Ex Vivo Lung Perfusion with β-Nicotinamide Adenine Dinucleotide (NAD+) Improves Ischemic Lung Function
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals
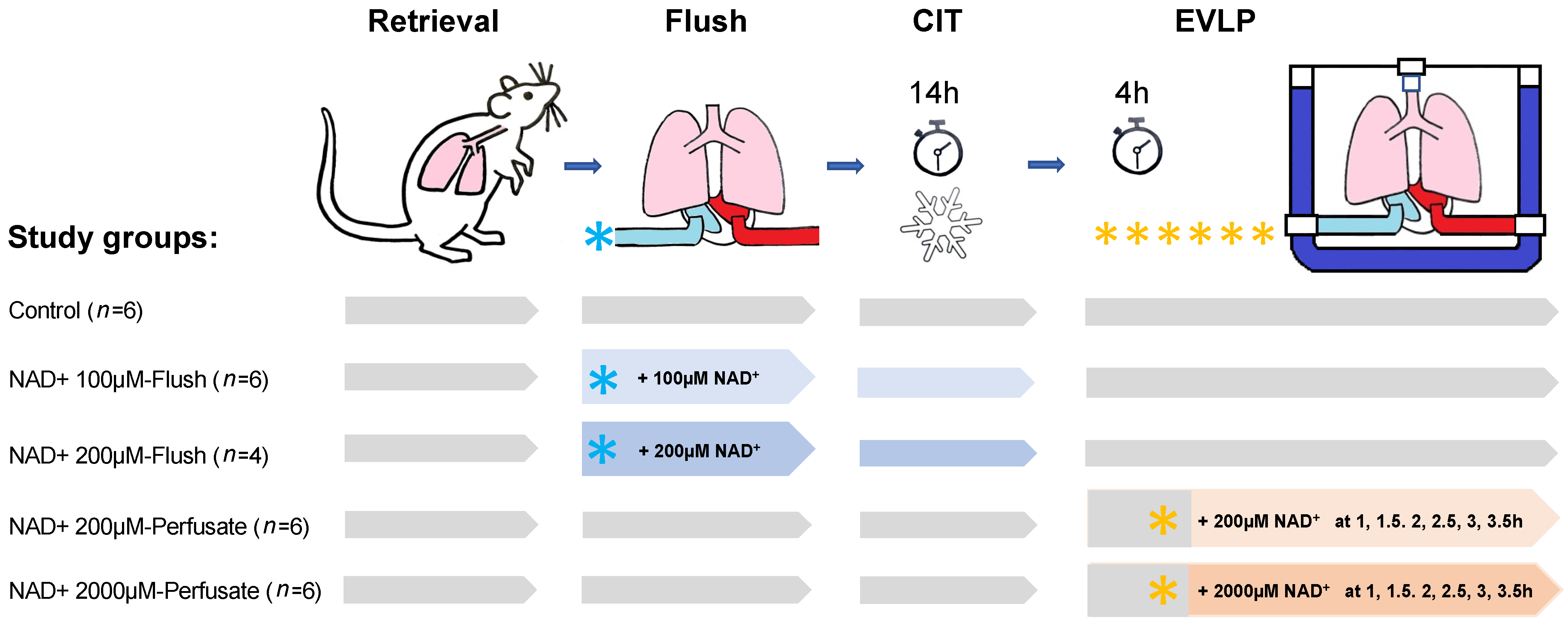
2.2. Surgical Techniques for Procurement of Lungs and EVLP Model
2.3. EVLP Procedure and Physiological Variables
2.4. Methodology for NAD+ Application
2.5. Clinical Biochemistry Parameters
2.6. Thiazolyl Blue Tetrazolium Bromide Viability Assay (MTT) with a Rat Epithelial Cell Line for Non-Toxic Dosage of NAD+ Concentrations
2.7. Detection of Cytokines and Chemokines in Perfusate and BAL
2.8. Estimates of Myeloperoxidase Activity in Lung Tissues
2.9. Statistical Method
3. Results
3.1. In Vitro Assay to Determine the Non-Toxic Concentration of NAD+ for Use in the Preservation Solution
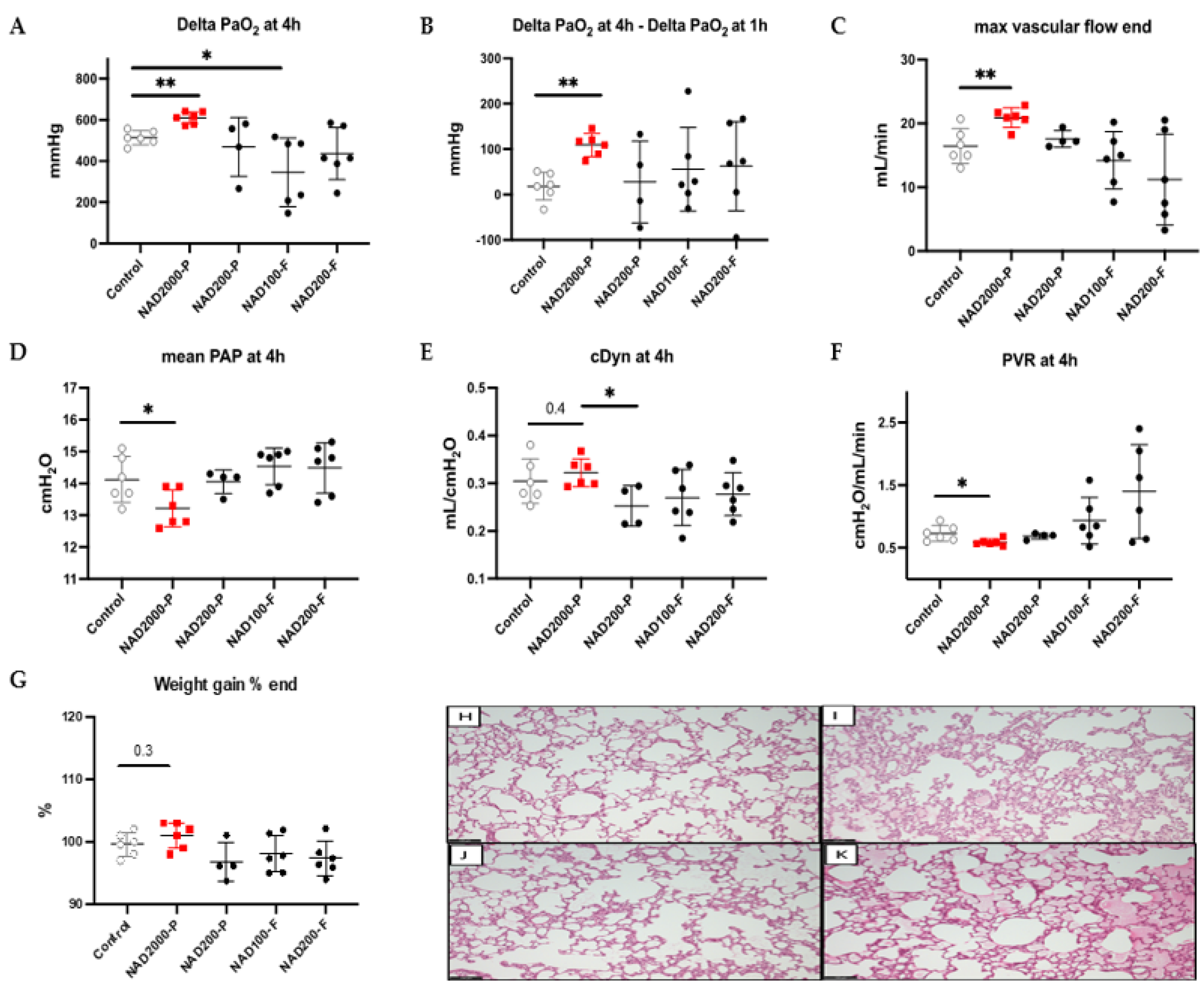
3.2. Lung Physiology with NAD+ in the Preservation Solution during EVLP
3.3. NAD+ in the Perfusate
3.3.1. Lung Physiology during EVLP with NAD+ 200 µM Perfusate
3.3.2. Lung Physiology during EVLP with NAD+ 2000 µM Perfusate
Lung Tissue Biochemical Measurements in NAD+ 2000 µM Perfusate
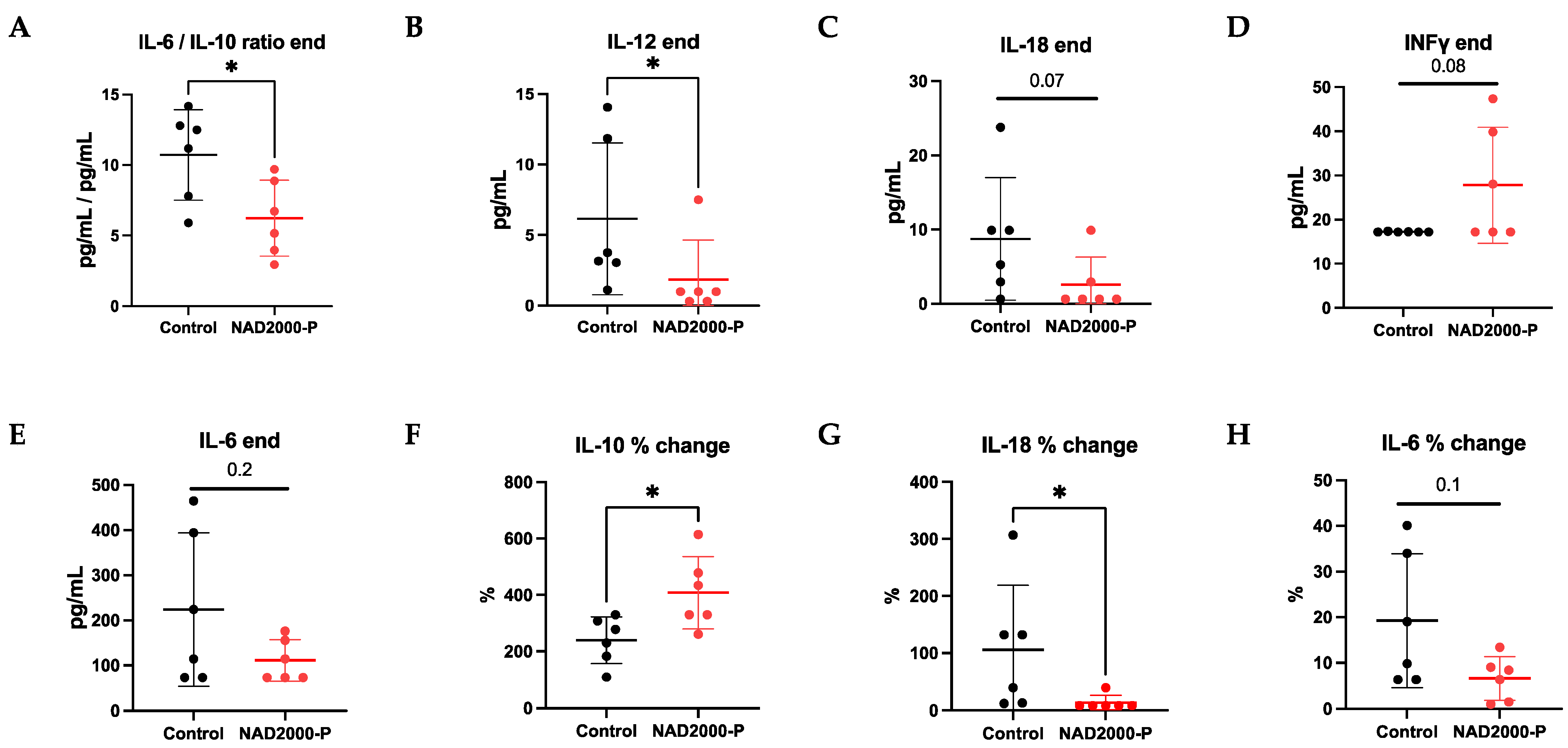
Perfusate Cytokines and Chemokines in NAD+ 2000 µM Perfusate
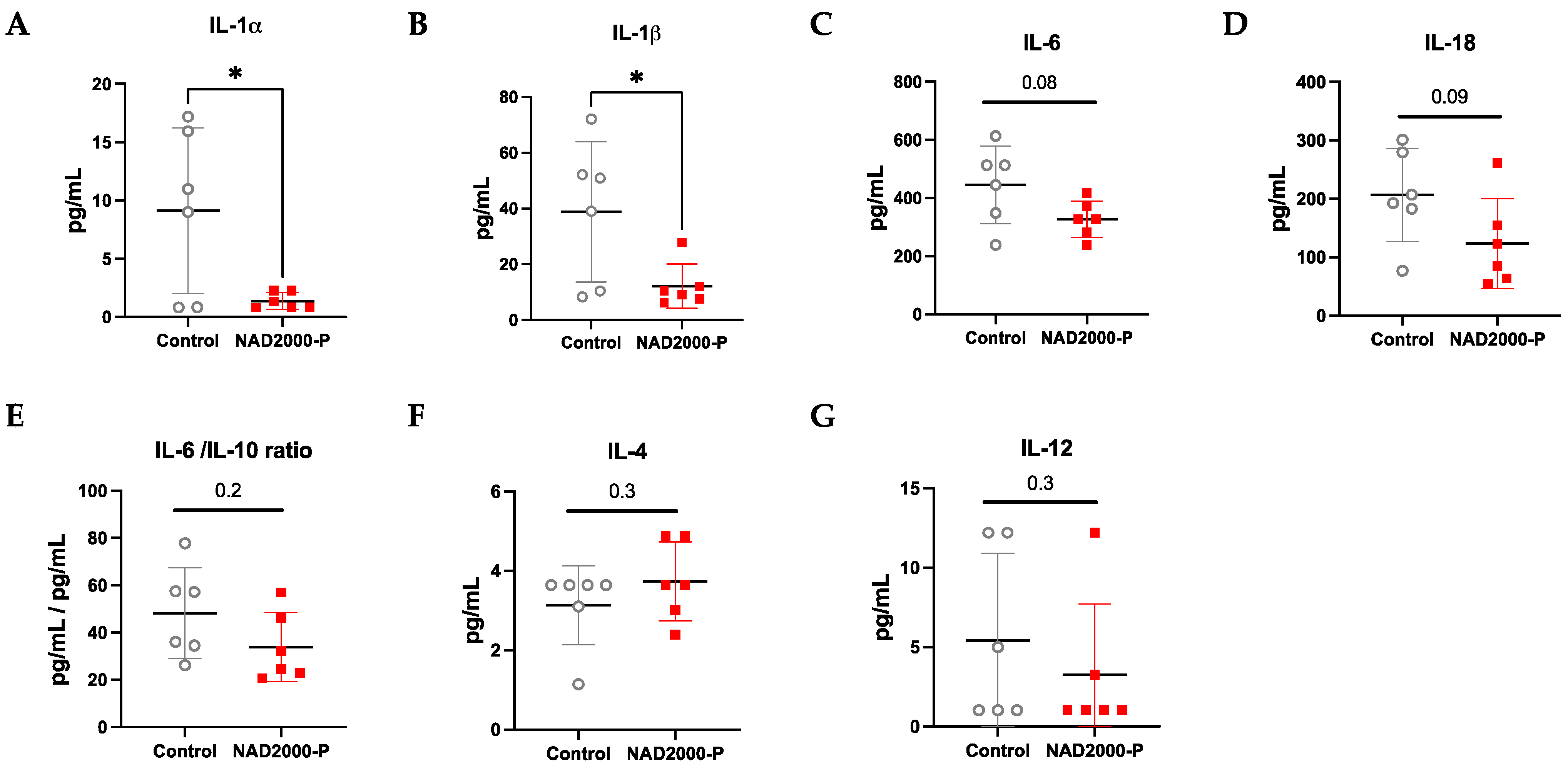
BAL Cytokines and Chemokines in NAD+ 2000 µM Perfusate
Biochemical Measurements in NAD+ 2000 µM Perfusate
4. Discussion
4.1. NAD+ May Reduce Oxidative Stress and Reset Cell Homeostasis
4.2. NAD+ May Directly Act against Hypoxia-Induced Vasoconstriction
4.3. NAD+ Reduces the Pro-Inflammatory Milieu
4.4. No Beneficial Impact of NAD+ in the Preservation Solution
4.5. Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| BAL | bronchoalveolar lavage |
| cDyn | dynamic lung compliance |
| CIT | cold ischemic time |
| DAMPS | damage-associated molecular patterns |
| EVLP | ex vivo lung perfusion |
| FiO2 | fraction of inspired oxygen |
| IRI | ischemia-reperfusion injury |
| MPO | myeloperoxidase |
| MTT | Thiazolyl Blue Tetrazolium Bromide Viability Assay |
| NAD+ | β-nicotinamide adenine dinucleotide |
| PAP | pulmonary arterial pressure |
| PEEP | positive end-expiratory pressure |
| PVR | pulmonary vascular resistance |
| ROS | reactive oxygen species |
References
- Chambers, D.C.; Yusen, R.D.; Cherikh, W.S.; Goldfarb, S.B.; Kucheryavaya, A.Y.; Khusch, K.; Levvey, B.J.; Lund, L.H.; Meiser, B.; Rossano, J.W.; et al. The Registry of the International Society for Heart and Lung Transplantation: Thirty-fourth Adult Lung And Heart-Lung Transplantation Report-2017; Focus Theme: Allograft ischemic time. J. Heart Lung Transpl. 2017, 36, 1047–1059. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Laubach, V.E.; Sharma, A.K. Mechanisms of lung ischemia-reperfusion injury. Curr. Opin. Organ Transpl. 2016, 21, 246–252. [Google Scholar] [CrossRef] [Green Version]
- Ferrari, R.S.; Andrade, C.F. Oxidative Stress and Lung Ischemia-Reperfusion Injury. Oxid. Med. Cell Longev. 2015, 2015, 590987. [Google Scholar] [CrossRef] [PubMed]
- den Hengst, W.A.; Gielis, J.F.; Lin, J.Y.; Van Schil, P.E.; De Windt, L.J.; Moens, A.L. Lung ischemia-reperfusion injury: A molecular and clinical view on a complex pathophysiological process. Am. J. Physiol. Heart Circ. Physiol. 2010, 299, H1283–H1299. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Talaie, T.; DiChiacchio, L.; Prasad, N.K.; Pasrija, C.; Julliard, W.; Kaczorowski, D.J.; Zhao, Y.; Lau, C.L. Ischemia-reperfusion Injury in the Transplanted Lung: A Literature Review. Transpl. Direct 2021, 7, e652. [Google Scholar] [CrossRef]
- Porteous, M.K.; Diamond, J.M.; Christie, J.D. Primary graft dysfunction: Lessons learned about the first 72 h after lung transplantation. Curr. Opin. Organ. Transpl. 2015, 20, 506–514. [Google Scholar] [CrossRef] [Green Version]
- Bharat, A.; Kuo, E.; Steward, N.; Aloush, A.; Hachem, R.; Trulock, E.P.; Patterson, G.A.; Meyers, B.F.; Mohanakumar, T. Immunological link between primary graft dysfunction and chronic lung allograft rejection. Ann. Thorac. Surg. 2008, 86, 189–195; discussion 196–187. [Google Scholar] [CrossRef] [Green Version]
- Prasad, N.K.; Pasrija, C.; Talaie, T.; Krupnick, A.S.; Zhao, Y.; Lau, C.L. Ex Vivo Lung Perfusion: Current Achievements and Future Directions. Transplantation 2021, 105, 979–985. [Google Scholar] [CrossRef]
- Xie, N.; Zhang, L.; Gao, W.; Huang, C.; Huber, P.E.; Zhou, X.; Li, C.; Shen, G.; Zou, B. NAD+ metabolism: Pathophysiologic mechanisms and therapeutic potential. Signal Transduct. Target Ther. 2020, 5, 227. [Google Scholar] [CrossRef]
- Navarro, M.N.; Gomez de Las Heras, M.M.; Mittelbrunn, M. Nicotinamide adenine dinucleotide metabolism in the immune response, autoimmunity and inflammageing. Br. J. Pharmacol. 2021, 179, 1839–1856. [Google Scholar] [CrossRef]
- Dunham-Snary, K.J.; Wu, D.; Sykes, E.A.; Thakrar, A.; Parlow, L.R.G.; Mewburn, J.D.; Parlow, J.L.; Archer, S.L. Hypoxic Pulmonary Vasoconstriction: From Molecular Mechanisms to Medicine. Chest 2017, 151, 181–192. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tejero, J.; Stuehr, D. Tetrahydrobiopterin in nitric oxide synthase. IUBMB Life 2013, 65, 358–365. [Google Scholar] [CrossRef] [PubMed]
- Smith, B.C.; Underbakke, E.S.; Kulp, D.W.; Schief, W.R.; Marletta, M.A. Nitric oxide synthase domain interfaces regulate electron transfer and calmodulin activation. Proc. Natl. Acad. Sci. USA 2013, 110, E3577–E3586. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tullius, S.G.; Biefer, H.R.; Li, S.; Trachtenberg, A.J.; Edtinger, K.; Quante, M.; Krenzien, F.; Uehara, H.; Yang, X.; Kissick, H.T.; et al. NAD+ protects against EAE by regulating CD4+ T-cell differentiation. Nat. Commun. 2014, 5, 5101. [Google Scholar] [CrossRef]
- Rodriguez Cetina Biefer, H.; Heinbokel, T.; Uehara, H.; Camacho, V.; Minami, K.; Nian, Y.; Koduru, S.; El Fatimy, R.; Ghiran, I.; Trachtenberg, A.J.; et al. Mast cells regulate CD4+ T-cell differentiation in the absence of antigen presentation. J. Allergy Clin. Immunol. 2018, 142, 1894–1908.e7. [Google Scholar] [CrossRef] [Green Version]
- Elkhal, A.; Rodriguez Cetina Biefer, H.; Heinbokel, T.; Uehara, H.; Quante, M.; Seyda, M.; Schuitenmaker, J.M.; Krenzien, F.; Camacho, V.; de la Fuente, M.A.; et al. NAD+ regulates Treg cell fate and promotes allograft survival via a systemic IL-10 production that is CD4+ CD25+ Foxp3+ T cells independent. Sci. Rep. 2016, 6, 22325. [Google Scholar] [CrossRef] [Green Version]
- Yamamoto, T.; Byun, J.; Zhai, P.; Ikeda, Y.; Oka, S.; Sadoshima, J. Nicotinamide mononucleotide, an intermediate of NAD+ synthesis, protects the heart from ischemia and reperfusion. PLoS ONE 2014, 9, e98972. [Google Scholar] [CrossRef] [Green Version]
- Biefer, H.R.C.; Elkhal, A.; Cesarovic, N.; Emmert, M.Y. NAD+ the disregarded molecule in cardiac metabolism. Eur. Heart J. 2020, 41, 983–986. [Google Scholar] [CrossRef] [Green Version]
- Canto, C.; Menzies, K.J.; Auwerx, J. NAD+ Metabolism and the Control of Energy Homeostasis: A Balancing Act between Mitochondria and the Nucleus. Cell Metab. 2015, 22, 31–53. [Google Scholar] [CrossRef] [Green Version]
- Council, N.R. Guide for the Care and Use of Laboratory Animals, 8th ed.; The National Academies Press: Washington, DC, USA, 2011; p. 246. [Google Scholar] [CrossRef]
- Ohsumi, A.; Kanou, T.; Ali, A.; Guan, Z.; Hwang, D.M.; Waddell, T.K.; Juvet, S.; Liu, M.; Keshavjee, S.; Cypel, M. A method for translational rat ex vivo lung perfusion experimentation. Am. J. Physiol. Lung Cell Mol. Physiol. 2020, 319, L61–L70. [Google Scholar] [CrossRef]
- Arni, S.; Maeyashiki, T.; Citak, N.; Opitz, I.; Inci, I. Subnormothermic Ex Vivo Lung Perfusion Temperature Improves Graft Preservation in Lung Transplantation. Cells 2021, 10, 748. [Google Scholar] [CrossRef] [PubMed]
- Arni, S.; Maeyashiki, T.; Latshang, T.; Opitz, I.; Inci, I. Ex Vivo Lung Perfusion with K(ATP) Channel Modulators Antagonize Ischemia Reperfusion Injury. Cells 2021, 10, 2296. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Ma, Y.; Kong, X.; Ding, X.; Gu, H.; Chu, T.; Ying, W. NAD+ administration decreases doxorubicin-induced liver damage of mice by enhancing antioxidation capacity and decreasing DNA damage. Chem. Biol. Interact. 2014, 212, 65–71. [Google Scholar] [CrossRef]
- Baixauli, F.; Acin-Perez, R.; Villarroya-Beltri, C.; Mazzeo, C.; Nunez-Andrade, N.; Gabande-Rodriguez, E.; Ledesma, M.D.; Blazquez, A.; Martin, M.A.; Falcon-Perez, J.M.; et al. Mitochondrial Respiration Controls Lysosomal Function during Inflammatory T Cell Responses. Cell Metab. 2015, 22, 485–498. [Google Scholar] [CrossRef] [Green Version]
- Dunham-Snary, K.J.; Wu, D.; Potus, F.; Sykes, E.A.; Mewburn, J.D.; Charles, R.L.; Eaton, P.; Sultanian, R.A.; Archer, S.L. Ndufs2, a Core Subunit of Mitochondrial Complex I, Is Essential for Acute Oxygen-Sensing and Hypoxic Pulmonary Vasoconstriction. Circ. Res. 2019, 124, 1727–1746. [Google Scholar] [CrossRef] [PubMed]
- Weigt, S.S.; Palchevskiy, V.; Belperio, J.A. Inflammasomes and IL-1 biology in the pathogenesis of allograft dysfunction. J. Clin. Investig. 2017, 127, 2022–2029. [Google Scholar] [CrossRef]
- Hecker, A.; Kullmar, M.; Wilker, S.; Richter, K.; Zakrzewicz, A.; Atanasova, S.; Mathes, V.; Timm, T.; Lerner, S.; Klein, J.; et al. Phosphocholine-Modified Macromolecules and Canonical Nicotinic Agonists Inhibit ATP-Induced IL-1beta Release. J. Immunol. 2015, 195, 2325–2334. [Google Scholar] [CrossRef]
- Hiller, S.D.; Heldmann, S.; Richter, K.; Jurastow, I.; Kullmar, M.; Hecker, A.; Wilker, S.; Fuchs-Moll, G.; Manzini, I.; Schmalzing, G.; et al. beta-Nicotinamide Adenine Dinucleotide (beta-NAD) Inhibits ATP-Dependent IL-1beta Release from Human Monocytic Cells. Int. J. Mol. Sci. 2018, 19, 1126. [Google Scholar] [CrossRef] [Green Version]
- Minhas, P.S.; Liu, L.; Moon, P.K.; Joshi, A.U.; Dove, C.; Mhatre, S.; Contrepois, K.; Wang, Q.; Lee, B.A.; Coronado, M.; et al. Macrophage de novo NAD+ synthesis specifies immune function in aging and inflammation. Nat. Immunol. 2019, 20, 50–63. [Google Scholar] [CrossRef]
- Cameron, A.M.; Castoldi, A.; Sanin, D.E.; Flachsmann, L.J.; Field, C.S.; Puleston, D.J.; Kyle, R.L.; Patterson, A.E.; Hassler, F.; Buescher, J.M.; et al. Inflammatory macrophage dependence on NAD+ salvage is a consequence of reactive oxygen species-mediated DNA damage. Nat. Immunol. 2019, 20, 420–432. [Google Scholar] [CrossRef]
- Adriouch, S.; Hubert, S.; Pechberty, S.; Koch-Nolte, F.; Haag, F.; Seman, M. NAD+ released during inflammation participates in T cell homeostasis by inducing ART2-mediated death of naive T cells in vivo. J. Immunol. 2007, 179, 186–194. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haag, F.; Freese, D.; Scheublein, F.; Ohlrogge, W.; Adriouch, S.; Seman, M.; Koch-Nolte, F. T cells of different developmental stages differ in sensitivity to apoptosis induced by extracellular NAD. Dev. Immunol. 2002, 9, 197–202. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, Z.X.; Azhipa, O.; Okamoto, S.; Govindarajan, S.; Dennert, G. Extracellular nicotinamide adenine dinucleotide induces t cell apoptosis in vivo and in vitro. J. Immunol. 2001, 167, 4942–4947. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hubert, S.; Rissiek, B.; Klages, K.; Huehn, J.; Sparwasser, T.; Haag, F.; Koch-Nolte, F.; Boyer, O.; Seman, M.; Adriouch, S. Extracellular NAD+ shapes the Foxp3+ regulatory T cell compartment through the ART2-P2X7 pathway. J. Exp. Med. 2010, 207, 2561–2568. [Google Scholar] [CrossRef] [PubMed]
- Van Gool, F.; Galli, M.; Gueydan, C.; Kruys, V.; Prevot, P.P.; Bedalov, A.; Mostoslavsky, R.; Alt, F.W.; De Smedt, T.; Leo, O. Intracellular NAD levels regulate tumor necrosis factor protein synthesis in a sirtuin-dependent manner. Nat. Med. 2009, 15, 206–210. [Google Scholar] [CrossRef] [Green Version]
- Sigma-Aldrich. Media Formulations. 2022. Available online: https://www.sigmaaldrich.com/deepweb/assets/sigmaaldrich/product/documents/345/899/n3014pis.pdf (accessed on 24 April 2022).
- Noda, K.; Tane, S.; Haam, S.J.; D’Cunha, J.; Hayanga, A.J.; Luketich, J.D.; Shigemura, N. Targeting Circulating Leukocytes and Pyroptosis During Ex Vivo Lung Perfusion Improves Lung Preservation. Transplantation 2017, 101, 2841–2849. [Google Scholar] [CrossRef]


| Perfusate | BAL | |||
|---|---|---|---|---|
| Control | NAD+ 2000-P | Control | NAD+ 2000-P | |
| (n = 6) | (n = 6) | (n = 6) | (n = 6) | |
| EGF | 0.041 (0.029) | 0.027 (0.001) | 0.612 (0.516) | 0.621 (0.415) |
| Eotaxin | 0 | 0 | 0 | 0 |
| Fractalkine | 19.03 (9.53) | 19.87 (10.00) | 225.1 (138.5) | 349.2 (235.2) |
| G-CSF | 0 | 0 | 0 | 0 |
| GM-CSF | 6.751 (7.84) | 14.80 (13.38) | 0 | 0 |
| GRO/KC | 3100 (3509) | 1780 (1936) | 3913 (1599) | 2854 (1870) |
| IFN-γ | 17.24 (0.07) | 27.80 (13.16) | 0 | 0 |
| IL1-α | 4.913 (4.721) | 9.237 (10.63) | 9.128 (7.104) * | 1.377 (0.701) * |
| IL1-β | 9.662 (4.117) | 9.062 (5.177) | 38.81 (25.18) * | 12.17 (7.93) * |
| IL-2 | 0 | 0 | 0 | 0 |
| IL-4 | 3.222 (2.030) | 3.430 (1.285) | 3.135 (0.997) | 3.740 (0.998) |
| IL-5 | 7.678 (3.174) | 7.582 (3.520) | 0 | 0 |
| IL-6 | 223.9 (169.9) | 110.9 (45.95) | 445.4 (133.7) | 327.3 (63.0) |
| IL-10 | 18.93 (10.43) | 18.38 (3.69) | 9.962 (3.401) | 10.67 (3.249) |
| IL-12 (p70) | 6.160 (5.377) * | 1.848 (2.784) * | 5.418 (5.481) | 3.263 (4.472) |
| IL-13 | 2.771 (2.313) | 1.708 (1.771) | 2.417 (2.468) | 1.185 (0.886) |
| IL-17A | 0 | 0 | 0 | 0 |
| IL-18 | 8.740 (8.247) | 2.565 (3.711) | 206.8 (79.9) | 123.6 (77.0) |
| IP10 | 543.8 (62.6) | 588.5 (101.2) | 783.8 (271.2) | 893.8 (277.2) |
| Leptin | 412.8 (106.8) | 425.5 (171.8) | 2.233 (1.164) | 4.426 (4.622) |
| LIX | 35.91 (3.56) | 29.84 (7.65) | 353.2 (197.7) | 309.9 (215.0) |
| MCP-1 | 202.3 (86.2) | 247.6 (65.3) | 174.8 (117.1) | 192.8 (123.8) |
| MIP1-α | 482.3 (265.3) | 409.0 (165.0) | 685.2 (224.0) | 552.3 (299.8) |
| MIP-2 | 756.5 (843.8) | 493.9 (478.5) | 5740 (3263) | 6107 (3281) |
| Rantes | 44.33 (23.94) | 33.24 (8.94) | 5.457 (4.735) | 4.130 (1.584) |
| TNF-α | 29.73 (19.56)(0.0) * | 22.10 (4.79) | 18.91 (14.68) | 23.40 (19.58) |
| VEGF | 2.850 (3.231) | 3.175 (2.229) | 554.9 (294.4) | 826.4 (361.3) |
| Control | 2000 µM NAD+ | ||
|---|---|---|---|
| (n = 6) | (n = 6) | p-value | |
| Potassium | 3.296 (0.078) | 3.217 (0.062) | ns |
| Calcium | 0.677 (0.003) | 0.691 (0.003) | ns |
| Chlorine | 92.96 (2.30) | 93.75 (1.66) | ns |
| Lactate | <0.3 | <0.3 | ns |
| Glucose | 143.7 (8.5) | 140.0 (5.7) | ns |
| pH | 7.093 (0.023) | 7.069 (0.010) | ns |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ehrsam, J.P.; Chen, J.; Rodriguez Cetina Biefer, H.; Opitz, I.; Arni, S.; Inci, I. Ex Vivo Lung Perfusion with β-Nicotinamide Adenine Dinucleotide (NAD+) Improves Ischemic Lung Function. Antioxidants 2022, 11, 843. https://doi.org/10.3390/antiox11050843
Ehrsam JP, Chen J, Rodriguez Cetina Biefer H, Opitz I, Arni S, Inci I. Ex Vivo Lung Perfusion with β-Nicotinamide Adenine Dinucleotide (NAD+) Improves Ischemic Lung Function. Antioxidants. 2022; 11(5):843. https://doi.org/10.3390/antiox11050843
Chicago/Turabian StyleEhrsam, Jonas Peter, Jin Chen, Hector Rodriguez Cetina Biefer, Isabelle Opitz, Stephan Arni, and Ilhan Inci. 2022. "Ex Vivo Lung Perfusion with β-Nicotinamide Adenine Dinucleotide (NAD+) Improves Ischemic Lung Function" Antioxidants 11, no. 5: 843. https://doi.org/10.3390/antiox11050843
APA StyleEhrsam, J. P., Chen, J., Rodriguez Cetina Biefer, H., Opitz, I., Arni, S., & Inci, I. (2022). Ex Vivo Lung Perfusion with β-Nicotinamide Adenine Dinucleotide (NAD+) Improves Ischemic Lung Function. Antioxidants, 11(5), 843. https://doi.org/10.3390/antiox11050843







