Analysis of the Antioxidant Composition of Low Molecular Weight Metabolites from the Agarolytic Bacterium Alteromonas macleodii QZ9-9: Possibilities for High-Added Value Utilization of Macroalgae
Abstract
1. Introduction
2. Materials and Methods
2.1. Screening and Identification of Agarolytic Bacteria
2.2. Detection of Agar-Degrading Activity
2.3. Enzyme Activity Assay
2.4. The Curves of Growth and Agarase Production
2.5. Antioxidant Activity Assay
2.6. Sample Preparation for Metabolomics of Antioxidant Fermentation
2.7. Metabolomics Analysis by UHPLC–QE/MS
2.8. Maculosin Specific Antioxidant Activity
2.9. Statistical Analysis
3. Results
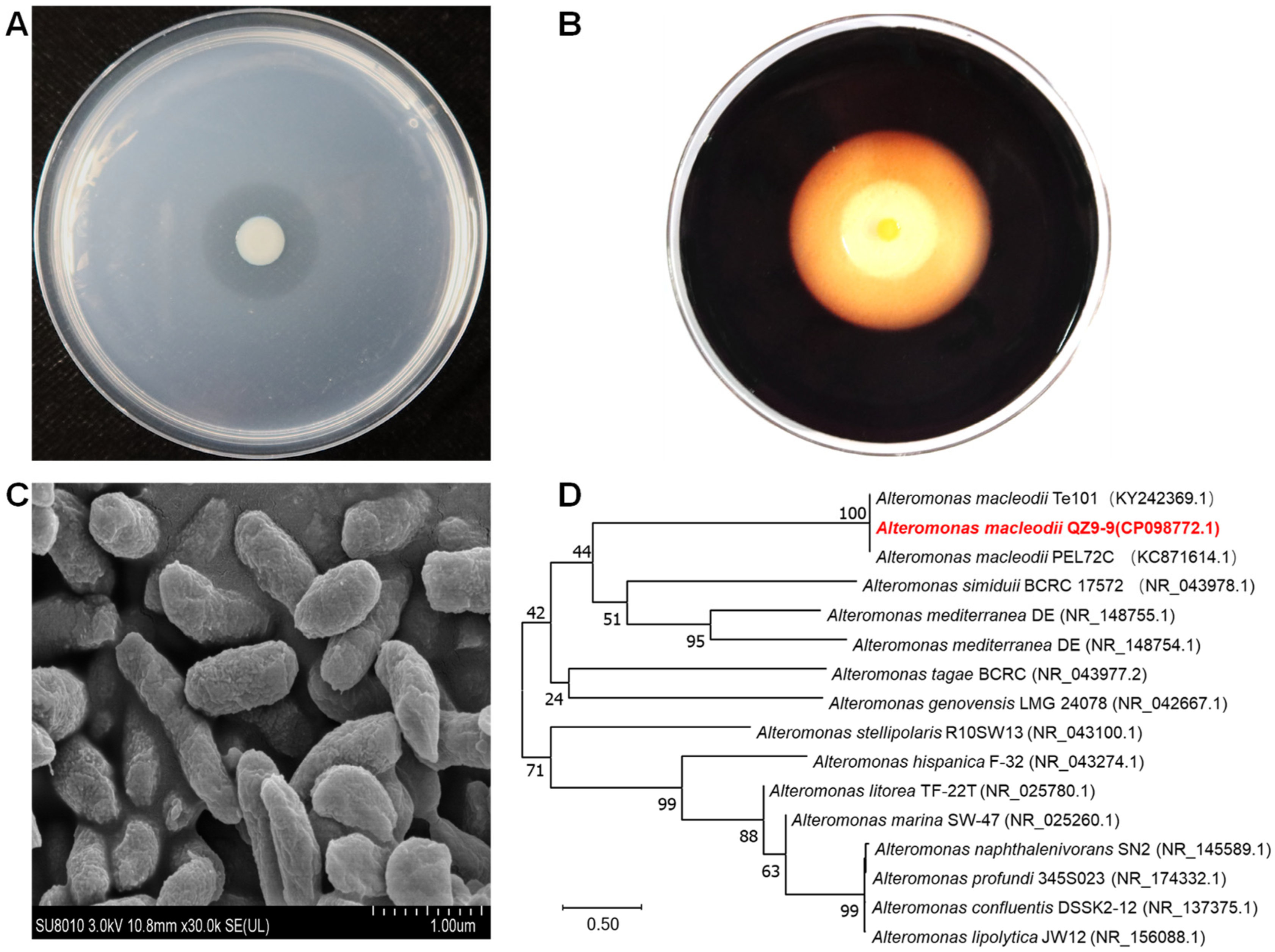
3.1. Characterization and Identification of Agarolytic Bacteria
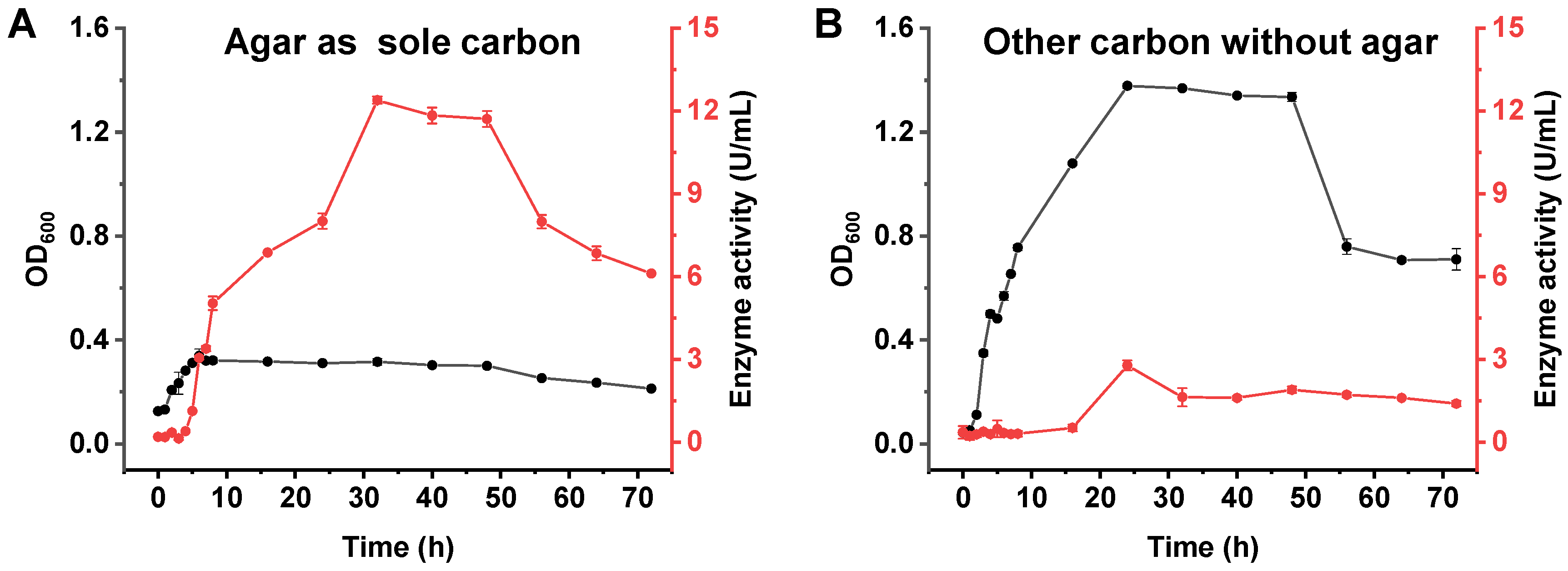
3.2. The Curves of Growth and Agarase Production of A. macleodii QZ9-9
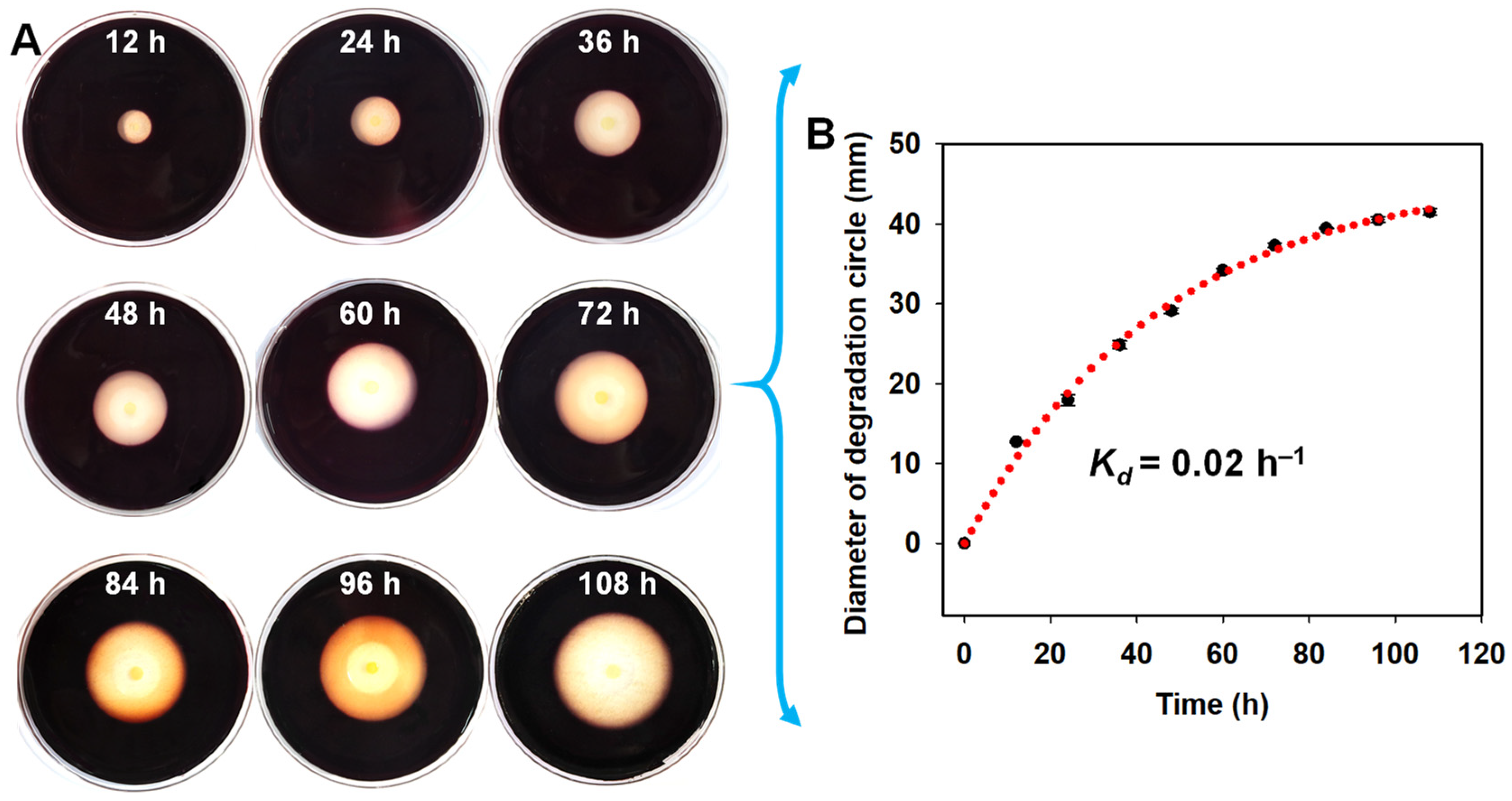
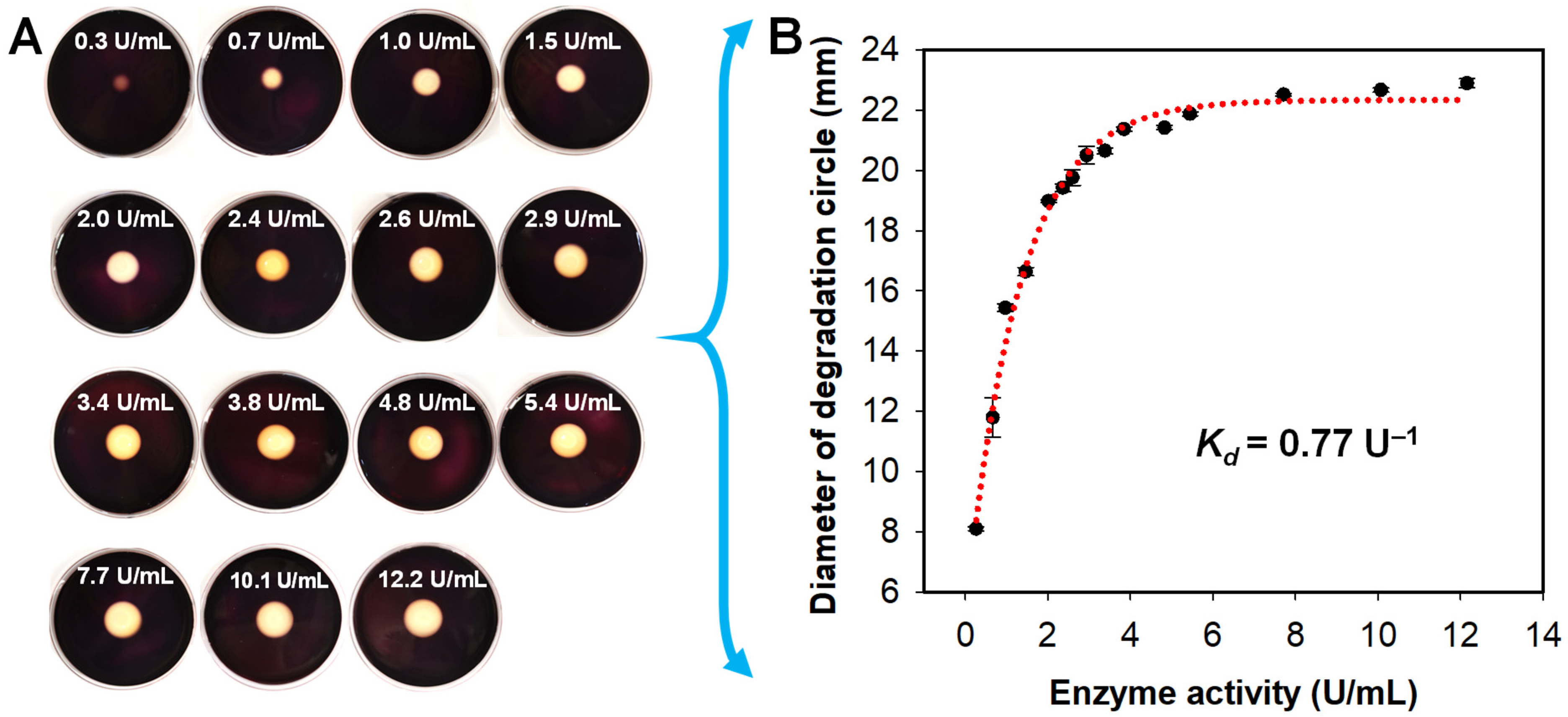
3.3. Analysis of the Agar-Degrading Activity of A. macleodii QZ9-9
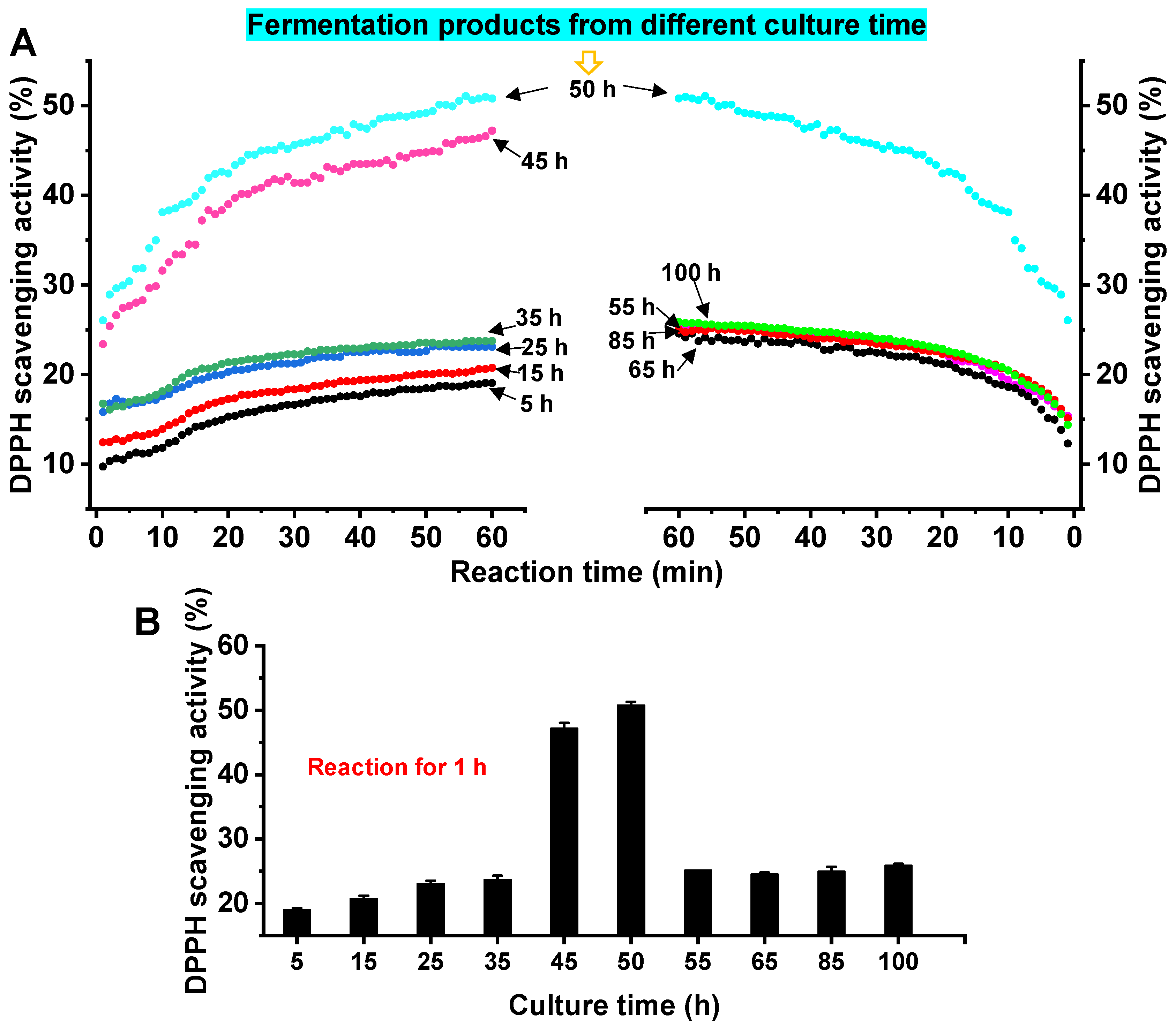
3.4. Analysis of the Antioxidant Activity of Fermentation Products of A. macleodii QZ9-9
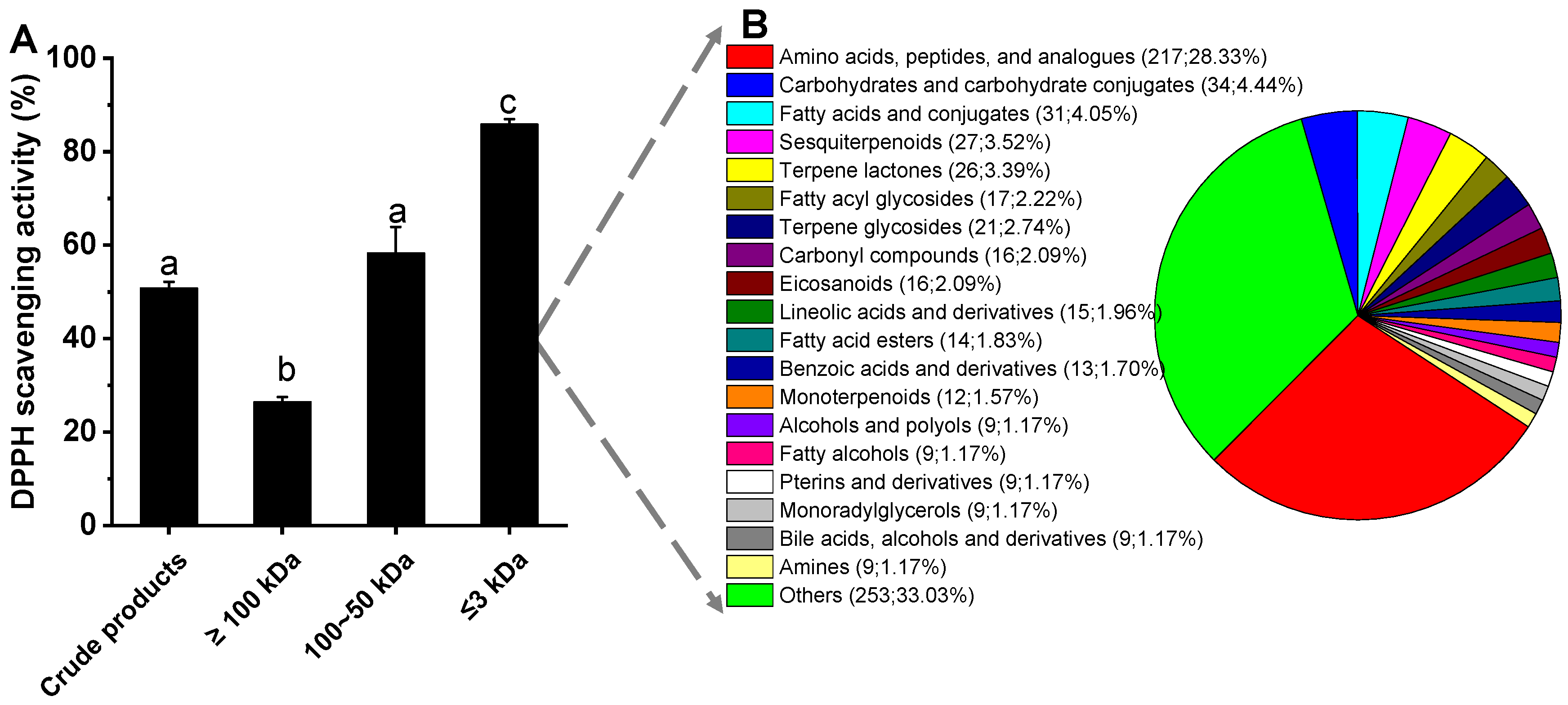
3.5. Overview of the Composition of the Low-Molecule Metabolites
3.6. Maculosin Specific Antioxidant Activity
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ross, A.B.; Jones, J.M.; Kubacki, M.L.; Bridgeman, T. Classification of macroalgae as fuel and its thermochemical behaviour. Bioresour. Technol. 2008, 99, 6494–6504. [Google Scholar] [CrossRef]
- Chi, W.-J.; Seo, J.W.; Hong, S.-K. Characterization of Two Thermostable β-agarases from a Newly Isolated Marine Agarolytic Bacterium, Vibrio sp. S1. Biotechnol. Bioprocess Eng. 2019, 24, 799–809. [Google Scholar] [CrossRef]
- Khalifa, A.; Aldayel, M. Isolation and characterisation of the agarolytic bacterium Pseudoalteromonas ruthenica. Open Life Sci. 2019, 14, 588–594. [Google Scholar] [CrossRef] [PubMed]
- Geng, Y.; Wang, K.; Chen, D.; Huang, X.; He, M.; Yin, Z. Stenotrophomonas maltophilia, an emerging opportunist pathogen for cultured channel catfish, Ictalurus punctatus, in China. Aquaculture 2010, 308, 132–135. [Google Scholar] [CrossRef]
- Schroeder, D.C.; Jaffer, M.A.; Coyne, V.E. Investigation of the role of a β (1–4) agarase produced by Pseudoalteromonas gracilis B9 in eliciting disease symptoms in the red alga Gracilaria gracilis. Microbiology 2003, 149 Pt 10, 2919–2929. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Chi, W.-J.; Chang, Y.-K.; Hong, S.-K. Agar degradation by microorganisms and agar-degrading enzymes. Appl. Microbiol. Biotechnol. 2012, 94, 917–930. [Google Scholar] [CrossRef] [PubMed]
- Yun, E.J.; Yu, S.; Kim, K.H. Current knowledge on agarolytic enzymes and the industrial potential of agar-derived sugars. Appl. Microbiol. Biotechnol. 2017, 101, 5581–5589. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Jiang, X.; Mou, H.; Guan, H. Anti-oxidation of agar oligosaccharides produced by agarase from a marine bacterium. J. Appl. Phycol. 2004, 16, 333–340. [Google Scholar] [CrossRef]
- Ren, W.; Cai, R.; Yan, W.; Lyu, M.; Fang, Y.; Wang, S. Purification and characterization of a biofilm-degradable dextranase from a marine bacterium. Mar. Drugs 2018, 16, 51. [Google Scholar] [CrossRef]
- Kim, J.H.; Yun, E.J.; Yu, S.; Kim, K.H.; Kang, N.J. Different Levels of Skin Whitening Activity among 3,6-Anhydro-l-galactose, Agarooligosaccharides, and Neoagarooligosaccharides. Mar. Drugs 2017, 15, 321. [Google Scholar] [CrossRef]
- Mussatto, S.I.; Mancilha, I.M. Non-digestible oligosaccharides: A review. Carbohydr. Polym. 2007, 68, 587–597. [Google Scholar] [CrossRef]
- Bhattarai, Y.; Kashyap, P.C. Agaro-oligosaccharides: A new frontier in the fight against colon cancer? Am. J. Physiol.-Gastrointest. Liver Physiol. 2016, 310, G335–G336. [Google Scholar] [CrossRef] [PubMed]
- Ren, W.; Wang, S.; Lü, M.; Wang, X.; Fang, Y.; Jiao, Y.; Hu, J. Optimization of four types of antimicrobial agents to increase the inhibitory ability of marine Arthrobacter oxydans KQ11 dextranase mouthwash. Chin. J. Oceanol. Limnol. 2016, 34, 354–366. [Google Scholar] [CrossRef]
- Ren, W.; Li, P.; Wang, X.; Che, Y.; Long, H.; Zhang, X.; Cai, X.; Huang, A.; Zeng, Y.; Xie, Z. Cross-habitat distribution pattern of Bacillus communities and their capacities of producing industrial hydrolytic enzymes in Paracel Islands: Habitat-dependent differential contributions of the environment. J. Environ. Manag. 2022, 323, 116252. [Google Scholar] [CrossRef]
- Jin, M.; Liu, H.; Hou, Y.; Chan, Z.; Di, W.; Li, L.; Zeng, R. Preparation, characterization and alcoholic liver injury protective effects of algal oligosaccharides from Gracilaria lemaneiformis. Food Res. Int. 2017, 100, 186–195. [Google Scholar] [CrossRef] [PubMed]
- Shieh, W.Y.; Simidu, U.; Maruyama, Y. Nitrogen Fixation by Marine Agar-degrading Bacteria. Microbiology 1988, 134, 1821–1825. [Google Scholar] [CrossRef][Green Version]
- Ivanova, G.; Rákhely, G.; Kovács, K.L. Hydrogen production from biopolymers by Caldicellulosiruptor saccharolyticus and stabilization of the system by immobilization. Int. J. Hydrogen Energy 2008, 33, 6953–6961. [Google Scholar] [CrossRef]
- Ren, W.; Liu, L.; Gu, L.; Yan, W.; Feng, Y.L.; Dong, D.; Wang, S.; Lyu, M.; Wang, C. Crystal structure of GH49 dextranase from Arthrobacter oxidans KQ11: Identification of catalytic base and improvement of thermostability using semirational design based on B-factors. J. Agric. Food Chem. 2019, 67, 4355–4366. [Google Scholar] [CrossRef]
- Miller, G.L. Use of Dinitrosalicylic Acid Reagent for Determination of Reducing Sugar. Anal. Chem. 1959, 31, 426–428. [Google Scholar] [CrossRef]
- Wang, X.; Lu, M.; Wang, S.; Fang, Y.; Wang, D.; Ren, W.; Zhao, G. The atmospheric and room-temperature plasma (ARTP) method on the dextranase activity and structure. Int. J. Biol. Macromol. 2014, 70, 284–291. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Ren, W.; Ly, M.; Li, H.; Wang, S. Characterization of an Alkaline GH49 Dextranase from Marine Bacterium Arthrobacter oxydans KQ11 and Its Application in the Preparation of Isomalto-Oligosaccharide. Mar. Drugs 2019, 17, 479. [Google Scholar] [CrossRef]
- Ren, W.; Ding, Y.; Gu, L.; Yan, W.; Wang, C.; Lyu, M.; Wang, C.; Wang, S. Characterization and mechanism of the effects of Mg–Fe layered double hydroxide nanoparticles on a marine bacterium: New insights from genomic and transcriptional analyses. Biotechnol. Biofuels 2019, 12, 196. [Google Scholar] [CrossRef]
- Ren, W.; Xu, X.; Long, H.; Zhang, X.; Cai, X.; Huang, A.; Xie, Z. Tropical Cellulolytic Bacteria: Potential Utilization of Sugarcane Bagasse as Low-Cost Carbon Source in Aquaculture. Front. Microbiol. 2021, 12, 745853. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.; Bu, J.; Long, H.; Zhang, X.; Cai, X.; Huang, A.; Ren, W.; Xie, Z. Community structure of protease-producing bacteria cultivated from aquaculture systems: Potential impact of a tropical environment. Front. Microbiol. 2021, 12, 638129. [Google Scholar] [CrossRef] [PubMed]
- Jiao, L.; Li, B.; Wang, M.; Liu, Z.; Zhang, X.; Liu, S. Antioxidant activities of the oligosaccharides from the roots, flowers and leaves of Panax ginseng CA Meyer. Carbohydr. Polym. 2014, 106, 293–298. [Google Scholar] [CrossRef]
- Wei, Y.; Wang, F.; Gao, J.; Huang, Y.; Ren, W.; Sheng, H. Culture-dependent and culture-independent characterization of bacterial community diversity in different types of sandy lands: The case of Minqin County, China. BMC Microbiol. 2021, 21, 87. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.; Long, Z.; Ren, M. Microbial community and functional prediction during the processing of salt production in a 1000-year-old marine solar saltern of South China. Sci. Total Environ. 2022, 819, 152014. [Google Scholar] [CrossRef]
- Gu, L.; Yan, W.; Wu, H.; Fan, S.; Ren, W.; Wang, S.; Lyu, M.; Liu, J. Selection of DNAzymes for Sensing Aquatic Bacteria: Vibrio Anguillarum. Anal. Chem. 2019, 91, 7887–7893. [Google Scholar] [CrossRef]
- De Jesus Raposo, M.F.; De Morais, A.M.B.; De Morais, R.M.S.C. Marine Polysaccharides from Algae with Potential Biomedical Applications. Mar. Drugs 2015, 13, 2967–3028. [Google Scholar] [CrossRef] [PubMed]
- Sakai, M.; Hosoda, A.; Ogura, K.; Ikenaga, M. The Growth of Steroidobacter agariperforans sp nov., a Novel Agar-Degrading Bacterium Isolated from Soil, is Enhanced by the Diffusible Metabolites Produced by Bacteria Belonging to Rhizobiales. Microbes Environ. 2014, 29, 89–95. [Google Scholar] [CrossRef] [PubMed]
- Pathiraja, D.; Christiansen, L.; Park, B.; Schultz-Johansen, M.; Bang, G.; Stougaard, P.; Choi, I.-G.; Stabb, E.V. A Novel Auxiliary Agarolytic Pathway Expands Metabolic Versatility in the Agar-Degrading Marine Bacterium Colwellia echini A3T. Appl. Environ. Microbiol. 2021, 87, e00230-21. [Google Scholar] [CrossRef] [PubMed]
- de Albuquerque, T.L.; de Sousa, M.; Silva, N.C.G.E.; Neto, C.A.C.G.; Gonçalves, L.R.B.; Fernandez-Lafuente, R.; Rocha, M.V.P. β-Galactosidase from Kluyveromyces lactis: Characterization, production, immobilization and applications—A review. Int. J. Biol. Macromol. 2021, 191, 881–898. [Google Scholar] [CrossRef]
- Spiridon, I.; Popescu, M.C.; Bodârlău, R.; Vasile, C. Enzymatic degradation of some nanocomposites of poly(vinyl alcohol) with starch. Polym. Degrad. Stab. 2008, 93, 1884–1890. [Google Scholar] [CrossRef]
- Feng, Z.; Peng, L.; Chen, M.; Li, M. Rhodococcus sp. Q5, a novel agarolytic bacterium isolated from printing and dyeing wastewater. Folia Microbiol. 2012, 57, 379–386. [Google Scholar] [CrossRef] [PubMed]
- Lakshmikanth, M.; Manohar, S.; Souche, Y.; Lalitha, J. Extracellular β-agarase LSL-1 producing neoagarobiose from a newly isolated agar-liquefying soil bacterium, Acinetobacter sp., AG LSL-1. World J. Microbiol. Biotechnol. 2006, 22, 1087–1094. [Google Scholar] [CrossRef]
- Chi, W.-J.; Lee, C.-R.; Dugerjonjuu, S.; Park, J.-S.; Kang, D.-K.; Hong, S.-K. Biochemical characterization of a novel iron-dependent GH16 β-agarase, AgaH92, from an agarolytic bacterium Pseudoalteromonas sp H9. FEMS Microbiol. Lett. 2015, 362, fnv035. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Wang, Y.; Liu, T.; Guo, S.; Zhang, P.; Sun, P.; Chen, M.; Ming, H. Characterization and overexpression of a glycosyl hydrolase family 16 β-agarase Aga0917 from Pseudoalteromonas fuliginea YTW1-15-1. J. Gen. Appl. Microbiol. 2018, 64, 276–283. [Google Scholar] [CrossRef]
- Wang, W.; Wang, J.; Yan, R.; Zeng, R.; Zuo, Y.; Wang, D.; Qu, W. Expression and Characterization of a Novel Cold-Adapted and Stable β-Agarase Gene agaW1540 from the Deep-Sea Bacterium Shewanella sp. WPAGA9. Mar. Drugs 2021, 19, 431. [Google Scholar] [CrossRef]
- Skerratt, J.H.; Bowman, J.P.; Nichols, P.D. Shewanella olleyana sp. nov., a marine species isolated from a temperate estuary which produces high levels of polyunsaturated fatty acids. Int. J. Syst. Evol. Microbiol. 2002, 52 Pt 6, 2101–2106. [Google Scholar] [CrossRef] [PubMed]
- Teramoto, M.; Nishijima, M. Agaribacter marinus gen. nov., sp nova, an agar-degrading bacterium from surface seawater. Int. J. Syst. Evol. Microbiol. 2014, 64, 2416–2423. [Google Scholar] [CrossRef]
- Wong, F.-C.; Xiao, J.; Wang, S.; Ee, K.-Y.; Chai, T.-T. Advances on the antioxidant peptides from edible plant sources. Trends Food Sci. Technol. 2020, 99, 44–57. [Google Scholar] [CrossRef]
- Cunha, S.A.; Pintado, M.E. Bioactive peptides derived from marine sources: Biological and functional properties. Trends Food Sci. Technol. 2022, 119, 348–370. [Google Scholar] [CrossRef]
- Vladkova, T.; Georgieva, N.; Staneva, A.; Gospodinova, D. Recent Progress in Antioxidant Active Substances from Marine Biota. Antioxidants 2022, 11, 439. [Google Scholar] [CrossRef]
- Tripathi, V.C.; Horam, S.; Singh, A.; Lata, M.; Reddy, T.J.; Arockiaraj, J.; Pasupuleti, M. The discovery of antioxidants in marine microorganisms and their protective effects on the hepatic cells from chemical-induced oxidative stress. Free Radic. Res. 2020, 54, 150–161. [Google Scholar] [CrossRef] [PubMed]
- Sonani, R.R.; Singh, N.K.; Kumar, J.; Thakar, D.; Madamwar, D. Concurrent purification and antioxidant activity of phycobiliproteins from Lyngbya sp. A09DM: An antioxidant and anti-aging potential of phycoerythrin in Caenorhabditis elegans. Process Biochem. 2014, 49, 1757–1766. [Google Scholar] [CrossRef]
- Abuine, R.; Rathnayake, A.U.; Byun, H.-G. Biological activity of peptides purified from fish skin hydrolysates. Fish. Aquat. Sci. 2019, 22, 10. [Google Scholar] [CrossRef]
- Cardellina, J.; Strobel, G. Maculosin, a host-specific phytotoxin for spotted knapweed from Alternaria alternata. Proc. Natl. Acad. Sci. USA 1988, 85, 8008–8011. [Google Scholar] [CrossRef]
- Biasetto, C.; Somensi, A.; Figueiro, F.; Moraes, L.; Silva, G.; Young, M.C.M.; Bolzani, V.; Araujo, A. Diketopiperazines and arylethylamides produced by Schizophyllum commune, an endophytic fungus in Alchornea glandulosa. Eclética Química J. 2019, 44, 36–42. [Google Scholar] [CrossRef]
- Ahil, S.B.; Hira, K.; Shaik, A.B.; Pal, P.P.; Kulkarni, O.P.; Araya, H.; Fujimoto, Y. L-Proline-based-cyclic dipeptides from Pseudomonas sp. (ABS-36) inhibit pro-inflammatory cytokines and alleviate crystal-induced renal injury in mice. Int. Immunopharmacol. 2019, 73, 395–404. [Google Scholar] [CrossRef]
- Chen, S.; Zhang, D.; Chen, M.; Zhang, Z.; Lian, X.-Y. A rare diketopiperazine glycoside from marine-sourced Streptomyces sp. ZZ446. Nat. Prod. Res. 2020, 34, 1046–1050. [Google Scholar] [CrossRef] [PubMed]
- Karanam, G.; Arumugam, M.K. Reactive oxygen species generation and mitochondrial dysfunction for the initiation of apoptotic cell death in human hepatocellular carcinoma HepG2 cells by a cyclic dipeptide Cyclo(-Pro-Tyr). Mol. Biol. Rep. 2020, 47, 3347–3359. [Google Scholar] [CrossRef]
- Paudel, B.; Maharjan, R.; Rajbhandari, P.; Aryal, N.; Aziz, S.; Bhattarai, K.; Baral, B.; Malla, R.; Bhattarai, H.D. Maculosin, a non-toxic antioxidant compound isolated from Streptomyces sp. KTM18. Pharm. Biol. 2021, 59, 933–936. [Google Scholar] [CrossRef]

| No. | Metabolite | m/z | Retention Time (min) | Formula | KEGG ID | Library ID | Area (Counts) | Relative Abundance (%) |
|---|---|---|---|---|---|---|---|---|
| 1 | Meso–Pristane | 332.33 | 10.87 | C19H40 | - | HMDB0034497 | 5.39 × 109 | 54.96% |
| 2 | Prolyl–Histidine | 252.00 | 2.91 | C11H16N4O3 | - | HMDB0029019 | 2.63 × 108 | 2.68% |
| 3 | Hexadecasphinganine | 273.27 | 10.20 | C16H35NO2 | C13915 | LMSP01040001 | 1.54 × 108 | 1.58% |
| 4 | Glycerol | 115.04 | 1.01 | C3H8O3 | C00116 | HMDB0000131; 56-81-5 | 1.17 × 108 | 1.20% |
| 5 | 6-Hydroxyoctadecanoic acid | 318.30 | 10.21 | C18H36O3 | - | HMDB0112193 | 1.10 × 108 | 1.12% |
| 6 | Maculosin | 261.12 | 5.90 | C14H16N2O3 | C10605 | - | 9.28 × 107 | 0.95% |
| 7 | Benzalkonium | 304.30 | 10.49 | C21H37N | - | - | 7.91 × 107 | 0.81% |
| 8 | MG(0:0/16:0/0:0) | 353.27 | 12.680 | C19H38O4 | - | HMDB0011533; LMGL01010025 | 5.28 × 107 | 0.54% |
| 9 | (2xi,6xi)-7-Methyl-3-methylene-1,2,6,7-octanetetrol | 227.13 | 9.63 | C10H20O4 | - | HMDB0033217; LMFA05000554 | 4.71 × 107 | 0.48% |
| 10 | 10,20-Dihydroxyeicosanoic acid | 362.33 | 10.22 | C20H40O4 | - | HMDB0031923 | 4.04 × 107 | 0.41% |
| 11 | (+/−)-Flurbiprofen | 245.10 | 4.43 | C15H13FO2 | - | - | 3.69 × 107 | 0.38% |
| 12 | 2-Isopropyl-3,5-dimethoxy-6-methylpyrazine | 197.13 | 6.26 | C10H16N2O2 | - | HMDB0029741 | 3.54 × 107 | 0.36% |
| 13 | Acrylamide | 181.04 | 16.30 | C3H5NO | C01659 | HMDB0004296 | 3.41 × 107 | 0.35% |
| 14 | Isoleucyl–Histidine | 251.15 | 3.94 | C12H20N4O3 | - | HMDB0028909 | 2.90 × 107 | 0.30% |
| 15 | 4-(3,7-dimethylocta-2,6-dien-1-yl)benzene-1,2,3,5-tetrol | 301.14 | 11.45 | C16H22O4 | - | - | 2.88 × 107 | 0.29% |
| 16 | Isoleucyl–proline | 211.14 | 7.34 | C11H20N2O3 | - | HMDB0011174 | 2.80 × 107 | 0.29% |
| 17 | Arginyl–Proline | 254.16 | 3.73 | C11H21N5O3 | - | HMDB0028717 | 2.62 × 107 | 0.27% |
| 18 | 1-(4-hydroxy-3-methoxyphenyl)-7-(3-hydroxyphenyl)heptane-3,5-dione | 365.14 | 11.38 | C20H22O5 | - | - | 2.45 × 107 | 0.25% |
| 19 | 2-Deoxyglucose | 187.06 | 1.04 | C6H12O5 | - | HMDB0062477 | 2.30 × 107 | 0.23% |
| 20 | Taurocholic acid | 538.28 | 11.42 | C26H45NO7S | C05122 | 81-24-3; LMST05040001; HMDB0000036 | 2.28 × 107 | 0.23% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, X.; Feng, Z.; Li, C.; Cai, X.; Long, H.; Zhang, X.; Huang, A.; Zeng, Y.; Ren, W.; Xie, Z. Analysis of the Antioxidant Composition of Low Molecular Weight Metabolites from the Agarolytic Bacterium Alteromonas macleodii QZ9-9: Possibilities for High-Added Value Utilization of Macroalgae. Antioxidants 2022, 11, 1977. https://doi.org/10.3390/antiox11101977
Wang X, Feng Z, Li C, Cai X, Long H, Zhang X, Huang A, Zeng Y, Ren W, Xie Z. Analysis of the Antioxidant Composition of Low Molecular Weight Metabolites from the Agarolytic Bacterium Alteromonas macleodii QZ9-9: Possibilities for High-Added Value Utilization of Macroalgae. Antioxidants. 2022; 11(10):1977. https://doi.org/10.3390/antiox11101977
Chicago/Turabian StyleWang, Xinyi, Ziqiao Feng, Chenhui Li, Xiaoni Cai, Hao Long, Xiang Zhang, Aiyou Huang, Yanhua Zeng, Wei Ren, and Zhenyu Xie. 2022. "Analysis of the Antioxidant Composition of Low Molecular Weight Metabolites from the Agarolytic Bacterium Alteromonas macleodii QZ9-9: Possibilities for High-Added Value Utilization of Macroalgae" Antioxidants 11, no. 10: 1977. https://doi.org/10.3390/antiox11101977
APA StyleWang, X., Feng, Z., Li, C., Cai, X., Long, H., Zhang, X., Huang, A., Zeng, Y., Ren, W., & Xie, Z. (2022). Analysis of the Antioxidant Composition of Low Molecular Weight Metabolites from the Agarolytic Bacterium Alteromonas macleodii QZ9-9: Possibilities for High-Added Value Utilization of Macroalgae. Antioxidants, 11(10), 1977. https://doi.org/10.3390/antiox11101977







