in productive animals [6,7,8]. In a study by [9], it was found that the yellowtail kingfish (Seriola lalandi) fed a diet without Se supplementation showed necrotic myopathy in fish muscles. As a result, including Se nanoparticles into the aquafeed sector is highly recommended for the purpose of enhancing the productivity and health of aquatic animals.
Microalgae have been added to aqua-diets as supplements to promote the growth and health performance of the host fish [10] as a consequence of their high nutritional value [11,12], capabilities for immunomodulation [13], enhancement of pigmentation [14], and as a great source of vitamins and micronutrients [15]. The blue-green microalgae spirulina has a high protein content (up to 70%), a balanced fatty acid profile, vitamins, minerals, and a high concentration of bioactive substances, which helps the aquaculture organisms resist pathogens and environmental stressors [16]. Spirulina has been studied in many aquaculture species and now established as a functional feed ingredient in aquaculture production to improve growth, welfare, and the disease resistance of fish. A study by [17] reported that juvenile seabass performance in terms of growth and health is unaffected when up to 20% of their diets are provisioned with spirulina instead of fishmeal. The growth performance was reported to be augmented at any incorporation level of S. platensis in Labeo rohita [18]. The addition of Spirulina in the diet, comprising of phenolic compounds, improves the antioxidant response of Oplegnathus fasciatus [19]. Enzymes, such as glutathione peroxidase (GPx) and catalase (CAT), are important biochemical criteria for the antioxidant defense systems, and dietary selenium and spirulina supplementation have been demonstrated to improve all of these enzymatic activities [20]. Spirulina were reported to lower the blood cholesterol levels and promote the production of white and red blood cells in Oncorhynchus mykiss, resulting in improved immunity [21].
Asian Seabass is a fast-growing highly carnivore fish and the higher metabolic rates associated with faster-growing fish require sufficient energy to maximize their welfare, resulting in a need to uptake more nutrients, particularly vitamins and minerals. For example, a study with minerals’ supplementation, particularly selenium, with commercial fishmeal improved the welfare of yellowtail kingfish [9]. In addition, the natural immunostimulants e.g., microalgae and carotenoid pigments along with selenium, provide an intriguing alternative approach to immunoprophylactic control, as these compounds play essential roles in modifying and boosting the immunological response of aquatic animals against various pathogens [22]. Therefore, the current study investigated how the dietary supplementation of spirulina, coupled with organic selenium, affects the growth performance, antioxidant response, immunity, and disease resistance of the juvenile Asian seabass.
2. Materials and Methods
The current study was performed at Nha Trang University (NTU), Khanh Hoa, Vietnam. The fresh spirulina were collected from the Northwest region of Vietnam and the selenium-enrichment was carried out in the laboratory. The animal care was fully compliant with the Vietnamese Code of Practice for the care of animals for scientific purposes.
2.1. Spirulina Enrichment with Selenium
The organic Se (Sel-Plex, Alltech, USA) was added to the spirulina biomass through biosorption. The enrichment procedure was carried out in tanks with 50 L of room-temperature tap water and a selenium solution. The solutions were prepared by dissolving selenium at a rate of 12 mg/L. The contact time was 4 h. The enhanced biomass was then separated, using a filter with a 6 mm pore diameter, dried at 50 °C and crushed. A respective sample of dried feed ingredient of spirulina biomass from each enrichment tank was sampled, stored at −4.0 °C for a week before biochemical analysis.
2.2. Dietary Ingredients and Diet Formulation
The feed ingredients, formulation, and their nutrient composition are presented in Table 1. The diets were formulated to contain 0, 5, 10, and 20% spirulina protein named control, SeE-SP5, SeE-SP10, and SeE-SP20 as replacement with fishmeal protein. The diet without selenium supplementation was regarded as the control. Except for the fishmeal, which was supplied by TC Union Vietnam (Tien Giang, Vietnam), all of the feed ingredients were obtained from Long Sinh Feed Company (Khanh Hoa, Vietnam). All of the ingredients were properly mixed together to create a homogeneous mixture. In a food mixer, fish oil and 30% distilled water were added into the premixed dry components and mixed for 15 min to make a dough. The dough was screw-pelletized into 2.0–3.0 mm pellets, using a laboratory pelletizer. These moist pellets were oven-dried for 12 h at 50 °C, and then chilled to an ambient temperature before being kept at −4°C until needed.

Table 1.
Formulation and proximate composition of the experimental diets for juvenile seabass.
2.3. Experimental Setup and Facilities
Juvenile seabass was obtained from a commercial marine fish hatchery (Nha Trang, Khanh Hoa, Vietnam) and transported to the wet laboratory in Nha Trang University, where they were acclimated to experimental condition for 2 weeks. During acclimation, the fish were fed Uni-President-Vietnam feed (46% protein and 13% fat). The fish were then graded, and those within the weight range of 9.0–10.0 g were selected and randomly stocked into 12 tanks of 350 L water capacity tank. Each dietary treatment was triplicated, and every tank was stocked with 40 fish. Fish were fed three times a day (8.00, 12.00 and 16.00) until satiation, which took around 20 minutes. Uneaten feed was siphoned off right after feeding to justify feed intake. During the feeding trial, the dissolved oxygen was maintained higher than 5.0 mg/L, average temperature was 29.0 °C, total ammonia was less than 0.25 mg/L and salinity ranged from 29.0 to 32.0 ppt. Following a day of feed deprivation, total numbers of fish in each tank were counted and individual body weights were measured at the end of the growth trial.
2.4. Blood and Serum Biochemical Analyses
At the termination of the feeding trial, the blood samples were taken from six fish per tank by puncturing the caudal vein with a 1 mL non-heparinized syringe. For the measurement of the blood hemoglobin, the white blood cells (WBC), red blood cells (RBC), and glucose, an aliquot of the extracted blood samples was collected in heparinized tubes. An automated blood analyzer was used to analyze blood hemoglobin, RBC, and leukocrite (Sysmex XT-162 1800i, Kobe, Japan). The blood was centrifuged in glass capillary tubes at 2000 rpm for five minutes to calculate the blood hematocrit percentage and to test the blood glucose using a blood glucose meter kit (Accu-Chek, Sydney, Australia) [23]. Another set of blood samples was drawn into non-heparinized tubes and left to sit for 24 h before being centrifuged for 10 min at 4 °C at 5000 g to extract the serum. The separated serum samples were maintained at −20 °C until use. The serum blood parameters encompassing alanine aminotransferase (ALT), aspartate aminotransferase (AST), total cholesterol (TC), triglyceride (TG), total protein, and albumin concentrations using an automated blood analyzer (SLIM; SEAC Inc, Florence, Italy), and following the methods of [24]. The serum lysozyme activity was evaluated following the previously described procedure of [25]. An enzyme-linked immunosorbent test was used to assess the immunoglobulin M (IgM), using a commercial kit (Cusabio, Wuhan, Hubei, China). Following the manufacturer’s instructions, the enzymatic activity of the serum glutathione peroxidase (GPx) and catalase (CAT) were measured, using kits (Cusabio Biotech Co., Ltd., Wuhan, China).
2.5. Histological Examination
Six fish per treatment (two fish from each replicate) were randomly selected for the histological analysis. The dissected liver and distal intestine were then fixed in 10% buffered formalin for 72 h, after being cleaned with normal saline solution. Following a series of alcohol washes to dry the samples, they were cleaned in xylene and imbedded in paraffin wax for sectioning. The serial sections were cut to the thickness of 5 μm. The sections were stained with hematoxylin and eosin (H&E), covered with a coverslip, and magnified 400 times under a light microscope (Olympus, Germany). Using the onboard camera, the histopathology pictures were obtained (BX40F4, Olympus, Tokyo, Japan). To determine the intestinal micromorphology, including intestinal fold height (IFh), fold width (IFw), goblet cell number (IGCn), and number of adipocyte cells, ten intact intestinal folds from each dietary treatment were studied, as described previously [25,26]. The sections were assessed for anomalies in structure (the relative area of vacuolation in the liver) using the ImageJ software.
2.6. Challenge Test with Vibrio Harveyi
Since the growth performance of the fish fed the SeE-SP20 diet was significantly lowered, this group was not considered for the challenge study. The bacterial challenge trial was carried out with SeE-SP5, SeE-SP10, and the control diets, in accordance with the specified protocol of [27]. In brief, 10 fish from each replicate were intraperitoneally injected using a 1-mL syringe fitted with 27-gauge needle with 0.1 mL of pathogenic V. harveyi suspension containing LD50 = 1.1 × 108 cfu/mL. The blood samples were collected at 24 h and 3 days after the fish were challenged with V. harveyi. The fish were considered for cumulative survival counting 14-days after the challenge trial with V. harveyi. The clinical symptoms of vibriosis, including a thick layer of mucus on the body’s surface, congested fins, hemorrhages, and ulceration of the skin and muscle tissue were assessed three times daily for 14 days. According to the established technique for fish euthanasia, the fish exhibiting these vibriosis symptoms were euthanized with AQUI-S at a concentration of 175 mg/L for 20 min.
2.7. Samples Analyses and Calculations
The growth performance parameters, such as fish weight gain, FCR, total feed intake, and survival were calculated using the following formulas:
2.8. Statistical Analysis
The normality and homogeneity of the variances were validated before any statistical analysis. One-way ANOVA was used with post-hoc Turkey’s HSD multiple comparison tests, to establish the differences among the dietary groups. The Kaplan–Meier method was used to construct the survival graph. The statistical significance was calculated at p < 0.05. All of the data were presented as mean ± standard error (SE). For the statistical analysis and graph construction, GraphPad PRISM version 8.0 (GraphPad Software, Inc., La Jolla, CA, USA) was used. The principal component analysis (PCA) was used separately on the datasets obtained from the four dietary groups and various parameters analyzed (growth, blood, antioxidant and histomorphology) at the end of feeding trial, to assess the overall covariation of their respective variables. The PCA analysis was performed in the open-source environment R version 3.6.2 (R Core Team, Vienna, Austria).
3. Results
3.1. Growth Performance and Feed Utilization
The growth performance and feed utilization of the juvenile seabass fed the SeE-SP diets at varied inclusion levels were significantly affected, except for survival (Table 2). The fish fed SeE-SP5 and SeE-SP10 diets revealed equivalent growth performance in terms of final body weight (FBW) and weight gain (WG) when compared to the control (Figure 1). Whereas, the SeE-SP20 diet produced significantly lower growth performance compared to the control and the other dietary groups. Although the dietary groups of SeE-SP5 and SeE-SP10 produced a significantly higher specific growth rate (SGR) compare to Se-SP20, they were equivalent to the control (p > 0.05). The SeE-SP5 diet produced a significantly higher feed intake compared to the control and SeE-SP20 (p < 0.05). The SeE-SP20 diet had a significantly higher FCR than the other experimental diets (p < 0.05). The survival rate of fish was not impacted by any of the dietary groups (p > 0.05). The quadratic regression analysis revealed the highest SeE-SP level of 7.40% for the maximum growth performance of juvenile Asian seabass.

Table 2.
Blood and serum biochemical parameters and enzymatic activities of juvenile seabass fed various levels of SeE-SP diets for 8 weeks.
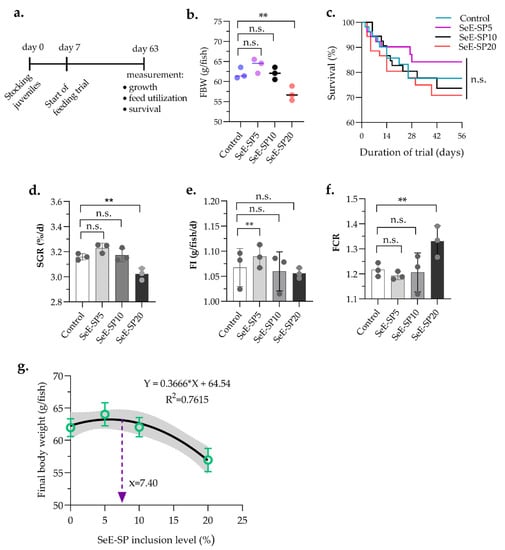
Figure 1.
Growth performance and feed intake of juvenile seabass fed various levels of selenium-enriched spirulina (SeE-SP). (a) Timeline of feeding trial followed by seven days of conditioning fish in feeding tank; (b) Final body weight (FBW); (c) Survival; (d) specific growth rate (SGR); (e) feed intake (FI); (f) feed conversion ratio (FCR) of fish after 56 days of feeding trial; (g) the highest SeE-SP level for the maximum FBW was 7.40% in the diet, as determined by quadratic regression analysis. Values are mean ± SE of three replicate tanks (indicated as dots on bars) per treatment. The bars with asterisks (**) are significantly different based on Tukey’s multiple range test (One-way ANOVA, p < 0.05). ns, non-significant at p < 0.05.
3.2. Blood and Serum Biochemical Responses
The serum biochemical parameters including hematocrit, RBC, hemoglobin, glucose, cholesterol, albumin, globulin, total protein, and triglyceride were not significantly (p > 0.05) influenced by the SeE-SP diets when compared to the control (Table 2). The enzymatic GPx activity was substantially (p < 0.05) increased with the SeE-SP5 and SeE-SP10 diets when compared to the control, while serum CAT activity was lowered in the fish fed the SeE-SP5 diet compared to the control and the rest of the diets (Figure 2). In terms of hepatic enzymatic activities, the serum AST level was found lowered in the fish fed the SeE-SP5 diet, whereas the ALT level was lowered both in the SeE-SP5 and SeE-SP10 diets when compared to the control (Figure 2).
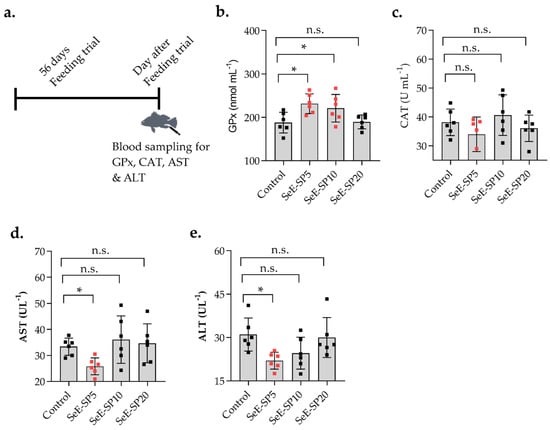
Figure 2.
Serum antioxidant response and liver enzymatic activities of juvenile seabass fed various levels of selenium-enriched spirulina (SeE-SP). (a) Timeline of feeding trial and blood collection; (b) glutathione peroxidase (GPx); (c) catalase (CAT); (d) aspartate aminotransferase (AST); and (e) alanine aminotransferase (ALT) of fish following 56 days of experimental feeding. Values are mean ± SE of three replicate tanks per treatment (values of individual fish per group are indicated as small squares on the bars). Bars holding asterisk (*) are significantly different based on Tukey’s multiple range test (One-way ANOVA, p < 0.05). ns, non-significant at p < 0.05.
3.3. Histological Examination
The liver, intestine, and fat cells micromorphology of the juvenile seabass fed the SeE-SP diets at various inclusion levels are shown in Figure 3. No aberrant hepatocytes were noticed in the livers of any of the dietary groups, except for the SeE-SP20 diet. The fish fed the SeE-SP20 diet produced higher lipid vacuolization when compared to the other dietary groups (p < 0.05). The IFh was not impacted up to 10% of the fishmeal replacement diets with SeE-SP diets, but was lowered at the 20% replacement level. Meanwhile, the IFw was neither increased or decreased by the inclusion of the selenium-enriched spirulina diets in the seabass. The IGCn was found to be significantly higher in the SeE-SP5 and SeE-SP10 diets when compared to the control. The fish fed the SeE-SP5 and SeE-SP10 diets were found to be have smaller and higher number of adipocyte cells when compared to the fish fed the control (p < 0.05).
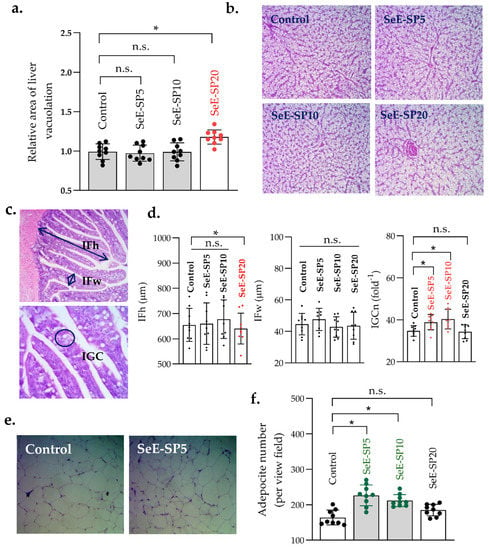
Figure 3.
Liver and fat cells histomorphology of juvenile seabass fed various levels of selenium-enriched spirulina (Se-SP). (a) Measurement of liver vacuolation; (b) representative hepatic images of fish fed various levels of SeE-SP diets (H&E, 40 × magnification); (c) Schematic representation of histometric measurement of intestinal fold height (IFh), fold width (IFw) and intestinal goblet cell number (IGCn) (H&E, 40 × magnification); (d) quantification of IFh, IFw, and IGCn of the distal intestine of seabass (values are mean ± SE of three replicate tanks per treatment, while values of individual fish per group are indicated as dots on the bars); (e) representative adipose tissue (fat cells) histology (H&E, 40 × magnification); and (f) quantification of adipocyte number in fish fed various levels of SeE-SP diets (values are mean ± SE of three replicate tanks per treatment, while values of individual fish per group are indicated as small dots on the bars). Bars holding asterisk (*) are significantly different based on Tukey’s multiple range test (One-way ANOVA, p < 0.05). ns, non-significant at p < 0.05.
3.4. Disease Resistance of Fish
The disease resistance of the fish fed the control and the SeE-SP diets was assessed using a pathogen challenge with V. harveyi. After 14 days of IP challenges, the fish fed the Se-SP5 diet had considerably lower mortalities than the fish fed the control and other Se-SP10 diets (Figure 4). The mortalities began from day 2 post-challenge, with a mean cumulative mortality of 78.25% in the control, 58.35% in the Se-SP10 and 24.55% in the Se-SP5, 14 days post-challenge.
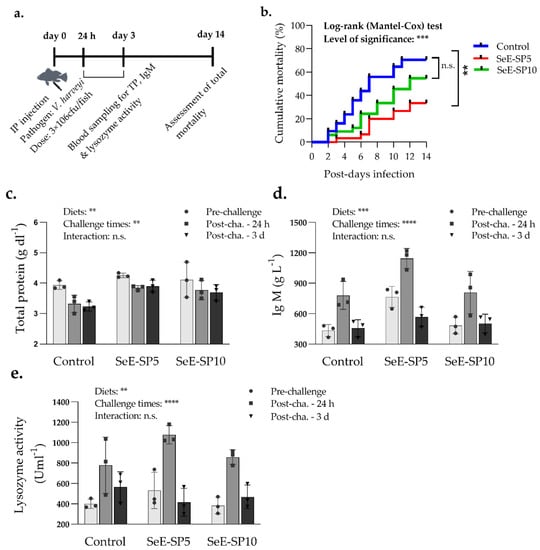
Figure 4.
Serum immunological response and mortalities of seabass juveniles challenged with the Vibrio harvei after 56 days of the feeding trial (a) Timeline of blood collection and mortality assessment followed by V. harveyi infection; (b) Survival curve of juvenile seabass after challenge with V. harveyi for a period of two weeks (Kaplan–Meier survival method, followed by Log-rank test, p < 0.05); (c) total protein; (d) immunoglobulin M (IgM); and (e) lysozyme activity of fish at pre-challenge and post-challenge of fish at 24 h and 3 day. Bars holding various asterisks (** ), (***) and (****) indicates whether any significant variation amongst diets, time of sampling and interaction between diets and sampling times (two-way ANOVA; Tukey post-hoc test) at p < 0.05, p < 0.01 and p < 0.001 respectively. n.s., indicates non-significant differences at p < 0.05.
3.5. Immunity of Fish against V. harveyi
The immunological indices including serum lysozyme activity, total protein and IgM of fish were affected both by the dietary groups and the blood sampling periods after challenge with V. harveyi (Figure 4). The fish in each dietary group had higher lysozyme activity at 24 h post-challenge in comparison to the pre-challenge condition and the post-challenge condition at 72 h. The IgM level was found to be significantly higher in the SeE-SP5 group, while total protein was higher in both the SeE-SP5 and SeE-SP10 groups compared to the control. The total protein level was unaffected by the challenge, however the IgM levels were greater at 24 h post-challenge compared to the pre-challenge and 72 h post-challenge conditions.
3.6. PCA Analysis
The principal component analysis (PCA) was performed to investigate the effects of the dietary groups on various growth, immunity, and health parameters in fish. The first two principal component axes (PC1 and PC2) explained more than 65.3% of the variation in the data, providing information about the predominant correlations between these data. The fish fed the control and the SeE-SP20 diets were grouped together in the PCA biplot’s negative site, where the FCR was negatively correlated with AST, and glucose (Figure 5a). On the other hand, the SeE-SP5 and SeE-SP10 diets grouped together in the PC1 positive zone, where FBW and SGR were found to be highly positively correlated with IFh, IGCn, ACn, TP, IgM, GPx, and lysozyme activity (Figure 5b). This arrangement of the variables demonstrates that the first principal component, which accounts for 52.6% of the variation in all of the parameters, reflects a composite perspective of the majority of the parameters. Additionally, the first principal component (PC1) predicts 52.6% variance, second principal component (PC2) 12.7%, and the third principal component (PC2) 10.52%, and so on. The bi-plot showed that the SeE-SP5 and SeE-SP10 diets were intermingled and overlapped with positive correlation, while the control and SeE-SP20 diets formed distinct groups that were negatively associated with each other.
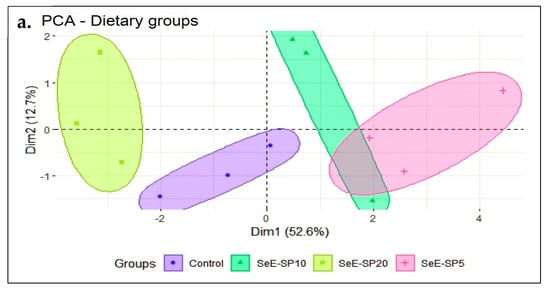
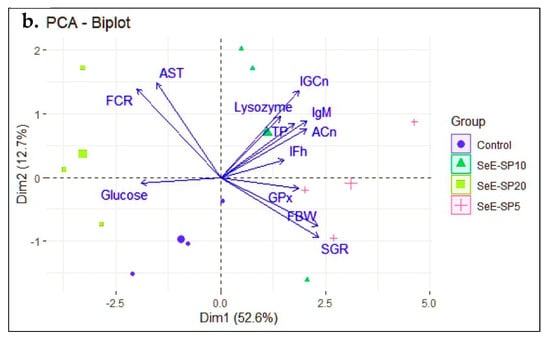
Figure 5.
PCA score and biplot with various dietary groups and some measured parameters at the end of the feeding trial. The graph shows positive and negative association between various dietary groups (a) and variables (b). Different markers with confidence eclipse indicate samples from various dietary groups utilized in the investigation, and loadings show how the variable contributed to the development of PC1 and PC2. FBW, final body weight; SGR, specific growth rate; FCR, feed conversion ratio; IFh, intestinal fold height; IGCn, intestinal goblet cell numbers; TP, total protein; IgM, immunoglobulin M; AST, aspartate aminotransferase, GPx, glutathione peroxidase; and ACn, adipocyte cell number.
4. Discussion
Spirulina has been put into practice as a fishmeal replacement ingredient in aquafeed due to it higher content of amino acids and micronutrients, such as potassium, magnesium, calcium, zinc, phosphorous, and iron in the dried biomass [28,29]. It also contains immunostimulatory and antiviral properties, ensuring the potential of increasing disease resistance in fish [30]. Meanwhile, selenium is an essential co-factor in the antioxidant enzyme system, whose intake has an impact on nutrition utilization, production performance, antioxidative mechanisms, reproductive function, and immune system responses in fish [31,32]. The past studies have indicated that the demand and bioavailability of dietary selenium is species-specific and its level is regulated by a range of factors, including type of feed, culture condition, age of species to be cultured and the type of selenium itself—whether it is organic or synthetic [33,34]. High mortalities, histological alterations in liver tissues, decreased reproductive performance, and reduction in feed intake, growth, and hematocrit levels are all indicators of an insufficiency of selenium in fish. On the other hand, excessive inclusion levels may result in selenium toxicity, which can damage fish growth and health [33]. A study by [35] found that higher doses of selenium cause an excessive build-up of Se in the liver and kidneys, which may result in oxidative stress in fish [35]. According to the present findings, the selenium-enriched spirulina had a positive influence on growth performance and feed utilization up to a 10% replacement level, however feeding at an exclusive level (>10%) inhibited the juvenile seabass growth. The spirulina supplementation in the range of 1 to 10% has been reported as a viable option for increasing the nutritional value of aquafeed [36], which validates our findings. The parrotfish, Oplegnathus fasciatus, treated with 5% Spirulina in formulated feed showed considerably higher weight increase, protein efficiency ratios, feed intake, and lower feed conversion ratios when compared to the fishmeal-fed control [19]. Similarly, rainbow trout, Oncorhynchus mykiss gained the most weight when the fishmeal was replaced with 7.5% spirulina [37]. An addition of up to 20% of the fishmeal replaced with spirulina was reported to be without adverse effect on the growth of the golden barb, Puntius gelius [38]. Conversely, the growth rate of the silver seabream, Rhabdosargus sarba, remained unaltered when 50% of the fishmeal was substituted by Spirulina maxima [39], whereas Atlantic cod, Gadus morhua, exhibited poor growth when the combination of dried Nannochloropsis sp. and Isochrysis sp. algae were added to the diet at a 30% inclusion level [29]. The weight gain and feed conversion ratio of juvenile barramundi were not negatively affected by an up to 20% fishmeal replacement with raw spirulina, while feeding at an exclusive level of 40% significantly reduced the growth performance [28]. The mode of action elucidating the variations in weight loss due to diets is unknown, but Refs. [11,40] reported that better lipid mobilization to produce energy delivered by microalgae in the muscle that is accessible from the algae could be the reason for the reduction in weight loss. The Atlantic cod, (Gadus morhua) a carnivorous species, when fed spirulina as a fishmeal substitute diet, had a decreased capacity to operate at optimum levels due to the diminished palatability of the meals [29].
The antioxidant defense system, which is maintained through enzymes and the antioxidant state, is closely linked to fish physiological functions and immunity [41]. In fish, there is a cellular equilibrium between the synthesis and clearance of reactive oxygen species (ROS) under normal conditions. When the fish are stressed, high quantities of reactive oxygen species (ROS) are created, which can cause considerable damage to the cell structures [42]. Under this circumstance, the fish activate an antioxidant response to prevent excessive ROS, which reduces the negative effects on the cells and tissues [41]. The antioxidant GPx activity of the blood serum was significantly increased in the selenium-enriched spirulina groups as compared to the control group, according to the findings of this study. This could be because the symbiotic-fed fish have a synchronized effect of selenium and spirulina supplementation, which leads to greater cellular competence against oxidative stress, a decrease in lipid peroxidation, and a higher number of healthy cells in an increasing GPx function. The selenium and spirulina include antioxidants that can suppress the generation of free radicals, boost the uptake mechanism of endogenous radicals, and promote cellular antioxidant enzymes, such as CAT and GPx, according to a number of studies [43,44].
The blood indices are used to determine metabolic activity, nutritional health, and the physiological state of the fish [25,32,45]. A higher quantity of serum protein, albumin, or globulin is associated with a stronger innate immune response, and is recognized as a reliable predictor of immune system activation [45,46]. Although these blood parameters were not significantly improved compared to the control group in this study, their apparent rise indicates that the selenium-enriched spirulina has a good impact on the health of the fish. The blood parameters of barramundi (Lates calcarifer) [47] were reported to be influenced by dietary canola meal, while an optimum growth performance was achieved as reported in barramundi [48] or grouper [49] which is in agreement with the present study. On the other hand, adding 10% S. platensis into rainbow trout, O. mykiss, meals considerably raised the levels of hemoglobin, total protein, albumin, white blood cells, and red blood cells, making it an immunostimulant [21]. The results of the present study revealed that, even at higher replacement levels (>10%), these blood and serum indices were not decreased in the Asian seabass fed selenium-enriched spirulina which confirm the role of selenium and spirulina in balancing the body homeostasis in this species. The overall increase in the serum lysozyme activity in the fish fed the SeE-SP5 diet suggests a stronger immunological response; because the antimicrobial peptides, such as lysozyme, were shown to aid in the inhibition of microorganism colonization in the host body, resulting in pathogen prevention and immune cells to fight infection [50,51]. Clinically, the serum AST and ALT levels are routinely used as indicators for liver health. The increase in the AST and ALT levels indicate hepatic cell damage or increased liver enzyme production [52]. In the current study, the AST and ALT levels were lowered in the fish with the SeE-SP5 diet indicates that the minimum inclusion of spirulina improves the liver condition of fish. It is reported in poultry that the dietary supplementation with the organic selenium improved the antioxidant status and decreased the level of these enzymes’ secretion in the laying hens [53]. The present results are in line with [54], who found the lowest AST and ALP levels in the fish supplemented with selenium and methionine.
The histological assay of the hepatic micromorphology of juvenile Asian seabass revealed abnormal hepatocytes in the dietary group of SeE-SP20, characterized by the formation of lipid droplets. In contrast, a study by [28] found that in the fish given up to 20% replacement of fishmeal Spirulina platensis no histological changes were induced in the liver of L. calcarifer, whereas a 40% replacement resulted in a higher number of vacuoles and necrotic cells in the liver. The fat accumulation in the hepatocytes occurs when the dietary lipids exceed the ability of the hepatic cells to break them down [55,56]. The available literature indicates that fat accumulation in the liver impedes the fish development and immunological response [55,56]. Regarding the intestine, the fish fed selenium-enriched spirulina diets up to 10% had a significantly higher number of goblet cells and enhanced fold heights, indicating a higher nutrient absorption and utilization in the fish at a moderate inclusion level. The intestinal fold heights were reduced in the SP20 diet, indicating that an overabundance of nutrients disrupted the intestinal homeostasis, resulting in reduced nutritional absorption and, as a result, reduced fish-growth performance. In a study on rainbow trout, it is reported that the goblet cell density, villus height, absorption surface area, and intraepithelial lymphocytes were modulated when the fish were fed a 5% spirulina-supplemented diet [30]. However, a higher inclusion level could lead to the loss of some amino acids, such as lysine, methionine, histidine, arginine, and threonine [57,58,59], which could affect the internal tissues integrity. In addition, the inadequate feeding and subsequent absorption in fish can result in changes in the intestinal structure, such as a decrease in the IGCn numbers and a shortening of the intestinal fold height, which can lead to impaired immune function [25]. On the other hand, increased fold height in the intestine may be due to improved nutrient absorption and utilization [25], which is in fair agreement with the present findings. The current study also reported that the fish given the SeE-SP5 and SeE-SP diets had a smaller but higher number of adipocyte cells, so that the number of adipocyte cells in the SeE-SP5 and SeE-SP10 groups were significantly higher than the control. There are no previous studies to compare with the current study on the effect of microalgae supplementation on adipocyte cell size and number in fish. More research is needed to confirm the effects of microalgae, i.e., spirulina on intraperitoneal fat cells and/or lipid metabolism in fish.
The spirulina extracts, namely, ethanol, acetone, diethyl ether, and methanol, have shown antibiotic efficacy against Escherichia coli, Staphylococcus aureus, and Pseudomonas aeruginosa and are reported to be efficient against Staphylococcus aureus, E. coli, Candida albicans, and Aspergillus niger [60,61]. In the present study, after 14 days of IP challenges, the fish given the SeE-SP5 and SeE-SP10 diets showed lower mortalities than the fish fed the control diet when challenged with V. harveyi. This suggests that supplementing fishmeal diets with spirulina and selenium could protect fish against bacterial infection. This research discovered a link between dietary SeE-SP and lysozyme activity, which led to increased disease resistance against infections. The therapeutic potential of garlic as a dietary supplement is reported to reduce the mortality of L. calcarifer after being challenged with V. harveyi [62]. Similarly, a number of studies utilizing spirulina and/or selenium have beem shown to enhance disease resistance against pathogenic bacteria in fish [63,64,65]. It has been reported that spirulina inclusion at an optimum level limits the growth of opportunistic pathogens by giving critical nutrients to the fish [17]. It is also worth highlighting that selenium plays a role in cellular antioxidant activities by increasing the synthesis of selenium-containing enzymes, such as GPx. In fish, dietary selenium can boost GPx activity, and play a key role in eliminating hydrogen peroxide (H2O2) from the host body to combat stress or infections [66]. An increased GPx scavenging capacity to eliminate hydrogen peroxide diffused from phagolysosomes could be one way by which the dietary selenium improves lysozyme activity in fish [67]. Increased GPx activity during phagocytosis may lower H2O2 generation and boost the circulation of NADPH oxidases [68].
The PCA analysis was applied to investigate the effect of SeE-SP diets on various growth, immunity, and health parameters in fish. The position of the dietary groups and variables in the plot showed that the SeE-SP5 and SeE-SP10 diets had a favorable influence on fish growth and wellbeing, whereas the SeE-SP20 diet had a negative association with fish performance. The PCA analysis was applied to investigate the effect of the SeE-SP dietary groups on various growth, immunity, and health parameters in fish. Previously, the PCA was also used to assess the data from the whole range of digestive enzyme activity in various species of annelida, in order to determine their dietary preferences [69]. In the present experiment, the position of the dietary groups and growth, immunity, and health parameters in the plot showed that the SeE-SP5 and SeE-SP10 diets had a favorable influence on fish growth and overlapped each other, whereas the SeE-SP20 diet had a negative association with fish performance. Indeed, the SeE-SP5 and SeE-SP10 diets significantly influenced IFh and IGCn, indicating that these diets enhanced intestinal health. The PCA revealed that the fish meal replacement with soy protein concentrate at a level of 50% did not affect the growth of the juvenile lumpfish (Cyclopterus lumpus) and up to 70% did not affect the IFH and IGCn [70]. However, in our study, the SeE-SP supplementation at rates of SeE-SP5 and SeE-SP10 was observed to positively connect to IFH and IGCn, while the control and SeE-SP20 negatively impacted these variables.
5. Conclusions
The optimum selenium-enriched spirulina (SeE-SP) level for juvenile Asian seabass was estimated to be 7.4%, based on the quadratic regression analysis of the final body weight of fish. Although the growth performance was not significantly enhanced by the SeE-SP inclusion in the diets, the increased antioxidant response, intestinal micro-morphological parameter, and immunological indices, such as lysozyme activity and immunoglobulin-M, were found to be significantly higher in the fish fed up to 10% SeE-SP in diet. Moreover, the fish fed a 5% SeE-SP diet showed significantly lower mortalities after 14-days of bacterial challenge with Vibrio harveyi. These outcomes indicate that up to 10% inclusion of SeE-SP in the practical diet does not compromise growth, whilst SeE-SP levels at 5% enhanced the disease resistance in Asian seabass.
Author Contributions
Conceptualization, M.A.B.S. and H.D.P.; methodology, M.A.B.S.; software, M.A.B.S.; validation, M.A.B.S., I.N.V., M.A.R. and H.D.P.; formal analysis, M.A.B.S.; investigation, H.D.P. and M.A.B.S.; resources, M.A.B.S. and H.D.P.; data curation, M.A.B.S. and H.D.P.; writing—original draft preparation, M.A.B.S. and M.A.R.; writing—review and editing, M.A.B.S., I.N.V., M.A.R. and H.D.P.; visualization, M.A.B.S.; supervision, I.N.V. and M.A.B.S.; project administration, M.A.B.S. and H.D.P.; funding acquisition, M.A.B.S., H.D.P. and I.N.V. All authors have read and agreed to the published version of the manuscript.
Funding
This research is funded by the Ministry of Education and Training, Vietnam under grant number B2019-TSN-562-13.
Institutional Review Board Statement
The animal care was fully compliant with the Vietnamese Code of practice for the care of animals for scientific purposes (Decree 32/2006/ND-CP, 2006).
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available in the article.
Conflicts of Interest
The authors have no conflict of interest to disclose.
References
- Béné, C.; Barange, M.; Subasinghe, R.; Pinstrup-Andersen, P.; Merino, G.; Hemre, G.-I.; Williams, M. Feeding 9 billion by 2050—Putting fish back on the menu. Food Secur. 2015, 7, 261–274. [Google Scholar] [CrossRef]
- Siddik, M.A.; Foysal, J.; Fotedar, R.; Francis, D.S.; Gupta, S.K. Probiotic yeast Saccharomyces cerevisiae coupled with Lactobacillus casei modulates physiological performance and promotes gut microbiota in juvenile barramundi, Lates calcarifer. Aquaculture 2021, 546, 737346. [Google Scholar] [CrossRef]
- Kiron, V. Fish immune system and its nutritional modulation for preventive health care. Anim. Feed Sci. Technol. 2012, 173, 111–133. [Google Scholar] [CrossRef]
- Cerezuela, R.; Guardiola, F.A.; Meseguer, J.; Esteban, M. Enrichment of gilthead seabream (Sparus aurata L.) diet with microalgae: Effects on the immune system. Fish Physiol. Biochem. 2012, 38, 1729–1739. [Google Scholar] [CrossRef]
- Hasani, M.; Djalalinia, S.; Khazdooz, M.; Asayesh, H.; Zarei, M.; Gorabi, A.M.; Ansari, H.; Qorbani, M.; Heshmat, R. Effect of selenium supplementation on antioxidant markers: A systematic review and meta-analysis of randomized controlled trials. Hormones 2019, 18, 451–462. [Google Scholar] [CrossRef] [PubMed]
- Cardoso, B.R.; Roberts, B.R.; Bush, A.I.; Hare, D.J. Selenium, selenoproteins and neurodegenerative diseases. Metallomics 2015, 7, 1213–1228. [Google Scholar] [CrossRef] [PubMed]
- Zoidis, E.; Seremelis, I.; Kontopoulos, N.; Danezis, G.P. Selenium-dependent antioxidant enzymes: Actions and properties of selenoproteins. Antioxidants 2018, 7, 66. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Zhang, Y.; Li, S. Effects of selenium deficiency on testis development and autophagy in chicks. Ital. J. Anim. Sci. 2020, 19, 753–761. [Google Scholar] [CrossRef]
- Le, K.; Fotedar, R.; Partridge, G. Selenium and vitamin E interaction in the nutrition of yellowtail kingfish (Seriola lalandi): Physiological and immune responses. Aquac. Nutr. 2013, 20, 303–313. [Google Scholar] [CrossRef]
- Xu, W.; Gao, Z.; Qi, Z.; Qiu, M.; Peng, J.Q.; Shao, R. Effect of dietary chlorella on the growth performance and physiological parameters of gibel carp, Carassius auratus gibelio. Turk. J. Fish. Aquat. Sci. 2014, 14, 53–57. [Google Scholar] [CrossRef]
- Güroy, D.; Güroy, B.; Merrifield, D.L.; Ergün, S.; Tekinay, A.A.; Yiğit, M. Effect of dietary Ulva and Spirulina on weight loss and body composition of rainbow trout, Oncorhynchus mykiss (Walbaum), during a starvation period. J. Anim. Physiol. Anim. Nutr. 2010, 95, 320–327. [Google Scholar] [CrossRef]
- Greenwell, H.C.; Laurens, L.M.L.; Shields, R.; Lovitt, R.; Flynn, K. Placing microalgae on the biofuels priority list: A review of the technological challenges. J. R. Soc. Interface 2009, 7, 703–726. [Google Scholar] [CrossRef] [PubMed]
- Cerezuela, R.; Guardiola, F.A.; González, P.; Meseguer, J.; Esteban, M. Effects of dietary Bacillus subtilis, Tetraselmis chuii, and Phaeodactylum tricornutum, singularly or in combination, on the immune response and disease resistance of sea bream (Sparus aurata L.). Fish Shellfish Immunol. 2012, 33, 342–349. [Google Scholar] [CrossRef]
- Gouveia, L. Evolution of pigment composition in Chlorella vulgaris. Bioresour. Technol. 1996, 57, 157–159. [Google Scholar] [CrossRef]
- Kay, R.A.; Barton, L.L. Microalgae as food and supplement. Crit. Rev. Food Sci. Nutr. 1991, 30, 555–573. [Google Scholar] [CrossRef] [PubMed]
- Maddaly, R.; Ravi, M.; De, S.L.; Azharuddin, S.; Paul, S.F.D. The beneficial effects of spirulina focusing on its immunomodulatory and antioxidant properties. Nutr. Diet. Suppl. 2010, 2, 73–83. [Google Scholar] [CrossRef]
- Van Vo, B.; Siddik, M.A.; Fotedar, R.; Chaklader, R.; Hanif, A.; Foysal, J.; Nguyen, H.Q. Progressive replacement of fishmeal by raw and enzyme-treated alga, Spirulina platensis influences growth, intestinal micromorphology and stress response in juvenile barramundi, Lates calcarifer. Aquaculture 2020, 529, 735741. [Google Scholar] [CrossRef]
- Nandeesha, M.C.; Gangadhara, B.; Manissery, J.K.; Venkataraman, L.V. Growth performance of two Indian major carps, catla (Catla catla) and rohu (Labeo rohita) fed diets containing different levels of Spirulina platensis. Bioresour. Technol. 2001, 80, 117–120. [Google Scholar] [CrossRef]
- Kim, S.-S.; Rahimnejad, S.; Kim, K.-W.; Lee, K.-J. Partial Replacement of Fish Meal with Spirulina pacifica in Diets for Parrot Fish (Oplegnathus fasciatus). Turk. J. Fish. Aquat. Sci. 2013, 13, 197–204. [Google Scholar]
- El-Kader, M.F.A.; El-Bab, A.F.F.; Abd-Elghany, M.F.; Abdel-Warith, A.-W.A.; Younis, E.M.; Dawood, M.A.O. Selenium Nanoparticles Act Potentially on the Growth Performance, Hemato-Biochemical Indices, Antioxidative, and Immune-Related Genes of European Seabass (Dicentrarchus labrax). Biol. Trace Element Res. 2020, 199, 3126–3134. [Google Scholar] [CrossRef] [PubMed]
- Yeganeh, S.; Teimouri, M.; Amirkolaie, A.K. Dietary effects of Spirulina platensis on hematological and serum biochemical parameters of rainbow trout (Oncorhynchus mykiss). Res. Veter- Sci. 2015, 101, 84–88. [Google Scholar] [CrossRef]
- Srivastava, P.K.; Pandey, A.K. Natural products and derivatives: Biological and pharmacological activities. Biochem. Cell. Arch. 2015, 15, 1–38. [Google Scholar]
- McLeay, D.J.; Gordon, M.R. Leucocrit: A Simple Hematological Technique for Measuring Acute Stress in Salmonid Fish, Including Stressful Concentrations of Pulpmill Effluent. J. Fish. Res. Board Can. 1977, 34, 2156–2163. [Google Scholar] [CrossRef]
- Blanc, M.-C.; Neveux, N.; Laromiguière, M.; Bérard, M.-P.; Cynober, L. Evaluation of a Newly Available Biochemical Analyzer: The Olympus AU 600. Clin. Chem. Lab. Med. (CCLM) 2000, 38, 465–475. [Google Scholar] [CrossRef] [PubMed]
- Siddik, M.A.B.; Howieson, J.; Partridge, G.; Fotedar, R.; GholipourKanani, H. Dietary tuna hydrolysate modulates growth performance, immune response, intestinal morphology and resistance to Streptococcus iniae in juvenile barramundi, Lates calcarifer. Sci. Rep. 2018, 8, 15942. [Google Scholar] [CrossRef] [PubMed]
- Chaklader, R.; Siddik, M.A.B.; Fotedar, R.; Howieson, J. Insect larvae, Hermetia illucens in poultry by-product meal for barramundi, Lates calcarifer modulates histomorphology, immunity and resistance to Vibrio harveyi. Sci. Rep. 2019, 9, 16703. [Google Scholar] [CrossRef] [PubMed]
- Siddik, M.A.B.; Howieson, J.; Fotedar, R. Beneficial effects of tuna hydrolysate in poultry by-product meal diets on growth, immune response, intestinal health and disease resistance to Vibrio harveyi in juvenile barramundi, Lates calcarifer. Fish Shellfish Immunol. 2019, 89, 61–70. [Google Scholar] [CrossRef] [PubMed]
- Van Vo, B.; Siddik, M.A.B.; Chaklader, R.; Fotedar, R.; Nahar, A.; Foysal, J.; Bui, D.P.; Nguyen, H.Q. Growth and health of juvenile barramundi (Lates calcarifer) challenged with DO hypoxia after feeding various inclusions of germinated, fermented and untreated peanut meals. PLoS ONE 2020, 15, e0232278. [Google Scholar] [CrossRef]
- Walker, A.B.; Berlinsky, D.L. Effects of partial replacement of fish meal protein by microalgae on growth, feed intake, and body composition of Atlantic cod. N. Am. J. Aquac. 2011, 73, 76–83. [Google Scholar] [CrossRef]
- Sheikhzadeh, N.; Mousavi, S.; Hamidian, G.; Firouzamandi, M.; Oushani, A.K.; Mardani, K. Role of dietary Spirulina platensis in improving mucosal immune responses and disease resistance of rainbow trout (Oncorhynchus mykiss). Aquaculture 2019, 510, 1–8. [Google Scholar] [CrossRef]
- Pham, H.D.; Siddik, M.A.B.; Fotedar, R.; Nguyen, C.M.; Nahar, A.; Gupta, S.K. Total Bioavailable Organic Selenium in Fishmeal-Based Diet Influences Growth and Physiology of Juvenile Cobia Rachycentron canadum (Linnaeus, 1766). Biol. Trace Element Res. 2018, 190, 541–549. [Google Scholar] [CrossRef] [PubMed]
- Ilham, I.; Siddik, M.A.B.; Fotedar, R. Effects of Organic Selenium Supplementation on Growth, Accumulation, Haematology and Histopathology of Juvenile Barramundi (Lates calcarifer) Fed High Soybean Meal Diets. Biol. Trace Element Res. 2016, 174, 436–447. [Google Scholar] [CrossRef] [PubMed]
- Le, K.T.; Fotedar, R. Toxic effects of excessive levels of dietary selenium in juvenile yellowtail king fi sh (Seriola lalandi). Aquaculture 2014, 433, 229–234. [Google Scholar] [CrossRef][Green Version]
- He, Y.; Chen, S.; Liu, Z.; Cheng, C.; Li, H.; Wang, M. Toxicity of selenium nanoparticles in male Sprague – Dawley rats at supranutritional and nonlethal levels. Life Sci. 2014, 115, 44–51. [Google Scholar] [CrossRef] [PubMed]
- Loeschner, K.; Hadrup, N.; Hansen, M.; Pereira, S.A.; Gammelgaard, B.; Møller, L.H.; Mortensen, A.; Lam, H.R.; Larsen, E.H. Absorption, distribution, metabolism and excretion of selenium following oral administration of elemental selenium nanoparticles or selenite in rats. Metallomics 2014, 6, 330–337. [Google Scholar] [CrossRef]
- Rosas, V.T.; Poersch, L.H.; Romano, L.A.; Tesser, M.B. Feasibility of the use of Spirulina in aquaculture diets. Rev. Aquac. 2018, 11, 1367–1378. [Google Scholar] [CrossRef]
- Teimouri, M.; Amirkolaie, A.K.; Yeganeh, S. The effects of dietary supplement of Spirulina platensis on blood carotenoid concentration and fillet color stability in rainbow trout (Oncorhynchus mykiss). Aquaculture 2013, 414–415, 224–228. [Google Scholar] [CrossRef]
- Hajiahmadian, M.; Vajargah, M.F.; Farsani, H.G.; Chorehi, M.M. Effect of Spirulina platensis Meal as Feed Additive on Growth Performance and Survival Rate in Golden Barb fish, Puntius gelius (Hamilton, 1822). J. Fish. Int. 2012, 7, 61–64. [Google Scholar]
- El-Sayed, A.-F.M. Evaluation of soybean meal, spirulina meal and chicken offal meal as protein sources for silver seabream (Rhabdosargus sarba) fingerlings. Aquaculture 1994, 127, 169–176. [Google Scholar] [CrossRef]
- Nakagawa, H.; Kasahara, S.; Sugiyama, T. Effect of Ulva meal supplementation on lipid metabolism of black sea bream, Acanthopagrus schlegeli (Bleeker). Aquaculture 1987, 62, 109–121. [Google Scholar] [CrossRef]
- Hoseinifar, S.H.; Yousefi, S.; Van Doan, H.; Ashouri, G.; Gioacchini, G.; Maradonna, F.; Carnevali, O. Oxidative Stress and Antioxidant Defense in Fish: The Implications of Probiotic, Prebiotic, and Synbiotics. Rev. Fish. Sci. Aquac. 2020, 29, 198–217. [Google Scholar] [CrossRef]
- Zhang, C.-N.; Li, X.-F.; Xu, W.-N.; Jiang, G.-Z.; Lu, K.-L.; Wang, L.-N.; Liu, W.-B. Combined effects of dietary fructooligosaccharide and Bacillus licheniformis on innate immunity, antioxidant capability and disease resistance of triangular bream (Megalobrama terminalis). Fish Shellfish Immunol. 2013, 35, 1380–1386. [Google Scholar] [CrossRef]
- Al-Deriny, S.H.; Dawood, M.A.O.; Elbialy, Z.I.; El-Tras, W.F.; Mohamed, R.A. Selenium Nanoparticles and Spirulina Alleviate Growth Performance, Hemato-Biochemical, Immune-Related Genes, and Heat Shock Protein in Nile Tilapia (Oreochromis niloticus). Biol. Trace Element Res. 2020, 198, 661–668. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Moneim, A.-M.E.; El-Saadony, M.T.; Shehata, A.M.; Saad, A.M.; Aldhumri, S.A.; Ouda, S.M.; Mesalam, N.M. Antioxidant and antimicrobial activities of Spirulina platensis extracts and biogenic selenium nanoparticles against selected pathogenic bacteria and fungi. Saudi J. Biol. Sci. 2021, 29, 1197–1209. [Google Scholar] [CrossRef]
- Siwicki, A.K.; Anderson, D.P.; Rumsey, G.L. Dietary intake of immunostimulants by rainbow trout affects non-specific immunity and protection against furunculosis. Veter. Immunol. Immunopathol. 1994, 41, 125–139. [Google Scholar] [CrossRef]
- Wiegertjes, G.; Stet, R.; Parmentier, H.K.; van Muiswinkel, W.B. Immunogenetics of disease resistance in fish: A comparative approach. Dev. Comp. Immunol. 1996, 20, 365–381. [Google Scholar] [CrossRef]
- Ngo, D.T.; Wade, N.M.; Pirozzi, I.; Glencross, B.D. Effects of canola meal on growth, feed utilisation, plasma biochemistry, histology of digestive organs and hepatic gene expression of barramundi (Asian seabass; Lates calcarifer). Aquaculture 2016, 464, 95–105. [Google Scholar] [CrossRef]
- Glencross, B.; Rutherford, N.; Jones, B. Evaluating options for fishmeal replacement in diets for juvenile barramundi (Lates calcarifer). Aquac. Nutr. 2010, 17, e722–e732. [Google Scholar] [CrossRef]
- Lin, Y.-H.; Shiau, S.-Y. Dietary lipid requirement of grouper, Epinephelus malabaricus, and effects on immune responses. Aquaculture 2003, 225, 243–250. [Google Scholar] [CrossRef]
- Misra, C.K.; Das, B.K.; Mukherjee, S.C.; Pattnaik, P. Effect of long term administration of dietary β-glucan on immunity, growth and survival of Labeo rohita fingerlings. Aquaculture 2006, 255, 82–94. [Google Scholar] [CrossRef]
- Chen, F.; Wei, Z.; Zhao, X.; Shao, Y.; Zhang, W. Molecular characteristics, expression, and antimicrobial activities of i-type lysozyme from the razor clam Sinonovacula constricta. Fish Shellfish Immunol. 2018, 79, 321–326. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.L.; Chen, H.C. Serum metabolic enzyme activities and hepatocyte ultrastructure of common carp after gallium exposure. Zool. Stud. 2003, 42, 455–461. [Google Scholar]
- Han, X.; Qin, P.; Li, W.; Ma, Q.; Ji, C.; Zhang, J.; Zhao, L. Effect of sodium selenite and selenium yeast on performance, egg quality, antioxidant capacity, and selenium deposition of laying hens. Poult. Sci. 2017, 96, 3973–3980. [Google Scholar] [CrossRef] [PubMed]
- Saffari, S.; Keyvanshokooh, S.; Zakeri, M.; Johari, S.; Pasha-Zanoosi, H. Effects of different dietary selenium sources (sodium selenite, selenomethionine and nanoselenium) on growth performance, muscle composition, blood enzymes and antioxidant status of common carp (Cyprinus carpio). Aquac. Nutr. 2016, 23, 611–617. [Google Scholar] [CrossRef]
- Lu, K.-L.; Xu, W.-N.; Wang, L.-N.; Zhang, D.-D.; Zhang, C.-N.; Liu, W.-B. Hepatic β-Oxidation and Regulation of Carnitine Palmitoyltransferase (CPT) I in Blunt Snout Bream Megalobrama amblycephala Fed a High Fat Diet. PLoS ONE 2014, 9, e93135. [Google Scholar] [CrossRef] [PubMed]
- Ye, H.; Zhou, Y.; Su, N.; Wang, A.; Tan, X.; Sun, Z.; Zou, C.; Liu, Q.; Ye, C. Effects of replacing fish meal with rendered animal protein blend on growth performance, hepatic steatosis and immune status in hybrid grouper (Epinephelus fuscoguttatus ♀ × Epinephelus lanceolatus♂). Aquaculture 2019, 511, 734203. [Google Scholar] [CrossRef]
- Olvera-Novoa, M.; Domínguez-Cen, L.J.; Olivera-Castillo, L.; A Martínez-Palacios, C. Effect of the use of the microalga Spirulina maxima as fish meal replacement in diets for tilapia, Oreochromis mossambicus (Peters), fry. Aquac. Res. 1998, 29, 709–715. [Google Scholar] [CrossRef]
- Jaime-Ceballos, B.J.; Hernández-Llamas, A.; Garcia-Galano, T.; Villarreal, H. Substitution of Chaetoceros muelleri by Spirulina platensis meal in diets for Litopenaeus schmitti larvae. Aquaculture 2006, 260, 215–220. [Google Scholar] [CrossRef]
- Macias-Sancho, J.; Poersch, L.H.; Bauer, W.; Romano, L.A.; Wasielesky, W.; Tesser, M.B. Fishmeal substitution with Arthrospira (Spirulina platensis) in a practical diet for Litopenaeus vannamei: Effects on growth and immunological parameters. Aquaculture 2014, 426–427, 120–125. [Google Scholar] [CrossRef]
- Abedin, R.M.A.; Taha, H.M. Antibacterial and antifungal activity of Cyanobacteria and green microalgae. Evaluation of medium components by Plackett-Burman design for antimicrobial activity of Spirulina platensis. Glob. J. Biotechnol. Biochem. 2008, 3, 22–31. [Google Scholar]
- Santoyo, S.; Herrero, M.; Señorans, F.J.; Cifuentes, A.; Ibáñez, E.; Jaime, L. Functional characterization of pressurized liquid extracts of Spirulina platensis. Eur. Food Res. Technol. 2006, 224, 75–81. [Google Scholar] [CrossRef]
- Siddik, M.A.; Howieson, J.; Islam, S.M.; Fotedar, R. Synbiotic feed supplementation improves antioxidant response and innate immunity of juvenile barramundi, Lates calcarifer subjected to bacterial infection. Aquaculture 2022, 552, 737965. [Google Scholar] [CrossRef]
- Wangkahart, E.; Bruneel, B.; Chantiratikul, A.; de Jong, M.; Pakdeenarong, N.; Subramani, P.A. Optimum dietary sources and levels of selenium improve growth, antioxidant status, and disease resistance: Re-evaluation in a farmed fish species, Nile tilapia (Oreochromis niloticus). Fish Shellfish Immunol. 2022, 121, 172–182. [Google Scholar] [CrossRef] [PubMed]
- Adel, M.; Yeganeh, S.; Dadar, M.; Sakai, M.; Dawood, M.A. Effects of dietary Spirulina platensis on growth performance, humoral and mucosal immune responses and disease resistance in juvenile great sturgeon (Huso huso Linnaeus, 1754). Fish Shellfish Immunol. 2016, 56, 436–444. [Google Scholar] [CrossRef] [PubMed]
- Xia, I.F.; Cheung, J.S.; Wu, M.; Wong, K.-S.; Kong, H.-K.; Zheng, X.-T.; Wong, K.-H.; Kwok, K.W. Dietary chitosan-selenium nanoparticle (CTS-SeNP) enhance immunity and disease resistance in zebrafish. Fish Shellfish Immunol. 2019, 87, 449–459. [Google Scholar] [CrossRef]
- Zhou, X.; Wang, Y.; Gu, Q.; Li, W. Effects of different dietary selenium sources (selenium nanoparticle and selenomethionine) on growth performance, muscle composition and glutathione peroxidase enzyme activity of crucian carp (Carassius auratus gibelio). Aquaculture 2009, 291, 78–81. [Google Scholar] [CrossRef]
- Placha, I.; Borutova, R.; Gresakova, L.; Petrovic, V.; Faix, S.; Leng, L. Effects of excessive selenium supplementation to diet contaminated with deoxynivalenol on blood phagocytic activity and antioxidative status of broilers. J. Anim. Physiol. Anim. Nutr. 2009, 93, 695–702. [Google Scholar] [CrossRef]
- Huang, Z.; Rose, A.H.; Hoffmann, P.R. The Role of Selenium in Inflammation and Immunity: From Molecular Mechanisms to Therapeutic Opportunities. Antioxidants Redox Signal. 2012, 16, 705–743. [Google Scholar] [CrossRef]
- Bielecki, A.; Świątek, P.; Jabłońska-Barna, I.; Kobak, J.; Hildebrand, J.; Dmitryjuk, M.; Strużyński, W.; Rost-Roszkowska, M. The activity of hydrolytic enzymes in the digestive system of Acanthobdellida, Branchiobdellida and Hirudinida (Annelida, Clitellata) – considerations on similarity and phylogeny. Eur. Zool. J. 2021, 88, 26–43. [Google Scholar] [CrossRef]
- Willora, F.P.; Vatsos, I.N.; Mallioris, P.; Bordignon, F.; Keizer, S.; Martınez-Llorens, S.; Sørensen, M.; Hagen, Ø. Replacement of fishmeal with plant protein in the diets of juvenile lumpfish (Cyclopterus lumpus, L. 1758): Effects on digestive enzymes and microscopic structure of the digestive tract. Aquaculture 2022, 561, 738601. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).