Improved Extraction Efficiency and Antioxidant Activity of Defatted Canola Meal Extract Phenolic Compounds Obtained from Air-Fried Seeds
Abstract
1. Introduction
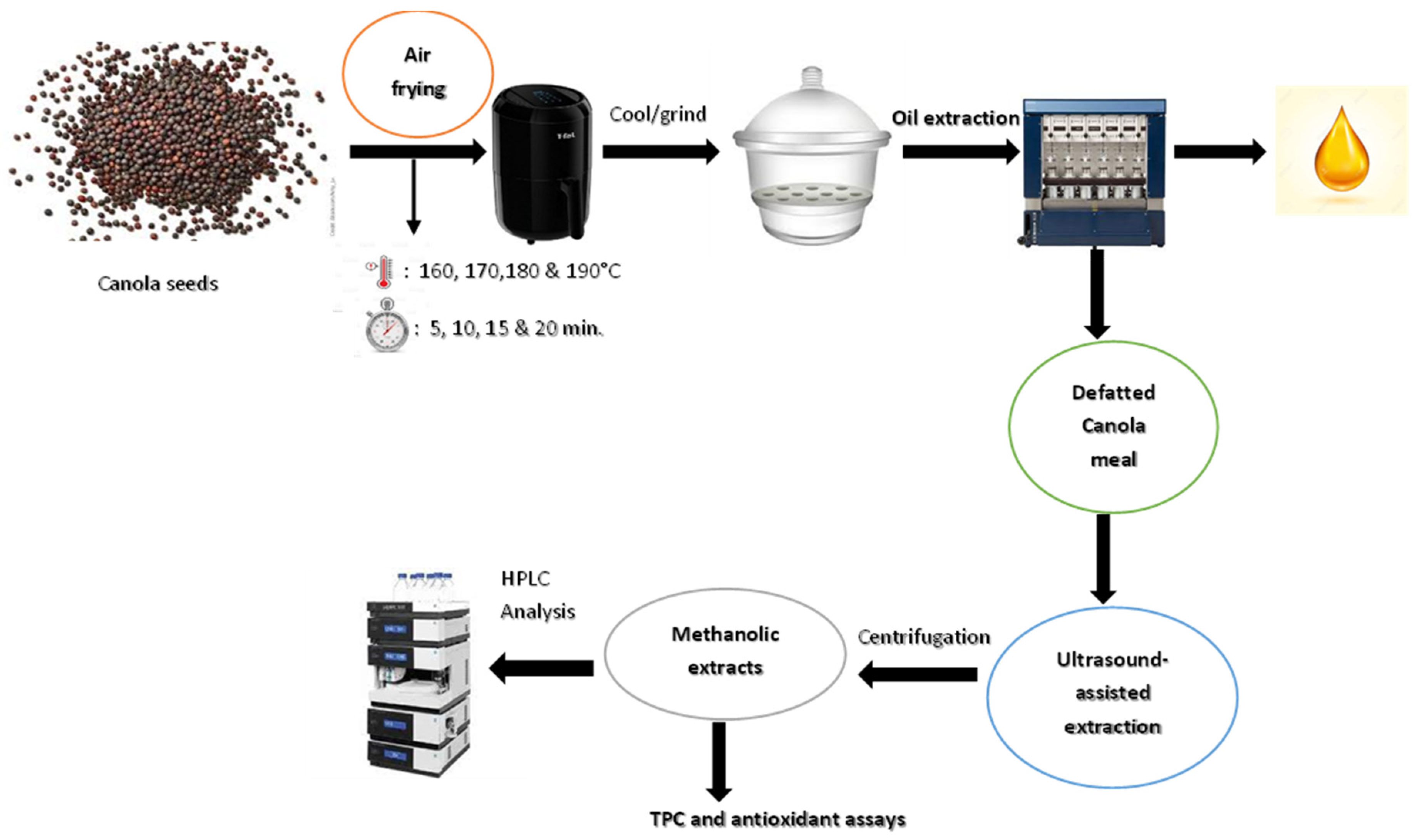
2. Materials and Methods
2.1. Roasting of Canola Seeds Using Air Fryer
2.2. Preparation of Canola Meal
2.3. Ultrasonic Extraction of Defatted Canola Meal
2.4. HPLC Analysis of Canola Meal-Derived Sinapic Acid Derivatives
2.5. Determination of Total Phenolic Content (TPC)
2.6. Evaluation of Antioxidant Properties
2.6.1. 2,2-Diphenyl-1-picrylhydrazyl Radical Scavenging Activity
2.6.2. Ferric Ion Reducing Antioxidant Power
2.6.3. Metal-Ion Chelation Properties
2.7. Statistical Analysis
3. Results and Discussion
3.1. Phenolic Composition of De-Oiled Canola Meal as Affected by Air Frying
3.2. Impact of Air Frying of Seeds on the TPC and Antioxidative Properties of Defatted Canola Meal Methanolic Extracts
3.2.1. Total Phenolic Content (TPC)
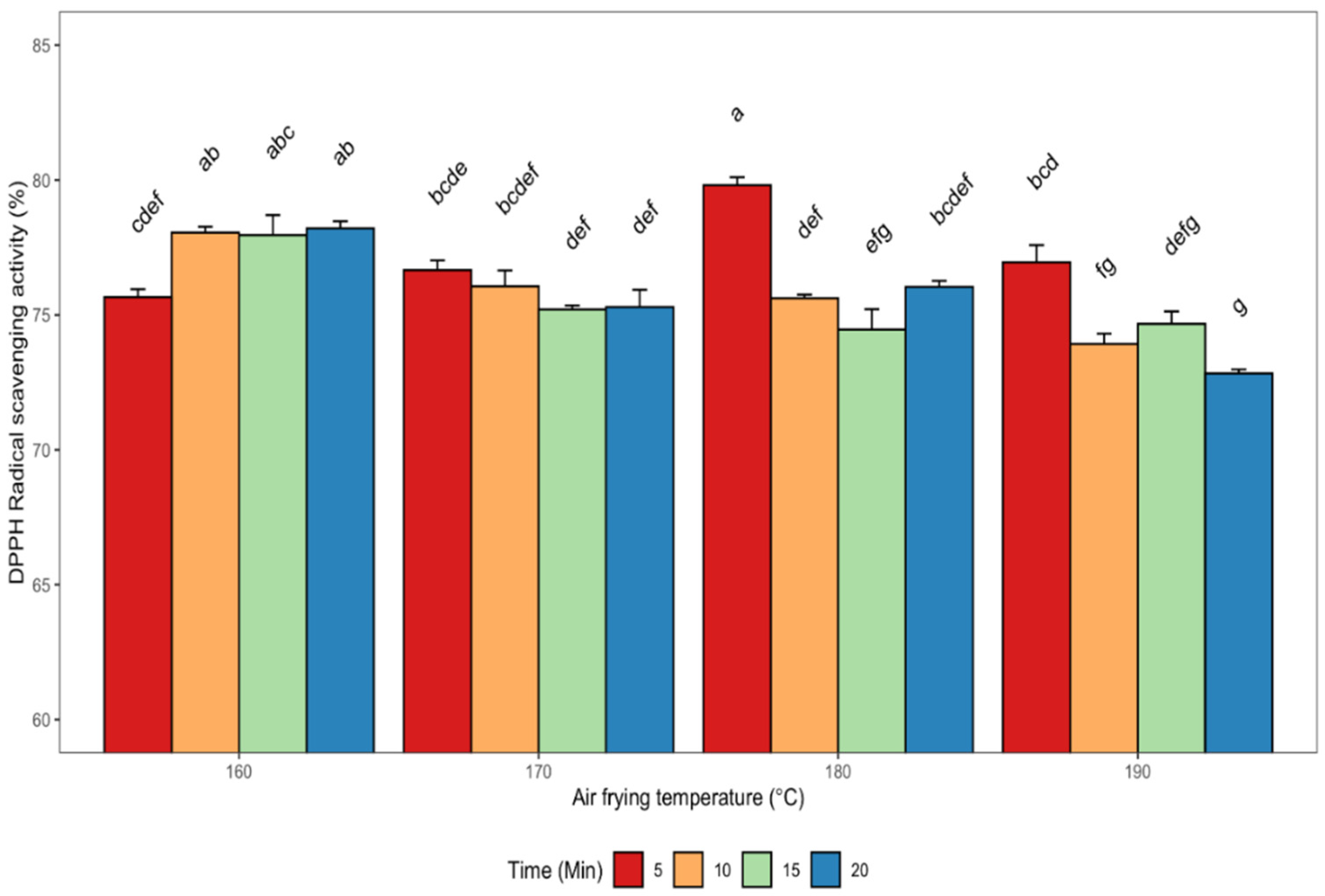
3.2.2. 2,2-Diphenyl-1-picrylhydrazyl (DPPH) Radical Scavenging Activity
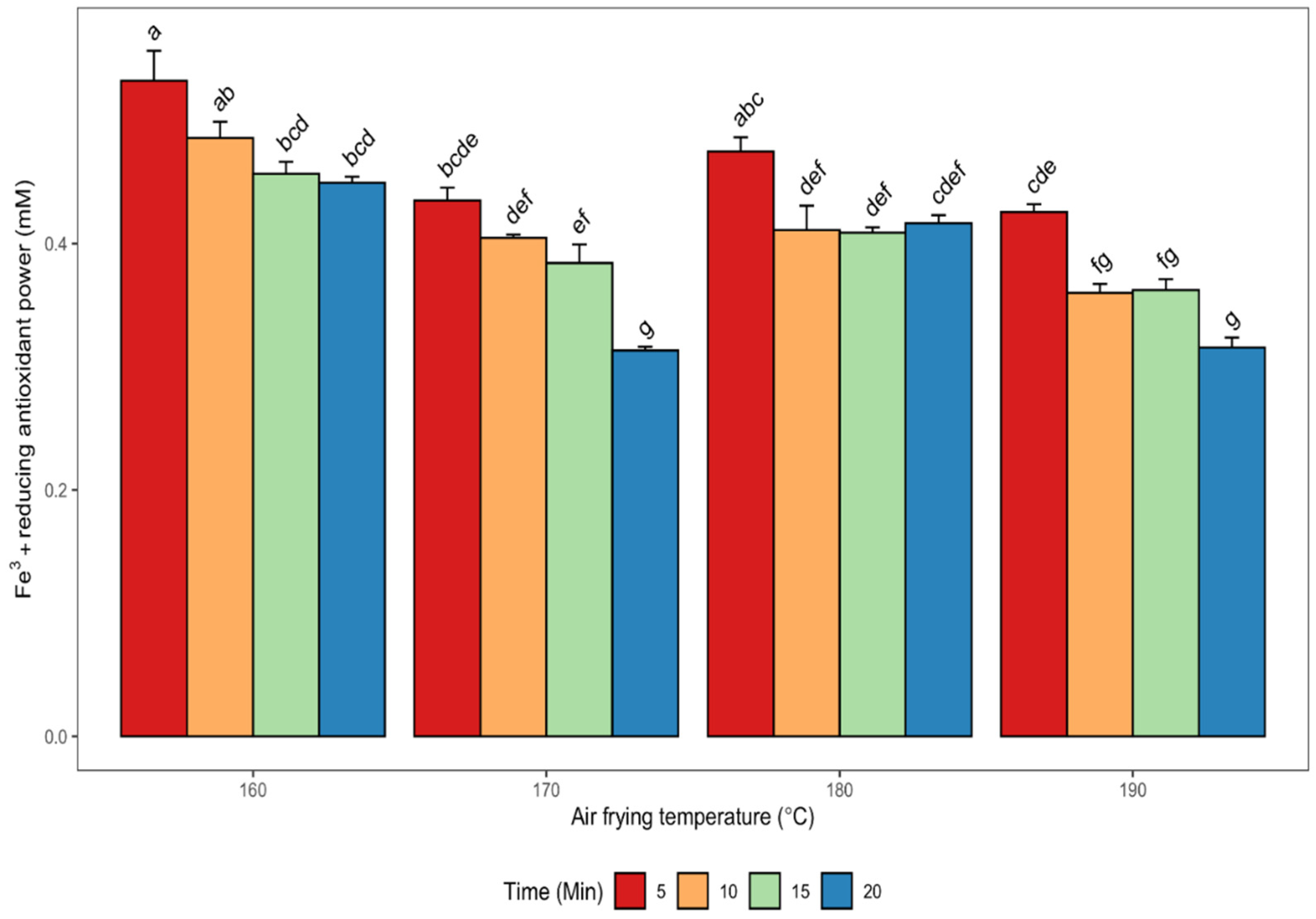
3.2.3. Ferric Reducing Antioxidant Power (FRAP)
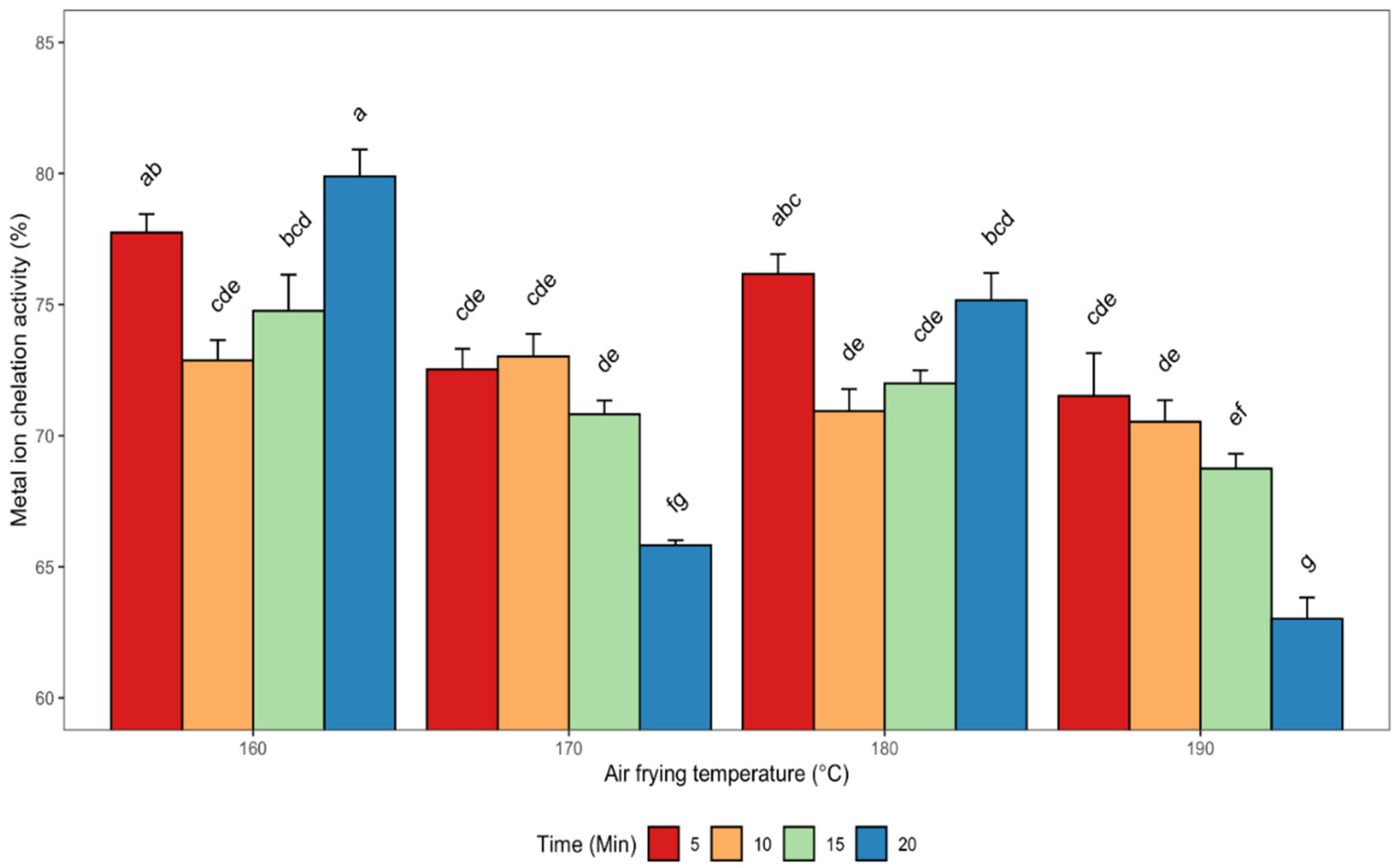
3.2.4. Metal Ion Chelating (MIC) Activity
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Eskin, M.N.; Aladedunye, F.; Unger, E.H.; Shah, S.; Chen, G.; Jones, P.J. Canola Oil. In Bailey’s Industrial Oil and Fat Products; John Wiley & Sons, Inc.: Chichester, UK, 2020; pp. 1–63. [Google Scholar] [CrossRef]
- Arrutia, F.; Binner, E.; Williams, P.; Waldron, K.W. Oilseeds beyond oil: Press cakes and meals supplying global protein requirements. Trends Food Sci. Technol. 2020, 100, 88–102. [Google Scholar] [CrossRef]
- Khattab, R.; Eskin, M.; Aliani, M.; Thiyam, U. Determination of Sinapic Acid Derivatives in Canola Extracts Using High-Performance Liquid Chromatography. J. Am. Oil Chem. Soc. 2010, 87, 147–155. [Google Scholar] [CrossRef]
- Alu’Datt, M.H.; Rababah, T.; Alhamad, M.N.; Al-Mahasneh, M.A.; Almajwal, A.; Gammoh, S.; Ereifej, K.; Johargy, A.; Alli, I. A review of phenolic compounds in oil-bearing plants: Distribution, identification, and occurrence of phenolic compounds. Food Chem. 2017, 218, 99–106. [Google Scholar] [CrossRef] [PubMed]
- Quinn, L.; Gray, S.G.; Meaney, S.; Finn, S.; Kenny, O.; Hayes, M. Sinapinic and protocatechuic acids found in rapeseed: Isolation, characterisation and potential benefits for human health as functional food ingredients. Ir. J. Agric. Food Res. 2017, 56, 104–119. [Google Scholar] [CrossRef]
- Hussain, S.; Rehman, A.U.; Obied, H.K.; Luckett, D.J.; Blanchard, C.L. Extraction, Chemical Characterization, In Vitro Antioxidant, and Antidiabetic Activity of Canola (Brassica napus L.) Meal. Separations 2022, 9, 38. [Google Scholar] [CrossRef]
- Nandasiri, R.; Imran, A.; Thiyam-Holländer, U.; Eskin, N.A.M. Rapidoxy® 100: A Solvent-Free Pre-treatment for Production of Canolol. Front. Nutr. 2021, 8, 687851. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.-C.; Arasu, M.V.; Chun, J.-H.; Jang, Y.-S.; Lee, Y.-H.; Kim, I.H.; Lee, K.-T.; Hong, S.-T.; Kim, S.-J. Identification and Determination of Phenolic Compounds in Rapeseed Meals (Brassica napus L.). J. Agric. Chem. Environ. 2015, 04, 14–23. [Google Scholar] [CrossRef]
- Li, J.; Guo, Z. Concurrent extraction and transformation of bioactive phenolic compounds from rapeseed meal using pressurized solvent extraction system. Ind. Crop. Prod. 2016, 94, 152–159. [Google Scholar] [CrossRef]
- Nandasiri, R.; Eskin, N.A.M.; Thiyam-Höllander, U. Antioxidative Polyphenols of Canola Meal Extracted by High Pressure: Impact of Temperature and Solvents. J. Food Sci. 2019, 84, 3117–3128. [Google Scholar] [CrossRef]
- Nandasiri, R.; Eskin, N.M. Canolol and its derivatives: A novel bioactive with antioxidant and anticancer properties. Adv. Food Nutr. Res. 2022, 100, 109–129. [Google Scholar] [CrossRef]
- Kraljić, K.; Brkan, V.; Škevin, D.; Srček, V.G.; Radošević, K. Canolol Dimer, a Biologically Active Phenolic Compound of Edible Rapeseed Oil. Lipids 2019, 54, 189–200. [Google Scholar] [CrossRef] [PubMed]
- Xia, X.; Xiang, X.; Huang, F.; Zheng, M.; Cong, R.; Han, L.; Zhang, Z. Dietary polyphenol canolol from rapeseed oil attenuates oxidative stress-induced cell damage through the modulation of the p38 signaling pathway. RSC Adv. 2018, 8, 24338–24345. [Google Scholar] [CrossRef] [PubMed]
- Harbaum-Piayda, B.; Oehlke, K.; Sönnichsen, F.D.; Zacchi, P.; Eggers, R.; Schwarz, K. New polyphenolic compounds in commercial deodistillate and rapeseed oils. Food Chem. 2010, 123, 607–615. [Google Scholar] [CrossRef]
- Thiyam-Holländer, U.; Aladedunye, F.; Logan, A.; Yang, H.; Diehl, B.W.K. Identification and quantification of canolol and related sinapate precursors in Indian mustard oils and Canadian mustard products. Eur. J. Lipid Sci. Technol. 2014, 116, 1664–1674. [Google Scholar] [CrossRef]
- Zago, E.; Lecomte, J.; Barouh, N.; Aouf, C.; Carré, P.; Fine, F.; Villeneuve, P. Influence of rapeseed meal treatments on its total phenolic content and composition in sinapine, sinapic acid and canolol. Ind. Crop. Prod. 2015, 76, 1061–1070. [Google Scholar] [CrossRef]
- Niu, Y.; Jiang, M.; Wan, C.; Yang, M.; Hu, S. Effect of Microwave Treatment on Sinapic Acid Derivatives in Rapeseed and Rapeseed Meal. J. Am. Oil Chem. Soc. 2013, 90, 307–313. [Google Scholar] [CrossRef]
- Fadairo, O.; Nandasiri, R.; Alashi, A.M.; Eskin, N.A.M.; Thiyam-Höllander, U. Air frying pretreatment and the recovery of lipophilic sinapates from the oil fraction of mustard samples. J. Food Sci. 2021, 86, 3810–3823. [Google Scholar] [CrossRef]
- Liang, J.; Zago, E.; Nandasiri, R.; Khattab, R.; Eskin, N.A.M.; Eck, P.; Thiyam-Holländer, U. Effect of Solvent, Preheating Temperature, and Time on the Ultrasonic Extraction of Phenolic Compounds from Cold-Pressed Hempseed Cake. J. Am. Oil Chem. Soc. 2018, 95, 1319–1327. [Google Scholar] [CrossRef]
- Cai, Y.; Luo, Q.; Sun, M.; Corke, H. Antioxidant activity and phenolic compounds of 112 traditional Chinese medicinal plants associated with anticancer. Life Sci. 2004, 74, 2157–2184. [Google Scholar] [CrossRef]
- Girgih, A.T.; Udenigwe, C.; Aluko, R.E. In Vitro Antioxidant Properties of Hemp Seed (Cannabis sativa L.) Protein Hydrolysate Fractions. J. Am. Oil Chem. Soc. 2010, 88, 381–389. [Google Scholar] [CrossRef]
- Benzie, I.F.F.; Strain, J.J. The Ferric Reducing Ability of Plasma (FRAP) as a Measure of “‘Antioxidant Power’”: The FRAP Assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef]
- Xie, Z.; Huang, J.; Xu, X.; Jin, Z. Antioxidant activity of peptides isolated from alfalfa leaf protein hydrolysate. Food Chem. 2008, 111, 370–376. [Google Scholar] [CrossRef] [PubMed]
- Nandasiri, R.; Eskin, N.A.M.; Komatsu, E.; Perreault, H.; Thiyam-Holländer, U. Valorization of canola by-products: Concomitance of flavor-active bitter phenolics using pressurized heat treatments. LWT 2020, 138, 110397. [Google Scholar] [CrossRef]
- Khattab, R.Y.; Eskin, M.N.A.; Thiyam-Hollander, U. Production of Canolol from Canola Meal Phenolics via Hydrolysis and Microwave-Induced Decarboxylation. J. Am. Oil Chem. Soc. 2014, 91, 89–97. [Google Scholar] [CrossRef]
- Thiyam, U.; Stöckmann, H.; Felde, T.Z.; Schwarz, K. Antioxidative effect of the main sinapic acid derivatives from rapeseed and mustard oil by-products. Eur. J. Lipid Sci. Technol. 2006, 108, 239–248. [Google Scholar] [CrossRef]
- Wang, W.; Yang, B.; Li, W.; Zhou, Q.; Liu, C.; Zheng, C. Effects of steam explosion pretreatment on the bioactive components and characteristics of rapeseed and rapeseed products. LWT 2021, 143, 111172. [Google Scholar] [CrossRef]
- Yang, M.; Zheng, C.; Zhou, Q.; Liu, C.; Li, W.; Huang, F. Influence of Microwaves Treatment of Rapeseed on Phenolic Compounds and Canolol Content. J. Agric. Food Chem. 2014, 62, 1956–1963. [Google Scholar] [CrossRef]
- Blair, R.; Reichert, R.D. Carbohydrate and phenolic constituents in a comprehensive range of rapeseed and canola fractions: Nutritional significance for animals. J. Sci. Food Agric. 1984, 35, 29–35. [Google Scholar] [CrossRef]
- Liu, Q.; Wu, L.; Pu, H.; Li, C.; Hu, Q. Profile and distribution of soluble and insoluble phenolics in Chinese rapeseed (Brassica napus). Food Chem. 2012, 135, 616–622. [Google Scholar] [CrossRef]
- Nandasiri, R.; Zago, E.; Thiyam-Holländer, U.; Eskin, N.A.M. Attenuation of sinapic acid and sinapine-derived flavor-active compounds using a factorial-based pressurized high-temperature processing. J. Am. Oil Chem. 2021, 98, 779–794. [Google Scholar] [CrossRef]
- Krygier, K.; Sosulski, F.; Hogge, L. Free, esterified, and insoluble-bound phenolic acids. 2. Composition of phenolic acids in rapeseed flour and hulls. J. Agric. Food Chem. 1982, 30, 334–336. [Google Scholar] [CrossRef]
- Kozlowska, H.; Naczk, M.; Shahidi, F.; Zadernowski, R. Phenolic Acids and Tannins in Rapeseed and Canola. In Canola and Rapeseed; Springer: Boston, MA, USA, 1990; pp. 193–210. [Google Scholar] [CrossRef]
- Siger, A.; Nogala-Kalucka, M.; Lampart-Szczapa, E. The Content and Antioxidant Activity of Phenolic Compounds in Cold-Pressed Plant Oils. J. Food Lipids 2008, 15, 137–149. [Google Scholar] [CrossRef]
- Cai, R.; Arntfield, S.D. A rapid high-performance liquid chromatographic method for the determination of sinapine and sinapic acid in canola seed and meal. J. Am. Oil Chem. Soc. 2001, 78, 903–910. [Google Scholar] [CrossRef]
- Szydłowska-Czerniak, A.; Karlovits, G.; Hellner, G.; Szłyk, E. Effect of enzymatic and hydrothermal treatments of rapeseeds on quality of the pressed rapeseed oils: Part II. Oil yield and oxidative stability. Process Biochem. 2010, 45, 247–258. [Google Scholar] [CrossRef]
- Teh, S.-S.; Birch, J. Physicochemical and quality characteristics of cold-pressed hemp, flax and canola seed oils. J. Food Compos. Anal. 2013, 30, 26–31. [Google Scholar] [CrossRef]
- Sørensen, A.M.; Friel, J.; Winkler-Moser, J.K.; Jacobsen, C.; Huidrom, D.; Reddy, N.; Thiyam-Holländer, U. Impact of endogenous canola phenolics on the oxidative stability of oil-in-water emulsions. Eur. J. Lipid Sci. Technol. 2013, 115, 501–512. [Google Scholar] [CrossRef]
- Spielmeyer, A.; Wagner, A.; Jahreis, G. Influence of thermal treatment of rapeseed on the canolol content. Food Chem. 2009, 112, 944–948. [Google Scholar] [CrossRef]
- Vuorela, S.; Kreander, K.; Karonen, M.; Nieminen, R.; Hämäläinen, M.; Galkin, A.; Laitinen, L.; Salminen, J.P.; Moilanen, E.; Pihlaja, K.; et al. Preclinical Evaluation of Rapeseed, Raspberry, and Pine Bark Phenolics for Health Related Effects. J. Agric. Food Chem. 2005, 53, 5922–5931. [Google Scholar] [CrossRef] [PubMed]
- Teh, S.-S.; Niven, B.E.; Bekhit, A.E.-D.; Carne, A.; Birch, E.J. Microwave and pulsed electric field assisted extractions of polyphenols from defatted canola seed cake. Int. J. Food Sci. Technol. 2015, 50, 1109–1115. [Google Scholar] [CrossRef]
- Cisneros, F.H.; Paredes, D.; Arana, A.; Cisneros-Zevallos, L. Chemical Composition, Oxidative Stability and Antioxidant Capacity of Oil Extracted from Roasted Seeds of Sacha-Inchi (Plukenetia volubilis L.). J. Agric. Food Chem. 2014, 62, 5191–5197. [Google Scholar] [CrossRef] [PubMed]
- Shrestha, K.; De Meulenaer, B. Effect of Seed Roasting on Canolol, Tocopherol, and Phospholipid Contents, Maillard Type Reactions, and Oxidative Stability of Mustard and Rapeseed Oils. J. Agric. Food Chem. 2014, 62, 5412–5419. [Google Scholar] [CrossRef] [PubMed]
- Kalın, P.; Gülçin, İ.; Gören, A.C. Antioxidant Activity and Polyphenol Content of Cranberries (Vaccinium macrocarpon). Rec. Nat. Prod. 2015, 9, 496–502. [Google Scholar]
- Kose, L.P.; Gulcin, I. Evaluation of the Antioxidant and Antiradical Properties of Some Phyto and Mammalian Lignans. Molecules 2021, 26, 7099. [Google Scholar] [CrossRef] [PubMed]

| Air Frying Condition | Sinapine (µg SPE/g DW) | Sinapic Acid (µg SAE/g DW) | Canolol (µg CE/g DW) | RT-26.6 min (µgSPE/g DW) | RT-32.4 min (µg SPE/g DW) | RT-27.28 min (µg SPE/g DW) | RT-23.81 min (µg SPE/g DW) |
|---|---|---|---|---|---|---|---|
| 160/5 min | 7572 ± 479 a | 727 ± 44 a | 84.92 ± 3.41 i | 1763 ± 74 a | 903 ± 26 efg | 375 ± 19 de | 458 ± 19 ab |
| 160/10 min | 7471 ± 198 a | 646 ± 22 b | 92.77 ± 0.88 hi | 1681 ± 50 abc | 909 ± 44 ef | 381 ± 22 cde | 430 ± 22 bcde |
| 160/15 min | 7187 ± 119 ab | 632 ± 46 bc | 96.81 ± 3.96 gh | 1678 ± 81 abc | 1103 ± 56 cd | 381 ± 23 cde | 406 ± 7 def |
| 160/20 min | 7193 ± 86 ab | 603 ± 18 bcd | 109.17 ± 1.61 def | 1753 ± 22 ab | 1077 ± 17 cd | 406 ± 4 abcd | 448 ± 6 abc |
| 170/5 min | 6772 ± 209 bc | 644 ± 72 b | 97.56 ± 0.08 fgh | 1668 ± 75 abcd | 1111 ± 33 bcd | 394 ± 17 abcd | 427 ± 16 bcde |
| 170/10 min | 6649 ± 110 cd | 598 ± 29 bcd | 103.03 ± 1.53 efg | 1650 ± 53 abcd | 1233 ± 73 ab | 401 ± 27 abcd | 440 ± 18 abcd |
| 170/15 min | 6231 ± 105 def | 563 ± 28 cde | 121.38 ± 6.25 c | 1576 ± 23 cde | 1305 ± 32 a | 393 ± 12 bcd | 410 ± 14 def |
| 170/20 min | 5417 ± 114 g | 422 ± 21 gh | 151.35 ± 7.65 a | 1359 ± 27 f | 1161 ± 64 bc | 348 ± 19 e | 376 ± 18 f |
| 180/5 min | 6791 ± 124 bc | 543 ± 3 def | 113.60 ± 1.26 cde | 1672 ± 30 abcd | 801 ± 55 fg | 438 ± 9 a | 472 ± 10 a |
| 180/10 min | 6066 ± 19 ef | 477 ± 27 fg | 115.55 ± 2.12 cd | 1458 ± 8 ef | 824 ± 39 fg | 380 ± 6 cde | 404 ± 12 ef |
| 180/15 min | 6208 ± 94 def | 403 ± 15 h | 134.76 ± 2.32 b | 1485 ± 38 ef | 796 ± 17 g | 387 ± 8 cd | 411 ± 11 cdef |
| 180/20 min | 6559 ± 42 cd | 374 ± 4 h | 149.59 ± 5.70 a | 1584 ± 9 cde | 806 ± 15 fg | 417 ± 5 abc | 449 ± 8 abc |
| 190/5 min | 6526 ± 33 cde | 523 ± 11 ef | 100.05 ± 0.72 fgh | 1636 ± 16 bcd | 994 ± 17 de | 428 ± 4 ab | 472 ± 4 a |
| 190/10 min | 6309 ± 56 cdef | 360 ± 11 hi | 139.75 ± 3.04 b | 1578 ± 423 cde | 898 ± 44 efg | 414 ± 8 abcd | 465 ± 14 ab |
| 190/15 min | 6241 ± 130 def | 295 ± 19 i | 151.92 ± 2.73 a | 1552 ± 44 de | 876 ± 41 efg | 439 ± 4 a | 470 ± 20 a |
| 190/20 min | 5940 ± 115 f | 214 ± 17 j | 151.62 ± 2.8 a | 1489 ± 31 e | 895 ± 38 efg | 414 ± 12 abcd | 466 ± 10 a |
| Canolol | Sinapine | Sinapic Acid | RT-26.6 min | RT-32.4 min | RT-27.38 min | RT-23.81 min | DPPH | FRAP | MIC | TPC | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Canolol | 1 | ||||||||||
| Sinapine | −0.79 *** | 1 | |||||||||
| Sinapic Acid | −0.93 *** | 0.75 *** | 1 | ||||||||
| 26.6 RT | −0.75 *** | 0.93 *** | 0.72 ** | 1 | |||||||
| 32.4 RT | −0.25 | −0.04 | 0.40 | 0.10 | 1 | ||||||
| 27.38 RT | 0.21 | 0.03 | −0.37 | 0.25 | −0.35 | 1 | |||||
| 23.81 RT | 0.00 | 0.29 | −0.19 | 0.46 | −0.42 | 0.85 *** | 1 | ||||
| DPPH | −0.63 ** | 0.63 ** | 0.67 ** | 0.62 * | 0.07 | 0.08 | 0.03 | 1 | |||
| FRAP | −0.83 *** | 0.91 *** | 0.81 *** | 0.81 *** | −0.17 | −0.04 | 0.17 | 0.70 ** | 1 | ||
| MIC | −0.63 ** | 0.80 *** | 0.70 ** | 0.80 ** | −0.05 | 0.07 | 0.17 | 0.70 ** | 0.85 *** | 1 | |
| TPC | 0.75 *** | −0.56 * | −0.91 *** | −0.49 | −0.57 * | 0.62 * | 0.43 | −0.54 * | −0.59 * | −0.51 * | 1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fadairo, O.S.; Nandasiri, R.; Nguyen, T.; Eskin, N.A.M.; Aluko, R.E.; Scanlon, M.G. Improved Extraction Efficiency and Antioxidant Activity of Defatted Canola Meal Extract Phenolic Compounds Obtained from Air-Fried Seeds. Antioxidants 2022, 11, 2411. https://doi.org/10.3390/antiox11122411
Fadairo OS, Nandasiri R, Nguyen T, Eskin NAM, Aluko RE, Scanlon MG. Improved Extraction Efficiency and Antioxidant Activity of Defatted Canola Meal Extract Phenolic Compounds Obtained from Air-Fried Seeds. Antioxidants. 2022; 11(12):2411. https://doi.org/10.3390/antiox11122411
Chicago/Turabian StyleFadairo, Olamide S., Ruchira Nandasiri, Thu Nguyen, N. A Michael Eskin, Rotimi E. Aluko, and Martin G. Scanlon. 2022. "Improved Extraction Efficiency and Antioxidant Activity of Defatted Canola Meal Extract Phenolic Compounds Obtained from Air-Fried Seeds" Antioxidants 11, no. 12: 2411. https://doi.org/10.3390/antiox11122411
APA StyleFadairo, O. S., Nandasiri, R., Nguyen, T., Eskin, N. A. M., Aluko, R. E., & Scanlon, M. G. (2022). Improved Extraction Efficiency and Antioxidant Activity of Defatted Canola Meal Extract Phenolic Compounds Obtained from Air-Fried Seeds. Antioxidants, 11(12), 2411. https://doi.org/10.3390/antiox11122411










