Supplementation with Oral Vitamin C Prior to and during Myeloablative Chemotherapy and Autologous Haematopoietic Stem Cell Transplantation: A Pilot Study
Abstract
1. Introduction
2. Materials and Methods
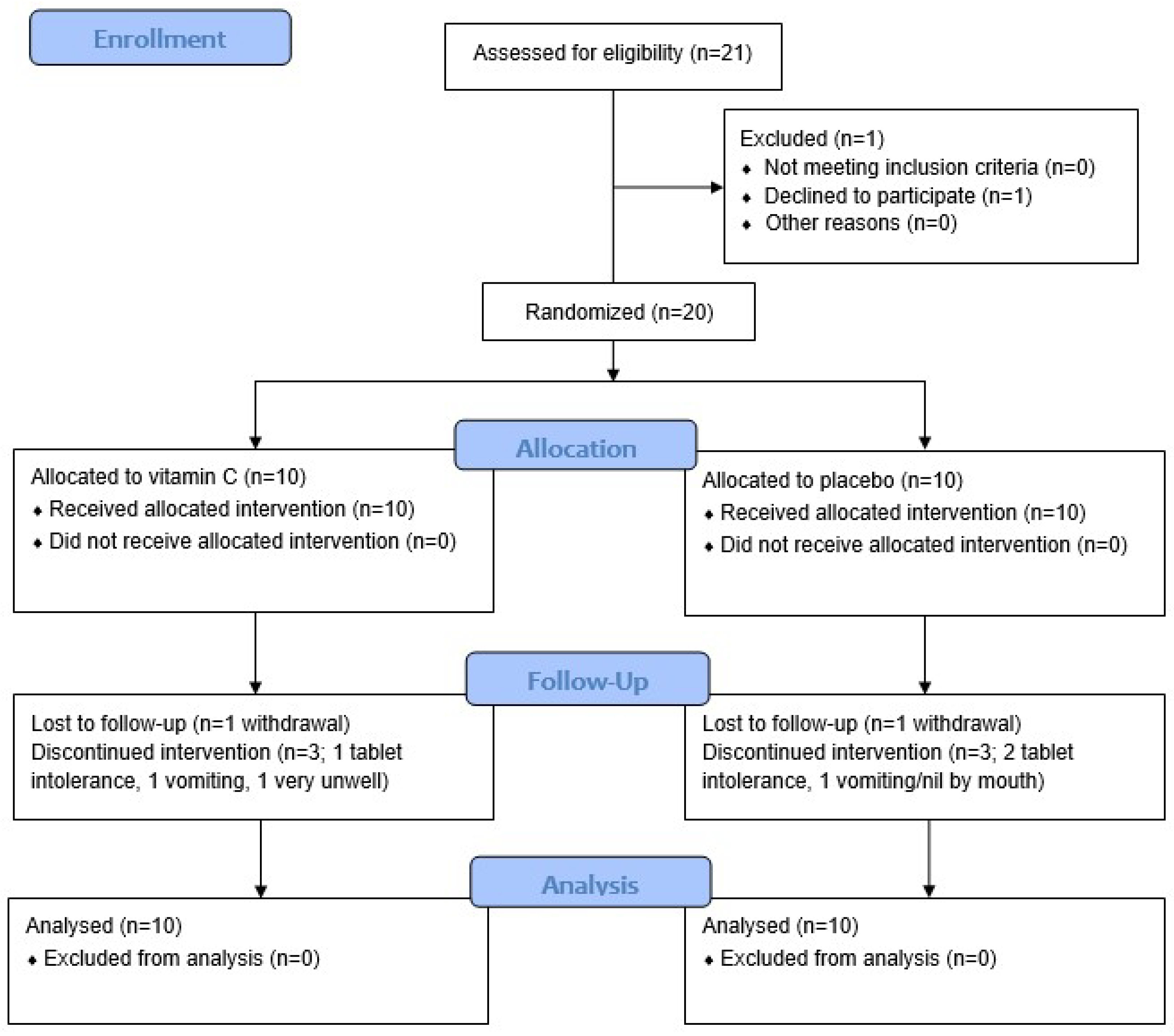
2.1. Study Design and Participants
2.2. Intervention
2.3. Collection and Analysis of Blood Samples
2.4. Assessment of Side Effects and Quality of Life
2.5. Statistical Analyses
3. Results
3.1. Participant Characteristics
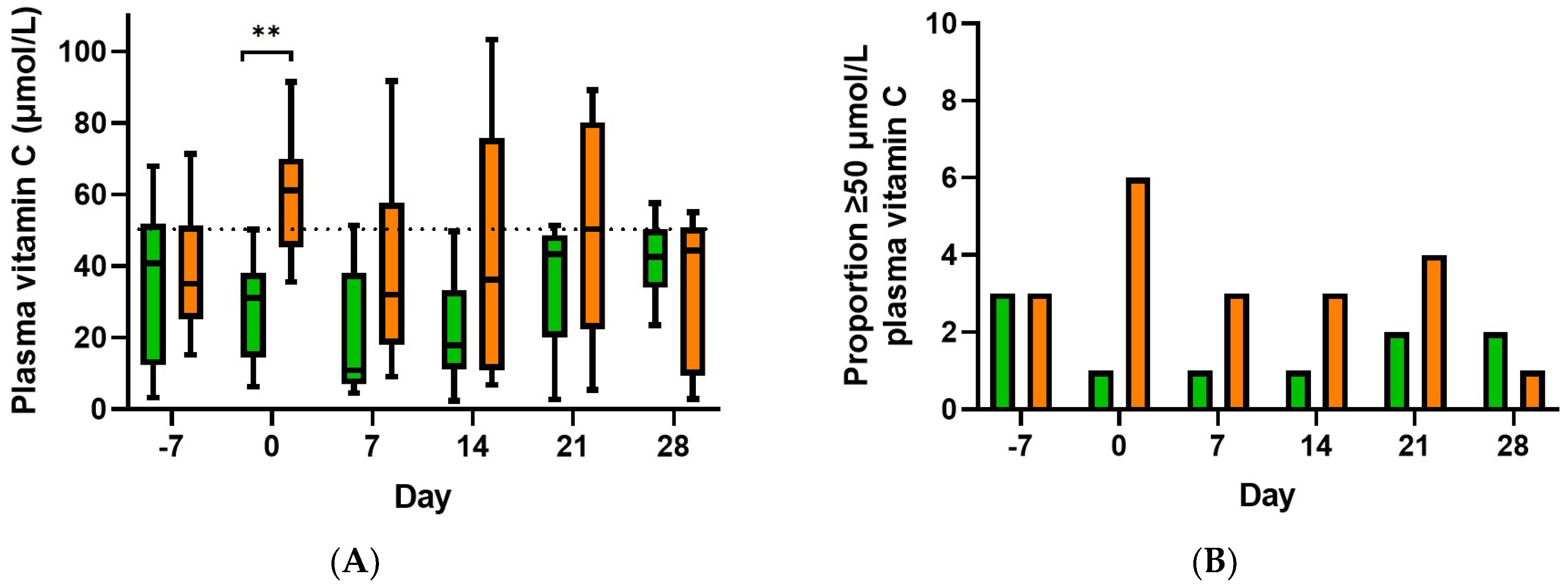
3.2. Effect of Supplementation on Plasma Vitamin C Concentrations
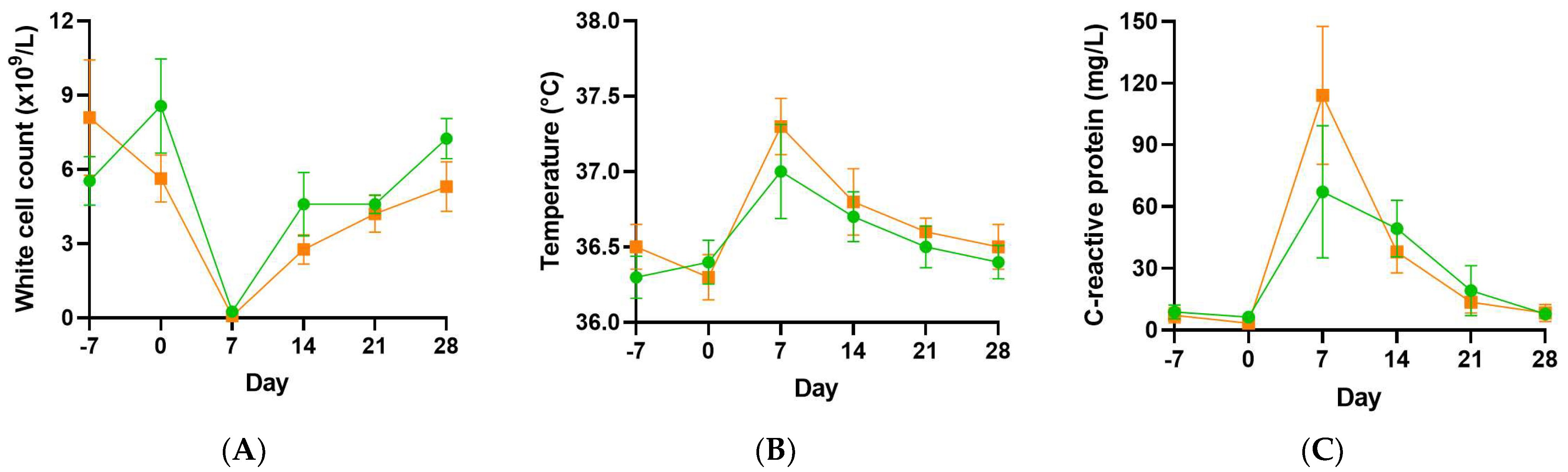
3.3. Infection Parameters
3.4. Gastrointestinal Symptoms
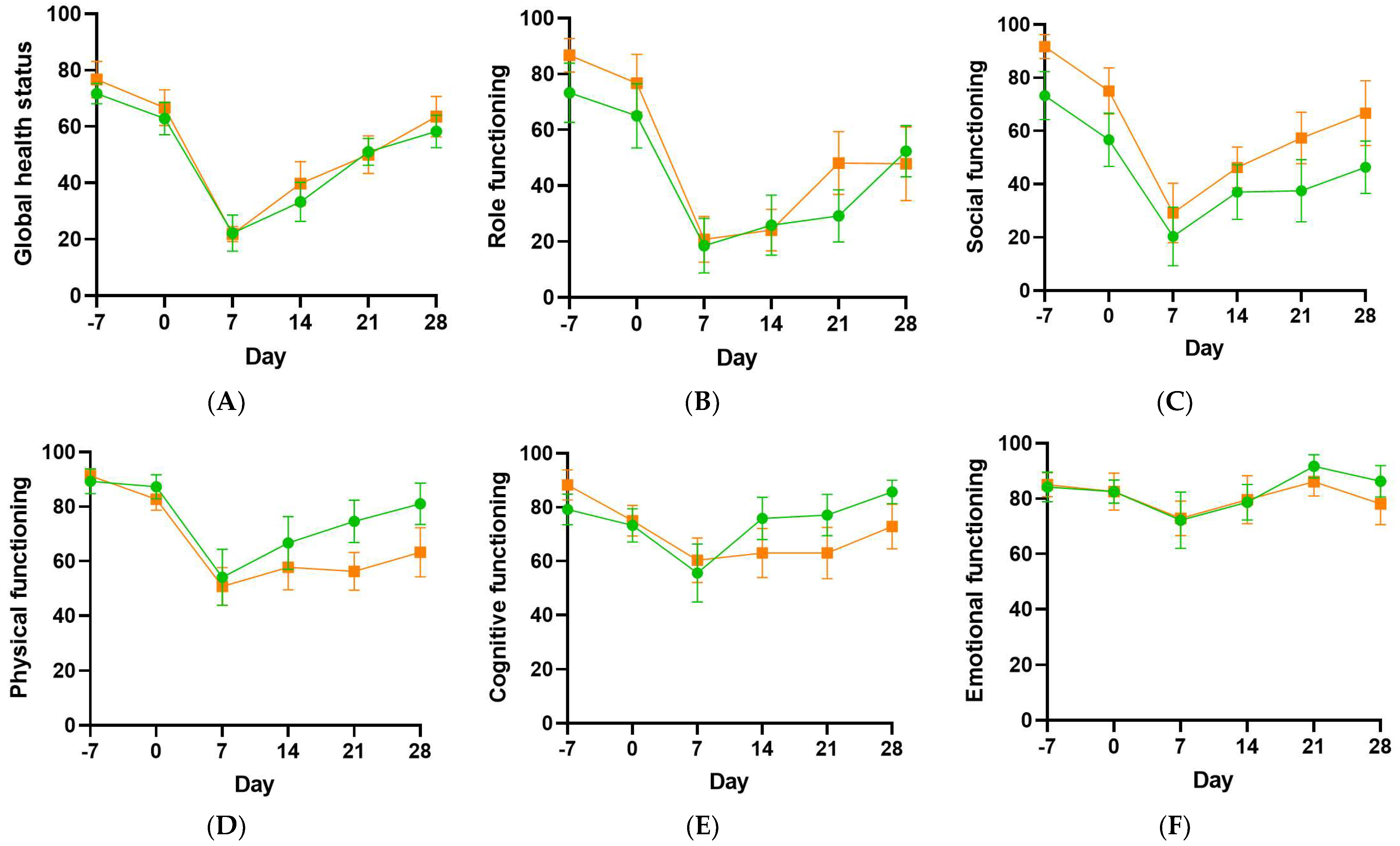
3.5. Health-Related Quality of Life
3.6. Association of Plasma Vitamin C Concentrations with Post-Transplant Symptoms
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bair, S.M.; Brandstadter, J.D.; Ayers, E.C.; A Stadtmauer, E. Hematopoietic stem cell transplantation for blood cancers in the era of precision medicine and immunotherapy. Cancer 2020, 126, 1837–1855. [Google Scholar] [CrossRef] [PubMed]
- Rubenstein, E.B.; Peterson, D.E.; Schubert, M.; Keefe, D.; McGuire, D.; Epstein, J.; Elting, L.S.; Fox, P.C.; Cooksley, C.; Sonis, S.T.; et al. Clinical practice guidelines for the prevention and treatment of cancer therapy-induced oral and gastrointestinal mucositis. Cancer 2004, 100, 2026–2046. [Google Scholar] [CrossRef] [PubMed]
- Sonis, S.T.; Elting, L.S.; Keefe, D.; Peterson, D.E.; Schubert, M.; Hauer-Jensen, M.; Bekele, B.N.; Raber-Durlacher, J.; Donnelly, J.P.; Rubenstein, E.B. Perspectives on cancer therapy-induced mucosal injury: Pathogenesis, measurement, epidemiology, and consequences for patients. Cancer 2004, 100 (Suppl. S9), 1995–2025. [Google Scholar] [CrossRef] [PubMed]
- Chaitanya, N.C.; Muthukrishnan, A.; Babu, D.B.G.; Kumari, C.S.; Lakshmi, M.A.; Palat, G.; Alam, K.S. Role of Vitamin E and Vitamin A in Oral Mucositis Induced by Cancer Chemo/Radiotherapy- A Meta-analysis. J. Clin. Diagn. Res. JCDR 2017, 11, ZE06–ZE09. [Google Scholar] [CrossRef]
- Carr, A.C.; Maggini, S. Vitamin C and Immune Function. Nutrients 2017, 9, 1211. [Google Scholar] [CrossRef]
- Carr, A.; Frei, B. Does vitamin C act as a pro-oxidant under physiological conditions? FASEB J. 1999, 13, 1007–1024. [Google Scholar] [CrossRef]
- Jafarnejad, S.; Boccardi, V.; Hosseini, B.; Taghizadeh, M.; Hamedifard, Z. A Meta-Analysis of Randomized Control Trials: The Impact of Vitamin C Supplementation on Serum CRP and Serum hs-CRP Concentrations. Curr. Pharm. Des. 2018, 24, 3520–3528. [Google Scholar] [CrossRef]
- Ma, Y.; Chapman, J.; Levine, M.; Polireddy, K.; Drisko, J.; Chen, Q. High-Dose Parenteral Ascorbate Enhanced Chemosensitivity of Ovarian Cancer and Reduced Toxicity of Chemotherapy. Sci. Transl. Med. 2014, 6, 222ra18. [Google Scholar] [CrossRef]
- Carr, A.C.; Vissers, M.C.M.; Cook, J.S. The Effect of Intravenous Vitamin C on Cancer- and Chemotherapy-Related Fatigue and Quality of Life. Front. Oncol. 2014, 4, 283. [Google Scholar] [CrossRef]
- Fritz, H.; Flower, G.; Weeks, L.; Cooley, K.; Callachan, M.; McGowan, J.; Skidmore, B.; Kirchner, L.; Seely, D. Intravenous Vitamin C and Cancer: A Systematic Review. Integr. Cancer Ther. 2014, 13, 280–300. [Google Scholar] [CrossRef]
- Carr, A.C.; Cook, J. Intravenous Vitamin C for Cancer Therapy—Identifying the Current Gaps in Our Knowledge. Front. Physiol. 2018, 9, 1182. [Google Scholar] [CrossRef] [PubMed]
- Rasheed, M.; Simmons, G.; Fisher, B.; Leslie, K.; Reed, J.; Roberts, C.; Natarajan, R.; Fowler, A.; Toor, A. Reduced plasma ascorbic acid levels in recipients of myeloablative conditioning and hematopoietic cell transplantation. Eur. J. Haematol. 2019, 103, 329–334. [Google Scholar] [CrossRef] [PubMed]
- Carr, A.C.; Spencer, E.; Das, A.; Meijer, N.; Lauren, C.; MacPherson, S.; Chambers, S.T. Patients Undergoing Myeloablative Chemotherapy and Hematopoietic Stem Cell Transplantation Exhibit Depleted Vitamin C Status in Association with Febrile Neutropenia. Nutrients 2020, 12, 1879. [Google Scholar] [CrossRef] [PubMed]
- Nannya, Y.; Shinohara, A.; Ichikawa, M.; Kurokawa, M. Serial Profile of Vitamins and Trace Elements during the Acute Phase of Allogeneic Stem Cell Transplantation. Biol. Blood Marrow Transplant. 2013, 20, 430–434. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Tripathi, M.; Satyam, A.; Kumar, L. Study of antioxidant levels in patients with multiple myeloma. Leuk. Lymphoma 2009, 50, 809–815. [Google Scholar] [CrossRef] [PubMed]
- Mehdi, W.A.; Zainulabdeen, J.A.; Mehde, A.A. Investigation of the Antioxidant Status in Multiple Myeloma Patients: Effects of Therapy. Asian Pac. J. Cancer Prev. 2013, 14, 3663–3667. [Google Scholar] [CrossRef]
- Hunnisett, A.; Davies, S.; McLaren-Howard, J.; Gravett, P.; Finn, M.; Gueret-Wardle, D. Lipoperoxides as an index of free radical activity in bone marrow transplant recipients. Biol. Trace Elem. Res. 1995, 47, 125–132. [Google Scholar] [CrossRef]
- Rasheed, M.; Roberts, C.H.; Gupta, G.; Fisher, B.J.; Leslie, K.; Simmons, G.L.; Wiedl, C.M.; Mccarty, J.M.; Clark, W.B.; Chung, H.M.; et al. Low Plasma Vitamin C Levels in Patients Undergoing Stem Cell Transplantation. Biol. Blood Marrow Transplant. 2017, 23, S286–S287. [Google Scholar] [CrossRef]
- Kletzel, M.; Powers, K.; Hayes, M. Scurvy: A new problem for patients with chronic GVHD involving mucous membranes; an easy problem to resolve. Pediatr. Transplant. 2014, 18, 524–526. [Google Scholar] [CrossRef]
- Carr, A.C.; Lykkesfeldt, J. Discrepancies in global vitamin C recommendations: A review of RDA criteria and underlying health perspectives. Crit. Rev. Food Sci. Nutr. 2020, 61, 742–755. [Google Scholar] [CrossRef]
- European Food Safety Authority Panel on Dietetic Products Nutrition and Allergies. Scientific opinion on dietary reference values for vitamin C. EFSA J. Eur. Food Saf. Auth. 2013, 11, 3418. [Google Scholar]
- Carr, A.C.; Bozonet, S.M.; Pullar, J.M.; Simcock, J.W.; Vissers, M.C. Human skeletal muscle ascorbate is highly responsive to changes in vitamin C intake and plasma concentrations. Am. J. Clin. Nutr. 2013, 97, 800–807. [Google Scholar] [CrossRef] [PubMed]
- Padayatty, S.J.; Sun, H.; Wang, Y.; Riordan, H.D.; Hewitt, S.M.; Katz, A.; Wesley, R.A.; Levine, M. Vitamin C Pharmacokinetics: Implications for Oral and Intravenous Use. Ann. Intern. Med. 2004, 140, 533–537. [Google Scholar] [CrossRef] [PubMed]
- Robitaille, L.; Hoffer, L.J. A simple method for plasma total vitamin C analysis suitable for routine clinical laboratory use. Nutr. J. 2015, 15, 40. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. WHO Handbook for Reporting Results of Cancer Treatment; World Health Organization: Geneva, Switzerland, 1979.
- Aaronson, N.K.; Ahmedzai, S.; Bergman, B.; Bullinger, M.; Cull, A.; Duez, N.J.; Filiberti, A.; Flechtner, H.; Fleishman, S.B.; De Haes, J.C.J.M.; et al. The European Organization for Research and Treatment of Cancer QLQ-C30: A Quality-of-Life Instrument for Use in International Clinical Trials in Oncology. J. Natl. Cancer Inst. 1993, 85, 365–376. [Google Scholar] [CrossRef]
- Cocks, K.; King, M.T.; Velikova, G.; de Castro, G., Jr.; St-James, M.M.; Fayers, P.M.; Brown, J.M. Evidence-Based guidelines for interpreting change scores for the European Organisation for the Research and Treatment of Cancer Quality of Life Questionnaire Core 30. Eur. J. Cancer 2012, 48, 1713–1721. [Google Scholar] [CrossRef]
- National Cancer Institute. Common Terminology Criteria for Adverse Events (CTCAE) Version 4.02; National Institues of Health: Bethesda, MD, USA, 2009; p. 196.
- Bakdash, J.Z.; Marusich, L.R. Repeated Measures Correlation. Front. Psychol. 2017, 8, 456. [Google Scholar] [CrossRef]
- Bland, J.M.; Altman, D.G. Statistics notes: Calculating correlation coefficients with repeated observations: Part 1—Correlation within subjects. BMJ 1995, 310, 446. [Google Scholar] [CrossRef]
- Levine, M.; Conry-Cantilena, C.; Wang, Y.; Welch, R.W.; Washko, P.W.; Dhariwal, K.R.; Park, J.B.; Lazarev, A.; Graumlich, J.F.; King, J.; et al. Vitamin C pharmacokinetics in healthy volunteers: Evidence for a recommended dietary allowance. Proc. Natl. Acad. Sci. USA 1996, 93, 3704–3709. [Google Scholar] [CrossRef]
- Carr, A.C.; Pullar, J.M.; Bozonet, S.M.; Vissers, M.C.M. Marginal Ascorbate Status (Hypovitaminosis C) Results in an Attenuated Response to Vitamin C Supplementation. Nutrients 2016, 8, 341. [Google Scholar] [CrossRef]
- Gillberg, L.; Ørskov, A.D.; Nasif, A.; Ohtani, H.; Madaj, Z.; Hansen, J.W.; Rapin, N.; Mogensen, J.B.; Liu, M.; Dufva, I.H.; et al. Oral vitamin C supplementation to patients with myeloid cancer on azacitidine treatment: Normalization of plasma vitamin C induces epigenetic changes. Clin. Epigenet. 2019, 11, 143. [Google Scholar] [CrossRef] [PubMed]
- Riley, P.; Glenny, A.-M.; Worthington, H.V.; Littlewood, A.; Clarkson, J.E.; McCabe, M.G. Interventions for preventing oral mucositis in patients with cancer receiving treatment: Oral cryotherapy. Cochrane Database Syst. Rev. 2015, CD011552. [Google Scholar] [CrossRef]
- Englard, S.; Seifter, S. The Biochemical Functions of Ascorbic Acid. Annu. Rev. Nutr. 1986, 6, 365–406. [Google Scholar] [CrossRef] [PubMed]
- Carr, A.C.; McCall, C. The role of vitamin C in the treatment of pain: New insights. J. Transl. Med. 2017, 15, 77. [Google Scholar] [CrossRef]


| Total Cohort (n = 20) | Placebo Group (n = 10) | Vitamin C Group (n = 10) | |
|---|---|---|---|
| Age, years | 55 (41, 64) | 52 (41, 63) | 59 (37, 68) |
| Gender, male | 12 (60) | 7 (70) | 5 (50) |
| Weight, kg | 79 (74, 84) | 79 (75, 83) | 77 (73, 93) |
| Smoker | 5 (25) | 3 (30) | 2 (20) |
| Cancer type | |||
| - myeloma - lymphoma | 14 (70) 6 (30) | 8 (80) 2 (20) | 6 (60) 4 (40) |
| Myeloablative regimen | |||
| - melphalan - BEAM 1 - carmustine + thiotepa | 11 (55) 8 (40) 1 (5) | 6 (60) 3 (30) 1 (10) | 5 (50) 5 (50) 0 (0) |
| RM Correlation (95% CI) a | p Value | ≤23 µmol/L Vitamin C Subgroup | ≥50 µmol/L Vitamin C Subgroup | |
|---|---|---|---|---|
| Infection parameter | ||||
| C-reactive protein (mg/L) | −0.53 (−0.72, −0.26) | <0.001 | 47 (14, 123) | 10 (4, 19) |
| Gastrointestinal symptoms (%) | ||||
| Nausea/vomiting | −0.52 (−0.71, −0.27) | <0.001 | 50 (17, 96) | 17 (17, 33) |
| Diarrhoea | −0.53 (−0.72, −0.28) | <0.001 | 67 (33, 100) | 33 (25, 42) |
| Other symptoms (%) | ||||
| Fatigue | −0.34 (−0.58, −0.05) | 0.02 | 78 (61, 97) | 56 (42, 92) |
| Pain | −0.40 (−0.63, −0.12) | 0.006 | 33 (0, 46) | 8 (0, 17) |
| Functioning (%) | ||||
| Global health status | 0.43 (0.15, 0.65) | 0.002 | 25 (17, 46) | 50 (29, 67) |
| Role functioning | 0.63 (0.40, 0.78) | <0.001 | 0 (0, 33) | 42 (29, 67) |
| Social functioning | 0.29 (−0.01, 0.54) | 0.055 | 25 (0, 67) | 50 (33, 67) |
| Physical functioning | 0.42 (0.13, 0.64) | 0.004 | 57 (27, 80) | 67 (45, 77) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Carr, A.C.; Vlasiuk, E.; Zawari, M.; Meijer, N.; Lauren, C.; MacPherson, S.; Williman, J.; Chambers, S.T. Supplementation with Oral Vitamin C Prior to and during Myeloablative Chemotherapy and Autologous Haematopoietic Stem Cell Transplantation: A Pilot Study. Antioxidants 2022, 11, 1949. https://doi.org/10.3390/antiox11101949
Carr AC, Vlasiuk E, Zawari M, Meijer N, Lauren C, MacPherson S, Williman J, Chambers ST. Supplementation with Oral Vitamin C Prior to and during Myeloablative Chemotherapy and Autologous Haematopoietic Stem Cell Transplantation: A Pilot Study. Antioxidants. 2022; 11(10):1949. https://doi.org/10.3390/antiox11101949
Chicago/Turabian StyleCarr, Anitra C., Emma Vlasiuk, Masuma Zawari, Natalie Meijer, Carolyn Lauren, Sean MacPherson, Jonathan Williman, and Stephen T. Chambers. 2022. "Supplementation with Oral Vitamin C Prior to and during Myeloablative Chemotherapy and Autologous Haematopoietic Stem Cell Transplantation: A Pilot Study" Antioxidants 11, no. 10: 1949. https://doi.org/10.3390/antiox11101949
APA StyleCarr, A. C., Vlasiuk, E., Zawari, M., Meijer, N., Lauren, C., MacPherson, S., Williman, J., & Chambers, S. T. (2022). Supplementation with Oral Vitamin C Prior to and during Myeloablative Chemotherapy and Autologous Haematopoietic Stem Cell Transplantation: A Pilot Study. Antioxidants, 11(10), 1949. https://doi.org/10.3390/antiox11101949








