Intermittent Hypoxia-Induced Cardiomyocyte Death Is Mediated by HIF-1 Dependent MAM Disruption
Abstract
1. Introduction
2. Materials and Methods
2.1. Atrial Biopsies from SDB Patients
2.2. Rodent Model of Intermittent Hypoxia
2.2.1. Animals
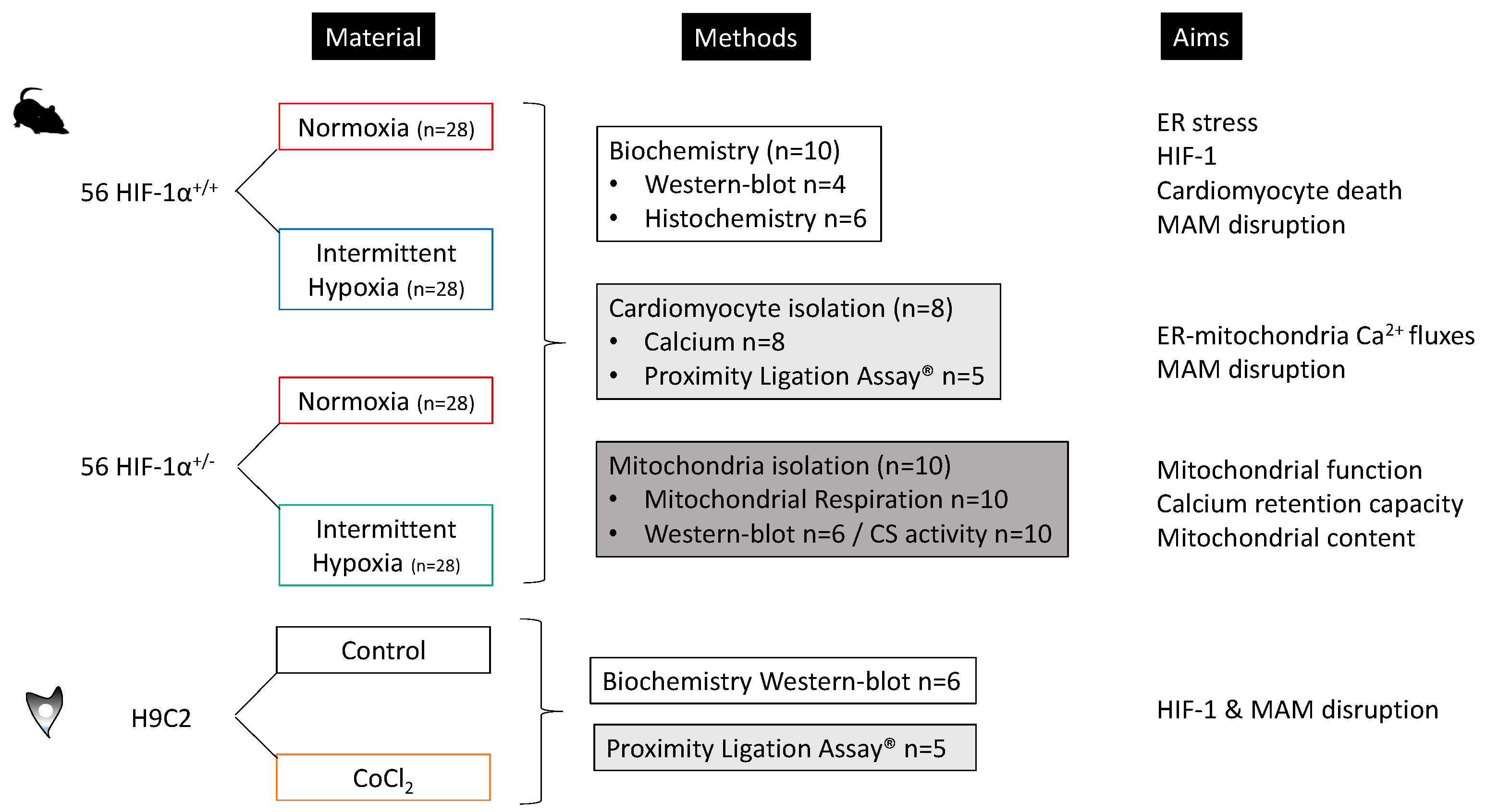
2.2.2. Experimental Design
2.2.3. Chronic Intermittent Hypoxia
2.2.4. In Vitro Model, Cell Culture and Design
2.2.5. HIF-1α, ER Stress Markers and Apoptosis
2.3. Ca2+ Inter-Organelle Fluxes and Assessment of MAM Integrity
2.3.1. Cardiomyocyte Isolation
2.3.2. Confocal Microcopy for Inter-Organelles Ca2+ Fluxes
2.3.3. Proximity Ligation Assay and Immunoprecipitation for MAM Integrity
2.4. Mitochondrial Exploration
2.4.1. Oxidative Phosphorylation
2.4.2. Calcium Retention Capacity
2.5. Statistical Analysis
3. Results
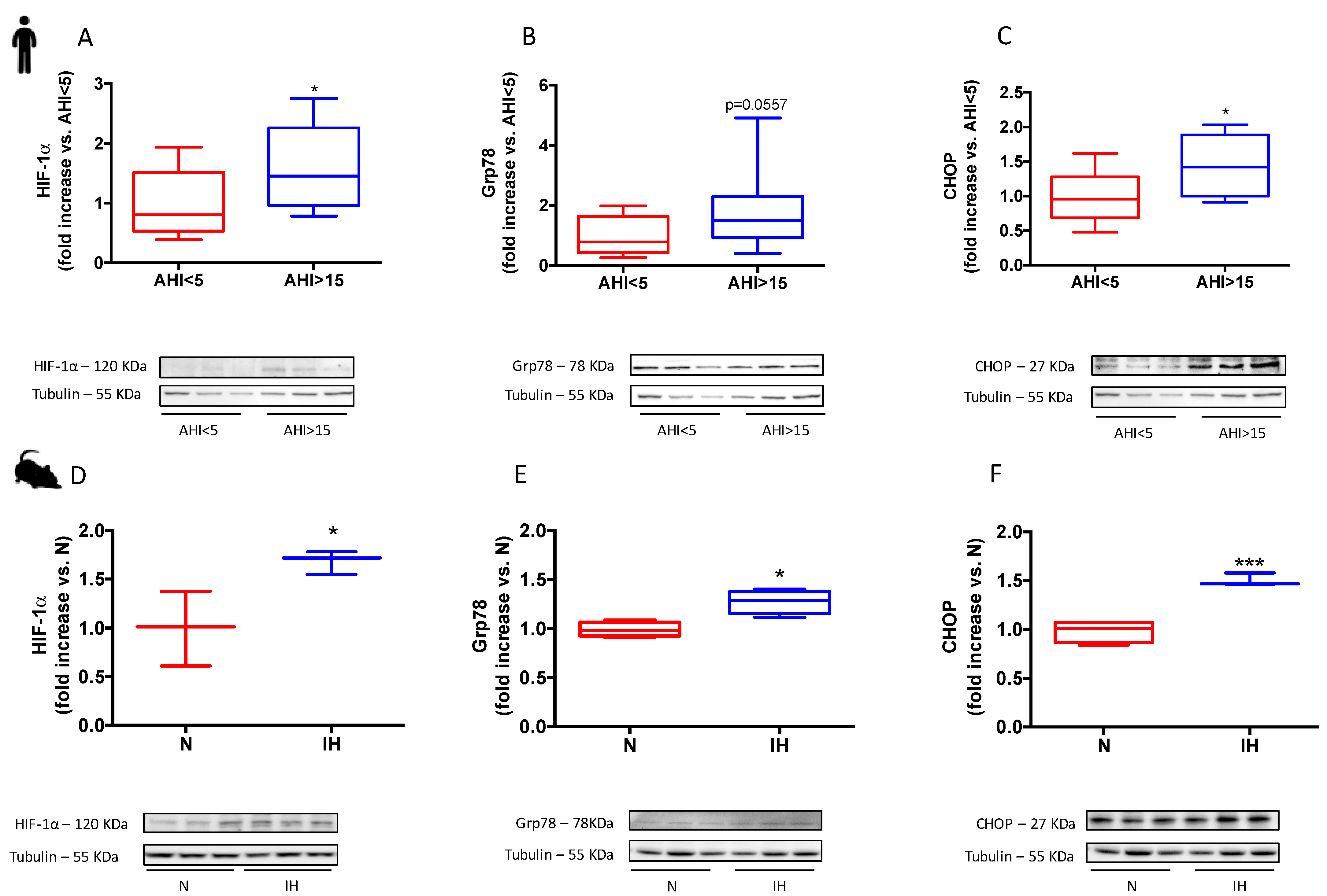
3.1. Sleep Apnea in Patients and Intermittent Hypoxia in Rodents Increase HIF-1α Expression and Induces a Pro-Apoptotic ER Stress
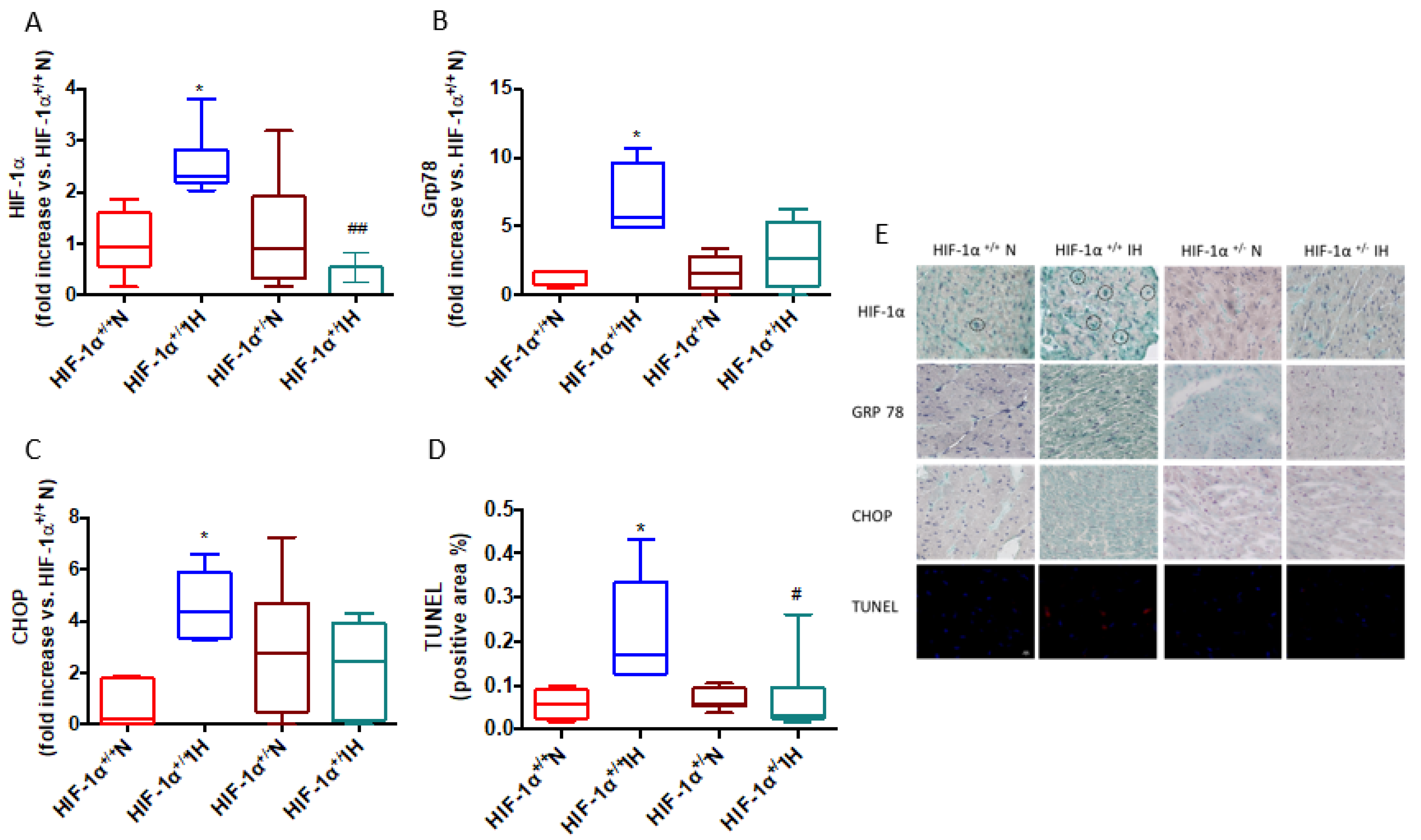
3.2. Intermittent Hypoxia Induces ER Stress and Apoptosis through HIF-1 Activation
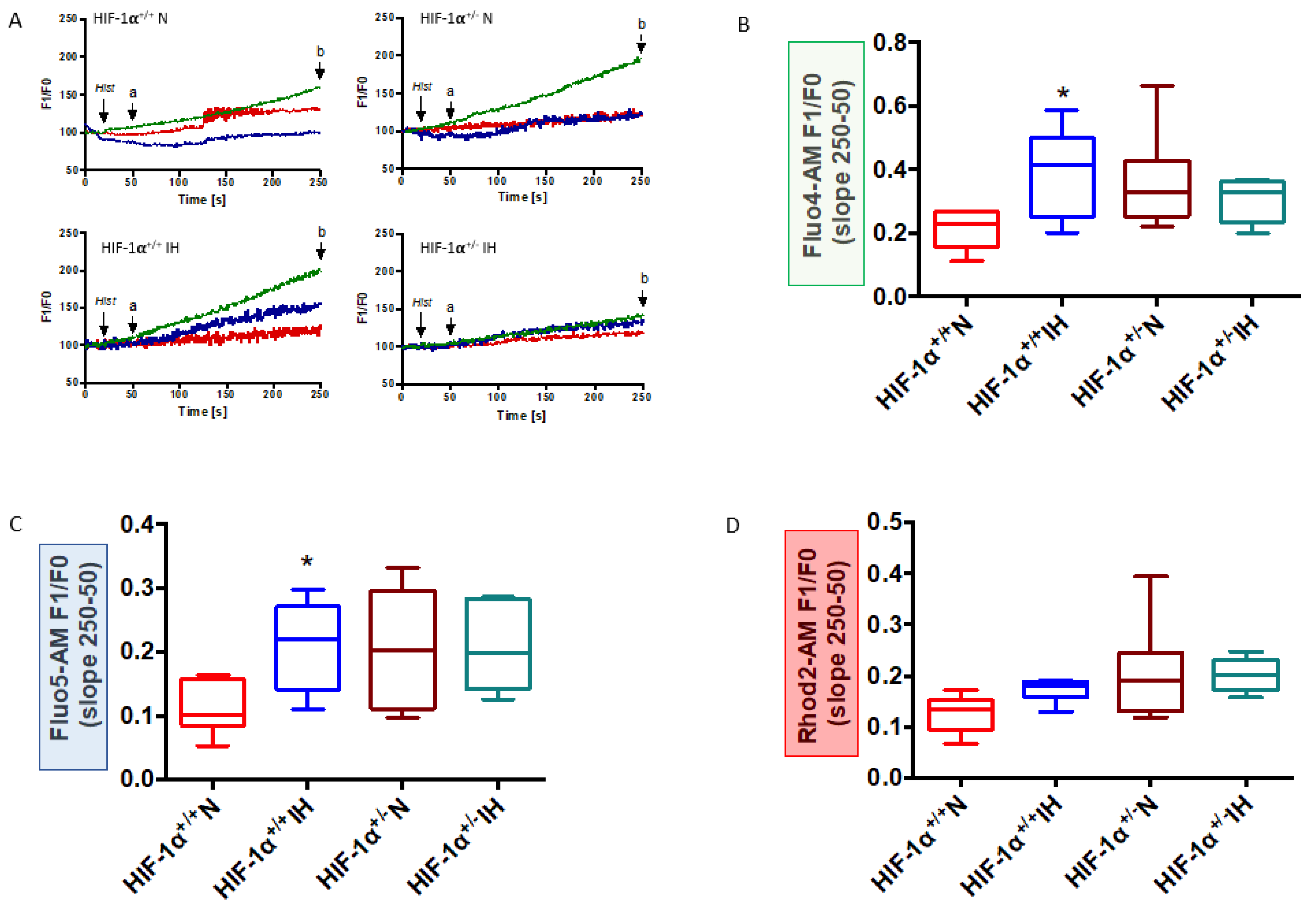
3.3. HIF-1 Is Involved in IH-Induced MAM Disruption and Alteration of Calcium Homeostasis
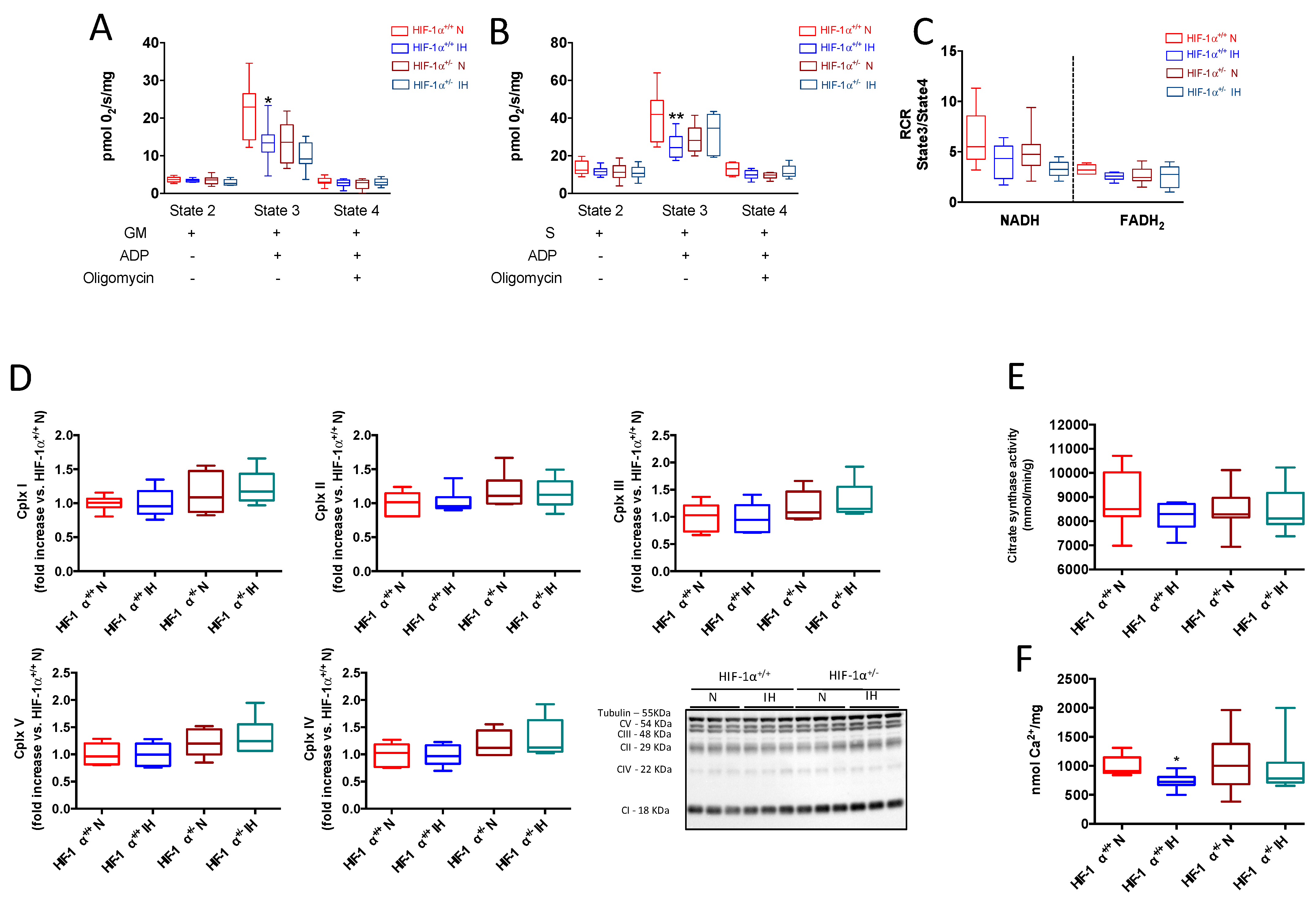
3.4. HIF-1 Is Involved in IH-Induced Mitochondrial Respiration Alteration
4. Discussion
4.1. HIF-1 Is Involved in IH-Induced ER Stress and Subsequent Cardiomyocyte Death
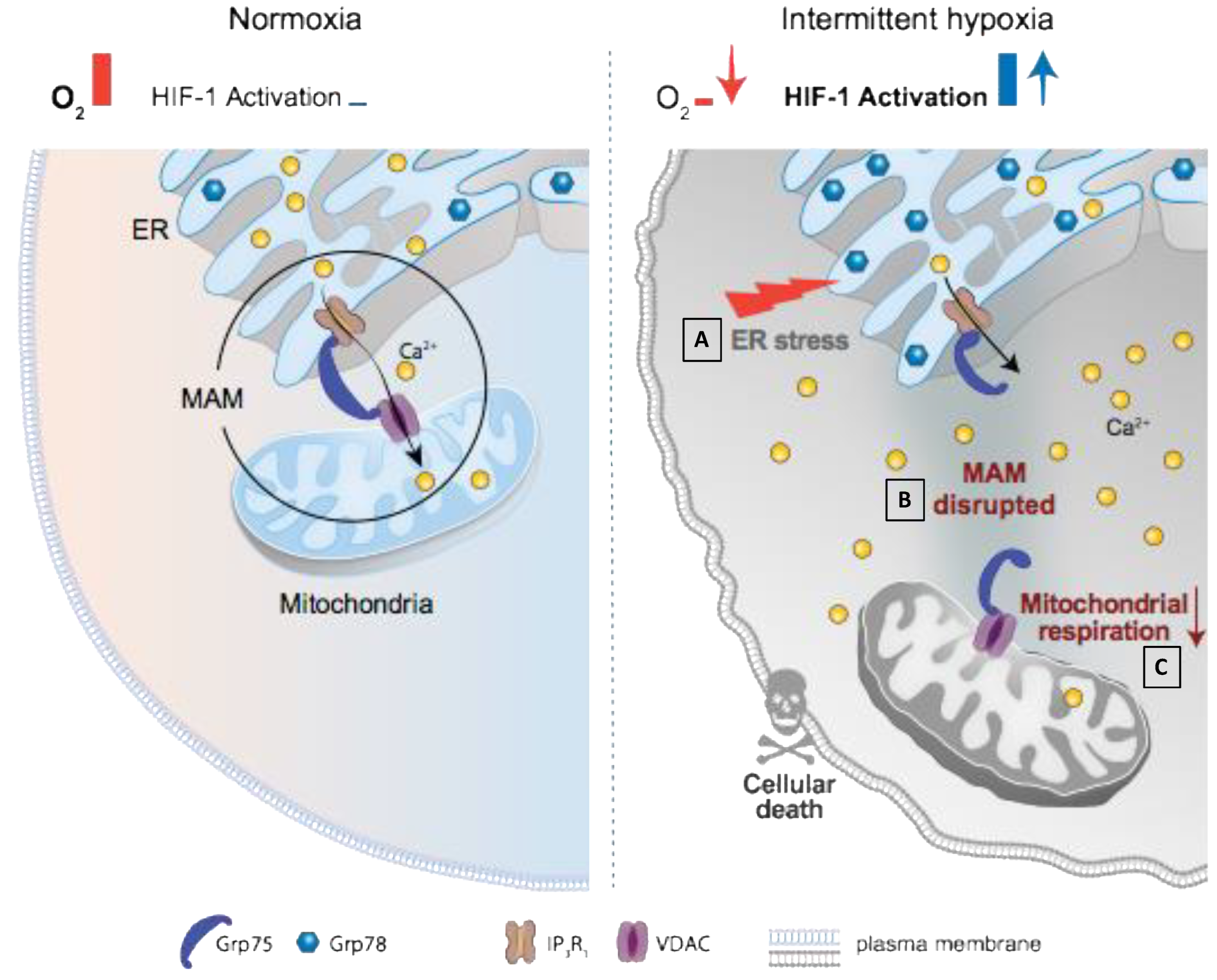
4.2. HIF-1 Is Involved in IH-Induced MAM and Ca2+ Homeostasis Alterations
4.3. HIF-1 Is Involved in IH-Induced Mitochondrial Respiration Alteration
5. Conclusions and Perspectives
6. Study limitations
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Aknowledgments
Conflicts of Interest
References
- Benjafield, A.V.; Ayas, N.T.; Eastwood, P.R.; Heinzer, R.; Ip, M.S.M.; Morrell, M.J.; Nunez, C.M.; Patel, S.R.; Penzel, T.; Pepin, J.L.; et al. Estimation of the global prevalence and burden of obstructive sleep apnoea: A literature-based analysis. Lancet Respir. Med. 2019, 7, 687–698. [Google Scholar] [CrossRef]
- Cuspidi, C.; Tadic, M. Obstructive sleep apnea and left ventricular strain: Useful tool or fancy gadget? J. Clin. Hypertens. 2020, 22, 120–122. [Google Scholar] [CrossRef]
- Ogilvie, R.P.; Genuardi, M.V.; Magnani, J.W.; Redline, S.; Daviglus, M.L.; Shah, N.; Kansal, M.; Cai, J.; Ramos, A.R.; Hurwitz, B.E.; et al. Association Between Sleep Disordered Breathing and Left Ventricular Function: A Cross-Sectional Analysis of the ECHO-SOL Ancillary Study. Circ. Cardiovasc. Imaging 2020, 13, e009074. [Google Scholar] [CrossRef]
- Buchner, S.; Satzl, A.; Debl, K.; Hetzenecker, A.; Luchner, A.; Husser, O.; Hamer, O.W.; Poschenrieder, F.; Fellner, C.; Zeman, F.; et al. Impact of sleep-disordered breathing on myocardial salvage and infarct size in patients with acute myocardial infarction. Eur. Heart J. 2014, 35, 192–199. [Google Scholar] [CrossRef] [PubMed]
- Barbe, F.; Sanchez-de-la-Torre, A.; Abad, J.; Duran-Cantolla, J.; Mediano, O.; Amilibia, J.; Masdeu, M.J.; Flores, M.; Barcelo, A.; de la Pena, M.; et al. Effect of obstructive sleep apnoea on severity and short-term prognosis of acute coronary syndrome. Eur. Respir. J. 2015, 45, 419–427. [Google Scholar] [CrossRef]
- Chen, T.I.; Lai, C.J.; Hsieh, C.J.; Tsai, K.L.; Yang, K.T. Differences in left ventricular cardiomyocyte loss induced by chronic intermittent hypoxia between spontaneously hypertensive and Wistar-Kyoto rats. Sleep Breath. 2011, 15, 845–854. [Google Scholar] [CrossRef]
- Zhou, S.; Yin, X.; Zheng, Y.; Miao, X.; Feng, W.; Cai, J.; Cai, L. Metallothionein prevents intermittent hypoxia-induced cardiac endoplasmic reticulum stress and cell death likely via activation of Akt signaling pathway in mice. Toxicol. Lett. 2014, 227, 113–123. [Google Scholar] [CrossRef]
- Belaidi, E.; Joyeux-Faure, M.; Ribuot, C.; Launois, S.H.; Levy, P.; Godin-Ribuot, D. Major role for hypoxia inducible factor-1 and the endothelin system in promoting myocardial infarction and hypertension in an animal model of obstructive sleep apnea. J. Am. Coll. Cardiol. 2009, 53, 1309–1317. [Google Scholar] [CrossRef]
- Moulin, S.; Thomas, A.; Arnaud, C.; Arzt, M.; Wagner, S.; Maier, L.S.; Pepin, J.L.; Godin-Ribuot, D.; Gaucher, J.; Belaidi, E. Cooperation Between Hypoxia-Inducible Factor 1alpha and Activating Transcription Factor 4 in Sleep Apnea-Mediated Myocardial Injury. Can. J. Cardiol. 2020, 36, 936–940. [Google Scholar] [CrossRef]
- Moulin, S.A.C.; Bouyon, S.; Pépin, J.L.; Godin-Ribuot, D.; Belaidi, E. Curcumin prevents chronic intermittent hypoxia-induced myocardial injury. Ther. Adv. Chronic Dis. 2020, 11, 1–13. [Google Scholar] [CrossRef]
- Bourdier, G.; Detrait, M.; Bouyon, S.; Lemarie, E.; Brasseur, S.; Doutreleau, S.; Pepin, J.L.; Godin-Ribuot, D.; Belaidi, E.; Arnaud, C. Intermittent Hypoxia Triggers Early Cardiac Remodeling and Contractile Dysfunction in the Time-Course of Ischemic Cardiomyopathy in Rats. J. Am. Heart Assoc. 2020, 9, e016369. [Google Scholar] [CrossRef] [PubMed]
- Du, Y.; Wang, X.; Li, L.; Hao, W.; Zhang, H.; Li, Y.; Qin, Y.; Nie, S.; Christopher, T.A.; Lopez, B.L.; et al. miRNA-Mediated Suppression of a Cardioprotective Cardiokine as a Novel Mechanism Exacerbating Post-MI Remodeling by Sleep Breathing Disorders. Circ. Res. 2020, 126, 212–228. [Google Scholar] [CrossRef] [PubMed]
- McEvoy, R.D.; Antic, N.A.; Heeley, E.; Luo, Y.; Ou, Q.; Zhang, X.; Mediano, O.; Chen, R.; Drager, L.F.; Liu, Z.; et al. CPAP for Prevention of Cardiovascular Events in Obstructive Sleep Apnea. N. Engl. J. Med. 2016, 375, 919–931. [Google Scholar] [CrossRef] [PubMed]
- Traaen, G.M.; Aakeroy, L.; Hunt, T.E.; Overland, B.; Bendz, C.; Sande, L.O.; Akhus, S.; Fagerland, M.W.; Steinshamn, S.; Anfinsen, O.G.; et al. Effect of Continuous Positive Airway Pressure on Arrhythmia in Atrial Fibrillation and Sleep Apnea: A Randomized Controlled Trial. Am. J. Respir. Crit. Care Med. 2021, 204, 573–582. [Google Scholar] [CrossRef]
- Semenza, G.L. Regulation of oxygen homeostasis by hypoxia-inducible factor 1. Physiology 2009, 24, 97–106. [Google Scholar] [CrossRef]
- Cai, Z.; Zhong, H.; Bosch-Marce, M.; Fox-Talbot, K.; Wang, L.; Wei, C.; Trush, M.A.; Semenza, G.L. Complete loss of ischaemic preconditioning-induced cardioprotection in mice with partial deficiency of HIF-1 alpha. Cardiovasc. Res. 2008, 77, 463–470. [Google Scholar] [CrossRef]
- Belaidi, E.; Beguin, P.C.; Levy, P.; Ribuot, C.; Godin-Ribuot, D. Prevention of HIF-1 activation and iNOS gene targeting by low-dose cadmium results in loss of myocardial hypoxic preconditioning in the rat. Am. J. Physiol. Heart Circ. Physiol. 2008, 294, H901–H908. [Google Scholar] [CrossRef]
- Ong, S.G.; Lee, W.H.; Theodorou, L.; Kodo, K.; Lim, S.Y.; Shukla, D.H.; Briston, T.; Kiriakidis, S.; Ashcroft, M.; Davidson, S.M.; et al. HIF-1 reduces ischaemia-reperfusion injury in the heart by targeting the mitochondrial permeability transition pore. Cardiovasc. Res. 2014, 104, 24–36. [Google Scholar] [CrossRef]
- Bekeredjian, R.; Walton, C.B.; MacCannell, K.A.; Ecker, J.; Kruse, F.; Outten, J.T.; Sutcliffe, D.; Gerard, R.D.; Bruick, R.K.; Shohet, R.V. Conditional HIF-1alpha expression produces a reversible cardiomyopathy. PLoS ONE 2010, 5, e11693. [Google Scholar] [CrossRef]
- Belaidi, E.; Morand, J.; Gras, E.; Pepin, J.L.; Godin-Ribuot, D. Targeting the ROS-HIF-1-endothelin axis as a therapeutic approach for the treatment of obstructive sleep apnea-related cardiovascular complications. Pharmacol. Ther. 2016, 168, 1–11. [Google Scholar] [CrossRef]
- Belaidi, E.; Khouri, C.; Harki, O.; Bailleuil, S.; Faury, G.; Briançon-Marjollet, A.; Pépin, J.L.; Arnaud, C. Cardiac consequences of intermittent hypoxia: A matter of dose? A systematic review and meta-analysis in rodent. Eur. Respir. Rev. 2022, 164, 210269. [Google Scholar] [CrossRef] [PubMed]
- Chou, Y.T.; Zhan, G.; Zhu, Y.; Fenik, P.; Panossian, L.; Li, Y.; Zhang, J.; Veasey, S. C/EBP homologous binding protein (CHOP) underlies neural injury in sleep apnea model. Sleep 2013, 36, 481–492. [Google Scholar] [CrossRef]
- Delbrel, E.; Soumare, A.; Naguez, A.; Label, R.; Bernard, O.; Bruhat, A.; Fafournoux, P.; Tremblais, G.; Marchant, D.; Gille, T.; et al. HIF-1alpha triggers ER stress and CHOP-mediated apoptosis in alveolar epithelial cells, a key event in pulmonary fibrosis. Sci. Rep. 2018, 8, 17939. [Google Scholar] [CrossRef] [PubMed]
- Delbrel, E.; Uzunhan, Y.; Soumare, A.; Gille, T.; Marchant, D.; Planes, C.; Boncoeur, E. ER Stress is Involved in Epithelial-To-Mesenchymal Transition of Alveolar Epithelial Cells Exposed to a Hypoxic Microenvironment. Int. J. Mol. Sci. 2019, 20, 1299. [Google Scholar] [CrossRef]
- Belaidi, E.; Thomas, A.; Bourdier, G.; Moulin, S.; Lemarie, E.; Levy, P.; Pepin, J.L.; Korichneva, I.; Godin-Ribuot, D.; Arnaud, C. Endoplasmic reticulum stress as a novel inducer of hypoxia inducible factor-1 activity: Its role in the susceptibility to myocardial ischemia-reperfusion induced by chronic intermittent hypoxia. Int. J. Cardiol. 2016, 210, 45–53. [Google Scholar] [CrossRef]
- Gao, P.; Yan, Z.; Zhu, Z. Mitochondria-Associated Endoplasmic Reticulum Membranes in Cardiovascular Diseases. Front. Cell Dev. Biol. 2020, 8, 604240. [Google Scholar] [CrossRef]
- Gustafsson, A.B.; Gottlieb, R.A. Heart mitochondria: Gates of life and death. Cardiovasc. Res. 2008, 77, 334–343. [Google Scholar] [CrossRef]
- Ding, W.; Zhang, X.; Zhang, Q.; Dong, Y.; Wang, W.; Ding, N. Adiponectin ameliorates lung injury induced by intermittent hypoxia through inhibition of ROS-associated pulmonary cell apoptosis. Sleep Breath. 2021, 25, 459–470. [Google Scholar] [CrossRef] [PubMed]
- Douglas, R.M.; Ryu, J.; Kanaan, A.; Del Carmen Rivero, M.; Dugan, L.L.; Haddad, G.G.; Ali, S.S. Neuronal death during combined intermittent hypoxia/hypercapnia is due to mitochondrial dysfunction. Am. J. Physiol. Cell Physiol. 2010, 298, C1594–C1602. [Google Scholar] [CrossRef]
- Laouafa, S.; Ribon-Demars, A.; Marcouiller, F.; Roussel, D.; Bairam, A.; Pialoux, V.; Joseph, V. Estradiol Protects against Cardiorespiratory Dysfunctions and Oxidative Stress in Intermittent Hypoxia. Sleep 2017, 40, zsx104. [Google Scholar] [CrossRef]
- Laouafa, S.; Roussel, D.; Marcouiller, F.; Soliz, J.; Gozal, D.; Bairam, A.; Joseph, V. Roles of oestradiol receptor alpha and beta against hypertension and brain mitochondrial dysfunction under intermittent hypoxia in female rats. Acta Physiol. 2019, 226, e13255. [Google Scholar] [CrossRef]
- Marchi, S.; Bittremieux, M.; Missiroli, S.; Morganti, C.; Patergnani, S.; Sbano, L.; Rimessi, A.; Kerkhofs, M.; Parys, J.B.; Bultynck, G.; et al. Endoplasmic Reticulum-Mitochondria Communication Through Ca2+ Signaling: The Importance of Mitochondria-Associated Membranes (MAMs). Adv. Exp. Med. Biol. 2017, 997, 49–67. [Google Scholar] [CrossRef]
- Paillard, M.; Tubbs, E.; Thiebaut, P.A.; Gomez, L.; Fauconnier, J.; Da Silva, C.C.; Teixeira, G.; Mewton, N.; Belaidi, E.; Durand, A.; et al. Depressing mitochondria-reticulum interactions protects cardiomyocytes from lethal hypoxia-reoxygenation injury. Circulation 2013, 128, 1555–1565. [Google Scholar] [CrossRef] [PubMed]
- Dia, M.; Gomez, L.; Thibault, H.; Tessier, N.; Leon, C.; Chouabe, C.; Ducreux, S.; Gallo-Bona, N.; Tubbs, E.; Bendridi, N.; et al. Reduced reticulum-mitochondria Ca2+ transfer is an early and reversible trigger of mitochondrial dysfunctions in diabetic cardiomyopathy. Basic Res. Cardiol. 2020, 115, 74. [Google Scholar] [CrossRef]
- Naon, D.; Scorrano, L. At the right distance: ER-mitochondria juxtaposition in cell life and death. Biochim. Biophys. Acta 2014, 1843, 2184–2194. [Google Scholar] [CrossRef]
- Arzt, M.; Woehrle, H.; Oldenburg, O.; Graml, A.; Suling, A.; Erdmann, E.; Teschler, H.; Wegscheider, K.; Schla, H.F.I. Prevalence and Predictors of Sleep-Disordered Breathing in Patients With Stable Chronic Heart Failure: The SchlaHF Registry. JACC Heart Fail. 2016, 4, 116–125. [Google Scholar] [CrossRef]
- Erman, M.K.; Stewart, D.; Einhorn, D.; Gordon, N.; Casal, E. Validation of the ApneaLink for the screening of sleep apnea: A novel and simple single-channel recording device. J. Clin. Sleep Med. 2007, 3, 387–392. [Google Scholar] [CrossRef]
- Dematteis, M.; Godin-Ribuot, D.; Arnaud, C.; Ribuot, C.; Stanke-Labesque, F.; Pepin, J.L.; Levy, P. Cardiovascular consequences of sleep-disordered breathing: Contribution of animal models to understanding the human disease. ILAR J. 2009, 50, 262–281. [Google Scholar] [CrossRef] [PubMed]
- Rieusset, J.; Fauconnier, J.; Paillard, M.; Belaidi, E.; Tubbs, E.; Chauvin, M.A.; Durand, A.; Bravard, A.; Teixeira, G.; Bartosch, B.; et al. Disruption of cyclophilin D-mediated calcium transfer from the ER to mitochondria contributes to hepatic ER stress and insulin resistance. Diabetologia 2016, 59, 614–623. [Google Scholar] [CrossRef]
- Cour, M.; Abrial, M.; Jahandiez, V.; Loufouat, J.; Belaidi, E.; Gharib, A.; Varennes, A.; Monneret, G.; Thibault, H.; Ovize, M.; et al. Ubiquitous protective effects of cyclosporine A in preventing cardiac arrest-induced multiple organ failure. J. Appl. Physiol. 2014, 117, 930–936. [Google Scholar] [CrossRef]
- Bourdier, G.; Flore, P.; Sanchez, H.; Pepin, J.L.; Belaidi, E.; Arnaud, C. High-intensity training reduces intermittent hypoxia-induced ER stress and myocardial infarct size. Am. J. Physiol. Heart Circ. Physiol. 2016, 310, H279–H289. [Google Scholar] [CrossRef]
- Hou, Y.; Yang, H.; Cui, Z.; Tai, X.; Chu, Y.; Guo, X. Tauroursodeoxycholic acid attenuates endoplasmic reticulum stress and protects the liver from chronic intermittent hypoxia induced injury. Exp. Ther. Med. 2017, 14, 2461–2468. [Google Scholar] [CrossRef] [PubMed]
- Cai, X.H.; Li, X.C.; Jin, S.W.; Liang, D.S.; Wen, Z.W.; Cao, H.C.; Mei, H.F.; Wu, Y.; Lin, Z.D.; Wang, L.X. Endoplasmic reticulum stress plays critical role in brain damage after chronic intermittent hypoxia in growing rats. Exp. Neurol. 2014, 257, 148–156. [Google Scholar] [CrossRef] [PubMed]
- Ding, W.; Cai, Y.; Wang, W.; Ji, L.; Dong, Y.; Zhang, X.; Su, M.; Liu, J.; Lu, G.; Zhang, X. Adiponectin protects the kidney against chronic intermittent hypoxia-induced injury through inhibiting endoplasmic reticulum stress. Sleep Breath. 2016, 20, 1069–1074. [Google Scholar] [CrossRef]
- Shi, Z.; Xu, L.; Xie, H.; Ouyang, R.; Ke, Y.; Zhou, R.; Yung, W.H. Attenuation of intermittent hypoxia-induced apoptosis and fibrosis in pulmonary tissues via suppression of ER stress activation. BMC Pulm. Med. 2020, 20, 92. [Google Scholar] [CrossRef] [PubMed]
- Ding, W.; Zhang, X.; Huang, H.; Ding, N.; Zhang, S.; Hutchinson, S.Z.; Zhang, X. Adiponectin protects rat myocardium against chronic intermittent hypoxia-induced injury via inhibition of endoplasmic reticulum stress. PLoS ONE 2014, 9, e94545. [Google Scholar] [CrossRef]
- Eisner, V.; Csordas, G.; Hajnoczky, G. Interactions between sarco-endoplasmic reticulum and mitochondria in cardiac and skeletal muscle—Pivotal roles in Ca2+ and reactive oxygen species signaling. J. Cell Sci. 2013, 126, 2965–2978. [Google Scholar] [CrossRef] [PubMed]
- Gong, Y.; Lin, J.; Ma, Z.; Yu, M.; Wang, M.; Lai, D.; Fu, G. Mitochondria-associated membrane-modulated Ca2+ transfer: A potential treatment target in cardiac ischemia reperfusion injury and heart failure. Life Sci. 2021, 278, 119511. [Google Scholar] [CrossRef]
- Csordas, A. Mitochondrial transfer between eukaryotic animal cells and its physiologic role. Rejuvenation Res. 2006, 9, 450–454. [Google Scholar] [CrossRef]
- Doroudgar, S.; Glembotski, C.C. New concepts of endoplasmic reticulum function in the heart: Programmed to conserve. J. Mol. Cell. Cardiol. 2013, 55, 85–91. [Google Scholar] [CrossRef]
- Giorgi, C.; Missiroli, S.; Patergnani, S.; Duszynski, J.; Wieckowski, M.R.; Pinton, P. Mitochondria-associated membranes: Composition, molecular mechanisms, and physiopathological implications. Antioxid. Redox Signal. 2015, 22, 995–1019. [Google Scholar] [CrossRef]
- Mylonis, I.; Kourti, M.; Samiotaki, M.; Panayotou, G.; Simos, G. Mortalin-mediated and ERK-controlled targeting of HIF-1alpha to mitochondria confers resistance to apoptosis under hypoxia. J. Cell Sci. 2017, 130, 466–479. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, T.; Rizzuto, R.; Hajnoczky, G.; Su, T.P. MAM: More than just a housekeeper. Trends Cell Biol. 2009, 19, 81–88. [Google Scholar] [CrossRef] [PubMed]
- Semenza, G.L. Hypoxia-inducible factor 1: Regulator of mitochondrial metabolism and mediator of ischemic preconditioning. Biochim. Biophys. Acta 2011, 1813, 1263–1268. [Google Scholar] [CrossRef] [PubMed]
- Semenza, G.L.; Jiang, B.H.; Leung, S.W.; Passantino, R.; Concordet, J.P.; Maire, P.; Giallongo, A. Hypoxia response elements in the aldolase A, enolase 1, and lactate dehydrogenase A gene promoters contain essential binding sites for hypoxia-inducible factor 1. J. Biol. Chem. 1996, 271, 32529–32537. [Google Scholar] [CrossRef]
- Thomas, L.W.; Ashcroft, M. Exploring the molecular interface between hypoxia-inducible factor signalling and mitochondria. Cell Mol. Life Sci. 2019, 76, 1759–1777. [Google Scholar] [CrossRef] [PubMed]
- Del Re, D.P.; Amgalan, D.; Linkermann, A.; Liu, Q.; Kitsis, R.N. Fundamental Mechanisms of Regulated Cell Death and Implications for Heart Disease. Physiol. Rev. 2019, 99, 1765–1817. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.D.; Miyamoto, S. Molecular Signaling to Preserve Mitochondrial Integrity against Ischemic Stress in the Heart: Rescue or Remove Mitochondria in Danger. Cells 2021, 10, 3330. [Google Scholar] [CrossRef]
- Valsecchi, V.; Pignataro, G.; Del Prete, A.; Sirabella, R.; Matrone, C.; Boscia, F.; Scorziello, A.; Sisalli, M.J.; Esposito, E.; Zambrano, N.; et al. NCX1 is a novel target gene for hypoxia-inducible factor-1 in ischemic brain preconditioning. Stroke 2011, 42, 754–763. [Google Scholar] [CrossRef]
- Wagner, S.; Seidler, T.; Picht, E.; Maier, L.S.; Kazanski, V.; Teucher, N.; Schillinger, W.; Pieske, B.; Isenberg, G.; Hasenfuss, G.; et al. Na+-Ca2+ exchanger overexpression predisposes to reactive oxygen species-induced injury. Cardiovasc. Res. 2003, 60, 404–412. [Google Scholar] [CrossRef][Green Version]
- Wang, Y.; Zhang, X.; Wen, Y.; Li, S.; Lu, X.; Xu, R.; Li, C. Endoplasmic Reticulum-Mitochondria Contacts: A Potential Therapy Target for Cardiovascular Remodeling-Associated Diseases. Front. Cell Dev. Biol. 2021, 9, 774989. [Google Scholar] [CrossRef] [PubMed]
- Holscher, M.; Schafer, K.; Krull, S.; Farhat, K.; Hesse, A.; Silter, M.; Lin, Y.; Pichler, B.J.; Thistlethwaite, P.; El-Armouche, A.; et al. Unfavourable consequences of chronic cardiac HIF-1alpha stabilization. Cardiovasc. Res. 2012, 94, 77–86. [Google Scholar] [CrossRef] [PubMed]
- Yeghiazarians, Y.; Jneid, H.; Tietjens, J.R.; Redline, S.; Brown, D.L.; El-Sherif, N.; Mehra, R.; Bozkurt, B.; Ndumele, C.E.; Somers, V.K. Obstructive Sleep Apnea and Cardiovascular Disease: A Scientific Statement From the American Heart Association. Circulation 2021, 144, e56–e67. [Google Scholar] [CrossRef]
- Yang, J.; AlTahan, A.; Jones, D.T.; Buffa, F.M.; Bridges, E.; Interiano, R.B.; Qu, C.; Vogt, N.; Li, J.L.; Baban, D.; et al. Estrogen receptor-alpha directly regulates the hypoxia-inducible factor 1 pathway associated with antiestrogen response in breast cancer. Proc. Natl. Acad. Sci. USA 2015, 112, 15172–15177. [Google Scholar] [CrossRef]
- Joseph, V.; Pagliardini, S.; Belaidi, E. Editorial: Causes and Consequences of Sleep Apnea: Spotlights on the Roles of Sex and Sex Hormones. Front. Physiol. 2022, 13, 857627. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moulin, S.; Thomas, A.; Wagner, S.; Arzt, M.; Dubouchaud, H.; Lamarche, F.; Bouyon, S.; Vial, G.; Godin-Ribuot, D.; Pépin, J.-L.; et al. Intermittent Hypoxia-Induced Cardiomyocyte Death Is Mediated by HIF-1 Dependent MAM Disruption. Antioxidants 2022, 11, 1462. https://doi.org/10.3390/antiox11081462
Moulin S, Thomas A, Wagner S, Arzt M, Dubouchaud H, Lamarche F, Bouyon S, Vial G, Godin-Ribuot D, Pépin J-L, et al. Intermittent Hypoxia-Induced Cardiomyocyte Death Is Mediated by HIF-1 Dependent MAM Disruption. Antioxidants. 2022; 11(8):1462. https://doi.org/10.3390/antiox11081462
Chicago/Turabian StyleMoulin, Sophie, Amandine Thomas, Stefan Wagner, Michael Arzt, Hervé Dubouchaud, Frédéric Lamarche, Sophie Bouyon, Guillaume Vial, Diane Godin-Ribuot, Jean-Louis Pépin, and et al. 2022. "Intermittent Hypoxia-Induced Cardiomyocyte Death Is Mediated by HIF-1 Dependent MAM Disruption" Antioxidants 11, no. 8: 1462. https://doi.org/10.3390/antiox11081462
APA StyleMoulin, S., Thomas, A., Wagner, S., Arzt, M., Dubouchaud, H., Lamarche, F., Bouyon, S., Vial, G., Godin-Ribuot, D., Pépin, J.-L., Arnaud, C., & Belaidi, E. (2022). Intermittent Hypoxia-Induced Cardiomyocyte Death Is Mediated by HIF-1 Dependent MAM Disruption. Antioxidants, 11(8), 1462. https://doi.org/10.3390/antiox11081462







