Modelling Virgin Olive Oil Potential Shelf-Life from Antioxidants and Lipid Oxidation Progress
Abstract
:1. Introduction
2. Materials and Methods
2.1. Virgin Olive Oil (VOO) Samples
2.2. Oxidation Experiments
2.3. Analytical Determinations
2.3.1. Peroxide Value (PV), K232 and K270
2.3.2. Phenolic Compounds
2.3.3. Tocopherols
2.3.4. Fatty Acid Composition
2.3.5. Chlorophyll and Carotenoid Compounds
2.3.6. Oxidative Stability
2.4. Statistical Analysis and Treatment of Experimental Data
3. Results and Discussion
3.1. Initial Characteristics of Virgin Olive Oils
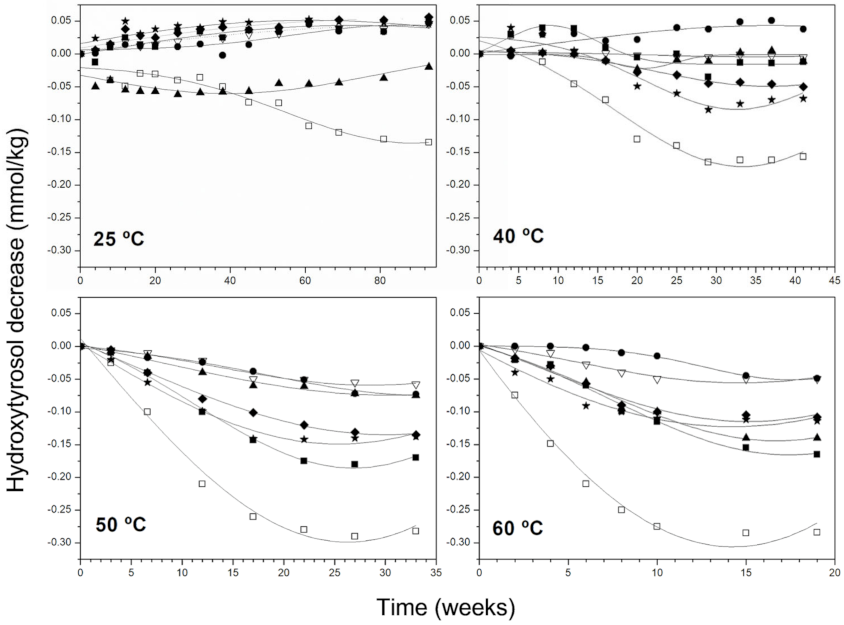
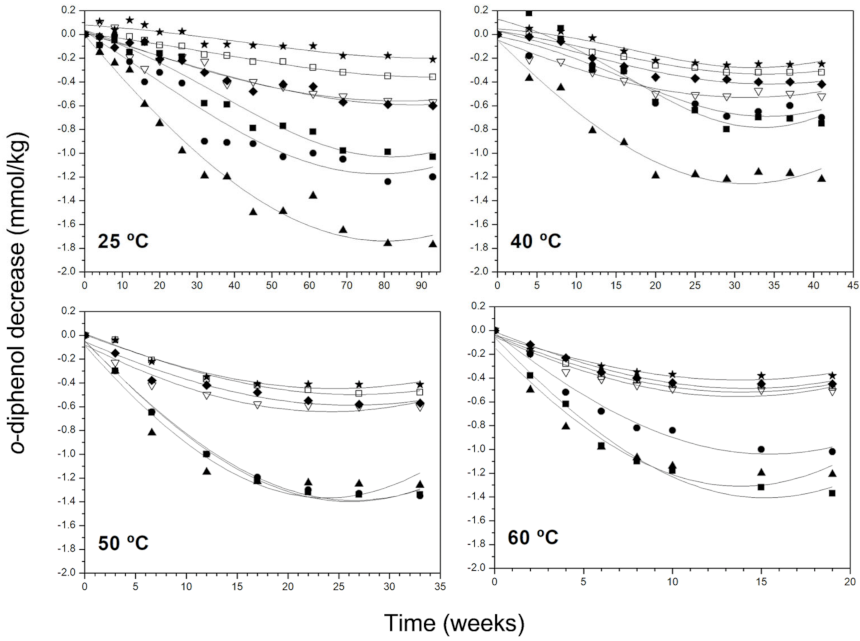
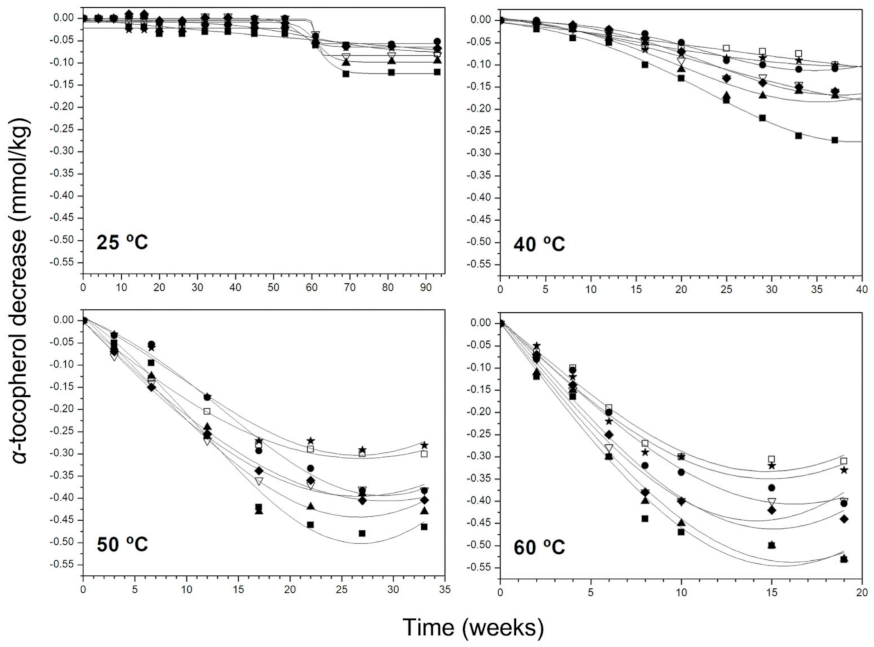
3.2. Kinetic Behaviour of Phenolic Compounds and Pigments Degradation
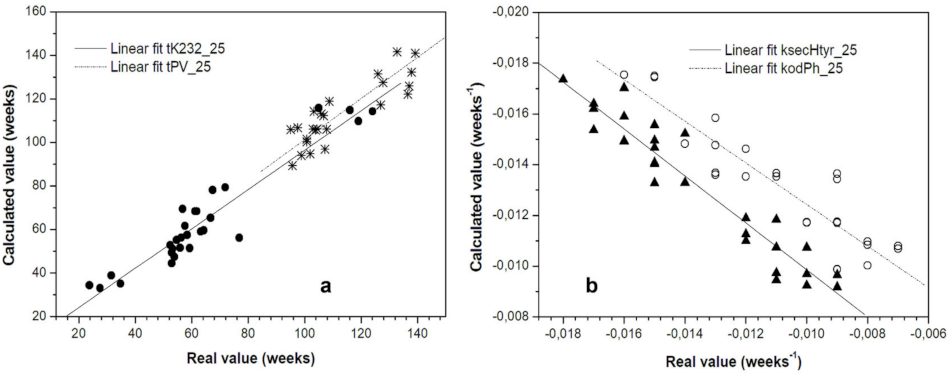
3.3. Feasibility of the Arrhenius Equation
3.4. Shelf-Life of Virgin Olive Oil Related to Its Initial Composition
- -
- tPV_25: time needed at 25 °C to reach the upper legal limit for peroxide value in extra virgin olive oils (20 meq/kg);
- -
- tK232_25: time needed at 25 °C to reach the upper legal limit for K232 in extra virgin olive oils (2.50);
- -
- tK270_25: time needed at 25 °C to reach the upper legal limit for K270 in extra virgin olive oils (0.22).
- -
- kodPh: rate of o-diphenols (hydroxytyrosol and derivatives) decrease at 25 °C;
- -
- ksecHTyr: rate of hydroxytyrosol secoiridoid derivatives decrease at 25 °C.
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gómez-Rico, A.; Inarejos-Garcia, A.; Salvador, M.D.; Fregapane, G. Effect of malaxation conditions on phenol and volatile profiles in olive paste and the corresponding virgin olive oils (Olea europaea L. cv. Cornicabra). J. Agric. Food Chem. 2009, 57, 3587–3595. [Google Scholar] [CrossRef] [PubMed]
- Lanza, B.; Ninfali, P. Antioxidants in Extra Virgin Olive Oil and Table Olives: Connections between Agriculture and Processing for Health Choices. Antioxidants 2020, 9, 41. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Servili, M.; Selvaggini, R.; Taticchi, A.; Esposto, S.; Montedoro, G.F. Air exposure time of olive pastes during the extraction process and phenolic and volatile composition of virgin olive oil. J. Am. Oils Chem. Soc. 2003, 80, 685–695. [Google Scholar] [CrossRef]
- Kiritsakis, A.; Kanavouras, A.; Kiritsakis, K. Review: Chemical analysis-quality control and packaging issues of olive oil. Eur. J. Lipids Sci. Technol. 2002, 104, 628–638. [Google Scholar] [CrossRef]
- Pristouri, G.; Badeka, A.; Kontominas, M.G. Effect of packaging material headspace, oxygen and light transmission, temperature and storage time on quality characteristics of extra virgin olive oil. Food Control 2010, 21, 412–418. [Google Scholar] [CrossRef]
- Coutelieris, F.A.; Kanavouras, A. Experimental and theoretical investigation of packaged olive oil: Development of a quality indicator based on mathematical predictions. J. Food Eng. 2006, 73, 85–92. [Google Scholar] [CrossRef]
- Guillaume, C.; Ravetti, L. Shelf-Life Prediction of Extra Virgin Olive Oils Using an Empirical Model Based on Standard Quality Tests. J. Chem. 2016, 2016, 6393962. [Google Scholar] [CrossRef] [Green Version]
- Servili, M.; Sordini, B.; Esposto, S.; Urbani, S.; Veneziani, G.; Di Maio, I.; Selvaggini, R.; Taticchi, A. Biological Activities of Phenolic Compounds of Extra Virgin Olive Oil. Antioxidants 2014, 3, 1–23. [Google Scholar] [CrossRef] [Green Version]
- EFSA Panel on Dietetic Products, Nutrition and Allergies (NDA). Scientific Opinion on the substantiation of health claims related to polyphenols in olive and protection of LDL particles from oxidative damage. EFSA J. 2011, 9, 1–25. [Google Scholar]
- Commision Regulation (EEC) No. 432/2012 Establishing a List of Permitted Health Claims Made on Foods, Other Than Those Referring to the Reduction of Disease Risk and to Children’s Development and Health Text with EEA Relevance. Available online: http://data.europa.eu/eli/reg/2012/432/oj (accessed on 28 January 2022).
- Commission Regulation (EEC) No. 2016/2095 amending Regulation (EEC) No 2568/91 on the Characteristics of Olive Oil and Olive-Residue Oil and on the Relevant Methods of Analysis. Available online: http://data.europa.eu/eli/reg_del/2016/2095/oj (accessed on 28 January 2022).
- Kaya, A.; Tekin, A.R.; Öner, D. Oxidative stability of sunflower and olive oils: Comparison between a modified active oxygen method and long-term storage. Lebensm. Wiss. Technol. 1993, 26, 464–468. [Google Scholar] [CrossRef]
- Farhoosh, R.; Hoseini-Yazdi, S.-Z. Shelf-life prediction of olive oils using empirical models developed at low and high temperatures. Food Chem. 2013, 141, 557–565. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, N.; Dias, L.G.; Veloso, A.C.A.; Pereira, J.A.; Peres, A.M. Evaluation of extra-virgin olive oils shelf-life using an electronic tongue—chemometric approach. Eur. Food Res. Technol. 2017, 243, 597–607. [Google Scholar] [CrossRef] [Green Version]
- Li, X.; Wang, S.C. Shelf Life of Extra Virgin Olive Oil and Its Prediction Models. J. Food Qual. 2018, 2018, 1–15. [Google Scholar] [CrossRef] [Green Version]
- Conte, L.; Milani, A.; Calligaris, S.; Rovellini, P.; Lucci, P.; Nicoli, M.C. Temperature Dependence of Oxidation Kinetics of Extra Virgin Olive Oil (EVOO) and Shelf-Life Prediction. Foods 2020, 9, 295. [Google Scholar] [CrossRef] [Green Version]
- Zanoni, B.; Bertuccioli, M.; Rovellini, P.; Marotta, F.; Mattei, A. A preliminary approach to predictive modelling of extra virgin olive oil stability. J. Sci. Food Agric. 2005, 85, 1492–1498. [Google Scholar] [CrossRef]
- Mancebo-Campos, V.; Fregapane, G.; Salvador, M.D. Kinetic study for the development of an accelerated oxidative stability test to estimate virgin olive oil potential shelf life. Eur. J. Lipid Sci. Technol. 2008, 110, 969–976. [Google Scholar] [CrossRef]
- Commission Regulation (EEC) No. 2568/91 on the Characteristics of Olive Oil and Olive-Residue Oil and on the Relevant Methods of Analysis. Available online: http://data.europa.eu/eli/reg/1991/2568/oj (accessed on 28 January 2022).
- Mateos, R.; Domínguez, M.M.; Espartero, J.L.; Cert, A. Antioxidant effect of phenolic compounds, tocopherol and other minor components in virgin olive oil. J. Agric. Food Chem. 2003, 51, 7170–7175. [Google Scholar] [CrossRef]
- Mancebo-Campos, V.; Salvador, M.D.; Fregapane, G. Comparative study of virgin olive oil behaviour under Rancimat accelerated oxidation conditions and long-term room temperature storage. J. Agric. Food Chem. 2007, 55, 8231–8236. [Google Scholar] [CrossRef]
- Dobarganes, M.C.; Pérez-Camino, M.C.; Márquez-Ruíz, G. High Performance Size Exclusion Chromatography of Polar Compounds in Heated and Non-Heated Fats. Eur. J. Lipid Sci. Technol. 1988, 90, 308–311. [Google Scholar] [CrossRef]
- Minguez-Mosquera, M.I.; Gandul-Rojas, B.; Garrido-Fernandez, J.; Gallardo-Guerrero, L. Pigments present in virgin olive oil. J. Am. Oils Chem. Soc. 1990, 67, 192–196. [Google Scholar] [CrossRef]
- Gutiérrez, F. Determinación de la estabilidad oxidativa de aceites de oliva vírgenes: Comparación entre el método del Oxígeno Activo (A.O.M.) y el método Rancimat. Grasas Aceites 1989, 40, 1–5. [Google Scholar]
- Arrhenius, S. Über die Reaktionsgeschwindigkeit bei der Inversion von Rohrzucker durch Säuren. Zeitschrift für Physikalische Chemie 1889, 4, 226–248. [Google Scholar] [CrossRef] [Green Version]
- IUPAC: IUPAC Compendium of Chemical Terminology. Electronic Version. Available online: http://goldbook.iupac.org/M03963.htlm (accessed on 28 January 2022).
- Heberger, K.; Kemeny, S.; Vidoczy, T. On the errors of the Arrhenius parameters and estimated rate constant values. Int. J. Chem. Kinet. 1987, 19, 171–181. [Google Scholar] [CrossRef]
- Commission Regulation (EEC) No. 1989/2003. Available online: http://data.europa.eu/eli/reg/2003/1989/oj (accessed on 28 January 2022).
- Baldioli, M.; Servili, M.; Perretti, G.; Montedoro, G. Antioxidant activity of tocopherols and phenolic compounds in virgin olive oil. J. Am. Oils Chem. Soc. 1996, 73, 1589–1593. [Google Scholar] [CrossRef]
- Gómez-Alonso, S.; Salvador, M.D.; Fregapane, G. Phenolic compounds profile of Cornicabra virgin olive oil. J. Agric. Food Chem. 2002, 50, 6812–6817. [Google Scholar] [CrossRef]
- Gómez-Alonso, S.; Mancebo-Campos, V.; Salvador, M.D.; Fregapane, G. Evolution of major and minor components and oxidation indices of virgin olive oil during 21 months storage at room temperature. Food Chem. 2007, 100, 36–42. [Google Scholar] [CrossRef]
- Brenes, M.; Garcia, A.; Garcia, P.; Garrido, A. Acid hydrolysis of secoiridoid aglycons during storage of virgin olive oil. J. Agric. Food Chem. 2001, 49, 5609–5614. [Google Scholar] [CrossRef]
- Mancebo-Campos, V.; Salvador, M.D.; Fregapane, G. Antioxidant capacity of individual and combined virgin olive oil minor compounds evaluated at mild temperature (25 and 40 °C) as compared to accelerated and antiradical assays. Food Chem. 2014, 150, 374–381. [Google Scholar] [CrossRef]
- Krichene, D.; Allalout, A.; Mancebo-Campos, V.; Salvador, M.D.; Zarrouk, M.; Fregapane, G. Stability of virgin olive oil and behaviour of its natural antioxidants under medium temperature accelerated storage conditions. Food Chem. 2010, 121, 171–177. [Google Scholar] [CrossRef]
- Krichene, D.; Salvador, M.D.; Fregapane, G. Stability of virgin olive oil phenolic compounds during long-term storage (18 months) at temperatures of 5−50 °C. J. Agric. Food Chem. 2015, 63, 6779–6786. [Google Scholar] [CrossRef]
- Lavelli, V.; Fregapane, G.; Salvador, M.D. Effect of storage on secoiridoid and tocopherol contents and antioxidant activity of monovarietal extra virgin olive oils. J. Agric. Food Chem. 2006, 54, 3002–3007. [Google Scholar] [CrossRef] [PubMed]
- Verleyen, T.; Kamal-Eldin, A.; Dobarganes, C.; Verhe, R.; Dewettinck, K.; Huyghebaert, A. Modelling of R-tocopherol loss and oxidation products formed during thermoxidation in triolein and tripalmitin mixtures. Lipids 2001, 36, 719–726. [Google Scholar] [CrossRef] [PubMed]
- Fakourelis, N.; Lee, E.C.; Min, D.B. Effects of chlorophyll and β-carotene on the oxidation stability of olive oil. J. Food Sci. 1987, 52, 234–235. [Google Scholar] [CrossRef]
- Yanishlieva, N.V.; Aitzetmüller, K.; Ravena, V.G. β-Carotene and lipid oxidation. Reviews. Fett. Lipid 1998, 100, 444–462. [Google Scholar] [CrossRef]
- Hrncirik, K.; Fritsche, S. Relation between the Endogenous Antioxidant System and the Quality of Extra Virgin Olive Oil under Accelerated Storage Conditions. J. Agric. Food Chem. 2005, 53, 2103–2110. [Google Scholar] [CrossRef]
- Subagio, A.; Morita, N. Instability of carotenoids is a reason for their promotion on lipid oxidation. Food Res. Int. 2001, 34, 183–188. [Google Scholar] [CrossRef]
- Ceballos, C.; Moyano, M.J.; Vicario, I.M.; Alba, J.; Heredia, F.J. Chromatic evaluation of virgin olive oils submitted to an accelerated test. J. Am. Oils Chem. Soc. 2003, 80, 257–262. [Google Scholar] [CrossRef]
- Endo, Y.; Usuki, R.; Kaneda, T. Antioxidant effects of chlorophyll and pheophytin on the autoxidation of oils in the dark. I. Comparison of the inhibitory effects. J. Am. Oils Chem. Soc. 1985, 62, 1375–1378. [Google Scholar] [CrossRef]
- Endo, Y.; Usuki, R.; Kaneda, T. Antioxidant effects of chlorophyll and pheophytin on the autoxidation of oils in the dark. II. The mechanism of antioxidative action of chlorophylls. J. Am. Oils Chem. Soc. 1985, 62, 1387–1390. [Google Scholar] [CrossRef]
- Gertz, C.; Fiebig, H.J. Pyropheophytin a: Determination of thermal degradation products of cholorohyll a in virgin olive oil. Eur. J. Lipid Sci. Technol. 2006, 108, 1062–1065. [Google Scholar] [CrossRef]
- Campanella, L.; Nuccilli, A.; Tomassetti, M.; Vecchio, S. Biosensor analysis for the kinetic study of polyphenols deterioration during the forced thermal oxidation of extra-virgin olive oil. Talanta 2008, 74, 1287–1298. [Google Scholar] [CrossRef]
- Uri, N. Physico-chemical aspects of autoxidation. In Autoxidation and Antioxidants; Lundberg, W.O., Ed.; Inter-Science Publishers: New York, NY, USA, 1961; pp. 55–106. [Google Scholar]
- Cho, H.Y. Reaction mechanisms and kinetics of antioxidant using Arrhenius equation in soybean oil oxidation. J. Food Sci. Nutr. 1997, 2, 6–10. [Google Scholar]
- Van Boekel, M.A.J.S. Kinetic modelling of reactions in food: A critical review. Compr. Rev. Food Sci. Food Saf. 2008, 7, 144–158. [Google Scholar] [CrossRef]
- Velasco, J.; Dobarganes, M.C. Oxidative stability of virgin olive oil. Eur. J. Lipid Sci. Technol. 2002, 104, 661–676. [Google Scholar] [CrossRef]
- Beltran, G.; Rio, C.D.; Sanchez, S.; Martinez, L. Influence of harvest date and crop yield on the fatty acid composition of virgin olive oils from Cv. Picual. J. Agric. Food Chem. 2004, 52, 3434–3440. [Google Scholar] [CrossRef] [PubMed]
| VOO Samples | |||||||
|---|---|---|---|---|---|---|---|
| I | II | III | IV | V | VI | VII | |
| PV (meq O2/kg) | 5.5 ± 0.04 d | 5.5 ± 0.01 d | 3.1 ± 0.01 b | 3.7 ± 0.01 c | 2.9 ± 0.01 a | 6.5 ± 0.01 f | 5.8 ± 0.00 e |
| K232 | 1.93 ± 0.03 f | 1.87 ± 0.04 e | 1.73 ± 0.01 d | 1.61 ± 0.01 a,b | 1.56 ± 0.01 a | 1.67 ± 0.03 b,c | 1.72 ± 0.01 c,d |
| K270 | 0.16 ± 0.01 e | 0.16 ± 0.01 e | 0.13 ± 0.00 c,d | 0.12 ± 0.01 b,c | 0.10 ± 0.01 a | 0.12 ± 0.00 b | 0.14 ± 0.00 c,d |
| C18:1 (%) | 79.00 ± 0.04 a | 81.52 ± 0.01 g | 81.02 ± 0.01 f | 79.37 ± 0.03 b | 80.92 ± 0.01 e | 80.07 ± 0.01 c | 80.75 ± 0.01 d |
| C18:2 (%) | 5.09 ± 0.01 d | 3.61 ± 0.01 a | 3.87 ± 0.01 b | 5.29 ± 0.01 e | 5.32 ± 0.01 f | 4.94 ± 0.01 c | 5.31 ± 0.01 e,f |
| C18:3 (%) | 0.71 ± 0.01 e | 0.59 ± 0.01 c | 0.65 ± 0.01 d | 0.58 ± 0.01 b | 0.58 ± 0.01 b | 0.54 ± 0.01 a | 0.57 ± 0.01 b |
| Chlorophyll (1) | 11.40 ± 0.10 e | 42.97 ± 0.09 g | 19.57 ± 0.04 f | 3.83 ± 0.07 c | 2.20 ± 0.04 a | 6.79 ± 0.17 d | 2.58 ± 0.02 b |
| Carotenoids (1) | 6.32 ± 0.01 e | 15.27 ± 0.01 g | 12.89 ± 0.01 f | 4.12 ± 0.00 c | 2.71 ± 0.00 a | 4.49 ± 0.01 d | 3.09 ± 0.01 b |
| Htyr (2) (3,4-DHPEA) | 0.16 ± 0.00 d | 0.10 ± 0.00 c | 0.19 ± 0.02 e | 0.03 ± 0.00 a | 0.06 ± 0.00 b | 0.27 ± 0.01 f | 0.07 ± 0.00 b |
| 3,4-DHPEA-EDA | 1.48 ± 0.00 d | 1.60 ± 0.21 d | 0.89 ± 0.03 c | 0.30 ± 0.03 b | 0.34 ± 0.01 b | 0.05 ± 0.01 a | 0.19 ± 0.00 a,b |
| 3,4-DHPEA-EA | 0.40 ± 0.00 c | 0.47 ± 0.04 d | 1.38 ± 0.05 e | 0.38 ± 0.01 c | 0.31 ± 0.01 b | 0.27 ± 0.00 a,b | 0.25 ± 0.01 a |
| Sec. Htyr (2) | 1.88 ± 0.00 d | 2.07 ± 0.25 d,e | 2.27 ± 0.02 e | 0.68 ± 0.05 c | 0.64 ± 0.02 b,c | 0.32 ± 0.01 a | 0.43 ± 0.00 a,b |
| Tyr (2) (p-HPEA) | 0.12 ± 0.00 c | 0.10 ± 0.01 b | 0.12 ± 0.01 c | 0.04 ± 0.00 a | 0.06 ± 0.01 a | 0.54 ± 0.01 d | 0.09 ± 0.01 b |
| p-HPEA-EDA | 1.29 ± 0.00 d | 1.36 ± 0.17 d | 0.73 ± 0.03 c | 0.47 ± 0.01 b | 0.45 ± 0.00 b | 0.06 ± 0.01 a | 0.33 ± 0.01 b |
| p-HPEA-EA | 0.24 ± 0.00 c,d | 0.26 ± 0.03 d | 0.58 ± 0.04 e | 0.21 ± 0.01 b,c | 0.17 ± 0.00 a,b | 0.18 ± 0.01 a,b | 0.16 ± 0.00 a |
| Sect. Tyr (2) | 1.53 ± 0.00 e | 1.61 ± 0.20 e | 1.30 ± 0.01 d | 0.68 ± 0.02 c | 0.63 ± 0.01 b,c | 0.24 ± 0.01 a | 0.49 ± 0.01 b |
| o-diphenols (2) | 2.05 ± 0.00 b | 2.16 ± 0.25 b | 2.46 ± 0.00 c | 0.71 ± 0.04 a | 0.71 ± 0.03 a | 0.58 ± 0.01 a | 0.51 ± 0.01 a |
| Total phenols (2) * | 3.70 ± 0.00 c | 3.88 ± 0.45 c | 3.88 ± 0.00 c | 1.41 ± 0.06 b | 1.38 ± 0.03 b | 1.35 ± 0.02 b | 1.08 ± 0.00 a |
| o-diphenols/Total phenols | 0.55 ± 0.03 d | 0.56 ± 0.11 d | 0.63 ± 0.06 e | 0.51 ± 0.07 c | 0.51 ± 0.03 c | 0.43 ± 0.03 a | 0.47 ± 0.02 b |
| Sec. Htyr/Free Htyr | 11.53 ± 0.11 d | 21.14 ± 0.12 e | 12.23 ± 0.10 d | 31.00 ± 0.18 f | 10.46 ± 0.12 c | 1.21 ± 0.03 a | 5.95 ±0.08 b |
| Complex/Simple Phenols | 11.99 ± 0.11 c | 18.43 ± 0.13 d | 11.55 ± 0.15 c | 25.30 ± 0.19 e | 11.42 ± 0.12 c | 0.69 ±0.02 a | 6.02 ± 0.09 b |
| α-Tocopherol (2) | 0.55 ± 0.01 e | 0.44 ± 0.01 c | 0.53 ± 0.01 e | 0.38 ± 0.01 b | 0.45 ± 0.01 d | 0.33 ± 0.01 a | 0.36 ± 0.01 b |
| α-Tocopherol/o-diphenols | 0.27 ± 0.05 b | 0.20 ± 0.03 a | 0.22 ± 0.02 a | 0.54 ± 0.05 c | 0.63 ± 0.04 e | 0.57 ± 0.04 d | 0.71 ± 0.03 f |
| Stability (h) | 133.2 ± 2.5 d | 158.0 ± 0.4 e | 138.7 ± 7.2 d | 80.6 ± 0.8 b,c | 82.2 ± 0.8 c | 69.9 ± 2.5 a | 75.7 ± 1.1 b |
| Hydroxytyrosol | Tyrosol | α-Tocopherol | ||||
|---|---|---|---|---|---|---|
| Ea (kJ/mol) | A (week−1) | Ea (kJ/mol) | A (week−1) | Ea (kJ/mol) | A (week−1) | |
| I | 81.7 ± 13.8 a | 9.4∙10+10 ± 1.9∙10+02 a | 43.3 ± 15.2 a,b | 3.0∙10+04 ± 3.3∙10+02 a | 85.3 ± 5.3 a | 1.4∙10+12 ± 7.6 b |
| II | 49.7 ± 8.4 a | 2.3∙10+05 ± 2.5∙10+01 b | 91.1 ± 37.7 b | 3.4∙10+12 ± 1.7∙10+06 b | 93.4 ± 10.7 a | 1.6∙10+13 ± 5.8∙10+01 a |
| III | 84.6 ± 46.5 a | 1.2∙10+11 ± 4.8∙10+07 c | 66.5 ± 11.3 a,b | 2.1∙10+08 ± 7.5∙10+01 c | 88.4 ± 9.9 a | 3.8∙10+12 ± 4.4∙10+01 c |
| IV | 64.2 ± 21 a | 5.4∙10+07 ± 2.9∙10+03 d | 57.0 ± 12.2 a,b | 6.4∙10+06 ± 1.0∙10+02 d | 92.5 ± 6.8 a | 1.6∙10+13 ± 1.3∙10+01 a |
| V | 73.3 ± 6.5 a | 3.7∙10+09 ± 1.2∙10+01 e | 27.4 ± 18.5 a | 3.5∙10+01 ± 1.1∙10+03 a | 92.0 ± 7.8 a | 1.3∙10+13 ± 1.9∙10+01 d |
| VI | 71.2 ± 4.8 a | 4.7∙10+09 ± 6.2 f | 55.9 ± 8.7 a,b | 1.3∙10+07 ± 2.7∙10+01 e | 85.1 ± 14.9 a | 7.0∙10+11 ± 2.9∙10+02 e |
| VII | 80.0 ± 14.7 a | 5.8∙10+10 ± 2.7∙10+02 g | 49.6 ± 14.9 a,b | 1.7∙10+05 ± 2.8∙10+02 a | 92.9 ± 12.1 a | 1.3∙10+13 ± 1.0∙10+02 f |
| 0.623 ≤ R2 ≤ 0.991 | 0.5240 ≤ R2 ≤ 0.954 | 0.942 ≤ R2 ≤ 0.992 | ||||
| o-Diphenols | Total Phenols | Sec. Hydroxytyrosol | Sec. Tyrosol | |||||
|---|---|---|---|---|---|---|---|---|
| Ea (kJ/mol) | A (week−1) | Ea (kJ/mol) | A (week−1) | Ea (kJ/mol) | A (week−1) | Ea (kJ/mol) | A (week−1) | |
| I | 66.9 ± 3.4 a,b | 4.9∙10+09 ± 4 a | 58.6 ± 7.8 a | 1.4∙10+08 ± 19 a | 65.7 ± 3.5 a,b | 3.6∙10+09 ± 4 a | 53.79 ± 14.1 a | 1.9∙10+07 ± 220 a |
| II | 59.4 ± 5.6 a | 2.5∙10+08 ± 8 b | 52.0 ± 11.3 a | 1.1∙10+07 ± 73 b | 60.7 ± 6.0 a | 4.8∙10+08 ± 10 b | 41.26 ± 7.9 a | 1.5∙10+05 ± 20 b |
| III | 58.6 ± 5.9 a | 3.1∙10+08 ± 9 b | 53.3 ± 8.2 a | 2.7∙10+07 ± 23 c | 61.2 ± 7.1 a | 1.0∙10+09 ± 15 c | 44.04 ± 3.6 a | 7.2∙10+05 ± 4 c |
| IV | 74.6 ± 7.1 a,b,c | 1.4∙10+11 ± 15 c | 56.1 ± 10.1 a | 6.1∙10+07 ± 46 d | 68.7 ± 6.3 a,b | 2.0∙10+10 ± 11 d | 56.85 ± 12.3 a | 7.6∙10+07 ± 110 d |
| V | 71.5 ± 6.1 a,b,c | 3.8∙10+10 ± 10 d | 51.3 ± 7.8 a | 9.8∙10+06 ± 19 e | 62.0 ± 5.3 a | 1.4∙10+09 ± 7 e | 43.50 ± 12.3 a | 4.6∙10+05 ± 110 e |
| VI | 77.0 ± 3.1 b,c | 3.2∙10+11 ± 3 e | 63.4 ± 2.5 a | 8.4∙10+08 ± 3 f | 63.4 ± 2.0 a | 2.2∙10+09 ± 2 f | 59.36 ± 4.7 a | 2.4∙10+08 ± 6 f |
| VII | 84.2 ± 8.4 c | 5.2∙10+12 ± 24 f | 67.8 ± 2.4 a | 4.5∙10+09 ± 3 g | 73.9 ± 6.1 b | 1.1∙10+11 ± 10 g | 55.74 ± 3.9 a | 3.6∙10+07 ± 4 g |
| 0.980 ≤ R2 ≤ 0.997 | 0.914 ≤ R2 ≤ 0.998 | 0.981 ≤ R2 ≤ 0.998 | 0.862 ≤ R2 ≤ 0.991 | |||||
| Variables Related to Initial State | Variables Related to Oxidation Progress | ||
|---|---|---|---|
| Abbreviations | Meaning | Abbreviations | Meaning |
| CAROT | Carotens initial content | kPV | Rate constant of peroxide value |
| CHLOR | Chlorophyls initial content | kK232 | Rate constant of K232 |
| HTyr | Hydroxytyrosol initial content | kK270 | Rate constant of K270 |
| Tyr | Tyrosol initial content | kTPh | Rate constant of total phenols |
| TPh | Total Phenols initial content | kodPh | Rate constant of o-diphenols |
| secHTyr | Hydroxytyrosol secoiridois initial content | ksecHTyr | Rate constant of Hydroxytyrosol secoiridois |
| secTyr | Tyrosol secoiridois initial content | kαToh | Rate constant of α-tocopherol |
| odPh | o-diphenols initial content | kUFAs | Rate constant of Unsaturated Fatty Acids |
| Tyr + Der | Tyrosol + Tyrosol secoiridois initial content | kPUFAs | Rate constant of Polyunsaturated Fatty Acids |
| αToh | α-tocopherol initial content | tPV | Time to reach PV = 20 meq/kg |
| UFAs | Unsaturated Fatty Acids initial content | tK232 | Time to reach K232 = 2.50 |
| PUFAs | Polyunsaturated Fatty Acids initial content | tK270 | Time to reach K270 = 0.22 |
| PV | Initial Peroxide Value | T | Temperature of the oxidation experiment |
| K232 | Initial K232 | ||
| K270 | Initial K270 | ||
| RancimatOS | Rancimat Oxidative Stability | ||
| Component Matrix a (Loadings) | Rotated Component Matrix a (Loadings) | Components Score Coeficient Matrix | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Component | Component | Component | |||||||
| 1 | 2 | 3 | 1: Non Oxidisable Substrate | 2: Initial Oxidation State and Conditions | 3: Simple Phenols | 1: Non Oxidisable Substrate | 2: Initial Oxidation State and Conditions | 3: Simple Phenols | |
| T | −0.183 | 0.538 | 0.627 | −0.178 | 0.822 | −0.094 | −0.020 | 0.183 | 0.423 |
| PV | −0.341 | 0.720 | 0.257 | −0.295 | 0.716 | 0.318 | −0.038 | 0.246 | 0.173 |
| K232 | 0.152 | 0.731 | 0.274 | 0.195 | 0.725 | 0.261 | 0.017 | 0.249 | 0.185 |
| K270 | 0.529 | 0.539 | 0.327 | 0.550 | 0.610 | 0.055 | 0.059 | 0.184 | 0.221 |
| CAROT | 0.864 | 0.052 | −0.002 | 0.863 | 0.021 | −0.055 | 0.096 | 0.018 | −0.001 |
| CHLOR | 0.778 | 0.105 | 0.057 | 0.778 | 0.101 | −0.055 | 0.086 | 0.036 | 0.038 |
| HTyr | 0.015 | 0.762 | −0.515 | 0.113 | 0.229 | 0.884 | 0.002 | 0.260 | −0.347 |
| Tyr | −0.312 | 0.691 | −0.635 | −0.210 | 0.102 | 0.961 | −0.035 | 0.236 | −0.429 |
| TPh | 0.968 | 0.010 | −0.148 | 0.973 | −0.110 | 0.015 | 0.107 | 0.003 | −0.100 |
| secHTyr | 0.974 | −0.138 | −0.040 | 0.959 | −0.149 | −0.163 | 0.108 | −0.047 | −0.027 |
| secTyr | 0.933 | −0.190 | 0.069 | 0.907 | −0.115 | −0.274 | 0.103 | −0.065 | 0.046 |
| odPh | 0.973 | −0.049 | −0.102 | 0.970 | −0.124 | −0.058 | 0.108 | −0.017 | −0.069 |
| Tyr + Der | 0.919 | 0.106 | −0.218 | 0.937 | −0.084 | 0.136 | 0.102 | 0.036 | −0.147 |
| αToh | 0.726 | −0.147 | 0.283 | 0.691 | 0.063 | −0.384 | 0.080 | −0.050 | 0.191 |
| UFAs | −0.686 | −0.297 | −0.020 | −0.706 | −0.222 | −0.108 | −0.076 | −0.101 | −0.014 |
| PUFAs | −0.832 | −0.221 | 0.047 | −0.849 | −0.118 | −0.093 | −0.092 | −0.075 | 0.031 |
| RancimatOS | 0.945 | −0.005 | −0.008 | 0.940 | −0.028 | −0.096 | 0.105 | −0.002 | −0.006 |
| Extraction method: Principal Components Analysis | Rotation method: Varimax. Highest loadings in grey color | Rotation method: Without rotation Components score | |||||||
| Dependent Variable | Models by Stepwise MLR | R2 |
|---|---|---|
| tPV_25 | 42.56 + 17.85 degradation rates −16.57 initial oxidation state and conditions −14.20 free simple phenols +8.51 non-oxidizable substrate | 0.575 |
| tK232_25 | 20.15 +7.19 degradation rates −8.74 initial oxidation state and conditions −9.53 free simple phenols +5.51 non-oxidizable substrate | 0.501 |
| kodPh_25 | −0.095 + 0.082 degradation rates + 0.011 non-oxidizable substrate | 0.937 |
| ksecHTyr_25 | −0.106 + 0.087 degradation rates +0.006 non-oxidizable substrate +0.004 initial oxidation state and conditions | 0.969 |
| tPV_25 | 71.78+ 4.92 tK232_40 + 1211.12 kodPh_40+ 8.47 tPV_60 | 0.881 |
| tK232_25 | 6.23 + 7.63 tK232_40 + 490.05 kodPh_40 | 0.904 |
| kodPh_25 | −0.008 −0.005 kPV_50 + 0.001PV − 0.001 tK232_50 | 0.901 |
| ksecHTyr_25 | −0.029 + 0.114 K270 + 8.104 × 10−5 PUFAs + 0.111 kTPh_50 + 0.004 kUFAs_50 | 0.915 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mancebo-Campos, V.; Salvador, M.D.; Fregapane, G. Modelling Virgin Olive Oil Potential Shelf-Life from Antioxidants and Lipid Oxidation Progress. Antioxidants 2022, 11, 539. https://doi.org/10.3390/antiox11030539
Mancebo-Campos V, Salvador MD, Fregapane G. Modelling Virgin Olive Oil Potential Shelf-Life from Antioxidants and Lipid Oxidation Progress. Antioxidants. 2022; 11(3):539. https://doi.org/10.3390/antiox11030539
Chicago/Turabian StyleMancebo-Campos, Vanessa, María Desamparados Salvador, and Giuseppe Fregapane. 2022. "Modelling Virgin Olive Oil Potential Shelf-Life from Antioxidants and Lipid Oxidation Progress" Antioxidants 11, no. 3: 539. https://doi.org/10.3390/antiox11030539
APA StyleMancebo-Campos, V., Salvador, M. D., & Fregapane, G. (2022). Modelling Virgin Olive Oil Potential Shelf-Life from Antioxidants and Lipid Oxidation Progress. Antioxidants, 11(3), 539. https://doi.org/10.3390/antiox11030539






