Serum α-Carotene, but Not Other Antioxidants, Is Positively Associated with Muscle Strength in Older Adults: NHANES 2001–2002
Abstract
1. Introduction
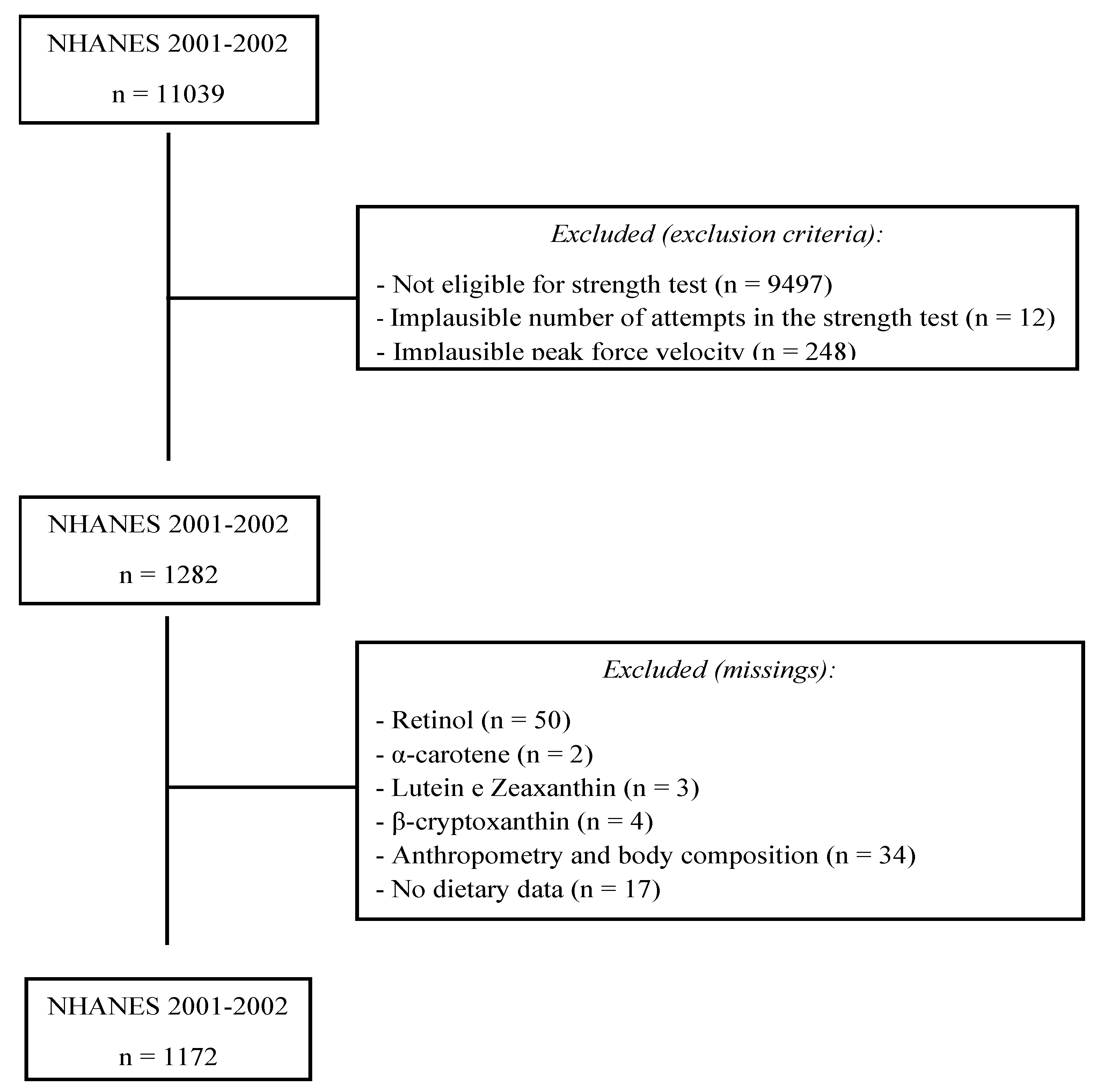
2. Methods
2.1. Participants
2.2. Muscle Strength
2.3. Serum Antioxidants
2.4. Anthropometry
2.5. Dietary Intake
2.6. Covariates of Interest
2.7. Statistical Analysis
3. Results
3.1. Individual’s Characteristics
3.2. Peak Force and Tertiles of Plasma Antioxidants
4. Discussions
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zammit, A.R.; Robitaille, A.; Piccinin, A.M.; Muniz-Terrera, G.; Hofer, S.M. Associations Between Aging-Related Changes in Grip Strength and Cognitive Function in Older Adults: A Systematic Review. J. Gerontol. Ser. A 2018, 74, 519–527. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Xia, J.; Zhang, X.; Gathirua-Mwangi, W.G.; Guo, J.; Li, Y.; Mckenzie, S.; Song, Y. Associations of Muscle Mass and Strength with All-Cause Mortality among US Older Adults. Med. Sci. Sports Exerc. 2018, 50, 458–467. [Google Scholar] [CrossRef]
- Loprinzi, P.D. Lower extremity muscular strength, sedentary behavior, and mortality. Age 2016, 38, 32. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.K.; Lyass, A.; Larson, M.G.; Massaro, J.M.; Wang, N.; D’Agostino, R.B.; Murabito, J.M. Biomarkers of oxidative stress are associated with frailty: The Framingham Offspring Study. Age 2016, 38, 1. [Google Scholar] [CrossRef] [PubMed]
- Fielding, R.A.; Vellas, B.; Evans, W.J.; Bhasin, S.; Morley, J.E.; Newman, A.B.; Zamboni, M. Sarcopenia: An undiagnosed condition in older adults. Current consensus definition: Prevalence, etiology, and consequences. International working group on sarcopenia. J. Am. Med. Dir. Assoc. 2011, 12, 249–256. [Google Scholar] [CrossRef]
- Uusi-Rasi, K.; Karinkanta, S.; Tokola, K.; Kannus, P.; Sievänen, H. Bone Mass and Strength and Fall-Related Fractures in Older Age. J. Osteoporos. 2019, 2019, 5134690. [Google Scholar] [CrossRef]
- Reid, K.F.; Naumova, E.; Carabello, R.J.; Phillips, E.M.; Fielding, R.A. Lower extremity muscle mass predicts functional performance in mobility-limited elders. J. Nutr. Health Aging 2008, 12, 493–498. [Google Scholar] [CrossRef]
- Granic, A.; Sayer, A.A.; Robinson, S.M. Dietary patterns, skeletal muscle health, and sarcopenia in older adults. Nutrients 2019, 11, 745. [Google Scholar] [CrossRef]
- Cuthbertson, D.; Smith, K.; Babraj, J.; Leese, G.; Waddell, T.; Atherton, P.; Rennie, M.J. Anabolic signaling deficits underlie amino acid resistance of wasting, aging muscle. FASEB J. 2005, 19, 422–424. [Google Scholar] [CrossRef]
- Volaklis, K.; Halle, M.; Thorand, B.; Peters, A.; Ladwig, K.; Schulz, H.; Koenig, W.; Meisinger, C. Handgrip strength is inversely and independently associated with multimorbidity among older women: Results from the KORA—Age study. Eur. J. Intern. Med. 2016, 31, 35–40. [Google Scholar] [CrossRef]
- Langhammer, B.; Bergland, A.; Rydwik, E. The Importance of Physical Activity Exercise among Older People. BioMed. Res. Int. 2018, 2018, 7856823. [Google Scholar] [CrossRef] [PubMed]
- Gregorio, L.; Brindisi, J.; Kleppinger, A.; Sullivan, R.; Mangano, K.; Bihuniak, J.D.; Kenny, A.M.; Kerstetter, J.E.; Insogna, K. Adequate dietary protein is associated with better physical performance among post-menopausal women 60–90 years. J. Nutr. Health Aging 2013, 18, 155–160. [Google Scholar] [CrossRef] [PubMed]
- Montero-Fernández, N.; Serra-Rexach, J.A. Role of exercise on sarcopenia in the elderly. Eur. J. Phys. Rehabil. Med. 2013, 49, 131–143. [Google Scholar] [PubMed]
- Fulle, S.; Protasi, F.; Di Tano, G.; Pietrangelo, T.; Beltramin, A.; Boncompagni, S.; Vecchiet, L.; Fanò, G. The contribution of reactive oxygen species to sarcopenia and muscle ageing. Exp. Gerontol. 2003, 39, 17–24. [Google Scholar] [CrossRef] [PubMed]
- Baumann, C.W.; Kwak, D.; Liu, H.M.; Thompson, L.V. Age-induced oxidative stress: How does it influence skeletal muscle quantity and quality? J. Appl. Physiol. 2016, 121, 1047–1052. [Google Scholar] [CrossRef] [PubMed]
- Sahni, S.; Dufour, A.B.; Fielding, R.A.; Newman, A.B.; Kiel, D.P.; Hannan, M.T.; Jacques, P.F. Total carotenoid intake is associated with reduced loss of grip strength and gait speed over time in adults: The Framingham Offspring Study. Am. J. Clin. Nutr. 2021, 113, 437–445. [Google Scholar] [CrossRef]
- Morley, J.E.; Mooradian, A.D.; Silver, A.J.; Heber, D.; Alfin-Slater, R.B. Nutrition in the elderly. Ann. Intern. Med. 1988, 109, 890–904. [Google Scholar] [CrossRef]
- Barrera-Mendoza, C.C.; Ayala-Mata, F.; Cortés-Rojo, C.; García-Pérez, M.E.; Rodríguez-Orozco, A.R. Antioxidant vitamins in asthma. Rev. Alerg. México 2018, 65, 61–77. [Google Scholar] [CrossRef]
- Harman, D. Aging: A Theory Based on Free Radical and Radiation Chemistry. J. Gerontol. 1956, 11, 298–300. [Google Scholar] [CrossRef]
- Jama, J.W.; Launer, L.J.; Witteman, J.C.M.; Den Breeijen, J.H.; Breteler, M.M.B.; Grobbee, D.E.; Hofman, A. Dietary antioxidants and cognitive function in a population-based sample of older persons: The Rotterdam Study. Am. J. Epidemiol. 1996, 144, 275–280. [Google Scholar] [CrossRef]
- Mecocci, P.; Polidori, M.C.; Troiano, L.; Cherubini, A.; Cecchetti, R.; Pini, G.; Senin, U. Plasma antioxidants and longevity: A study on healthy centenarians. Free. Radic. Biol. Med. 2000, 28, 1243–1248. [Google Scholar] [CrossRef] [PubMed]
- Nahas, P.C.; Rossato, L.T.; de Branco, F.M.; Azeredo, C.M.; Rinaldi, A.E.M.; de Oliveira, E.P. Serum uric acid is positively associated with muscle strength in older men and women: Findings from NHANES 1999–2002. Clin. Nutr. 2021, 40, 4386–4393. [Google Scholar] [CrossRef] [PubMed]
- Semba, R.D.; Blaum, C.; Guralnik, J.M.; Moncrief, D.T.; Ricks, M.O.; Fried, L.P. Carotenoid and vitamin E status are associated with indicators of sarcopenia among older women living in the community. Aging Clin. Exp. Res. 2003, 15, 482–487. [Google Scholar] [CrossRef] [PubMed]
- Rossato, L.T.; de Branco, F.M.; Azeredo, C.M.; Rinaldi, A.E.M.; de Oliveira, E.P. Association between omega-3 fatty acids intake and muscle strength in older adults: A study from National Health and Nutrition Examination Survey (NHANES) 1999–2002. Clin. Nutr. 2020, 39, 3434–3441. [Google Scholar] [CrossRef]
- Dodds, R.M.; Syddall, H.E.; Cooper, R.; Benzeval, M.; Deary, I.J.; Dennison, E.M.; Sayer, A.A. Grip strength across the life course: Normative data from twelve British studies. PLoS ONE 2014, 9, e113637. [Google Scholar] [CrossRef]
- Vargas, S.; Romance, R.; Petro, J.L.; Bonilla, D.A.; Galancho, I.; Espinar, S.; Kreider, R.B.; Benítez-Porres, J. Efficacy of ketogenic diet on body composition during resistance training in trained men: A randomized controlled trial. J. Int. Soc. Sports Nutr. 2018, 15, 31. [Google Scholar] [CrossRef]
- Lohman, T.G.; Roche, A.F.; Martorell, R. Anthropometric Standardization Reference Manual; Human Kinetics Books: Champaign, IL, USA, 1988. [Google Scholar]
- Papanikolaou, Y.; Brooks, J.; Reider, C.; Fulgoni, V.L. US adults are not meeting recommended levels for fish and omega-3 fatty acid intake: Results of an analysis using observational data from NHANES 2003–2008. Nutr. J. 2014, 13, 31. [Google Scholar] [CrossRef]
- Conway, J.M.; A Ingwersen, L.; Vinyard, B.T.; Moshfegh, A.J. Effectiveness of the US Department of Agriculture 5-step multiple-pass method in assessing food intake in obese and nonobese women. Am. J. Clin. Nutr. 2003, 77, 1171–1178. [Google Scholar] [CrossRef]
- Murakoshi, M.; Takayasu, J.; Kimura, O.; Kohmura, E.; Nishino, H.; Iwashima, A.; Iwasaki, R. Inhibitory effects of α-carotene on proliferation of the human neuroblastoma cell line GOTO. JNCI: J. Natl. Cancer Inst. 1989, 81, 1649–1652. [Google Scholar] [CrossRef]
- Murakoshi, M.; Nishino, H.; Satomi, Y.; Takayasu, J.; Hasegawa, T.; Tokuda, H.; Iwasaki, R. Potent preventive action of α-carotene against carcinogenesis: Spontaneous liver carcinogenesis and promoting stage of lung and skin carcinogenesis in mice are suppressed more effectively by α-carotene than by β-carotene. Cancer Res. 1992, 52, 6583–6587. [Google Scholar]
- Ziegler, R.G.; Colavito, E.A.; Hartge, P.; McAdams, M.J.; Schoenberg, J.B.; Mason, T.J.; Fraumeni Jr, J.F. Importance of α-carotene, β-carotene, and other phytochemicals in the etiology of lung cancer. JNCI J. Natl. Cancer Inst. 1996, 88, 612–615. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Ford, E.S.; Zhao, G.; Balluz, L.S.; Giles, W.H.; Liu, S. Serum α-carotene concentrations and risk of death among US adults: The Third National Health and Nutrition Examination Survey Follow-up Study. Arch. Intern. Med. 2011, 171, 507–515. [Google Scholar] [CrossRef] [PubMed]
- Hendrickson, S.J.; Willett, W.C.; Rosner, B.A.; Eliassen, A.H. Food Predictors of Plasma Carotenoids. Nutrients 2013, 5, 4051–4066. [Google Scholar] [CrossRef] [PubMed]
- Al-Delaimy, W.K.; Ferrari, P.; Slimani, N.; Pala, V.; Johansson, I.; Nilsson, S.; Riboli, E. Plasma carotenoids as biomarkers of intake of fruits and vegetables: Individual-level correlations in the European Prospective Investigation into Cancer and Nutrition (EPIC). Eur. J. Clin. Nutr. 2005, 59, 1387–1396. [Google Scholar] [CrossRef]
- Olmedilla-Alonso, B.; Rodríguez-Rodríguez, E.; Beltrán-de-Miguel, B.; Estévez-Santiago, R. Dietary β-cryptoxanthin and α-carotene have greater apparent bioavailability than β-carotene in subjects from countries with different dietary patterns. Nutrients 2020, 12, 2639. [Google Scholar] [CrossRef]
- Cesari, M.; Pahor, M.; Bartali, B.; Cherubini, A.; Penninx, B.W.J.H.; Williams, G.R.; Atkinson, H.; Martin, A.; Guralnik, J.M.; Ferrucci, L. Antioxidants and physical performance in elderly persons: The Invecchiare in Chianti (InCHIANTI) study. Am. J. Clin. Nutr. 2004, 79, 289–294. [Google Scholar] [CrossRef]
| Variables | Total | Men | Women | p-Value |
|---|---|---|---|---|
| Age, years | 61.4 (9.3) | 60.8 (9.0) | 62.0 (9.6) | 0.013 |
| Non-Hispanic white, % | 83.7 (77.6–88.3) | 84.9 (77.3–90.3) | 82.3 (77.0–86.6) | 0.171 |
| Sex, % | ||||
| Men | 51.4 (49.4–53.3) | |||
| Women | 48.6 (46.6–50.6) | |||
| Marital status, % | 0.930 | |||
| Single/divorced/widowed/never married | 26.1 (22.2–30.4) | 15.9 (11.8–21.0) | 36.9 (31.6–42.4) | |
| Married/living as married | 73.6 (69.3–77.6) | 83.7 (78.5–87.8) | 63.0 (57.5–68.3) | |
| Missing | 0.3 (0.04–1.5) | 0.4 (0.04–3.2) | 0.1 (0.01–0.7) | |
| Annual family income, % | 0.165 | |||
| $0–19.999 | 17.8 (14.6–21.4) | 14.3 (10.9–18.5) | 21.4 (17.4–26.1) | |
| $20.000–54.999 | 40.1 (35.3–45.1) | 40.2 (33.0–47.8) | 40.0 (36.5–43.7) | |
| $55.000–74.999 | 40.0 (33.4–47.1) | 44 (35.9–52.4) | 35.9 (30.1–42.1) | |
| Missing | 2.1 (1.2–3.4) | 1.5 (0.8–2.7) | 2.7 (1.4–4.7) | |
| Educational level, % | 0.287 | |||
| High school graduate or under | 41.7 (37.2–46.2) | 39.7 (33.2–46.5) | 43.7 (38.9–48.7) | |
| Some college or above | 58.3 (53.7–62.7) | 60.3 (53.5–66.7) | 56.2 (51.2–70.0) | |
| Health conditions and habits, % | ||||
| Hypertension | 37.7 (33.3–42.2) | 32.7 (27.8–38.1) | 42.9 (36.9–49.0) | 0.032 |
| Missing | 0.1 (0.02–0.43) | 0.2 (0.04–0.8) | ||
| Diabetes | 0.173 | |||
| Pre-diabetes | 2.4 (1.2–4.7) | 2.6 (1.05–6.5) | 2.1 (0.9–4.6) | |
| Yes | 9.0 (7.4–11.0) | 10.7 (8.1–14.0) | 7.3 (5.4–9.8) | |
| No | 88.6 (85.5–91.1) | 86.6 (81.5–90.5) | 90.6 (87.8–92.8) | |
| Smoking | 0.640 | |||
| Yes | 17.3 (14.6–20.4) | 18.1 (14.4–22.4) | 16.5 (13.4–20.2) | |
| No | 82.5 (79.6–85.2) | 81.8 (77.5–85.3) | 83.4 (79.7–86.5) | |
| Missing | 0.2 (0.03–0.45) | 0.1 (0.02–0.8) | 0.1 (0.01–0.7) | |
| Arthritis | 0.002 | |||
| Yes | 35.7 (31.1–40.7) | 30.9 (25.7–36.7) | 40.8 (35.5–46.4) | |
| No | 64.2 (59.3–68.9) | 69.1 (63.3–74.3) | 59.1 (53.6–64.5) | |
| Physical activity % | 0.775 | |||
| Moderate physical activity | ||||
| Yes | 51.4 (45.8–56.9) | 50.9 (44.7–57.1) | 51.8 (42.9–60.6) | |
| No | 48.6 (43.0–54.2) | 49.1 (42.8–55.3) | 48.1 (39.2–57.1) | |
| Vigorous physical activity | ||||
| Yes | 28.9 (23.9–34.4) | 32.1 (25.3–39.9) | 25.4 (20.3–31.2) | |
| No | 71.1 (65.5–76.1) | 67.8 (60.1–74.7) | 74.5 (68.6–79.6) | |
| Resistance physical activity | ||||
| Yes | 24.1 (19.2–29.7) | 23.4 (17.2–30.9) | 24.8 (19.0–31.7) | |
| No | 75.8 (70.2–80.7) | 76.6 (69.1–82.7) | 75.1 (68.2–80.9) | |
| Missing | 0.1 (0.06–0.1) | 0.1 (0.01–0.7) | ||
| Anthropometrics | ||||
| Weight, kg | 80.4 (18.1) | 87.2 (16.0) | 73.1 (17.4) | <0.001 |
| Height, m | 1.68 (0.10) | 1.75 (0.07) | 1.61 (0.06) | <0.001 |
| Body mass index, kg/m2 | 28.2 (5.5) | 28.25 (4.6) | 28.1 (6.4) | 0.813 |
| Strength | ||||
| Peak force, N | 384 (123,7) | 457 (111) | 307 (82.4) | <0.001 |
| Time to peak force, s | 1.03 (0.48) | 0.99 (0.39) | 1.06 (0.56) | 0.067 |
| Peak force velocity, degree/s | 60.7 (0.62) | 60.7 (0.67) | 60.6 (0.57) | 0.121 |
| Biochemical parameters | ||||
| Vitamin E (µmol/L) | 37.6 (15.7) | 36.1 (15.1) | 39.2 (16.2) | 0.013 |
| α-carotene (µmol/L) | 0.09 (0.13) | 0.08 (0.10) | 0.11 (0.16) | 0.008 |
| Trans-β-carotene (µmol/L) | 0.44 (0.43) | 0.37 (0.36) | 0.52 (0.48) | <0.001 |
| Cis-β-carotene (µmol/L) | 0.02 (0.02) | 0.02 (0.02) | 0.03 (0.03) | <0.001 |
| β-cryptoxanthin (µmol/L) | 0.18 (0.13) | 0.16 (0.12) | 0.19 (0.14) | 0.017 |
| Combined Lutein/zeaxanthin (µmol/L) | 0.31 (0.17) | 0.30 (0.16) | 0.32 (0.18) | 0.163 |
| Trans-lycopene (µmol/L) | 0.41 (0.21) | 0.42 (0.22) | 0.41 (0.20) | 0.834 |
| Carotenoids (µmol/L) | 1.46 (0.79) | 1.35 (0.70) | 1.58 (0.87) | 0.002 |
| Retinol (µg/dL) | 67.1 (1.02) | 69.2 (1.04) | 64.9 (1.20) | <0.001 |
| C-reactive protein (mg/dL) | 0.41 (0.03) | 0.35 (0.03) | 0.48 (0.04) | <0.001 |
| Uric acid (mg/dL) | 5.58 (1.41) | 6.10 (1.29) | 5.03 (1.31) | <0.001 |
| Dietary intake | ||||
| Energy, kcal | 1997 (960) | 2313 (1112) | 1662 (609) | <0.001 |
| Carbohydrate, g | 245 (126) | 278 (149) | 210 (82.1) | <0.001 |
| Protein, g | 75.7 (39.4) | 87.0 (44.8) | 63.7 (28.1) | <0.001 |
| Protein, g/kg | 0.97 (0.49) | 1.01 (0.51) | 0.92 (0.47) | 0.006 |
| Lipids, g | 77.5 (47,9) | 90.4 (56.6) | 63.9 (31.6) | <0.001 |
| Saturated fat, g | 24.1 (16.2) | 28.4 (19.1) | 19.5 (10.8) | <0.001 |
| Monounsaturated fat, g | 27.7 (18.7) | 32.7 (22.1) | 22.5 (12.3) | <0.001 |
| Polyunsaturated fat, g | 16.4 (12.1) | 18.5 (14.2) | 14.2 (8.9) | <0.001 |
| Total omega-3, g | 1.80 (1.50) | 2.04 (1.71) | 1.55 (1.18) | 0.001 |
| ALA, g | 1.50 (1.21) | 1.68 (1.33) | 1.32 (1.03) | 0.001 |
| EPA, g | 0.05 (0.19) | 0.06 (0.21) | 0.04 (0.16) | 0.198 |
| DHA, g | 0.09 (0.30) | 0.11 (0.36) | 0.07 (0.22) | 0.119 |
| Fiber, g | 16.2 (10.4) | 17.5 (12.0) | 14.8 (8.1) | 0.010 |
| Alcohol, g | 8.68 (24.1) | 12.4 (29.0) | 4.72 (16.8) | <0.001 |
| Vitamin A, RAE (mcg) | 629 (719) | 670 (886) | 586 (480) | 0.086 |
| Vitamin E, as α-tocopherol (mg) | 7.07 (5.75) | 7.85 (6.64) | 6.25 (4.51) | 0.002 |
| Retinol (mcg) | 416 (586) | 467 (757) | 361 (307) | 0.002 |
| Lycopene (mcg) | 5520 (10271) | 6108 (11183) | 4899 (9179) | 0.029 |
| Lutein + zeaxanthin (mcg) | 1717 (3118) | 1760 (3285) | 1672 (2934) | 0.705 |
| β-cryptoxanthin (mcg) | 146 (229) | 157 (253) | 134 (201) | 0.128 |
| β-carotene (mcg) | 2271 (4185) | 2125 (4391) | 2425 (3954) | 0.322 |
| α-carotene (mcg) | 412 (1480) | 428 (1766) | 395 (1100) | 0.738 |
| Model 1 β (95% CI) | Model 2 β (95% CI) | |||||||
|---|---|---|---|---|---|---|---|---|
| T1 | T2 | T3 | p-Trend | T1 | T2 | T3 | p-Trend | |
| α-carotene (µmol/L) | Ref | 2.37 (−17.7; 22.5) | −18.0 (−32.1; −3.90) | 0.016 | Ref | 15.1 (2.24; 28.0) | 16.0 (1.89; 30.1) | 0.027 |
| Trans-β-carotene (µmol/L) | Ref | 1.24 (−15.5; 18.0) | −42.6 (−64.8; −20.4) | 0.001 | Ref | 11.1 (−2.83; 25.1) | 9.13 (−9.41; 27.7) | 0.316 |
| Cis-β-carotene (µmol/L) | Ref | −17.6 (−30.8; −4.42) | −50.2 (−68.7; −31.6) | <0.001 | Ref | 4.70 (−7.61; 17.0) | 7.33 (−11.0; 25.7) | 0.399 |
| β-cryptoxanthin (µmol/L) | Ref | −17.1 (−39.6; 5.41) | −23.9 (−47.9; 0.05) | 0.042 | Ref | 6.73 (−10.2; 23.7) | −0.289 (−13.0; 12.4) | 0.990 |
| Combined Lutein/zeaxanthin (µmol/L) | Ref | 19.0 (−1.78; 39.7) | 1.25 (−24.1; 26.6) | 0.872 | Ref | 11.8 (−6.15; 29.7) | 6.95 (−14.0; 27.9) | 0.473 |
| Trans-lycopene (µmol/L) | Ref | 21.9 (4.25; 39.6) | 28.7 (9.0; 48.5) | 0.010 | Ref | 11.8 (−5.24; 28.9) | 3.32 (−9.20; 15.8) | 0.740 |
| Carotenoids (µmol/L) | Ref | 9.90 (−12.5; 32.3) | −14.1 (−37.7; 9.42) | 0.222 | Ref | 0.10 (−17.8; 18.0) | 0.17 (−18.3; 18.6) | 0.985 |
| Vitamin E (µmol/L) | Ref | 0.52 (−19.2; 20.2) | −22.9 (−41.9; −3.87) | 0.019 | Ref | 2.26 (−8.89; 13.4) | 8.71 (−8.00; 25.4) | 0.282 |
| Retinol (µg/dL) | Ref | −2.36 (−29.4; 24.7) | 21.4 (−10.1; 52.9) | 0.159 | Ref | 8.21 (−5.62; 22.0) | 11.1 (−12.8; 35.0) | 0.358 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bruno, R.R.; Rosa, F.C.; Nahas, P.C.; de Branco, F.M.S.; de Oliveira, E.P. Serum α-Carotene, but Not Other Antioxidants, Is Positively Associated with Muscle Strength in Older Adults: NHANES 2001–2002. Antioxidants 2022, 11, 2386. https://doi.org/10.3390/antiox11122386
Bruno RR, Rosa FC, Nahas PC, de Branco FMS, de Oliveira EP. Serum α-Carotene, but Not Other Antioxidants, Is Positively Associated with Muscle Strength in Older Adults: NHANES 2001–2002. Antioxidants. 2022; 11(12):2386. https://doi.org/10.3390/antiox11122386
Chicago/Turabian StyleBruno, Renata R., Fernanda C. Rosa, Paula C. Nahas, Flávia M. S. de Branco, and Erick P. de Oliveira. 2022. "Serum α-Carotene, but Not Other Antioxidants, Is Positively Associated with Muscle Strength in Older Adults: NHANES 2001–2002" Antioxidants 11, no. 12: 2386. https://doi.org/10.3390/antiox11122386
APA StyleBruno, R. R., Rosa, F. C., Nahas, P. C., de Branco, F. M. S., & de Oliveira, E. P. (2022). Serum α-Carotene, but Not Other Antioxidants, Is Positively Associated with Muscle Strength in Older Adults: NHANES 2001–2002. Antioxidants, 11(12), 2386. https://doi.org/10.3390/antiox11122386






