Short-Term Grape Consumption Diminishes UV-Induced Skin Erythema
Abstract
1. Introduction
2. Materials and Methods
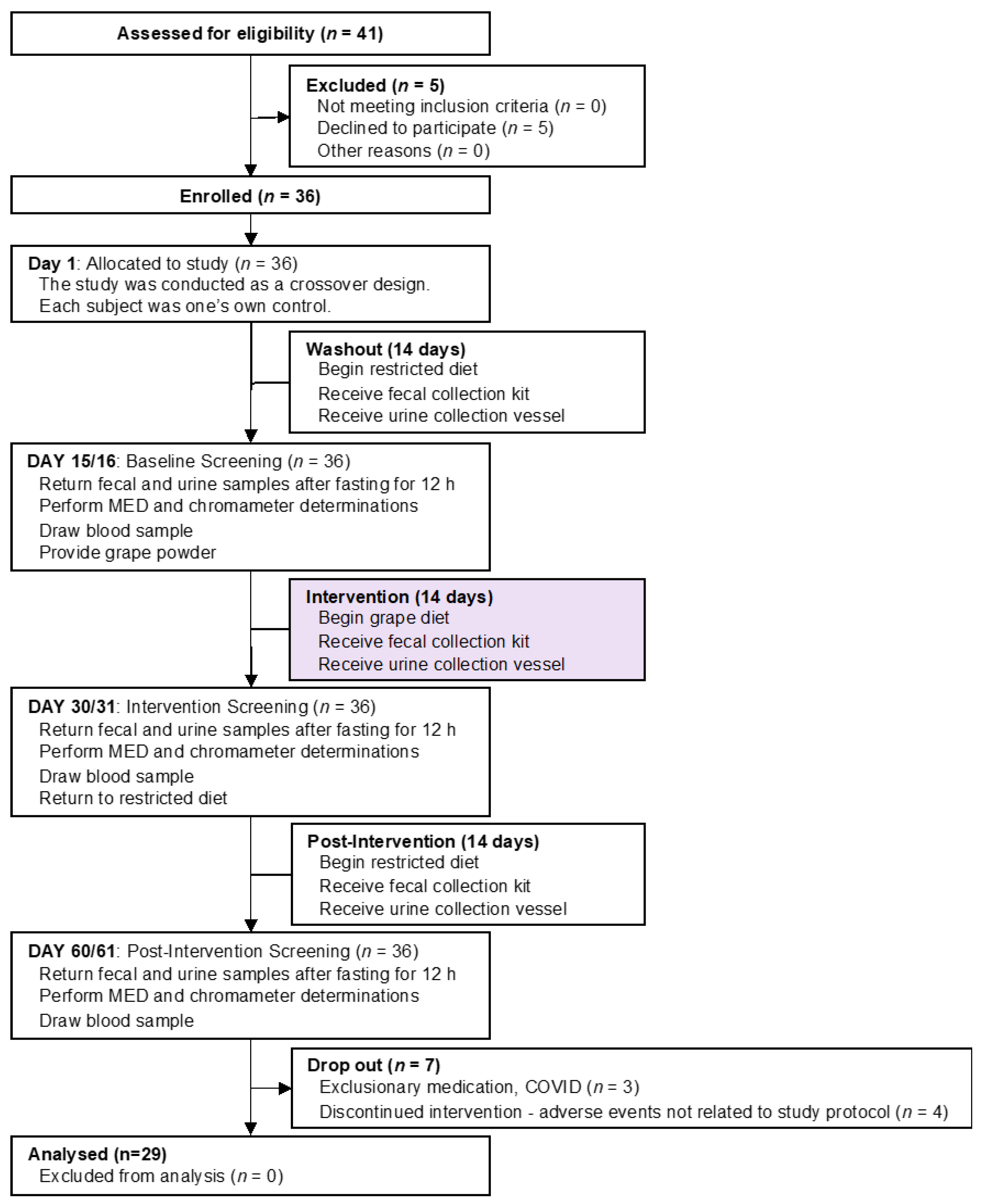
2.1. Human Subjects, Study Design and Specimen Procurement (Figure 1)
2.1.1. Subject Disposition
2.1.2. Subject Demographics
2.1.3. Subject Enrollment
2.1.4. Grape Powder
2.1.5. Study Procedure and Specimen Acquisition
2.1.6. Institutional Review Board
2.2. Dermatologic Treatments and Evaluations
2.2.1. Light Source
2.2.2. Minimal Erythema Dose (MED) Testing
2.2.3. Dermatologic Evaluations
2.2.4. Definition of MED
2.2.5. Chromameter Measurements
2.3. Treatment of Fecal Microbiota and Microbiome Analysis
2.3.1. DNA Extraction
2.3.2. DNA Quantification QC
2.3.3. Library Preparation and Sequencing
2.3.4. Sequence Quality Control
2.3.5. Taxonomic Annotation
2.3.6. Functional Annotation
2.3.7. Alpha- and Beta-Diversity
2.4. Urine and Plasma Metabolomics
2.4.1. Plasma GC-MS Metabolomics
2.4.2. Urine GC-MS Metabolomics
2.4.3. Orthogonal PLS-DA Analysis (OPLS-DA)
2.5. Sub-Analyses of UV-Resistant Study Participants
2.6. Statistical Analyses
3. Results
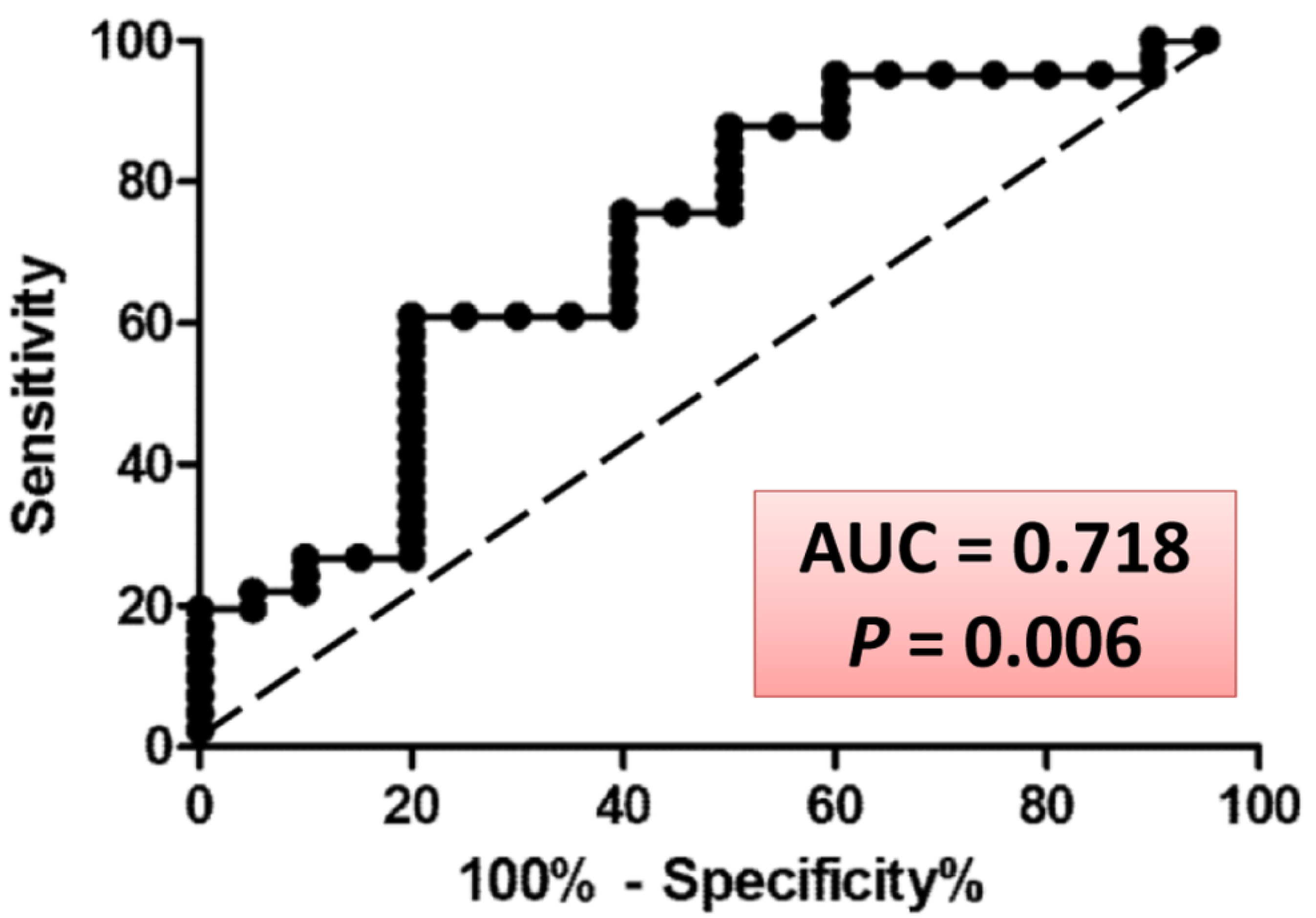
3.1. MED Testing
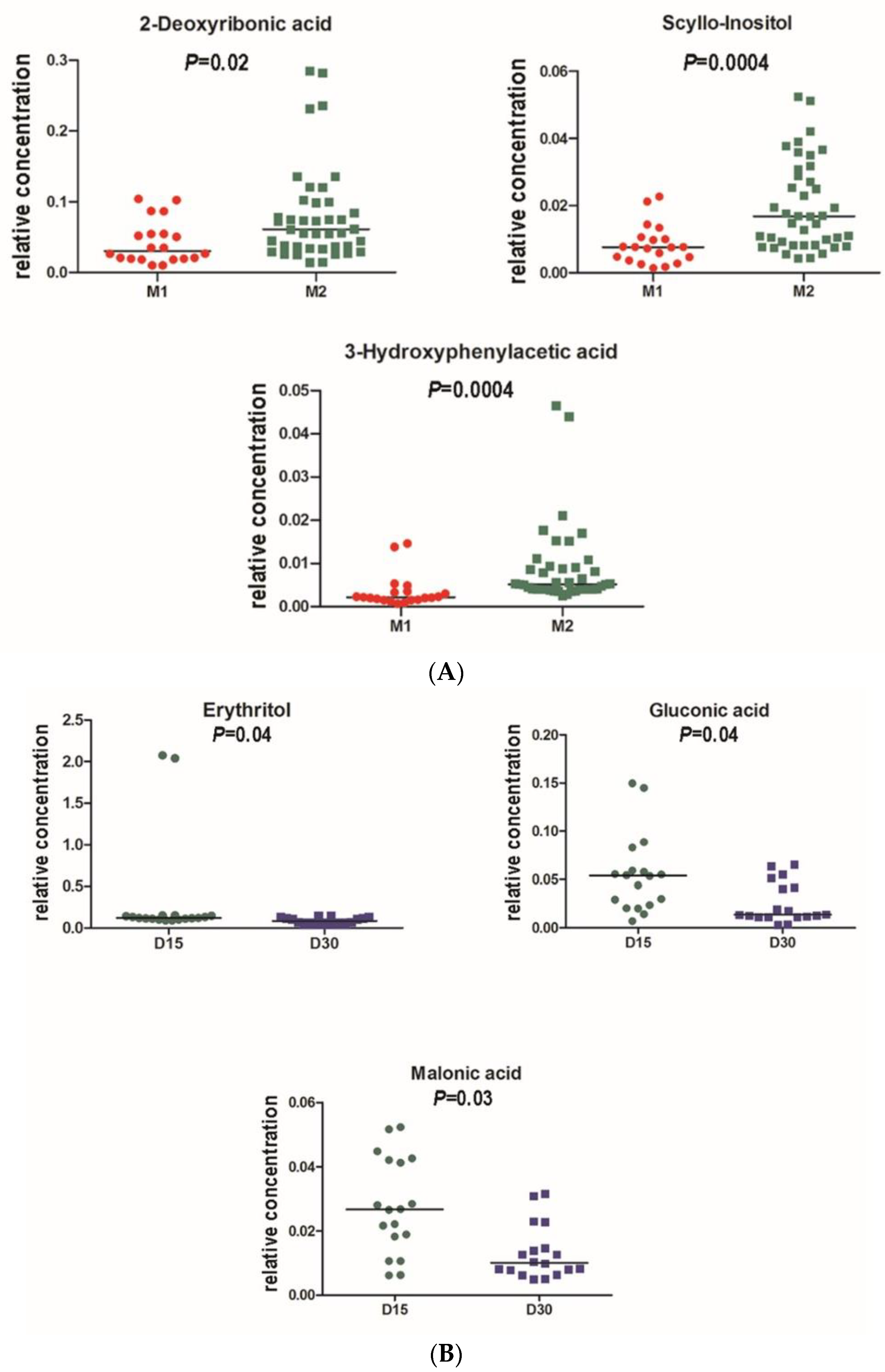
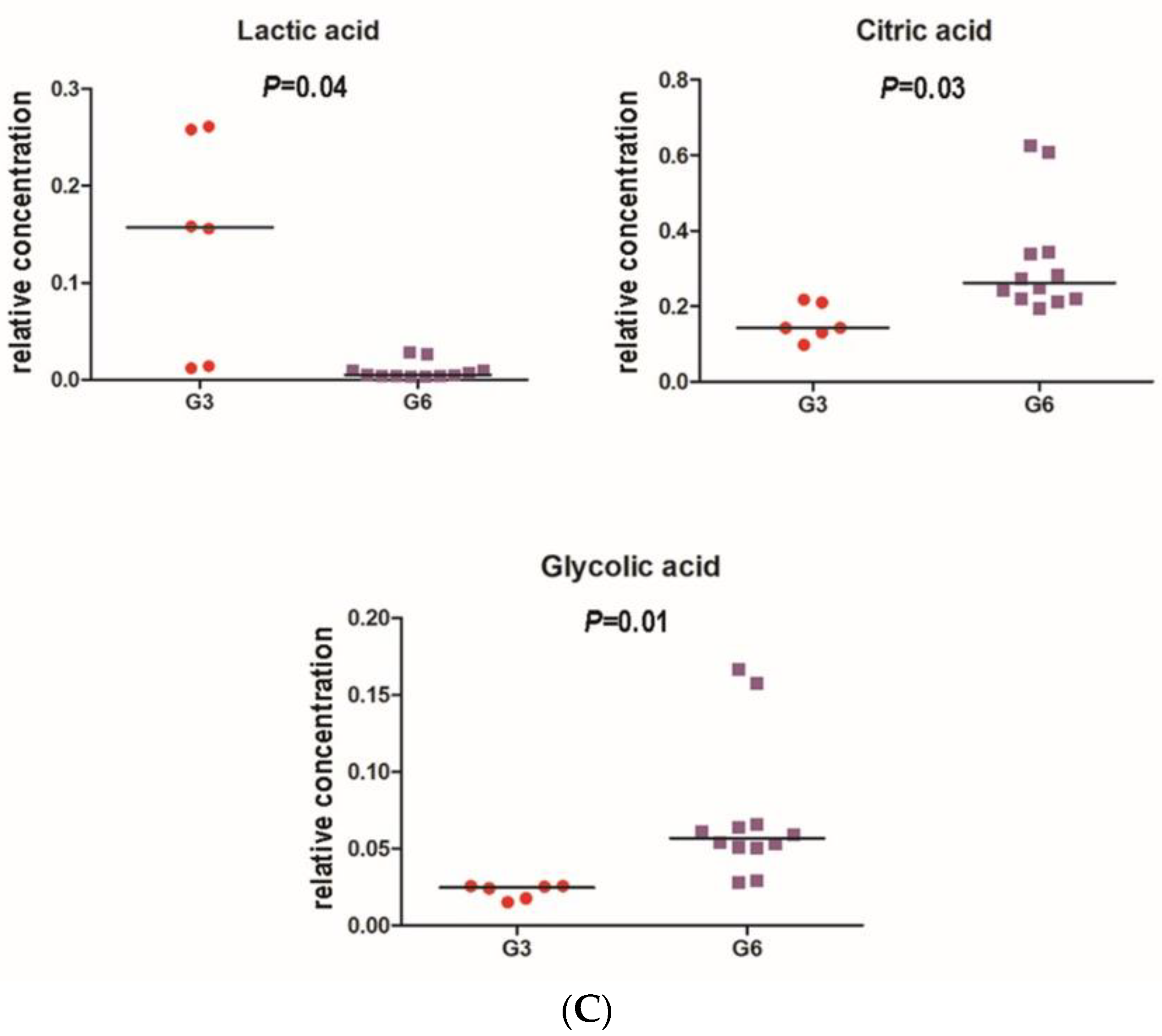
3.2. Chromameter Testing
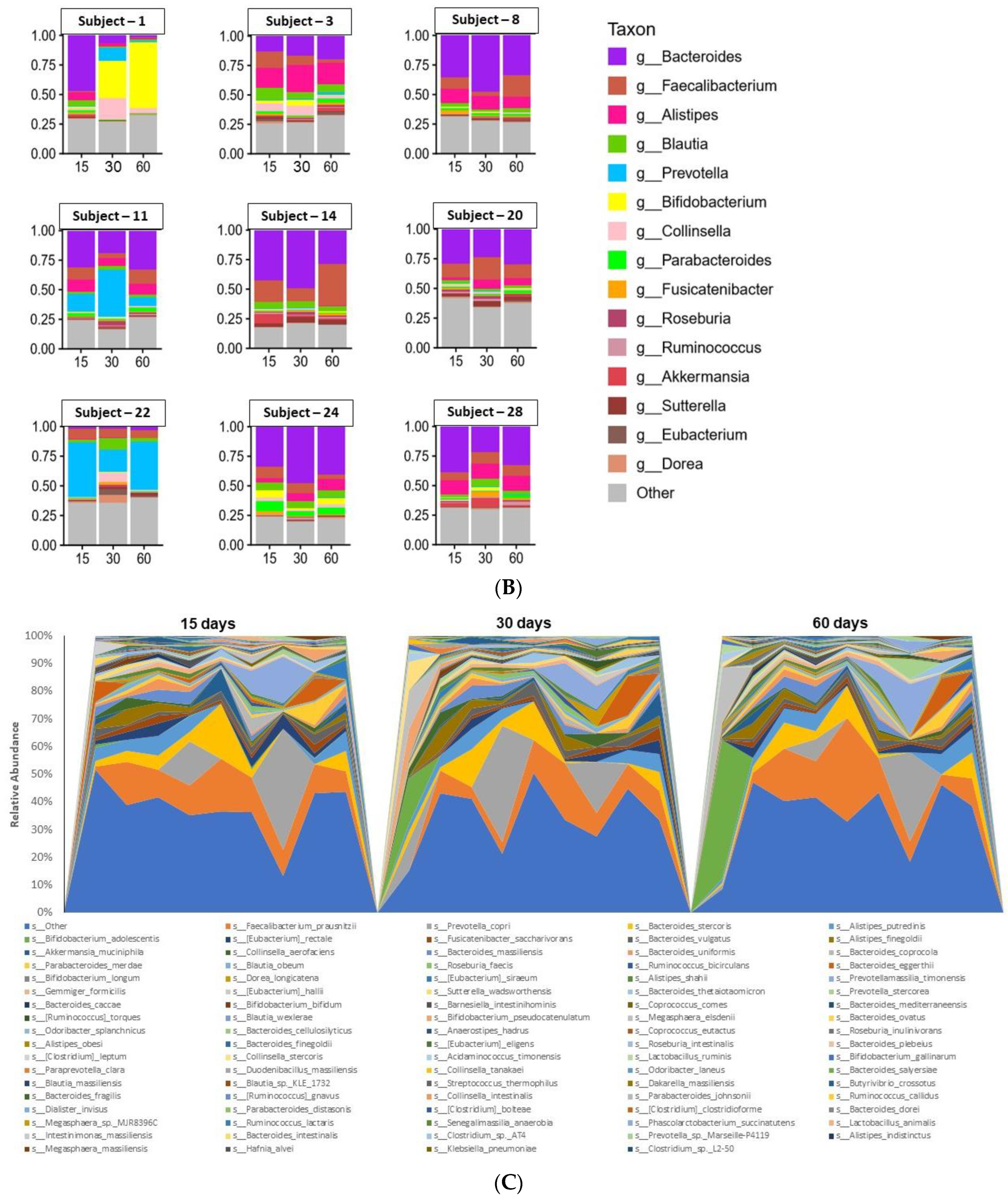
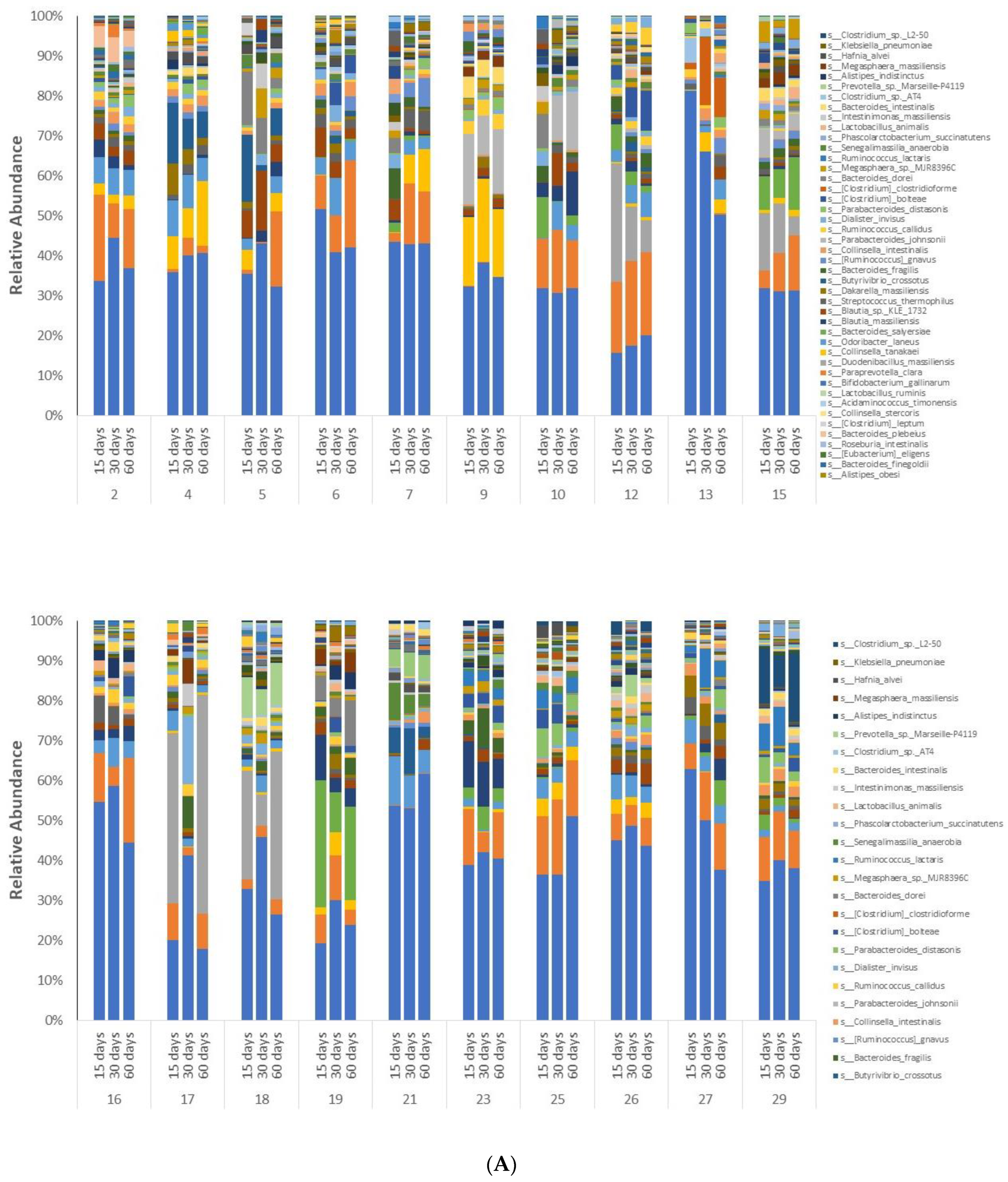
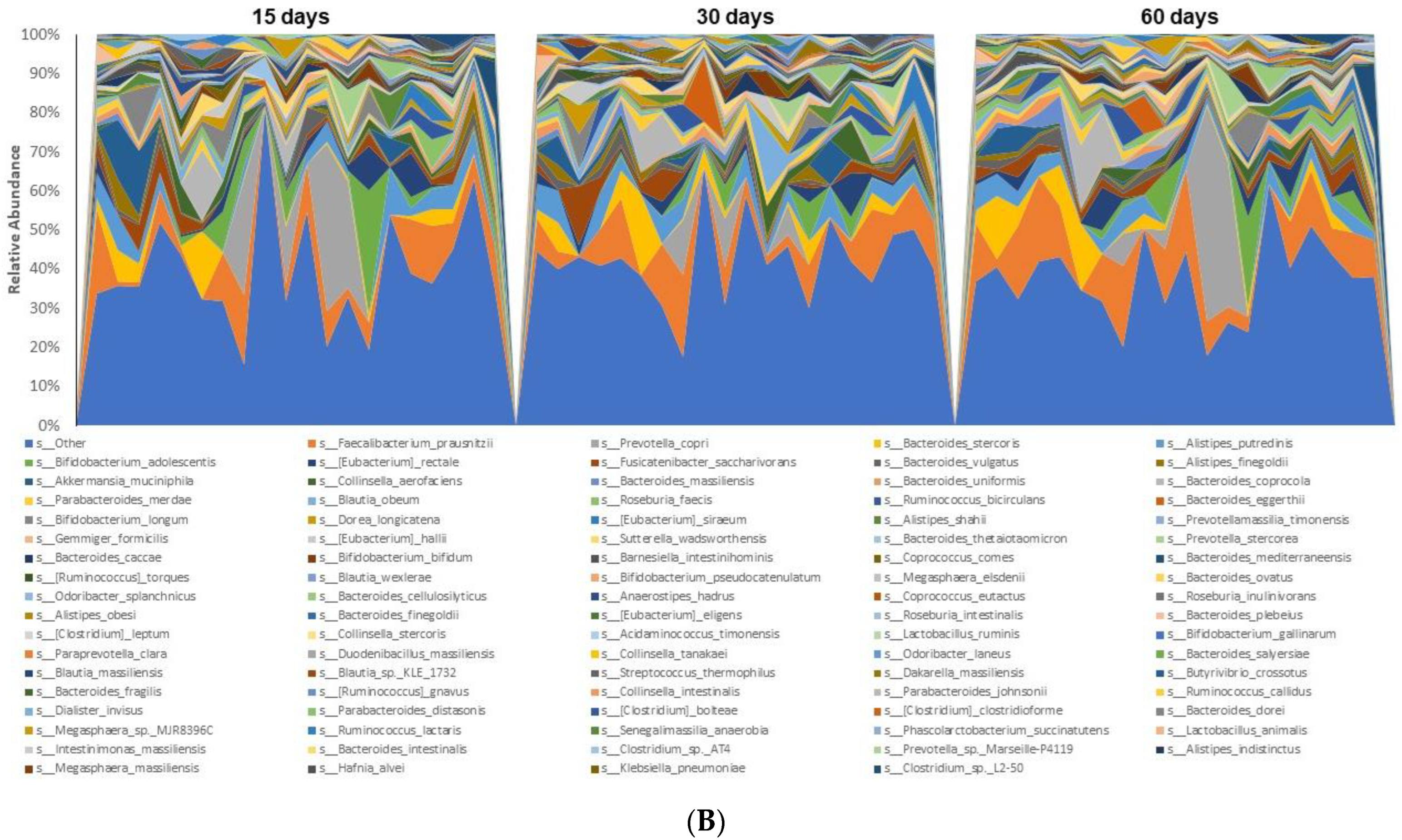
3.3. Microbiome Analyses
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Healthy Diet. Available online: https://www.who.int/news-room/fact-sheets/detail/healthy-diet (accessed on 14 November 2022).
- Jang, M.; Cai, L.; Udeani, G.O.; Slowing, K.V.; Thomas, C.F.; Beecher, C.W.; Fong, H.H.; Farnsworth, N.R.; Kinghorn, A.D.; Mehta, R.G.; et al. Cancer chemopreventive activity of resveratrol, a natural product derived from grapes. Science 1997, 275, 218–220. [Google Scholar] [CrossRef] [PubMed]
- Park, E.-J.; Pezzuto, J.M. The pharmacology of resveratrol in animals and humans. Biochim. Biophys. Acta 2015, 1852, 1071–1113. [Google Scholar] [CrossRef] [PubMed]
- Pezzuto, J.M. Grapes and human health: A perspective. J. Agric. Food Chem. 2008, 56, 6777–6784. [Google Scholar] [CrossRef] [PubMed]
- Pezzuto, J.M. Grapes and Health; Springer: Heidelberger, Germany, 2016. [Google Scholar]
- Slominski, A.T.; Zmijewski, M.A.; Plonka, P.M.; Szaflarski, J.P.; Paus, R. How UV light touches the brain and endocrine system through skin, and why. Endocrinology 2018, 159, 1992–2007. [Google Scholar] [CrossRef] [PubMed]
- Islami, F.; Sauer, A.G.; Miller, K.D.; Fedewa, S.A.; Minihan, A.K.; Geller, A.C.; Lichtenfeld, J.L.; Jemal, A. Cutaneous melanomas attributable to ultraviolet radiation exposure by state. Int. J. Cancer 2020, 147, 1385–1390. [Google Scholar] [CrossRef] [PubMed]
- Skin Cancer. Available online: https://www.aad.org/media/stats-skin-cancer (accessed on 15 October 2022).
- Pezzuto, J.M. Resveratrol: Twenty years of growth, development and controversy. Biomol. Ther. 2019, 27, 1–14. [Google Scholar] [CrossRef]
- Singh, C.K.; Mintie, C.A.; Ndiaye, M.A.; Chhabra, G.; Dakup, P.P.; Ye, T.; Yu, M.; Ahmad, N. Chemoprotective effects of dietary grape powder on UVB radiation-mediated skin carcinogenesis in SKH-1 hairless mice. J. Investig. Dermatol. 2019, 139, 552–561. [Google Scholar] [CrossRef]
- Mintie, C.A.; Singh, C.K.; Ndiaye, M.A.; Barrett-Wilt, G.A.; Ahmad, N. Identification of molecular targets of dietary grape-mediated chemoprevention of ultraviolet B skin carcinogenesis: A comparative quantitative proteomics analysis. J. Proteome Res. 2019, 18, 3741–3751. [Google Scholar] [CrossRef]
- Mintie, C.A.; Musarra, A.K.; Singh, C.K.; Ndiaye, M.A.; Sullivan, R.; Eickhoff, J.C.; Ahmad, N. Protective effects of dietary grape on UVB-mediated cutaneous damages and skin tumorigenesis in SKH-1 mice. Cancers 2020, 12, 1751. [Google Scholar] [CrossRef]
- Oak, A.S.W.; Shafi, R.; Elsayed, M.; Bae, S.; Saag, L.; Wang, C.L.; Athar, M.; Elmets, C.A. Dietary table grape protects against ultraviolet photodamage in humans: 1. Clinical evaluation. J. Am. Acad. Dermatol. 2021, 85, 1030–1032. [Google Scholar] [CrossRef]
- Oak, A.S.W.; Shafi, R.; Elsayed, M.; Mishra, B.; Bae, S.; Barnes, S.; Kashyap, M.P.; Slominski, A.T.; Wilson, L.S.; Athar, M.; et al. Dietary table grape protects against ultraviolet photodamage in humans: 2. Molecular biomarker studies. J. Am. Acad. Dermatol. 2021, 85, 1032–1034. [Google Scholar] [CrossRef] [PubMed]
- van Breemen, R.B.; Wright, B.; Li, Y.; Nosal, D.; Burton, T. Standardized Grape powder for basic and clinical research. In Grapes and Health; Pezzuto, J.M., Ed.; Springer International Publishing: Cham, Switzerland, 2016; pp. 17–26. ISBN 978-3-319-28995-3. [Google Scholar]
- Dave, A.; Beyoğlu, D.; Park, E.-J.; Idle, J.R.; Pezzuto, J.M. Influence of grape consumption on the human microbiome. submitted.
- Beyoğlu, D.; Park, E.-J.; Quiñones-Lombraña, A.; Dave, A.; Parande, F.; Pezzuto, J.M.; Idle, J.R. Addition of grapes to both a standard and a high-fat Western pattern diet modifies hepatic and urinary metabolite profiles in the mouse. Food Funct. 2022, 13, 8489–8499. [Google Scholar] [CrossRef] [PubMed]
- Dave, A.; Park, E.-J.; Kumar, A.; Parande, F.; Beyoğlu, D.; Idle, J.R.; Pezzuto, J.M. Consumption of grapes modulates gene expression, reduces non-alcoholic fatty liver disease, and extends longevity in female C57BL/6J mice provided with a high-fat Western-pattern diet. Foods 2022, 11, 1984. [Google Scholar] [CrossRef]
- Webb-Robertson, B.-J.; Kim, Y.-M.; Zink, E.M.; Hallaian, K.A.; Zhang, Q.; Madupu, R.; Waters, K.M.; Metz, T.O. A statistical analysis of the effects of urease pre-treatment on the measurement of the urinary metabolome by gas chromatography-mass spectrometry. Metabolomics 2014, 10, 897–908. [Google Scholar] [CrossRef]
- McGough, J.J.; Faraone, S.V. Estimating the size of treatment effects. Psychiatry 2009, 6, 21–29. [Google Scholar] [PubMed]
- Matias, A.R.; Ferreira, M.; Costa, P.; Neto, P. Skin colour, skin redness and melanin biometric measurements: Comparison study between Antera® 3D, Mexameter® and Colorimeter®. Skin Res. Technol. 2015, 21, 346–362. [Google Scholar] [CrossRef]
- Vacca, M.; Celano, G.; Calabrese, F.M.; Portincasa, P.; Gobbetti, M.; De Angelis, M. The Controversial role of human gut Lachnospiraceae. Microorganisms 2020, 8, 573. [Google Scholar] [CrossRef]
- Barrett, H.L.; Gomez-Arango, L.F.; Wilkinson, S.A.; McIntyre, H.D.; Callaway, L.K.; Morrison, M.; Dekker Nitert, M. A vegetarian diet is a major determinant of gut microbiota composition in early pregnancy. Nutrients 2018, 10, 890. [Google Scholar] [CrossRef]
- Creswell, R.; Tan, J.; Leff, J.W.; Brooks, B.; Mahowald, M.A.; Thieroff-Ekerdt, R.; Gerber, G.K. High-resolution temporal profiling of the human gut microbiome reveals consistent and cascading alterations in response to dietary glycans. Genome Med. 2020, 12, 59. [Google Scholar] [CrossRef]
- Kim, C.C.; Healey, G.R.; Kelly, W.J.; Patchett, M.L.; Jordens, Z.; Tannock, G.W.; Sims, I.M.; Bell, T.J.; Hedderley, D.; Henrissat, B.; et al. Genomic insights from Monoglobus pectinilyticus: A pectin-degrading specialist bacterium in the human colon. ISME J. 2019, 13, 1437–1456. [Google Scholar] [CrossRef]
- Koliarakis, I.; Messaritakis, I.; Nikolouzakis, T.K.; Hamilos, G.; Souglakos, J.; Tsiaoussis, J. Oral bacteria and intestinal dysbiosis in colorectal cancer. Int. J. Mol. Sci. 2019, 20, 4146. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Mao, B.; Gu, J.; Wu, J.; Cui, S.; Wang, G.; Zhao, J.; Zhang, H.; Chen, W. Blautia—A new functional genus with potential probiotic properties? Gut Microbes 2021, 13, 1875796. [Google Scholar] [CrossRef] [PubMed]
- Park, W. Gut microbiomes and their metabolites shape human and animal health. J. Microbiol. 2018, 56, 151–153. [Google Scholar] [CrossRef]
- Wang, K.; Liao, M.; Zhou, N.; Bao, L.; Ma, K.; Zheng, Z.; Wang, Y.; Liu, C.; Wang, W.; Wang, J.; et al. Parabacteroides distasonis alleviates obesity and metabolic dysfunctions via production of succinate and secondary bile acids. Cell Rep. 2019, 26, 222–235.e5. [Google Scholar] [CrossRef]
- Ilinskaya, O.N.; Ulyanova, V.V.; Yarullina, D.R.; Gataullin, I.G. Secretome of intestinal Bacilli: A natural guard against pathologies. Front. Microbiol. 2017, 8, 1666. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Wu, Y.; Wang, J.; Wu, G.; Long, W.; Xue, Z.; Wang, L.; Zhang, X.; Pang, X.; Zhao, Y.; et al. Accelerated dysbiosis of gut microbiota during aggravation of DSS-induced colitis by a butyrate-producing bacterium. Sci. Rep. 2016, 6, 27572. [Google Scholar] [CrossRef]
- Ting, N.L.-N.; Lau, H.C.-H.; Yu, J. Cancer pharmacomicrobiomics: Targeting microbiota to optimise cancer therapy outcomes. Gut 2022, 71, 1412–1425. [Google Scholar] [CrossRef]
- Huang, F.; Qiao, H.; Yin, J.; Gao, Y.; Ju, Y.; Li, Y. Early-life exposure to Clostridium leptum causes pulmonary immunosuppression. PLoS ONE 2015, 10, e0141717. [Google Scholar] [CrossRef]
- Davis-Richardson, A.G.; Ardissone, A.N.; Dias, R.; Simell, V.; Leonard, M.T.; Kemppainen, K.M.; Drew, J.C.; Schatz, D.; Atkinson, M.A.; Kolaczkowski, B.; et al. Bacteroides dorei dominates gut microbiome prior to autoimmunity in Finnish children at high risk for type 1 diabetes. Front. Microbiol. 2014, 5, 678. [Google Scholar] [CrossRef]
- Daillère, R.; Vétizou, M.; Waldschmitt, N.; Yamazaki, T.; Isnard, C.; Poirier-Colame, V.; Duong, C.P.M.; Flament, C.; Lepage, P.; Roberti, M.P.; et al. Enterococcus hirae and Barnesiella intestinihominis facilitate cyclophosphamide-induced therapeutic immunomodulatory effects. Immunity 2016, 45, 931–943. [Google Scholar] [CrossRef]
- Iljazovic, A.; Roy, U.; Gálvez, E.J.C.; Lesker, T.R.; Zhao, B.; Gronow, A.; Amend, L.; Will, S.E.; Hofmann, J.D.; Pils, M.C.; et al. Perturbation of the gut microbiome by Prevotella spp. enhances host susceptibility to mucosal inflammation. Mucosal Immunol. 2021, 14, 113–124. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Liu, Y.; Zheng, H.J.; Zhang, C.P. The oral microbiota may have influence on oral cancer. Front. Cell. Infect. Microbiol. 2020, 9, 476. [Google Scholar] [CrossRef] [PubMed]
- Muñiz Pedrogo, D.A.; Chen, J.; Hillmann, B.; Jeraldo, P.; Al-Ghalith, G.; Taneja, V.; Davis, J.M.; Knights, D.; Nelson, H.; Faubion, W.A.; et al. An increased abundance of Clostridiaceae characterizes arthritis in inflammatory bowel disease and rheumatoid arthritis: A cross-sectional study. Inflamm. Bowel Dis. 2019, 25, 902–913. [Google Scholar] [CrossRef] [PubMed]
- Haas, K.N.; Blanchard, J.L. Reclassification of the Clostridium clostridioforme and Clostridium sphenoides clades as Enterocloster gen. nov. and Lacrimispora gen. nov., including reclassification of 15 taxa. Int. J. Syst. Evol. Microbiol. 2020, 70, 23–34. [Google Scholar] [CrossRef] [PubMed]
- Manor, O.; Dai, C.L.; Kornilov, S.A.; Smith, B.; Price, N.D.; Lovejoy, J.C.; Gibbons, S.M.; Magis, A.T. Health and disease markers correlate with gut microbiome composition across thousands of people. Nat. Commun. 2020, 11, 5206. [Google Scholar] [CrossRef] [PubMed]
- Han, F.; Wu, G.; Zhang, Y.; Zheng, H.; Han, S.; Li, X.; Cai, W.; Liu, J.; Zhang, W.; Zhang, X.; et al. Streptococcus thermophilus attenuates inflammation in septic mice mediated by gut microbiota. Front. Microbiol. 2020, 11, 598010. [Google Scholar] [CrossRef] [PubMed]
- Bien, J.; Palagani, V.; Bozko, P. The intestinal microbiota dysbiosis and Clostridium difficile infection: Is there a relationship with inflammatory bowel disease? Therap. Adv. Gastroenterol. 2013, 6, 53–68. [Google Scholar] [CrossRef] [PubMed]
- Hasiba, W.; Xu, Y.; Sugiyama, H. Reactivity of 2′-deoxyuridin-1′-yl radical in various DNA structures. Nucleic Acids Symposium. Ser. 2005, 49, 179–180. [Google Scholar] [CrossRef]
- Kappen, L.S.; Goldberg, I.H. Identification of 2-deoxyribonolactone at the site of neocarzinostatin-induced cytosine release in the sequence d(AGC). Biochemistry 1989, 28, 1027–1032. [Google Scholar] [CrossRef]
- Heinrich, M.; Jalil, B.; Abdel-Tawab, M.; Echeverria, J.; Kulic, Ž.; McGaw, L.J.; Pezzuto, J.M.; Potterat, O.; Wang, J.-B. Best Practice in the chemical characterisation of extracts used in pharmacological and toxicological re-search—The ConPhyMP—Guidelines. Front. Pharmacol. 2022, 13, 953205. [Google Scholar] [CrossRef]
- Singh, R.K.; Chang, H.-W.; Yan, D.; Lee, K.M.; Ucmak, D.; Wong, K.; Abrouk, M.; Farahnik, B.; Nakamura, M.; Zhu, T.H.; et al. Influence of diet on the gut microbiome and implications for human health. J. Transl. Med. 2017, 15, 73. [Google Scholar] [CrossRef] [PubMed]
- Tomova, A.; Bukovsky, I.; Rembert, E.; Yonas, W.; Alwarith, J.; Barnard, N.D.; Kahleova, H. The Effects of vegetarian and vegan diets on gut microbiota. Front. Nutr. 2019, 6, 47. [Google Scholar] [CrossRef] [PubMed]
- Vyas, U.; Ranganathan, N. Probiotics, prebiotics, and synbiotics: Gut and beyond. Gastroenterol. Res. Pract. 2012, 2012, 872716. [Google Scholar] [CrossRef]
- Proctor, L.; LoTempio, J.; Marquitz, A.; Daschner, P.; Xi, D.; Flores, R.; Brown, L.; Ranallo, R.; Maruvada, P.; Regan, K.; et al. A review of 10 years of human microbiome research activities at the US National Institutes of Health, Fiscal Years 2007–2016. Microbiome 2019, 7, 31. [Google Scholar] [CrossRef]
- Mahmud, M.R.; Akter, S.; Tamanna, S.K.; Mazumder, L.; Esti, I.Z.; Banerjee, S.; Akter, S.; Hasan, M.R.; Acharjee, M.; Hossain, M.S.; et al. Impact of gut microbiome on skin health: Gut-skin axis observed through the lenses of therapeutics and skin diseases. Gut Microbes 2022, 14, 2096995. [Google Scholar] [CrossRef]
- Lefevere, M.F.; Verhaeghe, B.J.; Declerck, D.H.; Van Bocxlaer, J.F.; De Leenheer, A.P.; De Sagher, R.M. Metabolic profiling of urinary organic acids by single and multicolumn capillary gas chromatography. J. Chromatogr. Sci. 1989, 27, 23–29. [Google Scholar] [CrossRef] [PubMed]
- Tuchman, M.; Bowers, L.D.; Fregien, K.D.; Crippin, P.J.; Krivit, W. Capillary gas chromatographic separation of urinary organic acids. Retention indices of 101 urinary acids on a 5% phenylmethyl silicone capillary column. J. Chromatogr. Sci. 1984, 22, 198–202. [Google Scholar] [CrossRef]
- Urata, H.; Yamamoto, K.; Akagi, M.; Hiroaki, H.; Uesugi, S. A 2-deoxyribonolactone-containing nucleotide: Isolation and characterization of the alkali-sensitive photoproduct of the trideoxyribonucleotide d(ApCpA). Biochemistry 1989, 28, 9566–9569. [Google Scholar] [CrossRef]
- Dizdaroglu, M.; Schulte-Frohlinde, D.; von Sonntag, C. Isolation of 2-deoxy-d-erythro-pentonic acid from an alkali-labile site in γ-irradiated DNA. Int. J. Radiat. Biol. Relat. Stud. Phys. Chem. Med. 1977, 32, 481–483. [Google Scholar] [CrossRef]
- Furukawa, J.Y.; Martinez, R.M.; Morocho-Jácome, A.L.; Castillo-Gómez, T.S.; Pereda-Contreras, V.J.; Rosado, C.; Velasco, M.V.R.; Baby, A.R. Skin impacts from exposure to ultraviolet, visible, infrared, and artificial lights–a review. J. Cosmet. Laser Ther. 2021, 23, 1–7. [Google Scholar] [CrossRef]
- Sakuntabhai, A.; Matthews, J.N.; Farr, P.M. Improved prediction of the minimal phototoxic dose in PUVA therapy. Br. J. Dermatol. 1994, 130, 604–609. [Google Scholar] [CrossRef] [PubMed]
- Seidegård, J.; Vorachek, W.R.; Pero, R.W.; Pearson, W.R. Hereditary differences in the expression of the human glutathione transferase active on trans-stilbene oxide are due to a gene deletion. Proc. Natl. Acad. Sci. USA 1988, 85, 7293–7297. [Google Scholar] [CrossRef] [PubMed]
- Pemble, S.; Schroeder, K.R.; Spencer, S.R.; Meyer, D.J.; Hallier, E.; Bolt, H.M.; Ketterer, B.; Taylor, J.B. Human glutathione S-transferase theta (GSTT1): cDNA cloning and the characterization of a genetic polymorphism. Biochem. J. 1994, 300 Pt 1, 271–276. [Google Scholar] [CrossRef] [PubMed]
- Kerb, R.; Brockmöller, J.; Reum, T.; Roots, I. Deficiency of glutathione S-transferases T1 and M1 as heritable factors of increased cutaneous UV sensitivity. J. Investig. Dermatol. 1997, 108, 229–232. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Martinović, A.; Cocuzzi, R.; Arioli, S.; Mora, D. Streptococcus thermophilus: To survive, or not to survive the gastrointestinal tract, that is the question! Nutrients 2020, 12, 2175. [Google Scholar] [CrossRef] [PubMed]
- Benítez-Páez, A.; Gómez Del Pugar, E.M.; López-Almela, I.; Moya-Pérez, Á.; Codoñer-Franch, P.; Sanz, Y. Depletion of Blautia species in the microbiota of obese children relates to intestinal inflammation and metabolic phenotype worsening. MSystems 2020, 5, e00857-19. [Google Scholar] [CrossRef]
- Bag, S.; Ghosh, T.S.; Das, B. Complete genome sequence of Collinsella aerofaciens isolated from the gut of a healthy Indian subject. Genome Announc. 2017, 5, e01361-17. [Google Scholar] [CrossRef]
- Qin, Y.; Havulinna, A.S.; Liu, Y.; Jousilahti, P.; Ritchie, S.C.; Tokolyi, A.; Sanders, J.G.; Valsta, L.; Brożyńska, M.; Zhu, Q.; et al. Combined effects of host genetics and diet on human gut microbiota and incident disease in a single population cohort. Nat. Genet. 2022, 54, 134–142. [Google Scholar] [CrossRef]
- Antezack, A.; Boxberger, M.; La Scola, B.; Monnet-Corti, V. Isolation and description of catonella massiliensis sp. nov., a novel catonella species, isolated from a stable periodontitis subject. Pathogens 2021, 10, 367. [Google Scholar] [CrossRef]
- Meehan, C.J.; Beiko, R.G. A Phylogenomic View of Ecological Specialization in the Lachnospiraceae, A family of digestive tract-associated bacteria. Genome Biol. Evol. 2014, 6, 703–713. [Google Scholar] [CrossRef]
- Larsen, J.M. The immune response to Prevotella bacteria in chronic inflammatory disease. Immunology 2017, 151, 363–374. [Google Scholar] [CrossRef] [PubMed]
- Rajakovich, L.J.; Fu, B.; Bollenbach, M.; Balskus, E.P. Elucidation of an anaerobic pathway for metabolism of l-carnitine–derived γ-butyrobetaine to trimethylamine in human gut bacteria. Proc. Natl. Acad. Sci. USA 2021, 118, e2101498118. [Google Scholar] [CrossRef] [PubMed]
- Ganji, L.; Alebouyeh, M.; Shirazi, M.H.; Eshraghi, S.S.; Mirshafiey, A.; Ebrahimi Daryani, N.; Zali, M.R. Dysbiosis of fecal microbiota and high frequency of Citrobacter, Klebsiella spp., and Actinomycetes in patients with irritable bowel syndrome and gastroenteritis. Gastroenterol. Hepatol. Bed Bench 2016, 9, 325–330. [Google Scholar] [PubMed]
- Fakharian, F.; Asgari, B.; Nabavi-Rad, A.; Sadeghi, A.; Soleimani, N.; Yadegar, A.; Zali, M.R. The interplay between Helicobacter pylori and the gut microbiota: An emerging driver influencing the immune system homeostasis and gastric carcinogenesis. Front. Cell. Infect. Microbiol. 2022, 12. Available online: https://www.frontiersin.org/articles/10.3389/fcimb.2022.953718 (accessed on 15 October 2022). [CrossRef] [PubMed]
- Tamanai-Shacoori, Z.; Smida, I.; Bousarghin, L.; Loreal, O.; Meuric, V.; Fong, S.B.; Bonnaure-Mallet, M.; Jolivet-Gougeon, A. Roseburia spp.: A marker of health? Future Microbiol. 2017, 12, 157–170. [Google Scholar] [CrossRef] [PubMed]
- Forbes, J.D.; Van Domselaar, G.; Bernstein, C.N. The gut microbiota in immune-mediated inflammatory diseases. Front. Microbiol. 2016, 7, 1081. [Google Scholar] [CrossRef]
- Zakham, F.; Pillonel, T.; Brunel, A.-S.; Zambelli, P.-Y.; Greub, G.; Croxatto, A.; Bertelli, C. Molecular diagnosis and enrichment culture identified a septic pseudoarthrosis due to an infection with Erysipelatoclostridium ramosum. Int. J. Infect. Dis. 2019, 81, 167–169. [Google Scholar] [CrossRef]
- Rosario, D.; Benfeitas, R.; Bidkhori, G.; Zhang, C.; Uhlen, M.; Shoaie, S.; Mardinoglu, A. Understanding the representative gut microbiota dysbiosis in metformin-treated type 2 diabetes patients using genome-scale metabolic modeling. Front. Physiol. 2018, 9, 775. [Google Scholar] [CrossRef]
- Gryaznova, M.V.; Solodskikh, S.A.; Panevina, A.V.; Syromyatnikov, M.Y.; Dvoretskaya, Y.D.; Sviridova, T.N.; Popov, E.S.; Popov, V.N. Study of microbiome changes in patients with ulcerative colitis in the Central European part of Russia. Heliyon 2021, 7, e06432. [Google Scholar] [CrossRef]
- Bui, T.P.N.; Schols, H.A.; Jonathan, M.; Stams, A.J.M.; de Vos, W.M.; Plugge, C.M. Mutual metabolic interactions in co-cultures of the intestinal Anaerostipes Rhamnosivorans with an acetogen, methanogen, or pectin-degrader affecting butyrate production. Front. Microbiol. 2019, 10, 2449. Available online: https://www.frontiersin.org/articles/10.3389/fmicb.2019.02449 (accessed on 17 September 2022). [CrossRef]
- Cabral, L.; Persinoti, G.F.; Paixão, D.A.A.; Martins, M.P.; Morais, M.A.B.; Chinaglia, M.; Domingues, M.N.; Sforca, M.L.; Pirolla, R.A.S.; Generoso, W.C.; et al. Gut microbiome of the largest living rodent harbors unprecedented enzymatic systems to degrade plant polysaccharides. Nat. Commun. 2022, 13, 629. [Google Scholar] [CrossRef] [PubMed]
- Mekhalif, F.; Zgheib, R.; Akiana, J.; Bilen, M.; Ndombe, G.M.; Fenollar, F.; Fournier, P.-E.; Raoult, D.; Alibar, S.; Mediannikov, O.; et al. Peptostreptococcus faecalis sp. nov., new bacterial species isolated from healthy indigenous congolese volunteer. Heliyon 2022, 8, e09102. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Arango, L.F.; Barrett, H.L.; Wilkinson, S.A.; Callaway, L.K.; McIntyre, H.D.; Morrison, M.; Dekker Nitert, M. Low dietary fiber intake increases Collinsella abundance in the gut microbiota of overweight and obese pregnant women. Gut Microbes 2018, 9, 189–201. [Google Scholar] [CrossRef] [PubMed]
- Azad, M.; Kalam, A.; Sarker, M.; Li, T.; Yin, J. Probiotic species in the modulation of gut microbiota: An overview. Biomed Res. Int. 2018, 9478630. [Google Scholar] [CrossRef]
- Nie, K.; Ma, K.; Luo, W.; Shen, Z.; Yang, Z.; Xiao, M.; Tong, T.; Yang, Y.; Wang, X. Roseburia intestinalis: A beneficial gut organism from the discoveries in genus and species. Front. Cell. Infect. Microbiol. 2021, 11, 757718. Available online: https://www.frontiersin.org/articles/10.3389/fcimb.2021.757718 (accessed on 15 October 2022). [CrossRef]
- Lazar, V.; Ditu, L.-M.; Pircalabioru, G.G.; Gheorghe, I.; Curutiu, C.; Holban, A.M.; Picu, A.; Petcu, L.; Chifiriuc, M.C. Aspects of gut microbiota and immune system interactions in infectious diseases, Immunopathology, and Cancer. Front Immunol. 2018, 9, 1830. [Google Scholar] [CrossRef]
- Horvath, T.D.; Ihekweazu, F.D.; Haidacher, S.J.; Ruan, W.; Engevik, K.A.; Fultz, R.; Hoch, K.M.; Luna, R.A.; Oezguen, N.; Spinler, J.K.; et al. Bacteroides ovatus colonization influences the abundance of intestinal short chain fatty acids and neurotransmitters. iScience 2022, 25, 104158. Available online: https://www.sciencedirect.com/science/article/pii/S258900422200428X (accessed on 15 October 2022). [CrossRef]
- Bressa, C.; Bailén-Andrino, M.; Pérez-Santiago, J.; González-Soltero, R.; Pérez, M.; Montalvo-Lominchar, M.G.; Maté-Muñoz, J.L.; Domínguez, R.; Moreno, D.; Larrosa, M. Differences in gut microbiota profile between women with active lifestyle and sedentary women. PLOS ONE 2017, 12, e0171352. [Google Scholar] [CrossRef]
- Rizzatti, G.; Lopetuso, L.R.; Gibiino, G.; Binda, C.; Gasbarrini, A. Proteobacteria: A common factor in human diseases. Biomed Res. Int. 2017, 2017, 9351507. [Google Scholar] [CrossRef]
- Lai, C.-H.; Lin, T.-L.; Huang, M.-Z.; Li, S.-W.; Wu, H.-Y.; Chiu, Y.-F.; Yang, C.-Y.; Chiu, C.-H.; Lai, H.-C. Gut commensal Parabacteroides goldsteinii mts01 alters gut microbiota composition and reduces cholesterol to mitigate Helicobacter pylori-induced pathogenesis. Front. Immunol. 2022, 13. Available online: https://www.frontiersin.org/articles/10.3389/fimmu.2022.916848 (accessed on 2 October 2022). [CrossRef]
- Zamani, S.; Taslimi, R.; Sarabi, A.; Jasemi, S.; Sechi, L.A.; Feizabadi, M.M. Enterotoxigenic Bacteroides fragilis: A possible etiological candidate for bacterially-induced colorectal precancerous and cancerous lesions. Front. Cell. Infect. Microbiol. 2020, 9. Available online: https://www.frontiersin.org/articles/10.3389/fcimb.2019.00449 (accessed on 2 October 2022). [CrossRef] [PubMed]
- Thingholm, L.B.; Bang, C.; Rühlemann, M.C.; Starke, A.; Sicks, F.; Kaspari, V.; Jandowsky, A.; Frölich, K.; Ismer, G.; Bernhard, A.; et al. Ecology impacts the decrease of Spirochaetes and Prevotella in the fecal gut microbiota of urban humans. BMC Microbiol. 2021, 21, 276. [Google Scholar] [CrossRef] [PubMed]
- Wu, F.; Guo, X.; Zhang, J.; Zhang, M.; Ou, Z.; Peng, Y. Phascolarctobacterium faecium abundant colonization in human gastrointestinal tract. Exp. Ther. Med. 2017, 14, 3122–3126. [Google Scholar] [CrossRef] [PubMed]
- Scott, K.P.; Martin, J.C.; Chassard, C.; Clerget, M.; Potrykus, J.; Campbell, G.; Mayer, C.-D.; Young, P.; Rucklidge, G.; Ramsay, A.G.; et al. Substrate-driven gene expression in Roseburia inulinivorans: Importance of inducible enzymes in the utilization of inulin and starch. Proc. Natl. Acad. Sci. USA 2011, 108, 4672–4679. [Google Scholar] [CrossRef] [PubMed]
- Dai, Z.; Wu, Z.; Hang, S.; Zhu, W.; Wu, G. Amino acid metabolism in intestinal bacteria and its potential implications for mammalian reproduction. Mol. Hum. Reprod. 2015, 21, 389–409. [Google Scholar] [CrossRef]
- Wexler, H.M. Bacteroides: The good, the bad, and the nitty-gritty. Clin. Microbiol. Rev. 2007, 20, 593–621. [Google Scholar] [CrossRef]
- Huang, Y.; Shi, X.; Li, Z.; Shen, Y.; Shi, X.; Wang, L.; Li, G.; Yuan, Y.; Wang, J.; Zhang, Y.; et al. Possible association of Firmicutes in the gut microbiota of patients with major depressive disorder. Neuropsychiatr. Dis. Treat. 2018, 14, 3329–3337. [Google Scholar] [CrossRef]



| Subject | Age | Sex | Race | Ethnicity | Fitzpatrick | ΔMED3 | |
|---|---|---|---|---|---|---|---|
| Skin-Type | Day 30 | Day 60 | |||||
| 1 | 24.0 | Male | White/Caucasian | Non-Hispanic/Latino | III 1 | +9.90 | 0 |
| 2 | 30.0 | Male | White/Caucasian | Non-Hispanic/Latino | III | 0 | 0 |
| 3 | 37.0 | Female | White/Caucasian | Hispanic/Latino | III | +12.40 | +12.40 |
| 4 | 43.3 | Female | White/Caucasian | Non-Hispanic/Latino | III | 0 | 0 |
| 5 | 45.3 | Female | White/Caucasian | Non-Hispanic/Latino | III | 0 | −6.30 |
| 6 | 45.3 | Female | White/Caucasian | Non-Hispanic/Latino | III | 0 | 0 |
| 7 | 32.6 | Female | White/Caucasian | Hispanic/Latino | III | 0 | 0 |
| 8 | 33.9 | Male | White/Caucasian | Non-Hispanic/Latino | III | +8.00 | +8.00 |
| 9 | 29.4 | Female | White/Caucasian | Non-Hispanic/Latino | III | 0 | 0 |
| 10 | 40 | Male | White/Caucasian | Hispanic/Latino | III | 0 | 0 |
| 11 | 33.6 | Male | White/Caucasian | Non-Hispanic/Latino | III | +9.90 | +9.90 |
| 12 | 44.4 | Male | White/Caucasian | Non-Hispanic/Latino | II 2 | 0 | 0 |
| 13 | 34.9 | Female | White/Caucasian | Hispanic/Latino | III | 0 | 0 |
| 14 | 34.8 | Female | White/Caucasian | Non-Hispanic/Latino | III | +9.90 | 0 |
| 15 | 39.0 | Female | White/Caucasian | Hispanic/Latino | III | 0 | −9.90 |
| 16 | 44.3 | Female | White/Caucasian | Non-Hispanic/Latino | III | 0 | 0 |
| 17 | 36.2 | Female | White/Caucasian | Non-Hispanic/Latino | III | 0 | 0 |
| 18 | 42.9 | Female | White/Caucasian | Hispanic/Latino | III | 0 | 0 |
| 19 | 43.2 | Male | White/Caucasian | Non-Hispanic/Latino | III | 0 | 0 |
| 20 | 43.7 | Male | White/Caucasian | Hispanic/Latino | III | +8.00 | 0 |
| 21 | 52.9 | Female | White/Caucasian | Hispanic/Latino | III | 0 | 0 |
| 22 | 46.8 | Male | White/Caucasian | Non-Hispanic/Latino | III | +6.30 | 0 |
| 23 | 51.6 | Male | White/Caucasian | Non-Hispanic/Latino | II | 0 | −6.30 |
| 24 | 54.4 | Male | White/Caucasian | Non-Hispanic/Latino | II | +6.30 | 0 |
| 25 | 48.2 | Male | White/Caucasian | Non-Hispanic/Latino | III | 0 | 0 |
| 26 | 37.7 | Male | White/Caucasian | Non-Hispanic/Latino | III | 0 | 0 |
| 27 | 55.7 | Male | White/Caucasian | Non-Hispanic/Latino | II | 0 | 0 |
| 28 | 46.1 | Male | White/Caucasian | Non-Hispanic/Latino | III | +9.90 | 0 |
| 29 | 55.1 | Male | White/Caucasian | Non-Hispanic/Latino | III | 0 | 0 |
| Taxonomy 1 | Log2 (Fold-Change) | Q Value | D Value | Functional Connotations |
|---|---|---|---|---|
| g__Catonella | −2.994 | 0.083 | 1.013 | Associated with Lachnospiraceae family. Increases in Lachnospiraceae abundances are associated with aging [22]. |
| g__Holdemania | −2.168 | 0.083 | 0.929 | Leads to reduction in the vegetarian diet [23]. |
| g__Neglecta | 2.082 | 0.112 | 1.027 | Lead to increase in the abundance with the consumption of glycans [24]. |
| g__Monoglobus | 2.982 | 0.224 | 1.040 | Pectin is abundant in modern day diets, as it comprises the middle lamellae and one-third of the dry carbohydrate weight of fruit and vegetable cell walls [25]. |
| Taxonomy 1 | Log2 (Fold-Change) | Q Value | Functional Connotations |
|---|---|---|---|
| g__Tannerella | −1.281 | 0.074 | Associated with periodontal inflammation [26]. |
| g__Blautia | −0.916 | 0.077 | Alleviates inflammatory diseases and metabolic diseases [27]. |
| s__Blautia_massiliensis | −1.706 | 0.085 | Alleviates inflammatory diseases and metabolic diseases [27]. |
| s__Ruminococcus_bicirculans | −2.264 | 0.087 | Degrade dietary cellulosic biomass into nutritive short-chain fatty acids [28]. |
| s__Blautia_sp._KLE_1732 | −1.841 | 0.094 | Alleviates inflammatory diseases and metabolic diseases [27]. |
| s__Parabacteroides_distasonis | −0.765 | 0.094 | Alleviates obesity and metabolic dysfunctions [29]. |
| g__Intestinibacillus | −3.203 | 0.10 | Role in counteraction to infectious diseases [30]. |
| s__Anaerostipes_hadrus | −1.281 | 0.11 | Increased butyrate content in the gut [31]. |
| g__Eisenbergiella | −0.981 | 0.11 | Ketone diet could enrich Eisenbergiella massiliensis in human gut [32]. |
| s__[Clostridium]_leptum | −2.366 | 0.13 | Maintains the intestinal microecological balance, promotes immune maturation, and increases Treg numbers to alleviate airway inflammation [33]. |
| s__Bacteroides_dorei | −1.525 | 0.14 | Higher abundance is linked with Type I diabetes [34]. |
| Enzymes | Log2 (Fold-Change) | Q Value |
|---|---|---|
| 1.17.5.3 fdnG; formate dehydrogenase-N, alpha subunit | −2.339 | 0.027 |
| 5.5.1.2 pcaB; 3-carboxy-cis,cis-muconate cycloisomerase | −1.513 | 0.027 |
| 5.3.3.14 fabM; trans-2-decenoyl-[acyl-carrier protein] isomerase | −1.267 | 0.027 |
| 2.1.1.315 rif14; 27-O-demethylrifamycin SV methyltransferase | −1.334 | 0.027 |
| 6.5.1.1 ligD; bifunctional non-homologous end joining protein LigD | −1.527 | 0.028 |
| 3.4.21.62 aprE; subtilisin | −3.564 | 0.028 |
| 3.2.1.22 melA; alpha-galactosidase | −1.378 | 0.029 |
| 1.8.5.4 sqr; sulfide:quinone oxidoreductase | −1.297 | 0.029 |
| 2.1.1.265 tehB; tellurite methyltransferase | −1.874 | 0.029 |
| 1.5.1.24 ceo; N5-(carboxyethyl)ornithine synthase | −4.532 | 0.029 |
| 2.7.13.3 cpxA; two-component system, OmpR family, sensor histidine kinase CpxA | −1.211 | 0.030 |
| 1.8.4.10 cysH; phosphoadenosine phosphosulfate reductase | −1.865 | 0.030 |
| 1.8.4.8 cysH; phosphoadenosine phosphosulfate reductase | −1.865 | 0.030 |
| 6.3.2.14 entE, dhbE, vibE, mxcE; 2,3-dihydroxybenzoate-AMP ligase | −3.328 | 0.031 |
| 4.2.1.167 hgdA; (R)-2-hydroxyglutaryl-CoA dehydratase subunit alpha | 1.212 | 0.031 |
| 2.3.1.197 ftdC; dTDP-3-amino-3,6-dideoxy-alpha-D-galactopyranose 3-N-acetyltransferase | −1.387 | 0.038 |
| 2.7.7.58 entE, dhbE, vibE, mxcE; 2,3-dihydroxybenzoate-AMP ligase | −3.328 | 0.038 |
| 2.4.1.60 rfbV; abequosyltransferase | −3.127 | 0.042 |
| 5.3.1.22 hyi, gip; hydroxypyruvate isomerase | −1.407 | 0.049 |
| Pathways | Log2 (Fold-Change) | Q Value |
|---|---|---|
| 1. Oxidoreductases; 1.7 Acting on other nitrogenous compounds as donors; 1.7.2 With a cytochrome as acceptor | −1.119 | 0.038 |
| 1. Oxidoreductases; 1.3 Acting on the CH-CH group of donors; 1.3.99 With other acceptors | −0.259 | 0.039 |
| 3. Hydrolases; 3.4 Acting on peptide bonds (peptidases); 3.4.17 Metallocarboxypeptidases | 0.456 | 0.039 |
| 1. Oxidoreductases; 1.7 Acting on other nitrogenous compounds as donors; 1.7.99 With other acceptors | −0.437 | 0.040 |
| Nonribosomal peptide synthetase (NRPS); Iterative NRPS; Bacillibactin synthetase | −3.191 | 0.042 |
| OmpR family; BasS-BasR | −1.257 | 0.043 |
| OmpR family; PhoR-PhoB (phosphate) | −0.290 | 0.045 |
| OmpR family; CpxA-CpxR | −0.998 | 0.046 |
| ABC Transporters, Prokaryotic Type; Monosaccharide transporters; Ribose transporter [MD:M00212] | −0.703 | 0.048 |
| ABC Transporters, Prokaryotic Type; Metallic cation, iron-siderophore and vitamin B12 transporters; Manganese transporter [MD:M00316] | −0.793 | 0.050 |
| Nonribosomal peptide synthetase (NRPS); Nonlinear NRPS; Vibriobactin synthetase | −3.388 | 0.051 |
| Taxonomy 1 | Log2 (Fold-Change) | Q Value | Functional Connotations |
|---|---|---|---|
| s__Bacteroides_dorei | −3.881 | 0.030 | Higher abundance is linked with Type I diabetes [34]. |
| g__Barnesiella | −3.315 | 0.030 | Commensals reduced Treg cells in the tumor microenvironment (Foxp3 and/or γδT17 cells) [35]. |
| s__Prevotella_copri | −17.131 | 0.030 | Associated to colitis in mice, exacerbates intestinal inflammation [36]. |
| f__Prevotellaceae | −5.163 | 0.034 | Associated to colitis in mice, exacerbates intestinal inflammation [36]. |
| g__Prevotella | −7.316 | 0.036 | Associated to colitis in mice, exacerbates intestinal inflammation [36]. |
| f__Barnesiellaceae | −3.292 | 0.037 | Commensals reduced Treg cells in the tumor microenvironment (Foxp3 and/or γδT17 cells) [35]. |
| g__Catonella | −4.687 | 0.047 | Reside in the oral mucosa as commensals but may be opportunistic pathogens with potential correlations with oral squamous cell carcinoma (OSCC) [37]. |
| s__Clostridium_sp._AT4 | −4.576 | 0.047 | An increased abundance of Clostridiaceae was shared by both inflammatory bowel disease (IBD)-A and rheumatoid arthritis (RA) patients [38,39]. |
| g__Ruminiclostridium | −3.925 | 0.048 | Consistently present in the healthy human gut [28]. |
| s__Barnesiella_intestinihominis | −3.582 | 0.050 | Commensals reduced Treg cells in the tumor microenvironment (Foxp3 and/or γδT17 cells) [35]. |
| Taxonomy 1 | Log2 (Fold-Change) | Q Value | Functional Connotations |
|---|---|---|---|
| f_Acidaminococcaceae | −1.267 | 0.018 | Found to be higher in disease-related groups [40]. |
| s_Streptococcus_thermophilus | 0.643 | 0.020 | Anti-inflammatory potential in colitis [41]. |
| o_Acidaminococcales | −1.267 | 0.027 | Found to be higher in disease-related groups [40]. |
| s_Clostridium_sp._AT4 | −1.188 | 0.039 | Found to be higher in IBD [42]. |
| g__Acidaminococcus | −1.139 | 0.048 | Found to be higher in disease-related groups [40]. |
| Enzymes | Log2 (Fold-Change) | Q Value |
|---|---|---|
| 3.5.3.1 E3.5.3.1, rocF, arg; arginase | 1.031 | 0.011 |
| 3.1.1.17 E3.1.1.17, gnl, RGN; gluconolactonase | 0.845 | 0.011 |
| 2.7.13.3 cqsS; two-component system, CAI-1 autoinducer sensor kinase/phosphatase CqsS | 0.683 | 0.011 |
| 5.1.3.20 gmhD, rfaD; ADP-L-glycero-D-manno-heptose 6-epimerase | −0.867 | 0.022 |
| 3.4.21.50 E3.4.21.50; lysyl endopeptidase | 0.723 | 0.022 |
| 3.5.1.87 pydC; beta-ureidopropionase/N-carbamoyl-L-amino-acid hydrolase | −1.164 | 0.037 |
| 2.4.1.317 tylN; O-mycaminosyltylonolide 6-deoxyallosyltransferase | 0.950 | 0.038 |
| 2.4.2.44 mtiP; 5′-methylthioinosine phosphorylase | −1.080 | 0.039 |
| 3.5.1.6 pydC; beta-ureidopropionase/N-carbamoyl-L-amino-acid hydrolase | −1.164 | 0.041 |
| 1.7.1.7 E1.7.1.7, guaC; GMP reductase | −0.715 | 0.041 |
| 3.5.4.16 folE2; GTP cyclohydrolase IB | −0.735 | 0.048 |
| Pathways | Log2 (Fold-Change) | Q Value |
|---|---|---|
| 1. Oxidoreductases; 1.14 Acting on paired donors, with O2 as oxidant and incorporation or reduction of oxygen. The oxygen incorporated need not be derived from O2; 1.14.19 With oxidation of a pair of donors resulting in the reduction of O2 to two molecules of water | 0.897 | 0.044 |
| NarL family; RcsC-RcsD-RcsB | 1.165 | 0.048 |
| 2. Transferases; 2.7 Transferring phosphorus-containing groups; 2.7.9 Phosphotransferases with paired acceptors | −1.215 | 0.053 |
| 5. Isomerases; 5.3 Intramolecular oxidoreductases; 5.3.4 Transposing S-S bonds | −2.455 | 0.055 |
| Non-ion channels; Aquaglyceroporins or glycerol-uptake facilitators | 1.003 | 0.055 |
| ABC Transporters, Prokaryotic Type; Mineral and organic ion transporters; Putrescine transporter [MD:M00300] | 0.958 | 0.056 |
| 5. Isomerases; 5.3 Intramolecular oxidoreductases; 5.3.99 Other intramolecular oxidoreductases | 1.199 | 0.057 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pezzuto, J.M.; Dave, A.; Park, E.-J.; Beyoğlu, D.; Idle, J.R. Short-Term Grape Consumption Diminishes UV-Induced Skin Erythema. Antioxidants 2022, 11, 2372. https://doi.org/10.3390/antiox11122372
Pezzuto JM, Dave A, Park E-J, Beyoğlu D, Idle JR. Short-Term Grape Consumption Diminishes UV-Induced Skin Erythema. Antioxidants. 2022; 11(12):2372. https://doi.org/10.3390/antiox11122372
Chicago/Turabian StylePezzuto, John M., Asim Dave, Eun-Jung Park, Diren Beyoğlu, and Jeffrey R. Idle. 2022. "Short-Term Grape Consumption Diminishes UV-Induced Skin Erythema" Antioxidants 11, no. 12: 2372. https://doi.org/10.3390/antiox11122372
APA StylePezzuto, J. M., Dave, A., Park, E.-J., Beyoğlu, D., & Idle, J. R. (2022). Short-Term Grape Consumption Diminishes UV-Induced Skin Erythema. Antioxidants, 11(12), 2372. https://doi.org/10.3390/antiox11122372








