Abstract
Antioxidants have been widely studied in the fields of biology, medicine, food, and nutrition sciences. There has been extensive work on developing assays for foods and biological systems. The scientific communities have well-accepted the effectiveness of endogenous antioxidants generated in the body. However, the health efficacy and the possible action of exogenous dietary antioxidants are still questionable. This may be attributed to several factors, including a lack of basic understanding of the interaction of exogenous antioxidants in the body, the lack of agreement of the different antioxidant assays, and the lack of specificity of the assays, which leads to an inability to relate specific dietary antioxidants to health outcomes. Hence, there is significant doubt regarding the relationship between dietary antioxidants to human health. In this review, we documented the variations in the current methodologies, their mechanisms, and the highly varying values for six common food substrates (fruits, vegetables, processed foods, grains, legumes, milk, and dairy-related products). Finally, we discuss the strengths and weaknesses of the antioxidant assays and examine the challenges in correlating the antioxidant activity of foods to human health.
1. Introduction
By definition, antioxidants prevent/inhibit/reduce oxidation processes [1,2,3,4,5,6,7,8,9,10,11]. Historically, the industry has used antioxidants to prevent metal corrosion and rubber vulcanization [2]. More recently, antioxidants have been used as food preservatives, lubricants, and stabilizers [2]. Allied market research shows the worldwide industrial market value for natural and synthetic antioxidants in 2015 was USD 2.9 billion and forecasted more than 50% overall growth to over USD 4.5 billion by 2022. In another study, Grand View Research forecasted that the global market for natural antioxidants is expected to be USD 4.1 billion by 2022 [12]. In each case, the functions of these antioxidants meet the strict chemical definition and are not associated with bodily functions.
In recent years, there has been considerable interest in the impact of naturally occurring phytochemicals in foods that may function as antioxidants in the human body. This interest has arisen from popular literature that defines oxidants as harmful and antioxidants as the antithesis and, therefore, healthful. There have been many reviews on all aspects of antioxidant assays and the relationship of antioxidants to health, including their activity, classification, and applications [2,3,5,10,13,14,15,16,17,18]. Apak et al. [15,16,17] published reviews on various antioxidant assays, their mechanisms, advantages, and limitations. Recently, Gulcin et al. [18] published a detailed review on in vitro antioxidant methods used for the determination of the antioxidant capacity of food constituents. Similarly, Shahidi et al. [3] and Alam et al. [13] published review articles on in vitro and in vivo antioxidant assays and their experimental procedures.
There have also been numerous papers questioning the health benefits of the many compounds that are in vitro antioxidants and whether they exhibit similar in vivo activity [19,20,21,22,23,24]. The U.S. Food and Drug Administration (FDA) and the European Food Safety Authority (EFSA) allow health claims for vitamin antioxidants (i.e., vitamins A, C, and E, those with a recommended daily intake (RDI) [25,26]. However, even for these compounds, there is some controversy with respect to their performance as antioxidants in vivo [22].
In the current review, we briefly describe oxidative stress, types of antioxidants, and in vitro assays, their mechanisms, their strengths and weaknesses, bioaccessibility, and bioavailability. We have also compared the analytical variations between the reported methodologies and activities for six commonly consumed food substrates (fruits, vegetables, processed foods, grains, legumes, milk, and dairy-related products). It is important for consumers, nutritionists, and other healthcare professionals to understand the health benefits gained from the consumption of fruits and vegetables and to distinguish facts from commercial hype regarding antioxidants.
2. Oxidative Stress
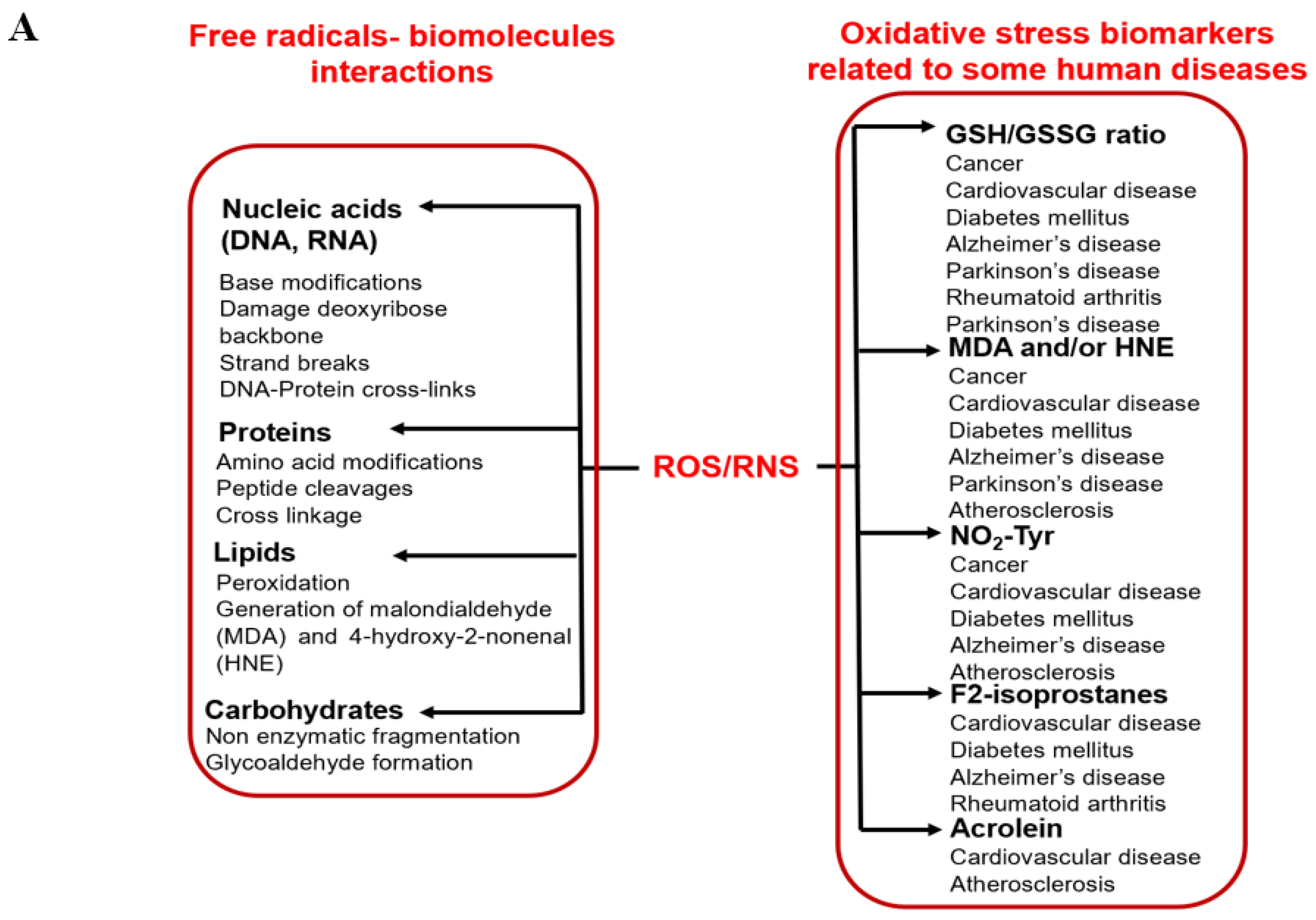
Oxidative stress is defined as the imbalance between the occurrence of reactive oxygen/nitrogen species (ROS/RNS) and cellular antioxidant defenses [1,4]. Oxidative stress is a result of excess ROS/RNS, which occurs due to a lack of counteraction by cellular antioxidant systems [1,5]. Increased oxidative stress can have severe consequences in biological systems, including molecular damage (such as nucleic acids, lipids, and proteins), which can severely impact health, as shown in Figure 1A [1,5]. Damage to biomolecules or the induction of several secondary reactive species due to oxidative stress ultimately leads to cell death (apoptosis or necrosis). It has been assessed that oxidative stress is associated with more than 100 diseases, including cardiovascular disease, cancer, hypertension, diabetes, neurogenerative diseases, aging, etc. [1,5].
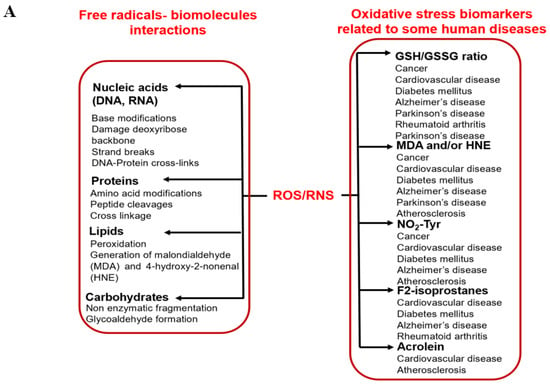
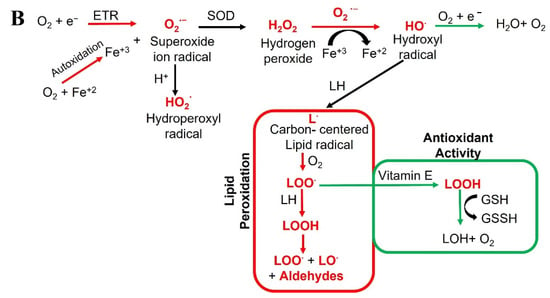
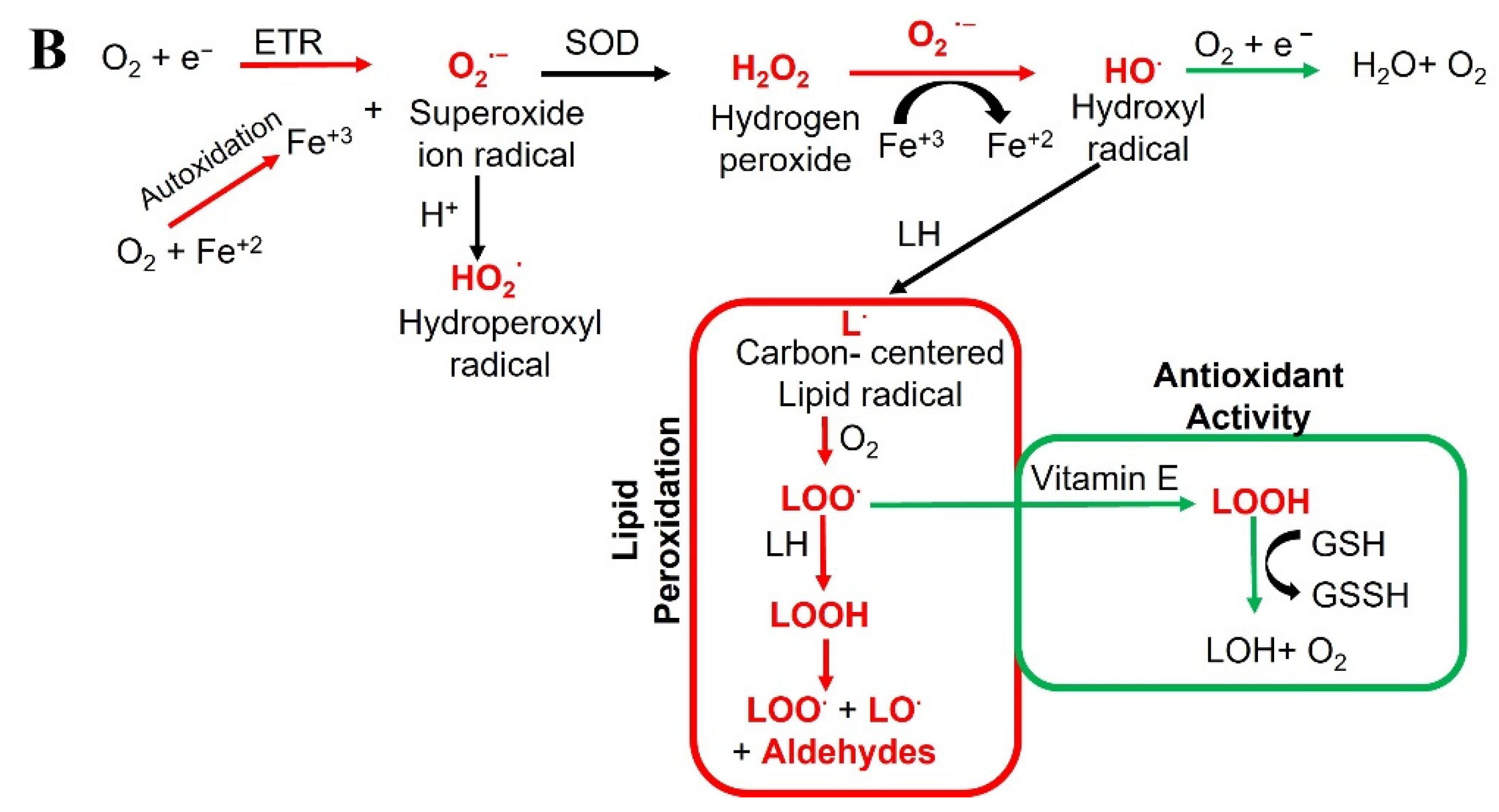
Figure 1.
Schematic representation of (A) radicals’ impact on human health, and (B) the generation of various radicals in vivo.
Contrary to their harmful effects on health, ROS/RNS can have beneficial effects depending on their function, location, and amount. For instance, superoxide (O2−•) and nitric oxide (•NO) radicals at low or medium concentrations are involved in cellular responses and participate in signaling pathways [1]. H2O2, formed by various oxidase enzymes, and the action of superoxide dismutase (SOD), allows its use as an important signaling molecule, also it is substrate for generating further reactive species such as HOCl [27,28]. ROS are also involved in immunological responses, degrading xeno compounds and organisms through phagocytosis.
ROS are oxygen-containing molecules, including radicals (like the superoxide anion) and non-radicals (like H2O2) that greatly vary in their chemical abilities, such as diffusion in living cells and chemical reactivity with biomolecules. ROS examples include singlet oxygen, superoxide, hydrogen peroxide, and hydroxyl radicals (Figure 1B). Singlet oxygen is the highest energy spin state of molecular oxygen. In contrast to molecular oxygen in ground state, the two valence electrons are paired in an anti-bonding orbital. Singlet oxygen is therefore only generated, when molecular oxygen is energized via radiation. Importantly, and in contrast to other ROS subspecies, no electron transfer does occur during this process. Singlet oxygen is very reactive towards organic compounds and plays a deleterious role in biological systems, for instance, by involving in the oxidation of LDL cholesterol, which can lead to cardiovascular diseases. Moreover, increased ROS can trigger mtDNA mutations as well as promote uncontrolled proliferation and carcinogenesis [29]. The delicate balance of harmful and beneficial effects of free radicals is crucial for life processes, and antioxidants play an essential role in achieving this balance.
3. Antioxidants
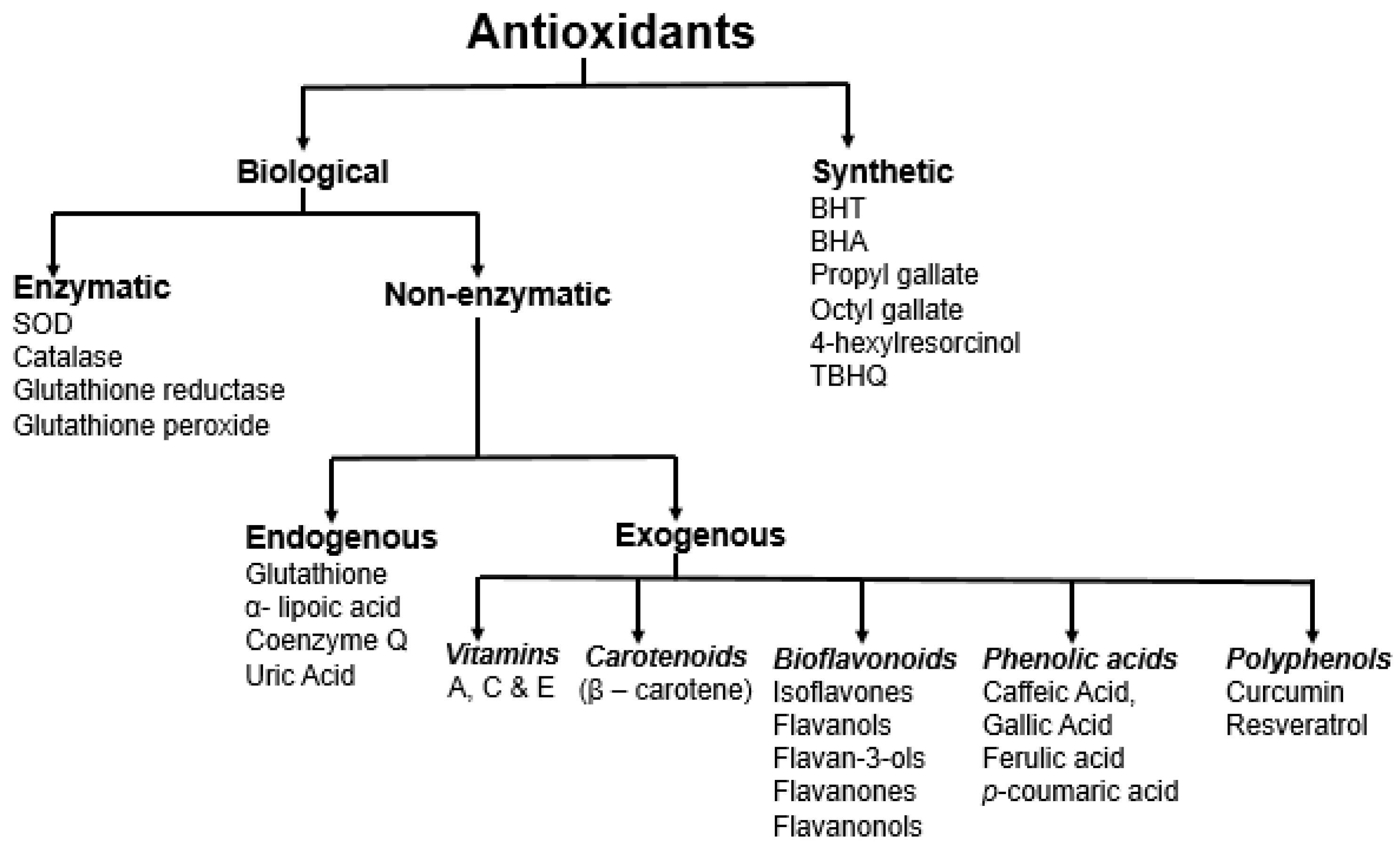
Antioxidants can be broadly categorized in many different ways: (i) natural and synthetic; (ii) polar and non-polar; (iii) enzymatic and non-enzymatic; (iv) endogenous and exogenous; and (v) by the mechanisms in which they are involved [30]. Antioxidants primarily exhibit activities based on three mechanisms, hydrogen atom transfer, single electron transfer, and metal chelation [10]. They show their activity through three different pathways: (i) preventive: prevention of free radical formation and derivatives; (ii) interruption: interrupt radical oxidation reactions; and (iii) inactivation: inactivate free radical/radical derivative reaction products [30]. Endogenous antioxidants are primarily enzymes, such as superoxide dismutase (SOD), catalase (CAT), glutathione reductase (GR), and glutathione peroxidase (GPx). On the other hand, non-enzymatic endogenous antioxidants, such as glutathione and lipoic acid, are products of the body’s metabolism [2,31]. The first-line defense antioxidants (enzymatic) convert reactive superoxide and hydrogen peroxide into water and oxygen. The non-enzymatic antioxidants can act as a second-line defense against ROS by rapidly inactivating radicals and oxidants. The enzymatic antioxidants further act as the third-line defense involved in the detoxifying and removal. Dietary antioxidants, such as vitamins, carotenoids, polyphenols, flavonoids, and bioflavonoids (Figure 2), are exogenous antioxidants that have in vivo activity [30].
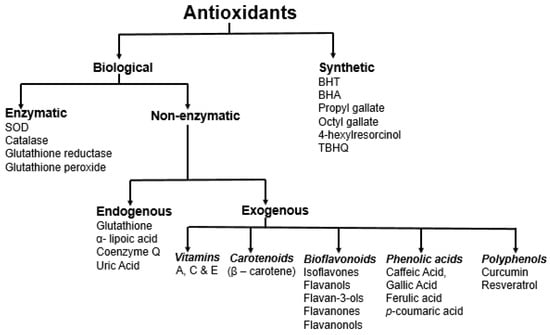
Figure 2.
Different approaches for the classification of antioxidants.
4. Antioxidant Assays
Various analytical methods have been developed to evaluate the antioxidant properties of plant-based phytochemicals [11,13,32,33]. The antioxidant activity depends on their chemical structure; specifically, it depends on their ability to donate hydrogen with electron, metal chelation, and their ability to delocalize the unpaired electron within the aromatic structure. Numerous analytical methods for evaluating each aspect of their antioxidant action, including either in vitro or in vivo, have been reported and discussed in the literature [34].
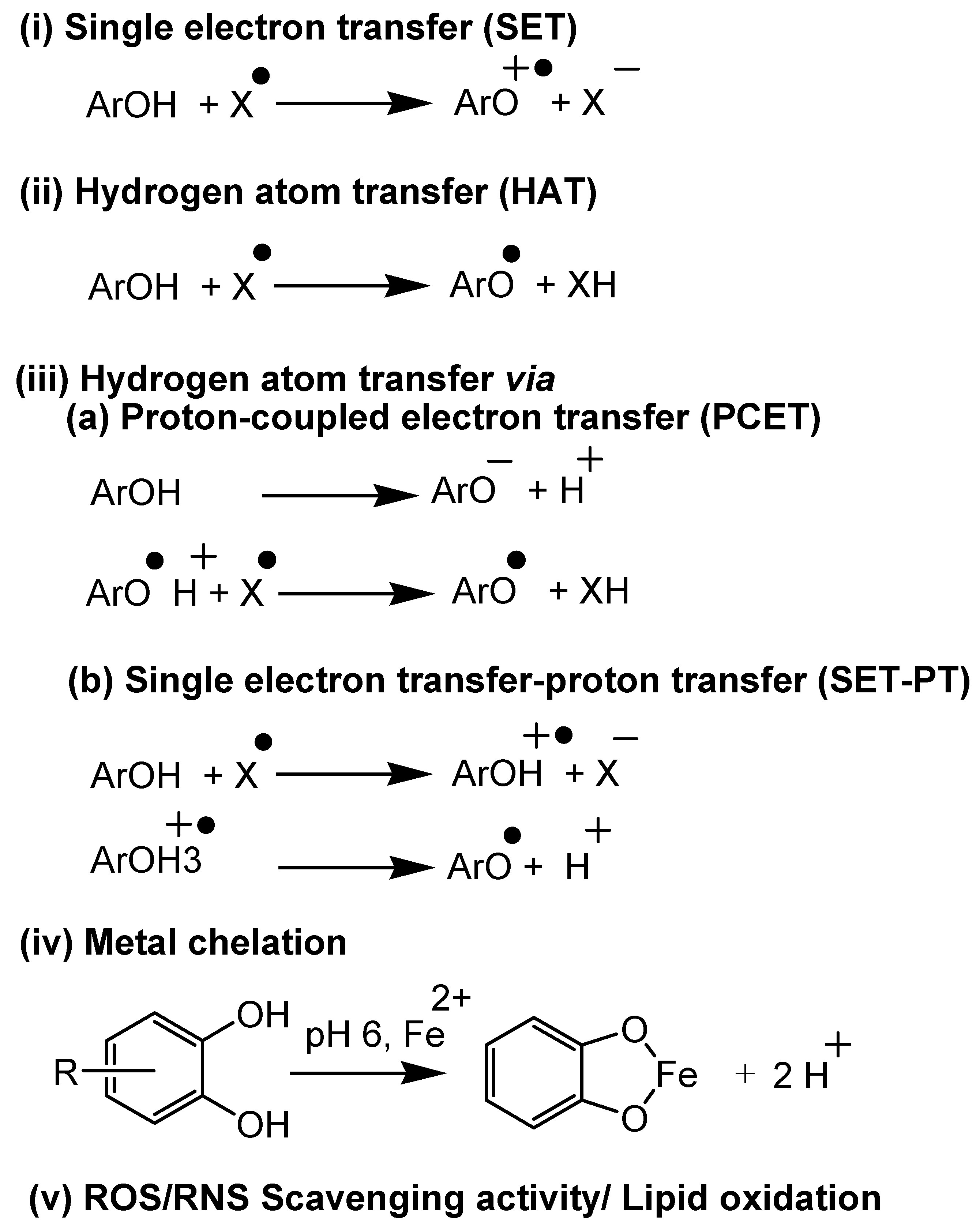
Antioxidant assays can be categorized into five mechanistic pathways, as summarized in Figure 3.
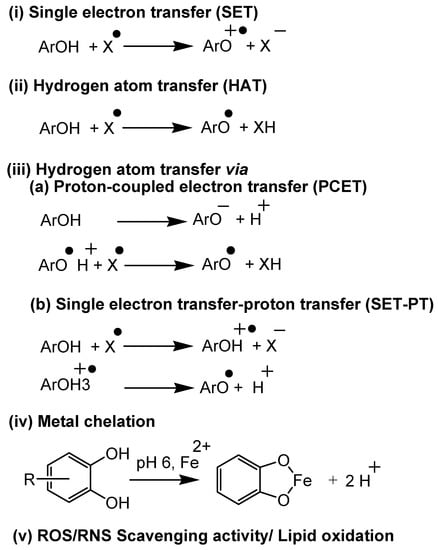
Figure 3.
Mechanistic aspects of antioxidant assays both in vitro and in vivo.
(i) Electron transfer-based assays: In these assays, a single electron transfer occurs between the antioxidant and substrate, which is measured to assess the potential of the plant’s secondary metabolites. Assays like cupric ion reducing antioxidant capacity (CUPRAC) [35,36,37], N,N-dimethyl-p-phenylenediamine dihydrochloride (DMPD) [38], ferric reducing-antioxidant power (FRAP) [39,40], Folin-Ciocalteu (FC), Trolox equivalent antioxidant capacity (TEAC) method/ABTS radical cation decolorization assay [41] come under this category.
(ii) Hydrogen atom transfer-based assays: In these assays, a hydrogen atom transfers from the antioxidant to the substrate. Assays such as oxygen radical absorbance capacity (ORAC) [42] and total radical-trapping antioxidant parameter (TRAP) [43] methods fall under this category.
(iii) Electron/hydrogen atom transfer (mixed) based assays: In these assays, the hydrogen atom transfer occurs via two-step mechanisms (Figure 3(iii)). Assays like DPPH scavenging activity [39,44,45] and TEAC follow this mechanism.
(iv) Metal chelation-based assays: In these assays, antioxidants chelate with transition metals like Fe(II) and Cu(II). Ferrous ion and cuprous ion chelating activity are examples of this category.
(v). Lipid oxidation and ROS/RNS scavenging activity assays: These assays are based on the ability of antioxidants in reducing/preventing lipid oxidation and scavenging ROS and RNS. β-carotene linoleic acid method/conjugated diene assay [45], ferric thiocyanate method (FTC) [39,46], thiobarbituric acid method (TBA), hydrogen peroxide (H2O2) scavenging assay [39,47], hydroxyl radical averting capacity method (HORAC) [42], nitric oxide scavenging activity [48], peroxynitrile radical scavenging activity, superoxide radical scavenging activity (SRSA/SOD), and xanthine oxidase methods come under this category.
(vi). Enzymatic antioxidant assays: Antioxidant enzyme systems that catalyze reactions to counterbalance free radicals and reactive oxygen species include superoxide dismutase and catalase. Catalase [49,50], ferric reducing ability of plasma [40,51,52], γ-glutamyl transpeptidase [53], glutathione peroxidase estimation [49,54], glutathione (reduced) GSH estimation [55], glutathione-S-transferase [56,57], LDL assay [58], lipid peroxidation assay [59], and superoxide dismutase method [60] can be categorized under enzymatic antioxidant assays.
Table 1 summarizes the various methods used to measure antioxidant activity. The principles, advantages, and limitations associated with each method, along with recent references, are also presented [13,14,18,36,46,51,61,62,63,64,65,66,67,68,69,70,71,72,73,74,75,76,77,78,79,80,81,82,83,84,85,86,87,88]. The advantages of electron transfer assays include faster reaction rates, ease of experimentation, sample throughput, and reproducibility. The main advantages associated with hydrogen atom transfer assays include close physiological resemblance, taking initiation and propagation into account, and uses of physiologically relevant radicals. Moreover, ORAC assay can be performed for antioxidants with a wide range of polarities, from lipophilic to hydrophilic [64]. Similarly, ROS/RNS scavenging activity/lipid oxidation assays, ET/HAT mixed, metal chelation, and lipid peroxidation inhibition assays have the advantages listed in Table 1.

Table 1.
Various in vitro and in vivo antioxidant assays, their principles, advantages, and limitations.
The major limitation associated with all these assays is their lack of specificity. However, specific limitations include the solubility of antioxidants in the extraction solvent, interferences with coloring substances and other reducing phytochemicals, ignoring reaction kinetics, and not representing the physiological radicals used in these assays.
A second major limitation is the lack of equivalence of the methods. It is not possible to convert ORAC to FRAP values with a simple proportionality factor. As shown in the next section, some foods high in FRAP values may be low in ORAC values, and the opposite can be true. This situation is best summed up by stating that the FRAP assay generates FRAP values, ORAC generates ORAC values, DPPH generates DPPH values, etc., and there are no equivalency factors.
5. Antioxidant Activity of Selected Prominent Foods
We have summarized (Table 2) [89,90,91,92,93,94,95,96,97,98,99,100,101,102,103,104,105,106,107,108,109,110,111,112,113,114,115,116,117,118,119,120,121] peer-reviewed literature reports on the antioxidant activity/capacity data for six prominent groups of food and related products that are consumed globally: fruits (apples and berries), vegetables (spinach and olives), processed products (wine, coffee, and tea), dairy products (milk and yogurt), legumes (soybeans, beans), and grains (wheat and corn), documented by different researchers using various assay procedures. Results from each group are separately presented below. In each case, antioxidant assay methods are used to document changes in composition and emphasize the impact of genetics and processing on composition. However, it must be remembered that a specific antioxidant activity can be achieved in literally multiple ways by different possible combinations of components. Without data for specific components, it is impossible to relate antioxidant values to composition or health outcomes.

Table 2.
Variations in the in vitro antioxidant activity for six prominent groups of food and related products that are consumed globally: fruits (apple and berries), vegetables (spinach and olives), processed products (wine, coffee, and tea), dairy products (milk and yogurt), legumes (soybeans, beans), and grains (wheat and corn), as documented in peer-reviewed published literature.
5.1. Fruits-Apples and Berries
Apples provide a rich source of phytochemicals, and epidemiological studies have shown that the consumption of apples reduces the risk of certain cancer types, cardiovascular diseases, asthma and diabetes. The antioxidant activity of apples has been attributed to various phytochemicals, particularly quercetin, catechin, phloridzin, and chlorogenic acid [122]. Antioxidant properties of different apple matrices (leaves, fresh fruit, pulp and peel, and pomace) have been investigated using various colorimetric assays (FC, DPPH, ABTS, and FRAP). The results from different matrices were expressed as gallic acid equivalents (GAE), ascorbic acid equivalents (AAE), Trolox equivalents (TE), and IC50 (50% inhibition concentration) (Table 2). For instance, Bahukhandi et al. [89] investigated the antioxidant activity of apples after pulverizing them to a fine texture. They studied the antioxidant activity of apples in terms of total phenolic contents using an FC reagent, and the results were expressed in GAE (10.87 mmol/kg). The antioxidant capacity for DPPH, ABTS, and FRAP was reported in AAE as 10.87, 24.57 mmol/kg, and 24.05 mmol/kg (of fresh apple), respectively. They also evaluated the correlation between total phenolic content, flavonoid, flavonol, TEACABTS, TEACFRAP and determined positive correlation values as 0.895, 0.843, 0.812, 0.856, and 0.830, respectively [89]. As noted, depending on the type of assay used, the reported values were significantly different. Additionally, significant variations in antioxidant activity were observed in different sample matrices (leaves, fresh fruit, pulp and peel, and pomace) even when the same assay was used. In a recent systematic review by Antonic et al. [123] on apple pomace, the authors showed that the high antioxidant content and dietary fibers present in apple pomace play an essential role as a food fortification ingredient in the food industry. The review highlights that fortified apple pomace increased the antioxidant activity and dietary fiber content in various food products. In a recent study, Li et al. [124] reported that red-fleshed apples showed greater antioxidant activities, phenolics, and flavonoid content than regular fuji apples. Particularly, one of the red-fleshed varieties, ‘A38’, showed about 3-fold higher FRAP, DPPH, and ABTS activities than the fuji apple [124]. The above results illustrate that proper documentation of genotypes is important. Unfortunately, none of these studies documented the difference in specific chemical components.
Berries, like apples, are considered to have several health benefits as they contain phenolic acids, flavonoids, and anthocyanins, which are localized mainly in berries, seeds, skins, and leaves [125]. For instance, blueberry anthocyanins are nature’s most potent antioxidants [125]. Similarly, blackberries show high antioxidant activity as they have highly abundant phenolic compounds, such as gallic acid, ellagic acid, ellagitannins, tannins, quercetin, cyanidins, and anthocyanins [126,127]. The antioxidant activities of berries are presented in Table 2. The data clearly shows significant variations in the results obtained from different assays. In another blueberry study, Liović et al. [128] studied the influence of freeze-drying, high-intensity ultra-sound, and pasteurization on gastrointestinal stability and antioxidant activity. The authors found that both total phenolic content and antioxidant capacities were improved for the freeze-dried and simulated gastric digested samples as compared to control untreated samples investigated in that study. These results suggested that external factors, such as drying and digestion, can also have a significant impact on bioactivity. Similarly, Dalmau and coworkers hypothesized that the drying process might alter the microstructures of vegetables as they found that both convective drying (CD) and freeze-drying (FD) decreased the TPC and antioxidant activity of beetroot samples [129]. The authors, however, claimed that these drying processes facilitate the better release of bioactive compounds during the digestion process and, in turn, lead to higher TPC and antioxidant activities [129].
5.2. Vegetables-Spinach and Olives
Spinach (Spinacia oleracea) is a vegetable with a wide array of phytoconstituents such as polyphenols, flavonoids, tocopherols, carotenoids, ascorbates, p-coumarins, vitamins, and polysaccharides, which are responsible for its nutritional properties [130,131]. The prominent antioxidants from spinach identified by different researchers include chlorogenic acid and spinacetins, and their analogs [132]. However, Mzoughi et al. reported the antioxidant activity of polysaccharides from spinach using DPPH, ABTS, and FRAP assays [90]. The water-soluble polysaccharides from Spinacia oleracea were extracted and characterized using FT-IR, UV–vis, 1H-NMR, SEC (Size Exclusion Chromatography)/MALS (multi-angle light scattering), and DRI (differential refractive index) techniques. The average molecular mass of the polysaccharide was 408 kDa composed of monosaccharides like arabinose, glucose, galactose, mannose, and rhamnose. Spinacia polysaccharide significantly prevented oxidation-induced Cd damage on HEK293 and HCT116 cells [90]. The results from both DPPH and ABTS assay were presented as percent inhibition (68.51 ± 0.89% and 70.12 ± 0.04%, respectively), whereas FRAP results were shown as reducing capacity in μmol/L (1590 ± 53.98 μmol/L at 10 mg/mL). On the other hand, Galla et al. [91] reported the antioxidant activity of the methanolic extract of spinach leaves assayed by DPPH, ABTS, and FRAP. In these two cases, one assay used water extraction to measure polysaccharides activity, and the other used methanol extraction to measure the activity of hydrophobic analytes. Hence, it becomes extremely challenging to identify the true antioxidant activity of foods using a single assay or extraction methodology. Another challenge involving these methods is the units used to report the activity. For instance, for the same assay (FRAP), Galla et al. [91] reported results in terms of percent inhibition, Hussain et al. [100] reported EC50, and Mzoughi et al. [90] reported reducing capacity in μmol/L [91]. Additional details on the antioxidant, antimicrobial activities, and clinical efficacy of Spinacia oleracea were presented in a recent review by Salehi et al. [131]. Recently, in 2020, Kamiloglu [133] reported the industrial freezing effects on the phenolic content and related antioxidant capacity of spinach. The results of both TPC and TAC (CUPRAC, ABTS, DPPH, and FRAP assays) showed that the freezing process increased both the TPC and TAC of spinach. Interestingly, undigested frozen samples have shown higher TPC and TAC results than digested (oral, gastric, and intestinal) samples [133]. These observations further illustrate that it is challenging to compare health claims just based on colorimetric assays commonly used for reporting antioxidant activities in foods.
Olives and olive oil have several health benefits due to the presence of phytochemicals [134,135]. Olives contain phenolic acids (caffeic acid, gallic acid) and their derivatives, phenolic alcohols (tyrosol, hydroxytyrosol), secoiridoids (oleuropein, oleocanthal), lignans (pinoresinol), and flavones (luteolin), which account for their antioxidant activity [134,135]. Hydroxytyrosol (HyT) is one of the main polyphenols found in virgin olive oil and olive mill waste [136] and has been shown to have strong ROS scavenging properties. HyT is the only phenolic compound that has received health claim approval from the European Food Safety Authority (EFSA). According to the EFSA, the consumption of olive oil polyphenols such as HyT and its derivatives play a protective role in preventing oxidative damage of blood lipids [136]. However, the antioxidant assays are not specific for HyT.
Recently, Fernández-Poyatos et al. [92] studied the antioxidant potential of table olives from Olea europaea L. They determined activity using conventional spectrophotometric methods ABTS and DPPH to be 308.68 μM and 228.46 μM Trolox equivalent per gram of dried extracts, respectively. Cheurfa et al. [105] investigated the antioxidant potential of the extract of olive leaves using water and aqueous ethanol separately. The ethanol extract of O. europeae leaves showed significant antioxidant activity (IC50 69.15 mg/mL, % inhibition 54.98, % inhibition 49.71, 82.63 mg ascorbic acid equivalent/g extract, and 7.53 mol of Fe2+/g extract for the DPPH, β-carotene bleaching, ferric thiocyanate, TAC, and FRAP assays, respectively) [105]. As noted above, there are significant variations in the antioxidant capacity values for olives depending on the assay used and the units for the results.
5.3. Processed Products-Wine, Coffee, and Tea
Wine is primarily made from grapes but is also made from several other fruits, including apple, cherry, pear, peach, plum, banana, mango, strawberry, blueberry, blackberry, and raspberry [137]. These fruits are widely known for their antioxidant activity. Similarly, coffee and tea are also considered to have significant antioxidant activity [138]. The coffee-brewed drink is prepared from coffee beans, and tea is prepared from fresh tea leaf extracts. Antioxidant activities of these three processed food products (wine, coffee, and tea) have been widely studied using different assays, including FRAP, ABTS, DPPH, ORAC, and TPC [110,111,112,113,139,140,141,142]. However, the data in Table 2 showed significant variations in the outcomes from different assays, despite using the same sample for analysis [110,111,112,113,139,140,141,142]. Table 2 presents DPPH and ABTS studies by Jung et al. [110] reported in percent radical scavenging activity (RSA), whereas, for the same assays, Bravo et al. [111] reported results in μM TE/g. Moreover, the results from either study (DPPH vs. ABTS) were distinctly different, which can be attributed to the mechanisms involved in the assays. Similar variations were observed for tea and wine antioxidant activities (Table 2).
5.4. Legumes-Bean, Soybean
Soybean (Glycine max (L.) Merr.) is one of the most important plant protein sources consumed by humans and animals and receives growing interest as a source of high-protein crops in Europe, North America, South Asia, and Japan [115]. The antioxidant activities of different matrices of soybeans have been studied, as shown in Table 2. However, for each assay, different units were reported, which makes it difficult to compare the antioxidant potential of different foods. For instance, Peiretti et al. [115] reported the TPC activity in catechin eq/g, whereas Handa et al. [93] reported in GA eq/g.
Common beans (Phaseolus vulgaris L.) are one of the most important legumes consumed globally and are used for nutritional and medical purposes [125]. Beans are rich in polyphenols, flavonoids, anthocyanidins, and procyanidins, which may explain their antioxidant activities. Zhao et al. [101] and Cid-Gallegos et al. [114] reported antioxidant activities for chickpeas. The results presented by the authors are not comparable as they used different solvents for extractions and expressed their results in different units (Table 2).
5.5. Grains-Corn, Wheat
Epidemiological studies have shown that the consumption of whole grains and grain-based products is associated with a reduced risk of oxidative stress-related chronic diseases and age-related disorders, such as cardiovascular diseases, carcinogenesis, type II diabetes, and obesity [143]. The health benefits of whole-grain flours are attributed to the presence of antioxidants, such as tocopherols and tocotrienols, vitamin E, carotenoids, phenolic acids, and flavonoids [144]. In 2019, Ranjbar et al. [117] studied the antioxidant potential of wheat flour with iron enrichment using DPPH and FRAP assays. However, the authors reported phenolic acid content as μg equivalent of ferulic acid/g, whereas, usually, the TAC is reported as either gallic acid or ascorbic acid equivalents/g. Hence, the comparison of antioxidant results becomes complicated for researchers and consumers.
Corn is among the largest produced staple foods across the globe. Corn is well known for its antioxidant properties, which may be attributed to the presence of high amounts of anthocyanins [145]. Antioxidant studies by Ramos-Escudero et al. [94] and Horvat et al. [95] showed no significant relationship between TPC and DPPH radical scavenging in corn samples (r = 0.202) [95]. This lack of correlation may be due to several reasons, such as experimental conditions, mechanisms, interferences, and types of the analytes assayed [95].
5.6. Dairy Products-Milk, Yogurt, and Others
Dairy products constitute about 25–30% of the average diet of an individual [146]. Milk and milk products are rich in essential nutrients such as vitamins (tocopherol, retinol, and carotenoids), minerals, oleic acid, omega-3 fatty acids, conjugated linoleic acid, antioxidants like milk caseins, ascorbate, low molecular weight thiols, and whey proteins, and other bioactive compounds [146,147].
Zulueta et al. [120] reported the antioxidant capacity of multiple commercial samples of pasteurized and ultra-high temperature (UHT) treated whole milk, whey, and deproteinized milk using ORACFL assay [120]. According to the study, the TAC of whole milk was attributed mainly to casein fractions, albumin, and whey protein, whereas hydrophilic antioxidant compounds, such as ascorbic acid and uric acid, were the main contributors to the total TAC of the deproteinized milk. A significant correlation was found between the fat% and the TAC of milk samples. In addition, pasteurized milk was found to have significantly higher TAC than UHT-treated milk for both whey and deproteinized milk samples. In contrast, the TAC values of pasteurized and UHT whole milk were not significantly different [120]. The reported antioxidant results were entirely based on a single ORACFL assay, which is the main limitation of this report, as no single assay is expected to provide an accurate measurement of total antioxidant capacity. In addition, this report also suggested that sample processing plays a very important role in the overall antioxidant activity/capacity of the food substrate.
In a recent study by de Carvalho et al. [121], yogurt samples were fortified with 0.25% and 0.5% freeze-dried stevia extract (FSE). The control and stevia-fortified yogurts were evaluated and compared for the TPC and antioxidant activity (using FRAP and ABTS). Table 2 shows the TPC, FRAP, and ABTS results for the yoghurt 0.14 ± 0.01 mg GAE/g, 0.40 ± 0.03 μmol Trolox/g, 0.40 ± 0.04 μmol Trolox/g, respectively. Upon adding 0.25% FSE, the antioxidant activity of the yoghurt significantly increased (TPC- 0.43 ± 0.02 mg GAE/g, FRAP- 2.57 ± 0.09 μmol Trolox/g, ABTS- 3.63 ± 0.08 μmol Trolox/g. Moreover, upon addition of 0.5% FSE, the antioxidant activity further increased TPC- 0.65 ± 0.02, FRAP- 4.19 ± 0.05 μmol Trolox/g, ABTS- 5.34 ± 0.23 μmol Trolox/g. Apart from FSE, the simulated digestion also increased the antioxidant activity of the fortified yogurts compared to the undigested fractions [121].
6. Strengths and Weaknesses of Antioxidant Assays
6.1. Strengths
In general, antioxidant assays provide rapid and inexpensive means of measuring the status of a sample. Changes in the composition of a food or supplement arising from genetics, environment, management, and processing are readily detected. Antioxidant assays are sensitive to the metadata associated with a sample. The assays cannot specify which components are changing, but changes are readily detected.
6.2. Weaknesses
Questions have been raised about the relationship between dietary/exogenous antioxidants and health. Lack of specificity, lack of harmonization, and the inability to correlate in vitro measurements with in vivo activity have been major factors. The U.S. Food and Drug Administration (FDA) and the European Food Safety Authority (EFSA) do allow health claims for vitamins (A, C, and E), which have shown ambiguous results in vivo. Several journals have banned papers whose primary measurements are antioxidant activity. This suggests the complexity of the issue [148]. The major limitations of currently used antioxidant assays are summarized below.
(a) There is a huge divergence in the results for food and dietary antioxidants because the assays are non-specific and are influenced by every component in a sample matrix. A change in an assay value cannot be correlated with a change in a specific component. Consequently, antioxidant assays cannot be correlated with health outcomes.
(b) Antioxidant assays cannot be compared or inter-converted. Antioxidant assays measure activities. A detailed explanation for these limitations has been described by Apak et al. [15]. Both AOAC International (Association of Analytical Communities) and the IUPAC (International Union of Pure and Applied Chemistry) reported that the results from different antioxidant methods could not be compared as each method employs different mechanisms, pH, temperature, and sample matrix [148]. Hence, the IUPAC concluded that there is no single universally accepted antioxidant assay available that accurately determines the antioxidant activity or the total antioxidant capacity [10,60,148]. In 2012, the U.S. Department of Agriculture (USDA) removed the ORAC database from online ‘because of increasing evidence that the data infers there is no relevance between antioxidant activity and the effects of bioactive compounds, including polyphenols on human health’ [10,148].
(c) Reporting results in multiple units for the same assay complicates data comparison [149]. For example, results for TPC have been reported as equivalents of gallic acid, ascorbic acid, or ferulic acid, which complicates the understanding of the results.
(d) The measurements in vitro experiments cannot be directly correlated with in vivo activity. This can be attributed to the complex physiological environment in vivo assays as compared with the controlled environments in vitro assays [149,150]. The activity of plant-based secondary metabolites such as polyphenols, phenolic acids, flavonoids, and proanthocyanidins, is little known [148].
(e) The interpretation of antioxidant assay results mainly focuses on the antioxidant-oxidant reactions and related kinetics; however, they often ignore the chromophore/luminescent interactions with the probe, which can cause interferences in the results [151].
7. Other Factors Influencing Antioxidant Activity
Unlike the in vitro assays, the measurements based on in vivo antioxidant assays are impacted by several factors relevant to physiological conditions. For instance, the fate of the antioxidant in vivo is determined by the pharmacokinetic phenomena ADME (absorption, distribution, metabolism, excretion) profiles, which are not evaluated in vitro [152]. Unlike in vitro, the actual concentrations of antioxidants at tissue levels are essentially depending on ADME profiles. Below are some of the critical limitations of in vivo antioxidant assays [15,152,153].
7.1. Bioaccessibility and Bioavailability of Antioxidants
Bioaccessibility and bioavailability are closely related, but they have different definitions. Bioaccessibility is the fraction of bioactive compounds that are released from a matrix during the digestion process and become available for absorption, whereas bioavailability is the fraction of compounds that are absorbed into the bloodstream, distributed by systemic circulation, and exert their effect after being metabolized then eliminated [154,155]. For phytochemicals, as with any food component, to exert their biological activity, they must be released from the matrix in an absorbable form into the stomach/intestine/colon (bioaccessible), followed by absorption into the bloodstream (bioavailable) [154,155]. Factors such as matrix interactions and chemical structures influence the bioaccessibility of phytochemicals, whereas bioavailability can be affected by multiple parameters, such as biological membranes (GI wall), the physicochemical properties of the phytochemicals, biological environment inside the GI, etc., The optimum properties of phytochemicals/drugs for GI absorption have been defined by Lipinski’s Rule [156], including appropriate molecular weight, hydrogen bonding capability, and partition coefficient (LogP) [156]. In addition, the presence of adjuvants, food processing techniques, sample preparation, and extraction methods can also significantly influence the bioavailability of the compounds by influencing their bioaccessibility [154,155].
The bioaccessibility and bioavailability of phytochemicals can be enhanced using processing techniques that induce physical or chemical modifications in the food [154]. These modifications include: (a) chemical modifications, such as cleavage of hydrophobic forces, hydrogen bonds, and bonds that attach phenolic compounds to matrix macromolecules, conjugation, and derivatization; (b) physical modifications, such as grinding, drying by different approaches; (c) disrupting the cell wall barriers so that phytochemicals can be released from the matrix; and (d) using encapsulation techniques/solubilization using nanotechnologies that protect phytochemicals until they are absorbed. One should note that these techniques can cause the degradation of phytochemicals; however, it is possible to reduce it by altering the operating conditions, which can facilitate increased bioaccessibility and bioavailability [154]. Techniques such as drying, freezing, thermal processing, sterilization and pasteurization, ultrasounds treatment, milling and grinding, chemical and enzymatic treatments, and encapsulation, which are commonly used for food processing and supplements, can influence the overall phytochemical availability [154]. The positive or adverse effects of processing techniques on bioaccessibility and bioavailability depend on compounds’ stability, matrix protective effects, and existing interactions.
Bioaccessibility, bioavailability, the metabolism of antioxidants, and their consequences, must be taken into consideration. For instance, flavonoids are structurally altered in vivo. Hence, the nutritional application of flavonoids requires extensive studies on their metabolism and controlled comparison of antioxidant activity of their structural isoforms. Additionally, the evidence shows that the removal of glycosidic substituents (sugar moiety) by enzymes or bacteria is likely to increase the antioxidant activity of flavonoids in vivo. In contrast, the methylation and glycosylation of OH groups in flavonoids reduce their prooxidant behavior by catechol-o-methyltransferase (COMT) and other enzymes. The impact of sugar moiety on the bioactivities of flavonoids has been discussed in recent reviews by different researchers [153,157,158].
7.2. Chelation
The efficacy of antioxidants’ function based on metal chelation mechanism, such as polyphenols, need to be investigated thoroughly in vivo. Though ascorbate is an antioxidant, it can also act as a pro-oxidant, especially in the presence of transition metals like iron and copper [159]. Hence, it is important to understand the actual factors contributing to the antioxidant activity in the presence of metals, ascorbate, and other possible interferences, such as uric acid, which is elevated upon consumption of polyphenol-rich foods and may cause increased plasma total antioxidant capacity in vivo. Finally, it has been suggested that the large increase in plasma total antioxidant capacity observed after the consumption of polyphenol-rich foods is not caused by the polyphenols themselves but is likely the consequence of increased uric acid levels [160].
7.3. In Vivo Assays
Over the years, researchers have discussed the validity of applying various assays to in vivo conditions. All of them provide an estimate of the antioxidant capacity of plasma/serum without distinguishing the contribution by exogenous molecules of dietary origin (i.e., ascorbic acid, vitamin E, polyphenols, and other phytochemicals) compared to endogenously-derived molecules such as enzymatic components (i.e., SOD, GSH-Px, and catalase, CAT) and small macromolecules (i.e., albumin, bilirubin, ceruloplasmin, ferritin, glutathione) [161]. In this regard, the EFSA remarked on the inappropriateness of the methods used to determine antioxidant capacity in humans and extrapolate possible effects on human health [162].
7.4. Sample Matrix
Sample/substrate matrix always has a great influence on the outcomes of antioxidant studies, both in vitro and in vivo. Along with matrix effects and multiple variable experimental conditions, performing the assays (radical generator, time of measure, expression of results, etc.) makes it difficult to compare the reported data.
7.5. Experimental Parameters
Most assays lack a detailed evaluation of important factors that affect the antioxidant activity measurements, such as concentration, pH, localization, distribution, metabolism, reactivity toward other non-targeted molecules, interaction with other antioxidants, and the fate of the radical that is derived from the antioxidant.
8. Perspectives/Recommendations
To achieve absolute efficacy of food antioxidants acceptable to various communities such as nutrition and clinical professionals and consumers, it is essential to consider the following factors:
(a) Matrix information must be presented in detail in the experimental section of the in vitro and in vivo antioxidant assays. The matrix effects should also be accounted for.
(b) Sample preparation (grinding, drying, processing, etc.) and the extraction procedures for antioxidants have a direct impact on the results. Hence, one should use optimized sample preparation and extraction methods to extract antioxidants with a wide range of polarities.
(c) Since there is no universal standard available currently, the development of universal multi-component standards is essential to address the variances associated with antioxidant capacity measurements.
(d) Both antioxidant activity and antioxidant capacity must be distinguished as these two terms are used interchangeably even though they are both measured completely differently, i.e., antioxidant activity is measured kinetically, and antioxidant capacity is measured thermodynamically. An ideal method for an antioxidant activity measurement requires: (i) usage of a biologically relevant radical source; (ii) determines actual chemistry that occurs in the assays; (iii) the use of a method with a defined endpoint and chemical mechanism involved in a particular assay; (iv) a suitable method for both hydrophilic and lipophilic antioxidants; and (v) instrumentation, which is readily available, simple, economical, rugged, user friendly, and facilitates high-throughput for routine analyses.
(e) The harmonization of methodology is required, which includes detailed standard operating procedures for different assays, test conditions, good internal/external standards, proper method validation, including intra- and inter-lab validation (reproducibility, accuracy, precision, and recovery), quality control, and quality assurance.
(f) All antioxidant results should be presented in a single international system of units (SI). Correlations tables between various assays should be established to promote easy comparison of results between different assay procedures.
(g) A better understanding of the bioaccessibility and bioavailability of antioxidants in vivo is essential. To achieve maximum efficacy from the antioxidants in vivo, their bioaccessibility and bioavailability must be increased by using appropriate delivery systems, such as liposomes, nanoparticles, etc.
Overall, it is not easy to accomplish the above recommendations altogether. However, focusing on each of the above steps will improve the credibility of antioxidants. This will enable nutrition professionals, and researchers to correlate accurately the role of antioxidants as it relates to nutrition and health.
9. Conclusions
The present review defines antioxidants and describes antioxidant assays, their mechanisms, and their strengths and weaknesses. These methods have been used to evaluate the “health benefits” of phytochemicals, as documented in the literature. Unfortunately, with antioxidant assays, it is not certain what has been measured. The lack of specificity of the assays creates confusion and ambiguity for consumers, healthcare professionals, and researchers. To have a wide acceptance and clear understanding of the health benefits of phytochemicals, there is a critical need to develop multi-omic approaches which measure specific food and supplement components. This will allow health outcomes to be correlated with specific food components.
Author Contributions
Conceptualization, D.L.L.; methodology, D.L.L., R.R.K., F.S.T. and E.Y.; formal analysis, D.L.L., R.R.K., F.S.T. and E.Y.; investigation, D.L.L., R.R.K., F.S.T. and E.Y.; resources, D.L.L., R.R.K., F.S.T. and E.Y.; data curation, D.L.L., R.R.K., F.S.T. and E.Y.; writing—original draft preparation, D.L.L., R.R.K., F.S.T. and E.Y.; writing—review and editing, D.L.L., R.R.K., F.S.T. and E.Y.; visualization, D.L.L., R.R.K., F.S.T. and E.Y.; supervision, D.L.L.; project administration, D.L.L.; funding acquisition, D.L.L. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Agricultural Research Service, US Department of Agriculture, Project # 1235-52000-066-00D.
Acknowledgments
We would like to thank James Harnly for his feedback on the preparation of this manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Valko, M.; Leibfritz, D.; Moncol, J.; Cronin, M.T.D.; Mazur, M.; Telser, J. Free radicals and antioxidants in normal physiological functions and human disease. Int. J. Biochem. Cell Biol. 2007, 39, 44–84. [Google Scholar] [CrossRef] [PubMed]
- Cömert, E.D.; Gökmen, V. Evolution of food antioxidants as a core topic of food science for a century. Food Res. Int. 2018, 105, 76–93. [Google Scholar] [CrossRef] [PubMed]
- Shahidi, F.; Zhong, Y. Measurement of antioxidant activity. J. Func. Foods 2015, 18, 757–781. [Google Scholar] [CrossRef]
- Waterman, K.C.; Adami, R.C.; Alsante, K.M.; Hong, J.; Landis, M.S.; Lombardo, F.; Roberts, C.J. Stabilization of pharmaceuticals to oxidative degradation. Pharm. Dev. Technol. 2002, 7, 1–32. [Google Scholar] [CrossRef]
- Carocho, M.; Ferreira, I.C. A review on antioxidants, prooxidants and related controversy: Natural and synthetic compounds, screening and analysis methodologies and future perspectives. Food Chem. Toxicol. 2013, 51, 15–25. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Chen, C.; Wang, X.; Sun, Y.; Zhang, J.; Chen, J.; Shi, Y. An Epigenetic Role of Mitochondria in Cancer. Cells 2022, 11, 2518. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.; Zhang, J.; Beeraka, N.M.; Tang, C.; Babayeva, Y.V.; Sinelnikov, M.Y.; Zhang, X.; Zhang, J.; Liu, J.; Reshetov, I.V.; et al. Advances in the Prevention and Treatment of Obesity-Driven Effects in Breast Cancers. Front. Oncol. 2022, 12, 820968. [Google Scholar] [CrossRef]
- Chen, K.; Lu, P.; Beeraka, N.M.; Sukocheva, O.A.; Madhunapantula, S.V.; Liu, J.; Sinelnikov, M.Y.; Nikolenko, V.N.; Bulygin, K.V.; Mikhaleva, L.M.; et al. Mitochondrial mutations and mitoepigenetics: Focus on regulation of oxidative stress-induced responses in breast cancers. Semin. Cancer Biol. 2022, 83, 556–569. [Google Scholar] [CrossRef] [PubMed]
- Niki, E. Assessment of antioxidant capacity in vitro and in vivo. Free Radic. Biol. Med. 2010, 49, 503–515. [Google Scholar] [CrossRef]
- Granato, D.; Shahidi, F.; Wrolstad, R.; Kilmartin, P.; Melton, L.D.; Hidalgo, F.J.; Miyashita, K.; Camp, J.V.; Alasalvar, C.; Ismail, A.B.; et al. Antioxidant activity, total phenolics, and flavonoids contents: Should we ban in vitro screening methods? Food Chem. 2018, 264, 471–475. [Google Scholar] [CrossRef]
- Finley, J.W.; Kong, A.N.; Hintze, K.J.; Jeffery, E.H.; Ji, L.L.; Lei, X.G. Antioxidants in foods: State of the science important to the food industry. J. Agric. Food Chem. 2011, 59, 6837–6846. [Google Scholar] [CrossRef] [PubMed]
- Natural Antioxidants Market Size Worth $ 4.14 Billion By 2022. Available online: grandviewresearch.com (accessed on 4 January 2021).
- Alam, M.N.; Bristi, N.J.; Rafiquzzaman, M. Review on in vivo and in vitro methods evaluation of antioxidant activity. Saudi Pharm. J. 2013, 21, 143–152. [Google Scholar] [CrossRef] [PubMed]
- Amorati, R.; Valgimigli, L. Advantages and limitations of common testing methods for antioxidants. Free Radic. Res. 2015, 49, 633–649. [Google Scholar] [CrossRef]
- Apak, R.; Özyürek, M.; Güçlü, K.; Çapanoğlu, E. Antioxidant Activity/Capacity Measurement. 1. Classification, Physicochemical Principles, Mechanisms, and Electron Transfer (ET)-Based Assays. J. Agric. Food Chem. 2016, 64, 997–1027. [Google Scholar] [CrossRef] [PubMed]
- Apak, R.; Özyürek, M.; Güçlü, K.; Çapanoğlu, E. Antioxidant Activity/Capacity Measurement. 2. Hydrogen Atom Transfer (HAT)-Based, Mixed-Mode (Electron Transfer (ET)/HAT), and Lipid Peroxidation Assays. J. Agric. Food Chem. 2016, 64, 1028–1045. [Google Scholar] [CrossRef] [PubMed]
- Apak, R.; Özyürek, M.; Güçlü, K.; Çapanoğlu, E. Antioxidant Activity/Capacity Measurement. 3. Reactive Oxygen and Nitrogen Species (ROS/RNS) Scavenging Assays, Oxidative Stress Biomarkers, and Chromatographic/Chemometric Assays. J. Agric. Food Chem. 2016, 64, 1046–1070. [Google Scholar] [CrossRef]
- Gulcin, İ. Antioxidants and antioxidant methods: An updated overview. Arch. Toxicol. 2020, 94, 651–715. [Google Scholar] [CrossRef]
- Frankel, E.N.; German, J.B. Antioxidants in foods and health: Problems and fallacies in the field. J. Sci. Food Agric. 2006, 86, 1999–2001. [Google Scholar] [CrossRef]
- Williams, R.J.; Spencer, J.P.; Rice-Evans, C. Flavonoids: Antioxidants or signaling molecules? Free Radic. Biol. Med. 2004, 36, 838–849. [Google Scholar] [CrossRef]
- Lotito, S.B.; Frei, B. Consumption of flavonoid-rich foods and increased plasma antioxidant capacity in humans: Cause, consequence, or epiphenomenon? Free Radic. Biol. Med. 2006, 41, 1727–1746. [Google Scholar] [CrossRef]
- Holst, B.; Williamson, G. Nutrients and phytochemicals: From bioavailability to bioefficacy beyond antioxidants. Curr. Opin. Biotechnol. 2008, 19, 73–82. [Google Scholar] [CrossRef]
- Hollman, P.C.; Cassidy, A.; Comte, B.; Heinonen, M.; Richelle, M.; Richling, E.; Serafini, M.; Scalbert, A.; Sies, H.; Vidry, S. The biological relevance of direct antioxidant effects of polyphenols for cardiovascular health in humans is not established. J. Nutr. 2011, 141, 989s–1009s. [Google Scholar] [CrossRef] [PubMed]
- Oxygen Radical Absorbance Capacity (ORAC) of Selected Foods. Available online: https://www.researchgate.net/publication/267379608_Oxygen_Radical_Absorbance_Capacity_ORAC_of_selected_foods (accessed on 1 October 2022).
- Food and Drug Administration (FDA); U.S. Department of Health and Human Services; Center for Food Safety and Applied Nutrition. Guidance for Industry, Food Labeling; Nutrient Content Claims; Definition for High Potency and Definition for Antioxidant for Use in Nutrient Content Claims for Dietary Supplements and Conventional Foods; U.S. Department of Health and Human Services: Atlanta, GA, USA, 2008.
- EFSA Panel on Dietetic Products, Nutrition and Allergies (NDA). Scientific Opinion on the substantiation of health claims related to various food(s)/food constituent(s) and protection of cells from premature aging, antioxidant activity, antioxidant content and antioxidant properties, and protection of DNA, proteins and lipids from oxidative damage pursuant to Article 13(1) of Regulation (EC) No 1924/2006. EFSA J. 2010, 8, 1489. [Google Scholar]
- Murphy, M.P.; Bayir, H.; Belousov, V.; Chang, C.J.; Davies, K.J.; Davies, M.J.; Halliwell, B. Guidelines for measuring reactive oxygen species and oxidative damage in cells and in vivo. Nat. Metab. 2022, 4, 651–662. [Google Scholar] [CrossRef] [PubMed]
- Sies, H.; Belousov, V.V.; Chandel, N.S.; Davies, M.J.; Jones, D.P.; Mann, G.E.; Winterbourn, C. Defining roles of specific reactive oxygen species (ROS) in cell biology and physiology. Nat. Rev. Mol. Cell Biol. 2022 23, 499–515. [CrossRef]
- Carew, J.S.; Huang, P. Mitochondrial defects in cancer. Mol. Cancer 2002, 1, 9. [Google Scholar] [CrossRef] [PubMed]
- Mirończuk-Chodakowska, I.; Witkowska, A.M.; Zujko, M.E. Endogenous non-enzymatic antioxidants in the human body. Adv. Med. Sci. 2018, 63, 68–78. [Google Scholar] [CrossRef]
- Fierascu, R.C.; Ortan, A.; Fierascu, I.C.; Fierascu, I. In vitro and in vivo evaluation of antioxidant properties of wild-growing plants. A short review. Curr. Opin. Food Sci. 2018, 24, 1–8. [Google Scholar] [CrossRef]
- Antolovich, M.; Prenzler, P.D.; Patsalides, E.; McDonald, S.; Robards, K. Methods for testing antioxidant activity. Analyst 2002, 127, 183–198. [Google Scholar] [CrossRef]
- Sanchez-Moreno, C. Review: Methods Used to Evaluate the Free Radical Scavenging Activity in Foods and Biological Systems. Food Sci. Technol. Int. 2002, 8, 121–137. [Google Scholar] [CrossRef]
- Aruoma, O.I. Methodological considerations for characterizing potential antioxidant actions of bioactive components in plant foods. Mutat. Res. 2003, 523, 9–20. [Google Scholar] [CrossRef]
- Apak, R.; Guclu, K.; Ozyurek, M.; Bektasoglu, B.; Bener, M. Cupric ion reducing antioxidant capacity assay for antioxidants in human serum and for hydroxyl radical scavengers. Methods Mol. Biol. 2010, 594, 215–239. [Google Scholar] [PubMed]
- Apak, R.; Güçlü, K.; Özyürek, M.; Çelik, S.E. Mechanism of antioxidant capacity assays and the CUPRAC (cupric ion reducing antioxidant capacity) assay. Microchim. Acta 2008, 160, 413–419. [Google Scholar] [CrossRef]
- Apak, R.; Güçlü, K.; Demirata, B.; Ozyürek, M.; Celik, S.E.; Bektaşoğlu, B.; Berker, K.I.; Ozyurt, D. Comparative evaluation of various total antioxidant capacity assays applied to phenolic compounds with the CUPRAC assay. Molecules 2007, 12, 1496–1547. [Google Scholar] [CrossRef]
- Mehdi, M.M.; Rizvi, S.I. N,N-Dimethyl-p-phenylenediamine dihydrochloride-based method for the measurement of plasma oxidative capacity during human aging. Anal. Biochem. 2013, 436, 165–167. [Google Scholar] [CrossRef]
- Aryal, S.; Baniya, M.K.; Danekhu, K.; Kunwar, P.; Gurung, R.; Koirala, N. Total Phenolic Content, Flavonoid Content and Antioxidant Potential of Wild Vegetables from Western Nepal. Plants 2019, 8, 96. [Google Scholar] [CrossRef]
- Benzie, I.F.; Strain, J.J. Ferric reducing/antioxidant power assay: Direct measure of total antioxidant activity of biological fluids and modified version for simultaneous measurement of total antioxidant power and ascorbic acid concentration. Methods Enzymol. 1999, 299, 15–27. [Google Scholar]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Dubost, N.J.; Ou, B.; Beelman, R.B. Quantification of polyphenols and ergothioneine in cultivated mushrooms and correlation to total antioxidant capacity. Food Chem. 2007, 105, 727–735. [Google Scholar] [CrossRef]
- Schlesier, K.; Harwat, M.; Bohm, V.; Bitsch, R. Assessment of antioxidant activity by using different in vitro methods. Free Radic. Res. 2002, 36, 177–187. [Google Scholar] [CrossRef] [PubMed]
- Kedare, S.B.; Singh, R.P. Genesis and development of DPPH method of antioxidant assay. J. Food Sci. Technol. 2011, 48, 412–422. [Google Scholar] [CrossRef] [PubMed]
- Tepe, B.; Sokmen, M.; Akpulat, H.A.; Sokmen, A. Screening of the antioxidant potentials of six Salvia species from Turkey. Food Chem. 2006, 95, 200–204. [Google Scholar] [CrossRef]
- Sharma, S.; Vig, A.P. Evaluation of in vitro antioxidant properties of methanol and aqueous extracts of Parkinsonia aculeata L. leaves. Sci. World J. 2013, 2013, 604865. [Google Scholar] [CrossRef]
- Sroka, Z.; Cisowski, W. Hydrogen peroxide scavenging, antioxidant and anti-radical activity of some phenolic acids. Food Chem. Toxicol. 2003, 41, 753–758. [Google Scholar] [CrossRef]
- Gardner, A.M.; Gardner, P.R. Flavohemoglobin detoxifies nitric oxide in aerobic, but not anaerobic, Escherichia coli. Evidence for a novel inducible anaerobic nitric oxide-scavenging activity. J. Biol. Chem. 2002, 277, 8166–8171. [Google Scholar] [CrossRef] [PubMed]
- Sarban, S.; Kocyigit, A.; Yazar, M.; Isikan, U.E. Plasma total antioxidant capacity, lipid peroxidation, and erythrocyte antioxidant enzyme activities in patients with rheumatoid arthritis and osteoarthritis. Clin. Biochem. 2005, 38, 981–986. [Google Scholar] [CrossRef]
- Weydert, C.J.; Cullen, J.J. Measurement of superoxide dismutase, catalase and glutathione peroxidase in cultured cells and tissue. Nat. Protoc. 2010, 5, 51–66. [Google Scholar] [CrossRef] [PubMed]
- Benzie, I.F.; Strain, J.J. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: The FRAP assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef]
- Rubio, C.P.; Hernandez-Ruiz, J.; Martinez-Subiela, S.; Tvarijonaviciute, A.; Ceron, J.J. Spectrophotometric assays for total antioxidant capacity (TAC) in dog serum: An update. BMC Vet. Res. 2016, 12, 166. [Google Scholar] [CrossRef]
- Brown, K.E.; Kinter, M.T.; Oberley, T.D.; Freeman, M.L.; Frierson, H.F.; Ridnour, L.A.; Tao, Y.; Oberley, L.W.; Spitz, D.R. Enhanced gamma-glutamyl transpeptidase expression and selective loss of CuZn superoxide dismutase in hepatic iron overload. Free Radic. Biol. Med. 1998, 24, 545–555. [Google Scholar] [CrossRef]
- Dadheech, G.; Mishra, S.; Gautam, S.; Sharma, P. Evaluation of antioxidant deficit in schizophrenia. Indian J. Psychiatry 2008, 50, 16–20. [Google Scholar] [PubMed]
- Gungor, N.; Ozyurek, M.; Guclu, K.; Cekic, S.D.; Apak, R. Comparative evaluation of antioxidant capacities of thiol-based antioxidants measured by different in vitro methods. Talanta 2011, 83, 1650–1658. [Google Scholar] [CrossRef] [PubMed]
- Pinkus, R.; Weiner, L.M.; Daniel, V. Role of oxidants and antioxidants in the induction of AP-1, NF-kappaB, and glutathione S-transferase gene expression. J. Biol. Chem. 1996, 271, 13422–13429. [Google Scholar] [CrossRef] [PubMed]
- Singhal, S.S.; Singh, S.P.; Singhal, P.; Horne, D.; Singhal, J.; Awasthi, S. Antioxidant role of glutathione S-transferases: 4-Hydroxynonenal, a key molecule in stress-mediated signaling. Toxicol. Appl. Pharmacol. 2015, 289, 361–370. [Google Scholar] [CrossRef]
- Anderson, K.J.; Teuber, S.S.; Gobeille, A.; Cremin, P.; Waterhouse, A.L.; Steinberg, F.M. Walnut polyphenolics inhibit in vitro human plasma and LDL oxidation. J. Nutr. 2001, 131, 2837–2842. [Google Scholar] [CrossRef]
- Romero, F.J.; Bosch-Morell, F.; Romero, M.J.; Jareño, E.J.; Romero, B.; Marín, N.; Romá, J. Lipid peroxidation products and antioxidants in human disease. Environ. Health Perspect. 1998, 106 (Suppl. 5), 1229–1234. [Google Scholar]
- Nelson, S.K.; Bose, S.K.; Grunwald, G.K.; Myhill, P.; McCord, J.M. The induction of human superoxide dismutase and catalase in vivo: A fundamentally new approach to antioxidant therapy. Free Radic. Biol. Med. 2006, 40, 341–347. [Google Scholar] [CrossRef]
- Apak, R.; Gorinstein, S.; Böhm, V.; Schaich, K.M.; Özyürek, M.; Güçlü, K. Methods of measurement and evaluation of natural antioxidant capacity/activity (IUPAC Technical Report). Pure Appl. Chem. 2013, 85, 957–998. [Google Scholar] [CrossRef]
- Fogliano, V.; Verde, V.; Randazzo, G.; Ritieni, A. Method for Measuring Antioxidant Activity and Its Application to Monitoring the Antioxidant Capacity of Wines. J. Agric. Food Chem. 1999, 47, 1035–1040. [Google Scholar] [CrossRef]
- Seeram, N.P.; Henning, S.M.; Niu, Y.; Lee, R.; Scheuller, H.S.; Heber, D. Catechin and Caffeine Content of Green Tea Dietary Supplements and Correlation with Antioxidant Capacity. J. Agric. Food Chem. 2006, 54, 1599–1603. [Google Scholar] [CrossRef]
- Prior, R.L.; Hoang, H.; Gu, L.; Wu, X.; Bacchiocca, M.; Howard, L.; Hampsch-Woodill, M.; Huang, D.; Ou, B.; Jacob, R. Assays for Hydrophilic and Lipophilic Antioxidant Capacity (oxygen radical absorbance capacity (ORACFL)) of Plasma and Other Biological and Food Samples. J. Agric. Food Chem. 2003, 51, 3273–3279. [Google Scholar] [CrossRef] [PubMed]
- Ghiselli, A.; Serafini, M.; Maiani, G.; Azzini, E.; Ferro-Luzzi, A. A fluorescence-based method for measuring total plasma antioxidant capability. Free Radic. Biol. Med. 1995, 18, 29–36. [Google Scholar] [CrossRef] [PubMed]
- Manzocco, L.; Anese, M.; Nicoli, M.C. Antioxidant Properties of Tea Extracts as Affected by Processing. LWT-Food Sci. Technol. 1998, 31, 694–698. [Google Scholar] [CrossRef]
- Lissi, E.; Salim-Hanna, M.; Pascual, C.; del Castillo, M.D. Evaluation of total antioxidant potential (TRAP) and total antioxidant reactivity from luminol-enhanced chemiluminescence measurements. Free Radic. Biol. Med. 1995, 18, 153–158. [Google Scholar] [CrossRef] [PubMed]
- Pisoschi, A.M.; Pop, A.; Cimpeanu, C.; Predoi, G. Antioxidant Capacity Determination in Plants and Plant-Derived Products: A Review. Oxid. Med. Cell Longev. 2016, 2016, 9130976. [Google Scholar] [CrossRef]
- Kabouche, A.; Kabouche, Z.; Öztürk, M.; Kolak, U.; Topçu, G. Antioxidant abietane diterpenoids from Salvia barrelieri. Food Chem. 2007, 102, 1281–1287. [Google Scholar] [CrossRef]
- Moon, J.-K.; Shibamoto, T. Antioxidant Assays for Plant and Food Components. J. Agric. Food Chem. 2009, 57, 1655–1666. [Google Scholar] [CrossRef]
- Zheleva-Dimitrova, D.; Nedialkov, P.; Kitanov, G. Radical scavenging and antioxidant activities of methanolic extracts from Hypericum species growing in Bulgaria. Pharmacogn. Mag. 2010, 6, 74–78. [Google Scholar] [CrossRef]
- Fernando, C.D.; Preethi Soysa, P. Optimized enzymatic colorimetric assay for determination of hydrogen peroxide (H2O2) scavenging activity of plant extracts. MethodsX 2015, 2, 283–291. [Google Scholar] [CrossRef]
- Kunchandy, E.; Rao, M.N.A. Oxygen radical scavenging activity of curcumin. Int. J. Pharm. 1990, 58, 237–240. [Google Scholar] [CrossRef]
- Marcocci, L.; Maguire, J.J.; Droy-Lefaix, M.T.; Packer, L. The Nitric Oxide-Scavenging Properties of Ginkgo Biloba Extract EGb 761. Biochem. Biophys. Res. Commun. 1994, 201, 748–755. [Google Scholar] [CrossRef]
- Kooy, N.W.; Royall, J.A.; Ischiropoulos, H.; Beckman, J.S. Peroxynitrite-mediated oxidation of dihydrorhodamine 123. Free Radic. Biol. Med. 1994, 16, 149–156. [Google Scholar] [CrossRef]
- Chun, O.K.; Kim, D.-O.; Lee, C.Y. Superoxide Radical Scavenging Activity of the Major Polyphenols in Fresh Plums. J. Agric. Food Chem. 2003, 51, 8067–8072. [Google Scholar] [CrossRef] [PubMed]
- Robak, J.; Gryglewski, R.J. Flavonoids are scavengers of superoxide anions. Biochem. Pharmacol. 1988, 37, 837–841. [Google Scholar] [CrossRef] [PubMed]
- Aebi, H. Catalase in vitro. Methods Enzymol. 1984, 105, 121–126. [Google Scholar] [PubMed]
- Jagtap, U.B.; Panaskar, S.N.; Bapat, V.A. Evaluation of Antioxidant Capacity and Phenol Content in Jackfruit (Artocarpus heterophyllus Lam.) Fruit Pulp. Plant Foods Hum. Nutr. 2010, 65, 99–104. [Google Scholar] [CrossRef]
- Singal, P.K.; Kapur, N.; Dhillon, K.S.; Beamish, R.E.; Dhalla, N.S. Role of free radicals in catecholamine-induced cardiomyopathy. Can. J. Physiol. Pharmacol. 1982, 60, 1390–1397. [Google Scholar] [CrossRef]
- Kakkar, P.; Das, B.; Viswanathan, P.N. A modified spectrophotometric assay of superoxide dismutase. Indian J. Biochem. Biophys. 1984, 21, 130–132. [Google Scholar]
- Ellman, G.L. Tissue sulfhydryl groups. Arch. Biochem. Biophys. 1959, 82, 70–77. [Google Scholar] [CrossRef]
- Buege, J.A.; Aust, S.D. Microsomal lipid peroxidation. Methods Enzymol. 1978, 52, 302–310. [Google Scholar]
- El-Saadani, M.; Esterbauer, H.; El-Sayed, M.; Goher, M.; Nassar, A.Y.; Jürgens, G. A spectrophotometric assay for lipid peroxides in serum lipoproteins using a commercially available reagent. J. Lipid Res. 1989, 30, 627–630. [Google Scholar] [CrossRef] [PubMed]
- Ohkawa, H.; Ohishi, N.; Yagi, K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal. Biochem. 1979, 95, 351–358. [Google Scholar] [CrossRef] [PubMed]
- McCord, J.M.; Fridovich, I. Superoxide Dismutase: An enzymic function for erythrocuprein (Hemocuprein). J. Biol. Chem. 1969, 244, 6049–6055. [Google Scholar] [CrossRef]
- Berker, K.I.; Güçlü, K.; Demirata, B.; Apak, R. A novel antioxidant assay of ferric reducing capacity measurement using ferrozine as the colour forming complexation reagent. Anal. Methods 2010, 2, 1770–1778. [Google Scholar] [CrossRef]
- Santos, J.S.; Alvarenga Brizola, V.R.; Granato, D. High-throughput assay comparison and standardization for metal chelating capacity screening: A proposal and application. Food Chem. 2017, 214, 515–522. [Google Scholar] [CrossRef]
- Bahukhandi, A.; Dhyani, P.; Bhatt, I.D.; Rawal, R.S. Variation in Polyphenolics and Antioxidant Activity of Traditional Apple Cultivars from West Himalaya, Uttarakhand. Hortic. Plant J. 2018, 4, 151–157. [Google Scholar] [CrossRef]
- Mzoughi, Z.; Souid, G.; Timoumi, R.; Le Cerf, D.; Majdoub, H. Partial characterization of the edible Spinacia oleracea polysaccharides: Cytoprotective and antioxidant potentials against Cd induced toxicity in HCT116 and HEK293 cells. Int. J. Biol. Macromol. 2019, 136, 332–340. [Google Scholar] [CrossRef] [PubMed]
- Galla, N.R.; Pamidighantam, P.R.; Karakala, B.; Gurusiddaiah, M.R.; Akula, S. Nutritional, textural and sensory quality of biscuits supplemented with spinach (Spinacia oleracea L.). Int. J. Gastron. Food Sci. 2017, 7, 20–26. [Google Scholar] [CrossRef]
- Fernández-Poyatos, M.P.; Ruiz-Medina, A.; Llorent-Martínez, E.J. Phytochemical profile, mineral content, and antioxidant activity of Olea europaea L. cv. Cornezuelo table olives. Influence of in vitro simulated gastrointestinal digestion. Food Chem. 2019, 297, 124933. [Google Scholar] [CrossRef]
- Handa, C.L.; de Lima, F.S.; Guelfi MF, G.; Fernandes, M.d.S.; Georgetti, S.R.; Ida, E.I. Parameters of the fermentation of soybean flour by Monascus purpureus or Aspergillus oryzae on the production of bioactive compounds and antioxidant activity. Food Chem. 2019, 271, 274–283. [Google Scholar] [CrossRef]
- Ramos-Escudero, F.; Muñoz, A.M.; Alvarado-Ortíz, C.; Alvarado, Á.; Yáñez, J.A. Purple corn (Zea mays L.) phenolic compounds profile and its assessment as an agent against oxidative stress in isolated mouse organs. J. Med. Food 2012, 15, 206–215. [Google Scholar] [CrossRef] [PubMed]
- Horvat, D.; Šimić, G.; Drezner, G.; Lalić, A.; Ledenčan, T.; Tucak, M.; Plavšić, H.; Andrić, L.; Zdunić, Z. Phenolic Acid Profiles and Antioxidant Activity of Major Cereal Crops. Antioxidants 2020, 9, 527. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Jalao, I.; Sánchez-Moreno, C.; De Ancos, B. Effect of high-pressure processing on flavonoids, hydroxycinnamic acids, dihydrochalcones and antioxidant activity of apple ‘Golden Delicious’ from different geographical origin. Innov. Food Sci. Emer. 2019, 51, 20–31. [Google Scholar] [CrossRef]
- Mihailović, N.R.; Mihailović, V.B.; Kreft, S.; Ćirić, A.R.; Joksović, L.G.; Đurđević, P.T. Analysis of phenolics in the peel and pulp of wild apples (Malus sylvestris (L.) Mill.). J. Food Compos. Anal. 2018, 67, 1–9. [Google Scholar] [CrossRef]
- Nile, S.H.; Nile, A.; Liu, J.; Kim, D.H.; Kai, G. Exploitation of apple pomace towards extraction of triterpenic acids, antioxidant potential, cytotoxic effects, and inhibition of clinically important enzymes. Food Chem. Toxicol. 2019, 131, 110563. [Google Scholar] [CrossRef]
- Afonso, S.; Ribeiro, C.; Bacelar, E.; Ferreira, H.; Oliveira, I.; Silva, A.P.; Gonçalves, B. Influence of training system on physiological performance, biochemical composition and antioxidant parameters in apple tree (Malus domestica Borkh.). Sci. Hortic. 2017, 225, 394–398. [Google Scholar] [CrossRef]
- Basu, P.; Maier, C. In vitro antioxidant activities and polyphenol contents of seven commercially available fruits. Pharmacogn. Res. 2016, 8, 258–264. [Google Scholar]
- Zhao, Y.; Du, S.-k.; Wang, H.; Cai, M. In vitro antioxidant activity of extracts from common legumes. Food Chem. 2014, 152, 462–466. [Google Scholar] [CrossRef]
- Stanger, M.C.; Steffens, C.A.; Soethe, C.; Moreira, M.A.; do Amarante CV, T.; Both, V.; Brackmann, A. Phenolic compounds content and antioxidant activity of ‘Galaxy’ apples stored in dynamic controlled atmosphere and ultralow oxygen conditions. Postharvest Biol. Technol. 2018, 144, 70–76. [Google Scholar] [CrossRef]
- Grace, M.H.; Xiong, J.; Esposito, D.; Ehlenfeldt, M.; Lila, M.A. Simultaneous LC-MS quantification of anthocyanins and non-anthocyanin phenolics from blueberries with widely divergent profiles and biological activities. Food Chem. 2019, 277, 336–346. [Google Scholar] [CrossRef]
- Hussain, P.R.; Suradkar, P.; Javaid, S.; Akram, H.; Parvez, S. Influence of postharvest gamma irradiation treatment on the content of bioactive compounds and antioxidant activity of fenugreek (Trigonella foenum–graceum L.) and spinach (Spinacia oleracea L.) leaves. Innov. Food Sci. Emerg. Technol. 2016, 33, 268–281. [Google Scholar] [CrossRef]
- Cheurfa, M.; Abdallah, H.H.; Allem, R.; Noui, A.; Picot-Allain CM, N.; Mahomoodally, F. Hypocholesterolaemic and antioxidant properties of Olea europaea L. leaves from Chlef province, Algeria using in vitro, in vivo and in silico approaches. Food Chem. Toxicol. 2019, 123, 98–105. [Google Scholar] [CrossRef] [PubMed]
- Falcinelli, B.; Maranghi, S.; Paoletti, A.; Marconi, O.; Rosati, A.; Famiani, F.; Benincasa, P. Sprouting olive (Olea europaea L.) seeds as a source of antioxidants from residual whole stones. Sci. Hortic. 2018, 240, 558–560. [Google Scholar] [CrossRef]
- Xu, L.; Yue, Q.; Bian F, e.; Zhai, H.; Yao, Y. Melatonin Treatment Enhances the Polyphenol Content and Antioxidant Capacity of Red Wine. Hortic. Plant J. 2018, 4, 144–150. [Google Scholar] [CrossRef]
- Milat, A.M.; Boban, M.; Teissedre, P.-L.; Šešelja-Perišin, A.; Jurić, D.; Skroza, D.; Generalić-Mekinić, I.; Ljubenkov, I.; Volarević, J.; Rasines-Perea, Z.; et al. Effects of oxidation and browning of macerated white wine on its antioxidant and direct vasodilatory activity. J. Funct. Foods 2019, 59, 138–147. [Google Scholar] [CrossRef]
- Majkić, T.M.; Torović, L.D.; Lesjak, M.M.; Četojević-Simin, D.D.; Beara, I.N. Activity profiling of Serbian and some other European Merlot wines in inflammation and oxidation processes. Food Res. Int. 2019, 121, 151–160. [Google Scholar] [CrossRef]
- Jung, S.; Kim, M.H.; Park, J.H.; Jeong, Y.; Ko, K.S. Cellular Antioxidant and Anti-Inflammatory Effects of Coffee Extracts with Different Roasting Levels. J. Med. Food 2017, 20, 626–635. [Google Scholar] [CrossRef]
- Bravo, J.; Monente, C.; Juániz, I.; De Peña, M.P.; Cid, C. Influence of extraction process on antioxidant capacity of spent coffee. Food Res. Int. 2013, 50, 610–616. [Google Scholar] [CrossRef]
- Zhao, C.-N.; Tang, G.-Y.; Cao, S.-Y.; Xu, X.-Y.; Gan, R.-Y.; Liu, Q.; Mao, Q.-Q.; Shang, A.; Li, H.-B. Phenolic Profiles and Antioxidant Activities of 30 Tea Infusions from Green, Black, Oolong, White, Yellow and Dark Teas. Antioxidants 2019, 8, 215. [Google Scholar] [CrossRef]
- Almeida, T.S.D.; Araújo, M.E.M.; Rodríguez, L.G.; Júlio, A.; Mendes, B.G.; Santos, R.M.B.D.; Simões, J.A.M. Influence of preparation procedures on the phenolic content, antioxidant and antidiabetic activities of green and black teas. Braz. J. Pharm. Sci. 2019, 55, e17695. [Google Scholar] [CrossRef]
- Cid-Gallegos, M.S.; Sánchez-Chino, X.M.; Álvarez-González, I.; Madrigal-Bujaidar, E.; Vásquez-Garzón, V.R.; Baltiérrez-Hoyos, R.; Villa-Treviño, S.; Dávila-Ortíz, G.; Jiménez-Martínez, C. Modification of In Vitro and In Vivo Antioxidant Activity by Consumption of Cooked Chickpea in a Colon Cancer Model. Nutrients 2020, 12, 2572. [Google Scholar] [CrossRef] [PubMed]
- Peiretti, P.G.; Karamać, M.; Janiak, M.; Longato, E.; Giorgia, M.; Amarowicz, R.; Gai, F. Phenolic Composition and Antioxidant Activities of Soybean (Glycine max (L.) Merr.) Plant during Growth Cycle. Agronomy 2019, 9, 153. [Google Scholar] [CrossRef]
- Lee, A.L.; Yu, Y.P.; Hsieh, J.F.; Kuo, M.I.; Ma, Y.S.; Lu, C.P. Effect of germination on composition profiling and antioxidant activity of the polysaccharide-protein conjugate in black soybean [Glycinemax (L.) Merr.]. Int. J. Biol. Macromol. 2018, 113, 601–606. [Google Scholar] [CrossRef] [PubMed]
- Ranjbar, A.; Heshmati, A.; Momtaz, J.; Vahidinia, A. Effect of iron-enrichment on the antioxidant properties of wheat flour and bread. J. Cereal Sci. 2019, 87, 98–102. [Google Scholar] [CrossRef]
- Malunga, L.N.; Izydorczyk, M.; Beta, T. Antiglycemic Effect of Water Extractable Arabinoxylan from Wheat Aleurone and Bran. J. Nutr. Metab. 2017, 2017, 5784759. [Google Scholar] [CrossRef]
- Abdel-Aal, E.-S.M.; Hucl, P.; Rabalski, I. Compositional and antioxidant properties of anthocyanin-rich products prepared from purple wheat. Food Chem. 2018, 254, 13–19. [Google Scholar] [CrossRef]
- Zulueta, A.; Maurizi, A.; Frígola, A.; Esteve, M.J.; Coli, R.; Burini, G. Antioxidant capacity of cow milk, whey and deproteinized milk. Int. Dairy J. 2009, 19, 380–385. [Google Scholar] [CrossRef]
- de Carvalho, M.W.; Arriola, N.D.A.; Pinto, S.S.; Verruck, S.; Fritzen-Freire, C.B.; Prudêncio, E.S.; Amboni, R.D.d.M.C. Stevia-fortified yoghurt: Stability, antioxidant activity and in vitro digestion behaviour. Int. J. Dairy Technol. 2019, 72, 57–64. [Google Scholar] [CrossRef]
- Boyer, J.; Liu, R.H. Apple phytochemicals and their health benefits. Nutr. J. 2004, 3, 5. [Google Scholar] [CrossRef]
- Antonic, B.; Jancikova, S.; Dordevic, D.; Tremlova, B. Apple pomace as food fortification ingredient: A systematic review and meta-analysis. J. Food Sci. 2020, 85, 2977–2985. [Google Scholar] [CrossRef]
- Li, C.X.; Zhao, X.H.; Zuo, W.F.; Zhang, T.L.; Zhang, Z.Y.; Chen, X.S. Phytochemical profiles, antioxidant, and antiproliferative activities of four red-fleshed apple varieties in China. J. Food Sci. 2020, 85, 718–726. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.-Y.; Zhang, H.-C.; Liu, W.-X.; Li, C.-Y. Survey of antioxidant capacity and phenolic composition of blueberry, blackberry, and strawberry in Nanjing. J. Zhejiang Univ. Sci. B 2012, 13, 94–102. [Google Scholar] [CrossRef]
- Hager, T.J.; Howard, L.R.; Prior, R.L. Processing and Storage Effects on Monomeric Anthocyanins, Percent Polymeric Color, and Antioxidant Capacity of Processed Blackberry Products. J. Agric. Food Chem. 2008, 56, 689–695. [Google Scholar] [CrossRef] [PubMed]
- Vega-Galvez, A.; Rodríguez, A.; Stucken, K. Antioxidant, functional properties and health-promoting potential of native South American berries: A review. J. Sci. Food Agric. 2021, 101, 364–378. [Google Scholar] [CrossRef] [PubMed]
- Liović, N.; Bratanić, A.; Zorić, Z.; Pedisić, S.; Režek Jambrak, A.; Krešić, G.; Bilušić, T. The effect of freeze-drying, pasteurisation and high-intensity ultrasound on gastrointestinal stability and antioxidant activity of blueberry phenolics. Int. J. Food Sci. 2021, 56, 1996–2008. [Google Scholar] [CrossRef]
- Dalmau, M.E.; Llabrés, P.J.; Eim, V.S.; Rosselló, C.; Simal, S. Influence of freezing on the bioaccessibility of beetroot (Beta vulgaris) bioactive compounds during in vitro gastric digestion. J. Sci. Food Agric. 2019, 99, 1055–1065. [Google Scholar] [CrossRef]
- Fiorito, S.; Preziuso, F.; Epifano, F.; Scotti, L.; Bucciarelli, T.; Taddeo, V.A.; Genovese, S. Novel biologically active principles from spinach, goji and quinoa. Food Chem. 2019, 276, 262–265. [Google Scholar] [CrossRef]
- Salehi, B.; Tumer, T.B.; Ozleyen, A.; Peron, G.; Dall’Acqua, S.; Rajkovic, J.; Naz, R.; Nosheen, A.; Mudau, F.N.; Labanca, F.; et al. Plants of the genus Spinacia: From bioactive molecules to food and phytopharmacological applications. Trends. Food Sci. Technol. 2019, 88, 260–273. [Google Scholar] [CrossRef]
- Tareq, F.S.; Kotha, R.R.; Ferreira JF, S.; Sandhu, D.; Luthria, D.L. Influence of Moderate to High Salinity on the Phytochemical Profiles of Two Salinity-Tolerant Spinach Genotypes. ACS Food Sci. Technol. 2021, 1, 205–214. [Google Scholar] [CrossRef]
- Kamiloglu, S. Industrial freezing effects on the content and bioaccessibility of spinach (Spinacia oleracea L.) polyphenols. J. Sci. Food Agric. 2020, 100, 4190–4198. [Google Scholar] [CrossRef]
- Rocha, J.; Borges, N.; Pinho, O. Table olives and health: A review. J. Nutr. Sci. 2020, 9, e57. [Google Scholar] [CrossRef] [PubMed]
- Lanza, B.; Ninfali, P. Antioxidants in Extra Virgin Olive Oil and Table Olives: Connections between Agriculture and Processing for Health Choices. Antioxidants 2020, 9, 41. [Google Scholar] [CrossRef]
- Lopez-Huertas, E.; Fonolla, J. Hydroxytyrosol supplementation increases vitamin C levels in vivo. A human volunteer trial. Redox Biol. 2017, 11, 384–389. [Google Scholar] [CrossRef]
- Pandeya, A.; Rayamajhi, S.; Pokhrel, P.; Giri, B. Evaluation of secondary metabolites, antioxidant activity, and color parameters of Nepali wines. Food Sci. Nutr. 2018, 6, 2252–2263. [Google Scholar] [CrossRef] [PubMed]
- Richelle, M.; Tavazzi, I.; Offord, E. Comparison of the antioxidant activity of commonly consumed polyphenolic beverages (coffee, cocoa, and tea) prepared per cup serving. J. Agric. Food Chem. 2001, 49, 3438–3442. [Google Scholar] [CrossRef] [PubMed]
- Sung, H.; Nah, J.; Chun, S.; Park, H.; Yang, S.E.; Min, W.K. In vivo antioxidant effect of green tea. Eur. J. Clin. Nutr. 2000, 54, 527–529. [Google Scholar] [CrossRef]
- Benzie, I.; Szeto, Y.T.; Strain, J.J.; Tomlinson, B. Consumption of Green Tea Causes Rapid Increase in Plasma Antioxidant Power in Humans. Nutr. Cancer 1999, 34, 83–87. [Google Scholar] [CrossRef]
- Bahorun, T.; Luximon-Ramma, A.; Neergheen-Bhujun, V.S.; Gunness, T.K.; Googoolye, K.; Auger, C.; Crozier, A.; Aruoma, O.I. The effect of black tea on risk factors of cardiovascular disease in a normal population. Prev. Med. 2012, 54, S98–S102. [Google Scholar] [CrossRef]
- Moura-Nunes, N.; Perrone, D.; Farah, A.; Donangelo, C.M. The increase in human plasma antioxidant capacity after acute coffee intake is not associated with endogenous non-enzymatic antioxidant components. Int. J. Food Sci. Nutr. 2009, 60 (Suppl. 6), 173–181. [Google Scholar] [CrossRef]
- Okarter, N.; Liu, C.-S.; Sorrells, M.E.; Liu, R.H. Phytochemical content and antioxidant activity of six diverse varieties of whole wheat. Food Chem. 2010, 119, 249–257. [Google Scholar] [CrossRef]
- Yu, L.; Nanguet, A.L.; Beta, T. Comparison of Antioxidant Properties of Refined and Whole Wheat Flour and Bread. Antioxidants 2013, 2, 370–383. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Zhai, W. Identification and antioxidant activity of anthocyanins extracted from the seed and cob of purple corn (Zea mays L.). Innov. Food Sci. Emer. 2010, 11, 169–176. [Google Scholar] [CrossRef]
- Khan, I.T.; Nadeem, M.; Imran, M.; Ullah, R.; Ajmal, M.; Jaspal, M.H. Antioxidant properties of Milk and dairy products: A comprehensive review of the current knowledge. Lipids Health Dis. 2019, 18, 41. [Google Scholar] [CrossRef]
- Alenisan, M.A.; Alqattan, H.H.; Tolbah, L.S.; Shori, A.B. Antioxidant properties of dairy products fortified with natural additives: A review. J. Assoc. Arab. Univ. Basic Appl. Sci. 2017, 24, 101–106. [Google Scholar] [CrossRef]
- Harnly, J. Antioxidant methods. J. Food Compos. Anal. 2017, 64, 145–146. [Google Scholar] [CrossRef]
- Abramovič, H.; Grobin, B.; Poklar Ulrih, N.; Cigić, B. Relevance and Standardization of In Vitro Antioxidant Assays: ABTS, DPPH, and Folin–Ciocalteu. J. Chem. 2018, 2018, 4608405. [Google Scholar] [CrossRef]
- Laguerre, M.; Lecomte, J.; Villeneuve, P. Evaluation of the ability of antioxidants to counteract lipid oxidation: Existing methods, new trends and challenges. Prog. Lipid Res. 2007, 46, 244–282. [Google Scholar] [CrossRef]
- González-Ruiz, V.; Rajesh, J.; Olives, A.I.; Rocchi, D.; Gómez-Carpintero, J.; González, J.F.; Sridharan, V.; Martín, M.A.; Menéndez, J.C. Antioxidants as molecular probes: Structurally novel dihydro-m-terphenyls as turn-on fluorescence chemodosimeters for biologically relevant oxidants. Antioxidants 2020, 9, 605. [Google Scholar] [CrossRef]
- Hermans, N.; Cos, P.; Maes, L.; De Bruyne, T.; Vanden Berghe, D.; Vlietinck, A.J.; Pieters, L. Challenges and pitfalls in antioxidant research. Curr. Med. Chem. 2007, 14, 417–430. [Google Scholar] [CrossRef]
- Heim, K.E.; Tagliaferro, A.R.; Bobilya, D.J. Flavonoid antioxidants: Chemistry, metabolism and structure-activity relationships. J. Nutr. Biochem. 2002, 13, 572–584. [Google Scholar] [CrossRef]
- Ribas-Agustí, A.; Martín-Belloso, O.; Soliva-Fortuny, R.; Elez-Martínez, P. Food processing strategies to enhance phenolic compounds bioaccessibility and bioavailability in plant-based foods. Crit. Rev. Food Sci. Nutr. 2018, 58, 2531–2548. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, J.; Ramos, R.; Luís, Â.; Rocha, S.; Rosado, T.; Gallardo, E.; Duarte, A.P. Assessment of the Bioaccessibility and Bioavailability of the Phenolic Compounds of Prunus avium L. by in Vitro Digestion and Cell Model. ACS Omega 2019, 4, 7605–7613. [Google Scholar] [CrossRef]
- Lipinski, C.A.; Lombardo, F.; Dominy, B.W.; Feeney, P.J. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv. Drug. Deliv. Rev. 2001, 46, 3–26. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Pandey, A.K. Chemistry and Biological Activities of Flavonoids: An Overview. Sci. World J. 2013, 2013, 162750. [Google Scholar] [CrossRef]
- Panche, A.N.; Diwan, A.D.; Chandra, S.R. Flavonoids: An overview. J. Nutr. Sci. 2016, 5, e47. [Google Scholar] [CrossRef]
- Kaźmierczak-Barańska, J.; Boguszewska, K.; Adamus-Grabicka, A.; Karwowski, B.T. Two Faces of Vitamin C-Antioxidative and Pro-Oxidative Agent. Nutrients 2020, 12, 1501. [Google Scholar] [CrossRef]
- Duplancic, D.; Kukoc-Modun, L.; Modun, D.; Radic, N. Simple and Rapid Method for the Determination of Uric Acid-Independent Antioxidant Capacity. Molecules 2011, 16, 7058–7067. [Google Scholar] [CrossRef]
- Lowe, F. Biomarkers of Oxidative Stress, in Systems Biology of Free Radicals and Antioxidants, 1st ed.; Springer: Berlin/Heidelberg, Germany, 2014; Volume 2. [Google Scholar]
- EFSA Panel on Dietetic Products, Nutrition and Allergies (EFSA NDA Panel); Turck, D.; Bresson, J.-L.; Burlingame, B.; Dean, T.; Fairweather-Tait, S.; Heinonen, M.; Hirsch-Ernst, K.I.; Mangelsdorf, I.; McArdle, H.J.; et al. Guidance for the scientific requirements for health claims related to antioxidants, oxidative damage and cardiovascular health: (Revision 1). EFSA J. 2018, 16, e05136. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).