Mycosynthesis of Silica Nanoparticles Using Aspergillus niger: Control of Alternaria solani Causing Early Blight Disease, Induction of Innate Immunity and Reducing of Oxidative Stress in Eggplant
Abstract
1. Introduction
2. Experimental Procedure
2.1. Chemical Used
2.2. Fungi Mediated Biosynthesis of SiO2-NPs
2.3. Characterization of Biosynthesized Silica Nanoparticles
2.4. Source of the Alternaria solani
2.5. In Vitro Antifungal Activity
2.6. In Vivo Experiment
2.7. Measurement of Growth Traits
2.8. Measurement of Photosynthetic Pigments
2.9. Determination of the Content of Proline
2.10. Determination of Total Phenol Contents
2.11. Antioxidant Enzyme Activity Assay
2.12. Statistical Analysis
3. Results and Discussion
3.1. Biosynthesis of SiO2-NPs Using Biomass Filtrate of A. niger
3.2. Characterization of Biosynthesized SiO2-NPs
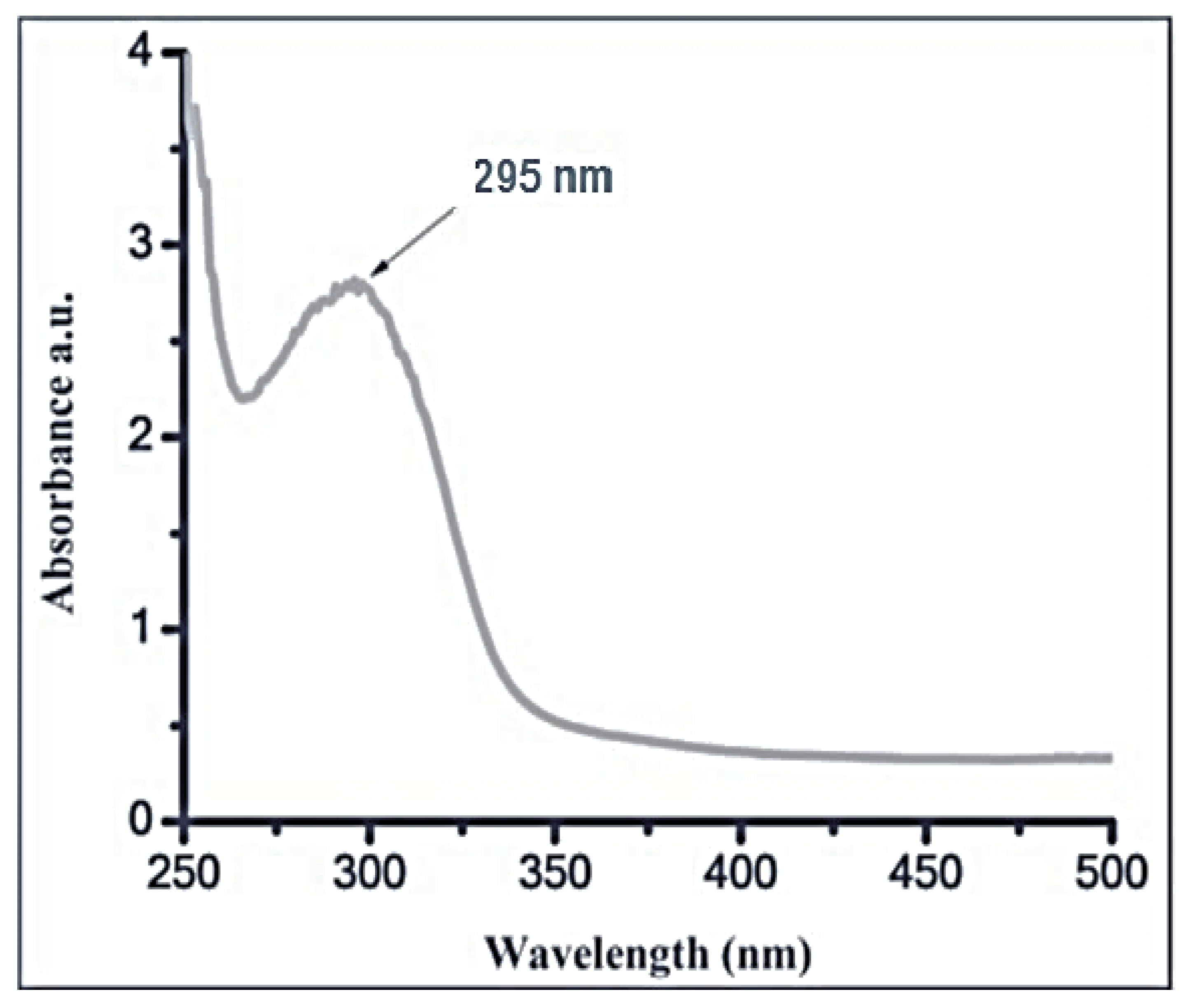
3.2.1. UV-Vis Spectroscopy
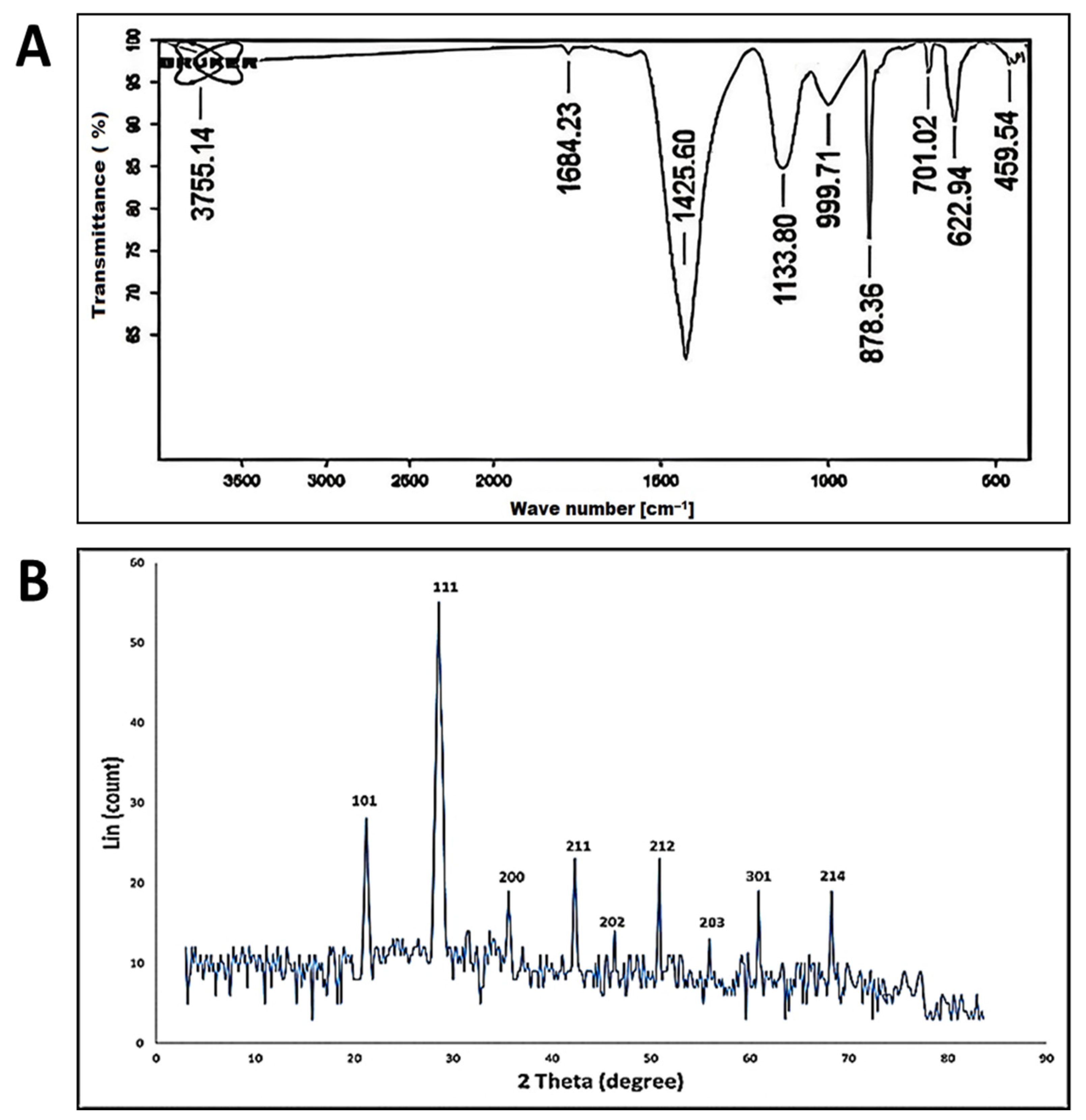
3.2.2. Fourier Transform Infrared (FT-IR) Spectroscopy
3.2.3. X-ray Diffraction (XRD) Pattern
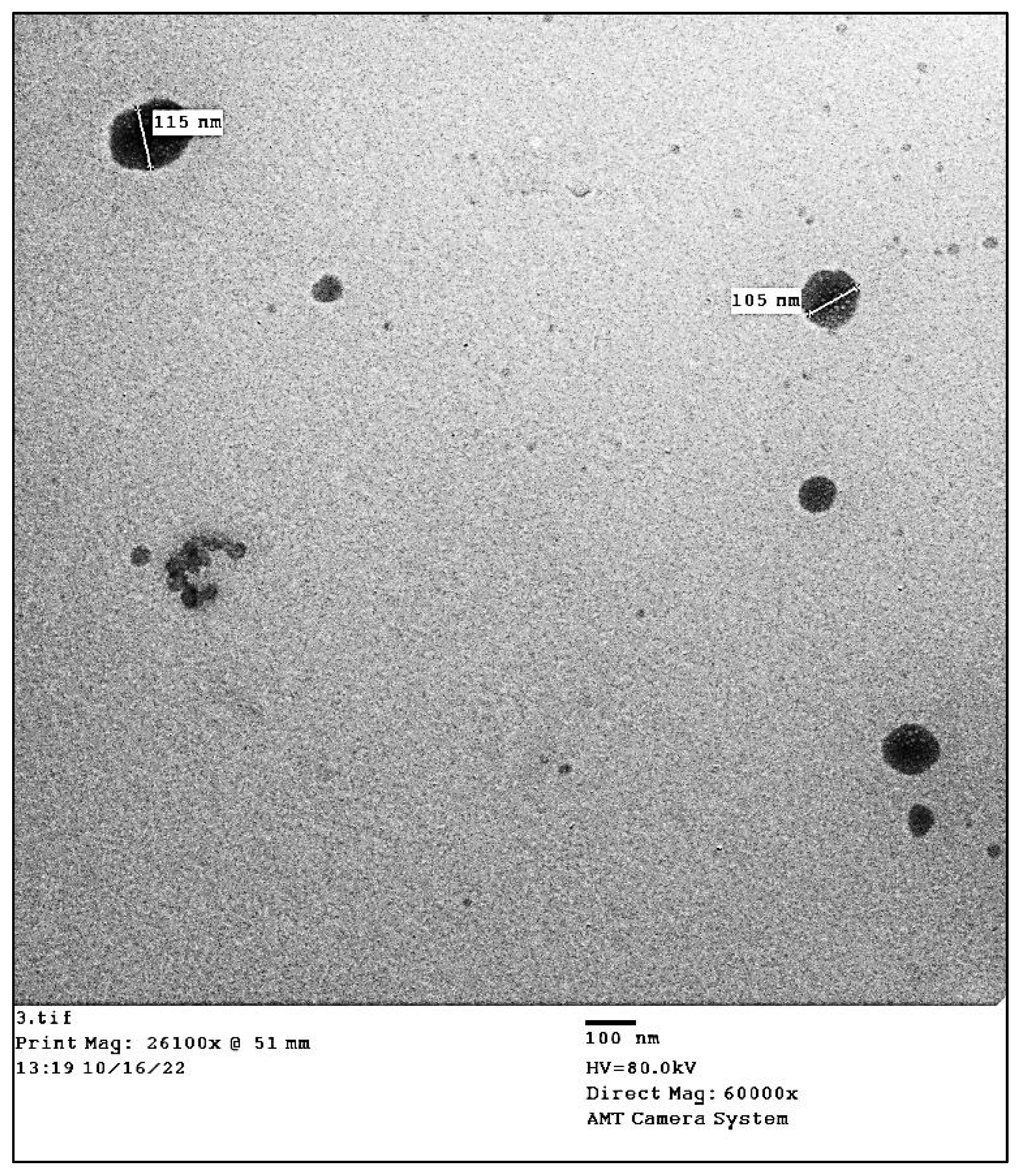
3.2.4. Transmission Electron Microscopy (TEM)
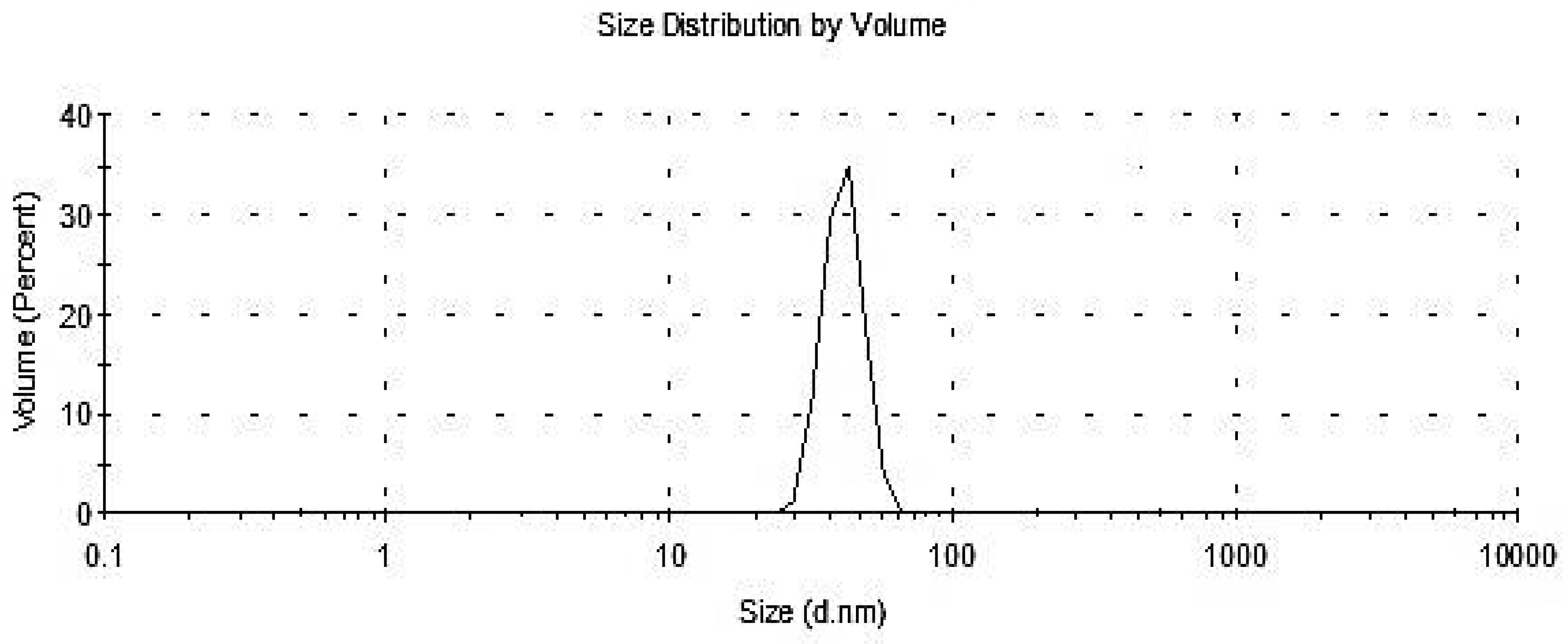
3.2.5. Particle Size Distribution DLS
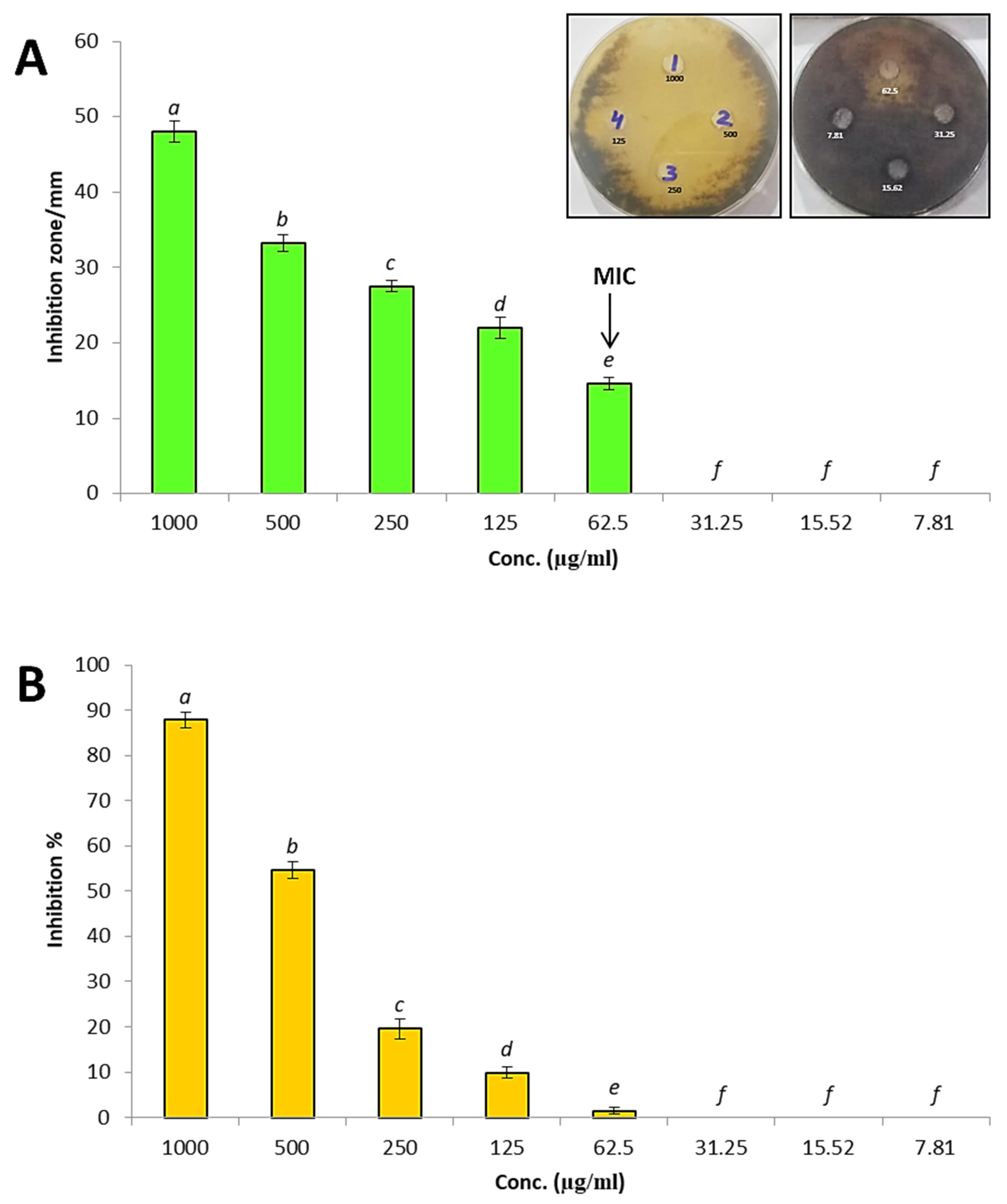
3.3. In Vitro Antifungal Activity of Silica Nanoparticles against A. solani
3.4. Disease Index
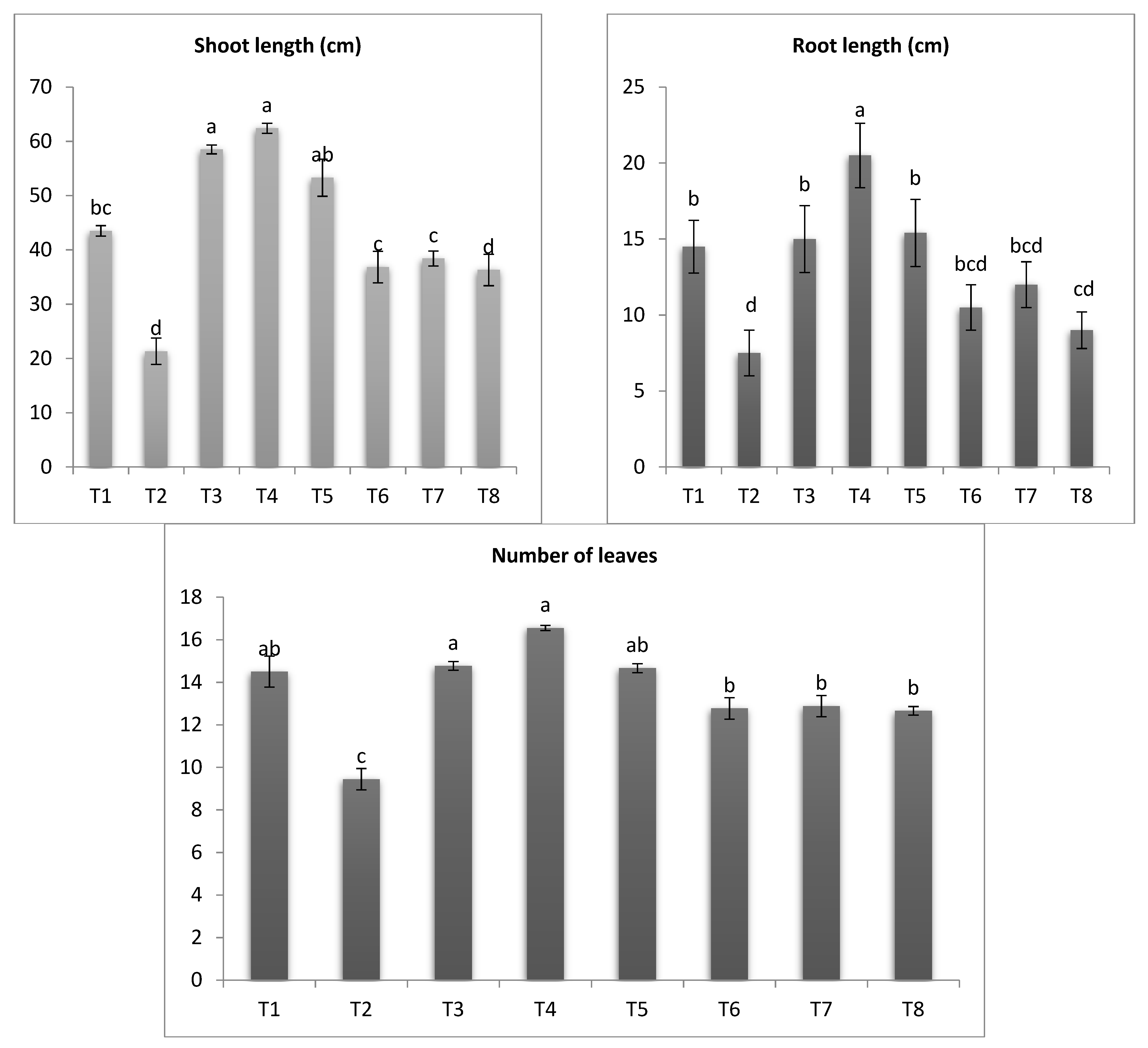
3.5. Measurement of Growth Traits
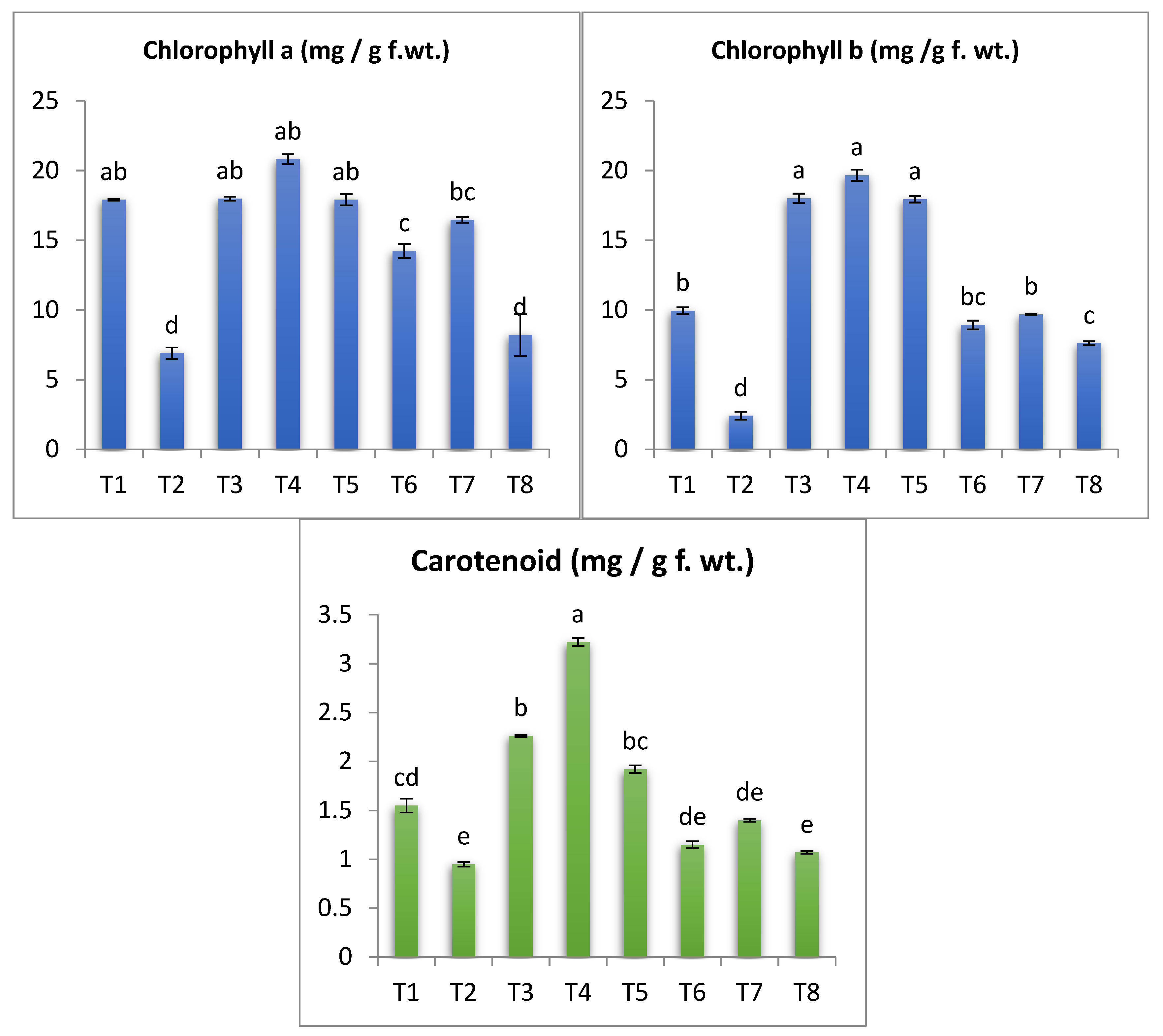
3.6. Photosynthetic Pigments
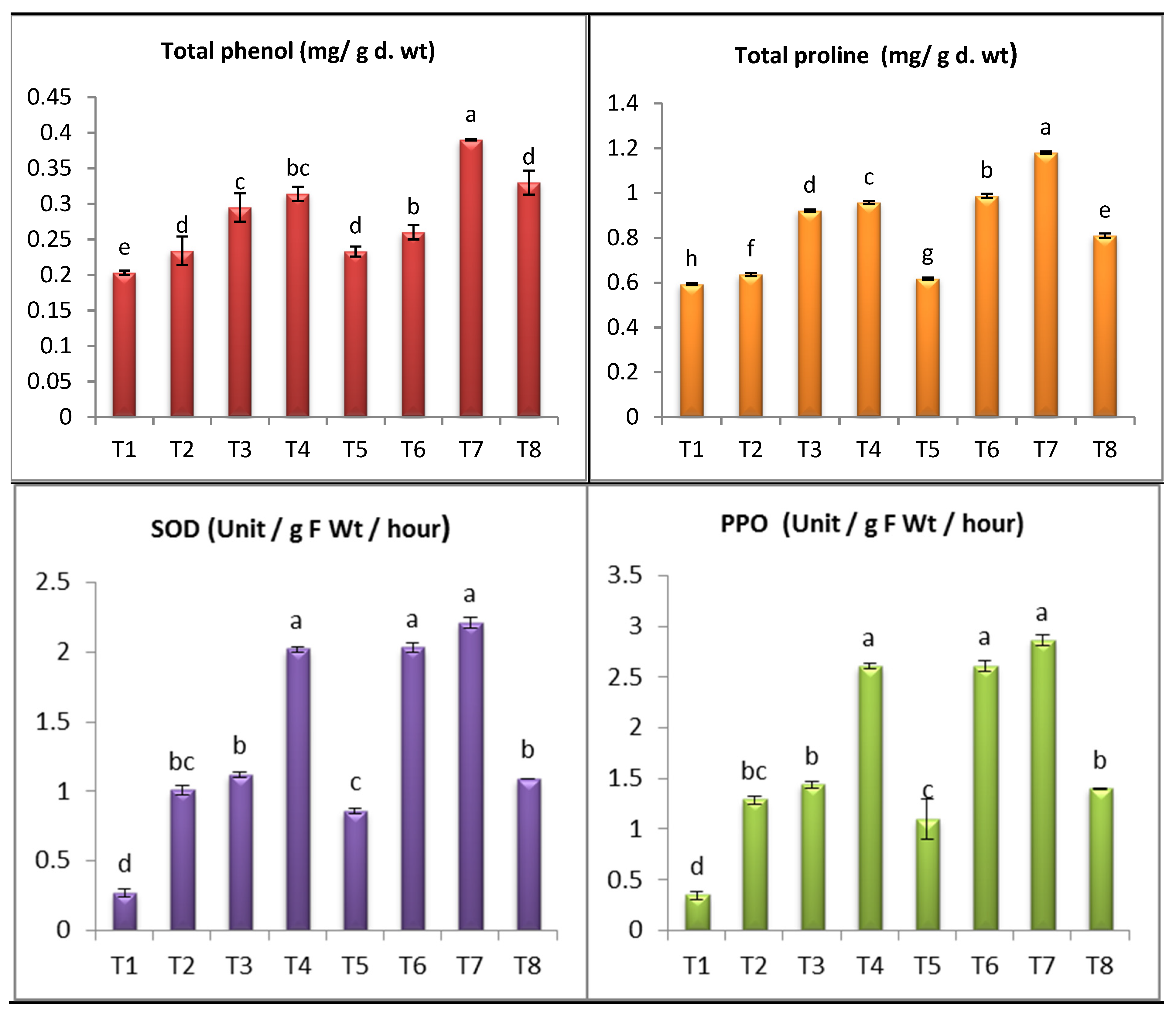
4. Oxidative Stress Markers
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Abdelaziz, A.M.; Salem, S.S.; Khalil, A.; El-Wakil, D.A.; Fouda, H.M.; Hashem, A.H. Potential of biosynthesized zinc oxide nanoparticles to control Fusarium wilt disease in eggplant (Solanum melongena) and promote plant growth. BioMetals 2022, 35, 601–616. [Google Scholar] [CrossRef]
- Attia, M.S.; El-Sayyad, G.S.; Abd Elkodous, M.; Khalil, W.F.; Nofel, M.M.; Abdelaziz, A.M.; Farghali, A.A.; El-Batal, A.I.; El Rouby, W.M. Chitosan and EDTA conjugated graphene oxide antinematodes in Eggplant: Toward improving plant immune response. Int. J. Biol. Macromol. 2021, 179, 333–344. [Google Scholar] [CrossRef]
- Khalil, A.M.; Ahmed, A.F.; Mahmoud, E.E.; Abdelaziz, A.M. Influence of organic farming system on microbial biomass and fungal communities of agricultural soil. Afr. J. Mycol. Biotechnol. 2015, 20, 23–40. [Google Scholar]
- Attia, M.S.; Abdelaziz, A.M.; Al-Askar, A.A.; Arishi, A.A.; Abdelhakim, A.M.; Hashem, A.H. Plant growth-promoting fungi as biocontrol tool against fusarium wilt disease of tomato plant. J. Fungi 2022, 8, 775. [Google Scholar] [CrossRef]
- Abd Alhakim, A.; Hashem, A.; Abdelaziz, A.M.; Attia, M.S. Impact of plant growth promoting fungi on biochemical defense performance of Tomato under Fusarial infection. Egypt. J. Chem. 2022. [Google Scholar] [CrossRef]
- Attia, M.S.; Hashem, A.H.; Badawy, A.A.; Abdelaziz, A.M. Biocontrol of early blight disease of eggplant using endophytic Aspergillus terreus: Improving plant immunological, physiological and antifungal activities. Bot. Stud. 2022, 63, 26. [Google Scholar] [CrossRef] [PubMed]
- Tsedaley, B. Review on early blight (Alternaria spp.) of potato disease and its management options. J. Biol. Agric. Healthc. 2014, 4, 191–199. [Google Scholar]
- Gupta, S.K.; Thind, T. Disease Problems in Vegetable Production; Scientific Publishers: Singapore, 2018. [Google Scholar]
- Opoku, B.A. Department of Crop and Soil Sciences; Cornell University Press: Ithacab, NY, USA, 2012. [Google Scholar]
- Rachidi, F.; Benhima, R.; Kasmi, Y.; Sbabou, L.; Arroussi, H.E. Evaluation of microalgae polysaccharides as biostimulants of tomato plant defense using metabolomics and biochemical approaches. Sci. Rep. 2021, 11, 930. [Google Scholar] [CrossRef]
- Attia, M.S.; Younis, A.M.; Ahmed, A.F.; Elaziz, A. Comprehensive management for wilt disease caused by Fusarium oxysporum in tomato plant. Int. J. Innov. Sci. Eng. Technol. 2016, 4, 2348–7968. [Google Scholar]
- Farrag, A.; Attia, M.S.; Younis, A.; Abd Elaziz, A. Potential impacts of elicitors to improve tomato plant disease resistance. Al Azhar. Bull. Sci. 2017, 9, 311–321. [Google Scholar]
- Sarwar, N.; Malhi, S.S.; Zia, M.H.; Naeem, A.; Bibi, S.; Farid, G. Role of mineral nutrition in minimizing cadmium accumulation by plants. J. Sci. Food Agric. 2010, 90, 925–937. [Google Scholar] [CrossRef] [PubMed]
- Souri, Z.; Khanna, K.; Karimi, N.; Ahmad, P. Silicon and plants: Current knowledge and future prospects. J. Plant Growth Regul. 2021, 40, 906–925. [Google Scholar] [CrossRef]
- Gogos, A.; Knauer, K.; Bucheli, T.D. Nanomaterials in plant protection and fertilization: Current state, foreseen applications, and research priorities. J. Agric. Food Chem. 2012, 60, 9781–9792. [Google Scholar] [CrossRef]
- Gall, H.L.; Philippe, F.; Domon, J.-M.; Gillet, F.; Pelloux, J.; Rayon, C. Cell wall metabolism in response to abiotic stress. Plants 2015, 4, 112–166. [Google Scholar] [CrossRef]
- El-Shetehy, M.; Moradi, A.; Maceroni, M.; Reinhardt, D.; Petri-Fink, A.; Rothen-Rutishauser, B.; Mauch, F.; Schwab, F. Silica nanoparticles enhance disease resistance in Arabidopsis plants. Nat. Nanotechnol. 2021, 16, 344–353. [Google Scholar] [CrossRef]
- Islam, W.; Tayyab, M.; Khalil, F.; Hua, Z.; Huang, Z.; Chen, H.Y. Silicon-mediated plant defense against pathogens and insect pests. Pestic. Biochem. Physiol. 2020, 168, 104641. [Google Scholar] [CrossRef] [PubMed]
- Ahire, M.; Mundada, P.; Nikam, T.; Bapat, V.; Penna, S. Multifaceted roles of silicon in mitigating environmental stresses in plants. Plant Physiol. Biochem. 2021, 169, 291–310. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Yu, S.; Gong, H.-J.; Zhao, H.-L.; Li, H.-L.; Hu, Y.-H.; Wang, Y.-C. Beneficial effects of silicon on photosynthesis of tomato seedlings under water stress. J. Integr. Agric. 2018, 17, 2151–2159. [Google Scholar] [CrossRef]
- Bakhat, H.F.; Bibi, N.; Zia, Z.; Abbas, S.; Hammad, H.M.; Fahad, S.; Ashraf, M.R.; Shah, G.M.; Rabbani, F.; Saeed, S. Silicon mitigates biotic stresses in crop plants: A review. Crop Prot. 2018, 104, 21–34. [Google Scholar] [CrossRef]
- Vasanthi, N.; Saleena, L.M.; Raj, S.A. Silicon in crop production and crop protection-A review. Agric. Rev. 2014, 35, 14–23. [Google Scholar] [CrossRef]
- Babu, T. Calibration and Categorization of Plant Available Silicon in Louisiana Soils; Louisiana State University and Agricultural and Mechanical College: Baton Rouge, LA, USA, 2015. [Google Scholar]
- Hashem, A.H.; Abdelaziz, A.M.; Attia, M.S.; Salem, S.S. Selenium and Nano-Selenium-Mediated Biotic Stress Tolerance in Plants. In Selenium and Nano-Selenium in Environmental Stress Management and Crop Quality Improvement; Hossain, M.A., Ahammed, G.J., Kolbert, Z., El-Ramady, H., Islam, M.T., Schiavon, M., Eds.; Springer International Publishing: Cham, Switzerland, 2022; pp. 209–226. [Google Scholar] [CrossRef]
- Saied, E.; Hashem, A.H.; Ali, O.M.; Selim, S.; Almuhayawi, M.S.; Elbahnasawy, M.A. Photocatalytic and Antimicrobial Activities of Biosynthesized Silver Nanoparticles Using Cytobacillus firmus. Life 2022, 12, 1331. [Google Scholar] [CrossRef] [PubMed]
- Saied, E.; Salem, S.S.; Al-Askar, A.A.; Elkady, F.M.; Arishi, A.A.; Hashem, A.H. Mycosynthesis of Hematite (α-Fe2O3) Nanoparticles Using Aspergillus niger and Their Antimicrobial and Photocatalytic Activities. Bioengineering 2022, 9, 397. [Google Scholar] [CrossRef]
- Hashem, A.H.; Selim, T.A.; Alruhaili, M.H.; Selim, S.; Alkhalifah, D.H.M.; Al Jaouni, S.K.; Salem, S.S. Unveiling Antimicrobial and Insecticidal Activities of Biosynthesized Selenium Nanoparticles Using Prickly Pear Peel Waste. J. Funct. Biomater. 2022, 13, 112. [Google Scholar] [CrossRef]
- Salem, S.S.; Hashem, A.H.; Sallam, A.-A.M.; Doghish, A.S.; Al-Askar, A.A.; Arishi, A.A.; Shehabeldine, A.M. Synthesis of Silver Nanocomposite Based on Carboxymethyl Cellulose: Antibacterial, Antifungal and Anticancer Activities. Polymers 2022, 14, 3352. [Google Scholar] [CrossRef]
- Shehabeldine, A.M.; Salem, S.S.; Ali, O.M.; Abd-Elsalam, K.A.; Elkady, F.M.; Hashem, A.H. Multifunctional Silver Nanoparticles Based on Chitosan: Antibacterial, Antibiofilm, Antifungal, Antioxidant, and Wound-Healing Activities. J. Fungi 2022, 8, 612. [Google Scholar] [CrossRef]
- El-Naggar, M.E.; Hasanin, M.; Hashem, A.H. Eco-Friendly Synthesis of Superhydrophobic Antimicrobial Film Based on Cellulose Acetate/Polycaprolactone Loaded with the Green Biosynthesized Copper Nanoparticles for Food Packaging Application. J. Polym. Environ. 2022, 30, 1820–1832. [Google Scholar] [CrossRef]
- Salem, S.S.; Ali, O.M.; Reyad, A.M.; Abd-Elsalam, K.A.; Hashem, A.H. Pseudomonas indica-Mediated Silver Nanoparticles: Antifungal and Antioxidant Biogenic Tool for Suppressing Mucormycosis Fungi. J. Fungi 2022, 8, 126. [Google Scholar] [CrossRef] [PubMed]
- Lashin, I.; Hasanin, M.; Hassan, S.A.M.; Hashem, A.H. Green biosynthesis of zinc and selenium oxide nanoparticles using callus extract of Ziziphus spina-christi: Characterization, antimicrobial, and antioxidant activity. Biomass Convers. Biorefinery 2021. [Google Scholar] [CrossRef]
- Hasanin, M.; Hashem, A.H.; Lashin, I.; Hassan, S.A.M. In vitro improvement and rooting of banana plantlets using antifungal nanocomposite based on myco-synthesized copper oxide nanoparticles and starch. Biomass Convers. Biorefinery 2021. [Google Scholar] [CrossRef]
- Vasanthi, N.; Saleena, L.M.; Raj, S.A. Silica Solubilization Potential of Certain Bacterial Species in the Presence of Different Silicate Minerals. Silicon 2018, 10, 267–275. [Google Scholar] [CrossRef]
- Raturi, G.; Sharma, Y.; Rana, V.; Thakral, V.; Myaka, B.; Salvi, P.; Singh, M.; Dhar, H.; Deshmukh, R. Exploration of silicate solubilizing bacteria for sustainable agriculture and silicon biogeochemical cycle. Plant Physiol. Biochem. PPB 2021, 166, 827–838. [Google Scholar] [CrossRef] [PubMed]
- Pieła, A.; Żymańczyk-Duda, E.; Brzezińska-Rodak, M.; Duda, M.; Grzesiak, J.; Saeid, A.; Mironiuk, M.; Klimek-Ochab, M. Biogenic synthesis of silica nanoparticles from corn cobs husks. Dependence of the productivity on the method of raw material processing. Bioorganic Chem. 2020, 99, 103773. [Google Scholar] [CrossRef] [PubMed]
- Tarshan, M. Digestibility of Fungi-Based Protein Products Fed to Broiler Chickens; Swedish University of Agricultural Sciences Library: Lomma, Sweden, 2017. [Google Scholar]
- Mahboubi, A.; Ferreira, J.A.; Taherzadeh, M.J.; Lennartsson, P.R. Value-added products from dairy waste using edible fungi. Waste Manag. 2016, 59, 518–525. [Google Scholar] [CrossRef] [PubMed]
- Mathur, P.; Roy, S. Nanosilica facilitates silica uptake, growth and stress tolerance in plants. Plant Physiol. Biochem. 2020, 157, 114–127. [Google Scholar] [CrossRef] [PubMed]
- Fouda, A.; Hassan, S.E.-D.; Saied, E.; Hamza, M.F. Photocatalytic degradation of real textile and tannery effluent using biosynthesized magnesium oxide nanoparticles (MgO-NPs), heavy metal adsorption, phytotoxicity, and antimicrobial activity. J. Environ. Chem. Eng. 2021, 9, 105346. [Google Scholar] [CrossRef]
- Alhazmi, H.A. FT-IR spectroscopy for the identification of binding sites and measurements of the binding interactions of important metal ions with bovine serum albumin. Sci. Pharm. 2019, 87, 5. [Google Scholar] [CrossRef]
- Goswami, P.; Mathur, J. Application of agro-waste-mediated silica nanoparticles to sustainable agriculture. Bioresour. Bioprocess. 2022, 9, 9. [Google Scholar] [CrossRef]
- Babick, F. Dynamic light scattering (DLS). In Characterization of Nanoparticles; Elsevier: Amsterdam, The Netherlands, 2020; pp. 137–172. [Google Scholar]
- Attia, M.S.; El-Sayyad, G.S.; Abd Elkodous, M.; El-Batal, A.I. The effective antagonistic potential of plant growth-promoting rhizobacteria against Alternaria solani-causing early blight disease in tomato plant. Sci. Hortic. 2020, 266, 109289. [Google Scholar] [CrossRef]
- Vernon, L.P.; Seely, G.R. The Chlorophylls; Academic Press: Cambridge, MA, USA, 2014. [Google Scholar]
- Bates, L.S.; Waldren, R.P.; Teare, I. Rapid determination of free proline for water-stress studies. Plant Soil 1973, 39, 205–207. [Google Scholar] [CrossRef]
- Dai, G.; Andary, C.; Cosson-Mondolot, L.; Boubals, D. Polyphenols and resistance of grapevines to downy mildew. Int. Symp. Nat. Phenols Plant Resist. 1993, 381, 763–766. [Google Scholar] [CrossRef]
- Bergmeyer, H. Determination with glucose oxidase and peroxidase. Methods Enzym. Anal. 1974, 1205–1215. [Google Scholar]
- Lavid, N.; Schwartz, A.; Yarden, O.; Tel-Or, E. The involvement of polyphenols and peroxidase activities in heavy-metal accumulation by epidermal glands of the waterlily (Nymphaeaceae). Planta 2001, 212, 323–331. [Google Scholar] [CrossRef]
- Snedecor, G.W. Comparisons of two samples. In Statistical Methods; Snedecor, G.W., Cochran, W.G., Eds.; Iowa State University Press: Ames, IA, USA, 1980. [Google Scholar]
- Zamani, H.; Jafari, A.; Mousavi, S.M.; Darezereshki, E. Biosynthesis of silica nanoparticle using Saccharomyces cervisiae and its application on enhanced oil recovery. J. Pet. Sci. Eng. 2020, 190, 107002. [Google Scholar] [CrossRef]
- Kannan, M.; Uma Sangareswari, K.; Suganya, P.; Ganesan, R.; Rajarathinam, K. Biobased approach for the synthesis, characterization, optimization and application of silica nanoparticles by fungus Fusarium oxysporum. Pharmaceut. Biol. Eval 2015, 2, 223–233. [Google Scholar]
- Bansal, V.; Rautaray, D.; Bharde, A.; Ahire, K.; Sanyal, A.; Ahmad, A.; Sastry, M. Fungus-mediated biosynthesis of silica and titania particles. J. Mater. Chem. 2005, 15, 2583–2589. [Google Scholar] [CrossRef]
- Singh, S.; Bhatta, U.M.; Satyam, P.; Dhawan, A.; Sastry, M.; Prasad, B. Bacterial synthesis of silicon/silica nanocomposites. J. Mater. Chem. 2008, 18, 2601–2606. [Google Scholar] [CrossRef]
- Biradar, A.I.; Sarvalkar, P.D.; Teli, S.B.; Pawar, C.; Patil, P.; Prasad, N.R. Photocatalytic degradation of dyes using one-step synthesized silica nanoparticles. Mater. Today Proc. 2021, 43, 2832–2838. [Google Scholar] [CrossRef]
- El-Gazzar, N.; Almanaa, T.N.; Reda, R.M.; El Gaafary, M.; Rashwan, A.; Mahsoub, F. Assessment the using of silica nanoparticles (SiO2NPs) biosynthesized from rice husks by Trichoderma harzianum MF780864 as water lead adsorbent for immune status of Nile tilapia (Oreochromis niloticus). Saudi J. Biol. Sci. 2021, 28, 5119–5130. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, S.; Kundu, M.; Dutta, S.; Mahalanobish, S.; Ghosh, N.; Das, J.; Sil, P.C. Enhancement of anti-neoplastic effects of cuminaldehyde against breast cancer via mesoporous silica nanoparticle based targeted drug delivery system. Life Sci. 2022, 298, 120525. [Google Scholar] [CrossRef]
- Olga, M.; Jana, M.; Anna, M.; Irena, K.; Jan, M.; Alena, Č. Antimicrobial properties and applications of metal nanoparticles biosynthesized by green methods. Biotechnol. Adv. 2022, 58, 107905. [Google Scholar]
- Lai, H.; Liu, Y.; Huang, G.; Chen, Y.; Song, Y.; Ma, Y.; Yue, P. Fabrication and antibacterial evaluation of peppermint oil-loaded composite microcapsules by chitosan-decorated silica nanoparticles stabilized Pickering emulsion templating. Int. J. Biol. Macromol. 2021, 183, 2314–2325. [Google Scholar] [CrossRef]
- Nieto-Ortega, B.; Bürgi, T. Vibrational properties of thiolate-protected gold nanoclusters. Acc. Chem. Res. 2018, 51, 2811–2819. [Google Scholar] [CrossRef]
- Foroutan, R.; Mohammadi, R.; Peighambardoust, S.J.; Jalali, S.; Ramavandi, B. Application of nano-silica particles generated from offshore white sandstone for cadmium ions elimination from aqueous media. Environ. Technol. Innov. 2020, 19, 101031. [Google Scholar] [CrossRef]
- Agusnar, H.; Sugita, P.; Nainggolan, I. Preparation sodium silicate from rice husk to synthesize silica nanoparticles by sol-gel method for adsorption water in analysis of methamphetamine. South Afr. J. Chem. Eng. 2022, 40, 80–86. [Google Scholar]
- Mohanraj, R.; Gnanamangai, B.M.; Poornima, S.; Oviyaa, V.; Ramesh, K.; Vijayalakshmi, G.; Nithya, M.; Karthik, N.; Ponmurugan, P.; Robinson, J.P. Decolourisation efficiency of immobilized silica nanoparticles synthesized by actinomycetes. Mater. Today Proc. 2020, 48, 129–135. [Google Scholar] [CrossRef]
- Wang, H.; Lu, J. A review on particle size effect in metal-catalyzed heterogeneous reactions. Chin. J. Chem. 2020, 38, 1422–1444. [Google Scholar] [CrossRef]
- Vetchinkina, E.; Loshchinina, E.; Kupryashina, M.; Burov, A.; Nikitina, V. Shape and size diversity of gold, silver, selenium, and silica nanoparticles prepared by green synthesis using fungi and bacteria. Ind. Eng. Chem. Res. 2019, 58, 17207–17218. [Google Scholar] [CrossRef]
- Stetefeld, J.; McKenna, S.A.; Patel, T.R. Dynamic light scattering: A practical guide and applications in biomedical sciences. Biophys. Rev. 2016, 8, 409–427. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Gaurav, S.S.; Shukla, G.; Rani, P. Assessment of mycogenic zinc nano-fungicides against pathogenic early blight (Alternaria solani) of potato (Solanum tuberosum L.). Mater. Today Proc. 2022, 49, 3528–3537. [Google Scholar] [CrossRef]
- Lahuf, A.A.; Abdullah, K.M.; Mohammadali, M.T. Assessment of the nanosized particles of ZnO and MgO and some cultivars in control of Alternaria solani causing tomato early blight. Ecol. Environ. Conserv. 2020, 26, 89–95. [Google Scholar]
- Quiterio-Gutiérrez, T.; Ortega-Ortiz, H. The Application of Selenium and Copper Nanoparticles Modifies the Biochemical Responses of Tomato Plants under Stress by Alternaria solani. Int. J. Mol. Sci. 2019, 20, 1950. [Google Scholar] [CrossRef]
- Ismail, A.-W.A.; Sidkey, N.M.; Arafa, R.A.; Fathy, R.M.; El-Batal, A.I. Evaluation of in vitro antifungal activity of silver and selenium nanoparticles against Alternaria solani caused early blight disease on potato. Br. Biotechnol. J. 2016, 12, 1. [Google Scholar] [CrossRef]
- Derbalah, A.; Shenashen, M.; Hamza, A.; Mohamed, A.; El Safty, S. Antifungal activity of fabricated mesoporous silica nanoparticles against early blight of tomato. Egypt. J. Basic Appl. Sci. 2018, 5, 145–150. [Google Scholar] [CrossRef]
- Ahamad, L.; Siddiqui, Z.A. Effects of silicon dioxide, zinc oxide and titanium dioxide nanoparticles on Meloidogyne incognita, Alternaria dauci and Rhizoctonia solani disease complex of carrot. Exp. Parasitol. 2021, 230, 108176. [Google Scholar] [CrossRef]
- Abdelrhim, A.S.; Mazrou, Y.S.; Nehela, Y.; Atallah, O.O.; El-Ashmony, R.M.; Dawood, M.F. Silicon Dioxide Nanoparticles Induce Innate Immune Responses and Activate Antioxidant Machinery in Wheat Against Rhizoctonia solani. Plants 2021, 10, 2758. [Google Scholar] [CrossRef]
- Khan, M.R.; Siddiqui, Z.A. Use of silicon dioxide nanoparticles for the management of Meloidogyne incognita, Pectobacterium betavasculorum and Rhizoctonia solani disease complex of beetroot (Beta vulgaris L.). Sci. Hortic. 2020, 265, 109211. [Google Scholar] [CrossRef]
- Matrood, A.A.; Rhouma, A. Evaluation of the efficiency of Paecilomyces lilacinus and Trichoderma harzianum as biological control agents against Alternaria solani causing early blight disease of eggplant. Pak. J. Phytopathol. 2021, 33, 171–176. [Google Scholar] [CrossRef]
- Barzman, M.; Bàrberi, P.; Birch, A.N.E.; Boonekamp, P.; Dachbrodt-Saaydeh, S.; Graf, B.; Hommel, B.; Jensen, J.E.; Kiss, J.; Kudsk, P. Eight principles of integrated pest management. Agron. Sustain. Dev. 2015, 35, 1199–1215. [Google Scholar] [CrossRef]
- Hashem, A.H.; Abdelaziz, A.M.; Askar, A.A.; Fouda, H.M.; Khalil, A.M.; Abd-Elsalam, K.A.; Khaleil, M.M. Bacillus megaterium-mediated synthesis of selenium nanoparticles and their antifungal activity against Rhizoctonia solani in Faba Bean Plants. J. Fungi 2021, 7, 195. [Google Scholar] [CrossRef] [PubMed]
- Abdelaziz, A.M.; Dacrory, S.; Hashem, A.H.; Attia, M.S.; Hasanin, M.; Fouda, H.M.; Kamel, S.; ElSaied, H. Protective role of zinc oxide nanoparticles based hydrogel against wilt disease of pepper plant. Biocatal. Agric. Biotechnol. 2021, 35, 102083. [Google Scholar] [CrossRef]
- Elbasuney, S.; El-Sayyad, G.S.; Attia, M.S.; Abdelaziz, A.M. Ferric oxide colloid: Towards green nano-fertilizer for tomato plant with enhanced vegetative growth and immune response against fusarium wilt disease. J. Inorg. Organomet. Polym. Mater. 2022, 32, 4270–4283. [Google Scholar] [CrossRef] [PubMed]
- Luyckx, M.; Hausman, J.-F.; Lutts, S.; Guerriero, G. Silicon and plants: Current knowledge and technological perspectives. Front. Plant Sci. 2017, 8, 411. [Google Scholar] [CrossRef] [PubMed]
- Mandlik, R.; Thakral, V.; Raturi, G.; Shinde, S.; Nikolić, M.; Tripathi, D.K.; Sonah, H.; Deshmukh, R. Significance of silicon uptake, transport, and deposition in plants. J. Exp. Bot. 2020, 71, 6703–6718. [Google Scholar] [CrossRef]
- Aldinary, A.M.; Abdelaziz, A.M.; Farrag, A.A.; Attia, M.S. Biocontrol of tomato Fusarium wilt disease by a new Moringa endophytic Aspergillus isolates. Mater. Today Proc. 2021. [Google Scholar] [CrossRef]
- Abdelaziz, A.M.; El-Wakil, D.A.; Attia, M.S.; Ali, O.M.; AbdElgawad, H.; Hashem, A.H. Inhibition of Aspergillus flavus Growth and Aflatoxin Production in Zea mays L. Using Endophytic Aspergillus fumigatus. J. Fungi 2022, 8, 482. [Google Scholar] [CrossRef]
- Abdelaziz, A.M.; Attia, M.S.; Salem, M.S.; Refaay, D.A.; Alhoqail, W.A.; Senousy, H.H. Cyanobacteria-mediated immune responses in pepper plants against fusarium wilt. Plants 2022, 11, 2049. [Google Scholar] [CrossRef]
- Attia, M.S.; Sharaf, A.; Zayed, A.S. Protective action of some bio-pesticides against early blight disease caused by Alternaria solani in tomato plant. JISET Int. J. Innov. Sci. Eng. Tech 2017, 4, 67–94. [Google Scholar]
- Alhaithloul, H.A.S.; Attia, M.S.; Abdein, M.A. Dramatic biochemical and anatomical changes in eggplant due to infection with Alternaria solani causing early blight disease. Int. J. Bot. Stud. 2019, 4, 55–60. [Google Scholar]
- Kruse, C.; Jost, R.; Lipschis, M.; Kopp, B.; Hartmann, M.; Hell, R. Sulfur-enhanced defence: Effects of sulfur metabolism, nitrogen supply, and pathogen lifestyle. Plant Biol. 2007, 9, 608–619. [Google Scholar] [CrossRef]
- Khalil, A.; Abdelaziz, A.; Khaleil, M.; Hashem, A. Fungal endophytes from leaves of Avicennia marina growing in semi-arid environment as a promising source for bioactive compounds. Lett. Appl. Microbiol. 2021, 72, 263–274. [Google Scholar] [CrossRef]
- Badawy, A.A.; Alotaibi, M.O.; Abdelaziz, A.M.; Osman, M.S.; Khalil, A.M.; Saleh, A.M.; Mohammed, A.E.; Hashem, A.H. Enhancement of seawater stress tolerance in barley by the endophytic fungus Aspergillus ochraceus. Metabolites 2021, 11, 428. [Google Scholar] [CrossRef]
- Sharaf, M.H.; Abdelaziz, A.M.; Kalaba, M.H.; Radwan, A.A.; Hashem, A.H. Antimicrobial, antioxidant, cytotoxic activities and phytochemical analysis of fungal endophytes isolated from ocimum basilicum. Appl. Biochem. Biotechnol. 2022, 194, 1271–1289. [Google Scholar] [CrossRef] [PubMed]
- Khattab, A.M.; Abo-Taleb, H.A.; Abdelaziz, A.M.; El-Tabakh, M.A.; El-Feky, M.M.; Abu-Elghait, M. Daphnia magna and Gammarus pulex, novel promising agents for biomedical and agricultural applications. Sci. Rep. 2022, 12, 13690. [Google Scholar] [CrossRef]
- Sahebi, M.; Hanafi, M.M.; Siti Nor Akmar, A.; Rafii, M.Y.; Azizi, P.; Tengoua, F.; Nurul Mayzaitul Azwa, J.; Shabanimofrad, M. Importance of silicon and mechanisms of biosilica formation in plants. BioMed Res. Int. 2015, 2015, 396010. [Google Scholar] [CrossRef]
- Puppe, D.; Sommer, M. Experiments, uptake mechanisms, and functioning of silicon foliar fertilization—A review focusing on maize, rice, and wheat. Adv. Agron. 2018, 152, 1–49. [Google Scholar]
- Zahedi, S.M.; Moharrami, F.; Sarikhani, S.; Padervand, M. Selenium and silica nanostructure-based recovery of strawberry plants subjected to drought stress. Sci. Rep. 2020, 10, 17672. [Google Scholar] [CrossRef] [PubMed]
- Gowayed, M.; Al-Zahrani, H.S.; Metwali, E.M. Improving the salinity tolerance in potato (Solanum tuberosum) by exogenous application of silicon dioxide nanoparticles. Int. J. Agric. Biol. 2017, 19, 183–194. [Google Scholar]
- Ali, S.; Mehmood, A.; Khan, N. Uptake, translocation, and consequences of nanomaterials on plant growth and stress adaptation. J. Nanomater. 2021, 2021, 6677616. [Google Scholar] [CrossRef]
- González-García, Y.; González-Moscoso, M.; Hernández-Hernández, H.; Méndez-López, A.; Juárez-Maldonado, A. Induction of Stress Tolerance in Crops by Applying Nanomaterials. In Nanotechnology in Plant Growth Promotion and Protection: Recent Advances and Impacts; Wiley Publishing: New York, NY, USA, 2021; pp. 129–169. [Google Scholar]
- Li, S.; Liu, J.; Wang, Y.; Gao, Y.; Zhang, Z.; Xu, J.; Xing, G. Comparative physiological and metabolomic analyses revealed that foliar spraying with zinc oxide and silica nanoparticles modulates metabolite profiles in cucumber (Cucumis sativus L.). Food Energy Secur. 2021, 10, e269. [Google Scholar] [CrossRef]
- Attia, M.S.; El-Naggar, H.A.; Abdel-Daim, M.M.; El-Sayyad, G.S. The potential impact of Octopus cyanea extracts to improve eggplant resistance against Fusarium-wilt disease: In vivo and in vitro studies. Environ. Sci. Pollut. Res. 2021, 28, 35854–35869. [Google Scholar] [CrossRef]
- Mohamed, A.H. Reactions of Starch Potato to Alternaria Leaf Spot; Leibniz Universität Hannover: Hannover, Germany, 2018. [Google Scholar]
- Sachdev, S.; Ansari, S.A.; Ansari, M.I.; Fujita, M.; Hasanuzzaman, M. Abiotic stress and reactive oxygen species: Generation, signaling, and defense mechanisms. Antioxidants 2021, 10, 277. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, F.; Kamal, A. Oxidative Stress Alleviation by Modulation of the Antioxidant System under Environmental Stressors. In Organic Solutes, Oxidative Stress, and Antioxidant Enzymes Under Abiotic Stressors; CRC Press: Boca Raton, FL, USA, 2021; pp. 191–212. [Google Scholar]
- Sanati, M.; Afshari, A.R.; Kesharwani, P.; Sukhorukov, V.N.; Sahebkar, A. Recent trends in the application of nanoparticles in cancer therapy: The involvement of oxidative stress. J. Control. Release 2022, 348, 287–304. [Google Scholar] [CrossRef]
- Hassanpour, H.; Ahmadi, N.; Hekmati, M.; Ghanbarzadeh, M. Effect of SiO2 nanoparticles on phytochemical and anatomical alterations in Anthemis gilanica. Iran. J. Plant Physiol. 2020, 10, 3223–3231. [Google Scholar]
- Khan, M.I.R.; Ashfaque, F.; Chhillar, H.; Irfan, M.; Khan, N.A. The intricacy of silicon, plant growth regulators and other signaling molecules for abiotic stress tolerance: An entrancing crosstalk between stress alleviators. Plant Physiol. Biochem. 2021, 162, 36–47. [Google Scholar] [CrossRef]
- Ahmad, Z.; Tahseen, S.; Wasi, A.; Ganie, I.B.; Shahzad, A.; Emamverdian, A.; Ramakrishnan, M.; Ding, Y. Nanotechnological Interventions in Agriculture. Nanomaterials 2022, 12, 2667. [Google Scholar] [CrossRef]
- El-Saadony, M.T.; Desoky, E.-S.M.; Saad, A.M.; Eid, R.S.; Selem, E.; Elrys, A.S. Biological silicon nanoparticles improve Phaseolus vulgaris L. yield and minimize its contaminant contents on a heavy metals-contaminated saline soil. J. Environ. Sci. 2021, 106, 1–14. [Google Scholar] [CrossRef]
- Ghorbanpour, M.; Mohammadi, H.; Kariman, K. Nanosilicon-based recovery of barley (Hordeum vulgare) plants subjected to drought stress. Environ. Sci. Nano 2020, 7, 443–461. [Google Scholar] [CrossRef]
- Kiadaliri, M.; Firouzbakhsh, F.; Deldar, H. Effects of feeding with red algae (Laurencia caspica) hydroalcoholic extract on antioxidant defense, immune responses, and immune gene expression of kidney in rainbow trout (Oncorhynchus mykiss) infected with Aeromonas hydrophila. Aquaculture 2020, 526, 735361. [Google Scholar] [CrossRef]
- Sharma, P.; Jha, A.B.; Dubey, R.S.; Pessarakli, M. Reactive oxygen species, oxidative damage, and antioxidative defense mechanism in plants under stressful conditions. J. Bot. 2012, 2012, 217037. [Google Scholar] [CrossRef]
- Babizhayev, M.A.; Strokov, I.A.; Nosikov, V.V.; Savel’yeva, E.L.; Sitnikov, V.F.; Yegorov, Y.E.; Lankin, V.Z. The role of oxidative stress in diabetic neuropathy: Generation of free radical species in the glycation reaction and gene polymorphisms encoding antioxidant enzymes to genetic susceptibility to diabetic neuropathy in population of type I diabetic patients. Cell Biochem. Biophys. 2015, 71, 1425–1443. [Google Scholar] [CrossRef]
| Treatment | Disease Symptoms Classes | DI (%) | Protection (%) | ||||
|---|---|---|---|---|---|---|---|
| 0 | 1 | 2 | 3 | 4 | |||
| Control Infected | 0 | 0 | 1 | 5 | 4 | 82.5 | 0 |
| Infected + SiO2-NPs 50 ppm | 4 | 0 | 4 | 2 | 0 | 35 | 57.57 |
| Infected + SiO2-NPs 100 ppm | 5 | 1 | 3 | 1 | 0 | 25 | 69.69 |
| Infected + sodium silicate 100 ppm | 2 | 1 | 1 | 3 | 3 | 60 | 27.27 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Albalawi, M.A.; Abdelaziz, A.M.; Attia, M.S.; Saied, E.; Elganzory, H.H.; Hashem, A.H. Mycosynthesis of Silica Nanoparticles Using Aspergillus niger: Control of Alternaria solani Causing Early Blight Disease, Induction of Innate Immunity and Reducing of Oxidative Stress in Eggplant. Antioxidants 2022, 11, 2323. https://doi.org/10.3390/antiox11122323
Albalawi MA, Abdelaziz AM, Attia MS, Saied E, Elganzory HH, Hashem AH. Mycosynthesis of Silica Nanoparticles Using Aspergillus niger: Control of Alternaria solani Causing Early Blight Disease, Induction of Innate Immunity and Reducing of Oxidative Stress in Eggplant. Antioxidants. 2022; 11(12):2323. https://doi.org/10.3390/antiox11122323
Chicago/Turabian StyleAlbalawi, Marzough A., Amer M. Abdelaziz, Mohamed S. Attia, Ebrahim Saied, Hussein H. Elganzory, and Amr H. Hashem. 2022. "Mycosynthesis of Silica Nanoparticles Using Aspergillus niger: Control of Alternaria solani Causing Early Blight Disease, Induction of Innate Immunity and Reducing of Oxidative Stress in Eggplant" Antioxidants 11, no. 12: 2323. https://doi.org/10.3390/antiox11122323
APA StyleAlbalawi, M. A., Abdelaziz, A. M., Attia, M. S., Saied, E., Elganzory, H. H., & Hashem, A. H. (2022). Mycosynthesis of Silica Nanoparticles Using Aspergillus niger: Control of Alternaria solani Causing Early Blight Disease, Induction of Innate Immunity and Reducing of Oxidative Stress in Eggplant. Antioxidants, 11(12), 2323. https://doi.org/10.3390/antiox11122323







