Inflammatory and Oxidative Stress Markers Related to Adherence to the Mediterranean Diet in Patients with Metabolic Syndrome
Abstract
:1. Introduction
2. Methods
2.1. Study Design and Participants
2.2. Anthropometric Measurements
2.3. Dietary Assessment and Dietary Inflammatory Index
2.4. Collection of Blood and Urine Samples
2.5. Immunoassay Kits
2.6. Enzymatic Determinations
2.7. Malondialdehyde Assay
2.8. Polyphenols Determination
2.9. 8-Oxo-7,8-Dihydro-Guanosine, and 8-Oxo-7,8-Dihydroguanosine Determination
2.10. PBMCs and Neutrophils ROS Production
2.11. Statistics
3. Results
4. Discussion
Strengths and Limitations of the Study
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Saklayen, M.G. The Global Epidemic of the Metabolic Syndrome. Curr. Hypertens. Rep. 2018, 20, 12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- O’Neill, S.; O’Driscoll, L. Metabolic syndrome: A closer look at the growing epidemic and its associated pathologies. Obes. Rev. 2015, 16, 1–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Salas, R.; Bibiloni, M.d.M.; Ramos, E.; Villarreal, J.Z.; Pons, A.; Tur, J.A.; Sureda, A. Metabolic Syndrome Prevalence among Northern Mexican Adult Population. PLoS ONE 2014, 9, e105581. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ford, E.S.; Li, C.; Zhao, G. Prevalence and correlates of metabolic syndrome based on a harmonious definition among adults in the US. J. Diabetes 2010, 2, 180–193. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bellia, A.; Giardina, E.; Lauro, D.; Tesauro, M.; Di Fede, G.; Cusumano, G.; Federici, M.; Rini, G.B.; Novelli, G.; Lauro, R.; et al. “The Linosa Study”: Epidemiological and heritability data of the metabolic syndrome in a Caucasian genetic isolate. Nutr. Metab. Cardiovasc. Dis. 2009, 19, 455–461. [Google Scholar] [CrossRef] [PubMed]
- Finicelli, M.; Squillaro, T.; Cristo, F.D.; Salle, A.D.; Melone, M.A.B.; Galderisi, U.; Peluso, G. Metabolic syndrome, Mediterranean diet, and polyphenols: Evidence and perspectives. J. Cell. Physiol. 2019, 234, 5807–5826. [Google Scholar] [CrossRef] [PubMed]
- Salas-Salvadó, J.; Guasch-Ferré, M.; Lee, C.H.; Estruch, R.; Clish, C.B.; Ros, E. Protective Effects of the Mediterranean Diet on Type 2 Diabetes and Metabolic Syndrome. J. Nutr. 2015, 146, 920S–927S. [Google Scholar] [CrossRef] [Green Version]
- Di Daniele, N.D.; Noce, A.; Vidiri, M.F.; Moriconi, E.; Marrone, G.; Annicchiarico-Petruzzelli, M.; D’Urso, G.; Tesauro, M.; Rovella, V.; De Lorenzo, A.D. Impact of Mediterranean diet on metabolic syndrome, cancer and longevity. Oncotarget 2017, 8, 8947–8979. [Google Scholar] [CrossRef] [Green Version]
- Widmer, R.J.; Flammer, A.J.; Lerman, L.O.; Lerman, A. The Mediterranean Diet, its Components, and Cardiovascular Disease. Am. J. Med. 2015, 128, 229–238. [Google Scholar] [CrossRef] [Green Version]
- Saura-Calixto, F.; Goñi, I. Definition of the Mediterranean diet based on bioactive compounds. Crit. Rev. Food Sci. Nutr. 2009, 49, 145–152. [Google Scholar] [CrossRef]
- Hernández Ruiz de Eguilaz, M.; Batlle, M.A.; Martínez de Morentin, B.; San-Cristóbal, R.; Pérez-Díez, S.; Navas-Carretero, S.; Martínez, J.A. Alimentary and lifestyle changes as a strategy in the prevention of metabolic syndrome and diabetes mellitus type 2: Milestones and perspectives. An. Sist. Sanit. Navar. 2016, 39, 269–289. [Google Scholar]
- Castro-Barquero, S.; Ruiz-León, A.M.; Sierra-Pérez, M.; Estruch, R.; Casas, R. Dietary Strategies for Metabolic Syndrome: A Comprehensive Review. Nutrients 2020, 12, 2983. [Google Scholar] [CrossRef] [PubMed]
- Hutcheson, R.; Rocic, P. The Metabolic Syndrome, Oxidative Stress, Environment, and Cardiovascular Disease: The Great Exploration. Exp. Diabetes Res. 2012, 2012, 271028. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, S.; Tan, H.Y.; Wang, N.; Zhang, Z.J.; Lao, L.; Wong, C.W.; Feng, Y. The Role of Oxidative Stress and Antioxidants in Liver Diseases. Int. J. Mol. Sci. 2015, 16, 26087–26124. [Google Scholar] [CrossRef] [Green Version]
- Pisoschi, A.M.; Pop, A. The role of antioxidants in the chemistry of oxidative stress: A review. Eur. J. Med. Chem. 2015, 97, 55–74. [Google Scholar] [CrossRef]
- Stocker, R.; Keaney, J.F., Jr. Role of oxidative modifications in atherosclerosis. Physiol. Rev. 2004, 84, 1381–1478. [Google Scholar] [CrossRef]
- Monteiro, R.; Azevedo, I. Chronic inflammation in obesity and the metabolic syndrome. Mediat. Inflamm. 2010, 2010, 289645. [Google Scholar] [CrossRef]
- Grandl, G.; Wolfrum, C. Hemostasis, endothelial stress, inflammation, and the metabolic syndrome. Semin. Immunopathol. 2017, 40, 215–224. [Google Scholar] [CrossRef] [Green Version]
- Elyasi, A.; Voloshyna, I.; Ahmed, S.; Kasselman, L.J.; Behbodikhah, J.; De Leon, J.; Reiss, A.B. The role of interferon-γ in cardiovascular disease: An update. Inflamm. Res. 2020, 69, 975–988. [Google Scholar] [CrossRef]
- Esser, N.; Legrand-Poels, S.; Piette, J.; Scheen, A.J.; Paquot, N. Inflammation as a link between obesity, metabolic syndrome and type 2 diabetes. Diabetes Res. Clin. Pract. 2014, 105, 141–150. [Google Scholar] [CrossRef] [Green Version]
- Engin, A.B. Adipocyte-Macrophage Cross-Talk in Obesity. Adv. Exp. Med. Biol. 2017, 960, 327–343. [Google Scholar] [PubMed]
- Lacraz, G.; Rakotoarivelo, V.; Labbé, S.M.; Vernier, M.; Noll, C.; Mayhue, M.; Stankova, J.; Schwertani, A.; Grenier, G.; Carpentier, A.; et al. Deficiency of Interleukin-15 Confers Resistance to Obesity by Diminishing Inflammation and Enhancing the Thermogenic Function of Adipose Tissues. PLoS ONE 2016, 11, e0162995. [Google Scholar]
- Klok, M.D.; Jakobsdottir, S.; Drent, M.L. The role of leptin and ghrelin in the regulation of food intake and body weight in humans: A review. Obes. Rev. 2007, 8, 21–34. [Google Scholar] [CrossRef] [PubMed]
- Alberti, K.G.M.M.; Eckel, R.H.; Grundy, S.M.; Zimmet, P.Z.; Cleeman, J.I.; Donato, K.A.; Fruchart, J.-C.; James, W.P.T.; Loria, C.M.; Smith, C.S., Jr.; et al. Harmonizing the metabolic syndrome: A joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation 2009, 120, 1640–1645. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bouzas, C.; Bibiloni, M.d.M.; Julibert, A.; Ruiz-Canela, M.; Salas-Salvadó, J.; Corella, D.; Zomeño, M.D.; Romaguera, D.; Vioque, J.; Alonso-Gómez, Á.M.; et al. Adherence to the Mediterranean Lifestyle and Desired Body Weight Loss in a Mediterranean Adult Population with Overweight: A PREDIMED-Plus Study. Nutrients 2020, 12, 2114. [Google Scholar] [CrossRef]
- Ainsworth, B.E.; Haskell, W.L.; Leon, A.S.; Jacobs, D.R., Jr.; Montoye, H.J.; Sallis, J.F.; Paffenbarger, R.S., Jr. Compendium of physical activities: Classification of energy costs of human physical activities. Med. Sci. Sports Exerc. 1993, 25, 71–74. [Google Scholar] [CrossRef]
- Fernández-Ballart, J.D.; Piñol, J.L.; Zazpe, I.; Corella, D.; Carrasco, P.; Toledo, E.; Perez-Bauer, M.; Martínez-González, M.Á.; Salas-Salvadó, J.; Martn-Moreno, J.M. Relative validity of a semi-quantitative food-frequency questionnaire in an elderly Mediterranean population of Spain. Br. J. Nutr. 2010, 103, 1808–1816. [Google Scholar] [CrossRef] [Green Version]
- Moreiras, O.; Carbajal, A.; Cabrera, L.; Cuadrado, C. Tablas de Composicion de Alimentos (Ciencia y Tecnica); Ediciones Pirámide: Madrid, Sapin, 2007; pp. 37–46. [Google Scholar]
- Shivappa, N.; Steck, S.E.; Hurley, T.G.; Hussey, J.R.; Hébert, J.R. Designing and developing a literature-derived, population-based dietary inflammatory index. Public Health Nutr. 2014, 17, 1689–1696. [Google Scholar] [CrossRef] [Green Version]
- Gallardo-Alfaro, L.; Del Mar Bibiloni, M.; Mascaró, C.M.; Montemayor, S.; Ruiz-Canela, M.; Salas-Salvad, J.; Corella, D.; Fitó, M.; Romaguera, D.; Vioque, J.; et al. Leisure-Time Physical Activity, Sedentary Behaviour and Diet Quality are Associated with Metabolic Syndrome Severity: The PREDIMED-Plus Study. Nutrients 2020, 12, 1013. [Google Scholar] [CrossRef] [Green Version]
- Bøyum, A. Separation of White Blood Cells. Nature 1964, 204, 793–794. [Google Scholar] [CrossRef]
- Capó, X.; Ferrer, M.D.; Olek, R.A.; Salaberry, E.; Gomila, R.M.; Martorell, G.; Sureda, A.; Tur, J.A.; Pons, A. Simultaneous analysis of saturated and unsaturated oxylipins in ‘ex vivo’ cultured peripheral blood mononuclear cells and neutrophils. J. Pharm. Biomed. Anal. 2020, 186, 113258. [Google Scholar] [CrossRef] [PubMed]
- Aebi, H. Catalase in Vitro. Methods Enzymol. 1984, 105, 121–126. [Google Scholar] [PubMed]
- McCord, J.M.; Fridovich, I. Superoxide dismutase. An enzymic function for erythrocuprein (hemocuprein). J. Biol. Chem. 1969, 244, 6049–6055. [Google Scholar] [CrossRef]
- Capeillère-Blandin, C. Oxidation of guaiacol by myeloperoxidase: A two-electron-oxidized guaiacol transient species as a mediator of NADPH oxidation. Biochem. J. 1998, 336, 395–404. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kubiliene, L.; Laugaliene, V.; Pavilonis, A.; Maruska, A.; Majiene, D.; Barcauskaite, K.; Kubilius, R.; Kasparaviciene, G.; Savickas, A. Alternative preparation of propolis extracts: Comparison of their composition and biological activities. BMC Complement. Altern. Med. 2015, 15, 156. [Google Scholar] [CrossRef] [Green Version]
- Poulsen, H.E.; Weimann, A.; Henriksen, T.; Kjær, L.K.; Larsen, E.L.; Carlsson, E.R.; Christensen, C.K.; Brandslund, I.; Fenger, M. Oxidatively generated modifications to nucleic acids in vivo: Measurement in urine and plasma. Free Radic. Biol. Med. 2019, 145, 336–341. [Google Scholar] [CrossRef] [PubMed]
- Fraga, C.G.; Celep, G.S.; Galleano, M. Biochemical actions of plant phenolics compounds: Thermodynamic and kinetic aspects. Plant Phenolics Hum. Health Biochem. Nutr. Pharmacol. 2009, 3, 91–106. [Google Scholar]
- Medina-Remón, A.; Casas, R.; Tressserra-Rimbau, A.; Ros, E.; Martínez-González, M.A.; Fitó, M.; Corella, D.; Salas-Salvadó, J.; Lamuela-Raventos, R.M.; Estruch, R. Polyphenol intake from a Mediterranean diet decreases inflammatory biomarkers related to atherosclerosis: A substudy of the PREDIMED trial. Br. J. Clin. Pharmacol. 2017, 83, 114–128. [Google Scholar] [CrossRef] [Green Version]
- Guo, X.; Tresserra-Rimbau, A.; Estruch, R.; Martínez-González, M.A.; Medina-Remón, A.; Castañer, O.; Corella, D.; Salas-Salvadó, J.; Lamuela-Raventós, R.M. Effects of Polyphenol, Measured by a Biomarker of Total Polyphenols in Urine, on Cardiovascular Risk Factors after a Long-Term Follow-Up in the PREDIMED Study. Oxid. Med. Cell. Longev. 2016, 2016, 2572606. [Google Scholar] [CrossRef] [Green Version]
- Krogholm, K.S.; Haraldsdóttir, J.; Knuthsen, P.; Rasmussen, S.E. Urinary total flavonoid excretion but not 4-pyridoxic acid or potassium can be used as a biomarker for the intake of fruits and vegetables. J. Nutr. 2004, 134, 445–451. [Google Scholar] [CrossRef]
- Mennen, L.I.; Sapinho, D.; Ito, H.; Bertrais, S.; Galan, P.; Hercberg, S.; Scalbert, A. Urinary flavonoids and phenolic acids as biomarkers of intake for polyphenol-rich foods. Br. J. Nutr. 2006, 96, 191–198. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Velázquez-López, L.; Santiago-Díaz, G.; Nava-Hernández, J.; Muñoz-Torres, A.V.; Medina-Bravo, P.; Torres-Tamayo, M. Mediterranean-style diet reduces metabolic syndrome components in obese children and adolescents with obesity. BMC Pediatr. 2014, 14, 175. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bakaloudi, D.R.; Chrysoula, L.; Kotzakioulafi, E.; Theodoridis, X.; Chourdakis, M. Impact of the Level of Adherence to Mediterranean Diet on the Parameters of Metabolic Syndrome: A Systematic Review and Meta-Analysis of Observational Studies. Nutrients 2021, 13, 1514. [Google Scholar] [CrossRef] [PubMed]
- Monserrat-Mesquida, M.; Quetglas-Llabrés, M.; Bouzas, C.; Capó, X.; Mateos, D.; Ugarriza, L.; Tur, J.A.; Sureda, A. Peripheral blood mononuclear cells oxidative stress and plasma inflammatory biomarkers in adults with normal weight, overweight and obesity. Antioxidants 2021, 10, 813. [Google Scholar] [CrossRef] [PubMed]
- Nair, A.R.; Mariappan, N.; Stull, A.J.; Francis, J. Blueberry supplementation attenuates oxidative stress within monocytes and modulates immune cell levels in adults with metabolic syndrome: A randomized, double-blind, placebo-controlled trial. Food Funct. 2017, 8, 4118–4128. [Google Scholar] [CrossRef] [PubMed]
- Luisi, M.L.E.; Lucarini, L.; Biffi, B.; Rafanelli, E.; Pietramellara, G.; Durante, M.; Vidali, S.; Provensi, G.; Madiai, S.; Gheri, C.F.; et al. Effect of Mediterranean diet enriched in high quality extra virgin olive oil on oxidative stress, inflammation and gut microbiota in obese and normal weight adult subjects. Front. Pharmacol. 2019, 10, 1366. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Feoli, A.M.P.; Macagnan, F.E.; Piovesan, C.H.; Bodanese, L.C.; Siqueira, I.R. Xanthine oxidase activity is associated with risk factors for cardiovascular disease and inflammatory and oxidative status markers in metabolic syndrome: Effects of a single exercise session. Oxid. Med. Cell. Longev. 2014, 2014, 587083. [Google Scholar] [CrossRef]
- Sureda, A.; Bibiloni, M.d.M.; Martorell, M.; Buil-Cosiales, P.; Marti, A.; Pons, A.; Tur, J.A.; Martinez-Gonzalez, M.Á. Mediterranean diets supplemented with virgin olive oil and nuts enhance plasmatic antioxidant capabilities and decrease xanthine oxidase activity in people with metabolic syndrome: The PREDIMED study. Mol. Nutr. Food Res. 2016, 60, 2654–2664. [Google Scholar] [CrossRef]
- Bekkouche, L.; Bouchenak, M.; Malaisse, W.J.; Yahia, D.A. The mediterranean diet adoption improves metabolic, oxidative, and inflammatory abnormalities in algerian metabolic syndrome patients. Horm. Metab. Res. 2014, 46, 274–282. [Google Scholar] [CrossRef]
- Chen, S.J.; Yen, C.H.; Huang, Y.C.; Lee, B.J.; Hsia, S.; Lin, P.T. Relationships between Inflammation, Adiponectin, and Oxidative Stress in Metabolic Syndrome. PLoS ONE 2012, 7, e45693. [Google Scholar] [CrossRef] [Green Version]
- Tur, J.A. Antioxidants in the Mediterranean Diet. Rev. Esp. Nutr. Comunitaria 2004, 10, 198–207. [Google Scholar]
- Liu, X.; Gan, W.; Zou, Y.; Yang, B.; Su, Z.; Deng, J.; Wang, L.; Cai, J. Elevated Levels of Urinary Markers of Oxidative DNA and RNA Damage in Type 2 Diabetes with Complications. Oxid. Med. Cell. Longev. 2016, 2016, 4323198. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Urpi-Sarda, M.; Casas, R.; Chiva-Blanch, G.; Romero-Mamani, E.S.; Valderas-Martínez, P.; Salas-Salvadó, J.; Covas, M.I.; Toledo, E.; Andres-Lacueva, C.; Llorach, R.; et al. The mediterranean diet pattern and its main components are associated with lower plasma concentrations of tumor necrosis factor receptor 60 in patients at high risk for cardiovascular disease. J. Nutr. 2012, 142, 1019–1025. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Esposito, K.; Marfella, R.; Ciotola, M.; Di Palo, C.; Giugliano, F.; Giugliano, G.; D’Armiento, M.; D’Andrea, F.; Giugliano, D. Effect of a Mediterranean-Style Diet on Endothelial Dysfunction and Markers of Vascular Inflammation in the Metabolic Syndrome: A Randomized Trial. JAMA 2004, 292, 1440–1446. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Urpi-Sarda, M.; Casas, R.; Sacanella, E.; Corella, D.; Andrés-Lacueva, C.; Llorach, R.; Garrabou, G.; Cardellach, F.; Sala-Vila, A.; Ros, E.; et al. The 3-year effect of the mediterranean diet intervention on inflammatory biomarkers related to cardiovascular disease. Biomedicines 2021, 9, 862. [Google Scholar] [CrossRef] [PubMed]
- Wuttge, D.M.; Eriksson, P.; Sirsjö, A.; Hansson, G.K.; Stemme, S. Expression of Interleukin-15 in Mouse and Human Atherosclerotic Lesions. Am. J. Pathol. 2001, 159, 417–423. [Google Scholar] [CrossRef] [Green Version]
- Sureda, A.; del Mar Bibiloni, M.; Julibert, A.; Bouzas, C.; Argelich, E.; Llompart, I.; Pons, A.; Tur, J.A. Adherence to the Mediterranean Diet and Inflammatory Markers. Nutrients 2018, 10, 62. [Google Scholar] [CrossRef] [Green Version]
- Giugliano, D.; Ceriello, A.; Esposito, K. The effects of diet on inflammation: Emphasis on the metabolic syndrome. J. Am. Coll. Cardiol. 2006, 48, 677–685. [Google Scholar] [CrossRef] [Green Version]
- Montefusco, L.; D’Addio, F.; Loretelli, C.; Ben Nasr, M.; Garziano, M.; Rossi, A.; Pastore, I.; Plebani, L.; Lunati, M.E.; Bolla, A.M.; et al. Anti-inflammatory effects of diet and caloric restriction in metabolic syndrome. J. Endocrinol. Investig. 2021, 44, 2407–2415. [Google Scholar] [CrossRef]
- Kojima, M.; Hosoda, H.; Date, Y.; Nakazato, M.; Matsuo, H.; Kangawa, K. Ghrelin is a growth-hormone-releasing acylated peptide from stomach. Nature 1999, 402, 656–660. [Google Scholar] [CrossRef]
- Garaulet, M.; Pérez De Heredia, F.; Garaulet, M. Behavioural therapy in the treatment of obesity (II): Role of the Mediterranean diet. Nutr. Hosp. 2010, 25, 9–17. [Google Scholar] [PubMed]
- Gahete, M.D.; Luque, R.M.; Yubero-Serrano, E.M.; Cruz-Teno, C.; Ibañez-Costa, A.; Delgado-Lista, J.; Gracia-Navarro, F.; Perez-Jimenez, F.; Castaño, J.P.; Lopez-Miranda, J. Dietary fat alters the expression of cortistatin and ghrelin systems in the PBMCs of elderly subjects: Putative implications in the postprandial inflammatory response. Mol. Nutr. Food Res. 2014, 58, 1897–1906. [Google Scholar] [CrossRef] [PubMed]
- Richard, C.; Royer, M.M.; Couture, P.; Cianflone, K.; Rezvani, R.; Desroches, S.; Lamarche, B. Effect of the Mediterranean diet on plasma adipokine concentrations in men with metabolic syndrome. Metabolism 2013, 62, 1803–1810. [Google Scholar] [CrossRef] [PubMed]
- Kaser, S.; Kaser, A.; Sandhofer, A.; Ebenbichler, C.F.; Tilg, H.; Patsch, J.R. Resistin messenger-RNA expression is increased by proinflammatory cytokines in vitro. Biochem. Biophys. Res. Commun. 2003, 309, 286–290. [Google Scholar] [CrossRef] [PubMed]
- Cabrera de León, A.; Almeida González, D.; González Hernández, A.; Domínguez Coello, S.; Marrugat, J.; Alemán Sánchez, J.J.; Brito Díaz, B.; Marcelino Rodríguez, I.; Rodríguez Pérez, M.d.C. Relationships between serum resistin and fat intake, serum lipid concentrations and adiposity in the general population. J. Atheroscler. Thromb. 2014, 21, 454–462. [Google Scholar] [CrossRef] [Green Version]
- Bédard, A.; Tchernof, A.; Lamarche, B.; Corneau, L.; Dodin, S.; Lemieux, S. Effects of the traditional Mediterranean diet on adiponectin and leptin concentrations in men and premenopausal women: Do sex differences exist? Eur. J. Clin. Nutr. 2014, 68, 561–566. [Google Scholar] [CrossRef]
- Tzima, N.; Pitsavos, C.; Panagiotakos, D.B.; Skoumas, J.; Zampelas, A.; Chrysohoou, C.; Stefanadis, C. Mediterranean diet and insulin sensitivity, lipid profile and blood pressure levels, in overweight and obese people; the Attica study. Lipids Health Dis. 2007, 6, 22. [Google Scholar] [CrossRef] [Green Version]
- Agnoli, C.; Sieri, S.; Ricceri, F.; Giraudo, M.T.; Masala, G.; Assedi, M.; Panico, S.; Mattiello, A.; Tumino, R.; Giurdanella, M.C.; et al. Adherence to a Mediterranean diet and long-term changes in weight and waist circumference in the EPIC-Italy cohort. Nutr. Diabetes 2018, 8, 22. [Google Scholar] [CrossRef]
- Regitz-Zagrosek, V.; Lehmkuhl, E.; Mahmoodzadeh, S. Gender aspects of the role of the metabolic syndrome as a risk factor for cardiovascular disease. Gend. Med. 2007, 4 (Suppl. B), S162–S177. [Google Scholar] [CrossRef]
- Gerdts, E.; Regitz-Zagrosek, V. Sex differences in cardiometabolic disorders. Nat. Med. 2019, 25, 1657–1666. [Google Scholar] [CrossRef]
- Henstridge, D.C.; Abildgaard, J.; Lindegaard, B.; Febbraio, M.A. Metabolic control and sex: A focus on inflammatory-linked mediators. Br. J. Pharmacol. 2019, 176, 4193–4207. [Google Scholar] [CrossRef] [PubMed] [Green Version]
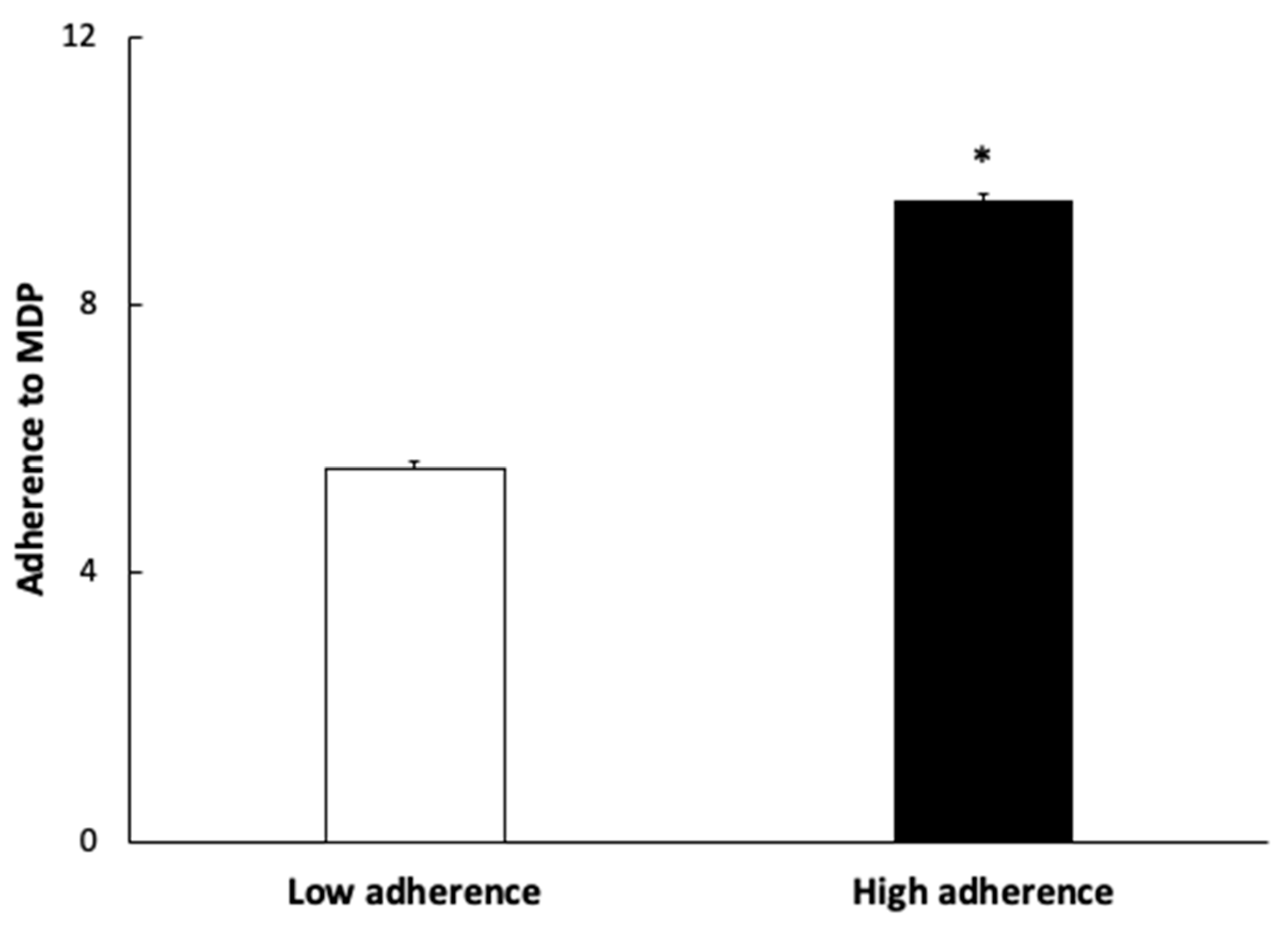

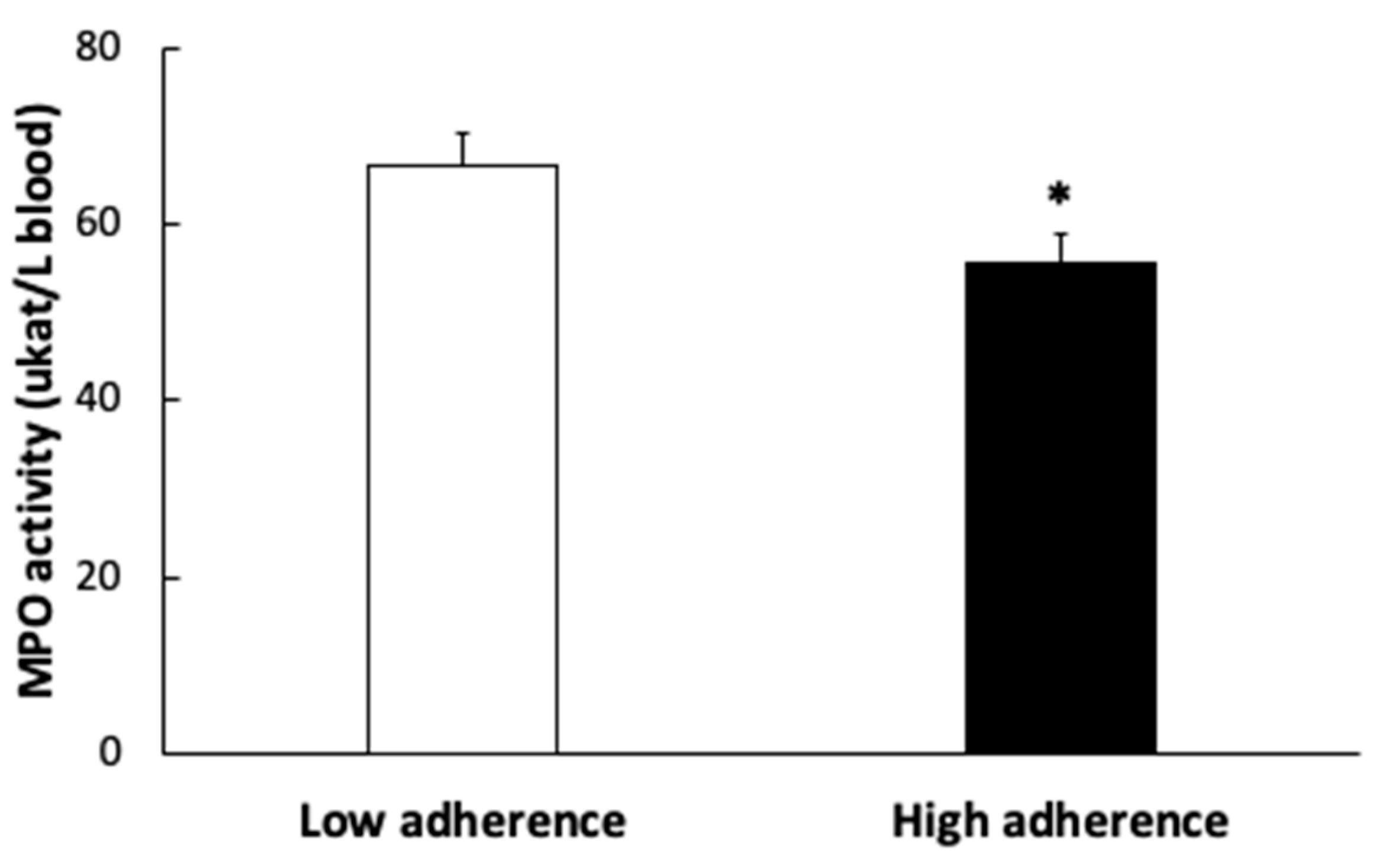
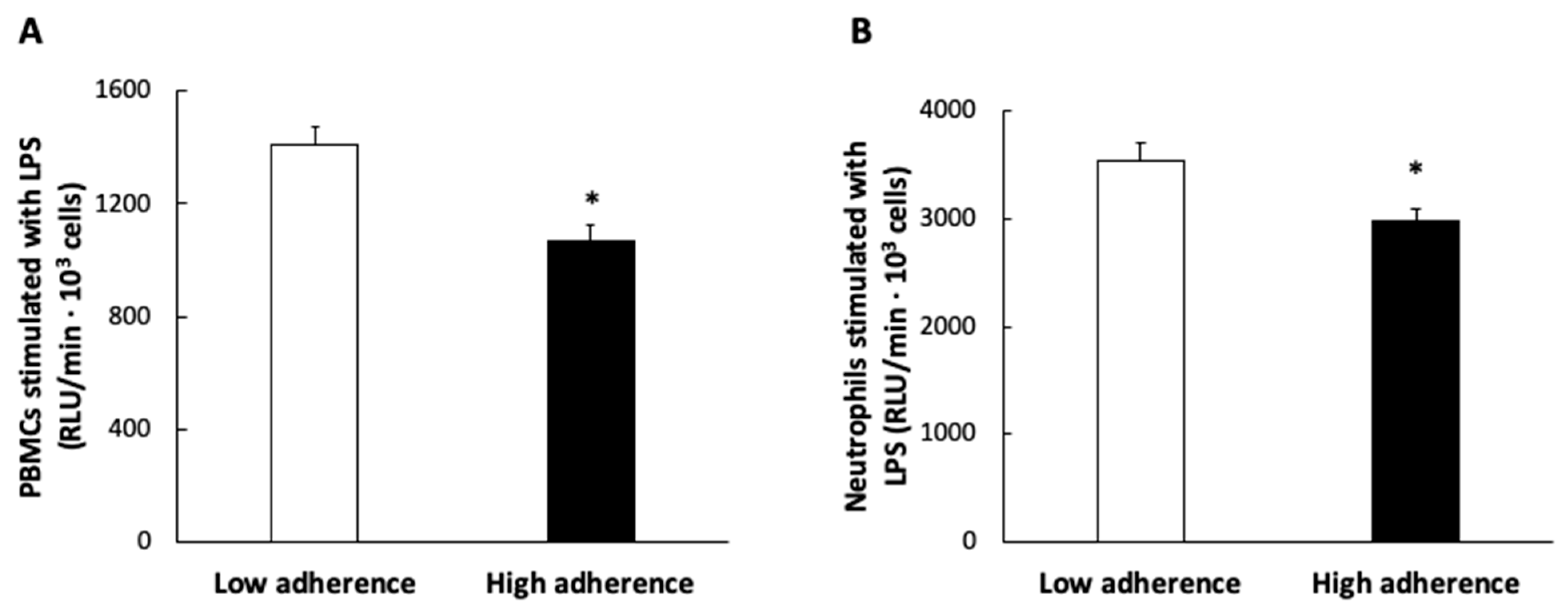
| Sociodemographic and Clinical Characteristics | Reference Values | Low Adherence n = 45 Mean ± SEM | High Adherence n = 45 Mean ± SEM | * p-Value |
|---|---|---|---|---|
| Age (years) | 64.2 ± 0.4 | 65.3 ± 0.4 | 0.074 | |
| Weight (kg) | 89.3 ± 1.1 | 85.9 ± 1.2 | 0.036 | |
| Height (cm) | 164.4 ± 0.8 | 161.0 ± 0.8 | 0.002 | |
| BMI (kg/m2) | 33.0 ± 0.3 | 32.9 ± 0.3 | 0.999 | |
| WHtR | 0.684 ± 0.005 | 0.683 ± 0.005 | 0.996 | |
| Abdominal obesity (cm) | 112.3 ± 0.8 | 110.1 ± 0.9 | 0.065 | |
| Systolic blood pressure (mmHg) | <130 | 143.1 ± 1.4 | 140.6 ± 1.6 | 0.115 |
| Diastolic blood pressure (mmHg) | <85 | 83.1 ± 0.8 | 81.9 ± 0.8 | 0.111 |
| Glucose (mg/dL) | 70–110 | 122.6 ± 3.8 | 113.3 ± 1.9 | 0.401 |
| HbA1c (%) | 3.8–6.2 | 6.44 ± 0.12 | 6.06 ± 0.07 | 0.048 |
| Triglycerides (mg/dL) | <149 | 158.7 ± 6.2 | 137.9 ± 5.2 | 0.021 |
| HDL-cholesterol (mg/dL) | ≥60 | 43.4 ± 0.9 | 45.3 ± 0.9 | 0.036 |
| LDL-cholesterol (mg/dL) | <100 | 109.3 ± 3.0 | 113.3 ± 3.0 | 0.470 |
| Cholesterol total (mg/dL) | <200 | 184.4 ± 3.2 | 186.3 ± 3.4 | 0.729 |
| Uric acid (mg/dL) | 3.5–7.2 | 6.24 ± 0.13 | 6.22 ± 0.10 | 0.808 |
| Total physical activity (MET·min/week) | 2972 ± 278 | 3027 ± 211 | 0.080 |
| Low Adherence n = 45 Mean ± SEM | High Adherence n = 45 Mean ± SEM | * p-Value | |
|---|---|---|---|
| Nutrients | |||
| Trans FA (g/day) | 0.759 ± 0.042 | 0.592 ± 0.033 | 0.002 |
| w-3 FA (g/day) | 0.694 ± 0.034 | 0.885 ± 0.038 | <0.001 |
| SFA (g/day) | 29.2 ± 1.0 | 26.2 ± 0.8 | 0.017 |
| MUFA (g/day) | 52.5 ± 1.5 | 53.8 ± 1.6 | 0.584 |
| PUFA (g/day) | 18.0 ± 0.6 | 18.2 ± 0.7 | 0.892 |
| Cholesterol (mg/day) | 411.3 ± 12.8 | 379.3 ± 10.3 | 0.053 |
| Fibre (g/day) | 23.4 ± 0.8 | 29.6 ± 0.9 | <0.001 |
| Vitamins | |||
| Vitamin A (μg/day) | 1166.5 ± 56.8 | 1255.1 ± 57.4 | 0.273 |
| Vitamin C (mg/day) | 188.5 ± 7.0 | 221.6 ± 8.5 | 0.003 |
| Vitamin D (μg/day) | 4.95 ± 0.25 | 6.13± 0.29 | 0.002 |
| Vitamin E (mg/day) | 10.9 ± 0.4 | 11.2 ± 0.36 | 0.511 |
| Minerals | |||
| Selenium (μg/day) | 108.6 ± 3.2 | 114.5 ± 3.0 | 0.179 |
| Zinc (mg/day) | 12.9 ± 0.3 | 13.5 ± 0.4 | 0.233 |
| Dietary Indices | |||
| DII | 0.951 ± 0.152 | −0.400 ± 0.175 | <0.001 |
| Reference Values | Low Adherence n = 45 Mean ± SEM | High Adherence n = 45 Mean ± SEM | * p-Value | |
|---|---|---|---|---|
| Haematocrit (%) | 40.0–50.0 | 42.9 ± 0.5 | 42.7 ± 0.3 | 0.323 |
| Erythrocytes (106/mm3) | 4.5–5.8 | 4.83 ± 0.04 | 4.74 ± 0.04 | 0.133 |
| Leukocytes (103/mm3) | 4.0–11.0 | 7.58 ± 0.17 | 7.27 ± 0.17 | 0.197 |
| Neutrophils (103/mm3) | 1.8–7.5 | 4.77 ± 0.41 | 4.01 ± 0.14 | 0.145 |
| Lymphocytes (103/mm3) | 1.0–4.5 | 2.63 ± 0.20 | 2.38 ± 0.07 | 0.463 |
| Monocytes (103/mm3) | 0.0–1.0 | 0.744 ± 0.074 | 0.674 ± 0.055 | 0.303 |
| Eosinophils (103/mm3) | 0.0–0.5 | 0.264 ± 0.034 | 0.273 ± 0.045 | 0.277 |
| Basophils (103/mm3) | 0.0–0.2 | 0.057 ± 0.008 | 0.085 ± 0.032 | 0.339 |
| Low Adherence n = 45 | High Adherence n = 45 | * p-Value | |
|---|---|---|---|
| Mean ± SEM | Mean ± SEM | ||
| Protein levels | |||
| XOD (ng/mL) | 0.444 ± 0.024 | 0.336 ± 0.021 | <0.001 |
| IL-1β (pg/mL) | 10.3 ± 0.5 | 8.33 ± 0.38 | 0.035 |
| IL-6 (pg/mL) | 6.71 ± 0.71 | 3.09 ± 0.41 | 0.001 |
| IL-15 (pg/mL) | 8.98 ± 0.44 | 5.98 ± 0.66 | 0.012 |
| TNFα (pg/mL) | 67.8 ± 4.4 | 56.3 ± 2.9 | 0.015 |
| Interferon-γ (pg/mL) | 5.91 ± 0.57 | 5.47 ± 0.40 | 0.316 |
| MCP-1 (pg/mL) | 259.1 ± 12.8 | 244.5 ± 35.7 | 0.063 |
| Resistin (ng/mL) | 5.45 ± 0.92 | 4.91 ± 0.78 | 0.643 |
| Ghrelin (pg/mL) | 327.2 ± 11.5 | 271.3 ± 8.6 | 0.002 |
| Leptin (ng/mL) | 10.4 ± 1.1 | 13.2 ± 2.8 | 0.631 |
| Plasma markers | |||
| MDA (nM) | 0.922 ± 0.071 | 0.928 ± 0.066 | 0.821 |
| Polyphenols (nM) | 0.058 ± 0.002 | 0.057 ± 0.002 | 0.392 |
| Urine markers | |||
| 8-oxoGuo/Creatinine (nM/mM) | 2.01 ± 0.05 | 1.93 ± 0.06 | 0.244 |
| 8-oxodG/Creatinine (nM/mM) | 1.47 ± 0.06 | 1.39 ± 0.06 | 0.509 |
| MDA/Creatinine (mM/mM) | 102.0 ± 9.2 | 88.0 ± 7.5 | 0.159 |
| Polyphenols/Creatinine (mM/mM) | 13.6 ± 1.0 | 16.0 ± 1.1 | 0.008 |
| Correlation | |
|---|---|
| Weight | −0.178 ** |
| Height | −0.182 * |
| Abdominal obesity | −0.173 ** |
| HbA1c | −0.137 * |
| Triglycerides | −0.197 ** |
| Plasma IL-1β | −0.230 * |
| Plasma XOD | −0.228 * |
| Plasma Ghrelin | −0.319 ** |
| ROS production in PBMCs by LPS | −0.220 * |
| Urine MDA | −0.165 * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Quetglas-Llabrés, M.M.; Monserrat-Mesquida, M.; Bouzas, C.; Gómez, C.; Mateos, D.; Ripoll-Vera, T.; Tur, J.A.; Sureda, A. Inflammatory and Oxidative Stress Markers Related to Adherence to the Mediterranean Diet in Patients with Metabolic Syndrome. Antioxidants 2022, 11, 901. https://doi.org/10.3390/antiox11050901
Quetglas-Llabrés MM, Monserrat-Mesquida M, Bouzas C, Gómez C, Mateos D, Ripoll-Vera T, Tur JA, Sureda A. Inflammatory and Oxidative Stress Markers Related to Adherence to the Mediterranean Diet in Patients with Metabolic Syndrome. Antioxidants. 2022; 11(5):901. https://doi.org/10.3390/antiox11050901
Chicago/Turabian StyleQuetglas-Llabrés, Maria Magdalena, Margalida Monserrat-Mesquida, Cristina Bouzas, Cristina Gómez, David Mateos, Tomàs Ripoll-Vera, Josep A. Tur, and Antoni Sureda. 2022. "Inflammatory and Oxidative Stress Markers Related to Adherence to the Mediterranean Diet in Patients with Metabolic Syndrome" Antioxidants 11, no. 5: 901. https://doi.org/10.3390/antiox11050901
APA StyleQuetglas-Llabrés, M. M., Monserrat-Mesquida, M., Bouzas, C., Gómez, C., Mateos, D., Ripoll-Vera, T., Tur, J. A., & Sureda, A. (2022). Inflammatory and Oxidative Stress Markers Related to Adherence to the Mediterranean Diet in Patients with Metabolic Syndrome. Antioxidants, 11(5), 901. https://doi.org/10.3390/antiox11050901










