Aerobic Exercise Training Reduces Atherogenesis Induced by Low-Sodium Diet in LDL Receptor Knockout Mice
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Experimental Protocol
2.3. Aerobic Exercise Training
2.4. Insulin Tolerance Test
2.5. Brachiocephalic Trunk Isolation and Histomorphometry
2.6. Oil Red O Staining
2.7. Immunofluorescence Staining
2.8. Real-Time Quantitative Polymerase Chain Reaction (RT-qPCR) in Brachiocephalic Trunk
2.9. Statistical Analyses
3. Results
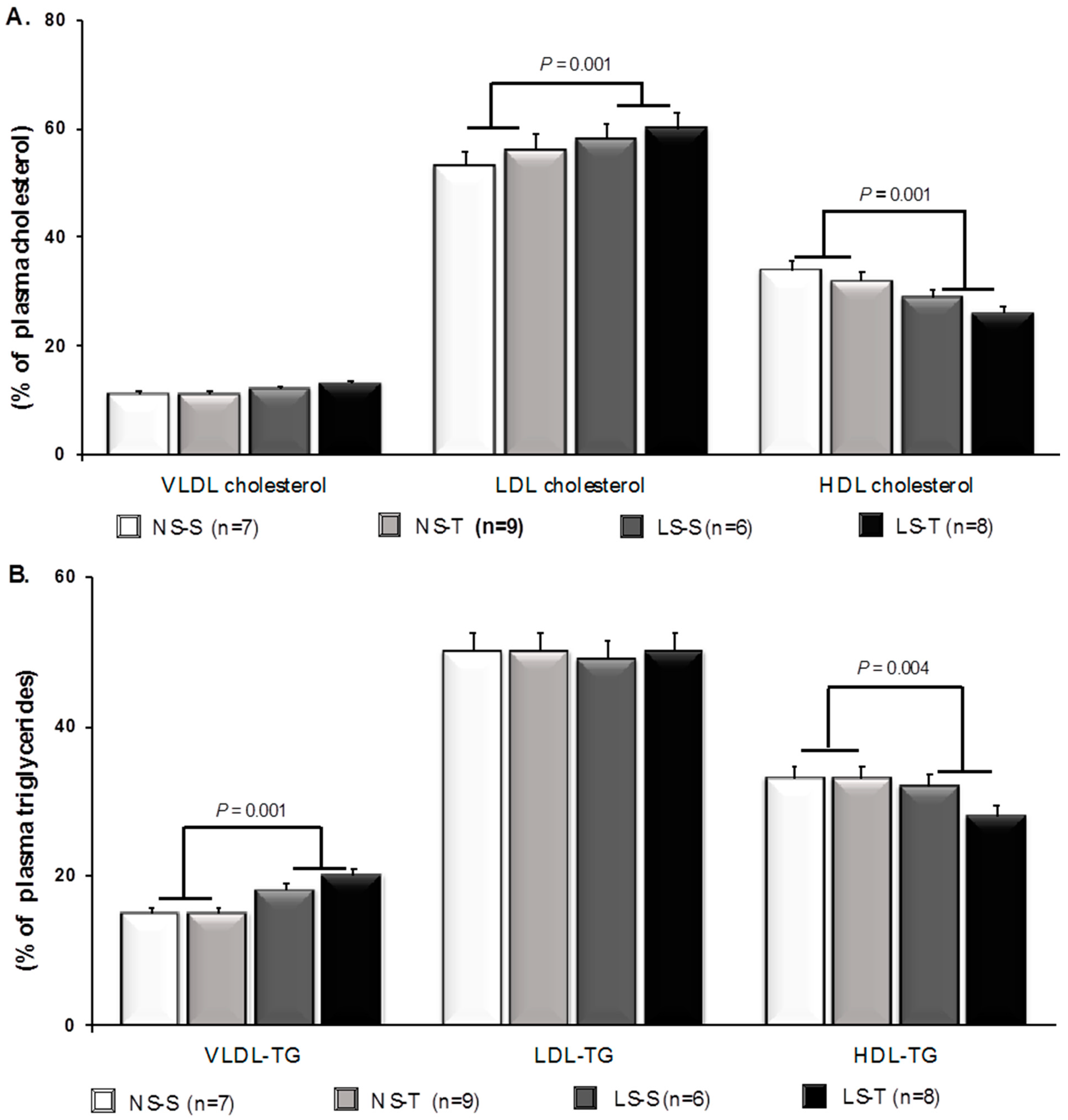
3.1. Impact of Dietary Sodium Restriction and Aerobic Exercise Training Effects on Plasma Lipids, Hematocrit, and Body Mass of LDLR KO Mice
3.2. Aerobic Exercise Training Prevented Dietary Sodium Restriction-Induced Insulin Resistance
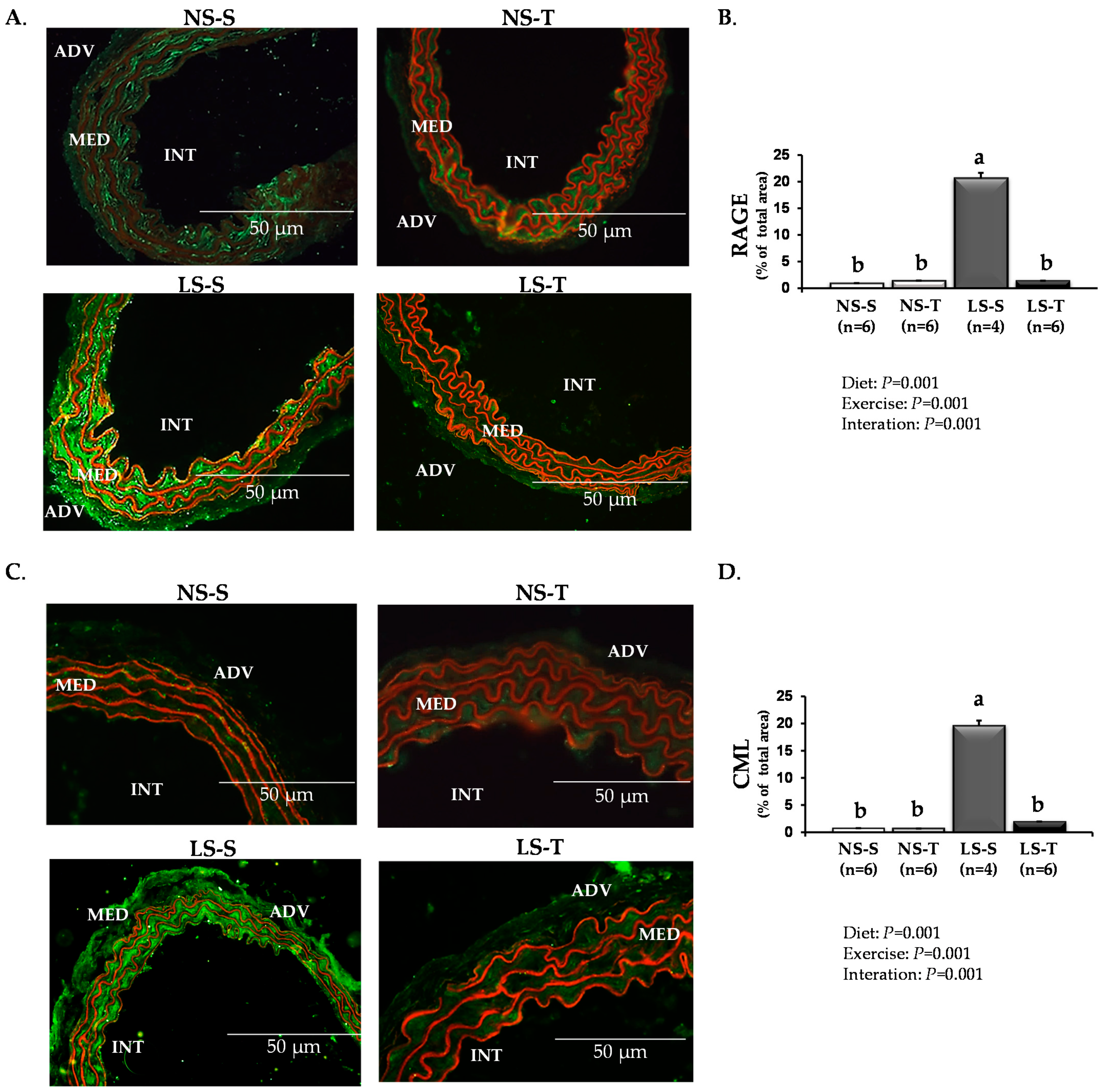
3.3. Aerobic Exercise Training Prevented Dietary Sodium Restriction-Induced Arterial Injury
3.4. Gene Expression
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jayedi, A.; Ghomashi, F.; Zargar, M.S.; Shab-Bidar, S. Dietary sodium, sodium-to-potassium ratio, and risk of stroke: A systematic review and nonlinear dose-response meta-analysis. Clin. Nutr. 2019, 38, 1092–1100. [Google Scholar] [CrossRef] [PubMed]
- Cook, N.R.; Appel, L.J.; Whelton, P.K. Sodium intake and all-cause mortality over 20 years in the trials of hypertension prevention. J. Am. Coll. Cardiol. 2016, 68, 1609–1617. [Google Scholar] [CrossRef] [PubMed]
- Alderman, M.H. Reducing dietary sodium: The case for caution. JAMA 2010, 303, 448–449. [Google Scholar] [CrossRef] [PubMed]
- Ishibashi, S.; Brown, M.S.; Goldstein, J.L.; Gerard, R.D.; Hammer, R.E.; Herz, J. Hypercholesterolemia in low density lipoprotein receptor knockout mice and its reversal by adenovirus-mediated gene delivery. J. Clin. Investig. 1993, 92, 883–893. [Google Scholar] [CrossRef]
- Catanozi, S.; Rocha, J.C.; Passarelli, M.; Guzzo, M.L.; Alves, C.; Furukawa, L.N.; Nunes, V.S.; Nakandakare, E.R.; Heimann, J.C.; Quintão, E.C. Dietary sodium chloride restriction enhances aortic wall lipid storage and raises plasma lipid concentration in LDL receptor knockout mice. J. Lipid Res. 2003, 44, 727–732. [Google Scholar] [CrossRef]
- Ivanovski, O.; Szumilak, D.; Nguyen-Khoa, T.; Dechaux, M.; Massy, Z.A.; Phan, O.; Mothu, N.; Lacour, B.; Drueke, T.B.; Muntzel, M. Dietary salt restriction accelerates atherosclerosis in apolipoprotein E-deficient mice. Atherosclerosis 2005, 180, 271–276. [Google Scholar] [CrossRef]
- Tikellis, C.; Pickering, R.J.; Tsorotes, D.; Huet, O.; Chin-Dusting, J.; Cooper, M.E.; Thomas, M.C. Activation of the renin-angiotensin system mediates the effects of dietary salt intake on atherogenesis in the apolipoprotein E knockout mouse. Hypertension 2012, 60, 98–105. [Google Scholar] [CrossRef]
- Fusco, F.B.; Gomes, D.J.; Bispo, K.C.; Toledo, V.P.; Barbeiro, D.F.; Capelozzi, V.L.; Furukawa, L.N.; Velosa, A.P.; Teodoro, W.R.; Heimann, J.C.; et al. Low-sodium diet induces atherogenesis regardless of lowering blood pressure in hypertensive hyperlipidemic mice. PLoS ONE 2017, 12, e0177086. [Google Scholar] [CrossRef]
- Gabriel, B.M.; Pugh, J.; Pruneta-Deloche, V.; Moulin, P.; Ratkevicius, A.; Gray, S.R. The effect of high intensity interval exercise on postprandial triacylglycerol and leukocyte activation--monitored for 48 h post exercise. PLoS ONE 2013, 8, e82669. [Google Scholar] [CrossRef]
- Kahn, B.B.; Alquier, T.; Carling, D.; Hardie, D.G. AMP-activated protein kinase: Ancient energy gauge provides clues to modern understanding of metabolism. Cell. Metab. 2005, 1, 15–25. [Google Scholar] [CrossRef]
- Hardie, D.G.; Ross, F.A.; Hawley, S.A. AMPK: A nutrient and energy sensor that maintains energy homeostasis. Nat. Rev. Mol. Cell. Biol. 2012, 13, 251–262. [Google Scholar] [CrossRef] [PubMed]
- Rocco, D.D.F.M.; Okuda, L.S.; Pinto, R.S.; Ferreira, F.D.; Kubo, S.K.; Nakandakare, E.R.; Quintão, E.C.R.; Catanozi, S.; Passarelli, M. Aerobic exercise improves reverse cholesterol transport in cholesteryl ester transfer protein transgenic mice. Lipids 2011, 46, 617–625. [Google Scholar] [CrossRef] [PubMed]
- Roque, F.R.; Hernanz, R.; Salaices, M.; Briones, A.M. Exercise training and cardiometabolic diseases: Focus on the vascular system. Curr. Hypertens. Rep. 2013, 15, 204–214. [Google Scholar] [CrossRef] [PubMed]
- Kadoglou, N.P.E.; Moustardas, P.; Kapelouzou, A.; Katsimpoulas, M.; Giagini, A.; Dede, E.; Kostomitsopoulos, N.; Karayannacos, P.E.; Kostakis, A.; Liapis, C.D. The anti-inflammatory effects of exercise training promote atherosclerotic plaque stabilization in apolipoprotein E knockout mice with diabetic atherosclerosis. Eur. J. Histochem. 2013, 57, e3. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, J.C.; Rolim, N.P.; Bartholomeu, J.B.; Gobatto, C.A.; Kokubun, E.; Brum, P.C. Maximal lactate steady state in running mice: Effect of exercise training. Clin. Exp. Pharmacol. Physiol. 2007, 34, 760–765. [Google Scholar] [CrossRef]
- Zheng, F.; Zhang, S.; Lu, W.; Wu, F.; Yin, X.; Yu, D.; Pan, Q.; Li, H. Regulation of insulin resistance and adiponectin signaling in adipose tissue by liver X receptor activation highlights a cross-talk with PPARγ. PLoS ONE 2014, 9, e101269. [Google Scholar] [CrossRef] [PubMed]
- Drinane, M.; Mollmark, J.; Zagorchev, L.; Moodie, K.; Sun, B.; Hall, A.; Shipman, S.; Morganelli, P.; Simons, M.; Mulligan-Kehoe, M.J. The antiangiogenic activity of rPAI-1(23) inhibits vasa vasorum and growth of atherosclerotic plaque. Circ. Res. 2009, 104, 337–345. [Google Scholar] [CrossRef]
- Centa, M.; Ketelhuth, D.F.J.; Malin, S.; Gisterå, A. Quantification of atherosclerosis in mice. J. Vis. Exp. 2019, 148, e59828. [Google Scholar] [CrossRef] [PubMed]
- Gomes, D.J.; Velosa, A.P.; Okuda, L.S.; Fusco, F.B.; da Silva, K.S.; Pinto, P.R.; Nakandakare, E.R.; Correa-Giannella, M.L.; Woods, T.; Brimble, M.A.; et al. Glycated albumin induces lipid infiltration in mice aorta independently of DM and RAS local modulation by inducing lipid peroxidation and inflammation. J. Diabetes Complicat. 2016, 30, 1614–1621. [Google Scholar] [CrossRef]
- Pinto, P.R.; Yoshinaga, M.Y.; Del Bianco, V.; Bochi, A.P.; Ferreira, G.S.; Pinto, I.F.; Rodrigues, L.G.; Nakandakare, E.R.; Okamoto, M.M.; Machado, U.F.; et al. Dietary sodium restriction alters muscle lipidomics that relates to insulin resistance in mice. J. Biol. Chem. 2021, 296, 100344. [Google Scholar] [CrossRef]
- da Silva Ferreira, G.; Bochi, A.P.G.; Pinto, P.R.; Del Bianco, V.; Rodrigues, L.G.; Morais, M.R.P.T.; Nakandakare, E.R.; Machado, U.F.; Catanozi, S.; Passarelli, M. Aerobic exercise training prevents insulin resistance and hepatic lipid accumulation in LDL receptor knockout mice chronically fed a low-sodium diet. Nutrients 2021, 13, 2174. [Google Scholar] [CrossRef]
- Catanozi, S.; Rocha, J.C.; Nakandakare, E.R.; Passarelli, M.; Mesquita, C.H.; Silva, A.A.; Dolnikoff, M.S.; Harada, L.M.; Quintao, E.C.; Heimann, J.C. The rise of the plasma lipid concentration elicited by dietary sodium chloride restriction in Wistar rats is due to an impairment of the plasma triacylglycerol removal rate. Atherosclerosis 2001, 158, 81–86. [Google Scholar] [CrossRef]
- Garg, R.; Williams, G.H.; Hurwitz, S.; Brown, N.J.; Hopkins, P.N.; Adler, G.K. Low-salt diet increases insulin resistance in healthy subjects. Metabolism 2011, 60, 965–968. [Google Scholar] [CrossRef]
- Olivares-Reyes, J.A.; Arellano-Plancarte, A.; Castillo-Hernandez, J.R. Angiotensin II and the development of insulin resistance: Implications for diabetes. Mol. Cell. Endocrinol. 2009, 302, 128–139. [Google Scholar] [CrossRef] [PubMed]
- Muscogiuri, G.; Chavez, A.O.; Gastaldelli, A.; Perego, L.; Tripathy, D.; Saad, M.J.; Velloso, L.; Folli, F. The crosstalk between insulin and renin-angiotensin-aldosterone signaling systems and its effect on glucose metabolism and diabetes prevention. Curr. Vasc. Pharmacol. 2008, 6, 301–312. [Google Scholar] [CrossRef]
- Ogihara, T.; Asano, T.; Ando, K.; Chiba, Y.; Sakoda, H.; Anai, M.; Shojima, N.; Ono, H.; Onishi, Y.; Fujishiro, M.; et al. Angiotensin II-induced insulin resistance is associated with enhanced insulin signaling. Hypertension 2002, 40, 872–879. [Google Scholar] [CrossRef] [PubMed]
- Röckl, K.S.; Witczak, C.A.; Goodyear, L.J. Signaling mechanisms in skeletal muscle: Acute responses and chronic adaptations to exercise. IUBMB Life 2008, 60, 145–153. [Google Scholar] [CrossRef]
- Stanford, K.I.; Goodyear, L.J. Exercise and type 2 diabetes: Molecular mechanisms regulating glucose uptake in skeletal muscle. Adv. Physiol. Educ. 2014, 38, 308–314. [Google Scholar] [CrossRef]
- Dikalov, S.I.; Nazarewicz, R.R.; Bikineyeva, A.; Hilenski, L.; Lassegue, B.; Griendling, K.K.; Harrison, D.G.; Dikalova, A.E. Nox2-induced production of mitochondrial superoxide in angiotensin II-mediated endothelial oxidative stress and hypertension. Antioxid. Redox Signal 2014, 20, 281–294. [Google Scholar] [CrossRef]
- Nickenig, G.; Strehlow, K.; Bäumer, A.T.; Baudler, S.; Waßmann, S.; Sauer, H.; Böhm, M. Negative feedback regulation of reactive oxygen species on AT1 receptor gene expression. Br. J. Pharmacol. 2000, 131, 795–803. [Google Scholar] [CrossRef] [PubMed]
- Linder, A.E.; Thakali, K.M.; Thompson, J.M.; Watts, S.W.; Webb, R.C.; Leite, R. Methyl-beta-cyclodextrin prevents angiotensin II-induced tachyphylactic contractile responses in rat aorta. J. Pharmacol. Exp. Ther. 2007, 323, 78–84. [Google Scholar] [CrossRef]
- Thomas, W.G. Regulation of angiotensin II type 1 (AT1) receptor function. Regul. Pept. 1999, 79, 9–23. [Google Scholar] [CrossRef]
- Lassègue, B.; Alexander, R.W.; Nickenig, G.; Clark, M.; Murphy, T.J.; Griendling, K.K. Angiotensin II down-regulates the vascular smooth muscle AT1 receptor by transcriptional and post-transcriptional mechanisms: Evidence for homologous and heterologous regulation. Mol. Pharmacol. 1995, 48, 601–609. [Google Scholar]
- Adams, V.; Linke, A.; Kränkel, N.; Erbs, S.; Gielen, S.; Möbius-Winkler, S.; Gummert, J.F.; Mohr, F.W.; Schuler, G.; Hambrecht, R. Impact of regular physical activity on the NAD(P)H oxidase and angiotensin receptor system in patients with coronary artery disease. Circulation 2005, 111, 555–562. [Google Scholar] [CrossRef] [PubMed]
- Gu, Q.; Wang, B.; Zhang, X.F.; Ma, Y.P.; Liu, J.D.; Wang, X.Z. Contribution of renin-angiotensin system to exercise-induced attenuation of aortic remodeling and improvement of endothelial function in spontaneously hypertensive rats. Cardiovasc. Pathol. 2014, 23, 298–305. [Google Scholar] [CrossRef]
- Pellegrin, M.; Alonso, F.; Aubert, J.F.; Bouzourene, K.; Braunersreuther, V.; Mach, F.; Haefliger, J.A.; Hayoz, D.; Berthelot, A.; Nussberger, J.; et al. Swimming prevents vulnerable atherosclerotic plaque development in hypertensive 2-kidney, 1-clip mice by modulating angiotensin II type 1 receptor expression independently from hemodynamic changes. Hypertension 2009, 53, 782–789. [Google Scholar] [CrossRef]
- Eto, H.; Miyata, M.; Shirasawa, T.; Akasaki, Y.; Hamada, N.; Nagaki, A.; Orihara, K.; Biro, S.; Tei, C. The long-term effect of angiotensin II type 1a receptor deficiency on hypercholesterolemia-induced atherosclerosis. Hypertens. Res. 2008, 31, 1631–1642. [Google Scholar] [CrossRef][Green Version]
- Sinzinger, H.; Virgolini, I. Effects of exercise on parameters of blood coagulation, platelet function and the prostaglandin system. Sports Med. 1988, 6, 238–245. [Google Scholar] [CrossRef]
- Yu, M.; Tsai, S.F.; Kuo, Y.M. The therapeutic potential of anti-inflammatory exerkines in the treatment of atherosclerosis. Int. J. Mol. Sci. 2017, 18, 1260. [Google Scholar] [CrossRef] [PubMed]
- Petersen, A.M.; Pedersen, B.K. The anti-inflammatory effect of exercise. J. Appl. Physiol. 2005, 98, 1154–1162. [Google Scholar] [CrossRef] [PubMed]
- Flynn, M.G.; McFarlin, B.K. Toll-like receptor 4: Link to the anti-inflammatory effects of exercise? Exerc. Sport. Sci. Rev. 2006, 34, 176–181. [Google Scholar] [CrossRef] [PubMed]
- Kawanishi, N.; Yano, H.; Yokogawa, Y.; Suzuki, K. Exercise training inhibits inflammation in adipose tissue via both suppression of macrophage infiltration and acceleration of phenotypic switching from M1 to M2 macrophages in high-fat-diet-induced obese mice. Exerc. Immunol. Rev. 2010, 16, 105–118. [Google Scholar]
- Tabas, I.; García-Cardeña, G.; Owens, G.K. Recent insights into the cellular biology of atherosclerosis. J. Cell. Biol. 2015, 209, 13–22. [Google Scholar] [CrossRef] [PubMed]
- Timmerman, K.L.; Flynn, M.G.; Coen, P.M.; Markofski, M.M.; Pence, B.D. Exercise training-induced lowering of inflammatory (CD14+CD16+) monocytes: A role in the anti-inflammatory influence of exercise? J. Leukoc. Biol. 2008, 84, 1271–1278. [Google Scholar] [CrossRef]
- Pedersen, B.K.; Febbraio, M.A. Muscles, exercise and obesity: Skeletal muscle as a secretory organ. Nat. Rev. Endocrinol. 2012, 8, 457–465. [Google Scholar] [CrossRef]
- Oliveira, M.; Gleeson, M. The influence of prolonged cycling on monocyte Toll-like receptor 2 and 4 expression in healthy men. Eur. J. Appl. Physiol. 2010, 109, 251–257. [Google Scholar] [CrossRef]
- Stefano, G.B.; Prevot, V.; Cadet, P.; Dardik, I. Vascular pulsations stimulating nitric oxide release during cyclic exercise may benefit health: A molecular approach (review). Int. J. Mol. Med. 2001, 7, 119–129. [Google Scholar] [CrossRef]
- Pedersen, B.K. The anti-inflammatory effect of exercise: Its role in diabetes and cardiovascular disease control. Essays Biochem. 2006, 42, 105–117. [Google Scholar]
- Vogel, C.; Marcotte, E.M. Insights into the regulation of protein abundance from proteomic and transcriptomic analyses. Nat. Rev. Genet. 2021, 13, 227–232. [Google Scholar] [CrossRef]
- Maier, T.; Güell, M.; Serrano, L. Correlation of mRNA and protein in complex biological samples. FEBS Lett. 2009, 583, 3966–3973. [Google Scholar] [CrossRef] [PubMed]
- Wassmann, S.; Nickenig, G. Pathophysiological regulation of the AT1-receptor and implications for vascular disease. J. Hypertens. Suppl. 2006, 24, S15–S21. [Google Scholar] [CrossRef]
- Lee, S.H.; Fujioka, S.; Takahashi, R.; Oe, T. Angiotensin II-Induced Oxidative Stress in Human Endothelial Cells: Modification of Cellular Molecules through Lipid Peroxidation. Chem. Res. Toxicol. 2019, 32, 1412–1422. [Google Scholar] [CrossRef]
- Yokoyama, S.; Kawai, T.; Yamamoto, K.; Yibin, H.; Yamamoto, H.; Kakino, A.; Takeshita, H.; Nozato, Y.; Fujimoto, T.; Hongyo, K.; et al. RAGE ligands stimulate angiotensin II type I receptor (AT1) via RAGE/AT1 complex on the cell membrane. Sci. Rep. 2021, 11, 5759. [Google Scholar] [CrossRef]
- Kamioka, M.; Ishibashi, T.; Sugimoto, K.; Uekita, H.; Nagai, R.; Sakamoto, N.; Ando, K.; Ohkawara, H.; Teramoto, T.; Maruyama, Y.; et al. Blockade of renin-angiotensin system attenuates advanced glycation end products-mediated signaling pathways. J. Atheroscler. Thromb. 2010, 17, 590–600. [Google Scholar] [CrossRef]
- Thomas, M.C.; Tikellis, C.; Burns, W.M.; Bialkowski, K.; Cao, Z.; Coughlan, M.T.; Jandeleit-Dahm, K.; Cooper, M.E.; Forbes, J.M. Interactions between renin angiotensin system and advanced glycation in the kidney. J. Am. Soc. Nephrol. 2005, 16, 2976–2984. [Google Scholar] [CrossRef]
- Pickering, R.J.; Tikellis, C.; Rosado, C.J.; Tsorotes, D.; Dimitropoulos, A.; Smith, M.; Huet, O.; Seeber, R.M.; Abhayawardana, R.; Johnstone, E.K.; et al. Transactivation of RAGE mediates angiotensin-induced inflammation and atherogenesis. J. Clin. Investig. 2019, 129, 406–421. [Google Scholar] [CrossRef]
- Ohgami, N.; Miyazaki, A.; Sakai, M.; Kuniyasu, A.; Nakayama, H.; Horiuchi, S. Advanced glycation end products (AGE) inhibit scavenger receptor class B type I-mediated reverse cholesterol transport: A new crossroad of AGE to cholesterol metabolism. J. Atheroscler. Thromb. 2003, 10, 1–6. [Google Scholar] [CrossRef]
- Kamtchueng Simo, O.; Ikhlef, S.; Berrougui, H.; Khalil, A. Advanced glycation end products affect cholesterol homeostasis by impairing ABCA1 expression on macrophages. Can. J. Physiol. Pharmacol. 2017, 95, 977–984. [Google Scholar] [CrossRef]
- Xu, L.; Wang, Y.R.; Li, P.C.; Feng, B. Atorvastatin blocks advanced glycation end products induced reduction in macrophage cholesterol efflux mediated with ATP-binding cassette transporters G 1. Circ. J. 2019, 83, 1954–1964. [Google Scholar] [CrossRef]
- Da Silva, K.S.; Pinto, P.R.; Fabre, N.T.; Gomes, D.J.; Thieme, K.; Okuda, L.S.; Iborra, R.T.; Freitas, V.G.; Shimizu, M.H.; Teodoro, W.R.; et al. N-acetylcysteine counteracts adipose tissue macrophage infiltration and insulin resistance elicited by advanced glycated albumin in healthy rats. Front. Physiol. 2017, 8, 723. [Google Scholar] [CrossRef]
- Esteves, J.V.; Yonamine, C.Y.; Pinto-Junior, D.C.; Gerlinger-Romero, F.; Enguita, F.J.; Machado, U.F. Advanced glycation end products-induced insulin resistance involves repression of skeletal muscle GLUT4 expression. Sci. Rep. 2018, 8, 8109. [Google Scholar]
- Bond, A.R.; Jackson, C.L. The fat-fed apolipoprotein E knockout mouse brachiocephalic artery in the study of atherosclerotic plaque rupture. J. Biomed. Biotechnol. 2011, 2011, 379069. [Google Scholar] [CrossRef] [PubMed]
- Williams, H.; Johnson, J.L.; Carson, K.G.; Jackson, C.L. Characteristics of intact and ruptured atherosclerotic plaques in brachiocephalic arteries of apolipoprotein E knockout mice. Arterioscler. Thromb. Vasc. Biol. 2002, 22, 788–792. [Google Scholar] [CrossRef] [PubMed]
- Rosenfeld, M.E.; Averill, M.M.; Bennett, B.J.; Schwartz, S.M. Progression and disruption of advanced atherosclerotic plaques in murine models. Curr. Drug. Targets 2008, 9, 210–216. [Google Scholar] [CrossRef]
- Emini, V.B.; Perrotta, P.; De Meyer, G.R.A.; Roth, L.; Van der Donckt, C.; Martinet, W.; De Meyer, G.R.Y. Animal models of atherosclerosis. Eur. J. Pharmacol. 2017, 816, 3–13. [Google Scholar] [CrossRef]


| NS | LS | p | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Baseline NS-S | Sedentary NS-S | Baseline NS-T | AET NS-T | Baseline LS-S | Sedentary LS-S | Baseline LS-T | AET LS-T | Diet | AET | |
| BM (g) (n = 15, 19, 15, 20) | 26 ± 2 | 25 ± 2 | 25 ± 1 | 24 ± 2 | 25 ± 2 | 24 ± 1 | 25 ± 2 | 27 ± 2 | 0.006 | 0.02 |
| TC (mmol/L) (n = 12, 14, 13, 13) | 6.9 ± 0.9 | 8.8 ± 3.7 | 6.7 ± 0.9 | 8.6 ± 2.8 | 7.4 ± 1.0 | 8.6 ± 2.8 | 6.9 ± 1.0 | 9.6 ± 2.7 | - | - |
| TG (mmol/L) (n = 12, 14, 13, 13) | 1.9 ± 0.4 | 1.4 ± 0.3 | 1.8 ± 0.4 | 1.5 ± 0.6 | 1.8 ± 0.3 | 1.7 ± 0.4 | 1.8 ± 0.4 | 2.1 ± 0.7 | 0.013 | - |
| Hematocrit (%) (n = 9, 7, 8, 9) | 49 ± 5.4 | 48 ± 2 | 50 ± 7.0 | 49 ± 2 | 49 ± 6.3 | 50 ± 2 | 49 ± 6.2 | 49 ± 3 | - | - |
| HR (bpm) (n = 8, 8, 8, 9) | 515 ± 76 | 552 ± 63 | 487 ± 56 | 531 ± 50 | 502 ± 63 | 541 ± 66 | 481 ± 80 | 544 ± 65 | - | - |
| SBP (mmHg) (n = 8, 8, 7, 9) | 108 ± 8 | 108 ± 7 | 113 ± 6 | 105 ± 5 | 111 ± 6 | 101 ± 5 | 109 ± 6 | 103 ± 5 | - | - |
| UNa (mEq/24 h) (n = 8, 7, 6, 6) | 0.157 ± 0.021 | 0.186 ± 0.034 | 0.027 ± 0.011 | 0.030 ± 0.020 | 0.001 | - | ||||
| Glucose decay rate (%/min) (n = 7, 8, 6, 5) | 3.46 ± 0.87 | 3.48 ± 0.76 | 1.89 ± 0.32 | 3.43 ± 1.01 | 0.015 | 0.018 | ||||
| Maximum exercise capacity (s) (n = 5, 6, 6, 7) | 947 ± 252 | 1844 ± 317 | 1165 ± 177 | 1740 ± 531 | - | 0.001 | ||||
| NS | LS | p | |||||
|---|---|---|---|---|---|---|---|
| Sedentary NS-S | AET NS-T | Sedentary LS-S | AET LS-T | Diet | AET | Interaction | |
| Agtr1a (2^−ΔΔCT) (n = 5) | 1.00 | 1.99 | 1.24 | 2.33 | 0.714 | 0.215 | 0.942 |
| Ager (2^−ΔΔCT) (n = 5) | 1.00 | 0.66 | 0.46 | 0.67 | 0.360 | 0.968 | 0.337 |
| Orl1 (2^−ΔΔCT) (n = 5) | 1.00 | 0.55 | 0.44 | 0.31 | 0.223 | 0.926 | 0.257 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bochi, A.P.G.; Ferreira, G.d.S.; Del Bianco, V.; Pinto, P.R.; Rodrigues, L.G.; Trevisani, M.d.S.; Furukawa, L.N.S.; Bispo, K.C.S.; da Silva, A.A.; Velosa, A.P.P.; et al. Aerobic Exercise Training Reduces Atherogenesis Induced by Low-Sodium Diet in LDL Receptor Knockout Mice. Antioxidants 2022, 11, 2023. https://doi.org/10.3390/antiox11102023
Bochi APG, Ferreira GdS, Del Bianco V, Pinto PR, Rodrigues LG, Trevisani MdS, Furukawa LNS, Bispo KCS, da Silva AA, Velosa APP, et al. Aerobic Exercise Training Reduces Atherogenesis Induced by Low-Sodium Diet in LDL Receptor Knockout Mice. Antioxidants. 2022; 11(10):2023. https://doi.org/10.3390/antiox11102023
Chicago/Turabian StyleBochi, Ana Paula Garcia, Guilherme da Silva Ferreira, Vanessa Del Bianco, Paula Ramos Pinto, Letícia Gomes Rodrigues, Mayara da Silva Trevisani, Luzia Naoko Shinohara Furukawa, Kely Cristina Soares Bispo, Alexandre Alves da Silva, Ana Paula Pereira Velosa, and et al. 2022. "Aerobic Exercise Training Reduces Atherogenesis Induced by Low-Sodium Diet in LDL Receptor Knockout Mice" Antioxidants 11, no. 10: 2023. https://doi.org/10.3390/antiox11102023
APA StyleBochi, A. P. G., Ferreira, G. d. S., Del Bianco, V., Pinto, P. R., Rodrigues, L. G., Trevisani, M. d. S., Furukawa, L. N. S., Bispo, K. C. S., da Silva, A. A., Velosa, A. P. P., Nakandakare, E. R., Machado, U. F., Teodoro, W. P. R., Passarelli, M., & Catanozi, S. (2022). Aerobic Exercise Training Reduces Atherogenesis Induced by Low-Sodium Diet in LDL Receptor Knockout Mice. Antioxidants, 11(10), 2023. https://doi.org/10.3390/antiox11102023








