The Cluster Transfer Function of AtNEET Supports the Ferredoxin–Thioredoxin Network of Plant Cells
Abstract
:1. Introduction
2. Materials and Methods
2.1. Vector Construction and Generation of Transgenic Plants
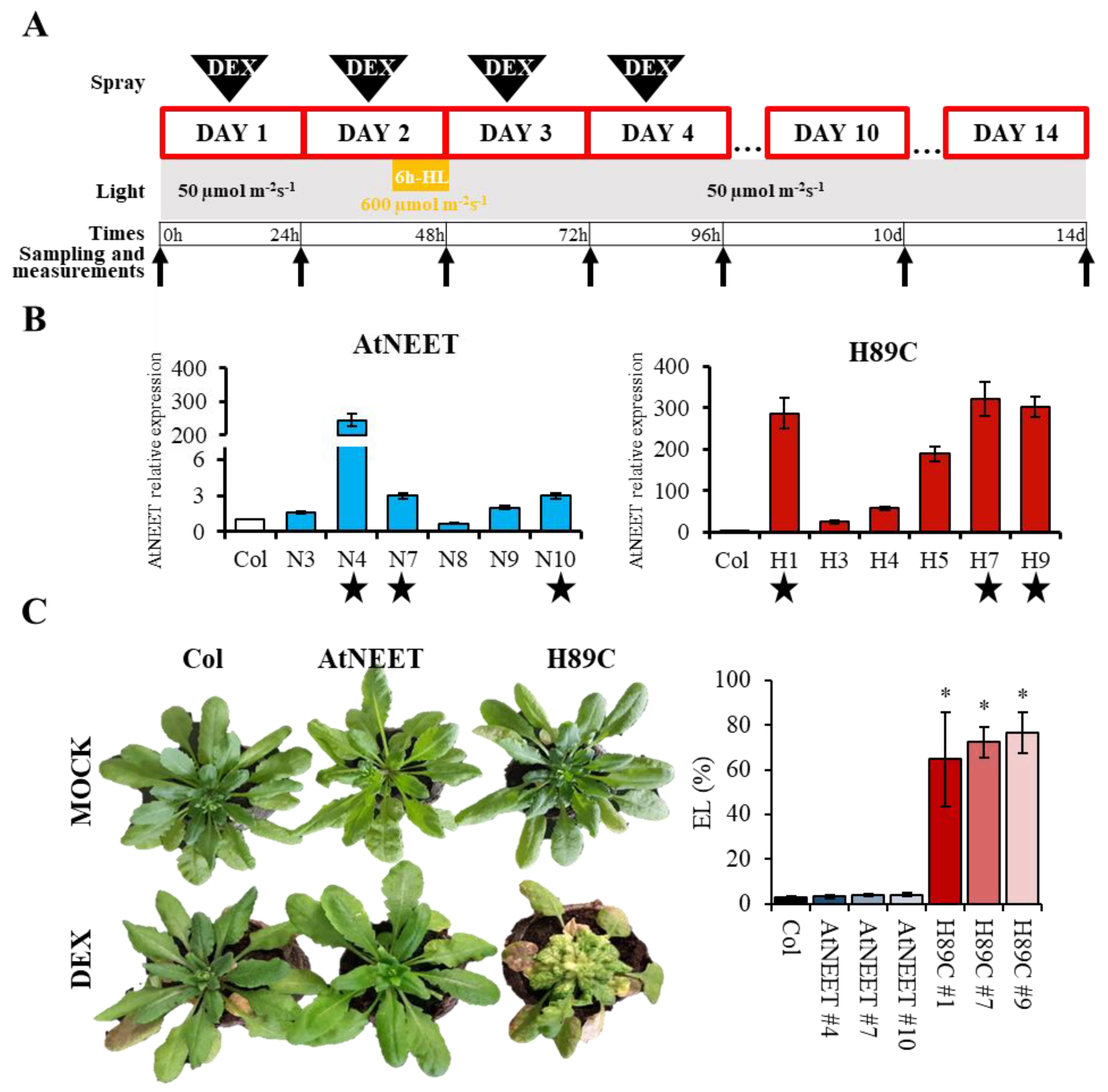
2.2. Growth Conditions and DEX Treatment
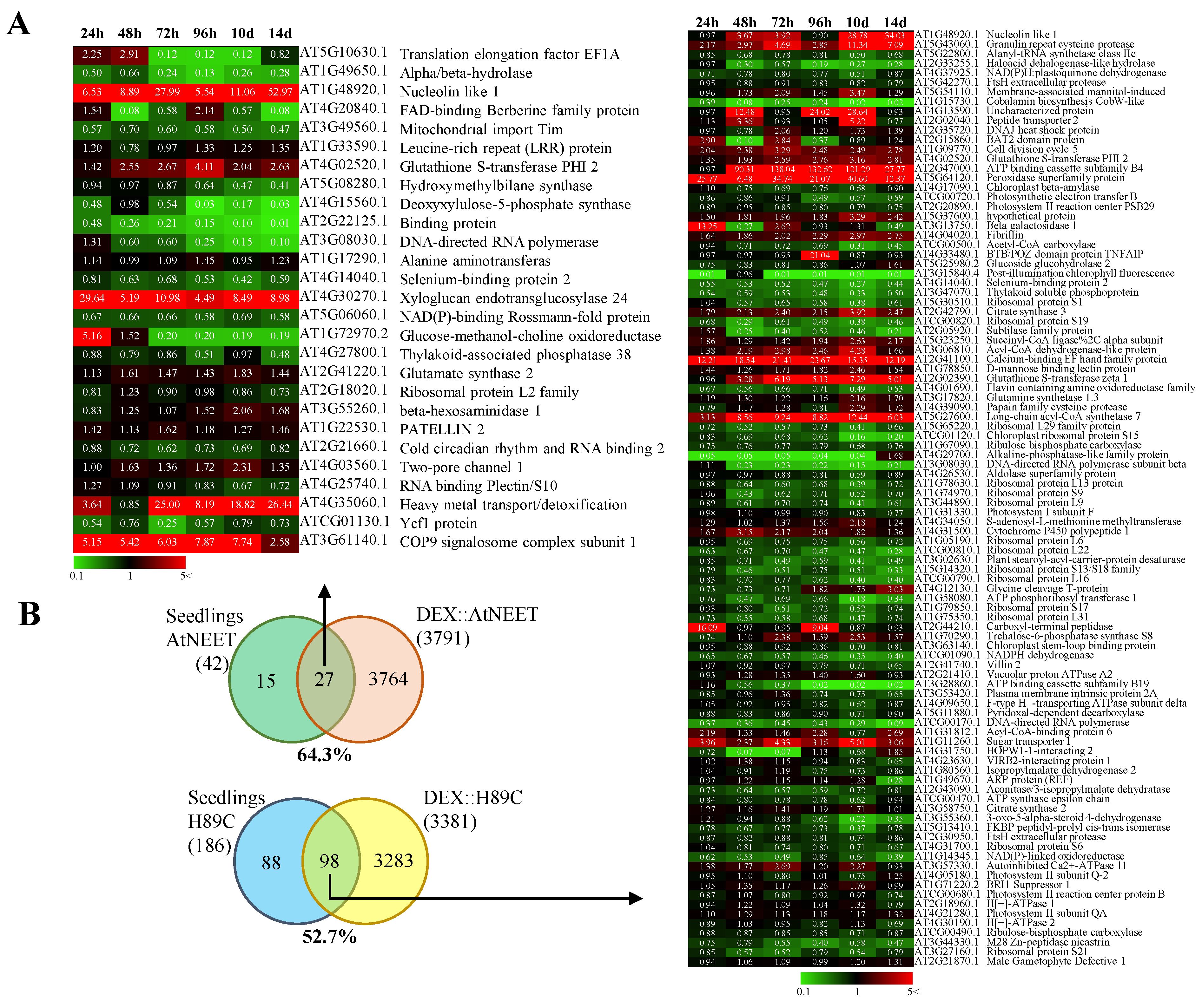
2.3. Proteomics Analysis
2.4. Electrolyte Leakage
2.5. RT-qPCR Analysis
2.6. Photosynthetic Parameters
2.7. Chlorophyll Measurements
2.8. Statistical Analysis
3. Results
3.1. Inducible Expression of H89C in Arabidopsis
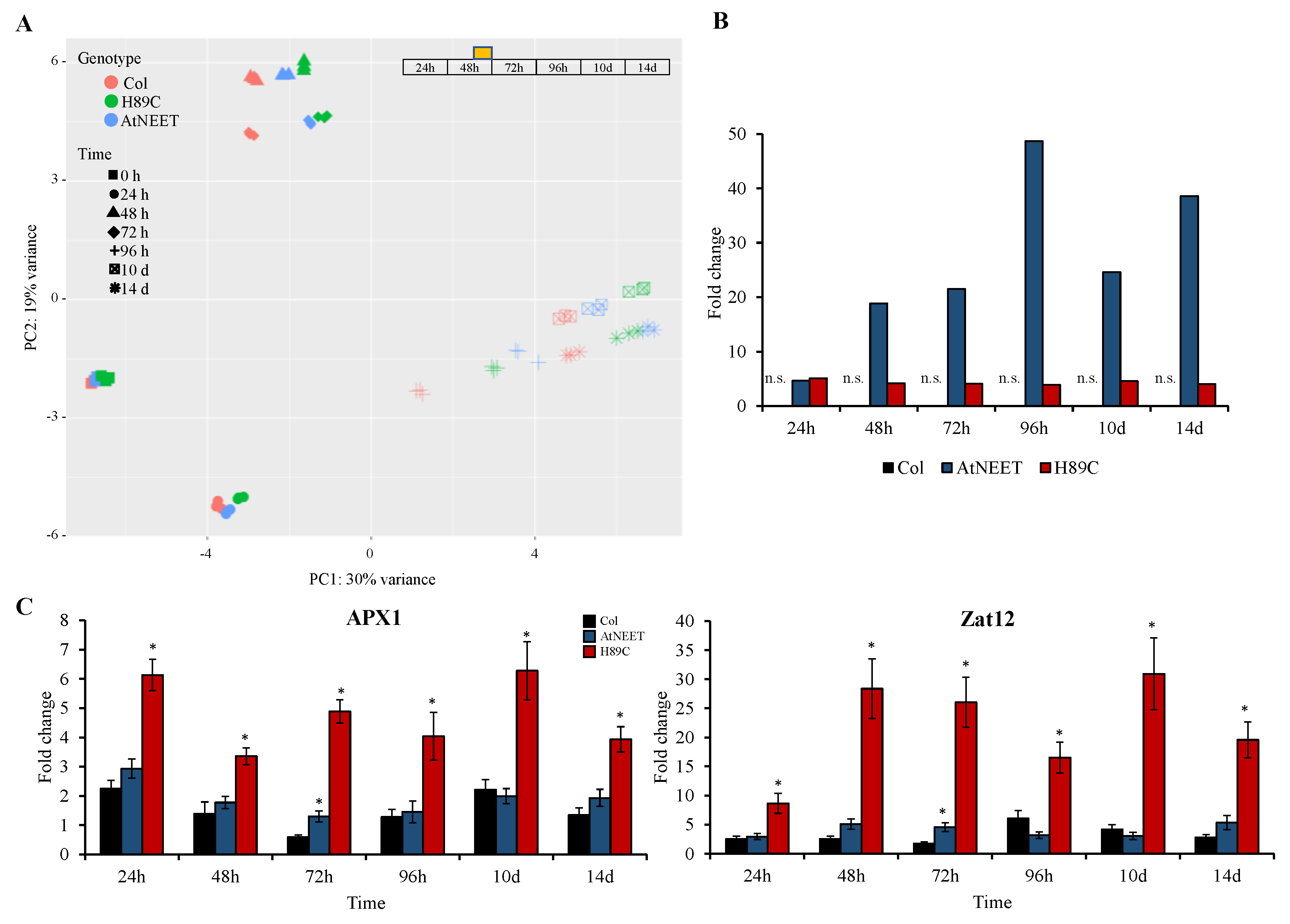
3.2. Proteomics Analysis of AtNEET and H89C Plants Following DEX Application
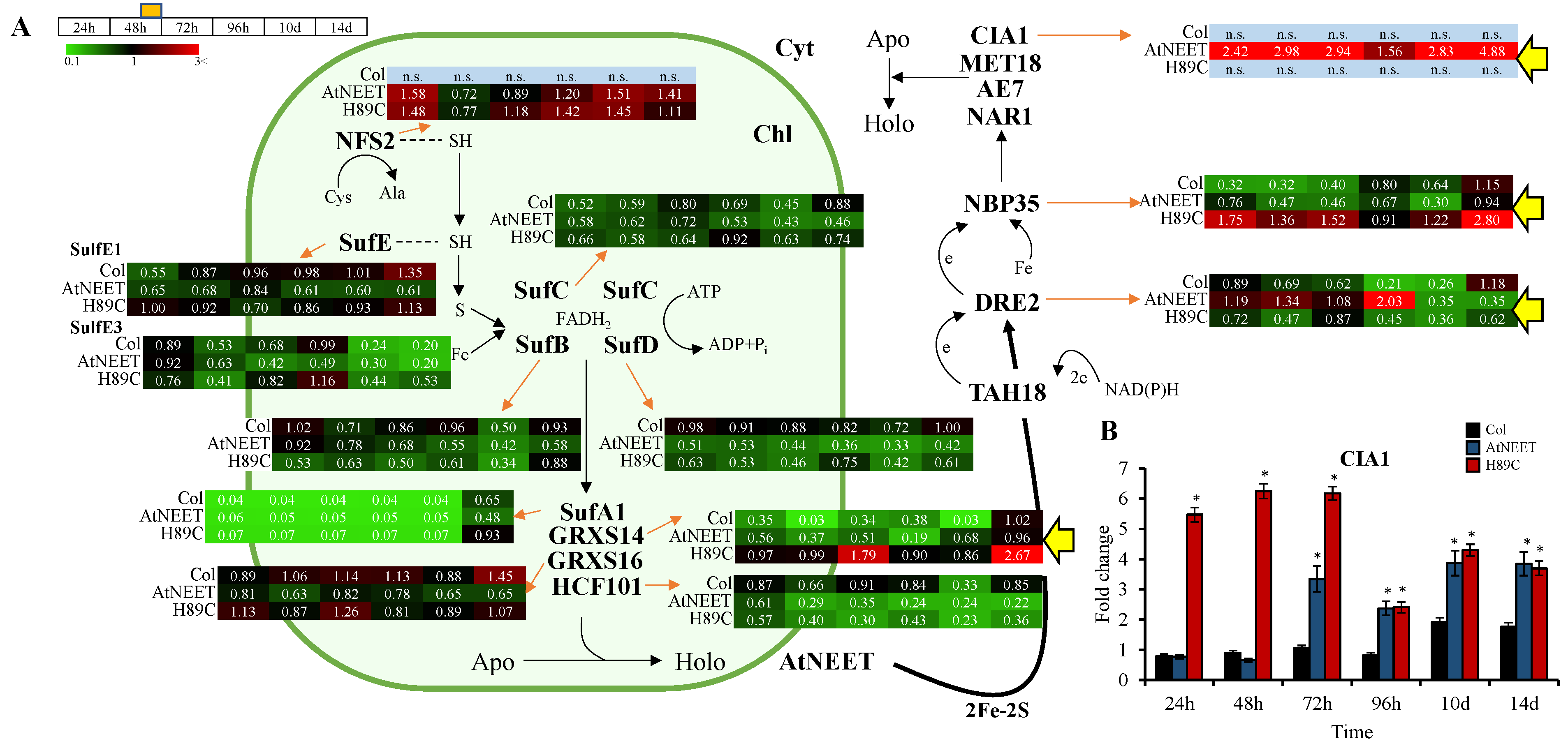
3.3. Altered Abundance of Different Components of the Cytosolic Iron-Sulfur Cluster Assembly (CIA) Pathway in AtNEET and H89C Plants Following DEX Application
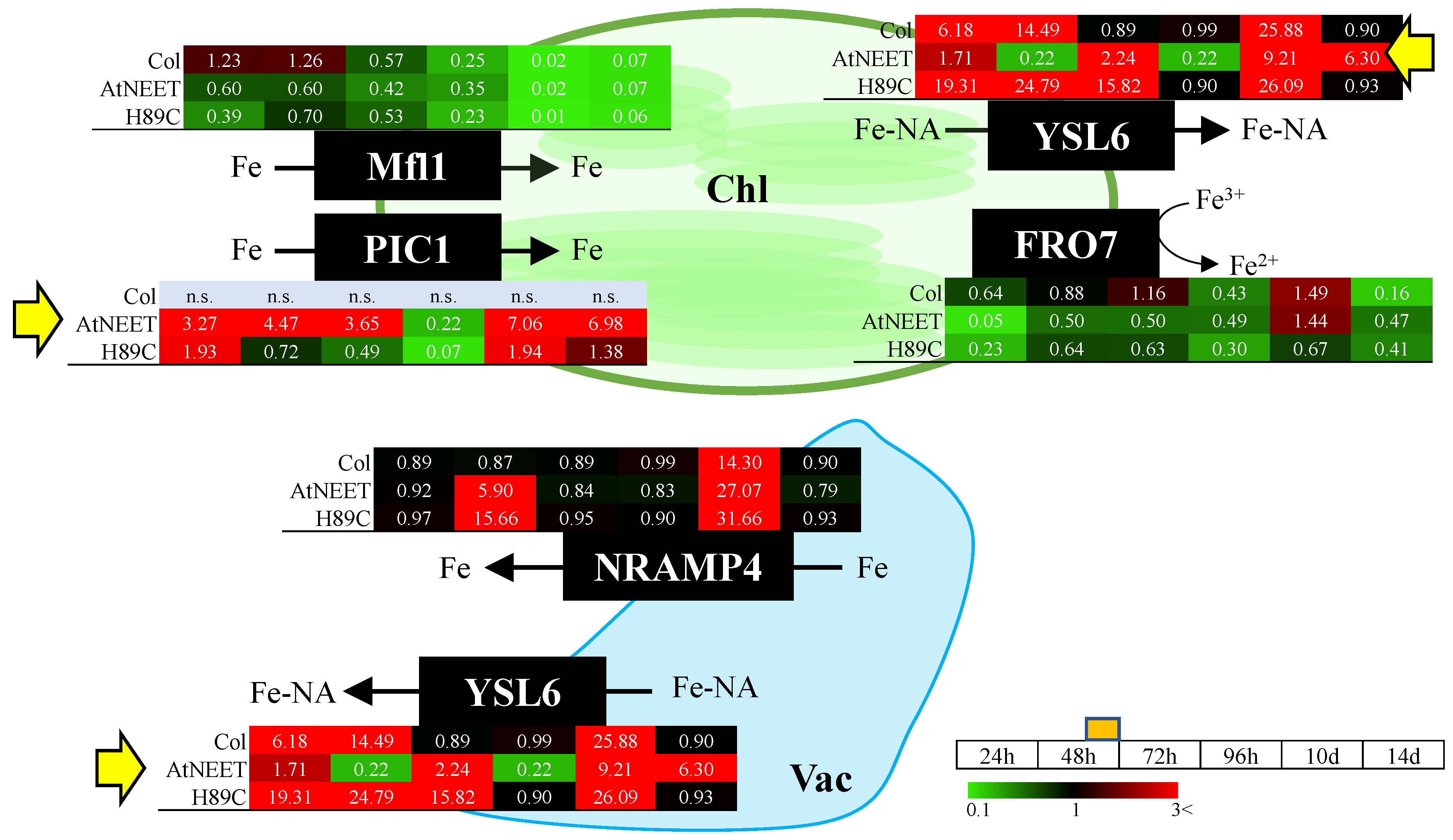
3.4. Altered Abundance of Iron Efflux Proteins Following Alterations in AtNEET Function
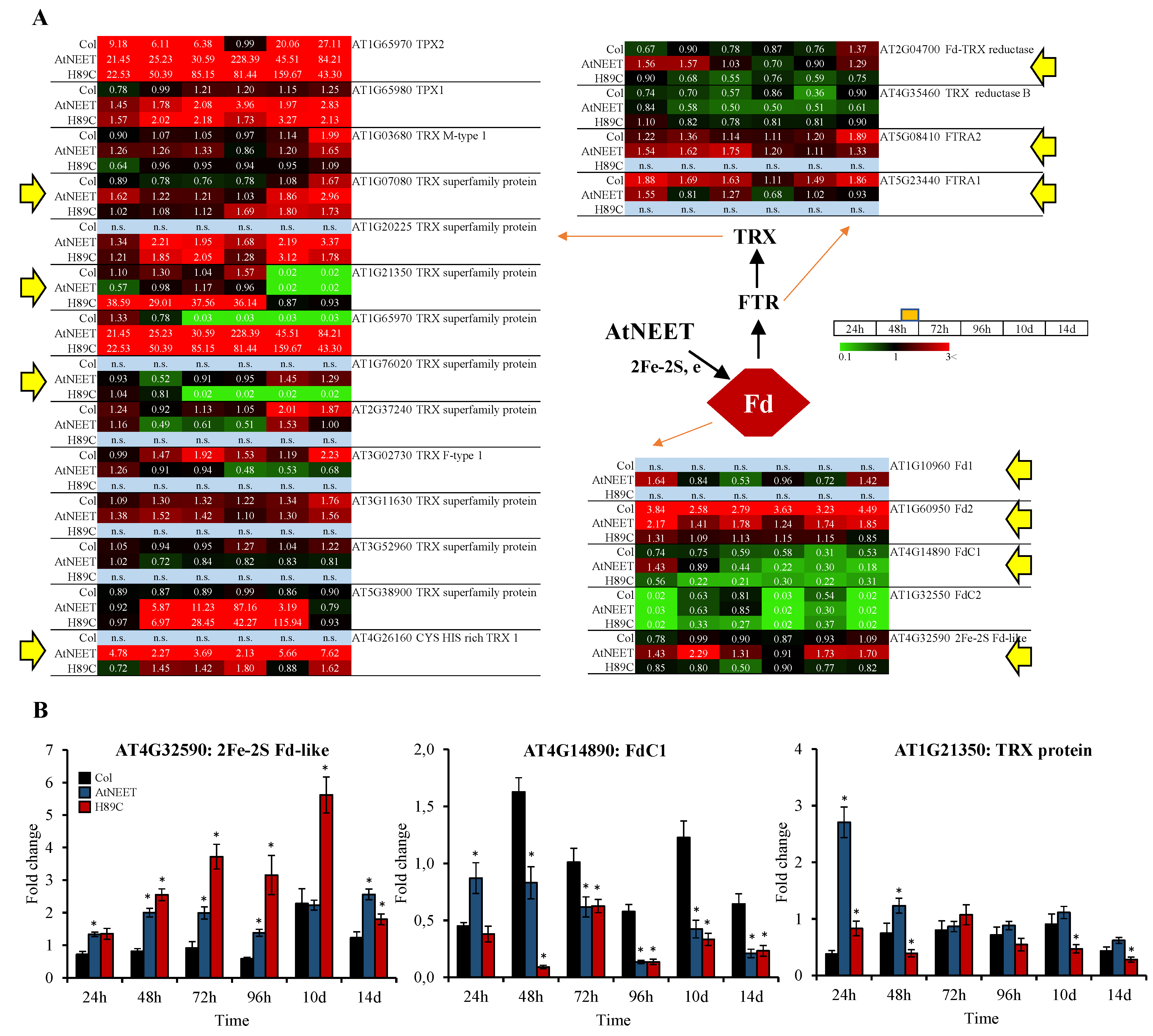
3.5. Alterations in the Fd-FTR-TRX Network of Arabidopsis Following the Inducible Expression of AtNEET or H89C
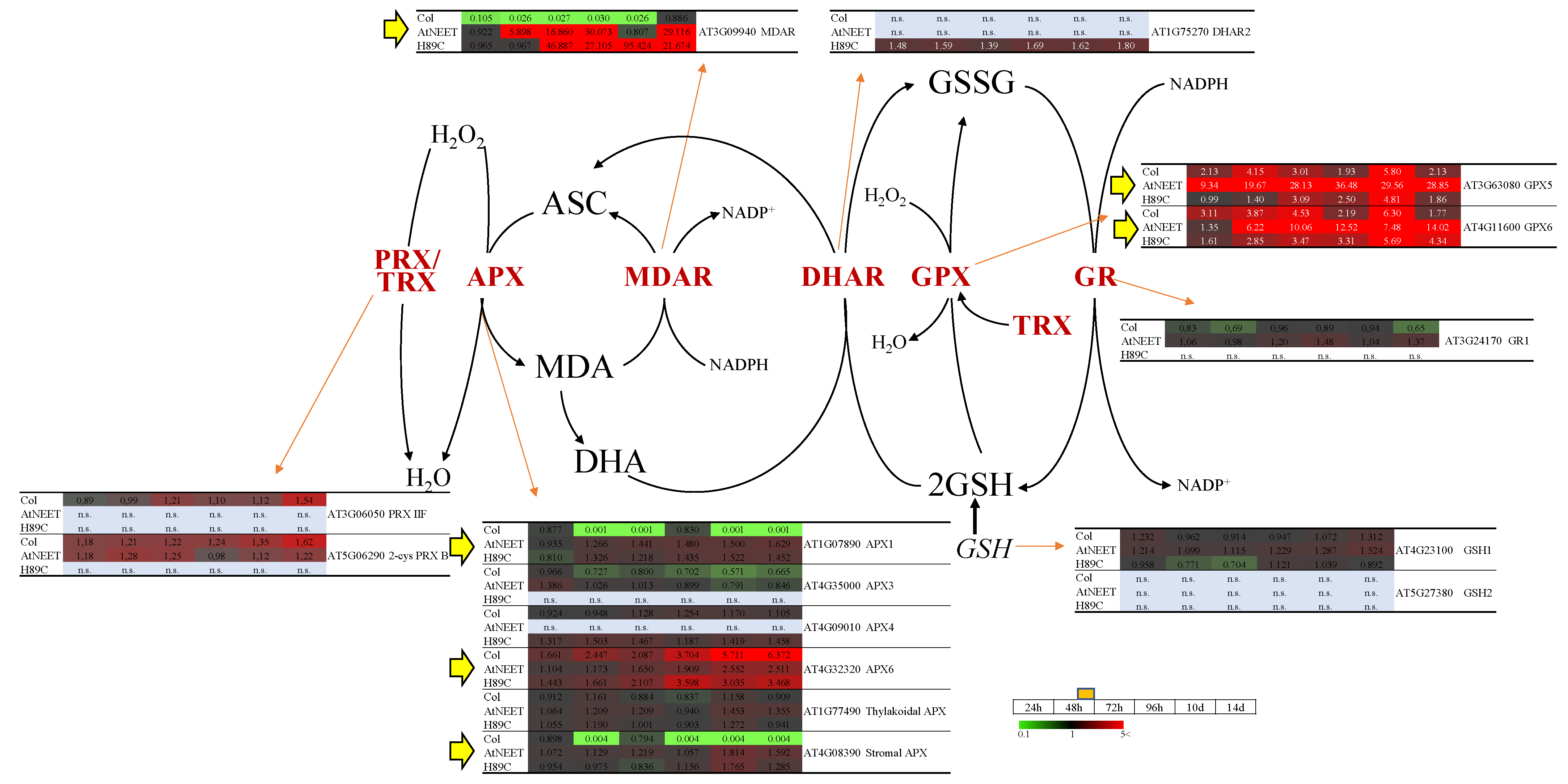
3.6. Changes in the Abundance of Different ROS Scavenging Enzymes Following Alterations in AtNEET Function
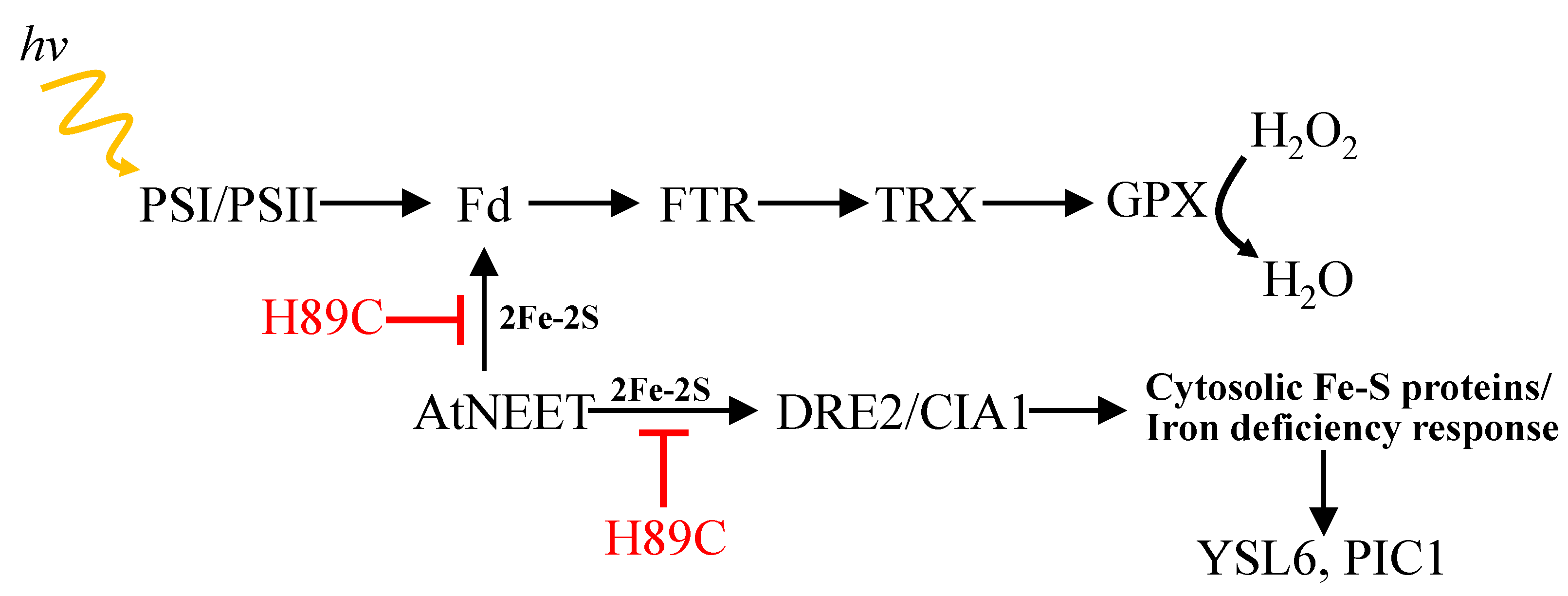
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nechushtai, R.; Conlan, A.R.; Harir, Y.; Song, L.; Yogev, O.; Eisenberg-Domovich, Y.; Livnah, O.; Michaeli, D.; Rosen, R.; Ma, V.; et al. Characterization of Arabidopsis NEET Reveals an Ancient Role for NEET Proteins in Iron Metabolism. Plant Cell 2012, 24, 2139–2154. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nechushtai, R.; Karmi, O.; Zuo, K.; Marjault, H.B.; Darash-Yahana, M.; Sohn, Y.S.; King, S.D.; Zandalinas, S.I.; Carloni, P.; Mittler, R. The Balancing Act of NEET Proteins: Iron, ROS, Calcium and Metabolism. Biochim. Biophys. Acta-Mol. Cell Res. 2020, 1867, 118805. [Google Scholar] [CrossRef] [PubMed]
- Inupakutika, M.A.; Sengupta, S.; Nechushtai, R.; Jennings, P.A.; Onuchic, J.N.; Azad, R.K.; Padilla, P.; Mittler, R. Phylogenetic Analysis of Eukaryotic NEET Proteins Uncovers a Link between a Key Gene Duplication Event and the Evolution of Vertebrates. Sci. Rep. 2017, 7, 42571. [Google Scholar] [CrossRef] [PubMed]
- Sengupta, S.; Nechushtai, R.; Jennings, P.A.; Onuchic, J.N.; Padilla, P.A.; Azad, R.K.; Mittler, R. Phylogenetic Analysis of the CDGSH Iron-Sulfur Binding Domain Reveals Its Ancient Origin. Sci. Rep. 2018, 8, 4840. [Google Scholar] [CrossRef]
- Molino, D.; Pila-Castellanos, I.; Marjault, H.; Dias Amoedo, N.; Kopp, K.; Rochin, L.; Karmi, O.; Sohn, Y.; Lines, L.; Hamaï, A.; et al. Chemical Targeting of NEET Proteins Reveals Their Function in Mitochondrial Morphodynamics. EMBO Rep. 2020, 21, e49019. [Google Scholar] [CrossRef]
- Mittler, R.; Darash-Yahana, M.; Sohn, Y.S.; Bai, F.; Song, L.; Cabantchik, I.Z.; Jennings, P.A.; Onuchic, J.N.; Nechushtai, R. NEET Proteins: A New Link between Iron Metabolism, Reactive Oxygen Species, and Cancer. Antioxid. Redox Signal. 2019, 30, 1083–1095. [Google Scholar] [CrossRef]
- Su, L.W.; Chang, S.H.; Li, M.Y.; Huang, H.Y.; Jane, W.N.; Yang, J.Y. Purification and Biochemical Characterization of Arabidopsis At-NEET, an Ancient Iron-Sulfur Protein, Reveals a Conserved Cleavage Motif for Subcellular Localization. Plant Sci. 2013, 213, 46–54. [Google Scholar] [CrossRef]
- Sohn, Y.-S.; Tamir, S.; Song, L.; Michaeli, D.; Matouk, I.; Conlan, A.R.; Harir, Y.; Holt, S.H.; Shulaev, V.; Paddock, M.L.; et al. NAF-1 and MitoNEET Are Central to Human Breast Cancer Proliferation by Maintaining Mitochondrial Homeostasis and Promoting Tumor Growth. Proc. Natl. Acad. Sci. USA 2013, 110, 14676–14681. [Google Scholar] [CrossRef] [Green Version]
- Zandalinas, S.I.; Song, L.; Sengupta, S.; McInturf, S.A.; Grant, D.G.; Marjault, H.-B.H.; Castro-Guerrero, N.A.; Burks, D.; Azad, R.K.; Mendoza Cozatl, D.G.; et al. Expression of a Dominant-negative AtNEET-H89C Protein Disrupts Iron–Sulfur Metabolism and Iron Homeostasis in Arabidopsis. Plant J. 2020, 101, 1152–1169. [Google Scholar] [CrossRef]
- Darash-Yahana, M.; Pozniak, Y.; Lu, M.; Sohn, Y.-S.; Karmi, O.; Tamir, S.; Bai, F.; Song, L.; Jennings, P.A.; Pikarsky, E.; et al. Breast Cancer Tumorigenicity Is Dependent on High Expression Levels of NAF-1 and the Lability of Its Fe-S Clusters. Proc. Natl. Acad. Sci. USA 2016, 113, 10890–10895. [Google Scholar] [CrossRef] [Green Version]
- Karmi, O.; Sohn, Y.S.; Zandalinas, S.I.; Rowland, L.; King, S.D.; Nechushtai, R.; Mittler, R. Disrupting CISD2 Function in Cancer Cells Primarily Impacts Mitochondrial Labile Iron Levels and Triggers TXNIP Expression. Free Radic. Biol. Med. 2021, 176, 92–104. [Google Scholar] [CrossRef] [PubMed]
- Aoyama, T.; Chua, N.H. A Glucocorticoid-Mediated Transcriptional Induction System in Transgenic Plants. Plant J. 1997, 11, 605–612. [Google Scholar] [CrossRef] [PubMed]
- Distéfano, A.M.; López, G.A.; Setzes, N.; Marchetti, F.; Cainzos, M.; Cascallares, M.; Zabaleta, E.; Pagnussat, G.C. Ferroptosis in Plants: Triggers, Proposed Mechanisms, and the Role of Iron in Modulating Cell Death. J. Exp. Bot. 2021, 72, 2125–2135. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Henriques, R.; Lin, S.S.; Niu, Q.W.; Chua, N.H. Agrobacterium-Mediated Transformation of Arabidopsis Thaliana Using the Floral Dip Method. Nat. Protoc. 2006, 1, 641–646. [Google Scholar] [CrossRef] [PubMed]
- Dahal, D.; Newton, K.J.; Mooney, B.P. Quantitative Proteomics of Zea Mays Hybrids Exhibiting Different Levels of Heterosis. J. Proteome Res. 2016, 15, 2445–2454. [Google Scholar] [CrossRef]
- Zandalinas, S.I.; Fichman, Y.; Devireddy, A.R.; Sengupta, S.; Azad, R.K.; Mittler, R. Systemic Signaling during Abiotic Stress Combination in Plants. Proc. Natl. Acad. Sci. USA 2020, 117, 13810–13820. [Google Scholar] [CrossRef]
- Zandalinas, S.I.; Balfagón, D.; Arbona, V.; Gómez-Cadenas, A.; Inupakutika, M.A.; Mittler, R. ABA Is Required for the Accumulation of APX1 and MBF1c during a Combination of Water Deficit and Heat Stress. J. Exp. Bot. 2016, 67, 5381–5390. [Google Scholar] [CrossRef] [Green Version]
- Davletova, S.; Schlauch, K.; Coutu, J.; Mittler, R. The Zinc-Finger Protein Zat12 Plays a Central Role in Reactive Oxygen and Abiotic Stress Signaling in Arabidopsis. Plant Physiol. 2005, 139, 847–856. [Google Scholar] [CrossRef] [Green Version]
- Davletova, S.; Rizhsky, L.; Liang, H.; Shengqiang, Z.; Oliver, D.J.; Coutu, J.; Shulaev, V.; Schlauch, K.; Mittler, R. Cytosolic Ascorbate Peroxidase 1 Is a Central Component of the Reactive Oxygen Gene Network of Arabidopsis. Plant Cell 2005, 17, 268–281. [Google Scholar] [CrossRef] [Green Version]
- Lill, R. Function and Biogenesis of Iron–Sulphur Proteins. Nature 2009, 460, 831–838. [Google Scholar] [CrossRef]
- Zhang, Y.; Lyver, E.R.; Nakamaru-Ogiso, E.; Yoon, H.; Amutha, B.; Lee, D.-W.; Bi, E.; Ohnishi, T.; Daldal, F.; Pain, D.; et al. Dre2, a Conserved Eukaryotic Fe/S Cluster Protein, Functions in Cytosolic Fe/S Protein Biogenesis. Mol. Cell. Biol. 2008, 28, 5569–5582. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Balk, J.; Pilon, M. Ancient and Essential: The Assembly of Iron-Sulfur Clusters in Plants. Trends Plant Sci. 2011, 16, 218–226. [Google Scholar] [CrossRef] [PubMed]
- Braymer, J.J.; Freibert, S.A.; Rakwalska-Bange, M.; Lill, R. Mechanistic Concepts of Iron-Sulfur Protein Biogenesis in Biology. Biochim. Biophys. Acta-Mol. Cell Res. 2021, 1868, 118863. [Google Scholar] [CrossRef] [PubMed]
- Divol, F.; Couch, D.; Conéjéro, G.; Roschzttardtz, H.; Mari, S.; Curie, C. The Arabidopsis YELLOW STRIPE LIKE4 and 6 Transporters Control Iron Release from the Chloroplast. Plant Cell 2013, 25, 1040–1055. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Conte, S.S.; Chu, H.H.; Chan-Rodriguez, D.; Punshon, T.; Vasques, K.A.; Salt, D.E.; Walker, E.L. Arabidopsis Thaliana Yellow Stripe1-Like4 and Yellow Stripe1-Like6 Localize to Internal Cellular Membranes and Are Involved in Metal Ion Homeostasis. Front. Plant Sci. 2013, 4, 283. [Google Scholar] [CrossRef] [Green Version]
- Duy, D.; Stube, R.; Wanner, G.; Philippar, K. The Chloroplast Permease PIC1 Regulates Plant Growth and Development by Directing Homeostasis and Transport of Iron. Plant Physiol. 2011, 155, 1709–1722. [Google Scholar] [CrossRef] [Green Version]
- Duy, D.; Wanner, G.; Meda, A.R.; Von Wirén, N.; Soll, J.; Philippar, K. PIC1, an Ancient Permease in Arabidopsis Chloroplasts, Mediates Iron Transport. Plant Cell 2007, 19, 986–1006. [Google Scholar] [CrossRef] [Green Version]
- Guan, X.; Chen, S.; Voon, C.P.; Wong, K.B.; Tikkanen, M.; Lim, B.L. FdC1 and Leaf-Type Ferredoxins Channel Electrons from Photosystem i to Different Downstream Electron Acceptors. Front. Plant Sci. 2018, 9, 410. [Google Scholar] [CrossRef]
- Bohrer, A.S.; Massot, V.; Innocenti, G.; Reichheld, J.P.; Issakidis-Bourguet, E.; Vanacker, H. New Insights into the Reduction Systems of Plastidial Thioredoxins Point out the Unique Properties of Thioredoxin z from Arabidopsis. J. Exp. Bot. 2012, 63, 6315–6323. [Google Scholar] [CrossRef] [Green Version]
- Nikkanen, L.; Toivola, J.; Diaz, M.G.; Rintamäki, E. Chloroplast Thioredoxin Systems: Prospects for Improving Photosynthesis. Philos. Trans. R. Soc. B Biol. Sci. 2017, 372, 20160474. [Google Scholar] [CrossRef] [Green Version]
- Kang, Z.; Qin, T.; Zhao, Z. Thioredoxins and Thioredoxin Reductase in Chloroplasts: A Review. Gene 2019, 706, 32–42. [Google Scholar] [CrossRef] [PubMed]
- Balsera, M.; Buchanan, B.B. Evolution of the Thioredoxin System as a Step Enabling Adaptation to Oxidative Stress. Free Radic. Biol. Med. 2019, 140, 28–35. [Google Scholar] [CrossRef] [PubMed]
- Cejudo, F.J.; González, M.C.; Pérez-Ruiz, J.M. Redox Regulation of Chloroplast Metabolism. Plant Physiol. 2021, 186, 9–21. [Google Scholar] [CrossRef]
- Ojeda, V.; Jiménez-López, J.; Romero-Campero, F.J.; Cejudo, F.J.; Pérez-Ruiz, J.M. A Chloroplast Redox Relay Adapts Plastid Metabolism to Light and Affects Cytosolic Protein Quality Control. Plant Physiol. 2021, 187, 88–102. [Google Scholar] [CrossRef] [PubMed]
- Nikkanen, L.; Rintamäki, E. Thioredoxin-Dependent Regulatory Networks in Chloroplasts under Fluctuating Light Conditions. Philos. Trans. R. Soc. B Biol. Sci. 2014, 369, 20130224. [Google Scholar] [CrossRef] [Green Version]
- Dangoor, I.; Peled-Zehavi, H.; Wittenberg, G.; Danon, A. A Chloroplast Light-Regulated Oxidative Sensor for Moderate Light Intensity in Arabidopsis. Plant Cell 2012, 24, 1894–1906. [Google Scholar] [CrossRef] [Green Version]
- Okegawa, Y.; Motohashi, K. M-Type Thioredoxins Regulate the PGR5/PGRL1-Dependent Pathway by Forming a Disulfide-Linked Complex with PGRL1. Plant Cell 2020, 32, 3866–3883. [Google Scholar] [CrossRef]
- Naranjo, B.; Diaz-Espejo, A.; Lindahl, M.; Cejudo, F.J. Type-f Thioredoxins Have a Role in the Short-Term Activation of Carbon Metabolism and Their Loss Affects Growth under Short-Day Conditions in Arabidopsis Thaliana. J. Exp. Bot. 2016, 67, 1951. [Google Scholar] [CrossRef] [Green Version]
- Serrato, A.J.; Pérez-Ruiz, J.M.; Spínola, M.C.; Cejudo, F.J. A Novel NADPH Thioredoxin Reductase, Localized in the Chloroplast, Which Deficiency Causes Hypersensitivity to Abiotic Stress in Arabidopsis Thaliana. J. Biol. Chem. 2004, 279, 43821–43827. [Google Scholar] [CrossRef] [Green Version]
- Meyer, A.J.; Dreyer, A.; Ugalde, J.M.; Feitosa-Araujo, E.; Dietz, K.J.; Schwarzländer, M. Shifting Paradigms and Novel Players in Cys-Based Redox Regulation and ROS Signaling in Plants—And Where to Go Next. Biol. Chem. 2020, 402, 399–423. [Google Scholar] [CrossRef]
- Foyer, C.H.; Baker, A.; Wright, M.; Sparkes, I.A.; Mhamdi, A.; Schippers, J.H.M.; Van Breusegem, F. On the Move: Redox-Dependent Protein Relocation in Plants. J. Exp. Bot. 2020, 71, 620–631. [Google Scholar] [CrossRef] [PubMed]
- Willems, P.; Mhamdi, A.; Stael, S.; Storme, V.; Kerchev, P.; Noctor, G.; Gevaert, K.; Van Breusegem, F. The ROS Wheel: Refining ROS Transcriptional Footprints. Plant Physiol. 2016, 171, 1720–1733. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Landry, A.P.; Cheng, Z.; Ding, H. Reduction of Mitochondrial Protein MitoNEET [2Fe-2S] Clusters by Human Glutathione Reductase. Free Radic. Biol. Med. 2015, 81, 119–127. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Landry, A.P.; Ding, H. Redox Control of Human Mitochondrial Outer Membrane Protein MitoNEET [2Fe-2S] Clusters by Biological Thiols and Hydrogen Peroxide. J. Biol. Chem. 2014, 289, 4307–4315. [Google Scholar] [CrossRef] [Green Version]
- Karmi, O.; Marjault, H.B.; Bai, F.; Roy, S.; Sohn, Y.S.; Yahana, M.D.; Morcos, F.; Ioannidis, K.; Nahmias, Y.; Jennings, P.A.; et al. A VDAC1-Mediated NEET Protein Chain Transfers [2Fe-2S] Clusters between the Mitochondria and the Cytosol and Impacts Mitochondrial Dynamics. Proc. Natl. Acad. Sci. USA 2022, 119, e2121491119. [Google Scholar] [CrossRef]
- Karmi, O.; Holt, S.H.; Song, L.; Tamir, S.; Luo, Y.; Bai, F.; Adenwalla, A.; Darash-Yahana, M.; Sohn, Y.-S.; Jennings, P.A.; et al. Interactions between MitoNEET and NAF-1 in Cells. PLoS ONE 2017, 12, e0175796. [Google Scholar] [CrossRef] [Green Version]
- Lipper, C.H.; Paddock, M.L.; Onuchic, J.N.; Mittler, R.; Nechushtai, R.; Jennings, P.A. Cancer-Related NEET Proteins Transfer 2Fe-2S Clusters to Anamorsin, a Protein Required for Cytosolic Iron-Sulfur Cluster Biogenesis. PLoS ONE 2015, 10, e0139699. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pearson, S.A.; Wachnowsky, C.; Cowan, J.A. Defining the Mechanism of the Mitochondrial Atm1p [2Fe-2S] Cluster Exporter. Metallomics 2020, 12, 902–915. [Google Scholar] [CrossRef]
- Lill, R. From the Discovery to Molecular Understanding of Cellular Iron-Sulfur Protein Biogenesis. Biol. Chem. 2020, 401, 855–876. [Google Scholar] [CrossRef]
- Talib, E.A.; Outten, C.E. Iron-Sulfur Cluster Biogenesis, Trafficking, and Signaling: Roles for CGFS Glutaredoxins and BolA Proteins. Biochim. Biophys. Acta Mol. Cell Res. 2021, 1868, 118847. [Google Scholar] [CrossRef]
- Kroh, G.E.; Pilon, M. Regulation of Iron Homeostasis and Use in Chloroplasts. Int. J. Mol. Sci. 2020, 21, 3395. [Google Scholar] [CrossRef] [PubMed]
- Vigani, G.; Solti, Á.; Thomine, S.; Philippar, K. Essential and Detrimental—An Update on Intracellular Iron Trafficking and Homeostasis. Plant Cell Physiol. 2019, 60, 1420–1439. [Google Scholar] [CrossRef] [PubMed]
- Hanke, G.; Mulo, P. Plant Type Ferredoxins and Ferredoxin-Dependent Metabolism. Plant Cell Environ. 2013, 36, 1071–1084. [Google Scholar] [CrossRef]
- Mittler, R.; Zandalinas, S.I.; Fichman, Y.; Van Breusegem, F. Reactive Oxygen Species Signalling in Plant Stress Responses. Nat. Rev. Mol. Cell Biol. 2022, in press. [Google Scholar] [CrossRef]
- Maiorino, M.; Bosello-Travain, V.; Cozza, G.; Miotto, G.; Roveri, A.; Toppo, S.; Zaccarin, M.; Ursini, F. Understanding Mammalian Glutathione Peroxidase 7 in the Light of Its Homologs. Free Radic. Biol. Med. 2015, 83, 352–360. [Google Scholar] [CrossRef]
- Jiang, X.; Stockwell, B.R.; Conrad, M. Ferroptosis: Mechanisms, Biology and Role in Disease. Nat. Rev. Mol. Cell Biol. 2021, 22, 266–282. [Google Scholar] [CrossRef] [PubMed]
- Zuris, J.A.; Harir, Y.; Conlan, A.R.; Shvartsman, M.; Michaeli, D.; Tamir, S.; Paddock, M.L.; Onuchic, J.N.; Mittler, R.; Cabantchik, Z.I.; et al. Facile Transfer of [2Fe-2S] Clusters from the Diabetes Drug Target MitoNEET to an Apo-Acceptor Protein. Proc. Natl. Acad. Sci. USA 2011, 108, 13047–13052. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tasnim, H.; Landry, A.P.; Fontenot, C.R.; Ding, H. Exploring the FMN Binding Site in the Mitochondrial Outer Membrane Protein MitoNEET. Free Radic. Biol. Med. 2020, 156, 11–19. [Google Scholar] [CrossRef]
- Li, X.; Wang, Y.; Tan, G.; Lyu, J.; Ding, H. Electron Transfer Kinetics of the Mitochondrial Outer Membrane Protein MitoNEET. Free Radic. Biol. Med. 2018, 121, 98–104. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zandalinas, S.I.; Song, L.; Nechushtai, R.; Mendoza-Cozatl, D.G.; Mittler, R. The Cluster Transfer Function of AtNEET Supports the Ferredoxin–Thioredoxin Network of Plant Cells. Antioxidants 2022, 11, 1533. https://doi.org/10.3390/antiox11081533
Zandalinas SI, Song L, Nechushtai R, Mendoza-Cozatl DG, Mittler R. The Cluster Transfer Function of AtNEET Supports the Ferredoxin–Thioredoxin Network of Plant Cells. Antioxidants. 2022; 11(8):1533. https://doi.org/10.3390/antiox11081533
Chicago/Turabian StyleZandalinas, Sara I., Luhua Song, Rachel Nechushtai, David G. Mendoza-Cozatl, and Ron Mittler. 2022. "The Cluster Transfer Function of AtNEET Supports the Ferredoxin–Thioredoxin Network of Plant Cells" Antioxidants 11, no. 8: 1533. https://doi.org/10.3390/antiox11081533
APA StyleZandalinas, S. I., Song, L., Nechushtai, R., Mendoza-Cozatl, D. G., & Mittler, R. (2022). The Cluster Transfer Function of AtNEET Supports the Ferredoxin–Thioredoxin Network of Plant Cells. Antioxidants, 11(8), 1533. https://doi.org/10.3390/antiox11081533






