Antioxidative Self-Assembling Nanoparticles Attenuate the Development of Steatohepatitis and Inhibit Hepatocarcinogenesis in Mice
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Preparation and Pharmacokinetics of SMAPoTN
2.3. Biochemical Analysis
2.4. Histological Analysis
2.5. Immunoblot Analysis
2.6. Real-Time Quantitative Polymerase Chain Reaction (qPCR)
2.7. RNA Sequencing (RNA-Seq) Analysis
2.8. TG and FFA Concentrations and FA Composition in Liver Tissues
2.9. Measurement of Serum and Fecal LPS Levels
2.10. Microbiome Analysis
2.11. Statistics
3. Results
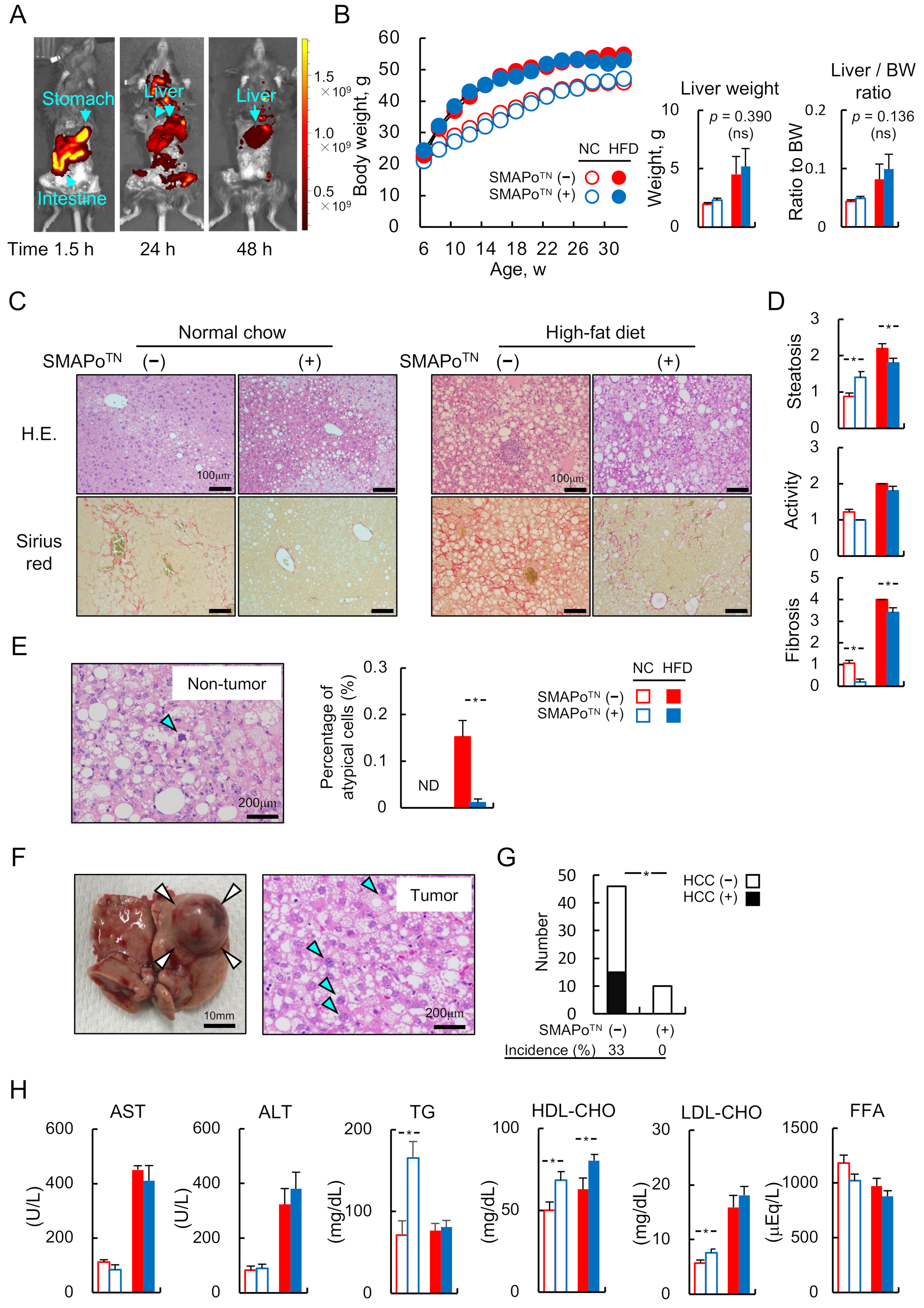
3.1. SMAPoTN Is Derived and Accumulated in the Livers through the Digestive Tract without Any Side Effects
3.2. SMAPoTN Inhibits the Development of Liver Fibrosis and HCC
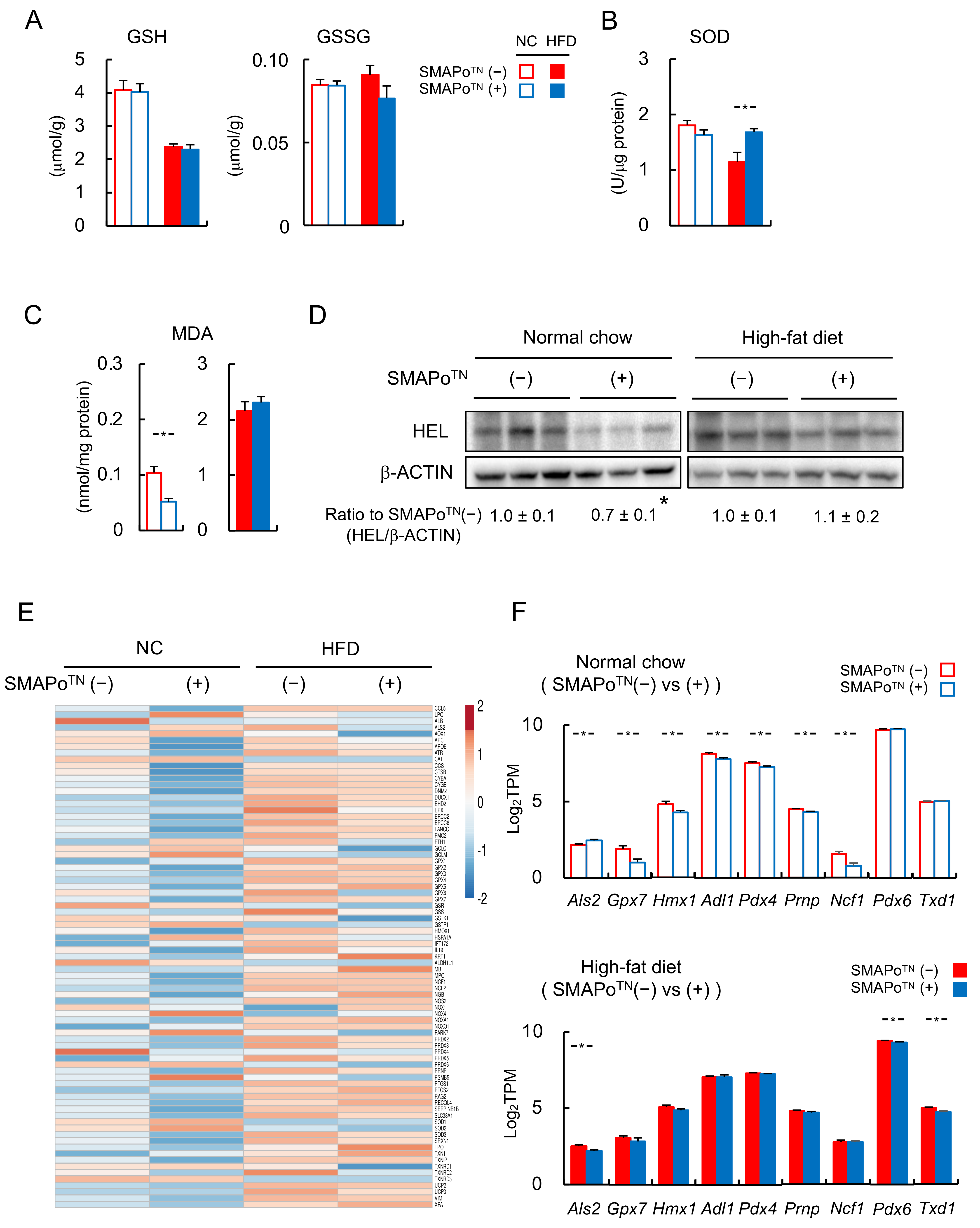
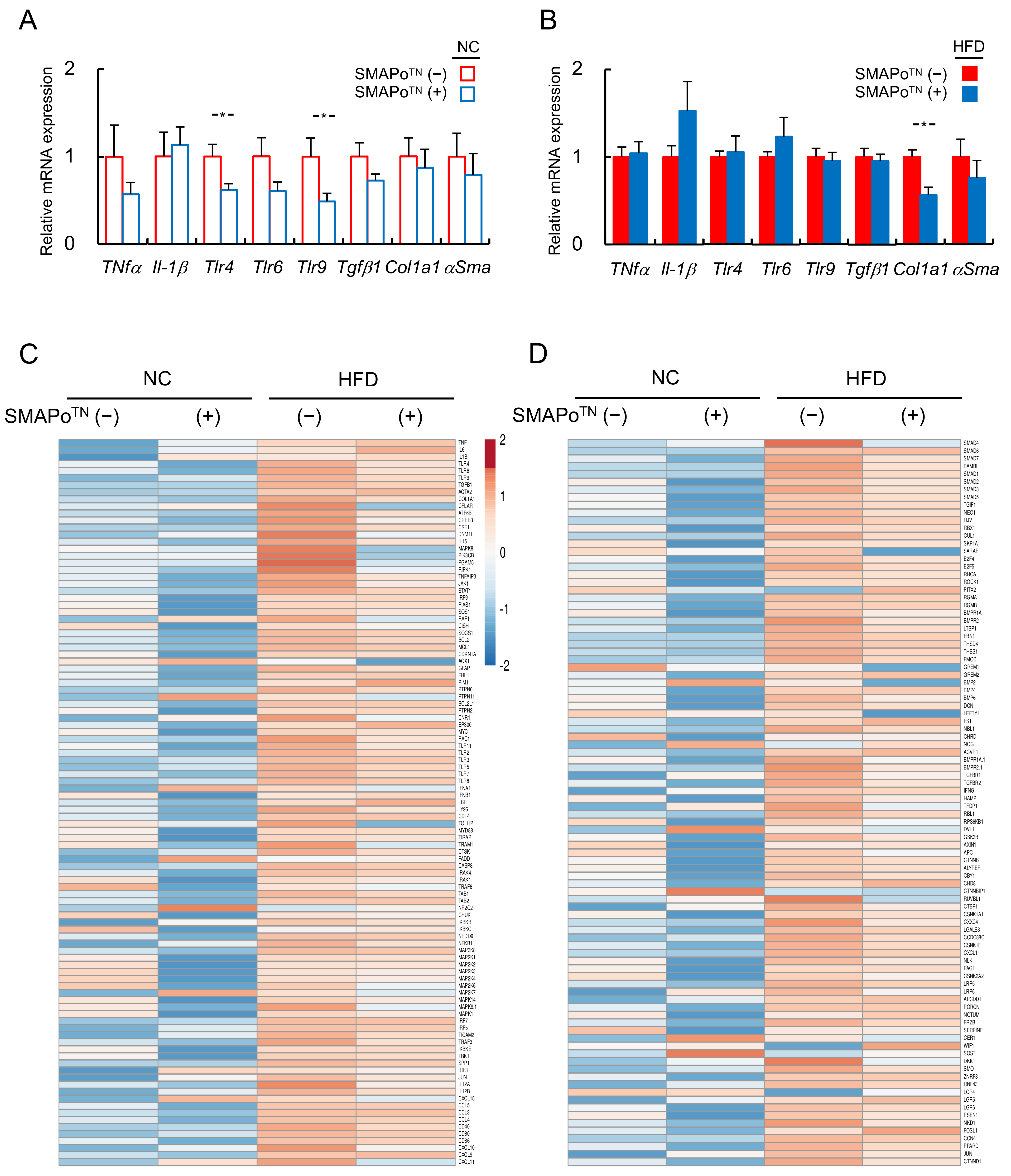
3.3. SMAPoTN Treatment Reduces OS, Inflammatory Signaling, and Fibrosis-Related Factors in the Liver
3.4. TG and FAs in the Livers of Mice with Steatohepatitis
3.5. RNA-Seq Analyses of the Livers to Evaluate the Efficacy of SMAPoTN against the Development of NASH and HCC
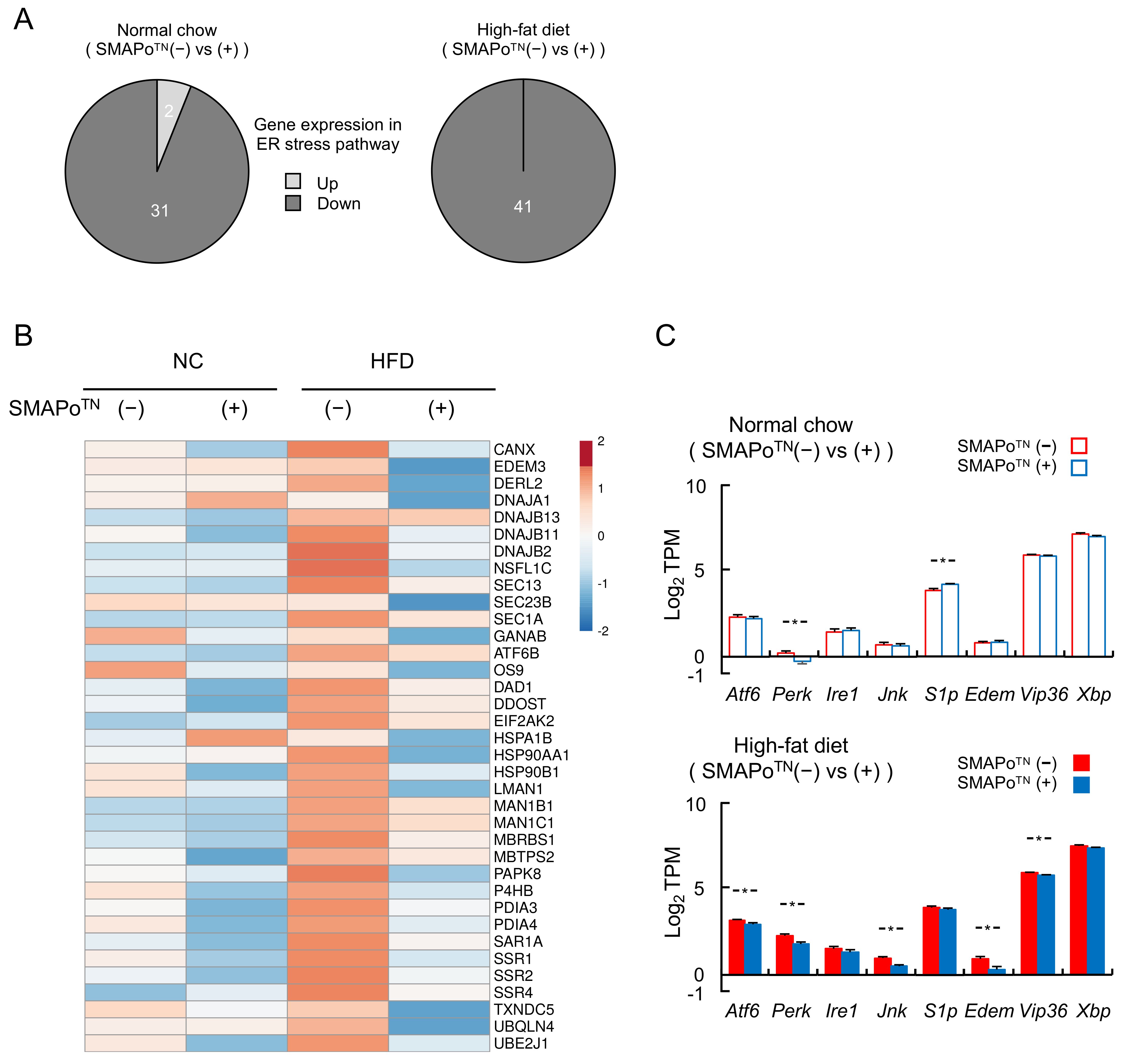
3.6. SMAPoTN Treatment Downregulates ER Stress Pathway Genes
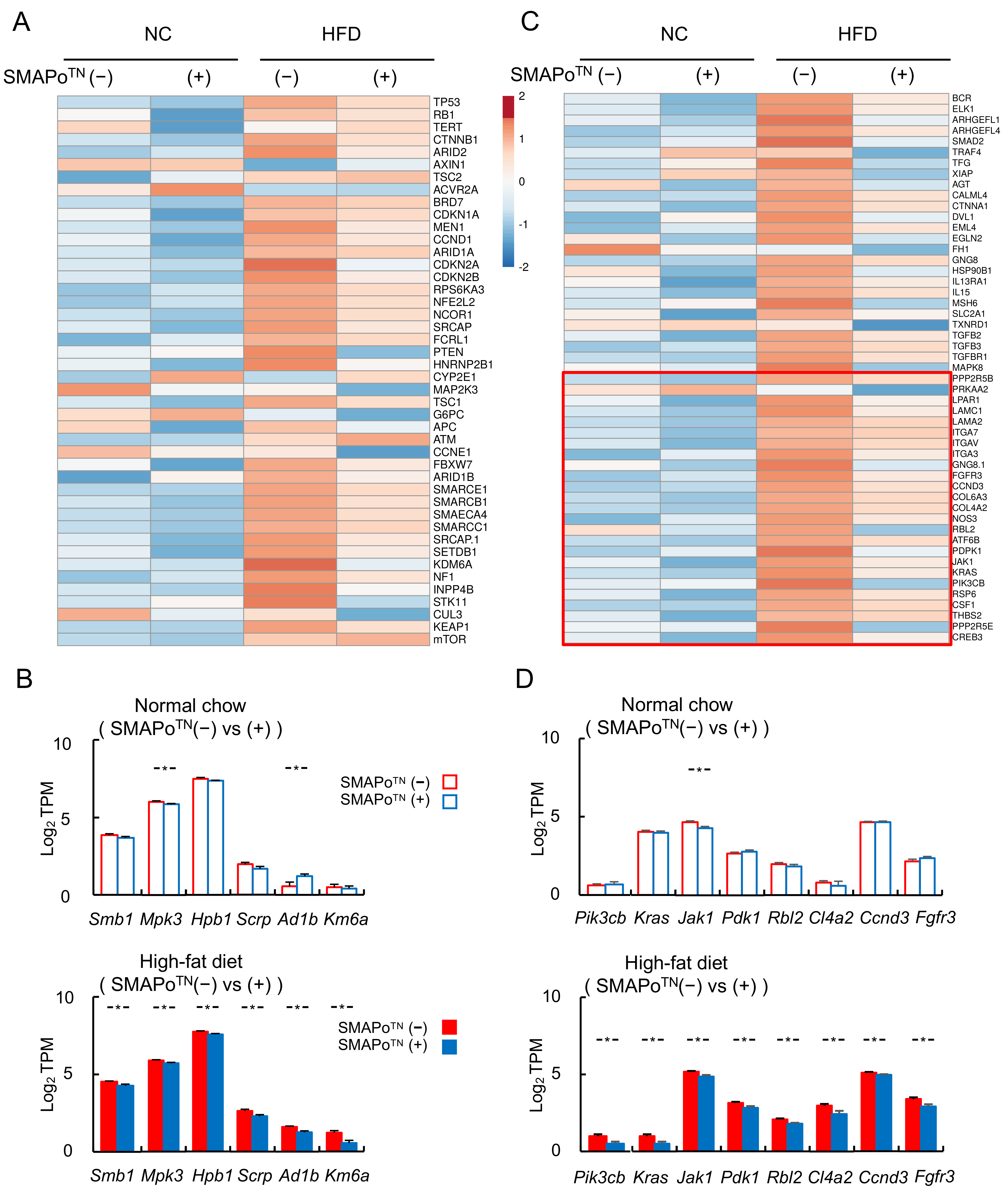
3.7. SMAPoTN Treatment Downregulates Cancer Driver Genes and Cancer Pathway Genes
3.8. SMAPoTN Treatment Decreases LPS by Improving Diversity and Changing the Microbiota Composition
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Malecka-Tendera, E.; Mazur, A. Childhood obesity: A pandemic of the twenty-first century. Int. J. Obes. 2006, 30, S1–S3. [Google Scholar] [CrossRef] [PubMed]
- Farrell, G.C.; Larter, C.Z. Nonalcoholic fatty liver disease: From steatosis to cirrhosis. Hepatology 2006, 43, 99–112. [Google Scholar] [CrossRef] [PubMed]
- Bugianesi, E.; Leone, N. Expanding the natural history of nonalcoholic steatohepatitis: From cryptogenic cirrhosis to hepatocellular carcinoma. Gastroenterology 2002, 123, 134–140. [Google Scholar] [CrossRef] [PubMed]
- Tilg, H.; Moschen, A.R. Evolution of inflammation in nonalcoholic fatty liver disease: The multiple parallel hits hypothesis. Hepatology 2010, 52, 1836–1846. [Google Scholar] [CrossRef] [PubMed]
- Brahma, M.K.; Gilglioni, E.H. Oxidative stress in obesity-associated hepatocellular carcinoma: Sources, signaling and therapeutic challenges. Oncogene 2021, 40, 5155–5167. [Google Scholar] [CrossRef] [PubMed]
- Borrelli, A.; Bonelli, P. Role of gut microbiota and oxidative stress in the progression of non-alcoholic fatty liver disease to hepatocarcinoma: Current and innovative therapeutic approaches. Redox Biol. 2018, 15, 467–479. [Google Scholar] [CrossRef]
- Lebeaupin, C.; Vallee, D. Endoplasmic reticulum stress signaling and the pathogenesis of non-alcoholic fatty liver disease. J. Hepatol. 2018, 69, 927–947. [Google Scholar] [CrossRef]
- Dapito, H.; Mencin, A. Promotion of Hepatocellular Carcinoma by the Intestinal Microbiota and TLR4. Cancer Cell 2012, 21, 504–516. [Google Scholar] [CrossRef]
- Zhou, A.; Tang, L. Gut microbiota: A new piece in understanding hepatocarcinogenesis. Cancer Lett. 2020, 474, 15–22. [Google Scholar] [CrossRef]
- Totoki, Y.; Tatsuno, K. Trans-ancestry mutational landscape of hepatocellular carcinoma genomes. Nat. Genmetics. 2014, 46, 1267–1276. [Google Scholar] [CrossRef]
- Schulze, K.; Imbeaud, S. Exome sequencing of hepatocellular carcinoma identifies new mutational signatures and potential therapeutic targets. Nat. Genetics. 2015, 47, 505–511. [Google Scholar] [CrossRef]
- Defour, J.E.; Caussy, C. Combibation Therapy for Non-Alcoholic Steathepatitis: Rationale, Opportunities and Challenges. Gut 2020, 69, 1877–1884. [Google Scholar] [CrossRef]
- Cardoso, A.C.; Sanyal, A.J. New Drugs for Non-Alcoholic Steatohepatitis. Liver Int. 2020, 40, 96–101. [Google Scholar] [CrossRef]
- Cusi, K. Role of obesity and lipotoxicity in the development of nonalcoholic steatohepatitis: Pathophysiology and clinical implications. Gastroenterology 2012, 142, 711–725. [Google Scholar] [CrossRef] [PubMed]
- Margini, C.; Dufour, J.F. The story of HCC in NAFLD: From epidemiology, across pathogenesis, to prevention and treatment. Liver Int. 2016, 36, 317–324. [Google Scholar] [CrossRef] [PubMed]
- Farazi, P.A.; DePinho, R.A. Hepatocellular carcinoma pathogenesis: From genes to environment. Nat. Rev. Cancer 2006, 6, 674–687. [Google Scholar] [CrossRef] [PubMed]
- Sanyal, A.J.; Chalasani, N. Pioglitazone, vitamin E, or placebo for nonalcoholic steatohepatitis. N. Engl. J. Med. 2010, 362, 1675–1685. [Google Scholar] [CrossRef]
- Lavine, J.E.; Schwimmer, J.B. Effect of vitamin E or metformin for treatment of nonalcoholic fatty liver disease in children and adolescents: The TONIC randomized controlled trial. JAMA 2011, 305, 1659–1668. [Google Scholar] [CrossRef]
- Italian Association for the Study of the Liver (AISF). AISF Position Paper on Nonalcoholic Fatty Liver Disease (NAFLD): Updates and Future Directions. Dig. Liver Dis. 2017, 49, 471–483. [Google Scholar] [CrossRef]
- Estes, C.; Anstee, Q.M. Modeling NAFLD disease burden in China, France, Germany, Italy, Spain, United Kingdom, and United States for the period 2016–2030. J. Hepatol. 2013, 69, 896–904. [Google Scholar] [CrossRef]
- Tateishi, R.; Uchino, K. A Nationwide survey on non-B, non-C hepatocellular Carcinoma in Japan: 2011–1015 update. J. Gastroenterol. 2019, 54, 367–376. [Google Scholar] [CrossRef]
- Reddy, J.K.; Rao, M.S. Lipid metabolism and liver inflammation. II. Fatty liver diseases and fatty acid oxidation. Am. J. Physiol. Gastrointest. Liver Pysiol. 2006, 29, G852–G858. [Google Scholar] [CrossRef]
- Neuschwander-Tetri, B.A.; Caldwell, S.H. Nonalcoholic steatohepatitis: Summary of an AASLD Single Topic Conference. Hepatology 2003, 37, 1202–1219. [Google Scholar] [CrossRef]
- Yuksel, B.C.; Serdar, S.E.; Tuncel, A. Effect of tempol, a membrane-permeable radical scavenger, on mesenteric blood flow and organ injury in a murine cecal ligation and puncture model of septic shock. Eur. Surg. Res. 2009, 43, 219–227. [Google Scholar] [CrossRef]
- Soule, B.P.; Hyodo, F.; Matsumoto, K. The chemistry and biology of nitroxide compounds. Free Radic. Biol. Med. 2007, 42, 1632–1650. [Google Scholar] [CrossRef]
- Nagasaki, Y. Nitroxide radicals and nanoparticles: A partnership for nanomedicine radical delivery. Ther. Deliv. 2012, 3, 165–179. [Google Scholar] [CrossRef]
- Shimizu, M.; Yoshitomi, T.; Nagasaki, Y. The behavior of ROS-scavenging nanoparticles in blood. J. Clin. Biochem. Nutr. 2014, 54, 166–173. [Google Scholar] [CrossRef][Green Version]
- Monti, E.; Supino, R.; Colleoni, M. Nitroxide TEMPOL impairs mitochondrial function and induces apoptosis in HL60 cells. J. Cell. Biochem. 2001, 82, 271–276. [Google Scholar] [CrossRef]
- Vong, L.B.; Nagasaki, Y. Oral nanotherapeutics: Effect of redox nanoparticle on. microflora in mice with dextran sodium sulfate-induced colitis. J. Gastroenterol. 2014, 49, 806–813. [Google Scholar] [CrossRef]
- Eguchi, A.; Ngasaki, Y. Redox nanoparticles as a novel treatment approach for inflammation and fibrosis associated with nonalcoholic steatohepatitis. Nanomedicine 2015, 10, 2697–2708. [Google Scholar] [CrossRef]
- Piguet, A.C.; Saran, U. Regular exercise decreases liver tumors development in hepatoicyte-specific PTEN-deficient mice independently of steatosis. J. Hepatol. 2015, 62, 1296–1303. [Google Scholar] [CrossRef]
- Arfianti, A.; Pok, S. Exercise retards hepatocarcinogenesis in obese mice independently of weight control. J. Hepatol. 2020, 73, 140–148. [Google Scholar] [CrossRef]
- Akiyama, K.; Warabi, E. Deletion of both p62 and Nrf2 spontaneously results in the development of nonalcoholic steatohepatitis. Exp. Anim. 2018, 67, 201–218. [Google Scholar] [CrossRef]
- Bedossa, P.; Poitou, C. Histopathological algorithm and scoring system for evaluation of liver lesions in morbidly obese patients. Hepatology 2012, 56, 1751–1759. [Google Scholar] [CrossRef]
- Okada, K.; Shoda, J. Nrf2 inhibits hepatic iron accumulation and counteracts oxidative stress-induced liver injury in nutritional steatohepatitis. J. Gastroenterol. 2012, 47, 924–935. [Google Scholar] [CrossRef]
- Gotoh, N.; Nagao, K. Effects of three different highly purified n-3 series highly unsaturated fatty acids on lipid metabolism in C57BL/KsJ-db/db mice. J. Agric. Food Chem. 2009, 57, 11047–11054. [Google Scholar] [CrossRef]
- Lin, Y.; Jiang, M. Cancer and ER stress: Mutual crosstalk between autophagy, oxidative stress and inflammatory response. Biomed. Pharmacother. 2019, 118, 109249. [Google Scholar] [CrossRef]
- Bidkhori, G.; Benfeitas, R. Metabolic network-based stratification of hepatocellular carcinoma reveals three distinct tumor subtypes. Proc. Natl. Acad. Sci. USA 2018, 115, 11874–11883. [Google Scholar] [CrossRef]
- Haddadi, N.; Lin, Y. PTEN/PTENP1: Regulating the regulator of RTK-dependent PI3K/Akt signaling, new targets for cancer therapy. Mol. Cancer 2018, 17, 37. [Google Scholar] [CrossRef]
- Tsuji, Y.; Yoshiji, H. Bile Acid Sequestrant, Sevelamer Ameliorates Hepatic Fibrosis with Reduced Overload of Endogenous Lipopolysaccharide in. Experimental Nonalcoholic Steatohepatitis. Microorganisms 2020, 8, 925. [Google Scholar] [CrossRef]
- Minicis, D.S.; Rychlicki, C. Dysbiosis Contributes to Fibrogenesis in Course of Chronic Liver Injury in Mice. Hepatology 2014, 59, 1738–1749. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Qi, C. Lactobacillus reuteri improves gut barrier function and affects diurnal variation of the gut microbiota in mice fed a high-fat diet. Food Funct. 2019, 10, 4705–4715. [Google Scholar] [CrossRef] [PubMed]
- Hutchinson, N.A.; Tingo, L. The Potentials Effects of Probiotics and ω-3 Fatty Acids on Chronic Low-Grade Inflammattion. Nutrients 2020, 12, 2402. [Google Scholar] [CrossRef] [PubMed]
- Rodes, L.; Khan, A. Effect of Probiotics Lactobacillus and Bifidobacterium on Gut-Derived Lipopolysaccharides and Inflammatory Cytokines: An In Vitro Study Using a Human Colonic Microbiota Model. J. Microbiol. Biotechnol. 2013, 23, 518–526. [Google Scholar] [CrossRef]

| Normal Chow | High-Fat Diet | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| SMAPoTN (−) | SMAPoTN (+) | SMAPoTN (−) | SMAPoTN (+) | |||||||||
| (n = 8) | (n = 8) | (n = 8) | (n = 8) | |||||||||
| TG content | 94.93 | ± | 18.31 | 117.13 | ± | 8.71 | 137.57 | ± | 7.39 | 112.75 | ± | 10.88 |
| (μmol/g liver) | ||||||||||||
| FFA content | 8.51 | ± | 1.16 | 10.60 | ± | 1.14 | 10.44 | ± | 0.50 | 9.65 | ± | 0.91 |
| (μmol/g liver) | ||||||||||||
| FFA composition (mg/g liver) | ||||||||||||
| C6:0 | ND | ND | ND | ND | ||||||||
| C8:0 | ND | ND | ND | ND | ||||||||
| C10:0 | ND | ND | ND | ND | ||||||||
| C11:0 | ND | ND | ND | ND | ||||||||
| C12:0 | ND | ND | 0.03 | ± | 0.01 | 0.04 | ± | 0.01 | ||||
| C13:0 | ND | ND | ND | ND | ||||||||
| C14:0 | 0.64 | ± | 0.14 | 0.73 | ± | 0.11 | 0.64 | ± | 0.07 | 0.74 | ± | 0.10 |
| C14:1 | 0.04 | ± | 0.00 | 0.05 | ± | 0.01 | 0.04 | ± | 0.01 | 0.06 | ± | 0.01 |
| C15:0 | 0.1 | ± | 0.0 | 0.1 | ± | 0.0 | 0.1 | ± | 0.0 | 0.1 | ± | 0.02 |
| C15:1 | ND | ND | ND | ND | ||||||||
| C16:0 | 29.12 | ± | 5.84 | 30.23 | ± | 3.95 | 28.82 | ± | 2.95 | 25.65 | ± | 3.90 |
| C16:1 | 6.66 | ± | 1.75 | 7.75 | ± | 1.45 | 5.16 | ± | 0.67 | 5.11 | ± | 0.86 |
| C17:0 | 1.43 | ± | 1.34 | 0.10 | ± | 0.01 | 0.11 | ± | 0.02 | 0.14 | ± | 0.02 |
| C17:1 | ND | ND | ND | ND | ||||||||
| C18:0 | ND | ND | ND | ND | ||||||||
| C18:1n9 | 49.04 | ± | 12.75 | 45.02 | ± | 13.05 | 57.16 | ± | 10.46 | 29.43 | ± | 10.22 |
| C18:2n6 | 12.68 | ± | 3.43 | 17.69 | ± | 2.44 | 6.54 | ± | 0.55 | 7.92 | ± | 0.87 |
| C18:3n6 | 0.21 | ± | 0.05 | 0.21 | ± | 0.03 | 0.16 | ± | 0.02 | 0.13 | ± | 0.02 |
| C18:3n3 | 0.53 | ± | 0.10 | 0.53 | ± | 0.06 | 0.25 | ± | 0.02 | 0.27 | ± | 0.04 |
| C20:0 | 0.26 | ± | 0.07 | 0.20 | ± | 0.03 | 0.06 | ± | 0.00 | 0.15 | ± | 0.05 * |
| C20:1n9 | 1.41 | ± | 0.26 | 1.34 | ± | 0.21 | 2.00 | ± | 0.25 | 1.80 | ± | 0.28 |
| C20:2 | 0.23 | ± | 0.03 | 0.24 | ± | 0.02 | 1.05 | ± | 0.10 | 0.77 | ± | 0.08 * |
| C20:3n6 | 0.46 | ± | 0.09 | 0.45 | ± | 0.06 | 0.41 | ± | 0.04 | 0.40 | ± | 0.03 |
| C20:4n6 | ND | ND | ND | ND | ||||||||
| C20:3n3 | ND | ND | ND | ND | ||||||||
| C20:5n3 | 0.26 | ± | 0.06 | 0.26 | ± | 0.04 | 0.09 | ± | 0.01 | 0.09 | ± | 0.01 |
| C21:0 | 1.45 | ± | 0.12 | 1.40 | ± | 0.11 | 1.80 | ± | 0.17 | 2.04 | ± | 0.08 |
| C22:0 | ND | ND | ND | ND | ||||||||
| C22:1n9 | ND | ND | ND | ND | ||||||||
| C22:2 | ND | ND | 0.35 | ± | 0.04 | 0.24 | ± | 0.03 | ||||
| C22:6n3 | 2.47 | ± | 0.36 | 2.36 | ± | 0.17 | 0.88 | ± | 0.13 | 0.98 | ± | 0.12 |
| C23:0 | ND | ND | ND | ND | ||||||||
| C24:0 | ND | ND | ND | ND | ||||||||
| C24:1n9 | ND | ND | ND | ND | ||||||||
| A | ||||||||
|---|---|---|---|---|---|---|---|---|
| Group | Normal Chow | High-Fat Diet | ||||||
| Gene number (TPM ≥ 1) | 11,529 | 13,072 | ||||||
| Differentially expressed | Up | Down | Total | % | Up | Down | Total | % |
| SMAPOTN (−) vs. (+) | 167 | 1019 | 1186 | 10.3 | 40 | 1037 | 1077 | 8.2 |
| B | ||||||||
| Group | Normal Chow (SMAPOTN (−) vs. (+)) | High-Fat Diet (SMAPOTN (−) vs. (+)) | ||||||
| Variable number of pathway | 42 | Variable number of pathway | 28 | |||||
| Pathway (KEGG, top three) | Genes (n) | % | Pathway (KEGG, top three) | Genes (n) | % | |||
| Metabolic pathways | 138 | 11.6 | Endoplasmic reticulum | 41 | 3.8 | |||
| Biosynthesis | 35 | 3.0 | Splicesome | 33 | 3.1 | |||
| Endoplasmic reticulum | 33 | 2.8 | Cancer pathway | 32 | 3.0 | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Watahiki, T.; Okada, K.; Miura, I.; To, K.; Tanaka, S.; Warabi, E.; Kanno, N.; Yamagata, K.; Gotoh, N.; Suzuki, H.; et al. Antioxidative Self-Assembling Nanoparticles Attenuate the Development of Steatohepatitis and Inhibit Hepatocarcinogenesis in Mice. Antioxidants 2022, 11, 1939. https://doi.org/10.3390/antiox11101939
Watahiki T, Okada K, Miura I, To K, Tanaka S, Warabi E, Kanno N, Yamagata K, Gotoh N, Suzuki H, et al. Antioxidative Self-Assembling Nanoparticles Attenuate the Development of Steatohepatitis and Inhibit Hepatocarcinogenesis in Mice. Antioxidants. 2022; 11(10):1939. https://doi.org/10.3390/antiox11101939
Chicago/Turabian StyleWatahiki, Takahisa, Kosuke Okada, Ikuru Miura, Keii To, Seiya Tanaka, Eiji Warabi, Naomi Kanno, Kenji Yamagata, Naohiro Gotoh, Hideo Suzuki, and et al. 2022. "Antioxidative Self-Assembling Nanoparticles Attenuate the Development of Steatohepatitis and Inhibit Hepatocarcinogenesis in Mice" Antioxidants 11, no. 10: 1939. https://doi.org/10.3390/antiox11101939
APA StyleWatahiki, T., Okada, K., Miura, I., To, K., Tanaka, S., Warabi, E., Kanno, N., Yamagata, K., Gotoh, N., Suzuki, H., Ariizumi, S., Tsuchiya, K., Nagasaki, Y., & Shoda, J. (2022). Antioxidative Self-Assembling Nanoparticles Attenuate the Development of Steatohepatitis and Inhibit Hepatocarcinogenesis in Mice. Antioxidants, 11(10), 1939. https://doi.org/10.3390/antiox11101939







