Potential Therapeutic Implication of Herbal Medicine in Mitochondria-Mediated Oxidative Stress-Related Liver Diseases
Abstract
1. Introduction
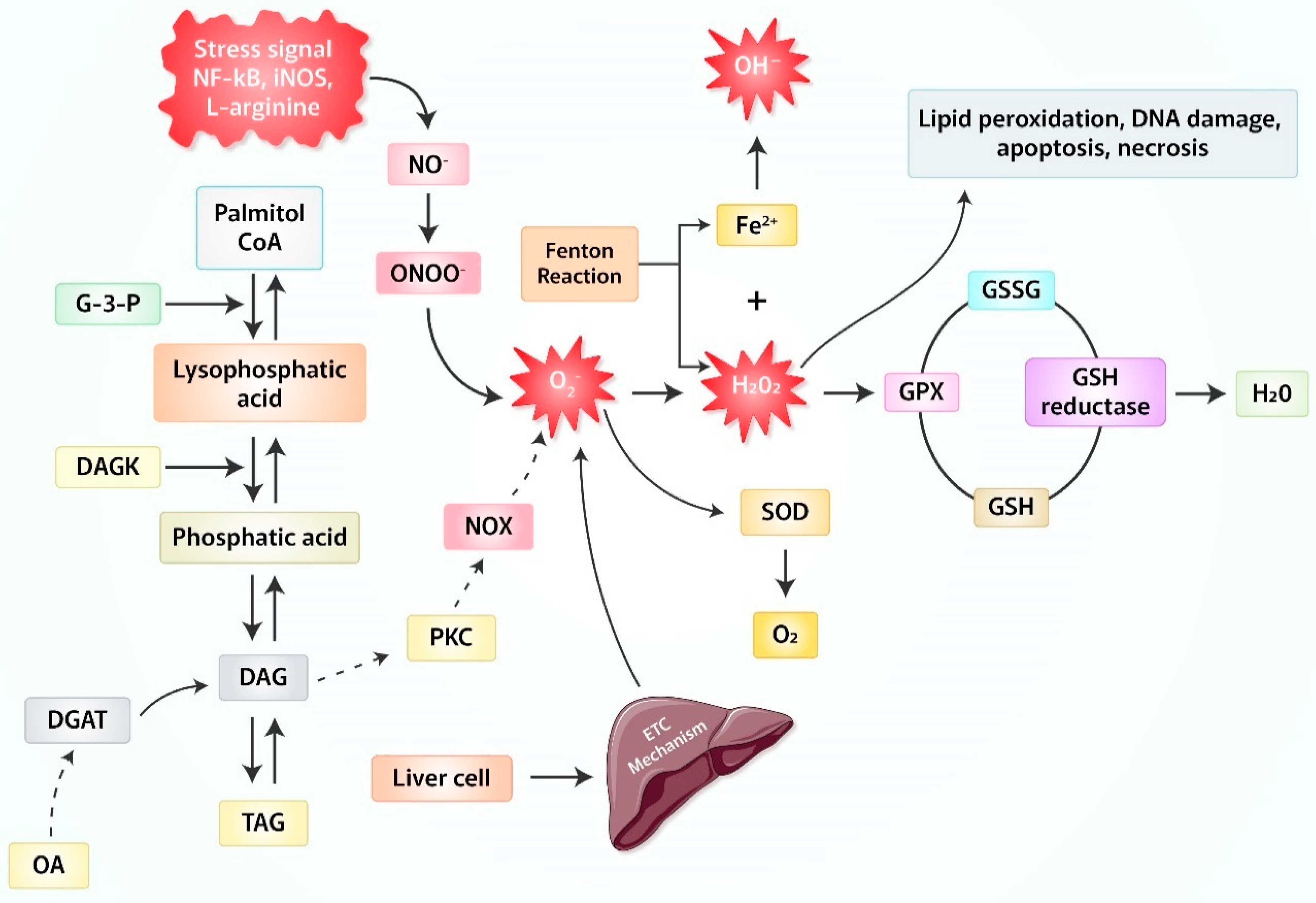
2. Production of Reactive Oxygen Species (ROS) Due to Oxidative Stress
3. Oxidative Stress-Related Mitochondrial Reactive Oxygen Species (ROS)/Signaling in Liver
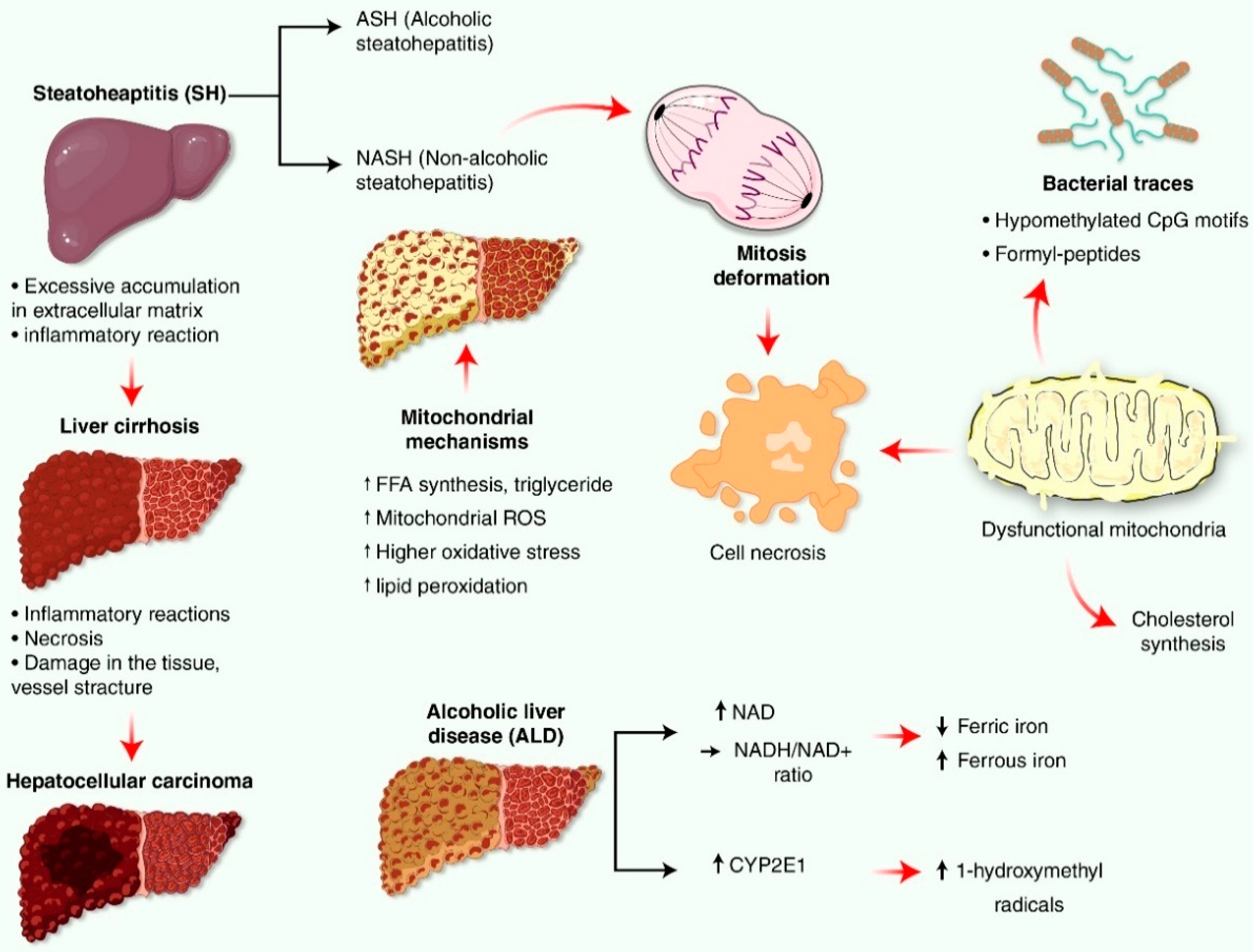
4. Liver Impairment Mediated by Mitochondrial Reactive Oxygen Species (ROS) Generation
5. Source and Defense System for Mitochondria-Mediated Oxidative Stress and Reactive Oxygen Species (ROS) Production in Liver Disease
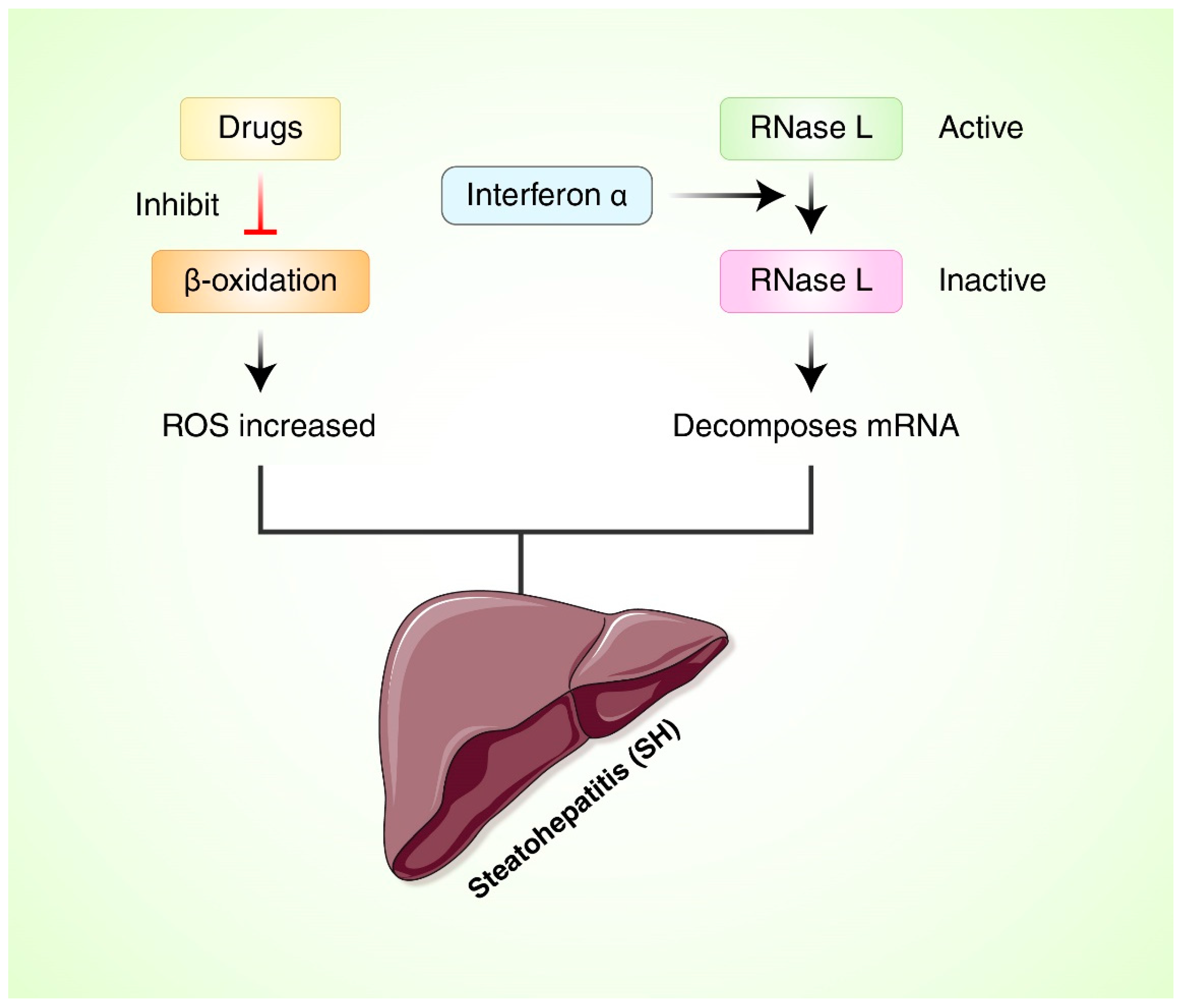
6. Oxidative Stress and Reactive Oxygen Species (ROS) in Nonalcoholic Fatty Liver Disease (NAFLD)
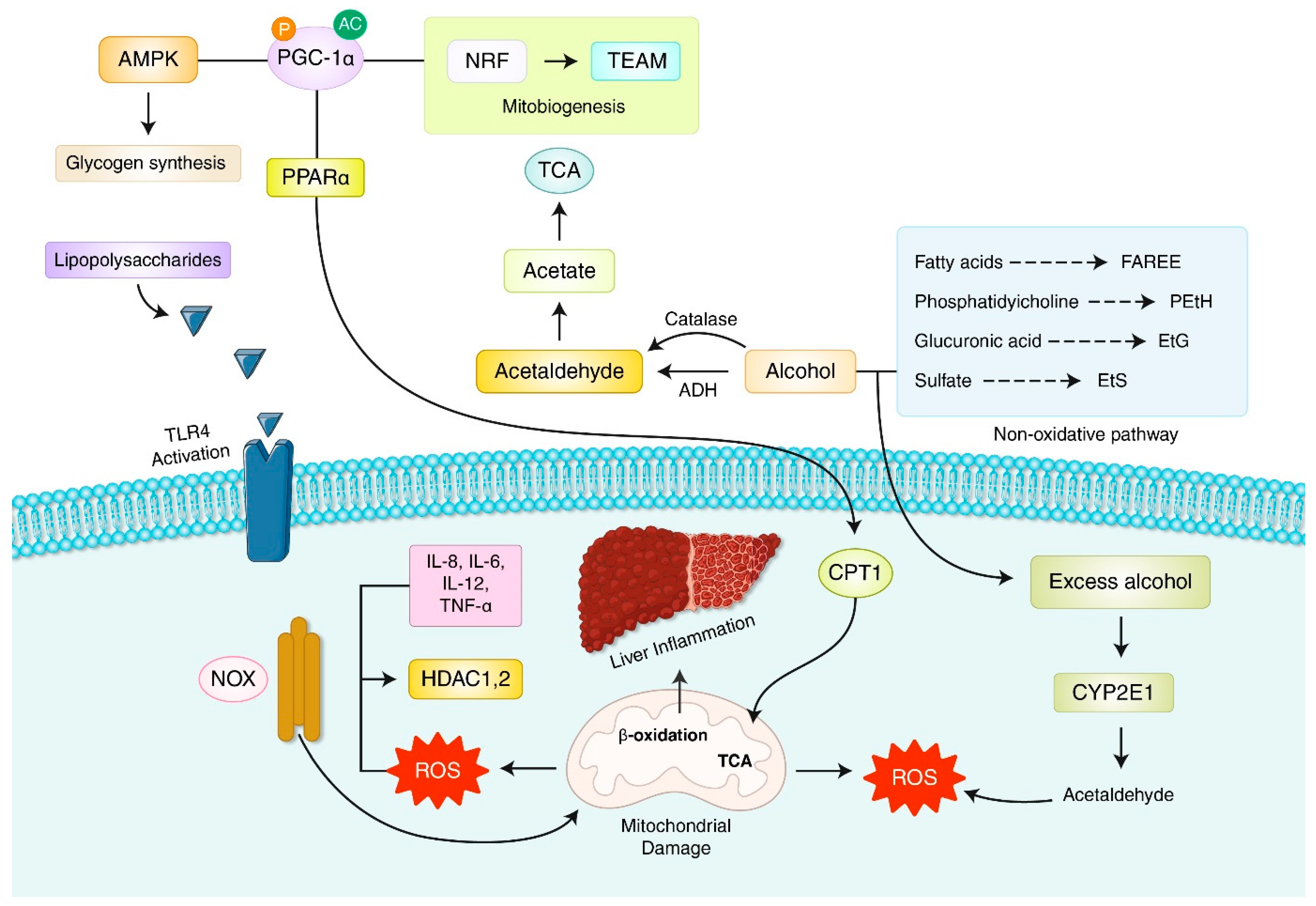
7. Oxidative Stress and Reactive Oxygen Species (ROS) in Alcoholic Liver Disease (ALD)
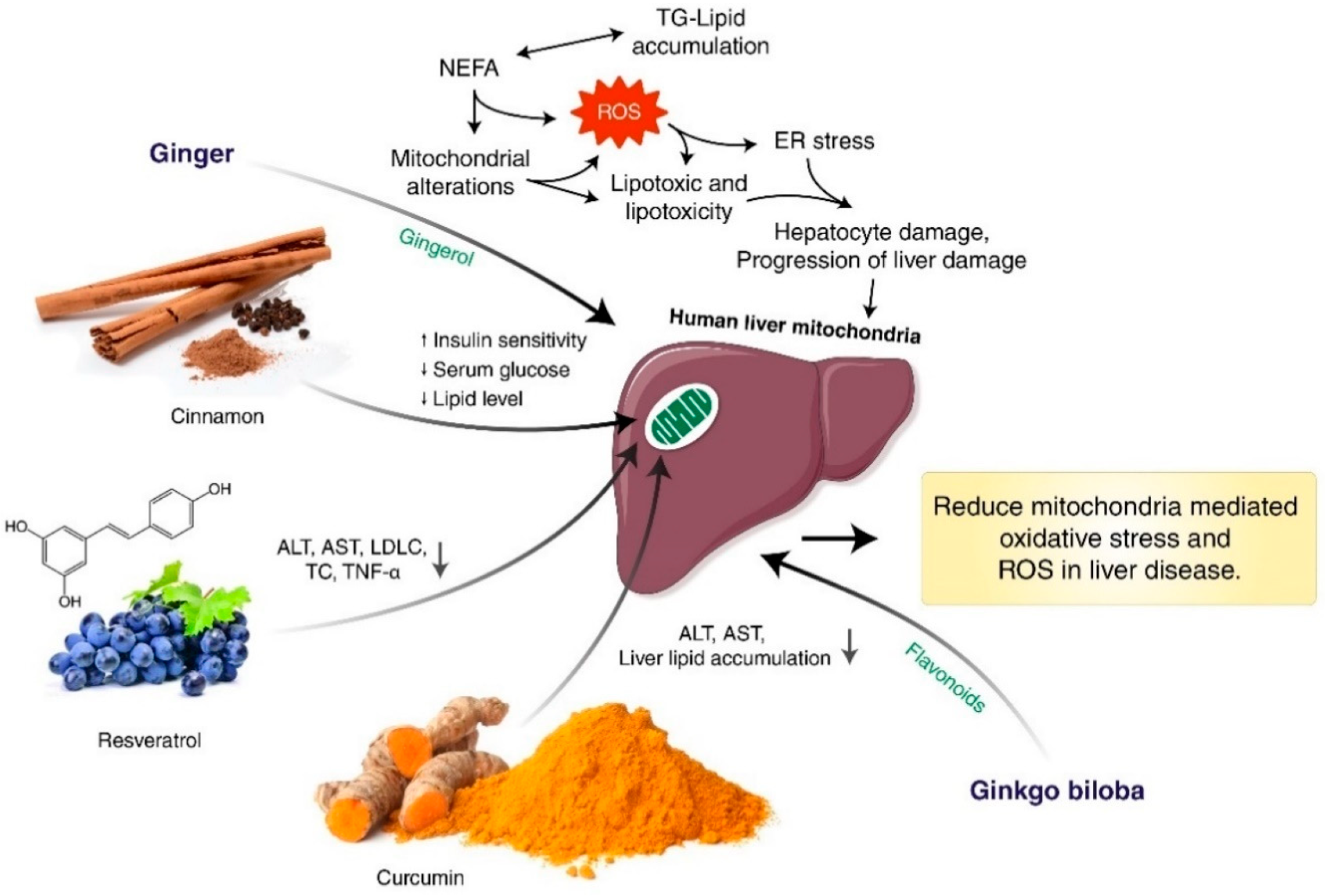
8. Herbal Medicine Targeting Mitochondria-Mediated Oxidative Stress and Reactive Oxygen Species (ROS) in Liver Disease
9. The Antioxidant Effect of Herbal Medicines via Suppression of Lipid Peroxidation to Thiolation Migration in Oxidative Damage
10. Drug Target and Clinical Use of Herbal Medicine to Reduce Mitochondria-Mediated Oxidative Stress
11. Conclusions and Perspectives
Author Contributions
Funding
Conflicts of Interest
References
- Annesley, S.J.; Fisher, P.R. Mitochondria in Health and Disease. Cells 2019, 8, 680. [Google Scholar] [CrossRef]
- Kim, J.; Wei, Y.; Sowers, J.R. Role of Mitochondrial Dysfunction in Insulin Resistance. Circ. Res. 2008, 102, 401–414. [Google Scholar] [CrossRef] [PubMed]
- St-Pierre, J.; Drori, S.; Uldry, M.; Silvaggi, J.M.; Rhee, J.; Jäger, S.; Handschin, C.; Zheng, K.; Lin, J.; Yang, W.; et al. Suppression of Reactive Oxygen Species and Neurodegeneration by the PGC-1 Transcriptional Coactivators. Cell 2006, 127, 397–408. [Google Scholar] [CrossRef] [PubMed]
- Du, K.; Ramachandran, A.; McGill, M.R.; Mansouri, A.; Asselah, T.; Farhood, A.; Woolbright, B.L.; Ding, W.-X.; Jaeschke, H. Induction of mitochondrial biogenesis protects against acetaminophen hepatotoxicity. Food Chem. Toxicol. 2017, 108, 339–350. [Google Scholar] [CrossRef] [PubMed]
- Venditti, P.; Di Stefano, L.; Di Meo, S. Mitochondrial metabolism of reactive oxygen species. Mitochondrion 2013, 13, 71–82. [Google Scholar] [CrossRef]
- García-Ruiz, C.; Fernández-Checa, J.C. Mitochondrial Oxidative Stress and Antioxidants Balance in Fatty Liver Disease. Hepatol. Commun. 2018, 2, 1425–1439. [Google Scholar] [CrossRef] [PubMed]
- Tao, F.; Zhang, Y.; Zhang, Z. The Role of Herbal Bioactive Components in Mitochondria Function and Cancer Therapy. Evid.-Based Complement. Altern. Med. 2019, 2019, 3868354. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Tan, H.-Y.; Wang, N.; Zhang, Z.-J.; Lao, L.; Wong, C.-W.; Feng, Y. The Role of Oxidative Stress and Antioxidants in Liver Diseases. Int. J. Mol. Sci. 2015, 16, 26087–26124. [Google Scholar] [CrossRef] [PubMed]
- Bonam, S.R.; Wu, Y.S.; Tunki, L.; Chellian, R.; Halmuthur, M.S.K.; Muller, S.; Pandy, V. What has come out from phytomedicines and herbal edibles for the treatment of cancer? ChemMedChem 2018, 13, 1854–1872. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.A.; Saha, S.K.; Rahman, M.S.; Uddin, M.J.; Uddin, M.S.; Pang, M.G.; Rhim, H.; Cho, S.G. Molecular Insights Into Therapeutic Potential of Autophagy Modulation by Natural Products for Cancer Stem Cells. Front. Cell Dev. Biol. 2020, 8, 283. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.A.; Rahman, M.R.; Zaman, T.; Uddin, M.; Islam, R.; Abdel-Daim, M.M.; Rhim, H. Emerging Potential of Naturally Occurring Autophagy Modulators Against Neurodegeneration. Curr. Pharm. Des. 2020, 26, 772–779. [Google Scholar] [CrossRef]
- Freeman, B.A.; Crapo, J.D. Biology of disease: Free radicals and tissue injury. Lab. Investig. 1982, 47, 412–426. [Google Scholar]
- Cheng, Z.; Yao, W.; Zheng, J.; Ding, W.; Wang, Y.; Zhang, T.; Zhu, L.; Zhou, F. A derivative of betulinic acid protects human Retinal Pigment Epithelial (RPE) cells from cobalt chloride-induced acute hypoxic stress. Exp. Eye Res. 2019, 180, 92–101. [Google Scholar] [CrossRef] [PubMed]
- Finkel, T. Signal Transduction by Mitochondrial Oxidants. J. Biol. Chem. 2012, 287, 4434–4440. [Google Scholar] [CrossRef]
- Perillo, B.; Di Donato, M.; Pezone, A.; Di Zazzo, E.; Giovannelli, P.; Galasso, G.; Castoria, G.; Migliaccio, A. ROS in cancer therapy: The bright side of the moon. Exp. Mol. Med. 2020, 52, 192–203. [Google Scholar] [CrossRef]
- Win, S.; Than, T.A.; Zhang, J.; Oo, C.; Min, R.W.M.; Kaplowitz, N. New insights into the role and mechanism of c-Jun-N-terminal kinase signaling in the pathobiology of liver diseases. Hepatology 2018, 67, 2013–2024. [Google Scholar] [CrossRef]
- Rahman, M.A.; Ahmed, K.R.; Rahman, M.H.; Parvez, M.A.K.; Lee, I.S.; Kim, B. Therapeutic Aspects and Molecular Targets of Autophagy to Control Pancreatic Cancer Management. Biomedicines 2022, 10, 1459. [Google Scholar] [CrossRef]
- Blas-Garcia, A.; Esplugues, J.V. Mitochondria Sentencing About Cellular Life and Death: A Matter of Oxidative Stress. Curr. Pharm. Des. 2011, 17, 4047–4060. [Google Scholar]
- Sharma, P.; Sampath, H. Mitochondrial DNA Integrity: Role in Health and Disease. Cells 2019, 8, 100. [Google Scholar] [CrossRef]
- Day, C.P.; James, O.F. Steatohepatitis: A tale of two “hits”? Gastroenterology 1998, 114, 842–845. [Google Scholar] [CrossRef]
- Pessayre, D.; Fromenty, B. NASH: A mitochondrial disease. J. Hepatol. 2005, 42, 928–940. [Google Scholar] [CrossRef]
- Nath, B.; Szabo, G. Alcohol-induced Modulation of Signaling Pathways in Liver Parenchymal and Nonparenchymal Cells: Implications for Immunity. Semin. Liver Dis. 2009, 29, 166–177. [Google Scholar] [CrossRef] [PubMed]
- Irshad, M. Oxidative stress in liver diseases. Trop. Gastroenterol. 2002, 23, 6–8. [Google Scholar] [PubMed]
- Arauz, J.; Ramos-Tovar, E.; Muriel, P. Redox state and methods to evaluate oxidative stress in liver damage: From bench to bedside. Ann. Hepatol. 2016, 15, 160–173. [Google Scholar]
- Li, G.; Scull, C.; Ozcan, L.; Tabas, I. NADPH oxidase links endoplasmic reticulum stress, oxidative stress, and PKR activation to induce apoptosis. J. Cell Biol. 2010, 191, 1113–1125. [Google Scholar] [CrossRef] [PubMed]
- Urtasun, R.; de la Rosa, L.C.; Nieto, N. Oxidative and Nitrosative Stress and Fibrogenic Response. Clin. Liver Dis. 2008, 12, 769–790. [Google Scholar] [CrossRef]
- Balaban, R.S.; Nemoto, S.; Finkel, T. Mitochondria, oxidants, and aging. Cell 2005, 120, 483–495. [Google Scholar] [CrossRef]
- Herzig, S.; Shaw, R.J. AMPK: Guardian of metabolism and mitochondrial homeostasis. Nat. Rev. Mol. Cell Biol. 2018, 19, 121–135. [Google Scholar] [CrossRef]
- Meakin, P.J.; Chowdhry, S.; Sharma, R.S.; Ashford, F.B.; Walsh, S.V.; McCrimmon, R.J.; Dinkova-Kostova, A.T.; Dillon, J.F.; Hayes, J.D.; Ashford, M.L.J. Susceptibility of Nrf2-Null Mice to Steatohepatitis and Cirrhosis upon Consumption of a High-Fat Diet Is Associated with Oxidative Stress, Perturbation of the Unfolded Protein Response, and Disturbance in the Expression of Metabolic Enzymes but Not with Insulin Resistance. Mol. Cell Biol. 2014, 34, 3305–3320. [Google Scholar]
- Pessayre, D.; Fromenty, B.; Berson, A.; Robin, M.-A.; Lettéron, P.; Moreau, R.; Mansouri, A. Central role of mitochondria in drug-induced liver injury. Drug Metab. Rev. 2012, 44, 34–87. [Google Scholar] [CrossRef]
- Fromenty, B.; Pessayre, D. Inhibition of mitochondrial beta-oxidation as a mechanism of hepatotoxicity. Pharmacol. Ther. 1995, 67, 101–154. [Google Scholar] [CrossRef]
- Koliaki, C.; Szendroedi, J.; Kaul, K.; Jelenik, T.; Nowotny, P.; Jankowiak, F.; Herder, C.; Carstensen, M.; Krausch, M.; Knoefel, W.T.; et al. Adaptation of Hepatic Mitochondrial Function in Humans with Non-Alcoholic Fatty Liver Is Lost in Steatohepatitis. Cell Metab. 2015, 21, 739–746. [Google Scholar] [CrossRef] [PubMed]
- Gusdon, A.M.; Song, K.-X.; Qu, S. Nonalcoholic Fatty Liver Disease: Pathogenesis and Therapeutics from a Mitochondria-Centric Perspective. Oxidat. Med. Cell Longev. 2014, 2014, 637027. [Google Scholar] [CrossRef]
- Canto, C.; Auwerx, J. PGC-1α, SIRT1 and AMPK, an energy sensing network that controls energy expenditure. Curr. Opin. Lipidol. 2009, 20, 98–105. [Google Scholar] [CrossRef]
- McGarry, J.D.; Foster, D.W. Regulation of Hepatic Fatty Acid Oxidation and Ketone Body Production. Annu. Rev. Biochem. 1980, 49, 395–420. [Google Scholar] [CrossRef]
- Mansouri, A.; Gattolliat, C.-H.; Asselah, T. Mitochondrial Dysfunction and Signaling in Chronic Liver Diseases. Gastroenterology 2018, 155, 629–647. [Google Scholar] [CrossRef] [PubMed]
- Begriche, K.; Igoudjil, A.; Pessayre, D.; Fromenty, B. Mitochondrial dysfunction in NASH: Causes, consequences and possible means to prevent it. Mitochondrion 2006, 6, 1–28. [Google Scholar] [CrossRef]
- Siegmund, S.E.; Grassucci, R.; Carter, S.D.; Barca, E.; Farino, Z.J.; Juanola-Falgarona, M.; Zhang, P.; Tanji, K.; Hirano, M.; Schon, E.A.; et al. Three-Dimensional Analysis of Mitochondrial Crista Ultrastructure in a Patient with Leigh Syndrome by In Situ Cryoelectron Tomography. iScience 2018, 6, 83–91. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.-C.; Chen, Y.-B.; Lin, H.; Pi, H.-F.; Zhang, N.-X.; Zhao, C.-C.; Shuai, L.; Zhong, M.; Yu, Z.-P.; Zhou, Z.; et al. Damage to mtDNA in liver injury of patients with extrahepatic cholestasis: The protective effects of mitochondrial transcription factor A. Free Radic. Biol. Med. 2012, 52, 1543–1551. [Google Scholar] [CrossRef] [PubMed]
- Kleniewska, P.; Piechota-Polanczyk, A.; Michalski, L.; Michalska, M.; Balcerczak, E.; Zebrowska, M.; Goraca, A. Influence of Block of NF-Kappa B Signaling Pathway on Oxidative Stress in the Liver Homogenates. Oxid. Med. Cell Longev. 2013, 2013, 308358. [Google Scholar] [CrossRef] [PubMed]
- Lu, S.C. Antioxidants in the treatment of chronic liver diseases: Why is the efficacy evidence so weak in humans? Hepatology 2008, 48, 1359–1361. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Vascotto, C.; Xu, S.; Dai, N.; Qing, Y.; Zhong, Z.; Tell, G.; Wang, D. Human AP endonuclease/redox factor APE1/ref-1 modulates mitochondrial function after oxidative stress by regulating the transcriptional activity of NRF1. Free Radic. Biol. Med. 2012, 53, 237–248. [Google Scholar] [CrossRef] [PubMed]
- Cichoż-Lach, H.; Michalak, A. Oxidative stress as a crucial factor in liver diseases. World J. Gastroenterol. 2014, 20, 8082–8091. [Google Scholar] [CrossRef] [PubMed]
- Ayasuriya, R.; Dhamodharan, U.; Ali, D.; Ganesan, K.; Xu, B.; Ramkumar, K.M. Targeting Nrf2/Keap1 signaling pathway by bioactive natural agents: Possible therapeutic strategy to combat liver disease. Phytomedicine 2021, 92, 153755. [Google Scholar] [CrossRef]
- Li, F.; Liao, X.; Jiang, L.; Zhao, J.; Wu, S.; Ming, J. Orientin Attenuated d-GalN/LPS-Induced Liver Injury through the Inhibition of Oxidative Stress via Nrf2/Keap1 Pathway. J. Agric. Food Chem. 2022, 70, 7953–7967. [Google Scholar] [CrossRef]
- Song, X.; Sun, W.; Cui, W.; Jia, L.; Zhang, J. A polysaccharide of PFP-1 from Pleurotus geesteranus attenuates alcoholic liver diseases via Nrf2 and NF-κB signaling pathways. Food Funct. 2021, 12, 4591–4605. [Google Scholar] [CrossRef]
- Aleksunes, L.M.; Manautou, J.E. Emerging Role of Nrf2 in Protecting Against Hepatic and Gastrointestinal Disease. Toxicol. Pathol. 2007, 35, 459–473. [Google Scholar] [CrossRef]
- Collins, A.R.; Gupte, A.A.; Ji, R.; Ramirez, M.R.; Minze, L.J.; Liu, J.Z.; Arredondo, M.; Ren, Y.; Deng, T.; Wang, J.; et al. Myeloid Deletion of Nuclear Factor Erythroid 2−Related Factor 2 Increases Atherosclerosis and Liver Injury. Arter. Thromb. Vasc. Biol. 2012, 32, 2839–2846. [Google Scholar] [CrossRef]
- D’Amico, G.; Garcia-Tsao, G.; Pagliaro, L. Natural history and prognostic indicators of survival in cirrhosis: A systematic review of 118 studies. J. Hepatol. 2006, 44, 217–231. [Google Scholar] [CrossRef]
- Rahman, M.A.; Akter, S.; Dorotea, D.; Mazumder, A.; Uddin, M.N.; Hannan, M.A.; Hossen, M.J.; Ahmed, M.S.; Kim, W.; Kim, B.; et al. Renoprotective potentials of small molecule natural products targeting mitochondrial dysfunction. Front. Pharmacol. 2022, 13, 925993. [Google Scholar] [CrossRef]
- Friedman, S.L. Mechanisms of hepatic fibrogenesis. Gastroenterology 2008, 134, 1655–1669. [Google Scholar] [CrossRef] [PubMed]
- Salvado, M.D.; Alfranca, A.; Haeggström, J.Z.; Redondo, J.M. Prostanoids in tumor angiogenesis: Therapeutic intervention beyond COX-2. Trends Mol. Med. 2012, 18, 233–243. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Stevens, J.; Hilton, M.B.; Seaman, S.; Conrads, T.P.; Veenstra, T.D.; Logsdon, D.; Morris, H.; Swing, D.A.; Patel, N.L.; et al. COX-2 Inhibition Potentiates Antiangiogenic Cancer Therapy and Prevents Metastasis in Preclinical Models. Sci. Transl. Med. 2014, 6, 242ra84. [Google Scholar] [CrossRef]
- Goos, J.A.; Hiemstra, A.C.; Coupé, V.M.; Diosdado, B.; Kooijman, W.; Delis-Van Diemen, P.M.; Karga, C.; Beliën, J.A.; Menke-van der Houven van Oordt, C.W.; Geldof, A.A.; et al. Epidermal growth factor receptor (egfr) and prostaglandin-endoperoxide synthase 2 (ptgs2) are prognostic biomarkers for patients with resected colorectal cancer liver metastases. Br. J. Cancer 2014, 111, 749–755. [Google Scholar] [CrossRef] [PubMed]
- Peglow, S.; Toledo, A.H.; Anaya-Prado, R.; Lopez-Neblina, F.; Toledo-Pereyra, L.H. Allopurinol and xanthine oxidase inhibition in liver ischemia reperfusion. J. Hepato-Biliary-Pancreat. Sci. 2011, 18, 137–146. [Google Scholar] [CrossRef] [PubMed]
- Taha, M.; Simões, M.; Noguerol, E.; Mendonça, F.; Pascoalick, H.; Alves, R.; Vivian, M.; Morales, F.; Campos, A.; Magalhães, K.; et al. Effects of Allopurinol on Ischemia and Reperfusion in Rabbit Livers. Transplant. Proc. 2009, 41, 820–823. [Google Scholar] [CrossRef] [PubMed]
- Hwang, C.; Sinskey, A.J.; Lodish, H.F. Oxidized redox state of glutathione in the endoplasmic reticulum. Science 1992, 257, 1496–1502. [Google Scholar] [CrossRef]
- Ray, R.; Shah, A.M. NADPH oxidase and endothelial cell function. Clin. Sci. 2005, 109, 217–226. [Google Scholar] [CrossRef]
- Marí, M.; Morales, A.; Colell, A.; García-Ruiz, C.; Kaplowitz, N.; Fernández-Checa, J.C. Mitochondrial glutathione: Features, regulation and role in disease. Biochim. Biophys. Acta 2013, 1830, 3317–3328. [Google Scholar] [CrossRef]
- Ribas, V.; García-Ruiz, C.; Fernández-Checa, J.C. Glutathione and mitochondria. Front. Pharmacol. 2014, 5, 151. [Google Scholar] [CrossRef]
- Zaghloul, R.A.; Zaghloul, A.M.; El-Kashef, D.H. Hepatoprotective effect of Baicalin against thioacetamide-induced cirrhosis in rats: Targeting NOX4/NF-κB/NLRP3 inflammasome signaling pathways. Life Sci. 2022, 295, 120410. [Google Scholar] [CrossRef] [PubMed]
- Yen, I.-C.; Tu, Q.-W.; Chang, T.-C.; Lin, P.-H.; Li, Y.-F.; Lee, S.-Y. 4-Acetylantroquinonol B ameliorates nonalcoholic steatohepatitis by suppression of ER stress and NLRP3 inflammasome activation. Biomed. Pharmacother. 2021, 138, 111504. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y.F.; Xu, Q.Q.; Chen, T.Q.; Ming, J.X.; Wang, Y.F.; Mao, L.N.; Zhou, J.J.; Sun, W.G.; Zhou, Q.; Ren, H.; et al. Kinsenoside alleviates inflammation and fibrosis in experimental NASH mice by suppressing the NF-κB/NLRP3 signaling pathway. Phytomedicine 2022, 104, 154241. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Sun, Y.; Zhu, Y.; Qiao, S.; Cai, J.; Zhang, Z. Melatonin relieves liver fibrosis induced by Txnrd3 knockdown and nickel exposure via IRE1/NF-κB/NLRP3 and PERK/TGF-β1 axis activation. Life Sci. 2022, 301, 120622. [Google Scholar] [CrossRef]
- Lv, Y.; Gao, X.; Luo, Y.; Fan, W.; Shen, T.; Ding, C.; Yao, M.; Song, S.; Yan, L. Apigenin ameliorates hfd-induced nafld through regulation of the xo/nlrp3 pathways. J. Nutr. Biochem. 2019, 71, 110–121. [Google Scholar] [CrossRef]
- Zhu, Z.; Hu, R.; Li, J.; Xing, X.; Chen, J.; Zhou, Q.; Sun, J. Alpinetin exerts anti-inflammatory, anti-oxidative and anti-angiogenic effects through activating the Nrf2 pathway and inhibiting NLRP3 pathway in carbon tetrachloride-induced liver fibrosis. Int. Immunopharmacol. 2021, 96, 107660. [Google Scholar] [CrossRef]
- Wang, A.; Gong, Y.; Pei, Z.; Jiang, L.; Xia, L.; Wu, Y. Paeoniflorin ameliorates diabetic liver injury by targeting the TXNIP-mediated NLRP3 inflammasome in db/db mice. Int. Immunopharmacol. 2022, 109, 108792. [Google Scholar]
- Friedman, S.L.; Neuschwander-Tetri, B.A.; Rinella, M.; Sanyal, A.J. Mechanisms of NAFLD development and therapeutic strategies. Nat. Med. 2018, 24, 908–922. [Google Scholar] [CrossRef]
- Bessone, F.; Razori, M.V.; Roma, M.G. Molecular pathways of nonalcoholic fatty liver disease development and progression. Cell Mol. Life Sci. 2019, 76, 99–128. [Google Scholar] [CrossRef]
- Schuster, S.; Cabrera, D.; Arrese, M.; Feldstein, A.E. Triggering and resolution of inflammation in NASH. Nat. Rev. Gastroenterol. Hepatol. 2018, 15, 349–364. [Google Scholar] [CrossRef]
- Dornas, W.; Schuppan, D. Mitochondrial oxidative injury: A key player in nonalcoholic fatty liver disease. Am. J. Physiol. Liver Physiol. 2020, 319, G400–G411. [Google Scholar] [CrossRef] [PubMed]
- Di Ciaula, A.; Passarella, S.; Shanmugam, H.; Noviello, M.; Bonfrate, L.; Wang, D.Q.H.; Portincasa, P. Nonalcoholic Fatty Liver Disease (NAFLD). Mitochondria as Players and Targets of Therapies? Int. J. Mol. Sci. 2021, 22, 5375. [Google Scholar] [CrossRef] [PubMed]
- Lindenmeyer, C.C.; McCullough, A.J. The Natural History of Nonalcoholic Fatty Liver Disease-An Evolving View. Clin. Liver Dis. 2018, 22, 11–21. [Google Scholar] [CrossRef]
- Rinella, M.E.; Sanyal, A.J. Management of NAFLD: A stage-based approach. Nat. Rev. Gastroenterol. Hepatol. 2016, 13, 196–205. [Google Scholar] [CrossRef] [PubMed]
- Eslam, M.; Newsome, P.N.; Sarin, S.K.; Anstee, Q.M.; Targher, G.; Romero-Gomez, M.; Zelber-Sagi, S.; Wong, V.W.-S.; Dufour, J.-F.; Schattenberg, J.M.; et al. A new definition for metabolic dysfunction-associated fatty liver disease: An international expert consensus statement. J. Hepatol. 2020, 73, 202–209. [Google Scholar] [CrossRef]
- Chen, Z.; Yu, Y.; Cai, J.; Li, H. Emerging Molecular Targets for Treatment of Nonalcoholic Fatty Liver Disease. Trends Endocrinol. Metab. 2019, 30, 903–914. [Google Scholar] [CrossRef]
- Di Ciaula, A.; Baj, J.; Garruti, G.; Celano, G.; De Angelis, M.; Wang, H.H.; Di Palo, D.M.; Bonfrate, L.; Wang, D.Q.-H.; Portincasa, P. Liver Steatosis, Gut-Liver Axis, Microbiome and Environmental Factors. A Never-Ending Bidirectional Cross-Talk. J. Clin. Med. 2020, 9, 2648. [Google Scholar] [CrossRef]
- Di Palo, D.M.; Garruti, G.; Di Ciaula, A.; Molina-Molina, E.; Shanmugam, H.; De Angelis, M.; Portincasa, P. Increased Colonic Permeability and Lifestyles as Contributing Factors to Obesity and Liver Steatosis. Nutrients 2020, 12, 564. [Google Scholar] [CrossRef]
- Di Ciaula, A.; Carbone, F.; Shanmugham, H.; Molina-Molina, E.; Bonfrate, L.; Ministrini, S.; Montecucco, F.; Portincasa, P. Adiponectin involved in portal flow hepatic extraction of 13C-methacetin in obesity and non-alcoholic fatty liver. Eur. J. Intern. Med. 2021, 89, 56–64. [Google Scholar] [CrossRef]
- Lauridsen, B.K.; Stender, S.; Kristensen, T.S.; Kofoed, K.F.; Køber, L.; Nordestgaard, B.G.; Tybjaerg-Hansen, A. Liver fat content, non-alcoholic fatty liver disease, and ischaemic heart disease: Mendelian randomization and meta-analysis of 279 013 individuals. Eur. Heart J. 2018, 39, 385–393. [Google Scholar] [CrossRef]
- Huang, Z.; Guo, X.; Zhang, G.; Liang, L.; Nong, B. Correlation between PNPLA3 rs738409 polymorphism and hepatocellular carcinoma: A meta-analysis of 10,330 subjects. Int. J. Biol. Mrk. 2019, 34, 117–122. [Google Scholar] [CrossRef]
- Krawczyk, M.; Bonfrate, L.; Portincasa, P. Nonalcoholic fatty liver disease. Best Pract. Res. Clin. Gastroenterol. 2010, 24, 695–708. [Google Scholar] [CrossRef]
- Thabet, K.; Chan, H.L.Y.; Petta, S.; Mangia, A.; Berg, T.; Boonstra, A.; Brouwer, W.P.; Abate, M.L.; Wong, V.W.-S.; Nazmy, M.; et al. The membrane-bound O-acyltransferase domain-containing 7 variant rs641738 increases inflammation and fibrosis in chronic hepatitis B. Hepatology 2017, 65, 1840–1850. [Google Scholar] [CrossRef] [PubMed]
- Meroni, M.; Longo, M.; Tria, G.; Dongiovanni, P. Genetics Is of the Essence to Face NAFLD. Biomedicines 2021, 9, 1359. [Google Scholar] [CrossRef] [PubMed]
- Petta, S.; Miele, L.; Bugianesi, E.; Camma’, C.; Rosso, C.; Boccia, S.; Cabibi, D.; Di Marco, V.; Grimaudo, S.; Grieco, A.; et al. Glucokinase Regulatory Protein Gene Polymorphism Affects Liver Fibrosis in Non-Alcoholic Fatty Liver Disease. PLoS ONE 2014, 9, e87523. [Google Scholar] [CrossRef]
- Luukkonen, P.K.; Tukiainen, T.; Juuti, A.; Sammalkorpi, H.; Haridas, P.N.; Niemelä, O.; Arola, J.; Orho-Melander, M.; Hakkarainen, A.; Kovanen, P.T.; et al. Hydroxysteroid 17-β dehydrogenase 13 variant increases phospholipids and protects against fibrosis in nonalcoholic fatty liver disease. JCI Insight 2020, 5, e132158. [Google Scholar] [CrossRef]
- Wei, Y.; Clark, S.E.; Thyfault, J.P.; Uptergrove, G.M.; Li, W.; Whaley-Connell, A.T.; Ferrario, C.M.; Sowers, J.R.; Ibdah, J.A. Oxidative Stress-Mediated Mitochondrial Dysfunction Contributes to Angiotensin II-Induced Nonalcoholic Fatty Liver Disease in Transgenic Ren2 Rats. Am. J. Pathol. 2009, 174, 1329–1337. [Google Scholar] [CrossRef]
- Yan, J.; Jiang, J.; He, L.; Chen, L. Mitochondrial superoxide/hydrogen peroxide: An emerging therapeutic target for metabolic diseases. Free Radic. Biol. Med. 2020, 152, 33–42. [Google Scholar] [CrossRef]
- Tell, G.; Vascotto, C.; Tiribelli, C. Alterations in the redox state and liver damage: Hints from the EASL Basic School of Hepatology. J. Hepatol. 2013, 58, 365–374. [Google Scholar] [CrossRef]
- Sanyal, A.J.; Campbell–Sargent, C.; Mirshahi, F.; Rizzo, W.B.; Contos, M.J.; Sterling, R.K.; Luketic, V.A.; Shiffman, M.L.; Clore, J.N. Nonalcoholic steatohepatitis: Association of insulin resistance and mitochondrial abnormalities. Gastroenterology 2001, 120, 1183–1192. [Google Scholar] [CrossRef]
- Cook, G.A.; Gamble, M.S. Regulation of carnitine palmitoyltransferase by insulin results in decreased activity and decreased apparent Ki values for malonyl-CoA. J. Biol. Chem. 1987, 262, 2050–2055. [Google Scholar] [CrossRef]
- Rautou, P.-E.; Cazals-Hatem, D.; Feldmann, G.; Mansouri, A.; Grodet, A.; Barge, S.; Martinot-Peignoux, M.; Duces, A.; Bieche, I.; Lebrec, D.; et al. Changes in Autophagic Response in Patients with Chronic Hepatitis C Virus Infection. Am. J. Pathol. 2011, 178, 2708–2715. [Google Scholar] [CrossRef]
- Madrigal-Matute, J.; Cuervo, A.M. Regulation of Liver Metabolism by Autophagy. Gastroenterology 2016, 150, 328–339. [Google Scholar] [CrossRef] [PubMed]
- Williams, J.A.; Ding, W.-X. A Mechanistic Review of Mitophagy and Its Role in Protection against Alcoholic Liver Disease. Biomolecules 2015, 5, 2619–2642. [Google Scholar] [CrossRef] [PubMed]
- von Montfort, C.; Matias, N.; Fernandez, A.; Fucho, R.; de la Rosa, L.C.; Martinez-Chantar, M.L.; Mato, J.M.; Machida, K.; Tsukamoto, H.; Murphy, M.P.; et al. Mitochondrial GSH determines the toxic or therapeutic potential of superoxide scavenging in steatohepatitis. J. Hepatol. 2012, 57, 852–859. [Google Scholar] [CrossRef]
- Marí, M.; Morales, A.; Colell, A.; García-Ruiz, C.; Fernández-Checa, J.C. Mitochondrial cholesterol accumulation in alcoholic liver disease: Role of ASMase and endoplasmic reticulum stress. Redox Biol. 2014, 3, 100–108. [Google Scholar] [CrossRef] [PubMed]
- Pessayre, D.; Berson, A.; Fromenty, B.; Mansouri, A. Mitochondria in Steatohepatitis. Semin. Liver Dis. 2001, 21, 057–070. [Google Scholar] [CrossRef]
- Pessayre, D. Role of mitochondria in non-alcoholic fatty liver disease. J. Gastroenterol. Hepatol. 2007, 22 (Suppl. 1), S20–S27. [Google Scholar] [CrossRef] [PubMed]
- Larosche, I.; Choumar, A.; Fromenty, B.; Lettéron, P.; Abbey-Toby, A.; Van Remmen, H.; Epstein, C.J.; Richardson, A.; Feldmann, G.; Pessayre, D.; et al. Prolonged ethanol administration depletes mitochondrial DNA in MnSOD-overexpressing transgenic mice, but not in their wild type littermates. Toxicol. Appl. Pharmacol. 2009, 234, 326–338. [Google Scholar] [CrossRef] [PubMed]
- Radi, R.; Cassina, A.; Hodara, R.; Quijano, C.; Castro, L. Peroxynitrite reactions and formation in mitochondria. Free Radic. Biol. Med. 2002, 33, 1451–1464. [Google Scholar] [CrossRef]
- Yin, X.; Zheng, F.; Pan, Q.; Zhang, S.; Yu, D.; Xu, Z.; Li, H. Glucose fluctuation increased hepatocyte apoptosis under lipotoxicity and the involvement of mitochondrial permeability transition opening. J. Mol. Endocrinol. 2015, 55, 169–181. [Google Scholar] [CrossRef] [PubMed]
- Navarro, C.D.; Figueira, T.R.; Francisco, A.; Dal’Bó, G.A.; Ronchi, J.A.; Rovani, J.C.; Escanhoela, C.A.; Oliveira, H.C.; Castilho, R.F.; Vercesi, A.E. Redox imbalance due to the loss of mitochondrial NAD(P)-transhydrogenase markedly aggravates high fat diet-induced fatty liver disease in mice. Free Radic. Biol. Med. 2017, 113, 190–202. [Google Scholar] [CrossRef]
- Mansouri, A.; Fromenty, B.; Berson, A.; Robin, M.-A.; Grimbert, S.; Beaugrand, M.; Erlinger, S.; Pessayre, D. Multiple hepatic mitochondrial DNA deletions suggest premature oxidative aging in alcoholic patients. J. Hepatol. 1997, 27, 96–102. [Google Scholar] [CrossRef]
- Begriche, K.; Massart, J.; Robin, M.-A.; Borgne-Sanchez, A.; Fromenty, B. Drug-induced toxicity on mitochondria and lipid metabolism: Mechanistic diversity and deleterious consequences for the liver. J. Hepatol. 2011, 54, 773–794. [Google Scholar] [CrossRef]
- Okuda, M.; Li, K.; Beard, M.R.; Showalter, L.A.; Scholle, F.; Lemon, S.M.; Weinman, S.A. Mitochondrial injury, oxidative stress, and antioxidant gene expression are induced by hepatitis C virus core protein. Gastroenterology 2002, 122, 366–375. [Google Scholar] [CrossRef] [PubMed]
- Fisicaro, P.; Barili, V.; Montanini, B.; Acerbi, G.; Ferracin, M.; Guerrieri, F.; Salerno, D.; Boni, C.; Massari, M.; Cavallo, M.C.; et al. Targeting mitochondrial dysfunction can restore antiviral activity of exhausted HBV-specific CD8 T cells in chronic hepatitis B. Nat. Med. 2017, 23, 327–336. [Google Scholar] [CrossRef]
- Mansouri, A.; Gaou, I.; Fromenty, B.; Berson, A.; Letteron, P.; Degott, C.; Erlinger, S.; Pessayre, D. Premature oxidative aging of hepatic mitochondrial DNA in wilson’s disease. Gastroenterology 1997, 113, 599–605. [Google Scholar] [CrossRef]
- Nahon, P.; Sutton, A.; Rufat, P.; Charnaux, N.; Mansouri, A.; Moreau, R.; Ganne-Carrié, N.; Grando-Lemaire, V.; N’Kontchou, G.; Trinchet, J.-C.; et al. A variant in myeloperoxidase promoter hastens the emergence of hepatocellular carcinoma in patients with HCV-related cirrhosis. J. Hepatol. 2012, 56, 426–432. [Google Scholar] [CrossRef] [PubMed]
- Altamirano, J.; Bataller, R. Alcoholic liver disease: Pathogenesis and new targets for therapy. Nat. Rev. Gastroenterol. Hepatol. 2011, 8, 491–501. [Google Scholar] [CrossRef]
- French, S.W. Chronic alcohol binging injures the liver and other organs by reducing NAD+ levels required for sirtuin’s deacetylase activity. Exp. Mol. Pathol. 2016, 100, 303–306. [Google Scholar] [CrossRef] [PubMed]
- Robin, M.-A.; Anandatheerthavarada, H.K.; Fang, J.-K.; Cudic, M.; Otvos, L.; Avadhani, N.G. Mitochondrial Targeted Cytochrome P450 2E1 (P450 MT5) Contains an Intact N Terminus and Requires Mitochondrial Specific Electron Transfer Proteins for Activity. J. Biol. Chem. 2001, 276, 24680–24689. [Google Scholar] [CrossRef] [PubMed]
- Abdelmegeed, M.A.; Ha, S.-K.; Choi, Y.; Akbar, M.; Song, B.-J. Role of CYP2E1 in Mitochondrial Dysfunction and Hepatic Injury by Alcohol and Non-Alcoholic Substances. Curr. Mol. Pharmacol. 2017, 10, 207–225. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Guo, W.; Zhang, C.; Chen, F.; Tan, H.Y.; Li, S.; Wang, N.; Feng, Y. Herbal Medicine in the Treatment of Non-Alcoholic Fatty Liver Diseases-Efficacy, Action Mechanism, and Clinical Application. Front. Pharmacol. 2020, 11, 601. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Xu, Y.; Guo, W.; Chen, F.; Zhang, C.; Tan, H.Y.; Wang, N.; Feng, Y. The Impacts of Herbal Medicines and Natural Products on Regulating the Hepatic Lipid Metabolism. Front. Pharmacol. 2020, 11, 351. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Meng, F.; Liao, X.; Wang, Y.; Sun, Z.; Guo, F.; Li, X.; Meng, M.; Li, Y.; Sun, C. Therapeutic Role of Ursolic Acid on Ameliorating Hepatic Steatosis and Improving Metabolic Disorders in High-Fat Diet-Induced Non-Alcoholic Fatty Liver Disease Rats. PLoS ONE 2014, 9, e86724. [Google Scholar] [CrossRef] [PubMed]
- Hong, X.; Tang, H.; Wu, L.; Li, L. Protective effects of the Alisma orientalis extract on the experimental nonalcoholic fatty liver disease. J. Pharm. Pharmacol. 2006, 58, 1391–1398. [Google Scholar] [CrossRef]
- Donnelly, K.L.; Smith, C.I.; Schwarzenberg, S.J.; Jessurun, J.; Boldt, M.D.; Parks, E.J. Sources of fatty acids stored in liver and secreted via lipoproteins in patients with nonalcoholic fatty liver disease. J. Clin. Investig. 2005, 115, 1343–1351. [Google Scholar] [CrossRef]
- Teli, M.R.; James, O.F.; Burt, A.D.; Bennett, M.K.; Day, C.P. The natural history of nonalcoholic fatty liver: A follow-up study. Hepatology 1995, 22, 1714–1719. [Google Scholar] [CrossRef]
- Jeong, H.-S.; Cho, Y.-H.; Kim, K.-H.; Kim, Y.; Kim, K.-S.; Na, Y.-C.; Park, J.; Lee, I.-S.; Lee, J.-H.; Jang, H.-J. Anti-lipoapoptotic effects of Alisma orientalis extract on non-esterified fatty acid-induced HepG2 cells. BMC Complement. Altern. Med. 2016, 16, 239. [Google Scholar] [CrossRef]
- Zhao, M.-G.; Sheng, X.-P.; Huang, Y.-P.; Wang, Y.-T.; Jiang, C.-H.; Zhang, J.; Yin, Z.-Q. Triterpenic acids-enriched fraction from Cyclocarya paliurus attenuates non-alcoholic fatty liver disease via improving oxidative stress and mitochondrial dysfunction. Biomed. Pharmacother. 2018, 104, 229–239. [Google Scholar] [CrossRef]
- Ekstedt, M.; Franzén, L.E.; Holmqvist, M.; Bendtsen, P.; Mathiesen, U.L.; Bodemar, G.; Kechagias, S. Alcohol consumption is associated with progression of hepatic fibrosis in non-alcoholic fatty liver disease. Scand. J. Gastroenterol. 2009, 44, 366–374. [Google Scholar] [CrossRef] [PubMed]
- Marra, F.; Lotersztajn, S. Pathophysiology of NASH: Perspectives for a targeted treatment. Curr. Pharm. Des. 2013, 19, 5250–5269. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Zhao, Y.; Zhang, D.-B.; Sun, L.-J. Effect of Sinai San decoction on the development of non-alcoholic steatohepatitis in rats. World J. Gastroenterol. 2005, 11, 1392–1395. [Google Scholar] [CrossRef] [PubMed]
- Tang, W.; Zeng, L.; Yin, J.; Yao, Y.; Feng, L.; Yao, X.; Sun, X.; Zhou, B. Hugan QingzhiExerts Anti-Inflammatory Effects in a Rat Model of Nonalcoholic Fatty Liver Disease. Evid. Based Complement. Altern. Med. 2015, 2015, 810369. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.-Y.; Kwon, E.-Y.; Choi, M.-S. Elucidation of the Metabolic and Transcriptional Responses of an Oriental Herbal Medicine, Bangpungtongseong-san, to Nonalcoholic Fatty Liver Disease in Diet-Induced Obese Mice. J. Med. Food 2019, 22, 928–936. [Google Scholar] [CrossRef] [PubMed]
- Han, H.-Y.; Lee, S.-K.; Choi, B.-K.; Lee, D.-R.; Lee, H.J.; Kim, T.-W. Preventive Effect of Citrus aurantium Peel Extract on High-Fat Diet-Induced Non-alcoholic Fatty Liver in Mice. Biol. Pharm. Bull. 2019, 42, 255–260. [Google Scholar] [CrossRef]
- Yang, L.; Ren, S.; Xu, F.; Ma, Z.; Liu, X.; Wang, L. Recent Advances in the Pharmacological Activities of Dioscin. BioMed Res. Int. 2019, 2019, 5763602. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, H.; Deng, X.; Zhang, N.; Liu, B.; Xin, S.; Li, G.; Xu, K. Baicalin attenuates non-alcoholic steatohepatitis by suppressing key regulators of lipid metabolism, inflammation and fibrosis in mice. Life Sci. 2018, 192, 46–54. [Google Scholar] [CrossRef]
- Li, B.; Cheng, Z.; Sun, X.; Si, X.; Gong, E.; Wang, Y.; Tian, J.; Shu, C.; Ma, F.; Li, D.; et al. Lonicera caerulea L. Polyphenols Alleviate Oxidative Stress-Induced Intestinal Environment Imbalance and Lipopolysaccharide-Induced Liver Injury in HFD-Fed Rats by Regulating the Nrf2/HO-1/NQO1 and MAPK Pathways. Mol. Nutr. Food Res. 2020, 64, e1901315. [Google Scholar] [CrossRef]
- Li, H.; Wang, X.; Liu, Y.; Pan, D.; Wang, Y.; Yang, N.; Xiang, L.; Cai, X.; Feng, Y. Hepatoprotection and hepatotoxicity of Heshouwu, a Chinese medicinal herb: Context of the paradoxical effect. Food Chem. Toxicol. 2017, 108 Pt B, 407–418. [Google Scholar] [CrossRef]
- Hwang, Y.H.; Kang, K.Y.; Kim, J.J.; Lee, S.J.; Son, Y.J.; Paik, S.H.; Yee, S.T. Effects of Hot Water Extracts from Polygonum multiflorum on Ovariectomy Induced Osteopenia in Mice. Evid. Based Complement. Alternat. Med. 2016, 2016, 8970585. [Google Scholar] [CrossRef] [PubMed]
- Ling, S.; Xu, J.W. Biological Activities of 2,3,5,4′-Tetrahydroxystilbene-2-O-β-D-Glucoside in Antiaging and Antiaging-Related Disease Treatments. Oxid. Med. Cell Longev. 2016, 2016, 4973239. [Google Scholar] [CrossRef]
- Wang, T.; Wang, J.; Jiang, Z.; Zhou, Z.; Li, Y.; Zhang, L.; Zhang, L. Study on hepatotoxicity of aqueous extracts of Polygonum multiforum in rats after 28-day oral administration-analysis on correlation of cholestasis. China J. Chin. Mater. Med. 2012, 37, 1445–1450. [Google Scholar]
- Yu, J.; Xie, J.; Mao, X.-J.; Wang, M.-J.; Li, N.; Wang, J.; Zhaori, G.-T.; Zhao, R.-H. Hepatoxicity of major constituents and extractions of Radix Polygoni Multiflori and Radix Polygoni Multiflori Praeparata. J. Ethnopharmacol. 2011, 137, 1291–1299. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Lin, L.; Lin, H.; Qu, C.; Yan, L.; Ni, J. Interpretation the Hepatotoxicity Based on Pharmacokinetics Investigated Through Oral Administrated Different Extraction Parts of Polygonum multiflorum on Rats. Front. Pharmacol. 2018, 9, 505. [Google Scholar] [CrossRef] [PubMed]
- Knebel, B.; Lehr, S.; Hartwig, S.; Haas, J.; Kaber, G.; Dicken, H.-D.; Susanto, F.; Bohne, L.; Jacob, S.; Nitzgen, U.; et al. Phosphorylation of sterol regulatory element-binding protein (SREBP)-1c by p38 kinases, ERK and JNK influences lipid metabolism and the secretome of human liver cell line HepG2. Arch. Physiol. Biochem. 2014, 120, 216–227. [Google Scholar] [CrossRef] [PubMed]
- Kotzka, J.; Knebel, B.; Janssen, O.E.; Schaefer, J.; Soufi, M.; Jacob, S.; Nitzgen, U.; Muller-Wieland, D. Identification of a gene variant in the master regulator of lipid metabolism SREBP-1 in a family with a novel form of severe combined hypolipidemia. Atherosclerosis 2011, 218, 134–143. [Google Scholar] [CrossRef] [PubMed]
- Chun, Y.-S.; Ku, S.-K.; Kim, J.-K.; Park, S.; Cho, I.-H.; Lee, N.-J. Hepatoprotective and anti-obesity effects of Korean blue honeysuckle extracts in high fat diet-fed mice. J. Exerc. Nutr. Biochem. 2018, 22, 39–54. [Google Scholar] [CrossRef]
- Musso, G.; Gambino, R.; Cassader, M. Recent insights into hepatic lipid metabolism in non-alcoholic fatty liver disease (NAFLD). Prog. Lipid Res. 2009, 48, 1–26. [Google Scholar] [CrossRef] [PubMed]
- Strable, M.S.; Ntambi, J.W. Genetic control of de novo lipogenesis: Role in diet-induced obesity. Crit. Rev. Biochem. Mol. Biol. 2010, 45, 199–214. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhao, J.; Zheng, H.; Zhong, X.; Zhou, J.; Hong, Z. Treatment of Nonalcoholic Fatty Liver Disease with Total Alkaloids in Rubus aleaefolius Poir through Regulation of Fat Metabolism. Evid. Based Complement. Altern. Med. 2014, 2014, 768540. [Google Scholar] [CrossRef]
- Kim, H.Y.; Kang, K.S.; Yamabe, N.; Yokozawa, T. Comparison of the Effects of Korean Ginseng and Heat-Processed Korean Ginseng on Diabetic Oxidative Stress. Am. J. Chin. Med. 2008, 36, 989–1004. [Google Scholar] [CrossRef]
- Wu, Y.L.; Wan, Y.; Jin, X.J.; OuYang, B.Q.; Bai, T.; Zhao, Y.Q.; Nan, J.X. 25-OCH3-PPD induces the apoptosis of activated t-HSC/Cl-6 cells via c-FLIP-mediated NF-κB activation. Chem. Biol. Interact. 2011, 194, 106–112. [Google Scholar] [CrossRef]
- Geng, J.; Peng, W.; Huang, Y.; Fan, H.; Li, S. Ginsenoside-Rg1 from Panax notoginseng prevents hepatic fibrosis induced by thioacetamide in rats. Eur. J. Pharmacol. 2010, 634, 162–169. [Google Scholar] [CrossRef]
- Ki, S.H.; Yang, J.H.; Ku, S.K.; Kim, S.C.; Kim, Y.W.; Cho, I.J. Red ginseng extract protects against carbon tetrachloride-induced liver fibrosis. J. Ginseng Res. 2013, 37, 45–53. [Google Scholar] [CrossRef]
- Qian, H.; Shi, J.; Fan, T.-T.; Lv, J.; Chen, S.-W.; Song, C.-Y.; Zheng, Z.-W.; Xie, W.-F.; Chen, Y.-X. Sophocarpine attenuates liver fibrosis by inhibiting the TLR4 signaling pathway in rats. World J. Gastroenterol. 2014, 20, 1822–1832. [Google Scholar] [CrossRef] [PubMed]
- Song, C.-Y.; Shi, J.; Zeng, X.; Zhang, Y.; Xie, W.-F.; Chen, Y.-X. Sophocarpine alleviates hepatocyte steatosis through activating AMPK signaling pathway. Toxicology 2013, 27, 1065–1071. [Google Scholar] [CrossRef]
- Choi, E.; Jang, E.; Lee, J.-H. Pharmacological Activities of Alisma orientale against Nonalcoholic Fatty Liver Disease and Metabolic Syndrome: Literature Review. Evid. Based Complement. Altern. Med. 2019, 2019, 2943162. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.-R.; Park, K.I.; Ma, J.Y. Leonurus japonicus Houtt Attenuates Nonalcoholic Fatty Liver Disease in Free Fatty Acid-Induced HepG2 Cells and Mice Fed a High-Fat Diet. Nutrients 2018, 10, 20. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Theriault, A.G.; Au, K.; Douglas, T.D.; Casaschi, A.; Kurowska, E.M.; Mukherjee, R. Citrus polymethoxylated flavones improve lipid and glucose homeostasis and modulate adipocytokines in fructose-induced insulin resistant hamsters. Life Sci. 2006, 79, 365–373. [Google Scholar] [CrossRef] [PubMed]
- Hui, Y.; Wang, X.; Yu, Z.; Fan, X.; Cui, B.; Zhao, T.; Mao, L.; Feng, H.; Lin, L.; Yu, Q.; et al. Scoparone as a therapeutic drug in liver diseases: Pharmacology, pharmacokinetics and molecular mechanisms of action. Pharmacol. Res. 2020, 160, 105170. [Google Scholar] [CrossRef]
- Liu, C.; Sun, M.; Yan, X.; Han, L.; Zhang, Y.; Liu, C.; El-Nezami, H.; Liu, P. Inhibition of hepatic stellate cell activation following Yinchenhao decoction administration to dimethylnitrosamine-treated rats. Hepatol. Res. 2008, 38, 919–929. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Sun, M.; Wang, L.; Wang, G.; Chen, G.; Liu, C.; Liu, P. Effects of Yinchenhao Tang and related decoctions on DMN-induced cirrhosis/fibrosis in rats. Chin. Med. 2008, 3, 1. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.-S.; Cha, B.-Y.; Saito, K.; Yamakawa, H.; Choi, S.-S.; Yamaguchi, K.; Yonezawa, T.; Teruya, T.; Nagai, K.; Woo, J.-T. Nobiletin improves hyperglycemia and insulin resistance in obese diabetic ob/ob mice. Biochem. Pharmacol. 2010, 79, 1674–1683. [Google Scholar] [CrossRef] [PubMed]
- Mulvihill, E.E.; Burke, A.C.; Huff, M.W. Citrus Flavonoids as Regulators of Lipoprotein Metabolism and Atherosclerosis. Annu. Rev. Nutr. 2016, 36, 275–299. [Google Scholar] [CrossRef]
- Li, S.; Li, X.; Chen, F.; Liu, M.; Ning, L.; Yan, Y.; Zhang, S.; Huang, S.; Tu, C. Nobiletin mitigates hepatocytes death, liver inflammation, and fibrosis in a murine model of NASH through modulating hepatic oxidative stress and mitochondrial dysfunction. J. Nutr. Biochem. 2021, 100, 108888. [Google Scholar] [CrossRef]
- Mahmoud, A.M.; Bautista, R.J.H.; Sandhu, M.A.; Hussein, O.E. Beneficial Effects of Citrus Flavonoids on Cardiovascular and Metabolic Health. Oxid. Med. Cell Longev. 2019, 2019, 5484138. [Google Scholar] [CrossRef]
- Lee, Y.-S.; Cha, B.-Y.; Choi, S.-S.; Choi, B.-K.; Yonezawa, T.; Teruya, T.; Nagai, K.; Woo, J.-T. Nobiletin improves obesity and insulin resistance in high-fat diet-induced obese mice. J. Nutr. Biochem. 2013, 24, 156–162. [Google Scholar] [CrossRef] [PubMed]
- Nie, Y.; Liu, Q.; Zhang, W.; Wan, Y.; Huang, C.; Zhu, X. Ursolic acid reverses liver fibrosis by inhibiting NOX4/NLRP3 inflammasome pathways and bacterial dysbiosis. Gut Microbes 2021, 13, 1972746. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Tang, K.; Deng, Y.; Chen, R.; Liang, S.; Xie, H.; He, Y.; Chen, Y.; Yang, Q. Effects of shenling baizhu powder herbal formula on intestinal microbiota in high-fat diet-induced NAFLD rats. Biomed. Pharmacother. 2018, 102, 1025–1036. [Google Scholar] [CrossRef]
- Li, Y.; Liu, T.; Yan, C.; Xie, R.; Guo, Z.; Wang, S.; Zhang, Y.; Li, Z.; Wang, B.; Cao, H. Diammonium Glycyrrhizinate Protects against Nonalcoholic Fatty Liver Disease in Mice through Modulation of Gut Microbiota and Restoration of Intestinal Barrier. Mol. Pharm. 2018, 15, 3860–3870. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.A.; Bishayee, K.; Habib, K.; Sadra, A.; Huh, S.O. 18α-Glycyrrhetinic acid lethality for neuroblastoma cells via de-regulating the Beclin-1/Bcl-2 complex and inducing apoptosis. Biochem. Pharmacol. 2016, 117, 97–112. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Aquino, E.; Muriel, P. Beneficial effects of naringenin in liver diseases: Molecular mechanisms. World J. Gastroenterol. 2018, 24, 1679–1707. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, M.S.; Uddin, M.J.; Hossen, M.J.; Rahman, M.A.; Mohibbullah, M.; Hannan, M.A.; Choi, J.S. Dendritic Cells (DCs)-Based Cancer Immunotherapy: A Review on the Prospects of Medicinal Plants and Their Phytochemicals as Potential Pharmacological Modulators. Appl. Sci. 2022, 12, 9452. [Google Scholar] [CrossRef]
- Aghemo, A.; Alekseeva, O.P.; Angelico, F.; Bakulin, I.G.; Bakulina, N.V.; Bordin, D.; Bueverov, A.O.; Drapkina, O.M.; Gillessen, A.; Kagarmanova, E.M.; et al. Role of silymarin as antioxidant in clinical management of chronic liver diseases: A narrative review. Ann. Med. 2022, 54, 1548–1560. [Google Scholar] [CrossRef]
- Sun, L.; Wen, S.; Li, Q.; Lai, X.; Chen, R.; Zhang, Z.; Li, D.; Sun, S. L-theanine relieves acute alcoholic liver injury by regulating the TNF-α/NF-κB signaling pathway in C57BL/6J mice. J. Funct. Foods 2021, 86, 104699. [Google Scholar] [CrossRef]
- Sukkasem, N.; Chatuphonprasert, W.; Jarukamjorn, K. Alteration of murine cytochrome p450 profiles in fatty liver disease by hesperidin and myricetin. Pharmacogn. Mag. 2022, 18, 89. [Google Scholar]
- Viswanathan, P.; Gupta, P.; Sharma, Y.; Maisuradze, L.; Bandi, S.; Gupta, S. Caffeine disrupts ataxia telangiectasia mutated gene-related pathways and exacerbates acetaminophen toxicity in human fetal immortalized hepatocytes. Toxicology 2021, 457, 152811. [Google Scholar] [CrossRef]
- Zhao, X.; Wang, J.; Deng, Y.; Liao, L.; Zhou, M.; Peng, C.; Li, Y. Quercetin as a protective agent for liver diseases: A comprehensive descriptive review of the molecular mechanism. Phytother. Res. 2021, 35, 4727–4747. [Google Scholar] [CrossRef]
- Akter, S.; Akhter, H.; Chaudhury, H.S.; Rahman, H.; Gorski, A.; Hasan, M.N.; Shin, Y.; Rahman, A.; Nguyen, M.N.; Choi, T.G.; et al. Dietary carbohydrates: Pathogenesis and potential therapeutic targets to obesity-associated metabolic syndrome. BioFactors 2022, 48, 1036–1059. [Google Scholar] [CrossRef] [PubMed]
- Hu, N.; Liu, J.; Xue, X.; Li, Y. The effect of emodin on liver disease—Comprehensive advances in molecular mechanisms. Eur. J. Pharmacol. 2020, 882, 173269. [Google Scholar] [CrossRef]
- Zheng, Y.Z.; Deng, G.; Guo, R.; Fu, Z.M.; Chen, D.F. The influence of the H5⋯OC4 intramolecular hydrogen-bond (IHB) on the antioxidative activity of flavonoid. Phytochemistry 2019, 160, 19–24. [Google Scholar] [CrossRef] [PubMed]
- Scalbert, A.; Johnson, I.; Saltmarsh, M. Polyphenols: Antioxidants and beyond. Am. J. Clin. Nutr. 2005, 81 (Suppl. 1), 215S–217S. [Google Scholar] [CrossRef]
- Zheng, Y.-Z.; Zhou, Y.; Liang, Q.; Chen, D.-F.; Guo, R.; Xiong, C.-L.; Xu, X.-J.; Zhang, Z.-N.; Huang, Z.-J. Solvent effects on the intramolecular hydrogen-bond and anti-oxidative properties of apigenin: A DFT approach. Dyes Pigment. 2017, 141, 179–187. [Google Scholar] [CrossRef]
- Masek, A.; Chrzescijanska, E.; Latos, M.; Zaborski, M. Influence of hydroxyl substitution on flavanone antioxidants properties. Food Chem. 2017, 215, 501–507. [Google Scholar] [CrossRef] [PubMed]
- Amić, A.; Marković, Z.; Klein, E.; Marković, J.M.D.; Milenković, D. Theoretical study of the thermodynamics of the mechanisms underlying antiradical activity of cinnamic acid derivatives. Food Chem. 2018, 246, 481–489. [Google Scholar] [CrossRef] [PubMed]
- Dimitrić Marković, J.M.; Pejin, B.; Milenković, D.; Amić, D.; Begović, N.; Mojović, M.; Marković, Z.S. Antiradical activity of delphinidin, pelargonidin and malvin towards hydroxyl and nitric oxide radicals: The energy requirements calculations as a prediction of the possible antiradical mechanisms. Food Chem. 2017, 218, 440–446. [Google Scholar] [CrossRef]
- Rice-Evans, C.A.; Miller, N.J.; Paganga, G. Structure-antioxidant activity relationships of flavonoids and phenolic acids. Free Radic. Biol. Med. 1996, 20, 933–956. [Google Scholar] [CrossRef]
- Heijnen, C.; Haenen, G.; van Acker, F.; van der Vijgh, W.; Bast, A. Flavonoids as peroxynitrite scavengers: The role of the hydroxyl groups. Toxicol. Vitr. 2001, 15, 3–6. [Google Scholar] [CrossRef]
- van Acker, S.A.; van den Berg, D.J.; Tromp, M.N.; Griffioen, D.H.; van Bennekom, W.P.; van der Vijgh, W.J.; Bast, A. Structural aspects of antioxidant activity of flavonoids. Free Radic. Biol. Med. 1996, 20, 331–342. [Google Scholar] [CrossRef]
- Bors, W.; Heller, W.; Michel, C.; Saran, M. [36] Flavonoids as antioxidants: Determination of radical-scavenging efficiencies. Methods Enzymol. 1990, 186, 343–355. [Google Scholar]
- Spiegel, M.; Andruniów, T.; Sroka, Z. Flavones’ and Flavonols’ Antiradical Structure-Activity Relationship-A Quantum Chemical Study. Antioxidants 2020, 9, 461. [Google Scholar] [CrossRef]
- Heijnen, C.G.; Haenen, G.; Vekemans, J.A.; Bast, A. Peroxynitrite scavenging of flavonoids: Structure activity relationship. Environ. Toxicol. Pharmacol. 2001, 10, 199–206. [Google Scholar] [CrossRef]
- Fitzgerald, P.A. Chapter 11. Adrenal Medulla and Paraganglia, in Greenspan’s Basic & Clinical Endocrinology, 9th ed.; Gardner, D.G., Shoback, D., Eds.; The McGraw-Hill Companies: New York, NY, USA, 2011. [Google Scholar]
- Ohshima, H.; Yoshie, Y.; Auriol, S.; Gilibert, I. Antioxidant and pro-oxidant actions of flavonoids: Effects on DNA damage induced by nitric oxide, peroxynitrite and nitroxyl anion. Free Radic. Biol. Med. 1998, 25, 1057–1065. [Google Scholar] [CrossRef]
- Awad, H.; Boersma, M.G.; Boeren, S.; van Bladeren, P.J.; Vervoort, A.J.; Rietjens, I. Structure−Activity Study on the Quinone/Quinone Methide Chemistry of Flavonoids. Chem. Res. Toxicol. 2001, 14, 398–408. [Google Scholar] [CrossRef]
- Metodiewa, D.; Jaiswal, A.K.; Cenas, N.; Dickancaité, E.; Segura-Aguilar, J. Quercetin may act as a cytotoxic prooxidant after its metabolic activation to semiquinone and quinoidal product. Free Radic. Biol. Med. 1999, 26, 107–116. [Google Scholar] [CrossRef]
- Ito, S.; Kato, T.; Fujita, K. Covalent binding of catechols to proteins through the sulphydryl group. Biochem. Pharmacol. 1988, 37, 1707–1710. [Google Scholar] [CrossRef]
- Tse, D.C.S.; McCreery, R.; Adams, R.N. Potential oxidative pathways of brain catecholamines. J. Med. Chem. 1976, 19, 37–40. [Google Scholar] [CrossRef] [PubMed]
- Haenen, G.R.; Jansen, F.P.; Vermeulen, N.P.; Bast, A. Activation of the microsomal glutathione S-transferase by metabolites of α-methyldopa. Arch. Biochem. Biophys. 1991, 287, 48–52. [Google Scholar] [CrossRef]
- van Acker, F.A.; Hulshof, J.W.; Haenen, G.R.; Menge, W.M.; van der Vijgh, W.J.; Bast, A. New synthetic flavonoids as potent protectors against doxorubicin-induced cardiotoxicity. Free Radic. Biol. Med. 2001, 31, 31–37. [Google Scholar] [CrossRef]
- Boots, A.W.; Haenen, G.R.; Hartog, G.J.D.; Bast, A. Oxidative damage shifts from lipid peroxidation to thiol arylation by catechol-containing antioxidants. Biochim. Biophys. Acta 2002, 1583, 279–284. [Google Scholar] [CrossRef]
- Chen, L.; Teng, H.; Xie, Z.; Cao, H.; Cheang, W.S.; Skalicka-Woniak, K.; Georgiev, M.I.; Xiao, J. Modifications of dietary flavonoids towards improved bioactivity: An update on structure–activity relationship. Crit. Rev. Food Sci. Nutr. 2017, 58, 513–527. [Google Scholar] [CrossRef] [PubMed]
- Amorati, R.; Valgimigli, L. Modulation of the antioxidant activity of phenols by non-covalent interactions. Org. Biomol. Chem. 2012, 10, 4147–4158. [Google Scholar] [CrossRef] [PubMed]
- Lucarini, M.; Pedulli, G.F.; Guerra, M. A Critical Evaluation of the Factors Determining the Effect of Intramolecular Hydrogen Bonding on the O-H Bond Dissociation Enthalpy of Catechol and of Flavonoid Antioxidants. Chemistry 2004, 10, 933–939. [Google Scholar] [CrossRef]
- Cano, A.; Arnao, M.; Williamson, G.; Garcia-Conesa, M.-T. Superoxide scavenging by polyphenols: Effect of conjugation and dimerization. Redox Rep. 2002, 7, 379–383. [Google Scholar] [CrossRef]
- Lyu, S.; Wang, W. Spectroscopic methodologies and computational simulation studies on the characterization of the interaction between human serum albumin and astragalin. J. Biomol. Struct. Dyn. 2020, 39, 2959–2970. [Google Scholar] [CrossRef] [PubMed]
- Jones, D.P.; Carlson, J.L.; Mody, V.C.; Cai, J.; Lynn, M.J.; Sternberg, P. Redox state of glutathione in human plasma. Free Radic. Biol. Med. 2000, 28, 625–635. [Google Scholar] [CrossRef]
- Varatharajalu, R.; Garige, M.; Leckey, L.C.; Arellanes-Robledo, J.; Reyes-Gordillo, K.; Shah, R.; Lakshman, M.R. Adverse Signaling of Scavenger Receptor Class B1 and PGC1s in Alcoholic Hepatosteatosis and Steatohepatitis and Protection by Betaine in Rat. Am. J. Pathol. 2014, 184, 2035–2044. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Guidot, D.M.; Brown, L.A.S. Mitochondrial glutathione replacement restores surfactant synthesis and secretion in alveolar epithelial cells of ethanol-fed rats. Alcohol. Clin. Exp. Res. 2000, 24, 1070–1076. [Google Scholar] [CrossRef] [PubMed]
- Nguyen-Khac, E.; Thevenot, T.; Piquet, M.-A.; Benferhat, S.; Goria, O.; Chatelain, D.; Tramier, B.; Dewaele, F.; Ghrib, S.; Rudler, M.; et al. Glucocorticoids plusN-Acetylcysteine in Severe Alcoholic Hepatitis. N. Engl. J. Med. 2011, 365, 1781–1789. [Google Scholar] [CrossRef]
- Tkachenko, P.; Maevskaya, M.; Pavlov, A.; Komkova, I.; Pavlov, C.; Ivashkin, V. Prednisolone plus S-adenosil-l-methionine in severe alcoholic hepatitis. Hepatol. Int. 2016, 10, 983–987. [Google Scholar] [CrossRef] [PubMed]
- Mato, J.M.; Martínez-Chantar, M.L.; Lu, S.C. S-adenosylmethionine metabolism and liver disease. Ann. Hepatol. 2013, 12, 183–189. [Google Scholar] [CrossRef]
- Salvemini, D.; Riley, D.P.; Cuzzocrea, S. Sod mimetics are coming of age. Nat. Rev. Drug Discov. 2002, 1, 367–374. [Google Scholar] [CrossRef] [PubMed]
- Batinić-Haberle, I.; Reboucas, J.; Spasojević, I. Superoxide Dismutase Mimics: Chemistry, Pharmacology, and Therapeutic Potential. Antioxid. Redox Signal. 2010, 13, 877–918. [Google Scholar] [CrossRef] [PubMed]
- Coudriet, G.M.; Delmastro-Greenwood, M.M.; Previte, D.M.; Marré, M.L.; O’Connor, E.C.; Novak, E.A.; Vincent, G.; Mollen, K.P.; Lee, S.; Dong, H.H.; et al. Treatment with a Catalytic Superoxide Dismutase (SOD) Mimetic Improves Liver Steatosis, Insulin Sensitivity, and Inflammation in Obesity-Induced Type 2 Diabetes. Antioxidants 2017, 6, 85. [Google Scholar] [CrossRef]
- Mansouri, A.; Tarhuni, A.; Larosche, I.; Reyl-Desmars, F.; Demeilliers, C.; Degoul, F.; Nahon, P.; Sutton, A.; Moreau, R.; Fromenty, B.; et al. MnSOD Overexpression Prevents Liver Mitochondrial DNA Depletion after an Alcohol Binge but Worsens This Effect after Prolonged Alcohol Consumption in Mice. Dig. Dis. 2010, 28, 756–775. [Google Scholar] [CrossRef] [PubMed]
- Larosche, I.; Letteron, P.; Berson, A.; Fromenty, B.; Huang, T.-T.; Moreau, R.; Pessayre, D.; Mansouri, A. Hepatic Mitochondrial DNA Depletion after an Alcohol Binge in Mice: Probable Role of Peroxynitrite and Modulation by Manganese Superoxide Dismutase. J. Pharmacol. Exp. Ther. 2010, 332, 886–897. [Google Scholar] [CrossRef] [PubMed]
- Larosche, I.; Lettéron, P.; Fromenty, B.; Vadrot, N.; Abbey-Toby, A.; Feldmann, G.; Pessayre, D.; Mansouri, A. Tamoxifen Inhibits Topoisomerases, Depletes Mitochondrial DNA, and Triggers Steatosis in Mouse Liver. J. Pharmacol. Exp. Ther. 2007, 321, 526–535. [Google Scholar] [CrossRef]
- Mansouri, A.; Haouzi, D.; Descatoire, V.; Demeilliers, C.; Sutton, A.; Vadrot, N.; Fromenty, B.; Feldmann, G.; Pessayre, D.; Berson, A. Tacrine inhibits topoisomerases and DNA synthesis to cause mitochondrial DNA depletion and apoptosis in mouse liver. Hepatology 2003, 38, 715–725. [Google Scholar] [CrossRef] [PubMed]
- Choumar, A.; Tarhuni, A.; Lettéron, P.; Reyl-Desmars, F.; Dauhoo, N.; Damasse, J.; Vadrot, N.; Nahon, P.; Moreau, R.; Pessayre, D.; et al. Lipopolysaccharide-Induced Mitochondrial DNA Depletion. Antioxid. Redox Signal. 2011, 15, 2837–2854. [Google Scholar] [CrossRef]
- El–Serag, H.B.; Everhart, J.E. Diabetes increases the risk of acute hepatic failure. Gastroenterology 2002, 122, 1822–1828. [Google Scholar] [CrossRef] [PubMed]
- Bruno, S.; Maisonneuve, P.; Castellana, P.; Rotmensz, N.; Rossi, S.; Maggioni, M.; Persico, M.; Colombo, A.; Monasterolo, F.; Casadei-Giunchi, D.; et al. Incidence and risk factors for non-alcoholic steatohepatitis: Prospective study of 5408 women enrolled in Italian tamoxifen chemoprevention trial. BMJ 2005, 330, 932. [Google Scholar] [CrossRef] [PubMed]
- Kent, P.D.; Luthra, H.S.; Michet, C., Jr. Risk factors for methotrexate-induced abnormal laboratory monitoring results in patients with rheumatoid arthritis. J. Rheumatol. 2004, 31, 1727–1731. [Google Scholar] [PubMed]
- Si, W.; Chen, Y.P.; Zhang, J.; Chen, Z.-Y.; Chung, H.Y. Antioxidant activities of ginger extract and its constituents toward lipids. Food Chem. 2018, 239, 1117–1125. [Google Scholar] [CrossRef]
- Berman, A.Y.; Motechin, R.A.; Wiesenfeld, M.Y.; Holz, M.K. The therapeutic potential of resveratrol: A review of clinical trials. NPJ Precis. Oncol. 2017, 1, 35. [Google Scholar] [CrossRef]
- Panahi, Y.; Kianpour, P.; Mohtashami, R.; Jafari, R.; Simental-Mendía, L.E.; Sahebkar, A. Efficacy and Safety of Phytosomal Curcumin in Non-Alcoholic Fatty Liver Disease: A Randomized Controlled Trial. Drug Res. 2017, 67, 244–251. [Google Scholar] [CrossRef]
- Aguirre, L.; Fernández-Quintela, A.; Arias, N.; Portillo, M.P. Resveratrol: Anti-Obesity Mechanisms of Action. Molecules 2014, 19, 18632–18655. [Google Scholar] [CrossRef]
- Zhang, C.; Wang, N.; Xu, Y.; Tan, H.-Y.; Li, S.; Feng, Y. Molecular Mechanisms Involved in Oxidative Stress-Associated Liver Injury Induced by Chinese Herbal Medicine: An Experimental Evidence-Based Literature Review and Network Pharmacology Study. Int. J. Mol. Sci. 2018, 19, 2745. [Google Scholar] [CrossRef]
- Schulz, T.J.; Zarse, K.; Voigt, A.; Urban, N.; Birringer, M.; Ristow, M. Glucose Restriction Extends Caenorhabditis elegans Life Span by Inducing Mitochondrial Respiration and Increasing Oxidative Stress. Cell Metab. 2007, 6, 280–293. [Google Scholar] [CrossRef]
- Uriho, A.; Tang, X.; Le, G.; Yang, S.; Harimana, Y.; Ishimwe, S.P.; Yiping, L.; Zhang, K.; Ma, S.; Muhoza, B. Effects of resveratrol on mitochondrial biogenesis and physiological diseases. Adv. Tradit. Med. 2020, 21, 1–14. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, M.N.; Rahman, M.A.; Rahman, M.H.; Kim, J.W.; Choi, M.; Kim, J.W.; Choi, J.; Moon, M.; Ahmed, K.R.; Kim, B. Potential Therapeutic Implication of Herbal Medicine in Mitochondria-Mediated Oxidative Stress-Related Liver Diseases. Antioxidants 2022, 11, 2041. https://doi.org/10.3390/antiox11102041
Park MN, Rahman MA, Rahman MH, Kim JW, Choi M, Kim JW, Choi J, Moon M, Ahmed KR, Kim B. Potential Therapeutic Implication of Herbal Medicine in Mitochondria-Mediated Oxidative Stress-Related Liver Diseases. Antioxidants. 2022; 11(10):2041. https://doi.org/10.3390/antiox11102041
Chicago/Turabian StylePark, Moon Nyeo, Md. Ataur Rahman, Md. Hasanur Rahman, Jong Woo Kim, Min Choi, Jeong Woo Kim, Jinwon Choi, Myunghan Moon, Kazi Rejvee Ahmed, and Bonglee Kim. 2022. "Potential Therapeutic Implication of Herbal Medicine in Mitochondria-Mediated Oxidative Stress-Related Liver Diseases" Antioxidants 11, no. 10: 2041. https://doi.org/10.3390/antiox11102041
APA StylePark, M. N., Rahman, M. A., Rahman, M. H., Kim, J. W., Choi, M., Kim, J. W., Choi, J., Moon, M., Ahmed, K. R., & Kim, B. (2022). Potential Therapeutic Implication of Herbal Medicine in Mitochondria-Mediated Oxidative Stress-Related Liver Diseases. Antioxidants, 11(10), 2041. https://doi.org/10.3390/antiox11102041








