Chondroprotective Effects of 4,5-Dicaffeoylquinic Acid in Osteoarthritis through NF-κB Signaling Inhibition
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Cell Culture
2.3. Cell Viability
2.4. Measurement of Nitric Oxide and PGE2 Production
2.5. Western Blotting Analysis
2.6. Aggrecan ELISA Analysis
2.7. Gelatin Zymography
2.8. Animals
2.9. DMM-Induced OA Model in Rats
2.10. Histology Analysis and Staining
2.11. Statistical Analysis
3. Results
3.1. Effect of 4,5-diCQA on the Viability of Rat Primary Chondrocytes
3.2. Effects of 4,5-diCQA on IL-1β-Induced Nitrite and PGE2 Expression in Rat Primary Chondrocytes
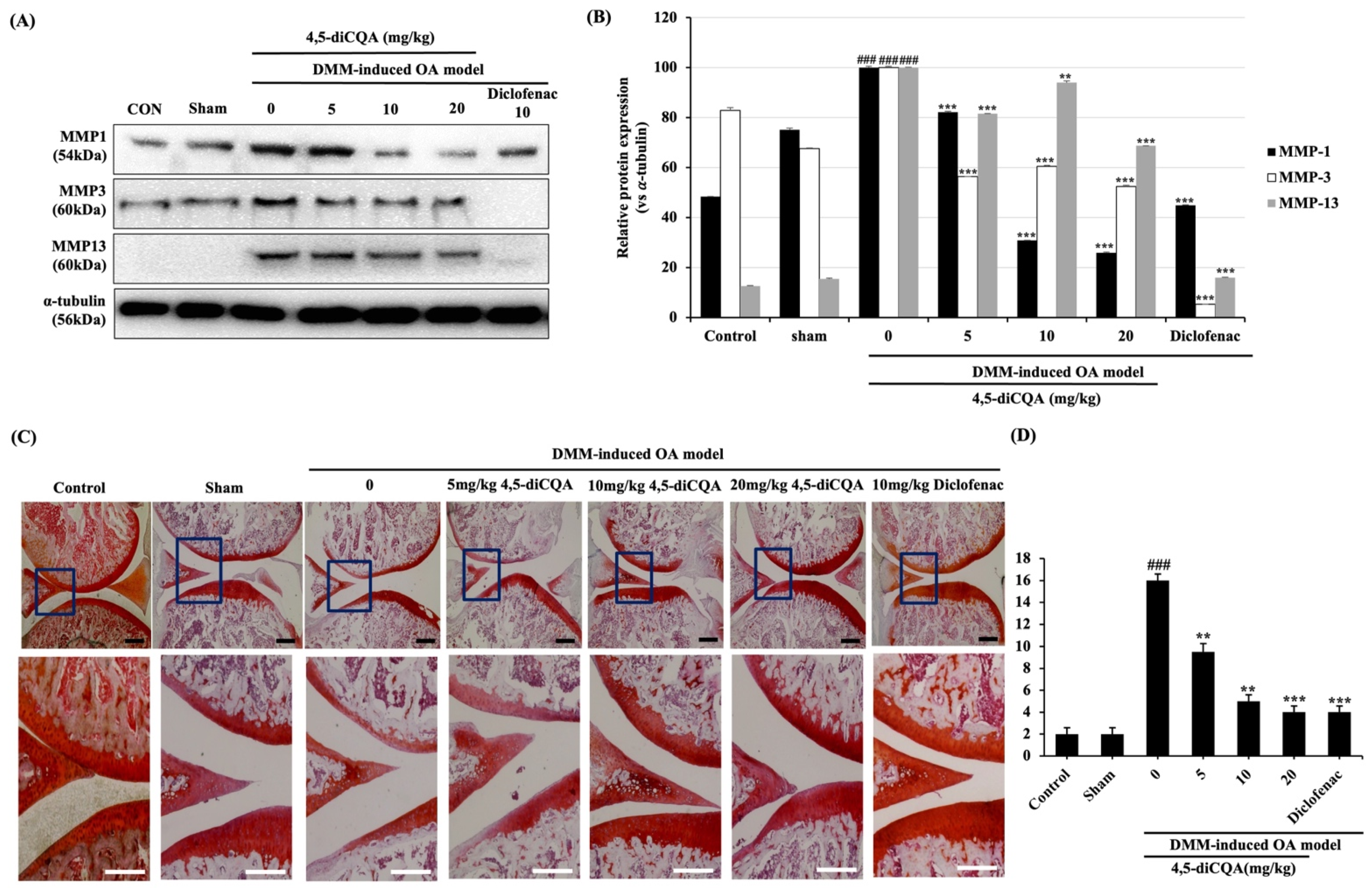
3.3. Effects of 4,5-diCQA on IL-1β-Induced Expression of Matrix-Degrading Enzymes in Rat Primary Chondrocytes
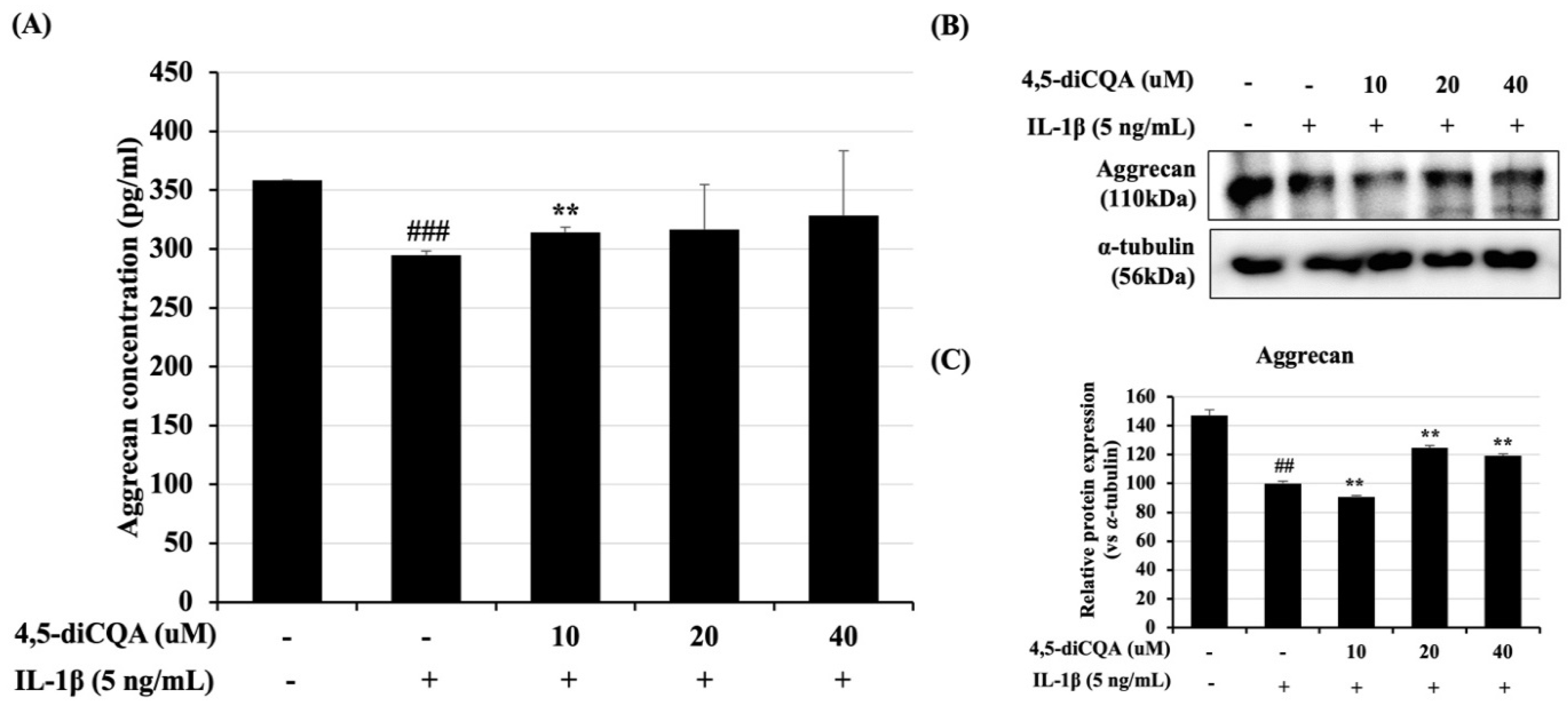
3.4. Effects of 4,5-diCQA on IL-1β-Induced ACAN Degradation in Rat Primary Chondrocytes
3.5. Effects of 4,5-diCQA on the NF-κB Signaling Pathway in IL-1β-Treated Rat Primary Chondrocytes
3.6. Effects of 4,5-diCQA Administration on Macroscopic and Histological Parameters in the Articular Cartilage of the Rat OA Model
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Glyn-Jones, S.; Palmer, A.J.R.; Agricola, R.; Price, A.J.; Vincent, T.; Weinans, H.; Carr, A.J. Osteoarthritis. Lancet 2015, 386, 376–387. [Google Scholar] [CrossRef]
- Loeser, R.F.; Goldring, S.R.; Scanzello, C.R.; Goldring, M.B. Osteoarthritis: A disease of the joint as an organ. Arthritis Rheum. 2012, 64, 1697–1707. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hunter, D.J.; Bierma-Zeinstra, S. Osteoarthritis. Lancet 2019, 393, 1745–1759. [Google Scholar] [CrossRef]
- Mehana, E.-S.E.; Khafaga, A.F.; El-Blehi, S.S. The role of matrix metalloproteinases in osteoarthritis pathogenesis: An updated review. Life Sci. 2019, 234, 116786. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.H.; Kim, S.J.; Kim, S.A.; Ju, G.-I. Past, present, and future of cartilage restoration: From localized defect to arthritis. Knee Surg. Relat. Res. 2022, 34, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Maniar, K.H.; Jones, I.A.; Gopalakrishna, R. Lowering side effects of NSAID usage in osteoarthritis: Recent attempts at minimizing dosage. Expert Opin. Pharmacother. 2017, 19, 93–102. [Google Scholar] [CrossRef] [PubMed]
- Goldring, M.B. Osteoarthritis and cartilage: The role of cytokines. Curr. Rheumatol. Rep. 2000, 2, 459–465. [Google Scholar] [CrossRef] [PubMed]
- Henrotin, Y.; Marty, M.; Mobasheri, A. What is the current status of chondroitin sulfate and glucosamine for the treatment of knee osteoarthritis? Maturitas 2014, 78, 184–187. [Google Scholar] [CrossRef]
- Pavone, V.; Vescio, A.; Turchetta, M.; Giardina, S.M.C.; Culmone, A.; Testa, G. Injection-Based Management of Osteoarthritis of the Knee: A Systematic Review of Guidelines. Front. Pharmacol. 2021, 12, 661805. [Google Scholar] [CrossRef]
- Peck, J.; Slovek, A.; Miro, P.; Vij, N.; Traube, B.; Lee, C.; Berger, A.A.; Kassem, H.; Kaye, A.D.; Sherman, W.F.; et al. A Comprehensive Review of Viscosupplementation in Osteoarthritis of the Knee. Orthop. Rev. 2021, 13, 25549. [Google Scholar] [CrossRef]
- Subramanyam, K.; Alguvelly, R.; Mundargi, A.; Khanchandani, P. Single versus multi-dose intra-articular injection of platelet rich plasma in early stages of osteoarthritis of the knee: A single-blind, randomized, superiority trial. Arch. Rheumatol. 2021, 36, 326. [Google Scholar] [CrossRef] [PubMed]
- Vilchez-Cavazos, F.; Blázquez-Saldaña, J.; Gamboa-Alonso, A.A.; Peña-Martínez, V.M.; Acosta-Olivo, C.A.; Sánchez-García, A.; Simental-Mendía, M. The use of platelet-rich plasma in studies with early knee osteoarthritis versus advanced stages of the disease: A systematic review and meta-analysis of 31 randomized clinical trials. Arch. Orthop. Trauma. Surg. 2022, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Ameye, L.G.; Chee, W.S. Osteoarthritis and nutrition. From nutraceuticals to functional foods: A systematic review of the scientific evidence. Arthritis Res. Ther. 2006, 8, R127. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Henrotin, Y.; Mobasheri, A. Natural Products for Promoting Joint Health and Managing Osteoarthritis. Curr. Rheumatol. Rep. 2018, 20, 72. [Google Scholar] [CrossRef] [PubMed]
- Pandey, M.; Verma, R.K.; Saraf, S.A. Nutraceuticals: New era of medicine and health. Asian J. Pharm. Clin Res. 2010, 3, 11–15. [Google Scholar]
- Parbat, A.Y.; Malode, G.P.; Shaikh, A.R.; Panchale, W.A.; Manwar, J.V.; Bakal, R.L. Ethnopharmacological review of traditional medicinal plants as immunomodulator. World J. Biol. Pharm. Heal. Sci. 2021, 6, 043–055. [Google Scholar] [CrossRef]
- Lee, S.A.; Moon, S.-M.; Han, S.H.; Hwang, E.J.; Hong, J.H.; Park, B.-R.; Choi, M.S.; Ahn, H.; Kim, J.-S.; Kim, H.-J.; et al. In Vivo and In Vitro Anti-Inflammatory Effects of Aqueous Extract of Anthriscus sylvestris Leaves. J. Med. Food 2018, 21, 585–595. [Google Scholar] [CrossRef] [Green Version]
- Lee, S.A.; Moon, S.-M.; Han, S.H.; Hwang, E.J.; Park, B.-R.; Kim, J.-S.; Kim, D.K.; Kim, C.S. Chondroprotective effects of aqueous extract of Anthriscus sylvestris leaves on osteoarthritis in vitro and in vivo through MAPKs and NF-κB signaling inhibition. Biomed. Pharmacother. 2018, 103, 1202–1211. [Google Scholar] [CrossRef]
- Iwai, K.; Kishimoto, N.; Kakino, Y.; Mochida, A.K.; Fujita, T. In Vitro Antioxidative Effects and Tyrosinase Inhibitory Activities of Seven Hydroxycinnamoyl Derivatives in Green Coffee Beans. J. Agric. Food Chem. 2004, 52, 4893–4898. [Google Scholar] [CrossRef]
- Lodise, O.; Patil, K.; Karshenboym, I.; Prombo, S.; Chukwueke, C.; Pai, S.B. Inhibition of Prostate Cancer Cells by 4,5-Dicaffeoylquinic Acid through Cell Cycle Arrest. Prostate Cancer 2019, 2019, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Trendafilova, A.; Ivanova, V.; Rangelov, M.; Todorova, M.; Ozek, G.; Yur, S.; Ozek, T.; Aneva, I.; Veleva, R.; Moskova-Doumanova, V.; et al. Caffeoylquinic Acids, Cytotoxic, Antioxidant, Acetylcholinesterase and Tyrosinase Enzyme Inhibitory Activities of Six Inula Species from Bulgaria. Chem. Biodivers. 2020, 17, e2000051. [Google Scholar] [CrossRef] [PubMed]
- Cao, S.; Wan, C.; Yu, Y.; Zhou, S.; Tian, S. Isolation and identification of phenolic compounds from Gynura divaricata leaves. Pharmacogn. Mag. 2011, 7, 101–108. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yin, X.-L.; Xu, B.-Q.; Zhang, Y.-Q. Gynura divaricata rich in 3, 5−/4, 5-dicaffeoylquinic acid and chlorogenic acid reduces islet cell apoptosis and improves pancreatic function in type 2 diabetic mice. Nutr. Metab. 2018, 15, 73. [Google Scholar] [CrossRef] [PubMed]
- Hong, S.; Joo, T.; Jhoo, J.-W. Antioxidant and anti-inflammatory activities of 3,5-dicaffeoylquinic acid isolated from Ligularia fischeri leaves. Food Sci. Biotechnol. 2015, 24, 257–263. [Google Scholar] [CrossRef]
- Jang, G.; Lee, S.; Hong, J.; Park, B.; Kim, D.; Kim, C. Anti-Inflammatory Effect of 4,5-Dicaffeoylquinic Acid on RAW264.7 Cells and a Rat Model of Inflammation. Nutrients 2021, 13, 3537. [Google Scholar] [CrossRef]
- Wang, S.; Li, X.; Niu, Y.; Liu, Y.; Zhu, Y.; Lu, X.; Fan, X.; Zhang, X.; Wang, Y. Identification and screening of chemical constituents with hepatoprotective effects from three traditional Chinese medicines for treating jaundice. J. Sep. Sci. 2016, 39, 3690–3699. [Google Scholar] [CrossRef]
- Kim, S.-S.; Park, R.-Y.; Jeon, H.-J.; Kwon, Y.-S.; Chun, W. Neuroprotective effects of 3,5-dicaffeoylquinic acid on hydrogen peroxide-induced cell death in SH-SY5Y cells. Phytother. Res. 2005, 19, 243–245. [Google Scholar] [CrossRef]
- Liu, W.; Li, J.; Zhang, X.; Zu, Y.; Yang, Y.; Liu, W.; Xu, Z.; Gao, H.; Sun, X.; Jiang, X.; et al. Current Advances in Naturally Occurring Caffeoylquinic Acids: Structure, Bioactivity, and Synthesis. J. Agric. Food Chem. 2020, 68, 10489–10516. [Google Scholar] [CrossRef]
- Kim, J.-S.; Ellman, M.B.; Yan, N.; An, H.S.; Kc, R.; Li, X.; Chen, D.; Xiao, G.; Cs-Szabo, G.; Hoskin, D.W.; et al. Lactoferricin mediates anti-inflammatory and anti-catabolic effects via inhibition of IL-1 and LPS activity in the intervertebral disc. J. Cell. Physiol. 2013, 228, 1884–1896. [Google Scholar] [CrossRef] [Green Version]
- National Research Council. Guide for the Care and Use of Laboratory Animals, 8th ed.; National Academies Press: Washington, DC, USA, 2011.
- Zhao, Y.; Liu, B.; Liu, C.-J. Establishment of a Surgically-induced Model in Mice to Investigate the Protective Role of Progranulin in Osteoarthritis. J. Vis. Exp. 2014, e50924. [Google Scholar] [CrossRef]
- Glasson, S.S.; Chambers, M.G.; Van Den Berg, W.B.; Little, C.B. The OARSI histopathology initiative—Recommendations for histological assessments of osteoarthritis in the mouse. Osteoarthr. Cartil. 2010, 18 (Suppl. 3), S17–S23. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, A.S.; Ellman, M.B.; Yan, D.; Kroin, J.S.; Cole, B.J.; van Wijnen, A.J.; Im, H.-J. A current review of molecular mechanisms regarding osteoarthritis and pain. Gene 2013, 527, 440–447. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Feng, Z.; Li, X.; Lin, J.; Zheng, W.; Hu, Z.; Xuan, J.; Ni, W.; Pan, X. Oleuropein inhibits the IL-1β-induced expression of inflammatory mediators by suppressing the activation of NF-κB and MAPKs in human osteoarthritis chondrocytes. Food Funct. 2017, 8, 3737–3744. [Google Scholar] [CrossRef] [PubMed]
- Jenei-Lanzl, Z.; Meurer, A.; Zaucke, F. Interleukin-1β signaling in osteoarthritis chondrocytes in focus. Cell. Signal. 2018, 53, 212–223. [Google Scholar] [CrossRef]
- Bauer, D.; Hunter, D.; Abramson, S.; Attur, M.; Corr, M.; Felson, D.; Heinegård, D.; Jordan, J.; Kepler, T.; Lane, N.; et al. Classification of osteoarthritis biomarkers: A proposed approach. Osteoarthr. Cartil. 2006, 14, 723–727. [Google Scholar] [CrossRef] [Green Version]
- Lane, N.; Brandt, K.; Hawker, G.; Peeva, E.; Schreyer, E.; Tsuji, W.; Hochberg, M. OARSI-FDA initiative: Defining the disease state of osteoarthritis. Osteoarthr. Cartil. 2011, 19, 478–482. [Google Scholar] [CrossRef] [Green Version]
- Bai, H.; Zhang, Z.; Li, Y.; Song, X.; Ma, T.; Liu, C.; Liu, L.; Yuan, R.; Wang, X.; Gao, L. L-Theanine Reduced the Development of Knee Osteoarthritis in Rats via Its Anti-Inflammation and Anti-Matrix Degradation Actions: In Vivo and In Vitro Study. Nutrients 2020, 12, 1988. [Google Scholar] [CrossRef]
- D’Adamo, S.; Cetrullo, S.; Panichi, V.; Mariani, E.; Flamigni, F.; Borzì, R.M. Nutraceutical Activity in Osteoarthritis Biology: A Focus on the Nutrigenomic Role. Cells 2020, 9, 1232. [Google Scholar] [CrossRef]
- Deligiannidou, G.-E.; Papadopoulos, R.-E.; Kontogiorgis, C.; Detsi, A.; Bezirtzoglou, E.; Constantinides, T. Unraveling Natural Products’ Role in Osteoarthritis Management—An Overview. Antioxidants 2020, 9, 348. [Google Scholar] [CrossRef] [Green Version]
- Kobayashi, M.; Squires, G.R.; Mousa, A.; Tanzer, M.; Zukor, D.J.; Antoniou, J.; Feige, U.; Poole, A.R. Role of interleukin-1 and tumor necrosis factor ? in matrix degradation of human osteoarthritic cartilage. Arthritis Care Res. 2005, 52, 128–135. [Google Scholar] [CrossRef]
- Berg, W.B.V.D. Pathophysiology of osteoarthritis. Jt. Bone Spine 2000, 67, 555–556. [Google Scholar] [CrossRef]
- Zheng, W.; Feng, Z.; You, S.; Zhang, H.; Tao, Z.; Wang, Q.; Chen, H.; Wu, Y. Fisetin inhibits IL-1β-induced inflammatory response in human osteoarthritis chondrocytes through activating SIRT1 and attenuates the progression of osteoarthritis in mice. Int. Immunopharmacol. 2017, 45, 135–147. [Google Scholar] [CrossRef] [PubMed]
- Majumdar, M.K.; Askew, R.; Schelling, S.; Stedman, N.; Blanchet, T.; Hopkins, B.; Morris, E.A.; Glasson, S.S. Double-knockout of ADAMTS-4 and ADAMTS-5 in mice results in physiologically normal animals and prevents the progression of osteoarthritis. Arthritis Rheum. 2007, 56, 3670–3674. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Qin, Z.; Zhao, J.; Hea, Y.; Rene, E.; ZhuF, Y.; Liue, G.; Maof, C.; Zhengab, L. Cartilage-targeting and dual MMP-13/pH responsive theranostic nanoprobes for osteoarthritis imaging and precision therapy. Biomaterials 2019, 225, 119520. [Google Scholar] [CrossRef]
- Zhou, L.; Ye, H.; Liu, L.; Chen, Y. Human Bone mesenchymal stem cell-derived exosomes inhibit IL-1β-induced inflammation in osteoarthritis chondrocytes. Cell J. 2021, 23, 485. [Google Scholar] [CrossRef]
- Johnson, G.L.; Lapadat, R. Mitogen-Activated Protein Kinase Pathways Mediated by ERK, JNK, and p38 Protein Kinases. Science 2002, 298, 1911–1912. [Google Scholar] [CrossRef] [Green Version]
- Loeser, R.F.; Erickson, E.A.; Long, D.L. Mitogen-activated protein kinases as therapeutic targets in osteoarthritis. Curr. Opin. Rheumatol. 2008, 20, 581–586. [Google Scholar] [CrossRef]
- Chen, N.; Gao, R.-F.; Yuan, F.-L.; Zhao, M.-D. Recombinant Human Endostatin Suppresses Mouse Osteoclast Formation by Inhibiting the NF-κB and MAPKs Signaling Pathways. Front. Pharmacol. 2016, 7, 145. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Pizzute, T.; Pei, M. A review of crosstalk between MAPK and Wnt signals and its impact on cartilage regeneration. Cell Tissue Res. 2014, 358, 633–649. [Google Scholar] [CrossRef] [Green Version]
- Min, G.-Y.; Park, J.-M.; Joo, I.-H.; Kim, D.-H. Inhibition effect of Caragana sinica root extracts on Osteoarthritis through MAPKs, NF-κB signaling pathway. Int. J. Med. Sci. 2021, 18, 861–872. [Google Scholar] [CrossRef]
- Ahmed, S.; Wang, N.; Bin Hafeez, B.; Cheruvu, V.K.; Haqqi, T.M. Punica granatum L. Extract Inhibits IL-1β–Induced Expression of Matrix Metalloproteinases by Inhibiting the Activation of MAP Kinases and NF-κB in Human Chondrocytes In Vitro. J. Nutr. 2005, 135, 2096–2102. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roman-Blas, J.; Jimenez, S. NF-κB as a potential therapeutic target in osteoarthritis and rheumatoid arthritis. Osteoarthr. Cartil. 2006, 14, 839–848. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rigoglou, S.; Papavassiliou, A.G. The NF-κB signalling pathway in osteoarthritis. Int. J. Biochem. Cell Biol. 2013, 45, 2580–2584. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Wang, X.; He, Y.-L.; Wang, Y.; Dong, L.; Ma, X.; Zheng, L.; Liu, C.-H.; Wang, G.-C.; Zheng, J.; et al. In Vivo and In Vitro Anti-Arthritic Effects of Cardenolide-Rich and Caffeoylquinic Acid-Rich Fractions of Periploca forrestii. Molecules 2018, 23, 1988. [Google Scholar] [CrossRef] [Green Version]
- Iijima, H.; Aoyama, T.; Ito, A.; Tajino, J.; Nagai, M.; Zhang, X.; Yamaguchi, S.; Akiyama, H.; Kuroki, H. Destabilization of the medial meniscus leads to subchondral bone defects and site-specific cartilage degeneration in an experimental rat model. Osteoarthr. Cartil. 2014, 22, 1036–1043. [Google Scholar] [CrossRef] [Green Version]
- Musumeci, G.; Castrogiovanni, P.; Mazzone, V.; Szychlinska, M.A.; Castorina, S.; Loreto, C. Histochemistry as a Unique Approach for Investigating Normal and Osteoarthritic Cartilage. Eur. J. Histochem. 2014, 58, 2371. [Google Scholar] [CrossRef] [Green Version]




| Grade | Osteoarthritic Damage |
|---|---|
| Grade 0 | Normal cartilage |
| Grade 1 | Threshold in cartilage for OA without loss of cartilage |
| Grade 2 | Discontinuity of the superficial layer |
| Grade 3 | Erosion of the matrix cracks by extending cartilage to <25% of the articular surface |
| Grade 4 | Erosion of the matrix cracks by extending cartilage to <25–50% of the articular surface |
| Grade 5 | Erosion of the matrix cracks by extending cartilage to <50–75% of the articular surface |
| Grade 6 | Erosion of the matrix cracks by extending cartilage to <75% of the articular surface |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jang, G.; Lee, S.A.; Hong, J.H.; Park, B.-R.; Kim, D.K.; Kim, C.S. Chondroprotective Effects of 4,5-Dicaffeoylquinic Acid in Osteoarthritis through NF-κB Signaling Inhibition. Antioxidants 2022, 11, 487. https://doi.org/10.3390/antiox11030487
Jang G, Lee SA, Hong JH, Park B-R, Kim DK, Kim CS. Chondroprotective Effects of 4,5-Dicaffeoylquinic Acid in Osteoarthritis through NF-κB Signaling Inhibition. Antioxidants. 2022; 11(3):487. https://doi.org/10.3390/antiox11030487
Chicago/Turabian StyleJang, Goeun, Seul Ah Lee, Joon Ho Hong, Bo-Ram Park, Do Kyung Kim, and Chun Sung Kim. 2022. "Chondroprotective Effects of 4,5-Dicaffeoylquinic Acid in Osteoarthritis through NF-κB Signaling Inhibition" Antioxidants 11, no. 3: 487. https://doi.org/10.3390/antiox11030487
APA StyleJang, G., Lee, S. A., Hong, J. H., Park, B.-R., Kim, D. K., & Kim, C. S. (2022). Chondroprotective Effects of 4,5-Dicaffeoylquinic Acid in Osteoarthritis through NF-κB Signaling Inhibition. Antioxidants, 11(3), 487. https://doi.org/10.3390/antiox11030487





