Inhibition of NADPH Oxidases Prevents the Development of Osteoarthritis
Abstract
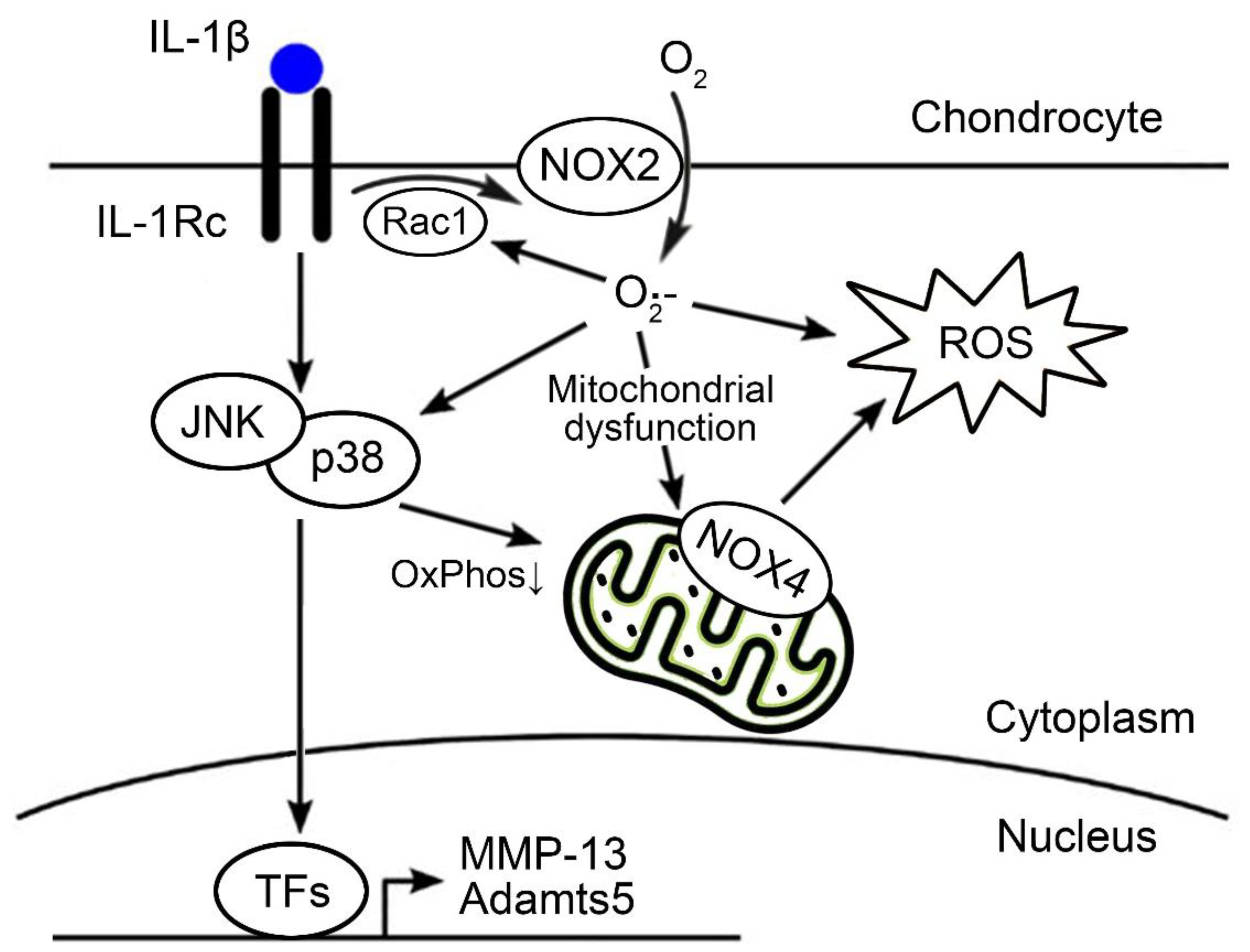
1. Introduction
2. Materials and Methods
2.1. Animal Experiments and Ethics
2.2. Primary Chondrocyte Cultures
2.3. Detection of Intracellular ROS and Oxidative DNA Damage
2.4. Quantitative Real-Time RT-PCR
2.5. Western Blot Analysis
2.6. Safranin-O Staining, Immunofluorescence, and Immunohistochemistry of Mouse Knee Joint
2.7. Extracellular Flux Analysis
2.8. Statistical Analysis
3. Results
3.1. NOX System Was Critically Involved in the IL-1β-Induced ROS Production and Oxidative DNA Damage in Chondrocytes
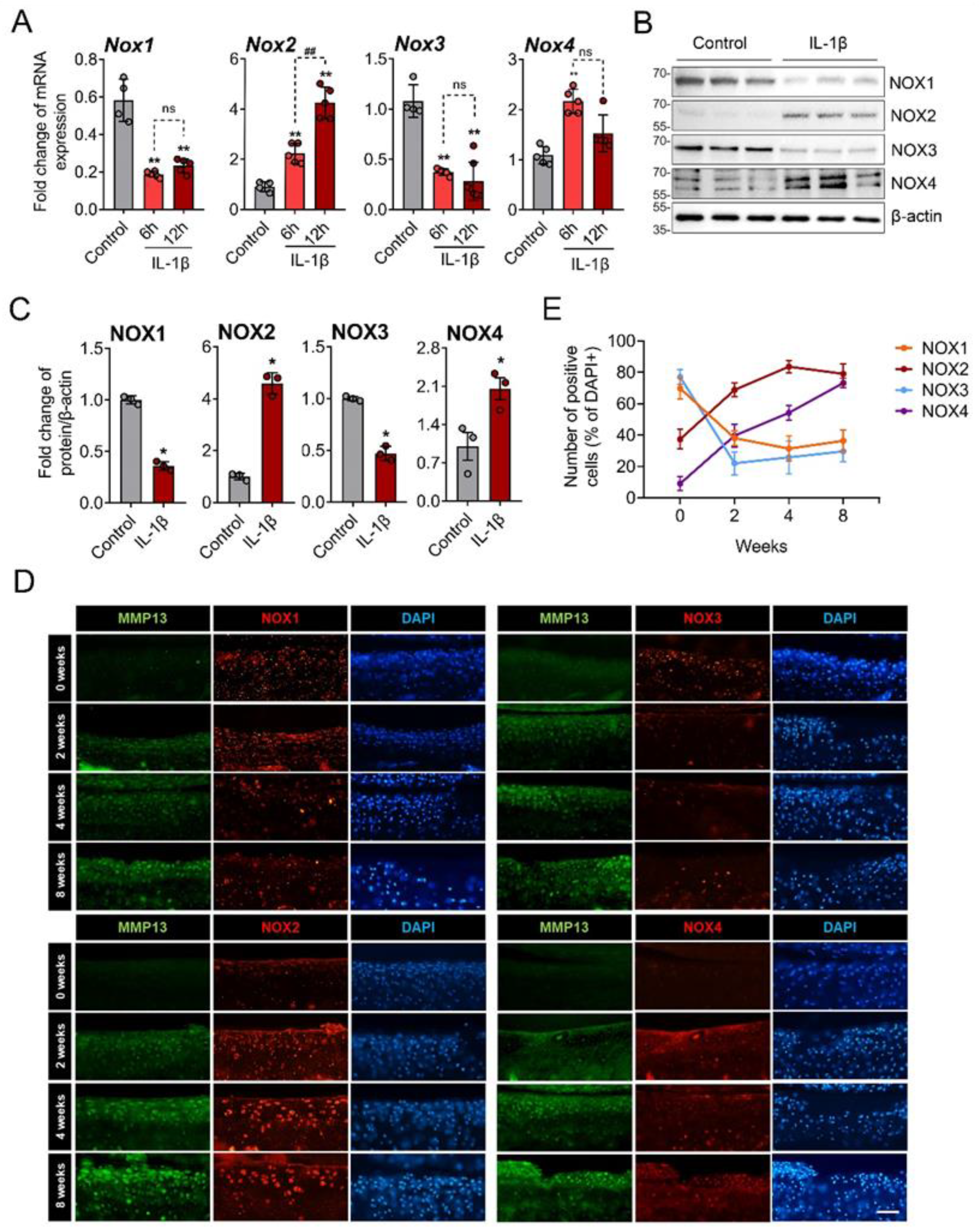
3.2. NOX2 and NOX4 Was Upregulated in IL-1β-Treated and OA Chondrocytes
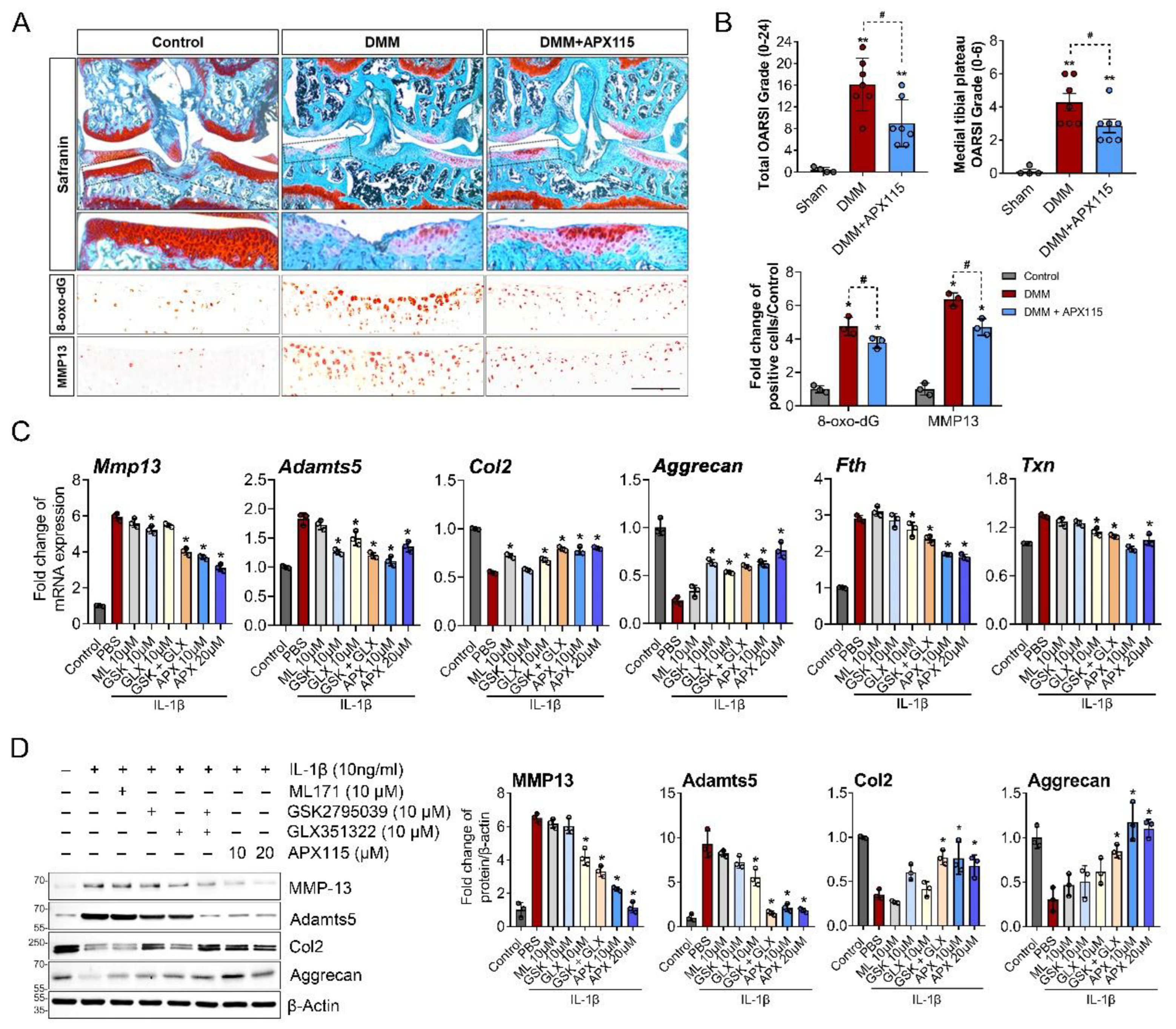
3.3. NOX Inhibition Attenuated OA Severity by Modulating Oxidative Damage and Expression of MMP-13 and Adamts5 in Chondrocytes
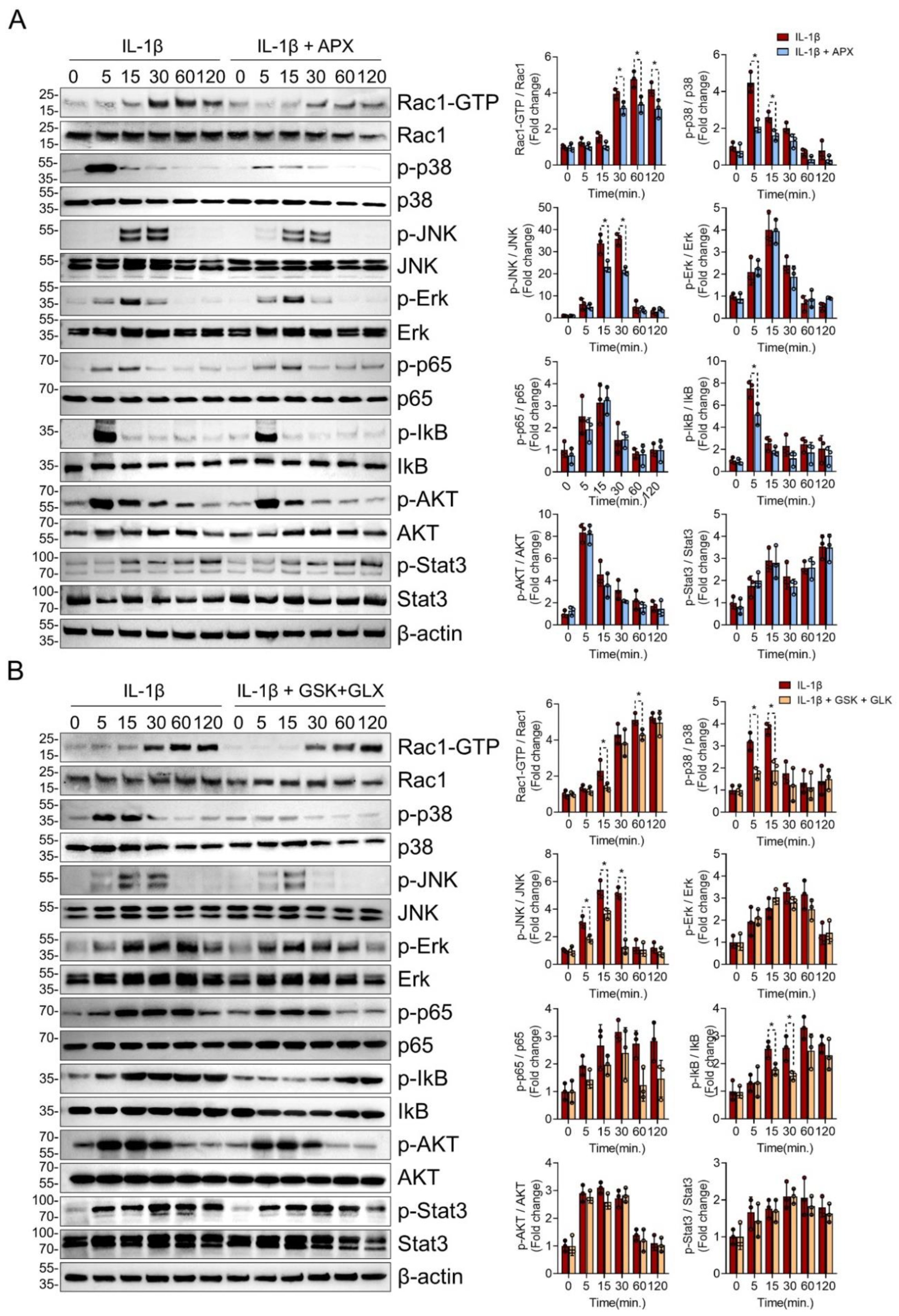
3.4. NOX Inhibition Consistently Suppressed Rac1, p38, and JNK MAPK Signaling in IL-1β-Treated Chondrocytes
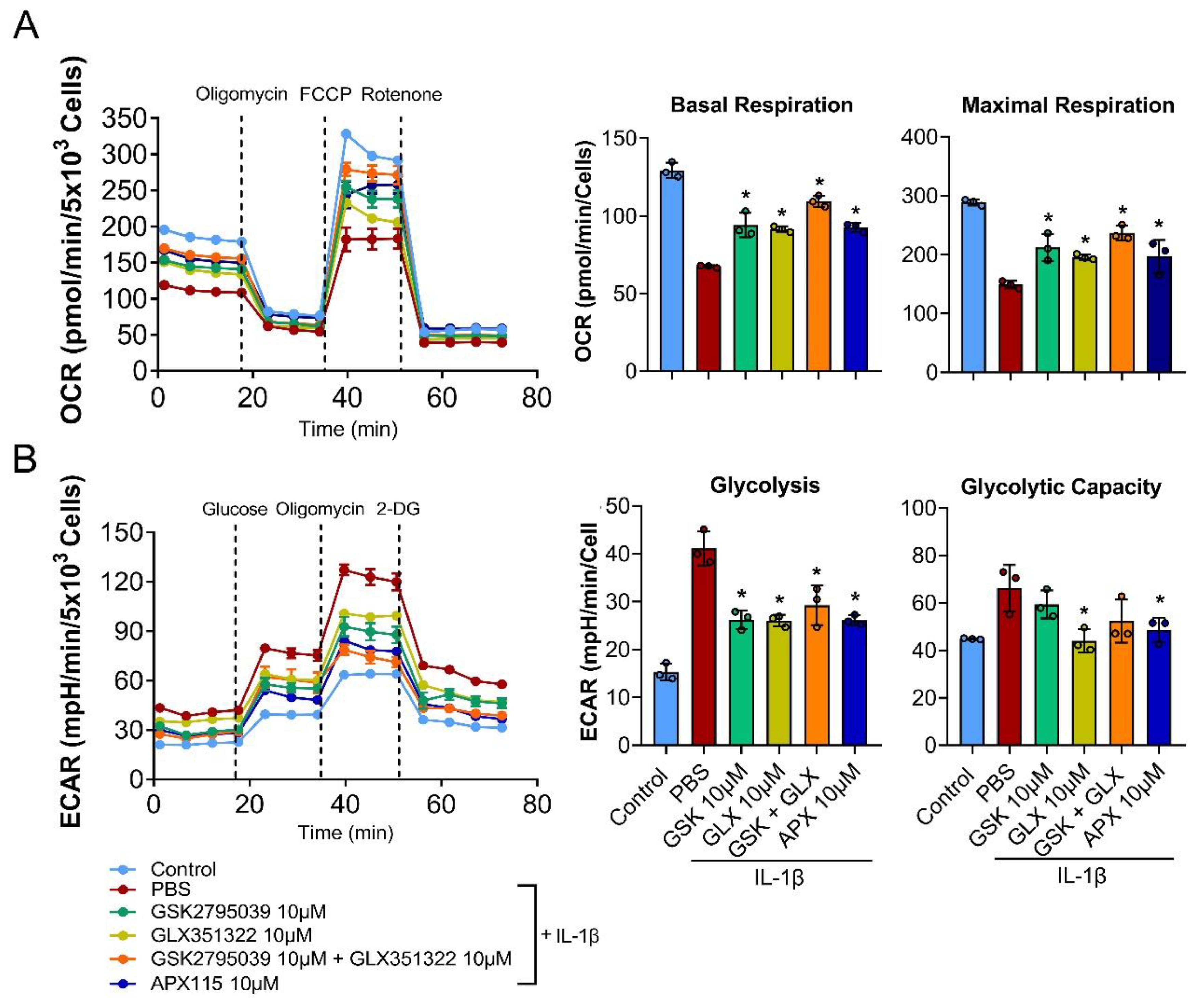
3.5. NOX Inhibition Restored Mitochondrial Respiration in IL-1β-Treated Chondrocytes
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Henrotin, Y.E.; Bruckner, P.; Pujol, J.-P. The role of reactive oxygen species in homeostasis and degradation of cartilage. Osteoarthr. Cartil. 2003, 11, 747–755. [Google Scholar] [CrossRef] [PubMed]
- Reed, K.N.; Wilson, G.; Pearsall, A.; Grishko, V.I. The role of mitochondrial reactive oxygen species in cartilage matrix destruction. Mol. Cell. Biochem. 2014, 397, 195–201. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.-S.; Hwang, C.Y.; Shin, S.-Y.; Kwon, K.-S.; Cho, K.-H. MLK3 Is Part of a Feedback Mechanism That Regulates Different Cellular Responses to Reactive Oxygen Species. Sci. Signal. 2014, 7, ra52. [Google Scholar] [CrossRef] [PubMed]
- Truong, T.H.; Carroll, K.S. Redox regulation of protein kinases. Crit. Rev. Biochem. Mol. Biol. 2013, 48, 332–356. [Google Scholar] [CrossRef]
- Hui, W.; Young, D.A.; Rowan, A.D.; Xu, X.; Cawston, T.E.; Proctor, C.J. Oxidative changes and signalling pathways are pivotal in initiating age-related changes in articular cartilage. Ann. Rheum. Dis. 2014, 75, 449–458. [Google Scholar] [CrossRef]
- Tang, Q.; Zheng, G.; Feng, Z.; Chen, Y.; Lou, Y.; Wang, C.; Zhang, X.; Zhang, Y.; Xu, H.; Shang, P.; et al. Trehalose ameliorates oxidative stress-mediated mitochondrial dysfunction and ER stress via selective autophagy stimulation and autophagic flux restoration in osteoarthritis development. Cell Death Dis. 2017, 8, e3081. [Google Scholar] [CrossRef]
- Balaban, R.S.; Nemoto, S.; Finkel, T. Mitochondria, oxidants, and aging. Cell 2005, 120, 483–495. [Google Scholar] [CrossRef]
- Cao, Y.; Zhang, X.; Shang, W.; Xu, J.; Wang, X.; Hu, X.; Ao, Y.; Cheng, H. Proinflammatory Cytokines Stimulate Mitochondrial Superoxide Flashes in Articular Chondrocytes In Vitro and In Situ. PLoS ONE 2013, 8, e66444. [Google Scholar] [CrossRef]
- Forrester, S.J.; Kikuchi, D.S.; Hernandes, M.S.; Xu, Q.; Griendling, K.K. Reactive Oxygen Species in Metabolic and Inflammatory Signaling. Circ. Res. 2018, 122, 877–902. [Google Scholar] [CrossRef]
- Vermot, A.; Petit-Härtlein, I.; Smith, S.; Fieschi, F. NADPH Oxidases (NOX): An Overview from Discovery, Molecular Mechanisms to Physiology and Pathology. Antioxidants 2021, 10, 890. [Google Scholar] [CrossRef]
- Belambri, S.A.; Rolas, L.; Raad, H.; Hurtado-Nedelec, M.; Dang, P.M.-C.; El-Benna, J. NADPH oxidase activation in neutrophils: Role of the phosphorylation of its subunits. Eur. J. Clin. Investig. 2018, 48, e12951. [Google Scholar] [CrossRef] [PubMed]
- Rousset, F.; Nguyen, M.V.C.; Grange, L.; Morel, F.; Lardy, B. Heme Oxygenase-1 Regulates Matrix Metalloproteinase MMP-1 Secretion and Chondrocyte Cell Death via Nox4 NADPH Oxidase Activity in Chondrocytes. PLoS ONE 2013, 8, e66478. [Google Scholar] [CrossRef]
- Kim, K.S.; Choi, H.W.; Yoon, H.E.; Kim, I.Y. Reactive Oxygen Species Generated by NADPH Oxidase 2 and 4 Are Required for Chondrogenic Differentiation. J. Biol. Chem. 2010, 285, 40294–40302. [Google Scholar] [CrossRef] [PubMed]
- Rousset, F.; Hazane-Puch, F.; Pinosa, C.; Nguyen, M.; Grange, L.; Soldini, A.; Rubens-Duval, B.; Dupuy, C.; Morel, F.; Lardy, B. IL-1beta mediates MMP secretion and IL-1beta neosynthesis via upregulation of p22phox and NOX4 activity in human articular chondrocytes. Osteoarthr. Cartil. 2015, 23, 1972–1980. [Google Scholar] [CrossRef] [PubMed]
- Jung, Y.-K.; Park, H.-R.; Cho, H.-J.; Jang, J.-A.; Lee, E.-J.; Han, M.-S.; Kim, G.-W.; Han, S. Degrading products of chondroitin sulfate can induce hypertrophy-like changes and MMP-13/ADAMTS5 production in chondrocytes. Sci. Rep. 2019, 9, 15846. [Google Scholar] [CrossRef]
- Cha, J.J.; Min, H.S.; Kim, K.T.; Kim, J.E.; Ghee, J.Y.; Kim, H.W.; Lee, J.E.; Han, J.-Y.; Lee, G.; Ha, H.; et al. APX-115, a first-in-class pan-NADPH oxidase (Nox) inhibitor, protects db/db mice from renal injury. Lab. Investig. 2017, 97, 419–431. [Google Scholar] [CrossRef]
- Al Shoyaib, A.; Archie, S.R.; Karamyan, V.T. Intraperitoneal Route of Drug Administration: Should it Be Used in Experimental Animal Studies? Pharm. Res. 2019, 37, 12. [Google Scholar] [CrossRef]
- Du Sert, N.P.; Hurst, V.; Ahluwalia, A.; Alam, S.; Avey, M.T.; Baker, M.; Browne, W.J.; Clark, A.; Cuthill, I.C.; Dirnagl, U.; et al. The ARRIVE guidelines 2.0: Updated guidelines for reporting animal research. PLoS Biol. 2020, 18, e3000410. [Google Scholar] [CrossRef]
- Coşkun, S.; Chao, H.; Vasavada, H.; Heydari, K.; Gonzales, N.; Zhou, X.; de Crombrugghe, B.; Hirschi, K.K. Development of the Fetal Bone Marrow Niche and Regulation of HSC Quiescence and Homing Ability by Emerging Osteolineage Cells. Cell Rep. 2014, 9, 581–590. [Google Scholar] [CrossRef]
- Gosset, M.; Berenbaum, F.; Thirion, S.; Jacques, C. Primary culture and phenotyping of murine chondrocytes. Nat. Protoc. 2008, 3, 1253–1260. [Google Scholar] [CrossRef]
- Glasson, S.S.; Chambers, M.G.; Van Den Berg, W.B.; Little, C.B. The OARSI histopathology initiative—Recommendations for histological assessments of osteoarthritis in the mouse. Osteoarthr. Cartil. 2010, 18 (Suppl. 3), S17–S23. [Google Scholar] [CrossRef] [PubMed]
- Siraj, M.A.; Mundil, D.; Beca, S.; Momen, A.; Shikatani, E.A.; Afroze, T.; Sun, X.; Liu, Y.; Ghaffari, S.; Lee, W.; et al. Cardioprotective GLP-1 metabolite prevents ischemic cardiac injury by inhibiting mitochondrial trifunctional protein-α. J. Clin. Investig. 2020, 130, 1392–1404. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wong, H.S. Are mitochondria the main contributor of reactive oxygen species in cells? J. Exp. Biol. 2021, 224. [Google Scholar] [CrossRef]
- Hordijk, P.L. Regulation of NADPH Oxidases: The role of Rac proteins. Circ. Res. 2006, 98, 453–462. [Google Scholar] [CrossRef] [PubMed]
- Gu, Y.; Jasti, A.C.; Jansen, M.; Siefring, J.E. RhoH, a hematopoietic-specific Rho GTPase, regulates proliferation, survival, migration, and engraftment of hematopoietic progenitor cells. Blood 2005, 105, 1467–1475. [Google Scholar] [CrossRef]
- Ahmed, S.; Prigmore, E.; Govind, S.; Veryard, C.; Kozma, R.; Wientjes, F.B.; Segal, A.W.; Lim, L. Cryptic Rac-binding and p21 -activated Kinase Phosphorylation Sites of NADPH Oxidase Component p67. J. Biol. Chem. 1998, 273, 15693–15701. [Google Scholar] [CrossRef]
- Matsushita, M.; Nakamura, T.; Moriizumi, H.; Miki, H.; Takekawa, M. Stress-responsive MTK1 SAPKKK serves as a redox sensor that mediates delayed and sustained activation of SAPKs by oxidative stress. Sci. Adv. 2020, 6, eaay9778. [Google Scholar] [CrossRef]
- Sakauchi, C.; Wakatsuki, H.; Ichijo, H.; Hattori, K. Pleiotropic properties of ASK1. Biochim. Biophys. Acta-Gen. Subj. 2016, 1861, 3030–3038. [Google Scholar] [CrossRef]
- Li, Z.; Dai, A.; Yang, M.; Chen, S.; Deng, Z.; Li, L. p38MAPK Signaling Pathway in Osteoarthritis: Pathological and Therapeutic Aspects. J. Inflamm. Res. 2022, 15, 723–734. [Google Scholar] [CrossRef]
- Ni, S.; Li, D.; Wei, H.; Miao, K.-S.; Zhuang, C. PPARγ Attenuates Interleukin-1β-Induced Cell Apoptosis by Inhibiting NOX2/ROS/p38MAPK Activation in Osteoarthritis Chondrocytes. Oxidative Med. Cell. Longev. 2021, 2021, 5551338. [Google Scholar] [CrossRef]
- Clavijo-Cornejo, D.; Martínez-Flores, K.; Silva-Luna, K.; Martínez-Nava, G.A.; Fernández-Torres, J.; Zamudio-Cuevas, Y.; Santamaría-Olmedo, M.G.; Granados-Montiel, J.; Pineda, C.; López-Reyes, A. The Overexpression of NALP3 Inflammasome in Knee Osteoarthritis Is Associated with Synovial Membrane Prolidase and NADPH Oxidase 2. Oxidative Med. Cell. Longev. 2016, 2016, 1472567. [Google Scholar] [CrossRef] [PubMed]
- Kruisbergen, N.N.L.; Di Ceglie, I.; van Gemert, Y.; Walgreen, B.; Helsen, M.M.A.; Slöetjes, A.W.; Koenders, M.I.; van de Loo, F.A.J.; Roth, J.; Vogl, T.; et al. Nox2 Deficiency Reduces Cartilage Damage and Ectopic Bone Formation in an Experimental Model for Osteoarthritis. Antioxidants 2021, 10, 1660. [Google Scholar] [CrossRef] [PubMed]
- Shin, H.J.; Park, H.; Shin, N.; Kwon, H.H.; Yin, Y.; Hwang, J.-A.; Kim, S.I.; Kim, S.R.; Kim, S.; Joo, Y.; et al. p47phox siRNA-Loaded PLGA Nanoparticles Suppress ROS/Oxidative Stress-Induced Chondrocyte Damage in Osteoarthritis. Polymers 2020, 12, 443. [Google Scholar] [CrossRef] [PubMed]
- Wegner, A.M.; Campos, N.R.; Robbins, M.A.; Haddad, A.F.; Cunningham, H.C.; Yik, J.H.N.; Christiansen, B.A.; Haudenschild, D.R. Acute Changes in NADPH Oxidase 4 in Early Post-Traumatic Osteoarthritis. J. Orthop. Res. 2019, 37, 2429–2436. [Google Scholar] [CrossRef] [PubMed]
- Liao, C.-R.; Wang, S.-N.; Zhu, S.-Y.; Wang, Y.-Q.; Li, Z.-Z.; Liu, Z.-Y.; Jiang, W.-S.; Chen, J.-T.; Wu, Q. Advanced oxidation protein products increase TNF-α and IL-1β expression in chondrocytes via NADPH oxidase 4 and accelerate cartilage degeneration in osteoarthritis progression. Redox Biol. 2019, 28, 101306. [Google Scholar] [CrossRef] [PubMed]
- Grange, L.; Nguyen, M.V.C.; Lardy, B.; Derouazi, M.; Campion, Y.; Trocme, C.; Paclet, M.; Gaudin, P.; Morel, F. NAD(P)H Oxidase Activity of Nox4 in Chondrocytes Is Both Inducible and Involved in Collagenase Expression. Antioxid. Redox Signal. 2006, 8, 1485–1496. [Google Scholar] [CrossRef]
- Morita, K.; Miyamoto, T.; Fujita, N.; Kubota, Y.; Ito, K.; Takubo, K.; Miyamoto, K.; Ninomiya, K.; Suzuki, T.; Iwasaki, R.; et al. Reactive oxygen species induce chondrocyte hypertrophy in endochondral ossification. J. Exp. Med. 2007, 204, 1613–1623. [Google Scholar] [CrossRef]
- Molavian, H.R.; Kohandel, M.; Sivaloganathan, S. High Concentrations of H2O2 Make Aerobic Glycolysis Energetically More Favorable for Cellular Respiration. Front. Physiol. 2016, 7, 362. [Google Scholar] [CrossRef]
- Trempolec, N.; Muñoz, J.P.; Slobodnyuk, K.; Marin, S.; Cascante, M.; Zorzano, A.; Nebreda, A.R. Induction of oxidative metabolism by the p38α/MK2 pathway. Sci. Rep. 2017, 7, 11367. [Google Scholar] [CrossRef]
- Papa, S.; Choy, P.M.; Bubici, C. The ERK and JNK pathways in the regulation of metabolic reprogramming. Oncogene 2018, 38, 2223–2240. [Google Scholar] [CrossRef]
- Gavriilidis, C.; Miwa, S.; von Zglinicki, T.; Taylor, R.W.; Young, D.A. Mitochondrial dysfunction in osteoarthritis is associated with down-regulation of superoxide dismutase 2. Arthritis Care Res. 2012, 65, 378–387. [Google Scholar] [CrossRef] [PubMed]
- Mobasheri, A.; Rayman, M.P.; Gualillo, O.; Sellam, J.; van der Kraan, P.; Fearon, U. The role of metabolism in the pathogenesis of osteoarthritis. Nat. Rev. Rheumatol. 2017, 13, 302–311. [Google Scholar] [CrossRef] [PubMed]
- Arra, M.; Swarnkar, G.; Ke, K.; Otero, J.E.; Ying, J.; Duan, X.; Maruyama, T.; Rai, M.F.; O’Keefe, R.J.; Mbalaviele, G.; et al. LDHA-mediated ROS generation in chondrocytes is a potential therapeutic target for osteoarthritis. Nat. Commun. 2020, 11, 3427. [Google Scholar] [CrossRef] [PubMed]
- Wall, J.A.; Wei, J.; Ly, M.; Belmont, P.; Martindale, J.J.; Tran, D.; Sun, J.; Chen, W.J.; Yu, W.; Oeller, P.; et al. Alterations in oxidative phosphorylation complex proteins in the hearts of transgenic mice that overexpress the p38 MAP kinase activator, MAP kinase kinase 6. Am. J. Physiol. Circ. Physiol. 2006, 291, H2462–H2472. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Han, J.; Park, D.; Park, J.Y.; Han, S. Inhibition of NADPH Oxidases Prevents the Development of Osteoarthritis. Antioxidants 2022, 11, 2346. https://doi.org/10.3390/antiox11122346
Han J, Park D, Park JY, Han S. Inhibition of NADPH Oxidases Prevents the Development of Osteoarthritis. Antioxidants. 2022; 11(12):2346. https://doi.org/10.3390/antiox11122346
Chicago/Turabian StyleHan, Jin, Donghwi Park, Ji Young Park, and Seungwoo Han. 2022. "Inhibition of NADPH Oxidases Prevents the Development of Osteoarthritis" Antioxidants 11, no. 12: 2346. https://doi.org/10.3390/antiox11122346
APA StyleHan, J., Park, D., Park, J. Y., & Han, S. (2022). Inhibition of NADPH Oxidases Prevents the Development of Osteoarthritis. Antioxidants, 11(12), 2346. https://doi.org/10.3390/antiox11122346




