Role and Potential Mechanism of Heme Oxygenase-1 in Intestinal Ischemia-Reperfusion Injury
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Materials
2.3. Experimental Design
2.4. Analysis Methods in Mice
2.4.1. Luminal Protein and Hemoglobin
2.4.2. Histologic Examination of Intestinal Tissues
2.4.3. Measurement of Myeloperoxidase (MPO) Activity
2.4.4. Analysis of mRNA Expression Levels in the Intestine
2.4.5. Sodium Dodecyl Sulfate-Polyacrylamide Gel Electrophoresis (SDS-PAGE) and Western Blotting
2.4.6. Preparations of Nuclear Extracts and Electrophoretic Mobility Shift Assay (EMSA)
2.5. Various Analysis Methods in Cells
2.5.1. Mesenteric Microvessel Endothelial Cells (MECs)
2.5.2. PMN Adhesion Assay
2.6. Statistical Analyses
3. Results
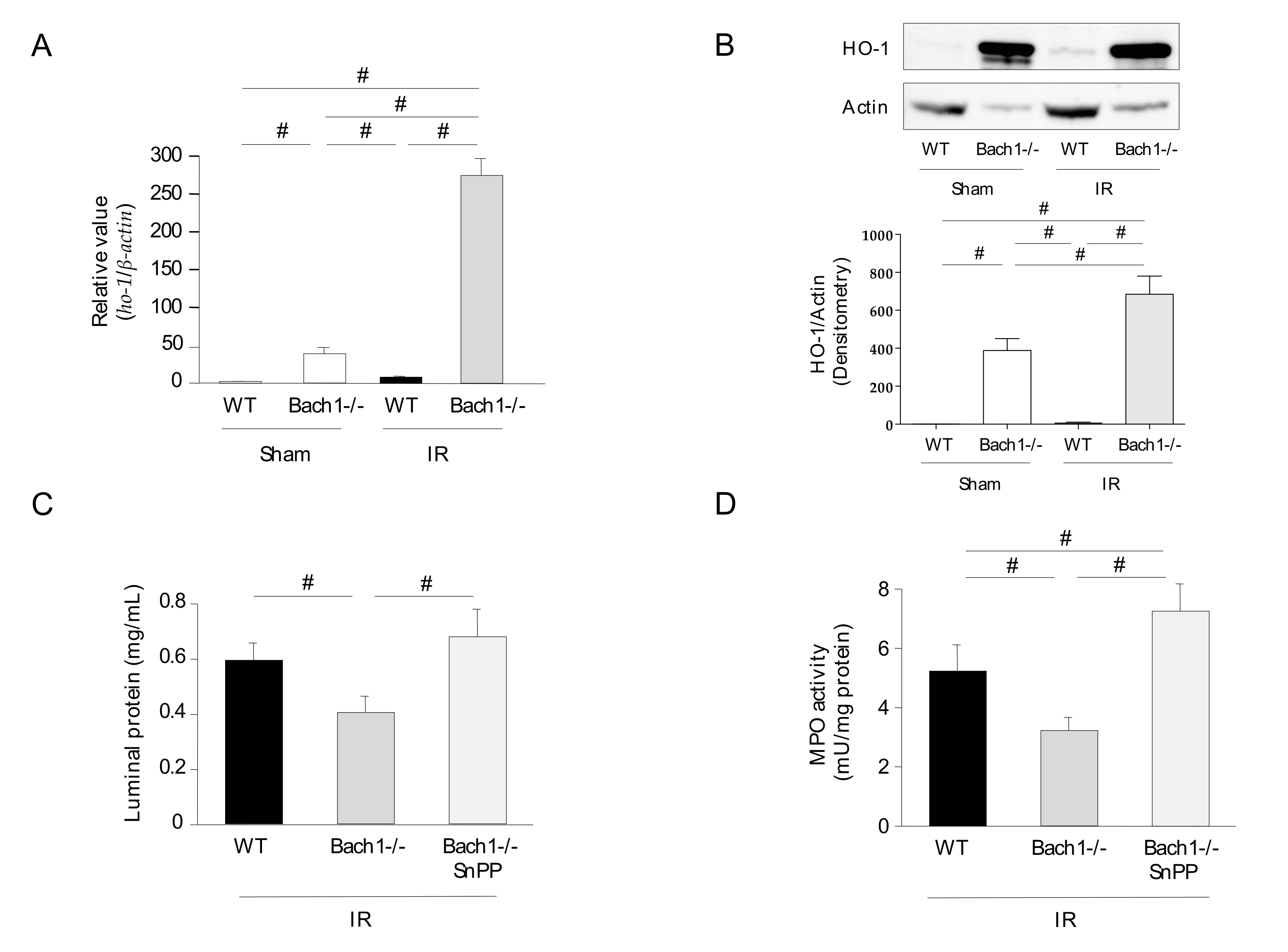
3.1. Effect of Bach1 Deficiency on the Inflammatory Response in Intestinal IR Injury
3.2. Effect of Bach1 Deficiency on HO-1 Upregulation in Intestinal IR Injury
3.3. Effects of Bach1 Deficiency on NF-κB Activation, Expression Levels of Adhesion Molecules, and Neutrophil-Endothelial Cell Interaction in Intestinal IR Injury
3.4. Effect of Nrf2 Deficiency on the Inflammatory Response in Intestinal IR Injury
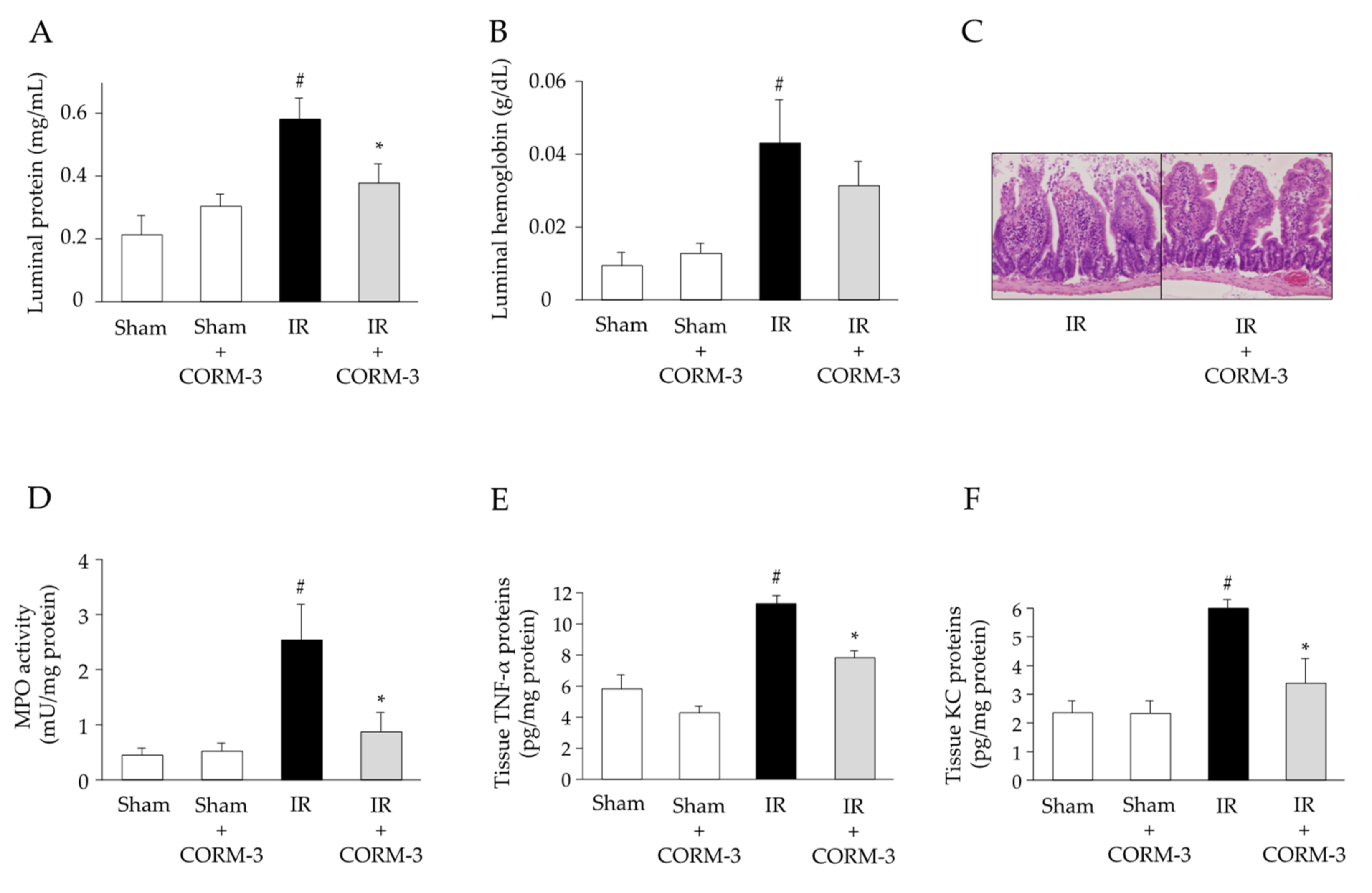
3.5. Effect of CO Derived from CORM-3 on the Inflammatory Response in Intestinal IR Injury
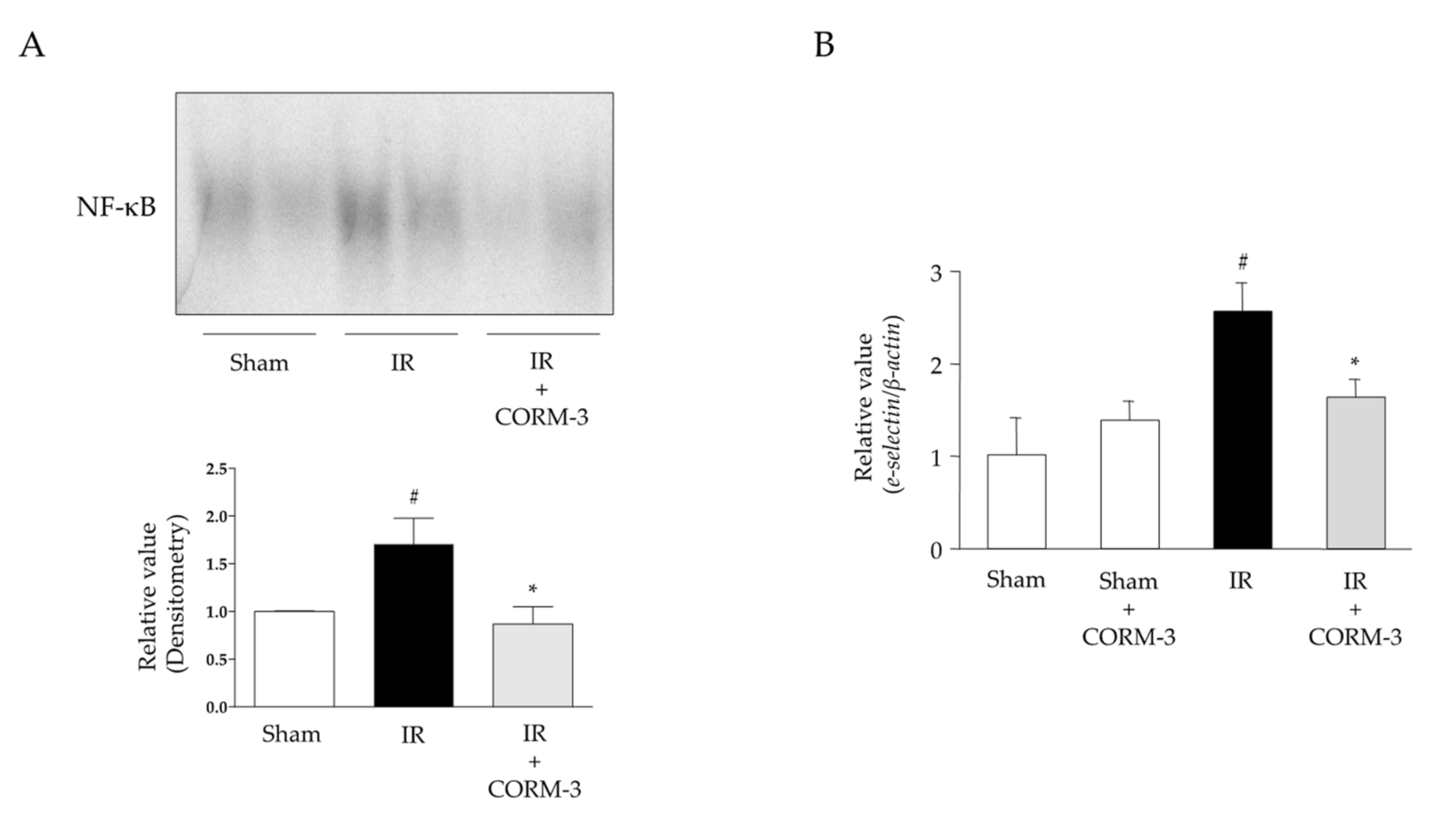
3.6. Effects of CO Derived from CORM-3 on NF-κB Activation and Expression Levels of Adhesion Molecules in Intestinal IR Injury
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Acosta, S. Epidemiology of mesenteric vascular disease: Clinical implications. Semin. Vasc. Surg. 2010, 23, 4–8. [Google Scholar] [CrossRef]
- Yasuhara, H. Acute mesenteric ischemia: The challenge of gastroenterology. Surg. Today 2005, 35, 185–195. [Google Scholar] [CrossRef] [PubMed]
- Hernandez, L.A.; Grisham, M.B.; Twohig, B.; Arfors, K.E.; Harlan, J.M.; Granger, D.N. Role of neutrophils in ischemia-reperfusion-induced microvascular injury. Am. J. Physiol. 1987, 253, 699–703. [Google Scholar] [CrossRef]
- Souza, D.G.; Vieira, A.T.; Pinho, V.; Sousa, L.P.; Andrade, A.A.; Bonjardim, C.A.; McMillan, M.; Kahn, M.; Teixeira, M.M. NF-kappaB plays a major role during the systemic and local acute inflammatory response following intestinal reperfusion injury. Br. J. Pharmacol. 2005, 145, 246–254. [Google Scholar] [CrossRef] [PubMed]
- Kubes, P.; Hunter, J.; Granger, D.N. Ischemia/reperfusion-induced feline intestinal dysfunction: Importance of granulocyte recruitment. Gastroenterology 1992, 103, 807–812. [Google Scholar] [CrossRef]
- Zou, L.; Attuwaybi, B.; Kone, B.C. Effects of NF-kappa B inhibition on mesenteric ischemia-reperfusion injury. Am. J. Physiol. Gastrointest. Liver Physiol. 2003, 284, 713–721. [Google Scholar] [CrossRef] [PubMed]
- Panes, J.; Perry, M.; Granger, D.N. Leukocyte-endothelial cell adhesion: Avenues for therapeutic intervention. Br. J. Pharmacol. 1999, 126, 537–550. [Google Scholar] [CrossRef]
- Colvin, B.L.; Thomson, A.W. Chemokines, their receptors, and transplant outcome. Transplantation 2002, 74, 149–155. [Google Scholar] [CrossRef][Green Version]
- Grisham, M.B.; Hernandez, L.A.; Granger, D.N. Xanthine oxidase and neutrophil infiltration in intestinal ischemia. Am. J. Physiol. 1986, 251, 567–574. [Google Scholar] [CrossRef]
- Takagi, T.; Naito, Y.; Uchiyama, K.; Yoshikawa, T. The role of heme oxygenase and carbon monoxide in inflammatory bowel disease. Redox. Rep. 2010, 15, 193–201. [Google Scholar] [CrossRef]
- Maines, M.D. The heme oxygenase system: A regulator of second messenger gases. Annu. Rev. Pharmacol. Toxicol. 1997, 37, 517–554. [Google Scholar] [CrossRef] [PubMed]
- Shibahara, S. Regulation of heme oxygenase gene expression. Semin. Hematol. 1988, 25, 370–376. [Google Scholar] [PubMed]
- Igarashi, K.; Sun, J. The heme-Bach1 pathway in the regulation of oxidative stress response and erythroid differentiation. Antioxid. Redox Signal. 2006, 8, 107–118. [Google Scholar] [CrossRef] [PubMed]
- Morse, D.; Choi, A.M. Heme oxygenase-1: From bench to bedside. Am. J. Respir. Crit. Care Med. 2005, 172, 660–670. [Google Scholar] [CrossRef] [PubMed]
- Katada, K.; Takagi, T.; Uchiyama, K.; Naito, Y. Therapeutic roles of carbon monoxide in intestinal ischemia-reperfusion injury. J. Gastroenterol. Hepatol. 2015, 30 (Suppl. S1), 46–52. [Google Scholar] [CrossRef] [PubMed]
- Katada, K.; Bihari, A.; Mizuguchi, S.; Yoshida, N.; Yoshikawa, T.; Fraser, D.D.; Potter, R.F.; Cepinskas, G. Carbon monoxide liberated from CO-releasing molecule (CORM-2) attenuates ischemia/reperfusion (I/R)-induced inflammation in the small intestine. Inflammation 2010, 33, 92–100. [Google Scholar] [CrossRef]
- Mizuguchi, S.; Stephen, J.; Bihari, R.; Markovic, N.; Suehiro, S.; Capretta, A.; Potter, R.F.; Cepinskas, G. CORM-3-derived CO modulates polymorphonuclear leukocyte migration across the vascular endothelium by reducing levels of cell surface-bound elastase. Am. J. Physiol. Heart Circ. Physiol. 2009, 297, 920–929. [Google Scholar] [CrossRef]
- Hervera, A.; Leanez, S.; Motterlini, R.; Pol, O. Treatment with carbon monoxide-releasing molecules and an HO-1 inducer enhances the effects and expression of micro-opioid receptors during neuropathic pain. Anesthesiology 2013, 118, 1180–1197. [Google Scholar] [CrossRef]
- Lush, C.W.; Cepinskas, G.; Kvietys, P.R. Regulation of intestinal nuclear factor-kappaB activity and E-selectin expression during sepsis: A role for peroxynitrite. Gastroenterology 2003, 124, 118–128. [Google Scholar] [CrossRef]
- Sugimoto, N.; Rui, T.; Yang, M.; Bharwani, S.; Handa, O.; Yoshida, N.; Yoshikawa, T.; Kvietys, P.R. Points of control exerted along the macrophage-endothelial cell-polymorphonuclear neutrophil axis by PECAM-1 in the innate immune response of acute colonic inflammation. J. Immunol. 2008, 181, 2145–2154. [Google Scholar] [CrossRef]
- Yoshida, N.; Granger, D.N.; Anderson, D.C.; Rothlein, R.; Lane, C.; Kvietys, P.R. Anoxia/reoxygenation-induced neutrophil adherence to cultured endothelial cells. Am. J. Physiol. 1992, 262, 1891–1898. [Google Scholar] [CrossRef] [PubMed]
- Cepinskas, G.; Savickiene, J.; Ionescu, C.V.; Kvietys, P.R. PMN transendothelial migration decreases nuclear NFkappaB in IL-1beta-activated endothelial cells: Role of PECAM-1. J. Cell Biol. 2003, 161, 641–651. [Google Scholar] [CrossRef] [PubMed]
- Janssen-Heininger, Y.M.; Poynter, M.E.; Baeuerle, P.A. Recent advances towards understanding redox mechanisms in the activation of nuclear factor kappaB. Free Radic. Biol. Med. 2000, 28, 1317–1327. [Google Scholar] [CrossRef]
- Zabalgoitia, M.; Colston, J.T.; Reddy, S.V.; Holt, J.W.; Regan, R.F.; Stec, D.E.; Rimoldi, J.M.; Valente, A.J.; Chandrasekar, B. Carbon monoxide donors or heme oxygenase-1 (HO-1) overexpression blocks interleukin-18-mediated NF-kappaB-PTEN-dependent human cardiac endothelial cell death. Free Radic. Biol. Med. 2008, 44, 284–298. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.D.; Zhang, F.; Shen, G.; Li, Y.B.; Li, Y.H.; Jing, H.R.; Ma, L.F.; Yao, J.H.; Tian, X.F. Sulforaphane protects liver injury induced by intestinal ischemia reperfusion through Nrf2-ARE pathway. World J. Gastroenterol. 2010, 16, 3002–3010. [Google Scholar] [CrossRef]
- Hassoun, H.T.; Kozar, R.A.; Kone, B.C.; Safi, H.J.; Moore, F.A. Intraischemic hypothermia differentially modulates oxidative stress proteins during mesenteric ischemia/reperfusion. Surgery 2002, 132, 369–376. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Zhu, C.; Wang, G.; Xu, R.; Zhu, Y. Higenamine regulates Nrf2-HO-1-Hmgb1 axis and attenuates intestinal ischemia-reperfusion injury in mice. Inflamm. Res. 2015, 64, 395–403. [Google Scholar] [CrossRef]
- Zu, G.; Zhou, T.; Che, N.; Zhang, X. Salvianolic acid A protects against oxidative stress and apoptosis induced by intestinal ischemia-reperfusion injury through activation of Nrf2/HO-1 pathways. Cell. Physiol. Biochem. 2018, 49, 2320–2332. [Google Scholar] [CrossRef]
- Gendy, A.; Soubh, A.; Al-Mokaddem, A.; Kotb El-Sayed, M. Dimethyl fumarate protects against intestinal ischemia/reperfusion lesion: Participation of Nrf2/HO-1, GSK-3beta and Wnt/beta-catenin pathway. Biomed. Pharmacother. 2021, 134, 111130. [Google Scholar] [CrossRef]
- Wang, Y.; Wen, J.; Almoiliqy, M.; Wang, Y.; Liu, Z.; Yang, X.; Lu, X.; Meng, Q.; Peng, J.; Lin, Y.; et al. Sesamin protects against and ameliorates rat intestinal ischemia/reperfusion injury with involvement of activating Nrf2/HO-1/NQO1 signaling pathway. Oxid. Med. Cell. Longev. 2021, 2021, 5147069. [Google Scholar] [CrossRef]
- Guo, X.; Hong, S.; He, H.; Zeng, Y.; Chen, Y.; Mo, X.; Li, J.; Li, L.; Steinmetz, R.; Liu, Q. NFkappaB promotes oxidative stress-induced necrosis and ischemia/reperfusion injury by inhibiting Nrf2-ARE pathway. Free Radic. Biol. Med. 2020, 159, 125–135. [Google Scholar] [CrossRef] [PubMed]
- Sun, M.; Guo, M.; Ma, G.; Zhang, N.; Pan, F.; Fan, X.; Wang, R. MicroRNA-30c-5p protects against myocardial ischemia/reperfusion injury via regulation of Bach1/Nrf2. Toxicol. Appl. Pharmacol. 2021, 426, 115637. [Google Scholar] [CrossRef]
- Yu, S.; Zhai, J.; Yu, J.; Yang, Q.; Yang, J. Downregulation of BACH1 protects against cerebral ischemia/reperfusion injury through the functions of HO-1 and NQO1. Neuroscience 2020, 436, 154–166. [Google Scholar] [CrossRef] [PubMed]
- Xing, Y.; Li, J.; Li, S.P.; Xi, J.; Ma, N.; Liu, L.; Wang, J.S.; Cai, J.Z. MiR-27a-5p regulates apoptosis of liver ischemia-reperfusion injury in mice by targeting Bach1. J. Cell. Biochem. 2018, 119, 10376–10383. [Google Scholar] [CrossRef]
- Otterbein, L.E.; Bach, F.H.; Alam, J.; Soares, M.; Tao Lu, H.; Wysk, M.; Davis, R.J.; Flavell, R.A.; Choi, A.M. Carbon monoxide has anti-inflammatory effects involving the mitogen-activated protein kinase pathway. Nat. Med. 2000, 6, 422–428. [Google Scholar] [CrossRef] [PubMed]
- Nakao, A.; Toyokawa, H.; Tsung, A.; Nalesnik, M.A.; Stolz, D.B.; Kohmoto, J.; Ikeda, A.; Tomiyama, K.; Harada, T.; Takahashi, T.; et al. Ex vivo application of carbon monoxide in University of Wisconsin solution to prevent intestinal cold ischemia/reperfusion injury. Am. J. Transplant. 2006, 6, 2243–2255. [Google Scholar] [CrossRef]
- Obara, T.; Yamamoto, H.; Aokage, T.; Igawa, T.; Nojima, T.; Hirayama, T.; Seya, M.; Ishikawa-Aoyama, M.; Nakao, A.; Motterlini, R.; et al. Luminal administration of a water-soluble carbon monoxide-releasing molecule (CORM-3) mitigates ischemia/reperfusion injury in rats following intestinal transplantation. Transplantation 2021. [Google Scholar] [CrossRef]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Katada, K.; Takagi, T.; Iida, T.; Ueda, T.; Mizushima, K.; Fukui, A.; Okayama, T.; Kamada, K.; Uchiyama, K.; Ishikawa, T.; et al. Role and Potential Mechanism of Heme Oxygenase-1 in Intestinal Ischemia-Reperfusion Injury. Antioxidants 2022, 11, 559. https://doi.org/10.3390/antiox11030559
Katada K, Takagi T, Iida T, Ueda T, Mizushima K, Fukui A, Okayama T, Kamada K, Uchiyama K, Ishikawa T, et al. Role and Potential Mechanism of Heme Oxygenase-1 in Intestinal Ischemia-Reperfusion Injury. Antioxidants. 2022; 11(3):559. https://doi.org/10.3390/antiox11030559
Chicago/Turabian StyleKatada, Kazuhiro, Tomohisa Takagi, Takaya Iida, Tomohiro Ueda, Katsura Mizushima, Akifumi Fukui, Tetsuya Okayama, Kazuhiro Kamada, Kazuhiko Uchiyama, Takeshi Ishikawa, and et al. 2022. "Role and Potential Mechanism of Heme Oxygenase-1 in Intestinal Ischemia-Reperfusion Injury" Antioxidants 11, no. 3: 559. https://doi.org/10.3390/antiox11030559
APA StyleKatada, K., Takagi, T., Iida, T., Ueda, T., Mizushima, K., Fukui, A., Okayama, T., Kamada, K., Uchiyama, K., Ishikawa, T., Naito, Y., & Itoh, Y. (2022). Role and Potential Mechanism of Heme Oxygenase-1 in Intestinal Ischemia-Reperfusion Injury. Antioxidants, 11(3), 559. https://doi.org/10.3390/antiox11030559







