1. Introduction
The blood–brain barrier (BBB) is a multicellular vascular structure that is composed of tightly bound cerebral endothelial cells, pericytes, and perivascular astrocytes, and its integrity is essential for neuroprotection from peripheral risk factors [
1]. As a unique feature of endothelial cells, tight junctions (TJs) contribute to sealing barriers for BBB integrity [
2]. Claudins are one of the major proteins of TJs, and they bind to scaffolding proteins such as zonula occludens-1/2/3 (ZO-1/2/3) and then indirectly link to the actin cytoskeleton via a PDZ-binding motif at the C-terminal [
3]. Loss of claudins is associated with BBB breakdown in neurodegenerative diseases, such as Huntington’s disease (HD) and Alzheimer’s disease (AD) [
4], as well as acute brain injury including ischemic stroke and depression [
5,
6]. Claudin 5 is regarded as the most important claudin to maintain TJs because it is mostly enriched in the endothelial layer of the brain [
7]. Mice with claudin 5 deficiency have a BBB permeability to small molecules, resulting in death within 10 h after birth [
7]. Based on this, claudin 5 has been considered a potential target for BBB protection during ischemic injury.
Although ischemia occurs locally in the affected brain area, it triggers peripheral stress responses, which drive oxidative and inflammatory damage to the microvascular endothelium and increase BBB permeability [
8]. In stressed cells, endotoxin or viral infection triggers oxidative stress to accumulate reactive oxygen species (ROS) and destroy the redox homeostasis, which induces the maturation and release of inflammatory factors and triggers inflammatory reactions. Particulate-matter exposure significantly enhanced the airway inflammatory response through ROS-mediated activation of MAPK and downstream NF-
κB signaling pathways [
9]. Mitochondrial ROS governed the proinflammatory response by regulating MAPK and NF-
κB pathways in microglia [
10]. There is evidence indicating that rising inflammatory cytokines in serum with ischemic stroke [
11,
12] and IL-1
β is known as the pivotal factor of BBB dysfunction, due to vascular leakage and inflammatory injury [
13,
14]. IL-1
β also increases intestinal permeability by degrading occludin in mice with colitis [
15]. In rat brain endothelial cells, IL-1
β induced relocation of ZO-1 and occludin from the plasma membrane to the cytosolic compartment, resulting in BBB permeability disruption [
16]. Hence, protection of claudin 5 from oxidation stress-mediated inflammation might be a feasible means to explore potential targets for ischemic stroke.
The Angong Niuhuang Pill (ANP) is a well-known classical prescription of traditional Chinese medicine (TCM), widely used for the management of acute cerebrovascular diseases such as ischemia stroke, intracerebral hemorrhage, and traumatic brain damage [
17,
18], having the role to prevent neural apoptosis and neurological deficits [
19]. Meanwhile, its protective effects on tight junction proteins have been documented [
20]. Importantly, the herbal pair of moschus-borneolum is regarded not only as a basic unit of ANP but also a vital component in Chinese traditional prescriptions [
21], making an irreplaceable contribution to the therapeutic effect of ANP on cerebrovascular diseases. The compatibility of moschus-borneolum is traditionally viewed as an effective means in clinical application. Notably, a study reported the neuroprotective effect of moschus compatible with borneolum on ischemia stroke [
22]. Muscone is a potent antioxidative and anti-inflammatory agent in moschus [
23], while (+)-borneol is a natural bicyclic monoterpene rich in borneolum with significant inhibitory effects on oxidative damage and inflammatory response [
24]. Chemical structures of muscone and (+)-borneol are shown in
Figure S1. Furthermore, muscone ameliorated lipopolysaccharide (LPS)-induced depressive-like behaviors by repressing neuroinflammation in the prefrontal cortex of mice [
25]. (+)-Borneol maintained the integrity of BBB via protecting TJs expression during ischemic injury [
26]. However, the effects of combined administration of muscone and (+)-borneol on ischemic brain injury have not been clarified.
Consistent with their effects on oxidation and inflammation inhibition and cerebral protection, in the present study, we further demonstrated that combined administration of muscone and (+)-borneol synergically protected claudin 5 stability and cerebral microvascular integrity by eliminating ROS accumulation and inhibiting IL-1β secretion in cerebral microvascular endothelial cells. ROS removed by muscone attenuated calcium overload-mediated Erk1/2 inflammatory pathway, while the scavenging effect of (+)-borneol on mitochondrial ROS inhibited SDH enzyme activity and blocked succinate/HIF-1α inflammatory pathway. (+)-Borneol played an anti-inflammatory role in terms of metabolic regulation, which was different from muscone. Hereafter, their roles focused on inhibiting IL-1β maturation and secretion, jointly enhancing claudin 5 expression, and protecting BBB function by activating the cAMP/CREB cascade. This study provided scientific data for revealing the pharmacological effect of a moschus-borneolum drug pair on improving cerebrovascular diseases and expanding its clinical application and provided a certain reference for further studying the synergistic effects of various active ingredients in TCM compounds.
2. Method
2.1. Chemicals and Reagents
Muscone and (+)-borneol (purity ≥ 98%) were purchased from Chengdu Must Biotechnology Co., Ltd. (Chengdu, China). Rose bengal (330000), 2,3,5-triphenyl tetrazolium chloride (TTC, T8877), Evans blue (E2129), lipopolysaccharide (LPS, L2880), dimethyl malonate (DMM, 136441), dimethyl succinate (W239607) and N-acetyl-L-cysteine (NAC, A9165) were purchased from Sigma (St. Louis, MO, USA), Cyclosporin A (CSA, HY-B0579), calcimycin (HY-N6687) and mito-TEMPO (HY-112879) were obtained from Med Chem Express (Brea, CA, USA). IL-1β (AF-211-11B) was purchased from Peprotech (Cranbury, NJ, USA).
2.2. Animals and Treatment
All animal experiments were approved by the Animal Ethics Committee of China Pharmaceutical University (protocol code: 2021-12-006 and date of approval: December 2021) following the National Institutes of Health and ARRIVE (Animal Research: Reporting of In Vivo Experiments) guidelines. Male C57BL/6J mice (six to eight-week-old, 20–22 g) were purchased from the Comparative Medical Center of Yangzhou University (Yangzhou, China). Animals were housed in a standard environment with constant temperature and humidity and a 12 h light–dark cycle. The mice were given a standard diet and water ad libitum.
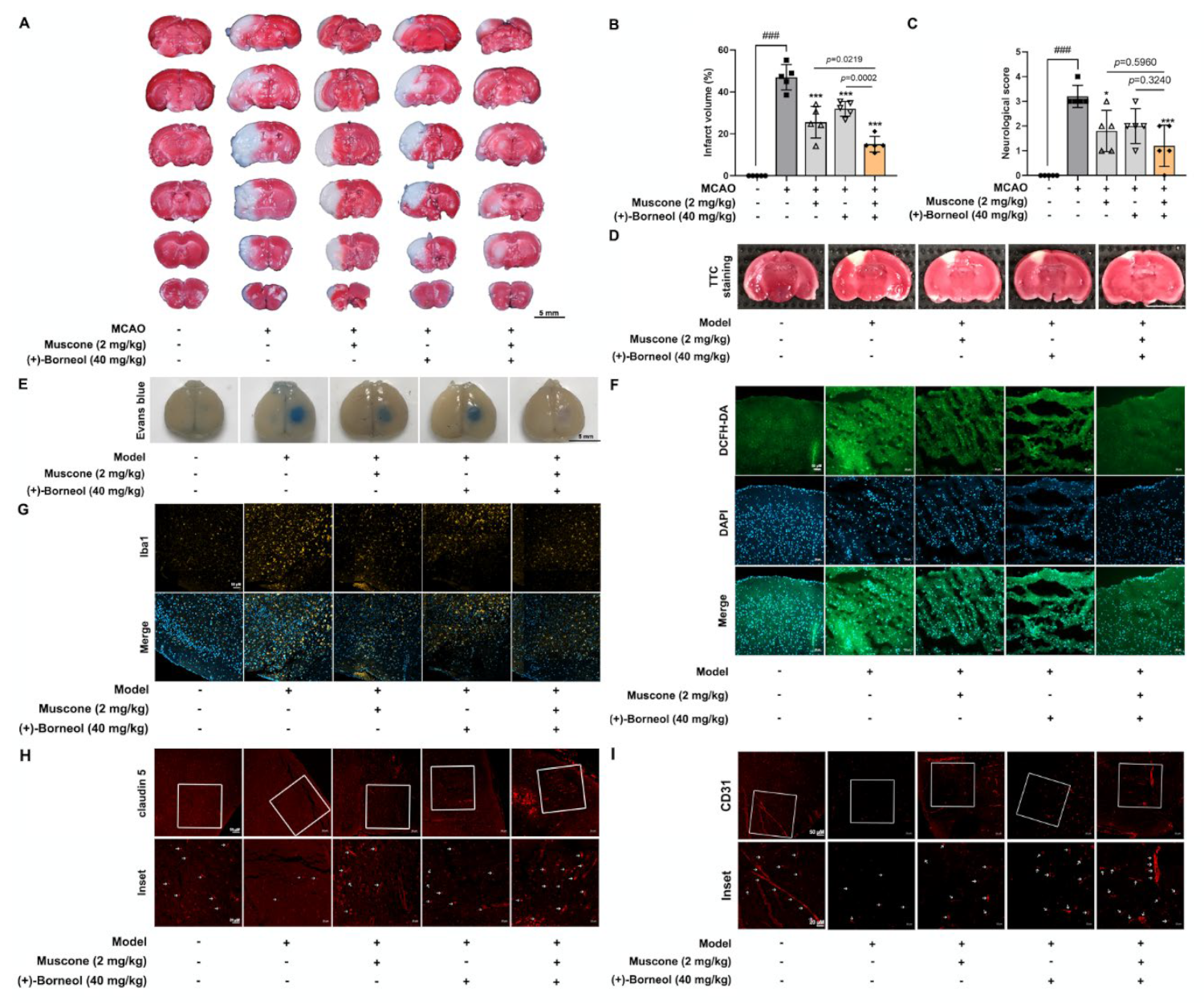
Cortical microvascular photothrombosis was employed for photothrombotic stroke (PT) in mice as previously described [
27]. Briefly, mice were anesthetized and then placed in a stereotactic frame (Stoelting, Wood Dale, IL, USA). After incising the midline of the skin to expose the skull, a cold light source (11,500 lux) with a 2 mm diameter was fixed on 1.5 mm to the right of the bregma point. Animals then received Rose Bengal solution (Sigma; 100 mg/kg) intraperitoneally and after 5 min the brain was illuminated for 15 min. The scalp was sutured and the mice were allowed to recover on a heating pad. Mice in the sham group were injected with the same dose of Rose Bengal without illumination. In this study, mice were arbitrarily and equally assigned into 5 groups: sham group, model group, model + muscone (2 mg/kg, i.g.) group, model + (+)-borneol (40 mg/kg, i.g.) group, model + muscone (2 mg/kg, i.g.) + (+)-borneol (40 mg/kg, i.g.) group. After photothrombotic stroke surgery, mice were given intragastric administration once a day for 3 consecutive days. Two hours after the last administration, the infarct volume and Evans Blue extravasation in the brain were measured. Meanwhile, brain tissues were harvested and fixed for immunofluorescence detection, and blood was collected for the analysis of inflammatory factors.
For the establishment of middle cerebral artery occlusion (MCAO), male C57BL/6J mice (weight 20–22 g) were anesthetized with isoflurane 3% for induction and 1.5% for maintenance (in a mixture of 30% oxygen and 70% nitrous oxide). Briefly, the carotid artery, external carotid artery (ECA), and internal carotid artery (ICA) were exposed, and a silicon-coated filament was inserted into ICA from ECA to occlude the MCA. After 1 h of ischemia, reperfusion was performed by removing the filament. To access whether the animals treated with MCAO had been successfully modeled, cerebral blood flow (CBF) was monitored via a laser speckle flow imaging system (FLPI2, Moor) before MCAO, after MCAO and reperfusion (
Figure S2). Before 30 min of occlusion and 4 h after occlusion, the MCAO mice were given muscone (2 mg/kg, i.g.), (+)-borneol (40 mg/kg), or combined administration of muscone and (+)-borneol. Mice in the sham and MCAO groups were given an equal volume of normal saline.
After 24 h reperfusion, the neurological deficit of the mice was accessed according to the Zea-Longa [
28]: 0 suggests no obvious deficit; 1 suggests a failure to stretch contralateral forelimb while tail pulled; 2 suggests spontaneous contralateral turning; 3 suggests spontaneous contralateral circling; 4 suggests loss of walking ability.
2.3. 2,3,5-Triphenyl-Tetrazolium Chloride (TTC) and Evans Blue Staining
Infarct volume was analyzed with TTC staining after photothrombotic stroke and middle cerebral artery occlusion (MCAO) surgery. After the mice were euthanized, the brains were rapidly isolated and frozen, and then the tissue was sectioned into 2 mm-thick coronal slices. Subsequently, these brain slices were stained with 1% TTC in a 37 °C water bath for 5–10 min and then fixed in 4% formaldehyde overnight. The unstained infarct area was quantified with Image J software 1.52a (NIH, Washington, USA).
BBB integrity was determined by the extravasation of Evans blue (EB) after photothrombotic stroke. Briefly, EB solution (2%, 4 mL/kg) was injected into the tail vein and circulated for 2 h. Subsequently, the mice were anesthetized and transcardially perfused with 0.9% saline and 4% paraformaldehyde sequentially. Finally, brains were collected and photographed.
2.4. Cell Culture
bEnd.3 cells were purchased from iCell Bioscience Inc (Shanghai, China) and incubated in Dulbecco’s modified eagle medium (DMEM, KeyGEN BioTECH, Nanjing, China) with 10% (v/v) fetal bovine serum (FBS, Gibco, New York, NY, USA). All cells were cultured at 37 °C in the 5% CO2 humidified incubator.
2.5. Transfection of bEnd.3 Cells
The bEnd.3 cells at 70–80% confluence were transfected with Creb siRNA, Cldn5 siRNA, or negative control (NC) to specifically suppress Creb or Cldn5 expression with Lipofectamine™ 3000 transfection reagent (Invitrogen™, L3000015, Carlsbad, CA, USA). The siRNA sequences were shown below. Creb siRNA: 5′-CGUAGAAAGAAGAAAGAAUTT-3′; Cldn5 siRNA: 5′-CUGCUAACCUGA AAGGGCA-3′. NC siRNA: 5′-UUC UCCGAA CGUGUC ACGU-3′. For CREB overexpression or CREB mutation, bEnd.3 cells were transfected with pcDNA3.1(+)-M_Creb, pcDNA3.1(+)-M_Creb(p.S133R)-HA (p.S133S→R) plasmids, or pc DNA3.1(+)-M_NC plasmids (Genomeditech Co., Ltd., Shanghai, China). After 48 h of transfection, cells were treated with a fresh medium for further experiments.
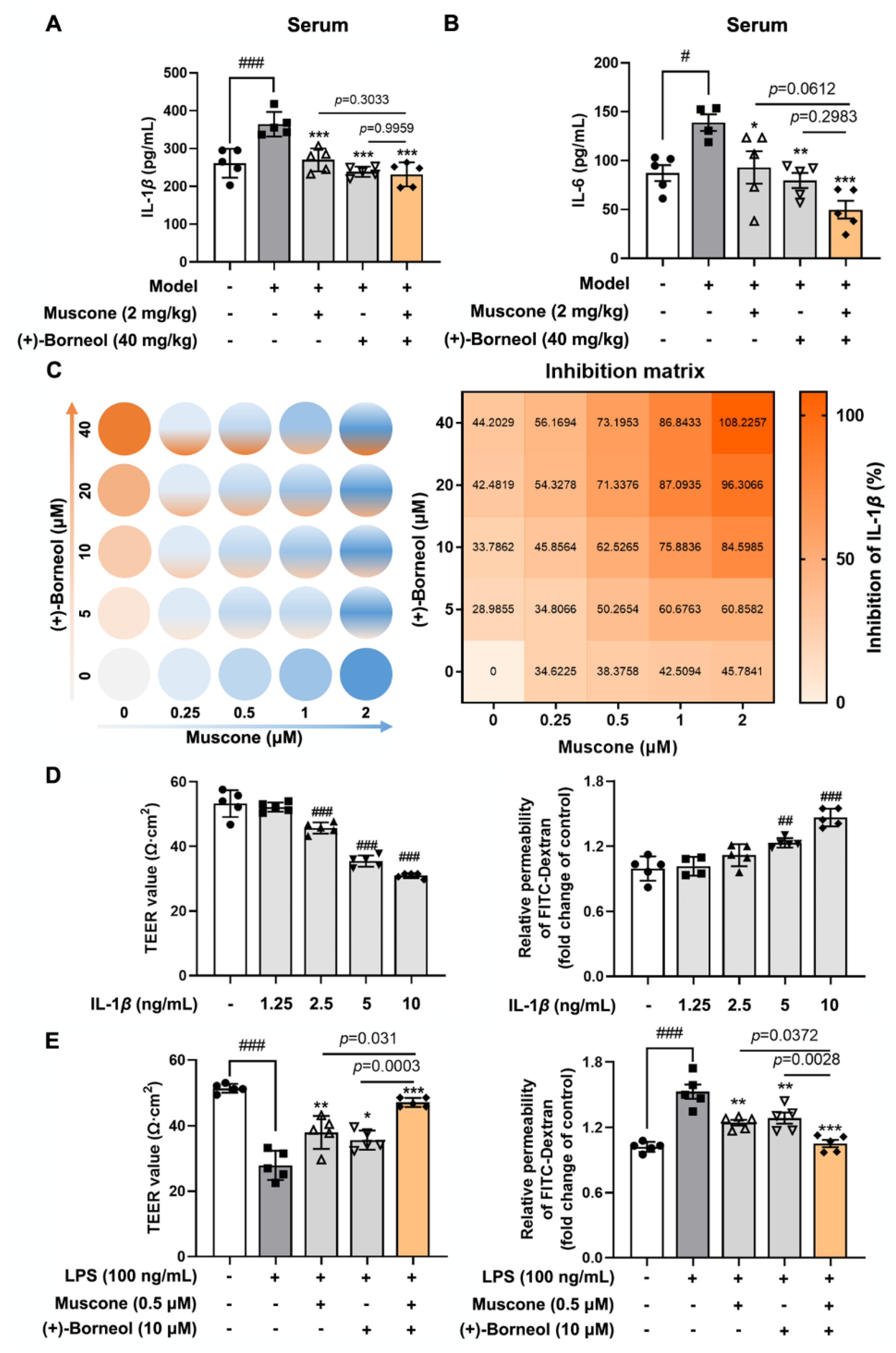
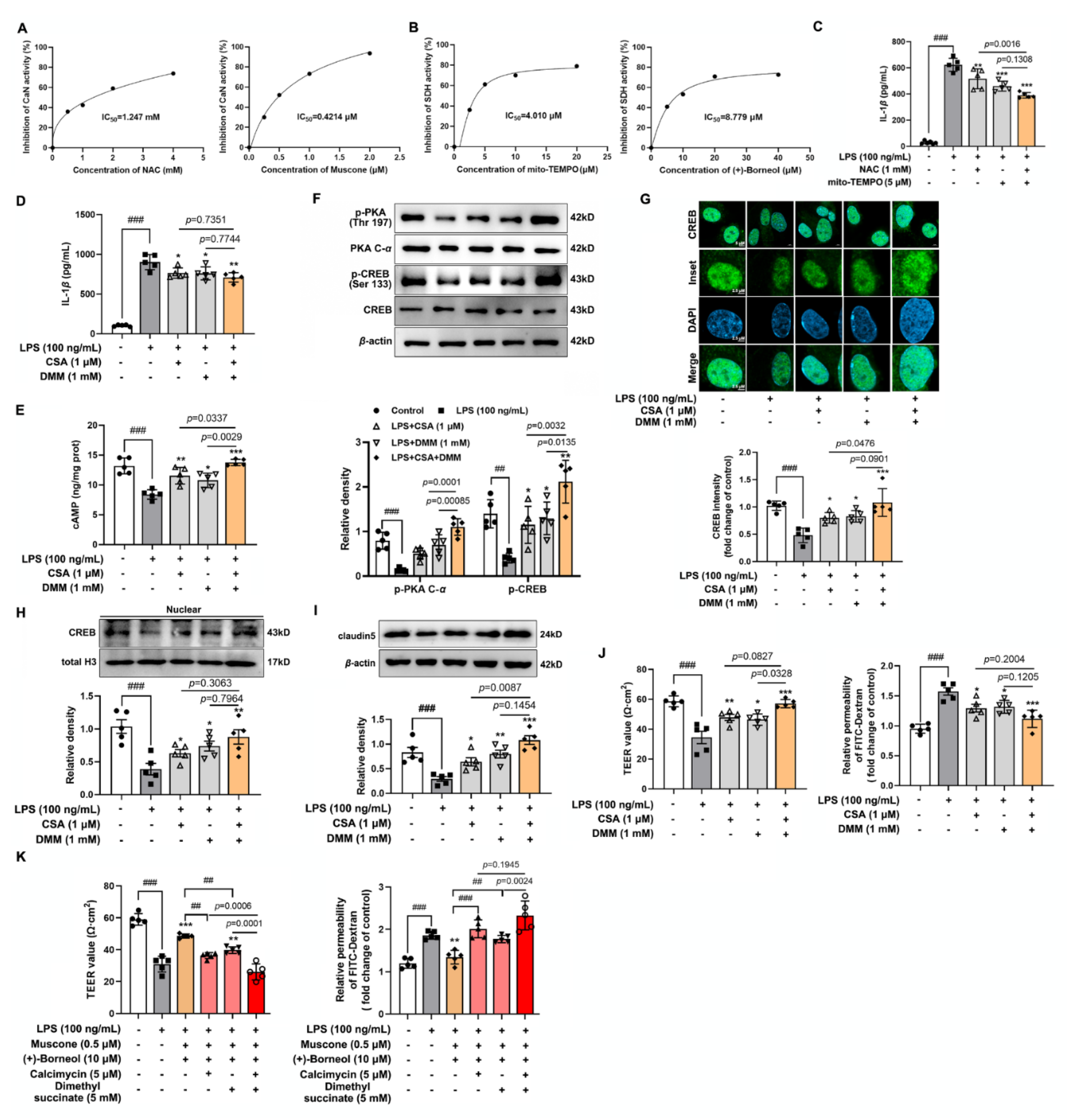
2.6. Transendothelial Electrical Resistance (TEER) Measurement and FITC-Dextran Paracellular Permeability Determination
TEER value is commonly used to assess endothelial barrier function in vitro. bEnd.3 cells suspension was seeded in the upper chamber of 12-well Transwell inserts (3460, CORNING, New York, NY, USA) at a volume of 600 μL/well, and 1.5 mL of DMEM medium was added to the lower chamber. The cells were incubated at 37 °C and 5% CO2 for 7 days until a confluent monolayer of cells was formed. After treating the cells with the indicated reagents for 16 h, the chambers were cleaned with Hanks’ Balanced Salt Solution (HBSS), and then TEER was measured with an R/V Meter of Epithelium (RE1600, Beijing KingTech Technology Co. Ltd., Beijing, China). The TEER values of the cell layers were obtained by subtracting the resistance of background TEER values in the cell-free insert and then multiplied by the total membrane surface area to obtain the resistance value in Ω·cm2.
For evaluating the paracellular permeability, 500 μL of DMEM containing 1 mg/mL FITC-Dextran (70 kDa, Sigma, St. Louis, MO, USA) was added to the upper chamber. After incubating at 37 °C for 1 h from light, the fluorescence intensity of FITC-Dextran transferred to the lower chamber was measured using a multimode microplate reader (BERTHOLD Technologies, Bad Wildbad, Germany) with excitation at 485 nm and emission at 530 nm. The permeability coefficient was calculated using the following equation: Pdextran = (RFUlower/RFUupper) (V) (1/time) (1/area), and normalized to control untreated cultures.
2.7. Human Interactome and Pathway-Enrichment Analysis
The human interactome used in this study contains 332,749 pairwise binding interactions between 18,508 human proteins [
29], assembled from 21 public databases that compile experimentally derived protein–protein interactions (PPI) data: (1) binary PPIs, derived from high-throughput yeast two-hybrid (Y2H) experiments and three-dimensional protein structures; (2) PPIs identified by affinity purification followed by mass spectrometry (AP-MS); (3) kinase-substrate pairs identified by literature-derived low-throughput or high-throughput experiments; (4) signaling interactions; (5) regulatory interactions. Kyoto Encyclopedia of Genes and Genomes (KEGG) enrichment analyses were performed to evaluate the functional pathways regulated by IL-1
β using cluster Profiler.
2.8. Quantitative Real-Time PCR (qRT-PCR)
RNA isolator (Vazyme, Nanjing, China) was used following the manufacturer’s protocol to extract the total RNA from bEnd.3 cells. The purity and concentration of isolated RNA were determined with a NANO-100 microspectrophotometer (ALLSHENG, Hangzhou, China), and an equal amount of RNA was reverse-transcribed into cDNA using an HiScript
® Q RT SuperMix for qPCR (Vazyme, Nanjing, China). Real-time PCR was then performed on LightCycler 480 II (Roche, Switzerland) with the primers and ChamQ SYBR Color qPCR Master Mix (Vazyme, Nanjing, China). The mRNA levels were normalized against
Actb and quantified using the 2
−ΔΔCt method. Primer sequences are listed in
Table S1.
2.9. Western Blotting
The total protein of bEnd.3 cells was lysed with RIPA buffer containing phosphatase and protease inhibitors (Roche, Switzerland). Nuclear extracts were prepared using a Nuclear and Cytoplasmic Protein Extraction Kit (Beyotime, P0028, Shanghai, China) following the manufacturer’s protocol. The concentration of protein was quantified using BCA protein assay kit (Beyotime, P0011, Shanghai, China). Equal amounts of protein samples were loaded on a 10% SDS-PAGE gel and then transferred to nitrocellulose filter (NC) membranes after being electrophoresed. Membranes were blocked by immersion in 5% non-fat milk for 2 h and then immunoblotted with primary antibody (anti-p-ERK1/2, 1:1000, Bioworld Technology Cat# BS4621, RRID:AB_1663587; anti-ERK1/2, 1:1000, Bioworld Technology Cat# BS3628, RRID:AB_1662299; anti-p-p38 MAPK, 1:1000, Cell Signaling Technology Cat# 9215, RRID:AB_331762; anti-p38, 1:1000, Bioworld Technology Cat# BS3566, RRID:AB_1663652; anti-p-SAPK/JNK (Thr183/Tyr185), 1:1000, Cell Signaling Technology Cat# 4668, RRID:AB_823588; anti-JNK1/2/3, 1:1000, Bioworld Technology Cat# BS1544, RRID:AB_1664025; anti-NLRP3, 1:1000, Abcam Cat# ab270449; anti-p-NF-κB p65 (Ser536), 1:1000, Cell Signaling Technology Cat# 3033, RRID:AB_331284; anti-NF-κB p65, 1:1000, Santa Cruz Biotechnology Cat# sc-8008, RRID:AB_628017; anti-TLR4, 1:1000, Santa Cruz Biotechnology Cat# sc-293072, RRID:AB_10611320; anti-Hif-1α, 1:1000, Cell Signaling Technology Cat# 36169, RRID: AB_2799095; anti-p-PKA C, 1:1000, Cell Signaling Technology Cat# 5661, RRID:AB_10707163; anti-PKA C, 1:1000, Cell Signaling Technology Cat# 5842, RRID:AB_10706172; anti-claudin 5, 1:1000, Bioworld Technology Cat# BS1069, RRID: AB_1664057; anti-p-CREB (Ser 133), 1:1000, Cell Signaling Technology Cat# 9198, RRID:AB_2561044; anti-CREB, 1:1000, Cell Signaling Technology Cat#9197, RRID:AB_331673; anti-ZO-1, 1:1000, Abcam Cat# ab216880, RRID:AB_2909434; anti-occludin, 1:1000, Bioworld Technology Cat# BS72035; β-actin, 1:2000, Proteintech Cat# 20536-1-AP, RRID: AB_10700003; anti-Histone H3, 1:1000, Cell Signaling Technology Cat#9715; RRID:AB_331563) overnight at 4 °C. After three washes with Tris-buffered saline-Tween (TBST) buffer, strips were incubated with goat anti-rabbit IgG (H + L) HRP (1:10,000, Bioworld Technology Cat# BS13278, RRID: AB_2773728) or Goat Anti-Mouse IgG (H + L) HRP, (1:10,000, Bioworld Technology Cat# BS12478, RRID: AB_2773727) at room temperature for 1 h. Finally, the signal was visualized by an ECL kit (Tanon, 180-5001, Shanghai, China) and the data were analyzed by Image-Pro Plus 6.0 (IPP 6.0) software (Media Cybernetics, Silver Spring, USA).
2.10. Immunofluorescence Staining
For detection of the protein expressions of CD31, claudin 5, and Iba1 in brain tissue of the mice treated with photothrombotic stroke, the brain tissue was fixed and embedded in Tissue-Tek O.C.T. Compound (Sakura Finetek, Tokyo, Japan), and then cut into 5 μm-thick slides. The slides fixed with 4% paraformaldehyde were permeabilized with 0.3% Triton X-100 and then blocked with goat serum. After that, the slides were incubated with primary antibodies (anti-CD31, 1 μg/mL, Abcam Cat# ab9498, RRID: AB_307284; anti-claudin 5, 1:200, Bioworld Technology Cat# BS1069, RRID: AB_1664057; anti-Iba1, 1:800, Abcam Cat# ab178847, RRID: AB_2832244) overnight at 4 °C, followed by incubation with second antibody (Goat Anti-Mouse IgG H&L (Alexa Fluor® 647), 1:500, Abcam Cat# ab150115, RRID: AB_2687948; Donkey Anti-Rabbit IgG H&L (Alexa Fluor® 647), 1:500, Abcam Cat# ab150075, RRID: AB_ 2752244) at room temperature for 2 h. After washing with PBS, the slides were stained with DAPI (Bioworld Technology, BD5010, St. Paul, MN, USA) and treated with an antifluorescence quenching agent (Beyotime, P0126, Shanghai, China). Fluorescence images were visualized and quantified by a confocal laser-scanning microscope (Zeiss, LSM 800, Jena, Germany).
For detection of intracellular immunofluorescence, the treated bEnd.3 cells were permeabilized with 0.1% Triton X-100 after fixation in 4% paraformaldehyde. After blocking with 5% bovine serum albumin (BSA), the cells were immunostained with anti-HIF-1α anti-HIF-1α (1:800, Cell Signaling Technology Cat# 36169, RRID: AB_2799095) or anti-CREB (1:500, Cell Signaling Technology Cat#9197, RRID: AB_331673) at 4 °C overnight, and subsequently incubated with Goat anti-Rabbit IgG H&L (Alexa Fluor® 488) (1:500, Abcam Cat# ab150077, RRID: AB_2630356) for 2 h in darkness. Finally, the nucleus was stained with DAPI (1:1000, Bioworld Technology, BD5010). Fluorescence images were visualized and quantified by a confocal laser-scanning microscope (Zeiss, LSM 800, Jena, Germany).
2.11. Measurement of ROS, Mitochondrial ROS, and Intracellular Calcium Contents
To observe ROS production in brain tissue of the mice from photothrombotic stroke and bEnd.3 cells, the tissue slices, and treated bEnd.3 cells were stained with 10 μM DCFH-DA (Beyotime, S0033S) and DAPI (Bioworld Technology, BD5010) at 37 °C for 20 min protected from light. Images of brain slices were viewed with the confocal laser-scanning microscope (Zeiss, LSM 800, Jena, Germany).
To observe the accumulation of mitochondrial ROS, the treated bEnd.3 cells were incubated with 5 μM MitoSOX™ red mitochondrial superoxide indicator (Invitrogen™, M36008, Carlsbad, CA, USA) reagent at 37 °C for 10 min, followed by 50 nM Mito-tracker green (Beyotime, C1048, Shanghai, China) reagent for 30 min in the dark. The nucleus was visualized by incubating with Hoechst (1:1000, Invitrogen™, H3570) for 10 min. Images were acquired and quantified by a confocal scanning microscope (Zeiss, LSM 800, Jena, Germany).
For the detection of intracellular calcium content, bEnd.3 cells with indicated treatment were incubated with Fluo-8AM (1 μg/mL, Abcam, ab142773, Cambridge, UK) for 30 min. After washing with PBS, the relative fluorescence intensity was detected using a multimode microplate reader (BERTHOLD Technologies, Bad Wildbad, Germany) at excitation of 490 nm and emission of 520 nm.
2.12. Dual-Luciferase Reporter Assay
The Mouse_Cldn5 promoter (starting from1978 bp upstream (position-1978) to 1 bp upstream (position-1) of the TSS) was synthesized and inserted in the pGL3-basic vector between Mlul and Xhol sites (Genomeditech Co., Ltd., Shanghai, China). Creb plasmids were transfected into bEnd.3 cells together with pcDNA3.1-M_Creb plasmids or pcDNA3.1(+)-M_NC plasmids with Lipofectamine™ 3000 transfection reagent (Invitrogen™, L3000015, Carlsbad, CA, USA) for 48 h. Cells were lysed with passive lysis buffer (Promega, E1941, Madison, WI, USA) and then the luciferase activities were measured by the dual-luciferase assay system (Promega, E2920, Madison, WI, USA).
2.13. Chromatin Immunoprecipitation (ChIP)
ChIP analysis was carried out using SimpleChIP Enzymatic Chromatin IP Kit (Cell Signaling, 9002, Danvers, MA, USA) following the manufacturer’s guidelines. Briefly, the treated bEnd.3 cells were cross-linked with 1% paraformaldehyde for 10 min at room temperature and then blocked with glycine. Chromatin was harvested using micrococcal nuclease digestion and sheared by sonication. The sheared chromatin was divided into two parts, one of which served as input control. The remaining portion was incubated with the primary antibody of CREB (1:50, Cell Signaling Technology Cat# 9197, RRID: AB_331673), at 4 °C overnight and then the complexes were captured by protein A/G plus agarose beads, with IgG as a negative control. After washing with ChIP elution buffer, the immunoprecipitated complex was treated with protease to release DNA fragments. Subsequently, DNA was purified with columns and amplified by quantitative polymerase chain reaction (qPCR) with site-specific primers. Primer sequences are listed in
Table S2.
2.14. Biochemical Assay
The blood samples collected from the experimental animal were allowed to stand and centrifuged at 1000× g for 15 min to obtain serum. The mouse IL-1β and IL-6 ELISA Kit (CUSABIO, Wuhan, China) were used to determine the levels of IL-1β and IL-6, respectively.
After treating bEnd.3 cells with the corresponding drugs, the supernatant was collected for the measurement of IL-1β (CUSABIO, Wuhan, China). In addition, the treated bEnd.3 cells were washed with PBS and collected with cell lysis buffer. After centrifuging at 12,000× g for 15 min at 4 °C, the supernatant was collected for the measurement of sAC, cAMP, and CaMKII according to the protocol of sAC ELISA Kit, cAMP ELISA Kit (Elabscience, Wuhan, China) and CaMKII ELISA Kit (Meimian, Yancheng, China). The quantification of succinate was measured by succinate colorimetric assay kit (Sigma, MAK184, St. Louis, MO, USA) The activity of CaN and SDH were detected according to the calcineurin assay kit (Jiancheng, A068-1-1, Nanjing, China) and succinate dehydrogenase assay kit (Jiancheng, A022-1-1, Nanjing, China).
2.15. Drug–Drug Synergy Determination
To determine drug synergy for inhibiting ROS and IL-1β production, bEnd.3 cells were plated in 96-well and 48-well plates, respectively. bEnd.3 cells were treated with muscone and (+)-borneol, which was serial twofold diluted in each step in the presence of LPS, resulting in four concentrations for each ingredient, combining a matrix of 25 different concentration ratios for both ingredients. After 16 h, bEnd.3 in 96-wells were detected ROS production by a microplate reader (BERTHOLD Technologies, Bad Wildbad, Germany). The supernate of bEnd.3 cells in a 48-well plate was used for measuring the IL-1β production.
2.16. Statistical Analysis
All statistical analyses were performed using GraphPad Prism 8.0 software. The data were presented as means ± SD (n = 5). All experiments were randomized and blinded. After being calculated for normal distribution with the Shapiro–Wilk test, statistical comparisons between different groups were evaluated by one-way ANOVA followed by Tukey’s test (>two groups) or t-test (two groups). Values of p < 0.05 were considered statistically significant.
4. Discussion
Traditional Chinese medicine is commonly prescribed in combination form, consisting of various herbs with dozens of active components, likely due to the advantage of solving complex diseases through the integration of multipathway or multitarget manners [
39]. Moschus and borneolum have been used as a common drug pair in traditional prescriptions, and the role is shown to ameliorate cerebral diseases dependent on different conditions. Although the synergistic effects on ischemia stroke in rats have been validated in the previous study [
22], our study presented that muscone and (+)-borneol worked together to increase claudin 5 expression and resultantly protected BBB integrity against ischemic brain injury in the context of oxidative stress and inflammatory activation. Muscone reduced ROS to block the Erk1/2 inflammatory pathway, while (+)-borneol removed mitochondrial ROS to weaken the inflammatory transcriptional regulation of Hif-1
α. Combined with their structural characteristics, muscone and (+)-borneol exerted antioxidant and anti-inflammatory effects through different pathways. Moreover, we revealed that CREB transcriptionally regulated claudin 5 induction, and inflammatory cytokine IL-1
β impaired cAMP/CREB signaling to reduce claudin 5 in endothelial cells, addressing that protection of claudin 5 stability was a way for pharmacological intervention to ensure the integrity of cerebral microvascular.
Cerebral thrombosis is a sequence of endothelial dysfunction devoted to ischemic injury. In line with previous studies of anti-ischemic effects [
26,
40], muscone and (+)-borneol reduced infarct volume after stroke in mice by protecting BBB integrity and barrier function. In patients who were suffering from acute stroke, the magnitude of inflammatory response was positively correlated with the extent of ischemic injury to a certain extent [
11]. Muscone relieved inflammatory pain by inhibiting microglial activation-mediated inflammatory response via inactivation of NOX4 and NLRP3 inflammasome [
23]. (+)-Borneol protected oxygen-glucose deprivation/reperfusion-induced cortical neurons via antioxidation and anti-inflammation through the NF-
κB signaling pathway [
24]. Consistently, in our study, we found that muscone and (+)-borneol reduced cerebral infarct volume and cerebrovascular leakage with claudin 5 protection in mice after stroke, largely due to inhibiting ROS accumulation and inflammatory microglia recruitment in the peripheral infarct area. Due to the superimposed antioxidative and anti-inflammatory pathways of muscone and (+)-borneol, the combined administration of the two drugs significantly enhanced cerebral protection. Thus, we addressed that the suppression of oxidation damage and inflammatory injury should be an important means for the synergistic effects of muscone and (+)-borneol in the protection of brain endothelium.
IL-1
β acts in a paracrine and autocrine manner through activating IL-1R1 in the endothelium, leading to increased recruitment of inflammatory cells into the brain parenchyma [
33]. Consistently, in our study, with the increase in IL-1
β concentration, the damage to BBB function was aggravated. Previous research confirmed that IL-1
β-mediated BBB damage largely depends on TJs’ destruction, including phosphorylation, degradation, and relocation in the context of ischemic injury [
41]. In the current study, IL-1
β downregulated the gene and protein level of claudin 5, contributing to cerebral microvessel damage. Muscone and (+)-borneol functioned to protect cerebral microvessels via preserving TJs stability against ischemic injury [
26,
42]. Owing to the combinative effect on restraining IL-1
β release, muscone and (+)-borneol mutually enhanced each other to protect claudin 5 expression from inflammatory insult. This suggests that targeting claudin 5 stability to increase the resistance to proinflammatory stimulation was an important strategy for the improvement of BBB function.
CREB is a transcription factor that binds to the cAMP-response element (CRE) to trigger gene transcription [
43]. Activated sAC catalyzes the formation of cAMP and then activates PKA to regulate CREB activity by phosphorylation [
43]. CREB is activated in the ischemic brain to promote functional recovery via transcriptional regulation of genes encoding cell survival-related molecules, growth factors, and structural proteins [
44]. TJ contains a variety of transmembrane proteins, including claudins and occludin, as well as intracellular scaffold proteins such as ZO-1, which play a key role in the regulation of endothelial monolayer resistance, paracellular solute permeability, and BBB function. Intriguingly, studies have shown that CREB binds to transcriptional promoters including ZO-1 and claudins [
45,
46], which maintains the integrity and function of brain microvascular. Our study was carried out under the background of LPS-stimulated inflammatory response activation to produce IL-1
β accumulation and accumulated IL-1
β disrupted cAMP/CREB activation and attenuated transcriptional regulation of CREB. CREB is known to bind preferentially with a canonical CRE sequence 5′-TGACGTCA-3′. JASPAR database predicted two potential CREB-binding sites in the
Cldn5 promoter region, which displayed significant homology with the canonical CREB recognition motif. ChIP-qPCR and luciferase reporter gene analysis confirmed the transcriptional regulation of claudin 5 by CREB. In LPS-stimulated bEnd.3 cells, muscone and (+)-borneol promoted claudin 5 expression through the activated cAMP/CREB pathway. Notably, the combined administration of the two drugs significantly increased the expression of claudin 5 by the up-regulated CREB transcription, which is closely related to the combined antioxidant and anti-inflammatory effects of muscone and (+)-borneol.
Given the important role of inflammatory factors in the destruction of the cerebrovascular barrier, it is particularly important to elucidate the pathway of IL-1
β production and to trace the action targets of muscone and (+)-borneol. Oxidative damage is closely related to inflammatory response and accumulated ROS activated MAPK and NF-
κB pathways to cause airway inflammation [
9]. In activated microglia, mitochondrial ROS activated the MAPK pathway to promote inflammatory response [
10]. In our study, muscone and (+)-borneol reduced intracellular ROS and mitochondrial ROS accumulation, thereby affecting different inflammatory pathways. Activated Erk1/2 signaling strengthened inflammatory response during cerebral ischemic injury [
47]. In LPS-stimulated bEnd.3 cells, muscone inhibited phosphorylation of Erk1/2 by inhibiting CaN activity. Meanwhile, (+)-borneol attenuated the HIF-1
α signaling pathway by inhibiting SDH activity, which was closely related to reduced mitochondrial ROS. Therefore, we proposed that regulation from different ways should be the reason for muscone and (+)-borneol combination’s potential role in IL-1
β suppression. However, we should note that the antioxidative and anti-inflammatory effects were not the only means of pharmacological intervention for ischemic brain injury. In the complex central nervous system, neurons, astrocytes, and microglia play a key role in brain homeostasis, inhibiting apoptosis and maintaining physiological functions of cells are also of great significance. Muscone and (+)-borneol, as active ingredients that can easily enter the BBB, may play a synergistic role in maintaining brain tissue homeostasis through multitarget and multi-pathway by acting on different cells.