Abstract
Antioxidants are compounds that normally prevent lipid and protein oxidation. They play a major role in preventing many adverse conditions in the human body, including inflammation and cancer. Synthetic antioxidants are widely used in the food industry to prevent the production of adverse compounds that harm humans. However, plant- and animal-based antioxidants are more appealing to consumers than synthetic antioxidants. Plant-based antioxidants are mainly phenolic compounds, carotenoids, and vitamins, while animal-based antioxidants are mainly whole protein or the peptides of meat, fish, egg, milk, and plant proteins. Plant-based antioxidants mainly consist of aromatic rings, while animal-based antioxidants mainly consist of amino acids. The phenolic compounds and peptides act differently in preventing oxidation and can be used in the food and pharmaceutical industries. Therefore, compared with animal-based antioxidants, plant-based compounds are more practical in the food industry. Even though plant-based antioxidant compounds are good sources of antioxidants, animal-based peptides (individual peptides) cannot be considered antioxidant compounds to add to food. However, they can be considered an ingredient that will enhance the antioxidant capacity. This review mainly compares plant- and animal-based antioxidants’ structure, efficacy, mechanisms, and applications.
1. Introduction
Many modern non-communicable diseases are related to oxidative stress, mainly generated by the imbalance between the formation and neutralization of the prooxidants [1]. Food proteins are good sources of antioxidants [2]. Antioxidants are natural or synthetic compounds that inhibit or delay the oxidation process at relatively low concentrations. They can be divided into primary and secondary compounds [3,4,5]. Various plants and vegetables are good sources of natural antioxidants such as vitamin C, carotenoids, anthocyanins, and phenols [4,6,7,8]. Antioxidants can interact with free radicals before damaging the host cells. The function of antioxidants can be explained in several mechanisms: scavenging free radicals that initiate and propagate peroxidation, chelating metal ions that accelerate the oxidation process, quenching ●O2− ions to prevent the formation of hydroxyl radical, breaking the auto-oxidation chain reaction, and lowering the localized O2 concentration in the product [7]. These antioxidants are considered safe products to add to any food product at the industrial level [9].
In the process, maintaining the nutritional value of the basic foods is very important. However, most food components containing fat and oils are subjected to oxidation during processing and storage. Lipid oxidation in processed food is one of the most common problems humans face. Various approaches have been taken to reduce oxidative stress and aging of the cells; adding antioxidants to foods is one of the main methods [7,10].
Phenolic compounds from plants can protect humans against cancer and many other diseases, including cardiovascular and inflammatory disorders. Therefore, adding the phenolic compounds to the food system is widely used to prevent various human diseases [1,8,11]. Vitamin C is a common natural antioxidant that reduces the serum uric acid level, which causes gout, and reduces the risk of having a stroke, and chronic and degenerative diseases. Vitamin E (tocopherol) is another antioxidant that protects the cell membrane, prevents lipid peroxidation, and chelates reactive oxygen species (ROS). Carotenoids, lutein, zeaxanthin, and lycopene reduce cancer and metabolic diseases. In addition to antioxidants, flavones also act as anti-inflammatory agents [7].
These natural antioxidants are used in many indigenous medicines and traditional foods. Natural antioxidants are widely used to prevent oxidative stress in meat and meat products. These natural compounds prevent lipid oxidation in meat products and improve the product’s physicochemical, chemical, and sensory properties [9]. Plants seeds rich in polyphenols have been used as natural antioxidants for a long time. The phenolic compounds are present in the coat of the seeds in many fruits, cereals, and legumes [10]. These natural antioxidants are good replacements for the synthetic antioxidants used in the food industry. Natural antioxidant compounds, such as anthocyanin, improve the color of the meat product while they are responsible for the oxidative stability in the meat product [9]. Furthermore, these natural antioxidant compounds can improve the sensory properties of meat and meat products. They are used to reduce the off-odor and off-flavor while improving the color of the processed meat [12]. These antioxidant compounds are used as anticancer agents with low toxicity and specificity and high availability. Combining these compounds with food components can have cytoprotective effects on normal cells and cytotoxic effects on cancer cells [10]. Many plant-based products are in traditional Chinese, Islamic, and ancient Rome medicines and the herbal teas they drink. Plant products taken from pine trees, including the bark, shoots, and resins, treat urinary tract diseases, digestive tract disorders, and problems associated with the nerve system, respiratory system, and skin problems [13].
A high level of reactive oxygen species (ROS) produced in the muscle tissues can increase the release of stress hormones into the bloodstream which stimulate the release of enzymes, cortisol, and catecholamines. Oxidation can lead to the formation of off-flavors and nutrient losses while reducing the shelf life of the products. Antioxidants are important to reduce oxidative stress in live animals and prevent the development of the off-flavor and rancid odor, discoloration, and accumulation of toxic compounds in meat during processing and storage.
Providing antioxidants to animals before transporting them to the slaughterhouse showed better quality meat production since it reduced the stress during loading and unloading and transportation even with poor road conditions [12]. Natural antioxidants can be used as antimicrobial agents to control foodborne pathogens such as Salmonella. These compounds can be used in several meat-based products, especially beef, poultry, and pork, to control foodborne pathogens [14]. Therefore, this review aims to compare the plant-based and animal-based antioxidants, their structure, and potential use in the food industry.
2. Antioxidants from Plant Sources
Plants contain many natural compounds that have antioxidant activity. These compounds can be categorized into vitamins (Vitamin C and E), polyphenols (flavonoids, phenolic acids, stilbenes, lignans), and terpenoid groups. Fruits and vegetables are rich sources of vitamin C and E. Among the fruits are the family Rosaceae (sour cherry, strawberry, blackberry), Empetraceae (cowberry), Ticaceae (blueberry), Asteraceae (sunflower seed), and Punicaceae (pomegranate), which are rich sources of these vitamins. Broccoli, brussels sprouts, green cabbage, tomatoes, cauliflowers, lettuce, and leeks are vegetable groups with high vitamin C and E. Vitamins in plants act as primary antioxidant substances [15]: Vitamin E acts as an essential lipid-soluble antioxidant, while vitamin C protects against oxidative stress-induced cellular damages [16,17]. Both vitamin E and vitamin C are used as antioxidants in foods, but the effect of vitamin C is marginal.
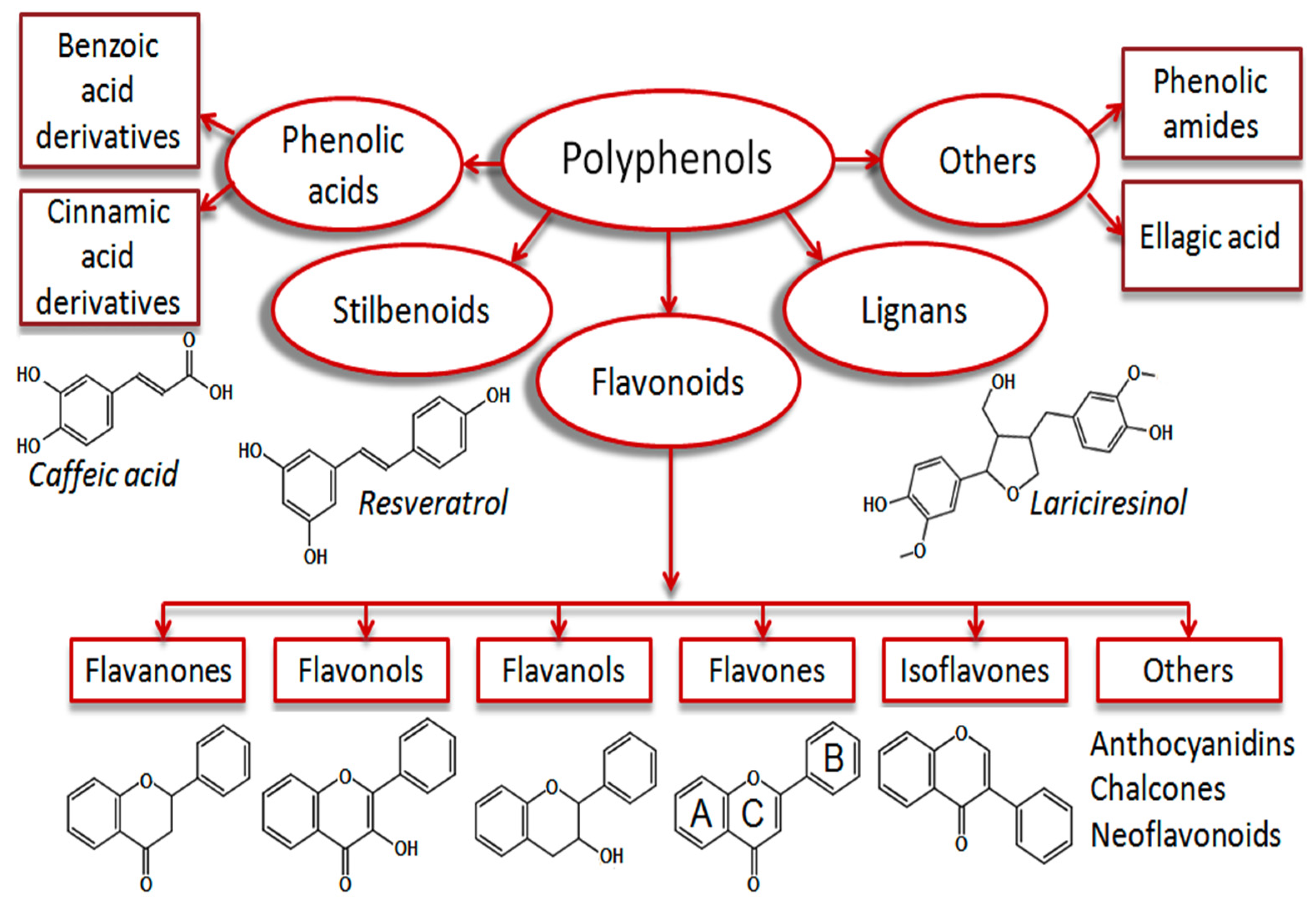
Polyphenols are the major plant antioxidants with various structural and functional characteristics and biological properties [11,18,19]. Phenolic compounds synthesize from phenylalanine or tyrosine through the shikimic acid pathway. They can vary from simple compounds to conjugated complex substances. These compounds vary from 500 to 4000 Da molecular weight, and over 12 phenolic hydroxyl groups are found among the phenolic compounds [4,20]. They can be subcategorized into phenolic acids, flavonoids, stilbenes, and lignans [6,21]. They are found in plant foods such as fruits, cereals, seeds, berries, and plant-based products such as wine, tea, and vegetable oils [22,23]. The summary of the breakdown of the phenolic acids is shown in Figure 1.
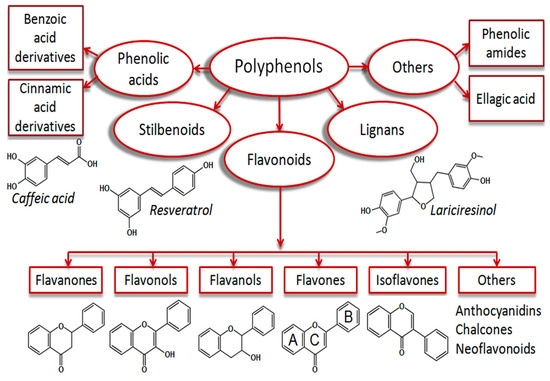
Figure 1.
Plant phenolic compound breakdown. This Figure is reproduced from ref. [23]. Copyright 2019 (Dirimanov & Högger, 2019).
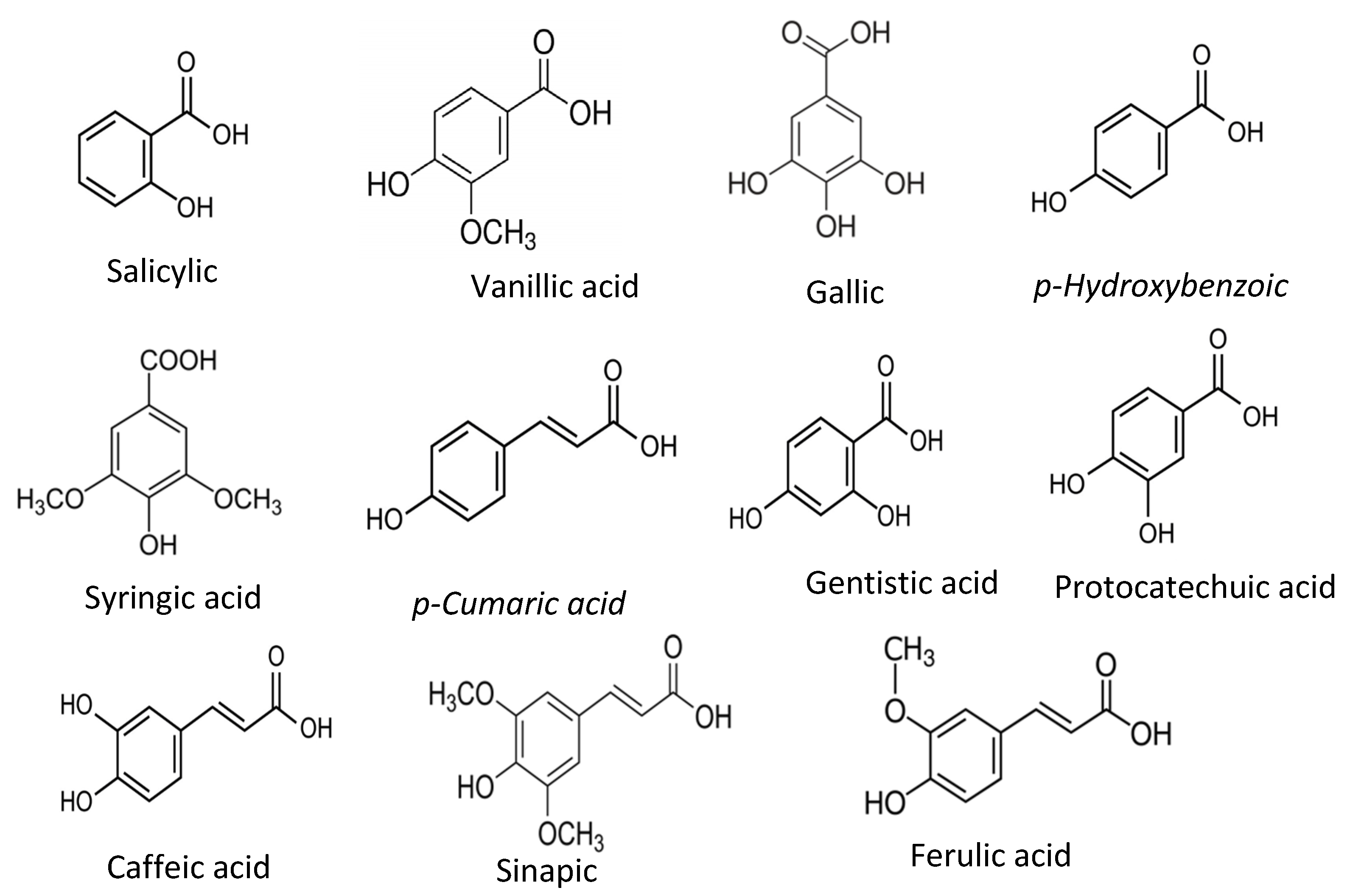
Phenolic acids are the derivatives of benzoic acids and cinnamic acids, and salicylic acid, gentisic acid, p-Hydoxybenzoic acid, protocatechuic acid, vanillic acid, syringic acid, gallic acid, p-coumaric acid, ferulic acid, caffeic acid, and sinapic acid are among the most common phenolic compounds present in the food plants and common phenolic acid compounds illustrated in Figure 2.
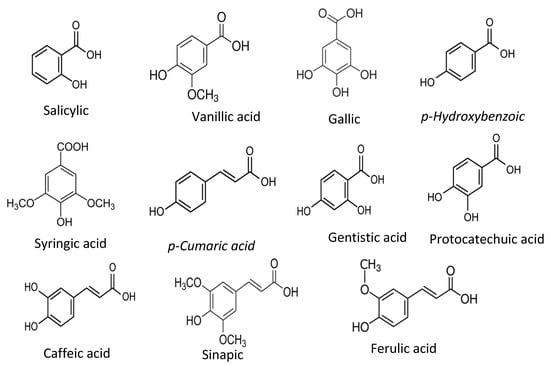
Figure 2.
Common phenolic acids found in food plants.
The antioxidant activities of those phenolic acids are confirmed by several antioxidant assays, including the DPPH radical scavenging assay, ABTS radical scavenging activity, and β-carotene assay [5]. The radical scavenging activities of these phenolic acids mainly depend on the hydroxy moieties attached to the phenyl rings of benzoic and cinnamic acids [24,25].
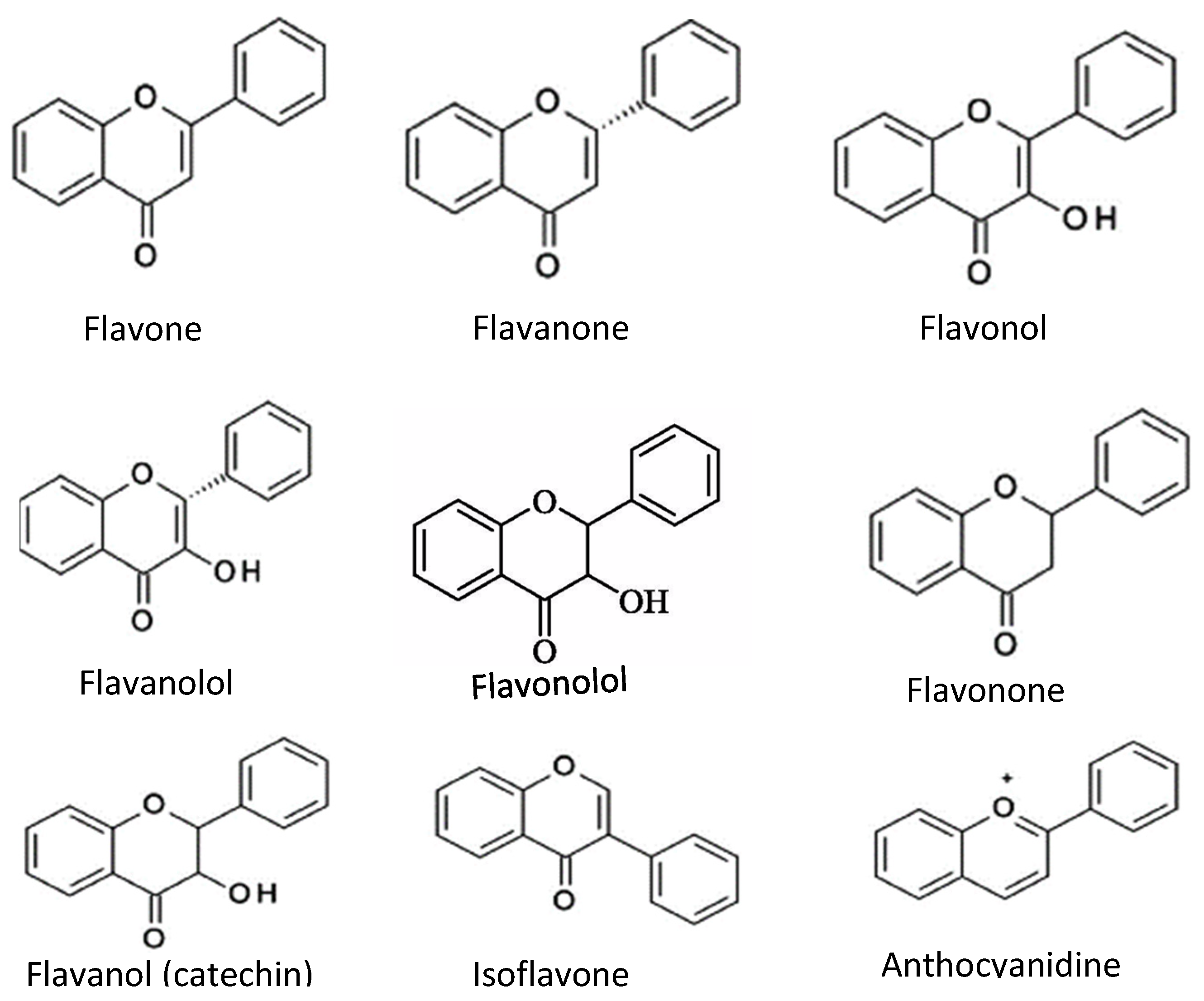
Flavonoids consist of two outer aromatic rings with three carbon rings. These flavonoids include flavone, flavanol, flavanone, flavanonol, flavonone, flavononol, flavanol (catechin), isoflavone, and anthocyanidin (Figure 3) [6]. The derivatives of the flavonoids have inhibitory properties against acetylcholinesterase [26].
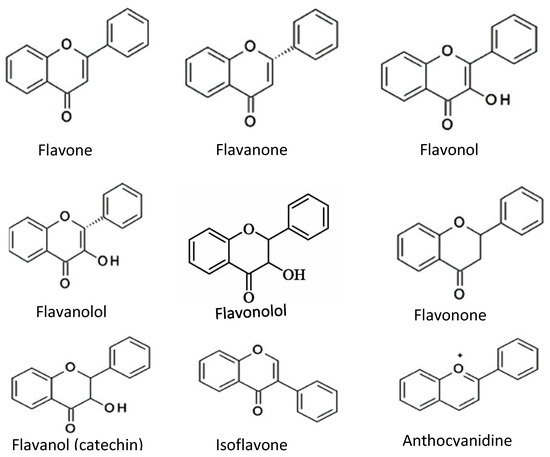
Figure 3.
Chemical structures of common flavonoids.
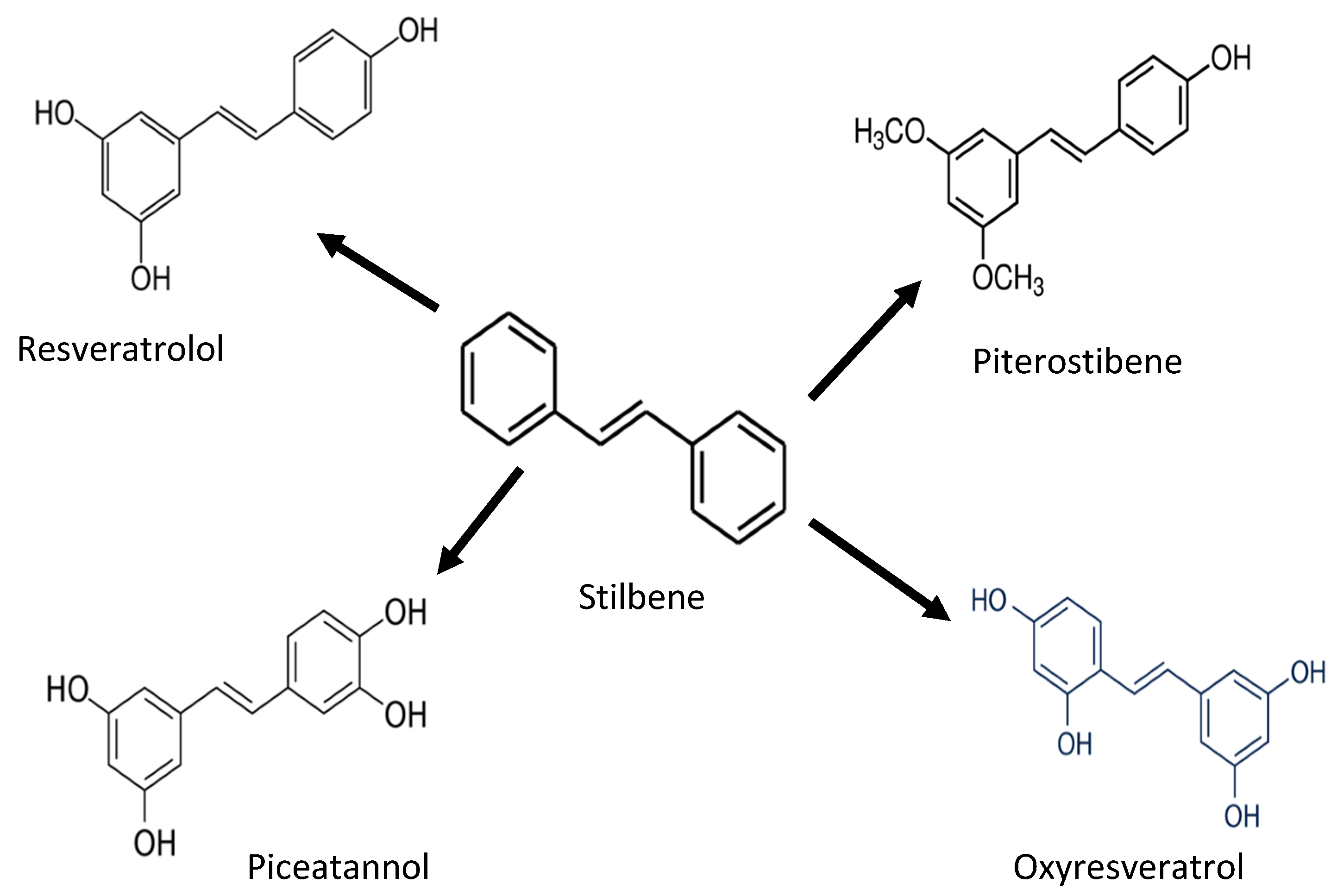
Stilbenoides/stilbenes are another phenolic compound commonly found in berries, grapevines, and peanuts. Stilbenoids are hydroxylated stilbene derivatives (i.e., resveratrol). The most common stilbenes are piceid, resveratrol, piceatannol, and pterostilbene (Figure 4). However, only resveratrol has antioxidant potential against proteins and lipids [27,28,29]. Endogenous compounds such as superoxide dismutase (SOD), catalase, and glutathione neutralize oxidative stress induced by UV radiation. Stilbenes, such as resveratrol, can increase the activity of antioxidant enzymes (glutathione S-transferase) and increase the SOD level. In addition, pterostilbenes reduce oxidative damage by activating the endogenous antioxidant enzymes [29].
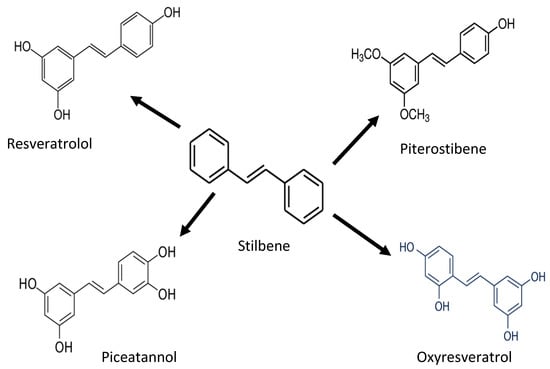
Figure 4.
Chemical structure of stilbene and its derivatives.
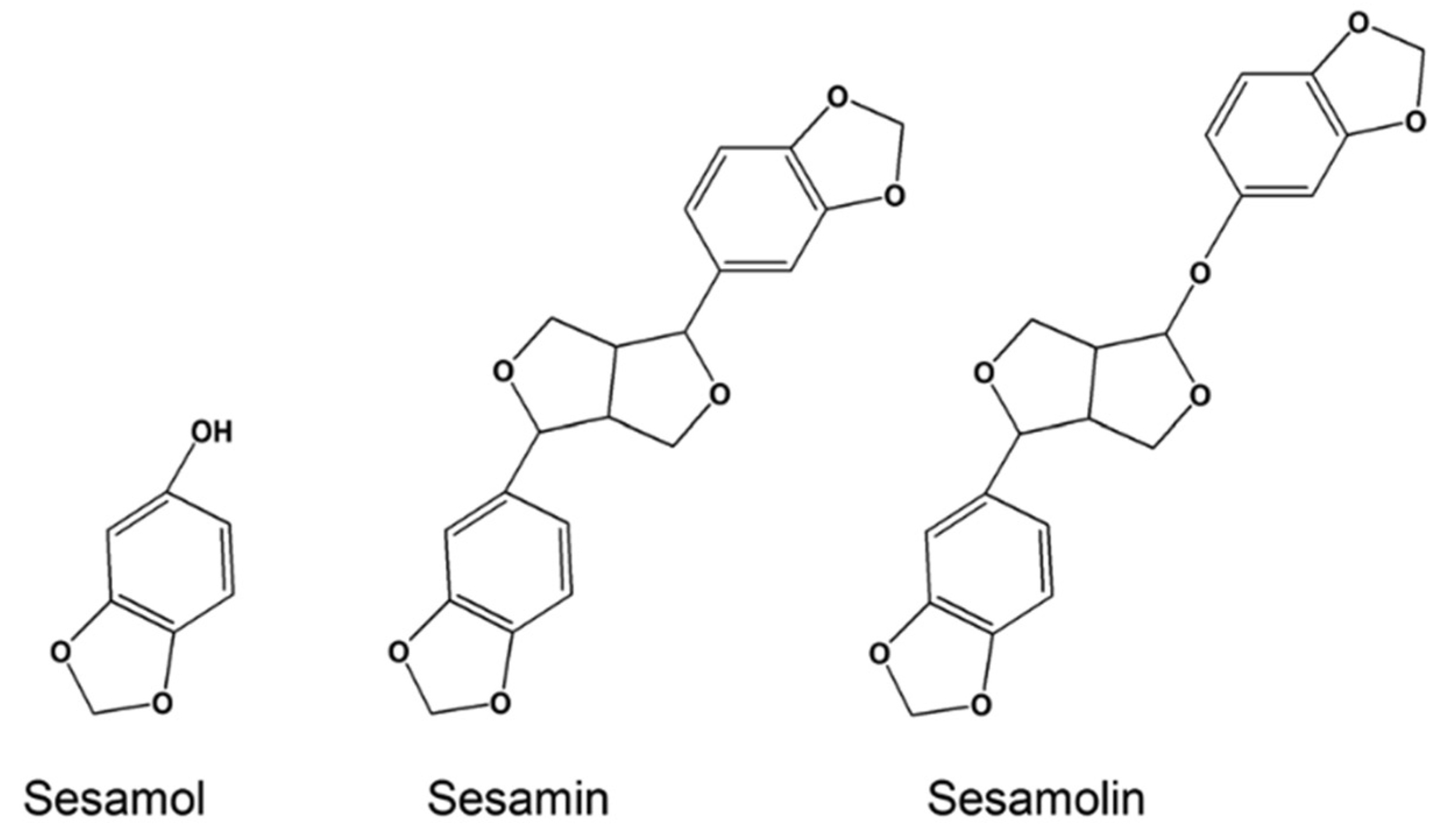
Lignans are precursors to phytoestrogens and synthesized from phenylalanine with dimerization of substituted cinnamic alcohols. Sesame seeds and flax seeds are the main sources of lignans. Secoisolariciresinol, matairesinol, pinoresinol, and lariciresinol are the common lignans from flax seeds and sesamin, sesamoiln, sesamolinol, and sesaminol are the common lignans from sesame seeds [30]. Sesamin and sesamolin possess antioxidant, neuroprotective, and anticancer activities, but sesamol, their decomposition product during the roasting process of sesame seeds (Figure 5), is the major antioxidant of the sesame seeds [31,32,33,34]. The common plant-based phenolic antioxidants are summarized in Table 1.
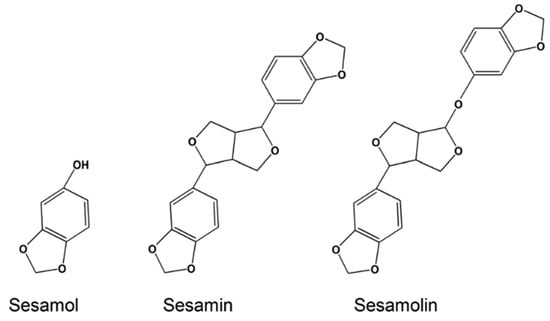
Figure 5.
Structure of sesamol, sesamin, and sesamolin. This Figure is reproduced from ref. [34]. Copyright 2016 Wiley.

Table 1.
Most common plant-based phenolic antioxidants and their potential applications.
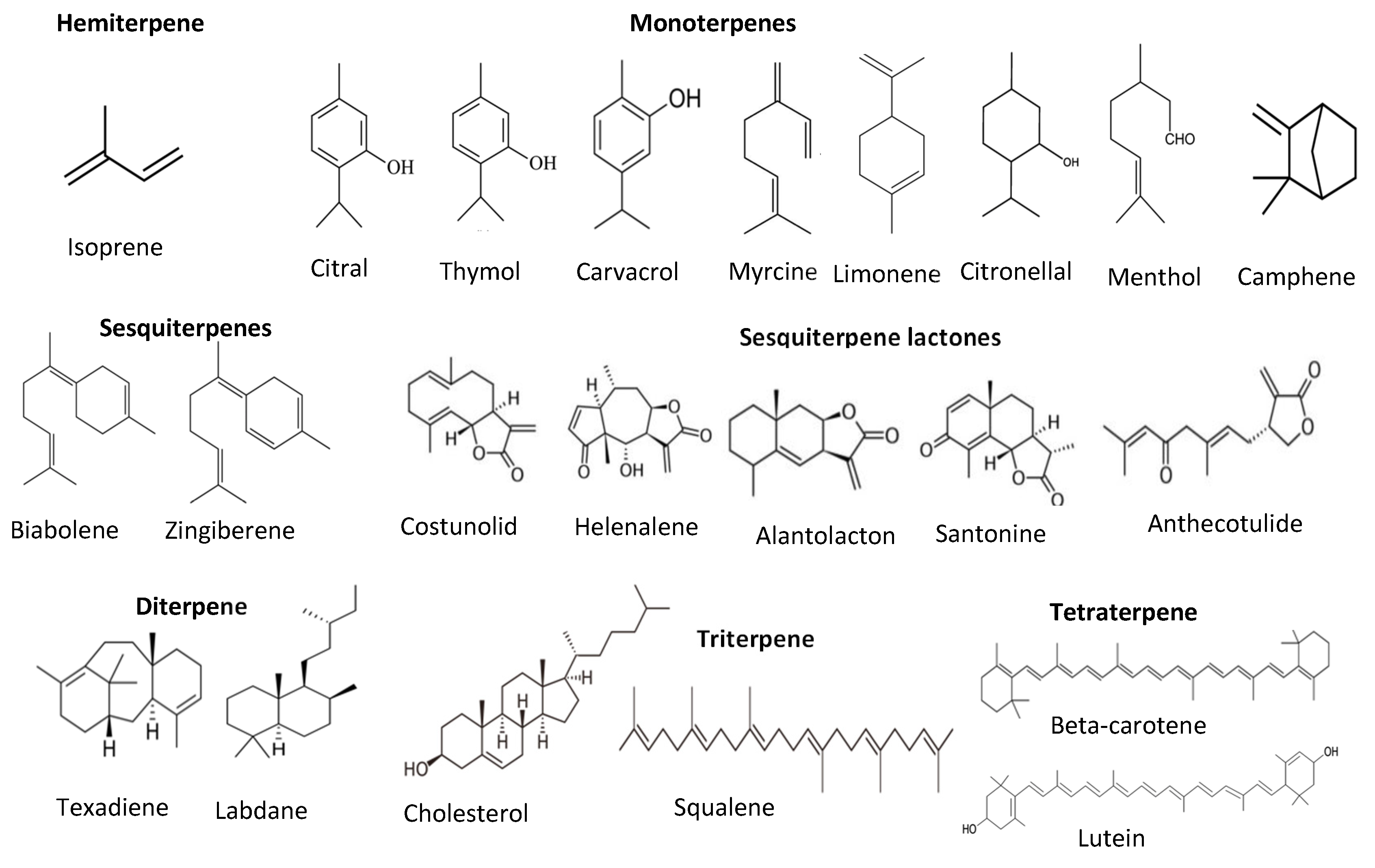
Terpenes and terpenoids are also good antioxidants from plant sources. They are the largest secondary metabolites of plants. Terpenes and terpenoids contain a hydrocarbon skeleton with five carbons (isoprene), and two or more isoprene molecules polymerize and form various terpenes. Most of them are non-polar compounds [45]. Plant oils such as pine oil, vegetables such as carrots, and some fruits such as lemon and orange are rich sources of terpenes and terpenoids. These compounds can further classify into monoterpenes (C-10), sesquiterpenes (C-15), diterpenes (C-20), triterpenes (C-30), tetraterpenes (C-40), or carotenoids, polyterpenes, norisopernoids, and sesquatreterpenes (Figure 6). These compounds have antioxidant and antimicrobial activities, contributing odor and flavor and other health-promoting properties such as relieving stress and depression, reducing depression and migraines, and antiaging and anticancer properties [35,46].
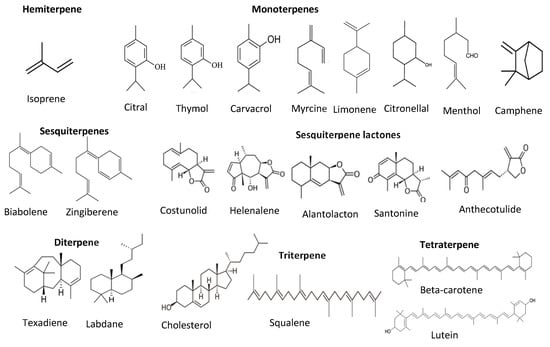
Figure 6.
Structure of different classes of terpenoids.
Tannins are another group of phenolic antioxidants in plants and can be divided into two main sub-classes: condensed tannins and hydrolyzable tannins. The condensed tannins are biopolymers based on flavan-3-ols, and gallic and ellagic acid derivatives (gallotannins and ellagitannins) are the main components with antioxidant properties [36,39]. Gallotannins are natural polymers formed by the esterification of D-glucose and gallic acid hydroxyl groups. Proanthocyanidins can donate hydrogen atoms/electrons and act as an antioxidant compound. Proanthocyanidins are abundant in green tea and bearberry [24,38]. Tannin extracts from red beans, adzuki beans, lentils, fava beans, and broad beans showed better antioxidant activity than the flavonoids and phenolic acids separated from the same plant materials [37,40,41]. The chemical structure of the tannic acid is shown in Figure 7.
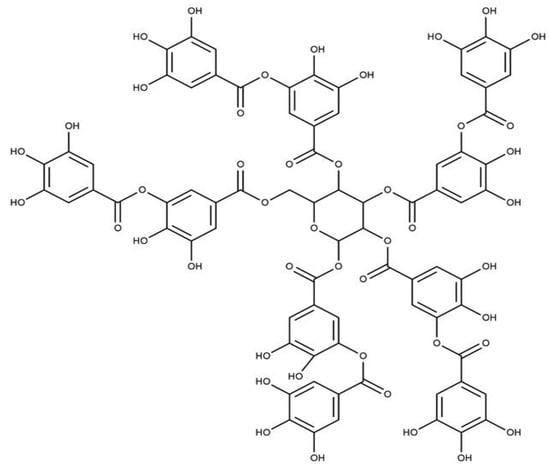
Figure 7.
Structure of Tannic acid.
Oilseeds, cereal grains, legumes, tea, coffee, tree nuts, fruits, and berries are excellent sources of plant antioxidants. Among the oilseeds, rapeseed and canola seeds have very high levels of phenolic compounds [24,47]. Sinapine (choline esters of sinapic acid) and sinapic acids are the main phenolic compounds in rapeseed [48]. Most cereals contain phenolic compounds, and ferulic acid is the most dominant type. However, some wheat cultivars contain high levels of p-coumaric, sinapic, and caffeic acids. Rice and rice bran contain γ-oryzanol, an ester of triterpene alcohols, and plant sterols [42] with strong antioxidant properties. Legumes also contain high phenolic acid, flavonoids, and tannins. Catechin, epicatechin glucosides, procyanidin dimers, quercetin glucoside, and p-coumaric are the main phenolic compounds in green lentils [24]. The leaves and seeds of legumes are considered a good source of lignans. Lamiaceae is a good source of phenolic acid, rosmarininc and caffeic acid, flavonoids, hispidulin, nepetin, luteolin, and apigenin [43]. Oregano (Origanum vulgare L.) is another plant that belongs to the family and is rich in phenolic antioxidant compounds such as rosmarinic acid and chlorogenic acid and flavonoids such as hyperoside and isoquercitrin [44].
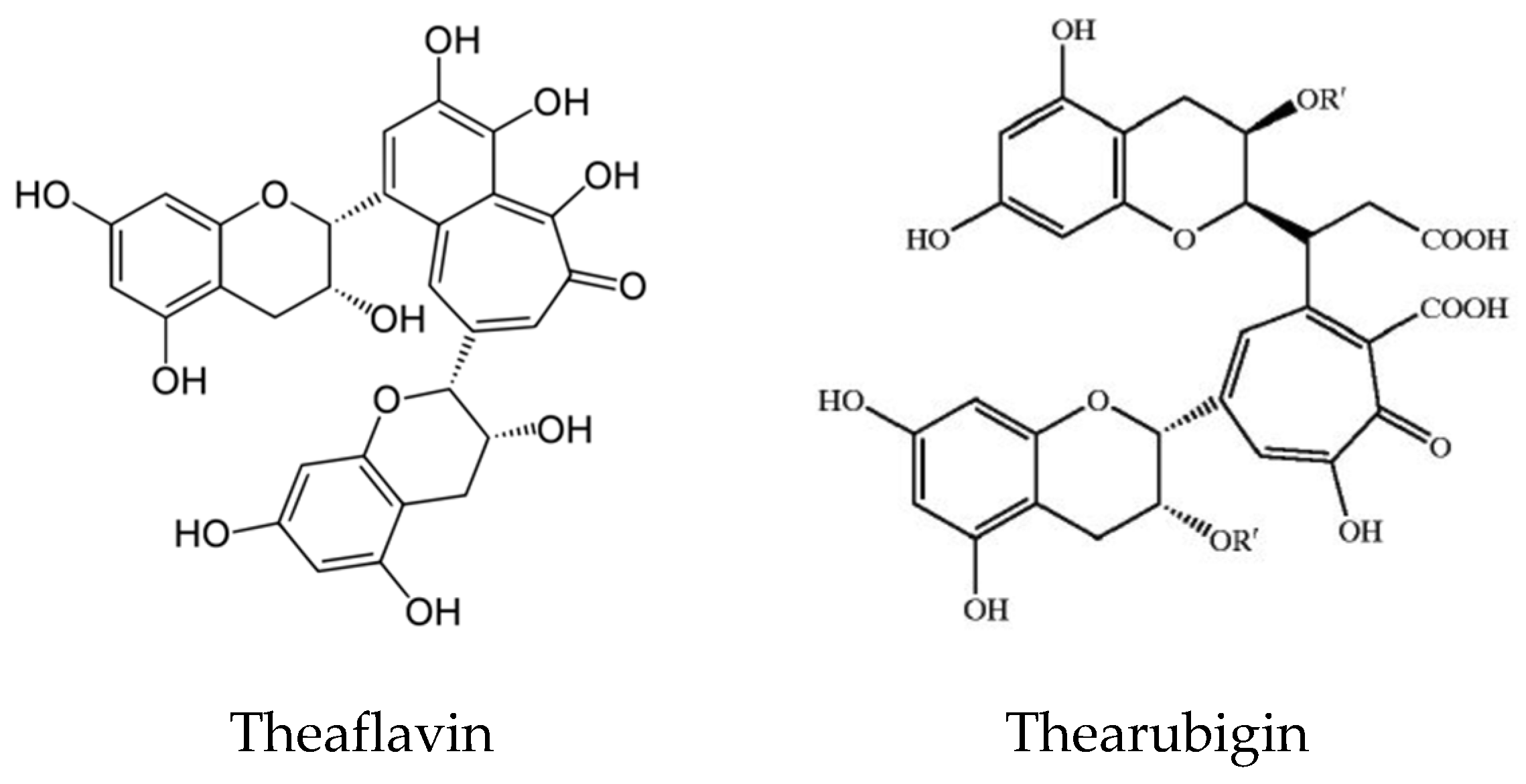
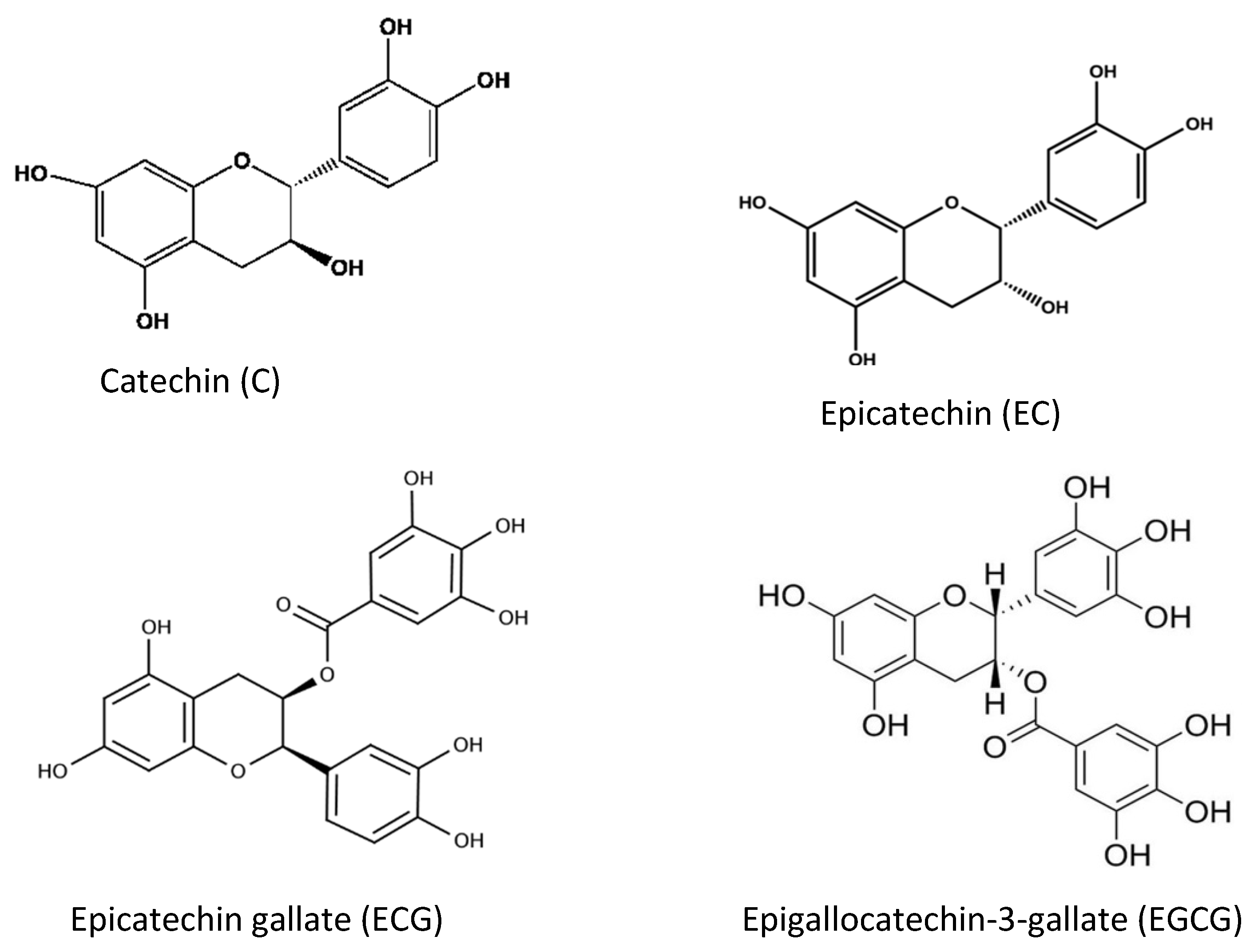
Tea and coffee are also rich sources of natural antioxidant compounds. Different antioxidants are found in green tea and black tea: theaflavin and thearubigin are the main antioxidant compounds in black tea (Figure 8), while the isomers of catechins are the main active antioxidant compounds in green tea (Figure 9). These are polyphenols, and the content varies among cultivars and by the method of processing [49,50,51].
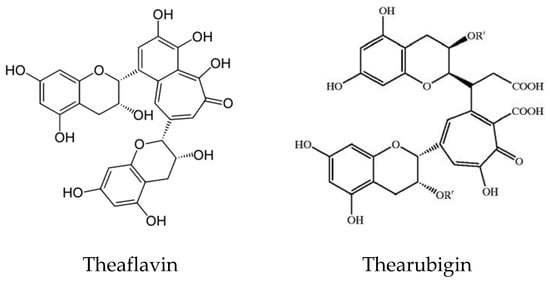
Figure 8.
Structure of Theaflavin and Thearubigin in black tea.
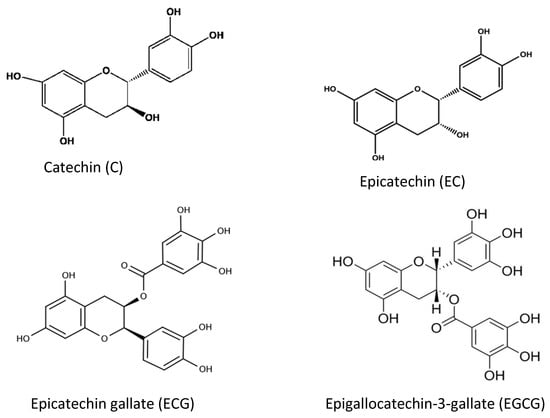
Figure 9.
Different chemical structures of catechins found in green tea.
Chlorogenic acids and their derivatives, including caffeoylquinic acids, caffeoylquinic acids, feruloyquinic acids, p-coumaroylquinic acids, caffeic acids, and ferulic acids, are the predominant phenolic compounds in coffee beans [52]. However, during heat processing, these phenolic compounds convert to quinolones and melanoidins [53]. Tree nuts are also rich sources of phenolic compounds: catechin, epicatechin, epicatechin 3-gallate, and procyanidins are rich in hazelnut kernels [54], and chlorogenic acid, caftaric acid, ferulic acid, gentisic acid, caffeic acid, p-coumaric acid, sinapic acid, isoquercitrin, rutozid, myricetin, fisetin, quercitrin, quercetin, luteolin, kaempferol, patuletin, hyperoside, and apigenin are rich in walnuts [55].
Fruits such as apples contain high amounts of polyphenols, hydroxycinnamates, flavonols, anthocyanins, and dihydrochalcones [56]. The main antioxidant compounds in plums are gallic acid, rutin, resorcinol, chlorogenic acid, catechin, and ellagic acid [8]. Berries, including strawberry, blackberry, blueberry, and cranberry, are rich in anthocyanins, flavonols, phenolic acids, and hydrolyzable tannins [57].
Phenolic compounds are mainly extracted using subcritical water. The extraction of phenolic compounds is based on hot water treatments called brewing. The temperature of the water and soaking duration determine the yield of the extracted phenolic compounds [53,58,59]. Solvent extraction is used to separate phenolic compounds from plant materials. Solvents such as methanol, acetone, ethanol, propanol, dimethylformamide, and ethyl acetate are mainly organic solvents. Different percentages of organic solvents separate the phenolic compounds [47]. The solvent and sample extraction time and ratio determine the yield and the purity of the phenolic compounds separated [60,61]. Furthermore, alkaline and acid hydrolysates separate these phenolic compounds [62].
The separated phenolic compounds incorporated into edible oils rich in unsaturated fatty acids effectively prevented lipid oxidation [63,64,65,66,67,68,69,70]. Combining the plant extracts rich in phenolic compounds, and synthetic antioxidants maintained the quality of many food products during storage [71]. Phenolic compounds are also widely used as natural antioxidants in meat and meat products. Among the plant extracts, almond seeds, grape seeds, and rosemary extracts are widely used as a natural preservative in beef and pork-based products [72,73,74]. Incorporating rosemary and green tea extracts into butter effectively prevented lipid oxidation [75]. Other plant extracts are also added to prevent lipid oxidation in seafood-based products [76,77].
3. Antioxidants from Animal Sources
Antioxidant Animal Proteins and Peptides
Egg white proteins such as ovotransferrin and phosvitins are potential antioxidant proteins [78,79,80]. The main antioxidant mechanism of these proteins is preventing metal-catalyzed lipid oxidation by chelating ionic irons [81]. Ovotransferrin, especially, exhibits a distinct thiol-linked self-cleavage activity with binding with the metal ions [80].
Many peptides produced from proteins by enzymatic hydrolysis or other physicochemical treatments showed antioxidant activities. The antioxidant activity of these peptides depends on their amino acid sequence, composition, and length [79,82,83]. These antioxidant peptides are produced from the proteins of milk, fish, egg, meat, and the by-products (head, skin, fins, intestine, blood). Bioactive peptides are produced from various proteins and have specific physiological effects [84,85,86]. Many bioactive peptides are used in therapeutic medicine, mostly in traditional medicines. The bioactive peptides should be non-toxic and can be easily excreted and destroyed, and they produce using enzymatic hydrolysis. The physiological activities of bioactive peptides have been proven in both in vitro and in vivo studies [87,88,89]. Many bioactive peptides from animal sources are produced during processing, especially fermentation. During fermentation, proteins hydrolyze into polypeptides, and further microbial enzymes are broken down into smaller peptides. Many exopeptidase enzymes can act on the polypeptides’ N or C terminals and break down into smaller dipeptides, tripeptides, or even single amino acids, all of which can act as antioxidative bioactive compounds [88].
Milk and milk by-products, such as whey, are very good sources of proteins to produce bioactive peptides, and they were the first animal protein sources used to produce peptides with antioxidant activities. Milk contains two endogenous protease enzymes that break down milk proteins [90], and these enzymes produce peptides with an amino acid sequence of VLPVPQK that show antioxidant activity [88,91]. Sheep milk also contains several peptides that have antioxidant activities. Those peptides were the products of αs1-casein and αs2 casein by Bacillus spp. [92]. The enzymatic hydrolysis of camel milk with pepsin, papain, and alcalase produced peptides with the amino acid sequences of RLDGQGRPRVWLGR, TPDNIDIWLGGIAEPQVKR, and VAYSDDGENWTEYRDQGAVEGK, and they showed strong antioxidant activities [93]. The hydrolysis of buffalo milk casein with pepsin, trypsin, and chymotrypsin produced bioactive peptides with the amino acid sequence of VLPVPQK, which showed strong antioxidant activity. The peptides’ activity was evaluated using DPPH radical scavenging activity and Trolox equivalent antioxidant capacity (TEAC) [94]. Hydrolysis of bovine and ovine milk proteins produced peptides with antioxidant activities; a peptide with the amino acid sequence of YFYPEL was produced from the bovine casein with pepsin, and the hydrolysis of bovine κ-casein with pepsin, trypsin, and chymotrypsin produced antioxidative peptides [95]. Milk peptides with amino acid sequences of ARHPHPHLSFM, AVPYPQR, NPYVPR, and KVLPVPEK showed strong antioxidant activity and inhibited lipid peroxidation in Caco-2 cells activating the Keap1-Nrf2 system [96].
Various cheese products, such as Cheddar, Gouda, cottage cheese, Pategrás, and Crescenza cheese, contain bioactive peptides that bind metal ions and slow lipid oxidation [91,97]. Peptides with antioxidant activity are also found in the water-soluble extracts of yogurt. These antioxidative peptides prevented the formation of free radicals or scavenged free radicals and active oxygen species. Cheddar cheese produced from buffalo milk contained high amounts of bioactive peptides derived from αs- and β-casein, and these peptides promoted glutathione synthesis [85,90,97,98].
Meat and meat products contain bioactive peptides because they are rich in high-quality proteins [91,95]. Postmortem aging of meat can produce bioactive peptides with a molecular weight of less than 3 kDa, but the amount and type of peptides produced can differ depending on the pH, temperature, and enzymes (e.g., trypsin, chymotrypsin, elastase, pepsin, and carboxypeptidase) involved. Many antioxidant peptides were produced from beef, pork, mutton, chicken, deer, duck, and marine species, and their sizes are 2–20 amino acids long and were produced mainly from the myofibrilla and sarcoplasmic proteins [85,91,99,100,101]. Papain hydrolysis of pig proteins produced peptides with a molecular weight between 3 and 10 kDa, and the peptides with amino acid sequences of DAQEKLE, AKHPSDFGADAQ, and AKHPSDFGADAQA showed strong antioxidant activities [102]. Peptides produced from myofibrillar proteins with the amino acid sequences of KRQKYD, EKERERQ, KAPVA, PTPVT, RPR, GLSDGEWQ, GFHI, DFHING, and FHG also showed strong antioxidant activities [91]. Hydrolysis of porcine myofibrilla proteins with papain and actinase E produced five antioxidant peptides with the amino acid sequences of DSGVT, IEAEGE, EELDNALN, VPSIDDQEELM, and DAQEKLE [103].
Some peptides with strong antioxidant activities were identified in fermented and cured meat products; the peptides had the amino acid sequences of DSGVT, IEAEGE, EELDNALN, VPSIDDQEELM, DAQEKLE, ATA, SLTA, VT, SAGNPN, GLAGA, DLEE, FGG, and DM [103,104]. Peptides produced from traditional Chinese chicken products, with the amino acid sequences of HVTEE and PVPVEGV, also showed antioxidant activities [105]. Three oligopeptides with two to four amino acids (ALTA, SLTA, and VT) showed antioxidant properties and antioxidant activity in both in vitro and in vivo studies [104,106]. These antioxidative peptides are suggested as good alternatives to the synthetic antioxidants used in the food industry [87,101,107,108], but without practicality.
Not only meat and meat-based products but also the by-products produced during meat processing can be used to produce peptides that have antioxidant activity [84,86,88]. Skin collagen and blood proteins are commonly used to produce bioactive peptides among the by-products. Hydrolyzing pig skin collagen produces a mixture of antioxidant peptides with the amino acid sequences of QGAR, LQGM, LQGMH, and HC. Furthermore, in a trial carried out with water buffalo horn proteins, three antioxidant peptides having the amino acid sequences of QYDQGV, YEDCTDCHN, and AADNANELFPPN were produced [101,109]. Hydrolysis of broiler hen skin with elastase produced peptides with amino acid sequences of GAHTHPRLPFKPR, GMPGFDVR, and ADASVLPK, which showed strong antioxidant activity against DPPH and ABTS radical scavenging activity [110]. Collagen extracted from pigs hydrolyzed with the pancreas, papain, and protease produced peptides with strong antioxidant activities. Most of the peptides produced were dipeptides, and proline, glycine, and hydroxyproline were the dominant free amino acids [111]. Blood plasma hydrolysates (with AlcalaseR 2.4 L) also showed strong antioxidant activities; the peptides responsible for the antioxidant activity were GAHQPSG and QQPVRDOQ [112]. Alcalase, pepsin, trypsin, papain, and flavorzyme are the most used enzymes to produce antioxidative peptides from the slaughterhouse blood [113]. Porcine liver proteins hydrolyzed using alcalase, papain, and pepsin produced fifteen oligopeptides with strong antioxidant activities [114].
Poultry eggs are also considered one of the best sources of bioactive peptides production, and many of the peptides showed health benefits [78,83,91]. Pepsin, trypsin, chymotrypsin, and proteases produced peptides with antioxidant activities from egg proteins. Among the egg white proteins, ovalbumin, ovotransferrin, lysozyme, ovomucin, and ovomucoid are commonly used to produce peptides with antioxidant activity [79,83,115]. Peptides (WNIP, GWNI, IRW, and LKP) from ovotransferrin showed strong antioxidant activity, and the peptides from ovalbumin using pepsin and protease showed strong antioxidant activities [116]. In addition, peptides produced from other egg white proteins (ovomucin and ovomucoid) using heat treatment at a high pH or enzymatic hydrolysis showed strong antioxidant activities [79,80]. These peptides derived from egg white proteins showed significant metal- (Fe2+ and Cu2+) chelating activities and prevented oxidation. Another study showed that egg white proteins hydrolyzed with different enzymes produced peptides (VYLPR, YLGAK, GGLEPINFN, ESKPV, DVYSF, and DSTRTQ) with antioxidant activity [83].
Hydrolysis of yolk proteins with pepsin and pancreatin produced peptides with amino acid sequences of WYGPD and KLSDW with antioxidant activity such as synthetic antioxidants [78,115]. Furthermore, the peptides (LMSYMWSTSM, LELHKLRSSHWFSRR, and LELHKLRSSHWFSRR) that are produced from yolk phosvitin showed strong antioxidant activities [115]. Egg yolk peptides with the amino acid sequences of WYGPD, KLSDW, KGLWE, YINQMPQKSRE; YINQMPQKSREA, VTGRFAGHPAAQ, LMSYMWSTSM, LELHKLRSSHWFSRR, RASDPLLSV, RNDDLNYIQ, LAPSLPGKPKPD, AGTTCLFTPLALPYDYSH, QSLVSVPGMS, and YIEAVNKVSPRAGQF showed antioxidant activities, verified with several antioxidant assays, including the DPPH radical scavenging assay [83].
Di- and tripeptides such as carnosine, anserine, glutathione, and ophidine found in meat, poultry, and fish showed good antioxidant activities [3,100,101,106]. Carnosine, anserine, and ophidine are similar in their structure and capable of scavenging free radicals. They can inhibit lipid oxidation catalyzed by ionic iron, hydrogen peroxide-activated hemoglobin, singlet oxygen, and other free radicals [82,117]. Glutathione also acts as an antioxidant by neutralizing (reducing) reactive oxygen species and preventing oxidant-mediated cell death [118]. However, the amount of these compounds present in meat depends on breed, age, gender, and breeding program [101]. Some amino acid monomers such as glutamine (Q), asparagine (N), leucine (L), phenylalanine (F), isoleucine (I), methionine (M), valine (V), alanine (A), cysteine (C), and tyrosine (Y) also show antioxidant activities [83]. The animal-based antioxidant proteins and peptides are listed in Table 2.

Table 2.
Most common animal-based antioxidant proteins and peptides.
4. Differences between the Antioxidants from Plant and Animal Sources
Most of the antioxidant compounds from plant sources are present in seeds, fruits, flowers, and leaves as active forms, while the animal-derived antioxidants are produced from proteins during digestion, or by hydrolyzing proteins using enzymes, and are functional only after the functional peptides are released from the proteins [72,91,113]. Some of the peptides and single amino acids have anti-inflammatory, hypoglycemic, antithrombotic, and ACE inhibitory activities, which are important for human health [88]. All the antioxidant compounds from plant sources (phenolic compounds, vitamins, and terpenoids) are produced as part of their metabolisms or tools for survival or defense against pests and diseases. These antioxidant compounds act as ROS scavengers in the plants, attractants for pollinations, protectants from insects or wounds, and improve metabolisms [44,72,91].
Different plant sources have different antioxidant compounds; most fruits and berries contain anthocyanins, catechins, flavanols, phenolic acids, and stilbenes. Tea and herbs are rich in tannins, catechins, and flavonoids [72,119]. The plant-derived antioxidant compounds are extracted using hot water, solvent, and alkaline water. On the other hand, enzymatic hydrolysis produces animal-based antioxidants after separating proteins from animal tissues. Pepsin, trypsin, elastase, chymotrypsin, proteases, and alcalase are some of the most common enzymes used to produce bioactive peptides with antioxidant activity. These bioactive peptides contain 3–20 amino acids [3,6,69,72,96,119] and are usually less than 6 kDa in molecular weight [82].
Plant-based natural antioxidants contain at least one phenyl ring with resonance double bonds and at least one OH- residue attached to the phenyl ring structure, whereas the animal protein-derived antioxidants (peptides) contain glutamine (Q), asparagine (N), leucine (L), phenylalanine (F), isoleucine (I), methionine (M), valine (V), alanine (A), cysteine (C), or tyrosine (Y) in their peptide sequences [6,82,83].
Plant-based antioxidant compounds counter-react with the reactive oxygen and nitrogen (ROS/RON) species and activate signal cascade systems inside the cells. The chain reactions of ROS/RON can take place with either hydrogen atom transfer (HAT) or single-electron transfer via proton transfer (SET-PT), or sequential electron transfer via proton transfer or transition metal chelation (TMC). Accordingly, these compounds stabilize the free radicals produced during the breakdown of lipids [4,82,120,121].
The stability of the animal-derived antioxidants depends on the temperature, pH, concentration of salts, and the enzymes used for hydrolysis. Since these peptides are small in molecular weight and stable structure, the activity and absorbance into the bloodstream are very high. These antioxidant peptides act on the lipid oxidation promoters, such as Fe2+, Cu2+, H2O2, lipid peroxides, NO, and other aldehydes, to prevent or delay lipid oxidation in food systems, which will prevent the damaging of vital compounds in the cells, such as DNA, proteins, lipids, and hormones. However, the ability of antioxidant peptides to prevent an oxidative reaction is not clear yet [120,122].
5. Applications of Antioxidants
Bioactive peptides are used as therapeutic agents to improve human health. In addition to their antioxidant activity, peptides can act as antimicrobial, anti-inflammatory, anticancer, or immunomodulatory agents and prevent type II diabetes. Many researchers suggested the potential of using antioxidant peptides as a natural antioxidant in food products while improving their sensory properties [120]. Plant-derived antioxidants are used extensively in the food industry to improve the foods’ shelf life, color, texture, and sensory properties. The production of plant-based antioxidants is simple and cheap, and their antioxidant effects have a stronger antioxidant capacity than the animal-derived ones [25,72]. These natural antioxidants derived from plant and animal sources are recommended as replacers for the synthetic antioxidants used in the food industry because natural antioxidants are considered safe and have fewer adverse health effects.
Compared with animal-based antioxidants, plant-based antioxidants are more practical for use in food processing because they are highly effective at low concentrations and a variety of antioxidant compounds can be extracted from a single plant source. Although some animal-derived antioxidants are known to have a comparable antioxidant capacity to synthetic ones, their production cost for separating specific antioxidant peptides is too expensive for practical use. Therefore, if animal- or plant-based antioxidants are used only to prevent lipid oxidation in foods, plant-based antioxidants are better. However, if the whole enzyme hydrolysate is used to produce animal-based antioxidants used in food, various peptides, including the specific antioxidant peptides, can provide many other biological properties that can benefit human health. Therefore, instead of separating and using a specific antioxidant peptide, whole protein hydrolysate is better to use as an ingredient in a food product. This concept is important because people are more concerned about their health, and including ingredients with natural antioxidants and functional peptides in their food products will satisfy consumers’ needs.
6. Conclusions
As a concluding remark, the authors would like to emphasize that plant-based antioxidants are a good source of antioxidants that can be used as food preservative agents. However, animal-based antioxidants (individual or multiple peptides) should not be considered as antioxidants or recommended as antioxidant agents in foods. Rather, they should be considered an ingredient to fulfill the nutritional requirements of the food with some antioxidant activities.
Funding
This work received no external funding.
Acknowledgments
The Iowa Agriculture and Home Economics Experiment Station, Ames, Iowa, supported this work.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Hazra, B.; Biswas, S.; Mandal, N. Antioxidant and free radical scavenging activity of Spondias pinnata. BMC Complement. Altern. Med. 2008, 8, 63. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mirzaei, M.; Mirdamadi, S.; Ehsani, M.R.; Aminlari, M. Antioxidant, ACE-inhibitory and antibacterial activities of Kluyeromyces marzianus protein hydrolysates and their peptide fractions. Funct. Foods Health Dis. 2016, 6, 425–439. [Google Scholar] [CrossRef]
- Kulczyński, B.; Sidor, A.; Gramza-Michałowska, A. Characteristics of selected antioxidative and bioactive compounds in meat and animal origin products. Antioxidants 2019, 8, 335. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zeb, A. Concept, mechanism, and applications of phenolic antioxidants in foods. J. Food Biochem. 2020, 44, e13394. [Google Scholar] [CrossRef]
- Abeyrathne, E.D.N.S.; Nam, K.C.; Ahn, D.U. Analytical methods for lipid oxidation and antioxidant capacity in food systems—A review. Antioxidants 2021, 10, 1587. [Google Scholar] [CrossRef]
- Abourashed, E.A. Bioavailability of plant-derived antioxidants. Antioxidants 2013, 2, 309–325. [Google Scholar] [CrossRef] [Green Version]
- Orian, M.; Escriche, I. Antioxidants: Characterization, natural sources, extraction, and analysis. Food Res. Int. 2015, 74, 10–36. [Google Scholar] [CrossRef]
- Hernández-Ruiz, K.L.; Ruiz-Cruz, S.; Cira-Chávez, L.A.; Gassos-Ortega, L.E.; Ornelas-Paz, J.J.; del-Toro-Sánchez, C.L.; Márquez-Ríos, E.; López-Mata, M.A.; Rodríguez-Félix, F. Evaluation of antioxidant capacity, protective effect on human erythrocytes and phenolic compounds identification in two varieties of plum fruit (Spondias spp.) by UPLC-MS. Molecules 2018, 23, 3200. [Google Scholar] [CrossRef] [Green Version]
- Munekata, P.E.S.; Gullón, B.; Pateiro, M.; Tomasevic, I.; Domínguez, R.; Lorenzo, J.S. Natural antioxidants from seeds and their applications in meat products. Antioxidants 2020, 9, 815. [Google Scholar] [CrossRef]
- Mojzer, E.B.; Hrnčič, M.K.; Škerget, M.; Knez, Ž.; Bren, U. Polyphenols: Extraction methods, Antioxidative action, bioavailability, and anticarcinogenic effects. Molecules 2016, 21, 901. [Google Scholar] [CrossRef]
- Alshkh, N.; de Camargo, A.D.; Shahidi, F. Phenolics of selected lentil cultivars: Antioxidant activities and inhibition of low-density lipoprotein and DNA damage. J. Funct. Foods 2015, 18, 1022–1038. [Google Scholar] [CrossRef]
- Falowo, A.B.; Fayemi, P.O.; Muchenje, V. Natural antioxidants against lipid-protein oxidative deterioration in meat and meat products. A review. Food Res. Int. 2014, 64, 171–181. [Google Scholar] [CrossRef] [PubMed]
- Dziedzinski, M.; Kobus-Cisowska, J.; Szymanowska, D.; Stuper-Szablewska, K.; Baranowska, M. Identification of polyphenols from coniferous shoots as natural antioxidants and antimicrobial compounds. Molecules 2020, 25, 3527. [Google Scholar] [CrossRef] [PubMed]
- Sant’Ana, A.S. Introduction to the special issue: Salmonella in foods: Evolution, strategies, and challenges. Food Res. Int. 2012, 45, 451–454. [Google Scholar] [CrossRef]
- Jideani, A.I.O.; Silungwe, H.; Takalani, T.; Omolola, A.O.; Udeh, H.O.; Anyashi, T.A. Antioxidant-rich natural fruit and vegetable products and human health. Int. J. Food Prop. 2021, 24, 41–67. [Google Scholar] [CrossRef]
- Chen, X.; Touyz, R.M.; Park, J.B.; Schiffrin, E.L. Antioxidant effects of vitamin C and E are associated with altered activation of vascular NADPH oxidase and superoxide dismutase in stroke-prone SHR. Hypertension 2001, 38, 606–611. [Google Scholar] [CrossRef] [Green Version]
- Traber, M.G.; Stevens, J.F. Vitamin C and E: Beneficial effects from a mechanistic perspective. Free Radic. Biol. Med. 2011, 51, 1000–1013. [Google Scholar] [CrossRef] [Green Version]
- Leopoldina, M.; Marino, T.; Russo, N.; Toscano, M. Antioxidant properties of phenolic compounds: H-atom versus electron transfer mechanism. J. Phys. Chem. A 2004, 108, 4916–4922. [Google Scholar] [CrossRef]
- Klein, E.; Lukeš, V. DFT/B3LYP study of the substituent effect on the reaction enthalpies of the individual steps of single-electron transfer-proton transfer and sequential proton loss electron transfer mechanisms of phenols antioxidant action. J. Phys. Chem. A 2006, 110, 12312–12320. [Google Scholar] [CrossRef]
- Abdel-Shafy, H.; Mansour, M.S.M. Polyphenols: Properties, occurrence, Content in food, and potential effects. In Environmental Science and Engineering; Volume 6: Toxicology; Chandra, R., Gurjar, B.R., Govil, J.N., Eds.; Studium Press LLC: Houston, TX, USA, 2017; pp. 232–261. [Google Scholar]
- Single, R.K.; Dubey, A.K.; Garg, A.; Sharma, R.K.; Fiorino, M. Natural polyphenols: Chemical Classification, definition of classes, subcategories, and structure. J. AOAC Int. 2019, 102, 1397–1400. [Google Scholar] [CrossRef]
- Dimitrios, B. Sources of natural phenolic antioxidants. Trends Food Sci. Technol. 2006, 17, 505–512. [Google Scholar] [CrossRef]
- Dirimanov, S.; Högger, P. Screening of inhibitory effects of polyphenols on Akt-phosphorylation in endothelial cells and determination of structure-activity features. Biomolecules 2019, 9, 219. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Amarowicz, R.; Pegg, R.B. Natural antioxidants of plant origin. In Advances in Food and Nutrition Research; Ferreira, I.C.F.R., Barros, L., Eds.; Academic Press: Cambridge, UK, 2019; pp. 1–81. [Google Scholar]
- Kumar, N.; Goel, N. Phenolic acids: Natural versatile molecules with promising therapeutic applications. Biotechnol. Rep. 2019, 24, e00370. [Google Scholar] [CrossRef]
- Shan, B.; Cai, Y.Z.; Brooks, J.D.; Corke, H. Antibacterial and antioxidant effects of five spice and herb extracts as natural preservatives of raw pork. J. Sci. Food Agric. 2009, 89, 1879–1885. [Google Scholar] [CrossRef]
- Markus, M.A.; Morris, B.J. Resveratrol in prevention and treatment of common clinical conditions of aging. Clin. Interv. Aging 2008, 3, 331–339. [Google Scholar] [PubMed]
- Pandey, K.B.; Rizvi, S.I. Resveratrol may protect plasma proteins from oxidation under conditions of oxidative stress in vitro. J. Braz. Chem. Soc. 2010, 21, 909–913. [Google Scholar] [CrossRef] [Green Version]
- Nagapan, T.S.; Ghazali, A.R.; Basri, D.F.; Lim, W.N. Photoprotective effect of stilbenes and its derivatives against ultraviolet radiation-induced skin disorders. Biomed. Pharmacol. J. 2018, 11, 1199–1208. [Google Scholar] [CrossRef]
- Liu, Z.; Sarrinen, N.M.; Thompson, L.U. Sesamin is one of the major precursors of mammalian lignans in sesame seed (Sesamum indicum), as observed in vitro and in rats. J. Nutr. 2006, 136, 906–912. [Google Scholar] [CrossRef] [Green Version]
- Wanasundara, P.K.J.P.D.; Shahidi, F. Process-induced change in edible oils. In Process-Induced Chemical Changes in Food; Shahidi, F., Ho, C.T., Chuyen, N.V., Eds.; Plenum Publishers: New York, NY, USA, 1998; pp. 135–160. [Google Scholar]
- Kumar, M.C.; Singh, S.A. Bioactive lignans from sesame (Sesamum indicum L.): Evaluation of their antioxidant and antibacterial effects for food applications. J. Food Sci. Technol. 2015, 52, 2934–2941. [Google Scholar] [CrossRef] [Green Version]
- Rosalina, R.; Weerapreeyakul, N. An Insight into sesamolin: Physicochemical properties, pharmacological activities, and future research prospects. Molecules 2021, 26, 5849. [Google Scholar] [CrossRef]
- Takahashi, M.; Nishizaki, Y.; Sugimoto, N.; Takeuchi, H.; Nakagawa, K.; Akiyama, H.; Sato, K.; Inoue, K. Determination and purification of sesamin and sesamolin in sesame seed oil unsaponified matter using reversed-phase liquid chromatography coupled with photodiode array and tandem mass spectrometry and high-speed counter-current chromatography. J. Sep. Sci. 2016, 39, 3898–3905. [Google Scholar] [CrossRef] [PubMed]
- Abdallah, I.I.; Quax, W.J. A Glimpse into the biosynthesis of terpenoids. In NRLS Conference Proceedings, International Conference on Natural Resources and Life Science (2016). KnE Life Sciences; Knoledge E Publisher: Dubai, United Arab Emirates, 2017; pp. 81–98. [Google Scholar]
- Hatano, T.; Kusuda, M.; Inada, K.; Ogawa, T.O.; Shiota, S.; Tsuchiya, T.; Yoshida, T. Effects of tannins and related polyphenols on methicillin-resistant Staphylococcus aureus. Phytochemistry 2005, 66, 2047–2055. [Google Scholar] [CrossRef] [PubMed]
- Amarowicz, R.; Estrella, I.; Hernández, T.; Troszynska, A. Antioxidant activity of extract of adzuki bean and its fractions. J. Food Lipids 2008, 15, 119–136. [Google Scholar] [CrossRef]
- Azman, N.A.M.; Gallego, M.G.; Segovia, F.; Abdullah, S.; Md Shaarani, S.; Pablos, M.P.A. Study of the properties of bearberry leaf extract as a natural antioxidant in model foods. Antioxidants 2016, 5, 11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jabri, M.-A.; Rtibi, K.; Ben-Said, A.; Aouadhi, C.; Hosni, K.; Sally, M.; Sebai, H. Antidiarrhoeal, antimicrobial and antioxidant effects of myrtle berries (Myrtus communis L.) seeds extract. J. Pharm. Pharmacol. 2016, 68, 264–274. [Google Scholar] [CrossRef]
- Amarowicz, R.; Karamać, M.; Dueňas, M.; Pegg, R.B. Antioxidant activity and phenolic composition of a red bean (Phaseolus Vulgaris) extract and its fractions. Nat. Prod. Commun. 2017, 12, 541–544. [Google Scholar]
- Amarowicz, R.; Shahidi, F. Antioxidant activity of Faba bean extract and fractions thereof. J. Food Bioact. 2018, 1, 112–118. [Google Scholar] [CrossRef] [Green Version]
- Patel, M.; Naik, S.N. Gamma-oryzanol from rice bran oil—A review. J. Sci. Ind. Res. 2004, 63, 569–578. [Google Scholar]
- Lee, S.H.; Kim, H.W.; Lee, M.K.; Lee, M.K.; Kim, Y.J.; Asamenew, G.; Cha, Y.S.; Kim, J.B. Phenolic profiling and quantitative determination of common sage (Salvia plebeian R. Br.) by UPLC-DAD-QTOF/MS. Eur. Food Res. Technol. 2018, 244, 1637–1646. [Google Scholar] [CrossRef] [Green Version]
- Oniga, I.; Puşcaş, C.; Silaghi-Dumitrescu, R.; Olah, N.-K.; Sevastre, B.; Marica, R.; Marcus, I.; Sevastre-Berghian, A.C.; Benedec, D.; Pop, C.E.; et al. Origanum vulgare ssp. vulgare: Chemical composition and biological studies. Molecules 2018, 23, 2077. [Google Scholar] [CrossRef] [Green Version]
- Jaeger, R.; Cuny, E. Terpenoids with special pharmacological significance: A review. Nat. Prod. Commun. 2016, 11, 1373–1390. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Prishtina, E.; Plyusnin, S.; Babak, T.; Lashmanova, E.; Maganova, F.; Koval, L.; Platonova, E.; Shaposhnikov, M.; Moskalev, A. Terpenoids as potential geroprotectors. Antioxidants 2020, 9, 529. [Google Scholar]
- Terpins, P.; Čeh, B.; Ulrich, N.P.; Abramovič, H. Studies of the correlation between antioxidant properties and the total phenolic content of different oil cake extracts. Ind. Crops Prod. 2012, 39, 210–217. [Google Scholar] [CrossRef]
- Szydłowska-Czerniak, A. Rapeseed and its products-sources of bioactive compounds: A review of their characteristics and analysis. Crit. Rev. Food Sci. Nutr. 2013, 53, 307–330. [Google Scholar] [CrossRef] [PubMed]
- Lee, L.S.; Kim, Y.C.; Park, J.D.; Kim, Y.B.; Kim, S.H. Changes in major polyphenolic compounds of tea (Camellia sinensis) leaves during the production of black tea. Food Sci. Biotechnol. 2016, 25, 1523–1527. [Google Scholar] [CrossRef] [PubMed]
- Koch, W.; Kukula-Koch, W.; Komsta, Ł.; Maezec, Z.; Szwarc, W.; Głowniak, K. Green tea quality evaluation based on its catechins and metals composition in combination with chemometric analysis. Molecules 2018, 23, 1689. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Takemoto, M.; Takemoto, H. Synthesis of theaflavins and their functions. Molecules 2018, 23, 918. [Google Scholar] [CrossRef] [Green Version]
- Moreira, A.S.P.; Nunes, F.M.; Domingues, M.R.; Coimbra, M.A. Coffee melanoidins: Structures, mechanisms of formation and potential health impacts. Food Funct. 2012, 3, 903–915. [Google Scholar] [CrossRef]
- Farah, A.; Donangelo, C.M. Phenolic compounds in coffee. Braz. J. Plant Biol. 2007, 18, 23–36. [Google Scholar] [CrossRef]
- Fanali, C.; Tripodo, G.; Russo, M.; Della Posta, S.; Pasqualetti, V.; de Gara, L. Effect of solvent on the extraction on the phenolic compounds and antioxidant capacity of the hazelnut kernel. Electrophoresis 2018, 39, 1683–1691. [Google Scholar] [CrossRef]
- Rusu, M.E.; Gheldiu, A.M.; Mocan, A.; Moldovan, C.; Popa, D.S.; Tomita, I.; Vlase, L. Process optimization for improved phenolic compounds recovery from walnut (Juglans regia L.) septum: Phytochemical profile and biological activities. Molecules 2018, 23, 2814. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vrhovsek, U.; Rigo, A.; Tonon, D.; Mattivi, F. Quantitation of polyphenols in different apple varieties. J. Agric. Food Chem. 2004, 52, 6532–6538. [Google Scholar] [CrossRef] [PubMed]
- Skrovankova, S.; Sumczynski, D.; Macek, J.; Jurikova, T.; Sochor, J. Bioactive compounds and antioxidant activity in different types of berries. Int. J. Mol. Sci. 2015, 16, 24673–24706. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khoshnoudi-Na, S.; Niakosari, M.; Tahiri, Z. Subcritical water technology for extraction phytochemical compound. J. Med. Plants 2017, 9, 94–107. [Google Scholar]
- Marchante, L.; Izquierdo-Caǹas, P.M.; Gómez-Alonso, S.; Alaǹón, S.; García-Romero, M.E.; Pérez-Coello, M.S.; Díaz-Maroto, M. Oenological potential of extracts from winery and cooperage by-products in combination with colloidal silver as natural substitutes to sulphur dioxide. Food Chem. 2019, 276, 485–493. [Google Scholar] [CrossRef]
- Nick, M.; Shahidi, F. Phenolics in cereals, fruits, and vegetables: Occurrence, extraction, and analysis. J. Pharm. Biomed. Anal. 2006, 41, 1523–1542. [Google Scholar]
- Liang, J.; Zago, E.; Nandasiri, R.; Khattab, R.; Eskin, N.A.M.M.; Eck, P.; Holländer, U.T. Effect of solvent preheating temperature and time on the ultrasonic extraction of phenolic compounds from cold-pressed hempseed cake. J. Am. Oil Chem. Soc. 2018, 95, 1319–1327. [Google Scholar] [CrossRef]
- Acosta-Estrada, B.A.; Gutierrez-Uribe, J.A.; Serna-Saldívar, S.O. Bound phenolics in foods. A review. Food Chem. 2014, 152, 46–55. [Google Scholar] [CrossRef]
- Mullen, W.; Nemzer, B.; Ou, B.; Stalmach, A.; Hunter, J.; Clifford, M.; Combet, E. The antioxidant and chlorogenic acid profiles of whole coffee fruits are influenced by extraction procedures. J. Agric. Food Chem. 2011, 59, 3754–3762. [Google Scholar] [CrossRef]
- Lee, S.G.; Terrence, M.; Vance, T.M.; Nam, T.G.; Kim, D.O.; Koo, S.I.; Chun, O.K. Contribution of anthocyanin composition of total antioxidant capacity of berries. Plant Food Hum. Nutr. 2005, 25, 523–1527. [Google Scholar] [CrossRef]
- Kozłowska, M.; Gruczynska, E. Comparison of the oxidative stability of soybean and sunflower oils enriched with herbal plant extracts. Chem. Pap. 2018, 72, 2607–2615. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dos Reis, L.C.R.; Facco, E.M.P.; Flôres, S.H.; Rios, A.D.O. Stability of functional compounds and antioxidant activity of fresh and pasteurized orange passion fruit (Passiflora caerulea) during cold storage. Food Res. Int. 2018, 106, 481–486. [Google Scholar] [CrossRef] [PubMed]
- İnan, Ö.; Özczn, M.M.; Aljuhaimi, F. Effect of location and Citrus species on total phenolic, antioxidant and radical scavenging activities of some Citrus seed and oil. J. Food Process. Preserv. 2018, 42, 1215–1219. [Google Scholar] [CrossRef]
- Do Couto, C.A.; de Souza, E.R.B.; Morgado, C.M.A.; Ogata, T.; Cunha Júnior, L.C. Citrus Sinensis cultivars: Alternatives for diversification of Brazilian orchards. Rev. Bras. Food Sci. 2018, 50, 905–991. [Google Scholar] [CrossRef] [Green Version]
- Konieczyski, P.; Viapiana, A.; Mark Wesolowski, M. Comparison of infusions from black and green teas (Camellia sinensis L. Kuntze) and erva-mate (Ilex paraguariensis A. St.-Hil) based on the content of essential elements, secondary metabolites, and antioxidant activity. Food Anal. Methods 2017, 10, 3063–3070. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.-F.; Oey, I.; Bremer, P.; Carne, A.; Silcock, P. Bioactive peptides derived from egg proteins: A review. Crit. Rev. Food Sci. Nutr. 2018, 58, 2508–2530. [Google Scholar] [CrossRef]
- Nowicka, A.; Kucharska, A.Z.; Sokół-Łętowska, A.; Fecka, I. Comparison of polyphenol content and antioxidant capacity of strawberry fruit from 90 cultivars of Fragaria × ananassa Duch. Food Chem. 2019, 270, 32–46. [Google Scholar] [CrossRef]
- Lourenço, S.C.; Moldão-Martins, M.; Alves, V.D. Antioxidants of natural plant origins: From sources to food industry applications. Molecules 2019, 24, 4132. [Google Scholar] [CrossRef] [Green Version]
- Wijeratne, S.S.K.; Amarowicz, R.; Shahidi, F. Antioxidant activity of almonds and their by-products in food model. J. Am. Oil Chem. Soc. 2006, 83, 223–230. [Google Scholar] [CrossRef]
- Rojas, M.C.; Brewer, M.S. Effect of natural antioxidants on oxidative stability of cooked, refrigerated beef and pork. J. Food Sci. 2007, 72, S282–S288. [Google Scholar] [CrossRef]
- Oswell, N.J.; Thippareddi, H.; Pegg, R.B. Practical use of natural antioxidants in meat products in the US. A Review. Meat Sci. 2017, 145, 469–479. [Google Scholar] [CrossRef] [PubMed]
- Gramza-Michałowska, A.; Korczak, J.; Reguła, J. Use of plant extracts in summer and winter season butter oxidative stability improvement. Asia Pac. J. Clin. Nutr. 2007, 16 (Suppl. 1), 85–88. [Google Scholar] [PubMed]
- Maqsood, S.; Benjakul, S.; Abushelaibi, A.; Alam, A. Phenolic compounds and plant phenolic extracts as natural antioxidants in prevention of lipid oxidation in seafood: A detailed review. Compr. Rev. Food Sci. Food Saf. 2014, 13, 1125–1140. [Google Scholar] [CrossRef]
- Your, M.; Howell, N. Antioxidant and ACE inhibitory bioactive peptides from purified egg yolk proteins. Int. J. Mol. Sci. 2015, 16, 29161–29178. [Google Scholar]
- Abeyrathne, E.D.N.S.; Huang, X.; Ahn, D.U. Antioxidant, angiotensin-converting enzyme inhibitory activity, and other functional properties of egg white proteins and their derived peptides. Poult. Sci. 2018, 97, 1462–1468. [Google Scholar] [CrossRef]
- Rathnapala, E.C.N.; Ahn, D.U.; Abeyrathne, E.D.N.S. Functional properties of ovotransferrin from chicken egg white and its derived peptides- A Review. Food Sci. Biotechnol. 2021, 30, 619–630. [Google Scholar] [CrossRef]
- Huang, X.; Ahn, D.U. Lipid oxidation and its implications to meat quality and human health. Food Sci. Biotechnol. 2019, 28, 1275–1285. [Google Scholar] [CrossRef]
- Sarmadi, B.; Ismail, A. Antioxidative peptides from food proteins: A review. Peptides 2010, 31, 1949–1956. [Google Scholar] [CrossRef]
- Benedé, S.; Molina, E. Chicken egg proteins and derived peptides with antioxidant properties. Foods 2020, 9, 735. [Google Scholar] [CrossRef]
- Shahidi, F.; Zhong, Y. Bioactive peptides. J. AOAC Int. 2008, 91, 914–931. [Google Scholar] [CrossRef] [Green Version]
- Bhat, Z.F.; Kumat, S.; Bhat, H.F. Bioactive peptides of animal origin: A review. J. Food Sci. Technol. 2015, 52, 5377–5392. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maestri, E.; Pavlicevic, M.; Montorsi, M.; Marmiroli, N. Meta-Analysis for correlating structure of bioactive peptides in foods of animal origin with regard to effect and stability. Compr. Rev. Food Sci. Food Saf. 2018, 18, 3–30. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xiang, L.J.; Hu, Y.Y.; Hu, H.Y.; Ge, Q.F.; Zhou, G.H.; Zhang, W.G. Purification and identification of antioxidative peptides from dry-cured Xuanwei ham. Food Chem. 2016, 194, 951–958. [Google Scholar] [CrossRef] [PubMed]
- Toldrá, F.; Gallego, M.; Reig, M.; Aristoy, M.C.; Mora, L. Recent progress in enzymatic release of peptides in foods of animal origin and assessment of bioactivity. J. Agric. Food Chem. 2020, 68, 12842–12855. [Google Scholar] [CrossRef] [PubMed]
- Akbarian, M.; Khani, A.; Eghbalpour, S.; Uversky, V.N. Bioactive peptides: Synthesis, source, application and proposed mechanisms of action. Int. J. Mol. Sci. 2022, 23, 1445. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.W.; Nam, M.S. Bioactive peptides in milk and dairy products: A review. Korean J. Food Sci. Anim. Resour. 2015, 35, 831–840. [Google Scholar] [CrossRef] [Green Version]
- Albenzio, M.; Santillo, A.; Caroprese, M.; della Malva, A.; Marino, R. Bioactive peptides in animal food proteins. Foods 2017, 6, 35. [Google Scholar] [CrossRef] [Green Version]
- Corrȇa, A.P.E.; Dariot, D.J.; Coelho, J.; Meirs, S.M.M.; Lopes, F.C.; Segalin, J. Antioxidant, antihypertensive and antimicrobial properties of ovine milk caseinate hydrolyzed with microbial proteinase. J. Sci. Food Agric. 2011, 91, 2247–2254. [Google Scholar]
- Wali, A.; Yanhua, G.; Ishimov, U.; Yili, A.; Aisa, H.A.; Salikhov, S. Therapeutics, Isolation, and identification of three novel antioxidant peptides from the Bactrian camel milk hydrolysates. Int. J. Pept. Res. Ther. 2020, 26, 641–650. [Google Scholar] [CrossRef]
- Shanmugam, V.P.; Kapila, S.; Sonfack, T.K.; Kapila, R. Antioxidative peptides derived from enzymatic digestion of buffalo casein. Int. Dairy J. 2015, 42, 1–5. [Google Scholar] [CrossRef]
- Chakrabarti, S.; Jahandideh, F.; Wu, J. Food-derived bioactive peptides on inflammation and oxidative stress. BioMed Res. Int. 2014, 2014, 608979. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tonolo, F.; Folda, A.; Cesaro, L.; Scalcon, V.; Marin, O.; Ferro, S.; Bindoli, A.; Rigobello, M.P. Milk-derived bioactive peptides exhibit antioxidant activity through the Keap1-Nrf2 signaling pathway. J. Funct. Foods 2020, 64, 103696. [Google Scholar] [CrossRef]
- Huma, N.; Rafiq, S.; Sameen, A.; Pasha, I.; Khan, M.I. Antioxidant potential of buffalo and cow milk cheddar cheeses to tackle human colon adenocarcinoma (Caco-2) cells. Asian-Australas. J. Anim. Sci. 2018, 31, 287–292. [Google Scholar] [CrossRef] [PubMed]
- Aloğlu, H.Ş.; Öner, Z. Determination of the antioxidant activity of bioactive peptides fractions obtained from yogurt. J. Dairy Sci. 2011, 94, 5305–5314. [Google Scholar] [CrossRef] [Green Version]
- Fu, Y.; Jette, F.Y.; Therkildsen, M. Bioactive peptides from meat muscle and by-products: Endogenous generation through postmortem aging. Meat Sci. 2017, 23, 134–142. [Google Scholar] [CrossRef]
- Susanto, E.; Fadlilah, A.; Amin, M.F. Synthesis, extraction, and identification of meat bioactive peptides: A review. IOP Conf. Series. Earth Environ. Sci. 2021, 888, 012058. [Google Scholar]
- Xing, L.; Liu, R.; Cao, S.; Zhang, W.; Zhu, G.H. Meat protein-based bioactive peptides and their potential functional activity: A review. Int. J. Food Sci. Technol. 2019, 54, 1956–1966. [Google Scholar] [CrossRef] [Green Version]
- Di Bernadini, R.; Mullen, A.M.; Bolton, D.; Kerry, J.; O’Neill, E.; Hayes, M. Assessment of angiotensin-I-converting enzyme (ACE-1) inhibitory and antioxidant activities of hydrolysates of bovine brisket sarcoplasmic proteins produced by papain and characterization of associated bioactive peptides fractions. Meat Sci. 2012, 90, 226–235. [Google Scholar] [CrossRef]
- Saiga, A.; Tanabe, S.; Nishimura, T. Antioxidant activity of peptides obtained from porcine myofibrilla proteins by protease treatment. J. Agric. Food Chem. 2003, 51, 3661–3667. [Google Scholar] [CrossRef]
- Ryan, J.T.; Ross, R.P.; Bolton, D.; Fitzgerald, G.F.; Stanton, C. Bioactive peptides from muscle sources: Meat and Fish. Nutrients 2011, 3, 765–791. [Google Scholar] [CrossRef] [Green Version]
- Wu, H.C.; Pan, B.S.; Chang, C.L.; Shiau, C.Y. Low-molecular-weight peptides as related to antioxidant properties of chicken essence. J. Food Drug Anal. 2005, 13, 176–183. [Google Scholar] [CrossRef]
- Stadnik, J.; Kęska, P. Meat and fermented meat products as a source of bioactive peptides. Acta Sci. Pol. Technol. Aliment. 2015, 14, 181–190. [Google Scholar] [CrossRef] [PubMed]
- Broncano, J.M.; Otte, J.; Petrón, M.J.; Parra, V.; Timón, M.L. Isolation and identification of low molecular weight antioxidant compounds from fermented “chorizo” sausage. Meat Sci. 2012, 90, 494–501. [Google Scholar] [CrossRef] [PubMed]
- Escudero, E.; Mora, L.; Fraser, P.D.; Aristory, M.C. Identification of novel antioxidant peptides generated in Spanish dry-cured ham. Food Chem. 2013, 138, 1282–1288. [Google Scholar] [CrossRef]
- Liu, R.; Wang, M.; Duan, J.A.; Guo, G.M.; Tang, Y.P. Purification and identification of three novel antioxidant peptides from Cornu bubali (water buffalo horn). Peptides 2010, 31, 786–793. [Google Scholar] [CrossRef]
- Nadalian, M.; Kamaruzaman, N.; Yusop, M.S.M.; Babji, A.S.; Yusop, S.M. Isolation, purification, and characterization of antioxidative bioactive Elastin peptides from poultry skin. Food Sci. Anim. Resour. 2019, 39, 966–979. [Google Scholar] [CrossRef] [Green Version]
- Ao, J.; Li, B. Amino acid composition and antioxidant activities of hydrolysates and peptides fractions from porcine collagen. Food Sci. Technol. Int. 2012, 18, 425–434. [Google Scholar] [CrossRef]
- Gómez-Sampedro, L.J.; Zapata-Montoya, J.E. Obtaining of antioxidant peptide from bovine plasma hydrolysates and effect of the degree of hydrolysis on antioxidant capacity. Rev. Mex. De Ing. Química 2016, 15, 101–109. [Google Scholar]
- Bah, C.S.F.; Bekhit, E.A.; Crne, A.; McConnell, M.A. Slaughterhouse blood: An emerging source of bioactive compounds. Compr. Rev. Food Sci. Food Saf. 2013, 12, 314–331. [Google Scholar] [CrossRef]
- Yu, H.C.; Hsu, J.L.; Chang, C.I.; Tan, F.J. Antioxidant properties of porcine liver proteins hydrolyzed using Monascus purpureus. Food Sci. Biotechnol. 2017, 26, 1217–1225. [Google Scholar] [CrossRef]
- Liu, Q.; Tang, G.Y.; Zhao, C.N.; Feng, X.L.; Xu, X.Y.; Cao, S.Y.; Meng, X.; Li, S.; Gan, R.Y.; Li, H.B. Comparison of antioxidant activities of different grape varieties. Molecules 2018, 23, 2432. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shen, S.; Chahal, B.; Majumder, K.; You, S.J.; Wu, J. Identification of novel antioxidative peptides derived from a thermolytic hydrolysate of ovotransferrin by LC-MS/MS. J. Agric. Food Chem. 2010, 58, 7664–7672. [Google Scholar] [CrossRef] [PubMed]
- Ayala, A.; Muñoz, M.F.; Argüelles, S. Lipid peroxidation: Production, metabolism and signaling mechanisms of malondialdehyde and 4-Hydroxy-2-Nonenal. Oxidative Med. Cell. Longev. 2014, 2014, 360438. [Google Scholar] [CrossRef] [PubMed]
- He, L.; He, T.; Farrar, S.; Ji, L.; Liu, T.; Ma, X. Antioxidants maintain cellular redox homeostasis by elimination of reactive oxygen species. Cell. Physiol. Biochem. 2017, 44, 532–553. [Google Scholar] [CrossRef]
- Jiang, H.; Tong, T.; Sun, J.; Xu, Y.; Zhao, Z.; Liao, D. Purification and characterization of antioxidative peptides from round scad (Decapterus maruadsi) muscle protein hydrolysate. Food Chem. 2014, 154, 158–163. [Google Scholar] [CrossRef]
- Lorenzo, J.M.; Munekata, P.E.S.; Gómez, B.; Barba, F.J.; Mora, L.; Pérez-Santaescolástica, C.; Toldrá, F. Bioactive peptides as natural antioxidants in food products—A review. Trends Food Sci. Technol. 2018, 79, 136–147. [Google Scholar] [CrossRef]
- Barreca, D. Mechanisms of Plant antioxidants actions. Plants 2021, 10, 35. [Google Scholar] [CrossRef]
- Zhu, C.Z.; Zhang, W.G.; Kanf, Z.L.; Zhou, G.H.; Xu, S.L. Stability of antioxidant peptides extracted from Jinhua ham. Meat Sci. 2014, 96, 783–789. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).