The Multifaceted Bacterial Cysteine Desulfurases: From Metabolism to Pathogenesis
Abstract
1. Introduction
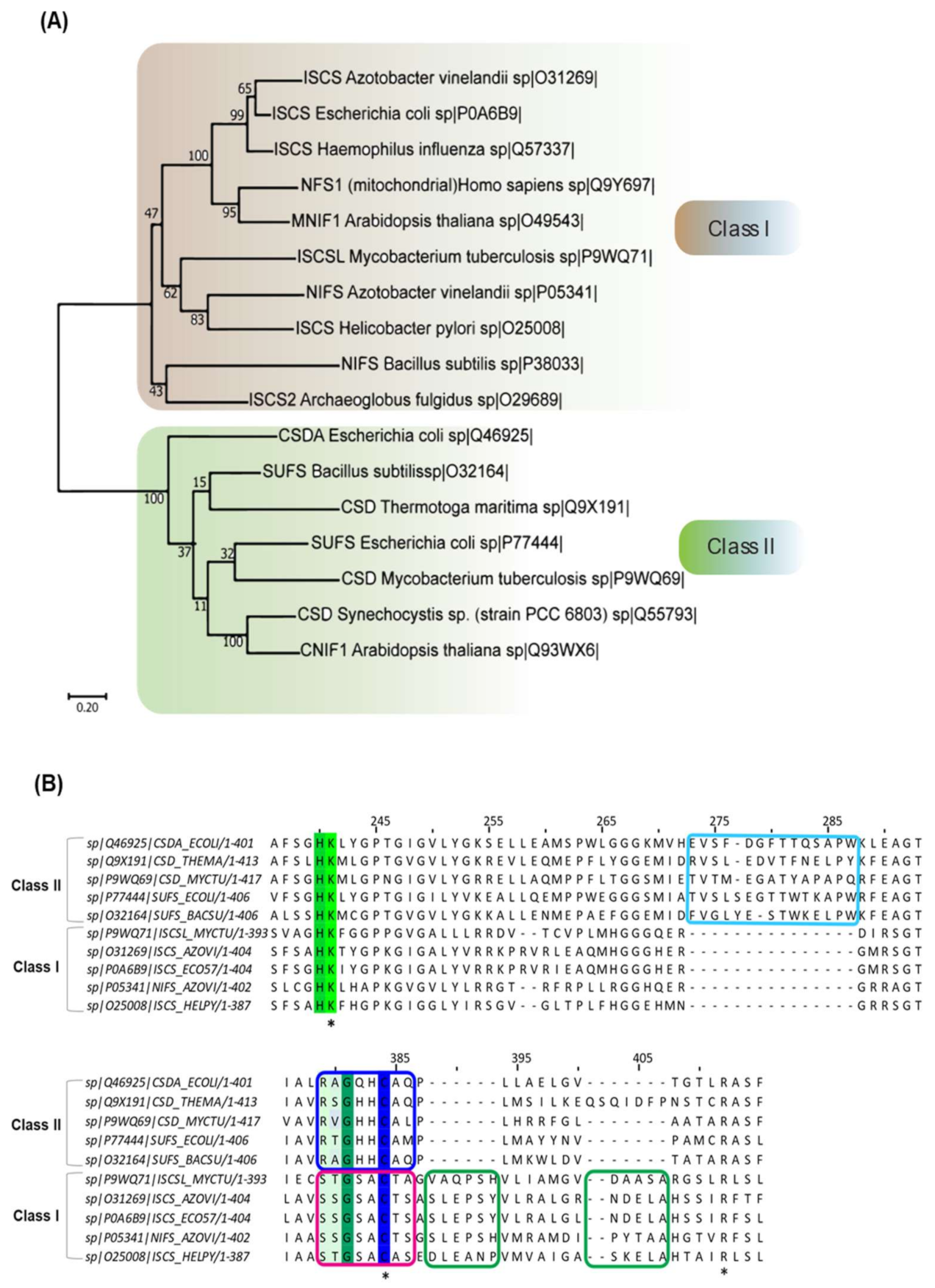
2. Classification and Distribution of Cysteine Desulfurases
2.1. Classification
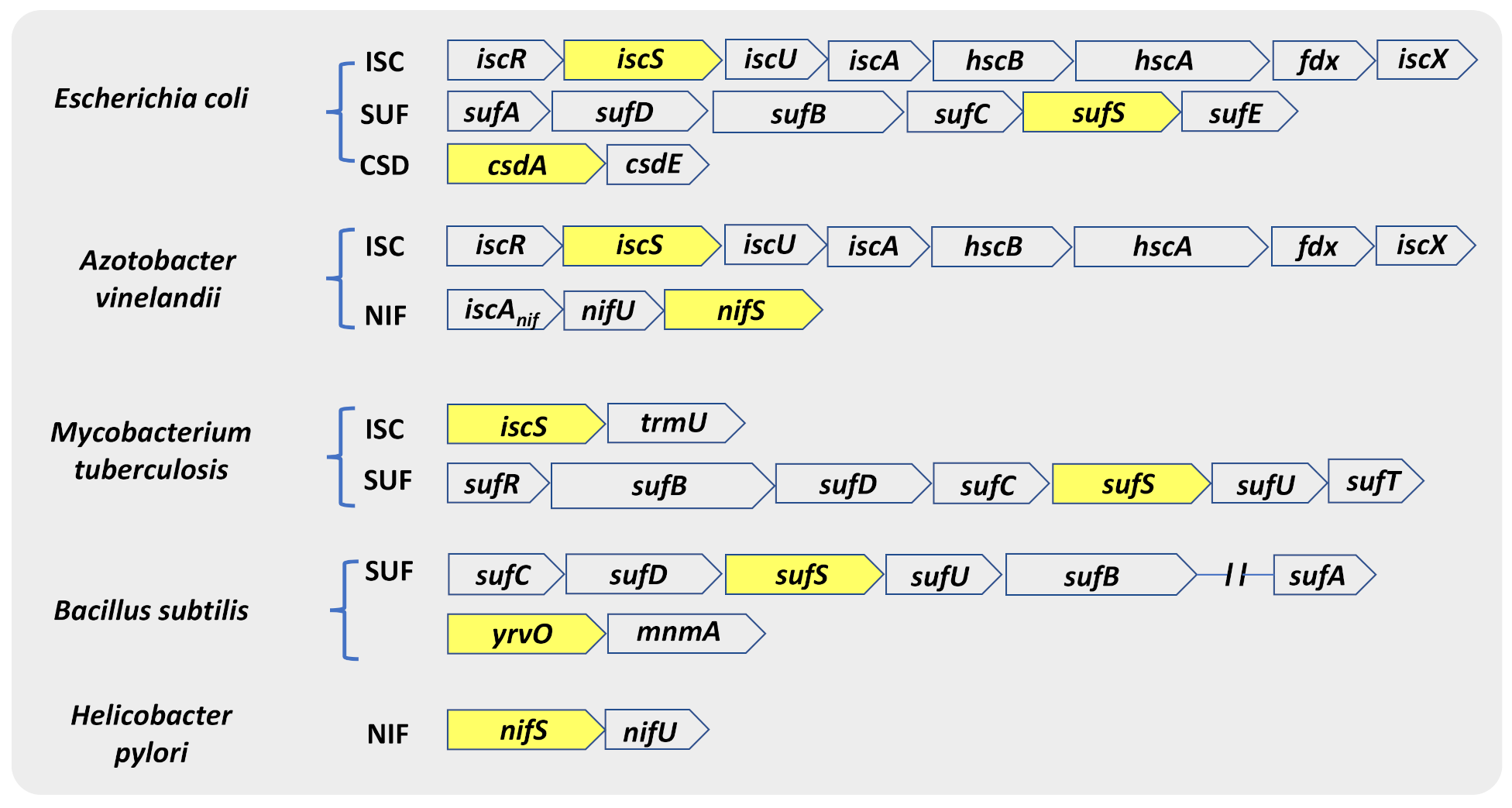
2.2. Distribution
3. Structure and Reaction Mechanism of Cysteine Desulfurase
3.1. Structure
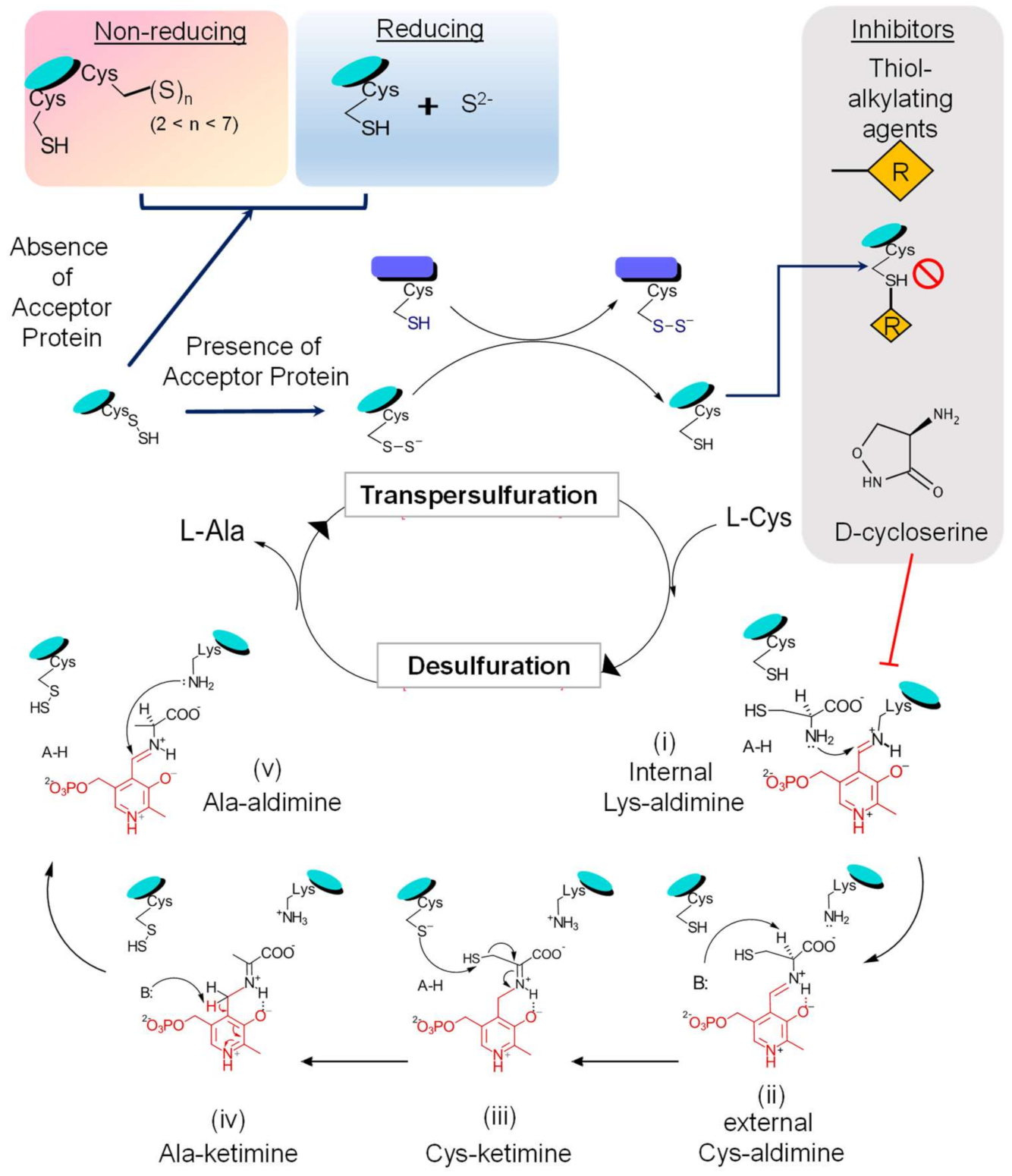
3.2. Reaction Mechanism
- (i)
- What is the mechanism of IscS mediated S-relay in Mtb?
- (ii)
- What scaffold protein participates in this process since IscS do not physically interact with Mtb SufU [51]?
- (iii)
- Is IscS alone sufficient to build Fe-S clusters?
- (iv)
- Does IscS contribute to stress tolerance and pathogenesis in Mtb?
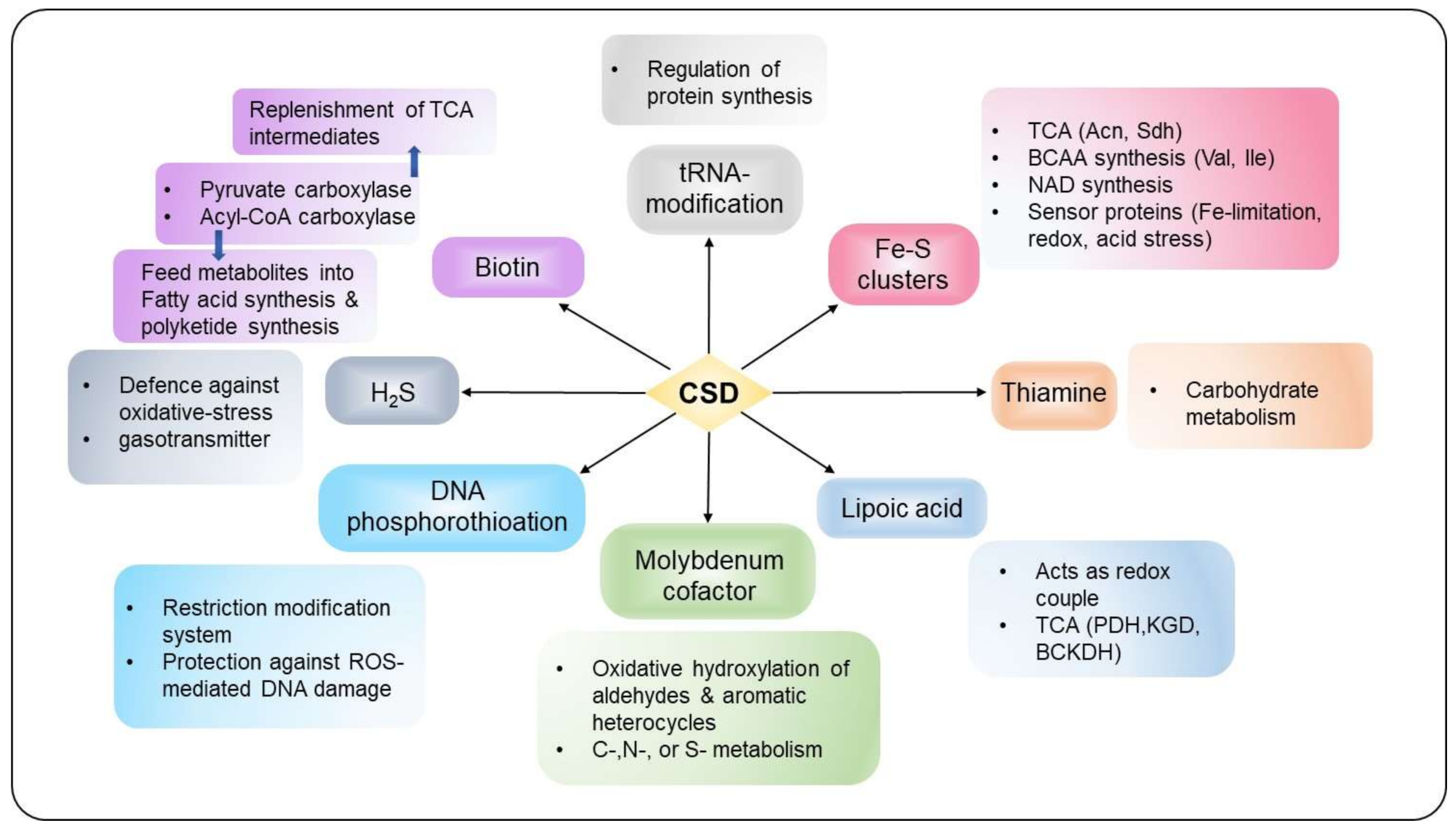
4. CSD Controls Basal Metabolism by Mobilizing S from Cysteine to Diverse Cellular Pathways
4.1. Fe-S Cluster Assembly
4.2. tRNA Modification (Thiolation)
4.3. Lipoic Acid Synthesis
4.4. Biotin Biogenesis
4.5. Thiamine Synthesis
4.6. Molybdopterin Synthesis
4.7. Hydrogen Sulfide Production
4.8. DNA Phosphorothioation
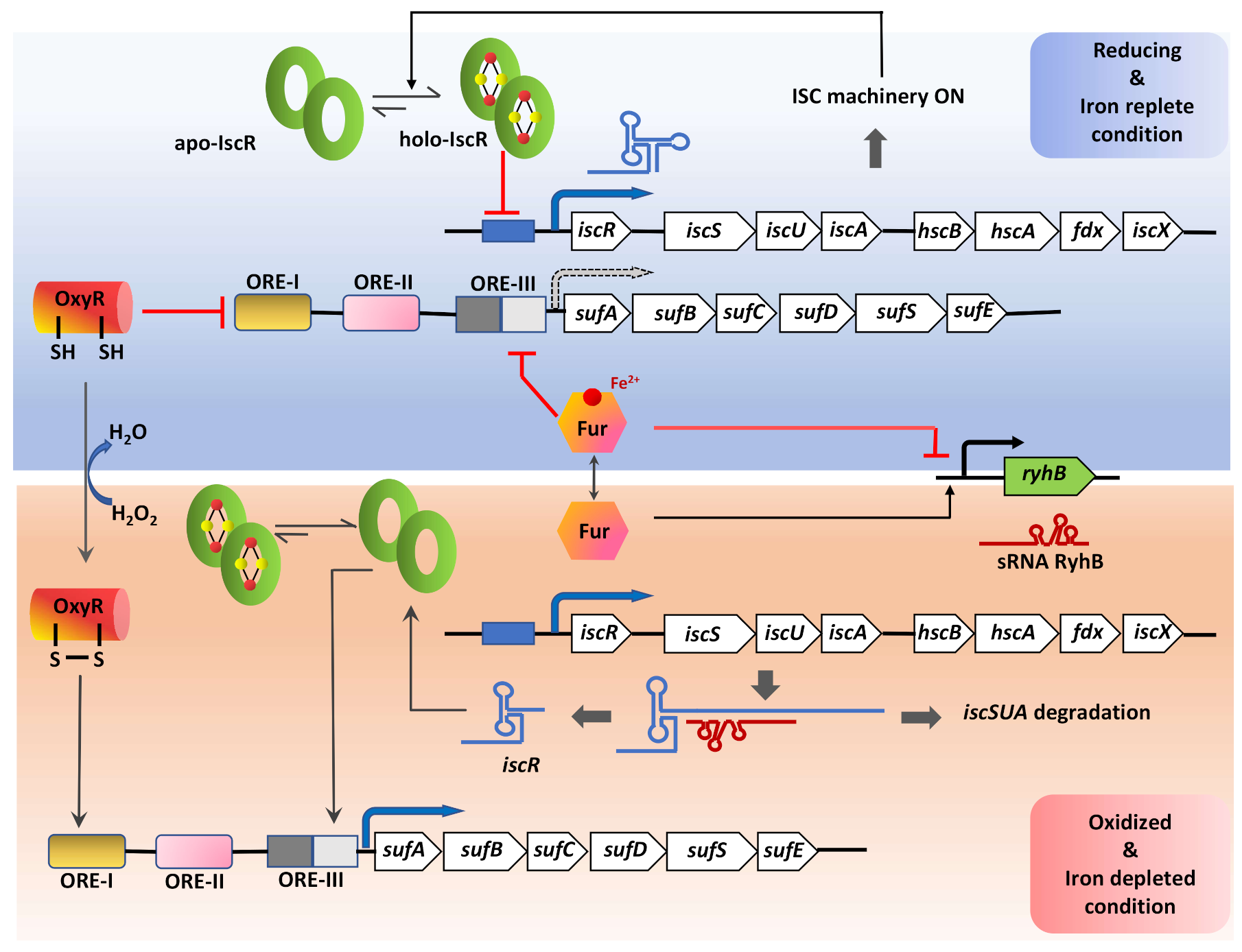
5. A Multi-Layered Regulation System Modulates the Expression and Activity of Cysteine Desulfurase in Bacteria
5.1. Transcriptional Level
5.2. Post-Transcriptional Level
5.3. Post-Translational/Enzymatic Level
6. Cysteine Desulfurase Maintain Intracellular Redox Homeostasis and Impart Oxidative-Stress Defense in Diverse Bacterial Species
6.1. Role in Sustaining Intracellular Redox Balance
6.2. Function of CSD in Neutralizing Exogenous Redox Stress
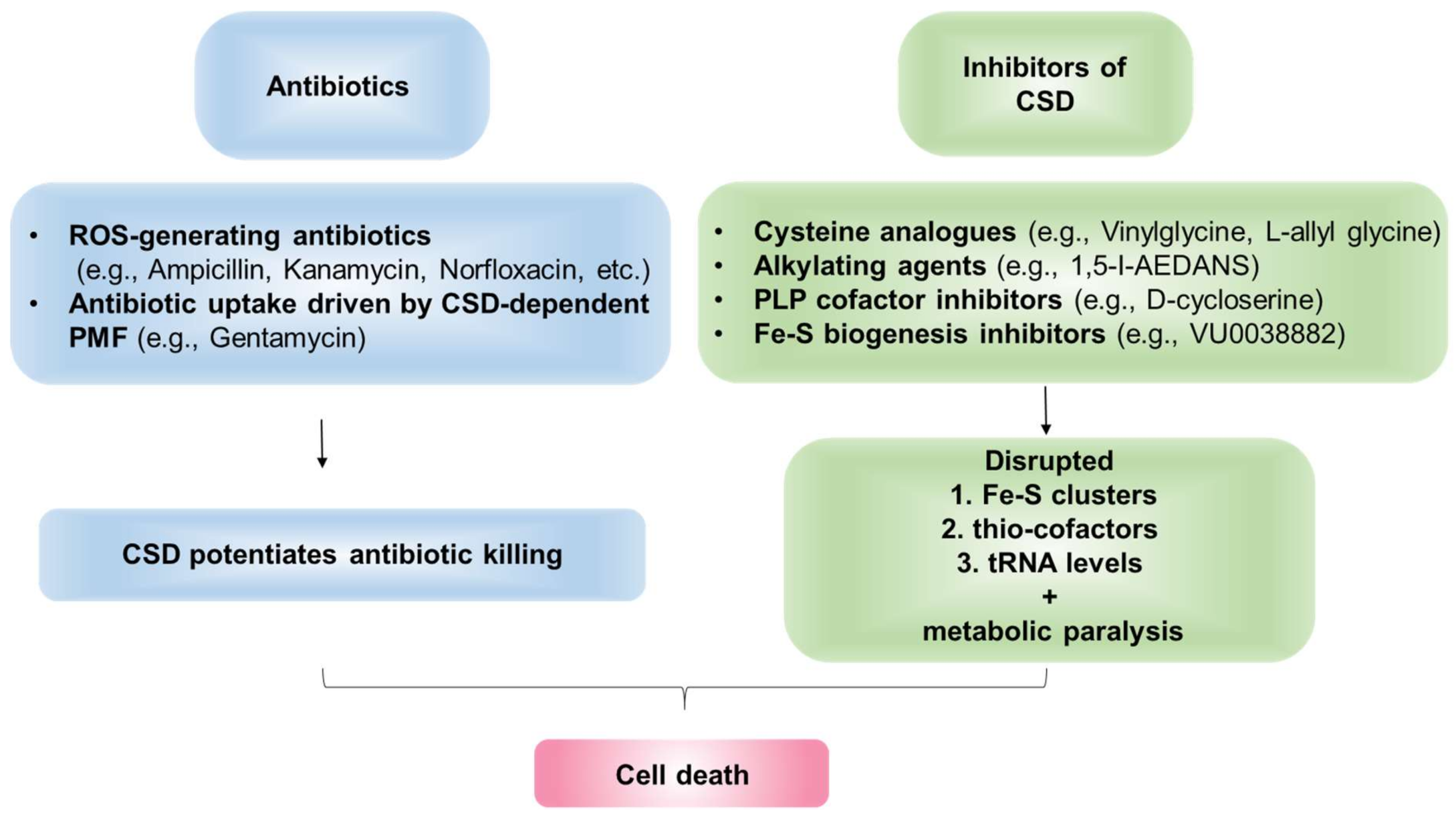
7. Cysteine Desulfurase Is a Potential Drug Target-Candidate
8. Conclusions and Future Directions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
References
- Kessler, D. Enzymatic Activation of Sulfur for Incorporation into Biomolecules in Prokaryotes. FEMS Microbiol. Rev. 2006, 30, 825–840. [Google Scholar] [CrossRef] [PubMed]
- Guédon, E.; Martin-Verstraete, I. Cysteine Metabolism and Its Regulation in Bacteria. In Amino Acid Biosynthesis~Pathways, Regulation and Metabolic Engineering; Springer: Berlin/Heidelberg, Germany, 2006; Volume 5, pp. 195–218. [Google Scholar]
- Paulsen, C.E.; Carroll, K.S. Cysteine-mediated Redox Signaling: Chemistry, Biology, and Tools for Discovery. Chem. Rev. 2013, 113, 4633–4679. [Google Scholar] [CrossRef] [PubMed]
- Mihara, H.; Esaki, N. Bacterial Cysteine Desulfurases: Their Function and Mechanisms. Appl. Microbiol. Biotechnol. 2002, 60, 12–23. [Google Scholar] [CrossRef] [PubMed]
- Hidese, R.; Mihara, H.; Esaki, N. Bacterial Cysteine Desulfurases: Versatile Key Players in Biosynthetic Pathways of Sulfur-Containing Biofactors. Appl. Microbiol. Biotechnol. 2011, 91, 47–61. [Google Scholar] [CrossRef] [PubMed]
- Black, K.A.; Santos, P.C.D. Shared-intermediates in the Biosynthesis of Thio-cofactors: Mechanism and Functions of Cysteine Desulfurases and Sulfur Acceptors. Biochim. Biophys. Acta Mol. Cell Res. 2015, 1853, 1470–1480. [Google Scholar] [CrossRef]
- Zheng, L.; White, R.H.; Cash, V.L.; Jack, R.F.; Dean, D.R. Cysteine Desulfurase Activity Indicates a Role for NIFS in Metallocluster Biosynthesis. Proc. Natl. Acad. Sci. USA 1993, 90, 2754–2758. [Google Scholar] [CrossRef]
- Zheng, L.; Cash, V.L.; Flint, D.H.; Dean, D.R. Assembly of Iron-Sulfur Clusters. Identification of an iscSUA-hscBA-fdx Gene Cluster from Azotobacter vinelandii. J. Biol. Chem. 1998, 273, 13264–13272. [Google Scholar] [CrossRef]
- Forouhar, F.; Arragain, S.; Atta, M.; Gambarelli, S.; Mouesca, J.M.; Hussain, M.; Xiao, R.; Kieffer-Jaquinod, S.; Seetharaman, J.; Acton, T.B.; et al. Two Fe-S clusters Catalyze Sulfur Insertion by Radical-SAM methylthiotransferases. Nat. Chem. Biol. 2013, 9, 333–338. [Google Scholar] [CrossRef]
- Roche, B.; Agrebi, R.; Huguenot, A.; Ollagnier-de-Choudens, S.; Barras, F.; Py, B. Turning Escherichia coli into a Frataxin-Dependent Organism. PLoS Genet. 2015, 11, e1005134. [Google Scholar] [CrossRef]
- Jacobson, M.R.; Cash, V.L.; Weiss, M.C.; Laird, N.F.; Newton, W.E.; Dean, D.R. Biochemical and Genetic Analysis of the nifUSVWZM Cluster from Azotobacter vinelandii. Mol. Gen. Genet. 1989, 219, 49–57. [Google Scholar] [CrossRef]
- Oishi, H.; Noto, T.; Sasaki, H.; Suzuki, K.; Hayashi, T.; Okazaki, H.; Ando, K.; Sawada, M. Thiolactomycin, a New Antibiotic: I. Taxonomy of the Producing Organism, Fermentation, and Biological Properties. J. Antibiot. 1982, 35, 391–395. [Google Scholar] [CrossRef] [PubMed]
- Noto, T.; Miyakawa, S.; Oishi, H.; Endo, H.; Okazaki, H. Thiolactomycin, a New Antibiotic III. In vitro Antibacterial Activity. J. Antibiot. 1982, 35, 401–410. [Google Scholar] [CrossRef] [PubMed]
- Tao, W.; Yurkovich, M.E.; Wen, S.; Lebe, K.E.; Samborskyy, M.; Liu, Y.; Yang, A.; Liu, Y.; Ju, Y.; Deng, Z.; et al. A Genomics-led Approach to Deciphering the Mechanism of Thiotetronate Antibiotic Biosynthesis. Chem. Sci. 2016, 7, 376–385. [Google Scholar] [CrossRef] [PubMed]
- Schaeffer, M.L.; Agnihotri, G.; Volker, C.; Kallender, H.; Brennan, P.J.; Lonsdale, J.T. Purification and Biochemical Characterization of the Mycobacterium tuberculosis Βeta-ketoacyl-acyl Carrier Protein Synthases KasA and KasB. J. Biol. Chem. 2001, 276, 47029–47037. [Google Scholar] [CrossRef] [PubMed]
- Waller, R.F.; Keeling, P.J.; Donald, R.G.; Striepen, B.; Handman, E.; Lang-Unnasch, N.; Cowman, A.F.; Besra, G.S.; Roos, D.S.; McFadden, G.I. Nuclear-encoded Proteins Target to the Plastid in Toxoplasma gondii and Plasmodium falciparum. Proc. Natl. Acad. Sci. USA 1998, 95, 12352–12357. [Google Scholar] [CrossRef] [PubMed]
- Slayden, R.A.; Lee, R.E.; Armour, J.W.; Cooper, A.M.; Orme, I.M.; Brennan, P.J.; Besra, G.S. Antimycobacterial Action of Thiolactomycin: An Inhibitor of Fatty Acid and Mycolic Acid Synthesis. Antimicrob. Agents Chemother. 1996, 40, 2813–2819. [Google Scholar] [CrossRef]
- Jones, S.M.; Urch, J.E.; Brun, R.; Harwood, J.L.; Berry, C.; Gilbert, I.H. Analogues of Thiolactomycin as Potential Anti-malarial and Anti-trypanosomal Agents. Bioorganic Med. Chem. 2004, 12, 683–692. [Google Scholar] [CrossRef] [PubMed]
- Novaes, R.D.; Teixeira, A.L.; de Miranda, A.S. Oxidative Stress in Microbial Diseases: Pathogen, Host, and Therapeutics. Oxidative Med. Cell. Longev. 2019, 2019, 10–12. [Google Scholar] [CrossRef]
- Kohanski, M.A.; Dwyer, D.J.; Hayete, B.; Lawrence, C.A.; Collins, J.J. A Common Mechanism of Cellular Death Induced by Bactericidal Antibiotics. Cell 2007, 130, 797–810. [Google Scholar] [CrossRef] [PubMed]
- Giordano, N.; Hastie, J.L.; Smith, A.D.; Foss, E.D.; Gutierrez-Munoz, D.F.; Carlson, P.E. Cysteine Desulfurase IscS2 Plays a Role in Oxygen Resistance in Clostridium difficile. Infect. Immun. 2018, 86, 1–8. [Google Scholar] [CrossRef]
- Banerjee, M.; Chakravarty, D.; Ballal, A. Molecular Basis of Function and the Unusual Antioxidant Activity of a Cyanobacterial Cysteine Desulfurase. Biochem. J. 2017, 474, 2435–2447. [Google Scholar] [CrossRef]
- Bhubhanil, S.; Niamyim, P.; Sukchawalit, R.; Mongkolsuk, S. Cysteine Desulphurase-encoding Gene sufS2 is Required for the Repressor Function of RirA and Oxidative Resistance in Agrobacterium tumefaciens. Microbiology 2014, 160, 79–90. [Google Scholar] [CrossRef]
- Singh, K.P.; Zaidi, A.; Anwar, S.; Bimal, S.; Das, P.; Ali, V. Reactive Oxygen Species Regulates Expression of Iron-sulfur Cluster Assembly Protein IscS of Leishmania donovani. Free Radic. Biol. Med. 2014, 75, 195–209. [Google Scholar] [CrossRef] [PubMed]
- Jacobson, M.R.; Brigle, K.E.; Bennett, L.T.; Setterquist, R.A.; Wilson, M.S.; Cash, V.L.; Beynon, J.; Newton, W.E.; Dean, D.R. Physical and Genetic Map of the Major nif Gene Cluster from Azotobacter vinelandii. J. Bacteriol. 1989, 171, 1017–1027. [Google Scholar] [CrossRef]
- Zheng, L.; White, R.H.; Cash, V.L.; Dean, D.R. Mechanism for the Desulfurization of L-Cysteine Catalyzed by the nifS Gene Product. Biochemistry 1994, 33, 4714–4720. [Google Scholar] [CrossRef]
- Flint, D.H. Escherichia coli Contains a Protein that is Homologous in Function and N-terminal Sequence to the Protein Encoded by the nifS Gene of Azotobacter vinelandii and that can Participate in the Synthesis of the Fe-S Cluster of Dihydroxy-acid Dehydratase. J. Biol. Chem. 1996, 271, 16068–16074. [Google Scholar] [CrossRef]
- Mihara, H.; Maeda, M.; Fujii, T.; Kurihara, T.; Hata, Y.; Esaki, N. A nifS-like Gene, csdB, Encodes an Escherichia coli Counterpart of Mammalian Selenocysteine Lyase: Gene cloning, Purification, Characterization, and Preliminary X-ray Crystallo-graphic Studies. J. Biol. Chem. 1999, 274, 14768–14772. [Google Scholar] [CrossRef] [PubMed]
- Mihara, H.; Kurihara, T.; Yoshimura, T.; Soda, K.; Esaki, N. Cysteine Sulfinate Desulfinase, a NIFS-like Protein of Escherichia coli with Selenocysteine Lyase and Cysteine Desulfurase Activities: Gene cloning, Purification, and Characterization of a Novel Pyridoxal Enzyme. J. Biol. Chem. 1997, 272, 22417–22424. [Google Scholar] [CrossRef] [PubMed]
- Patzer, S.I.; Hantke, K. SufS is a NifS-like Protein, and SufD is Necessary for Stability of the [2Fe-2S] FhuF Protein in Escherichia coli. J. Bacteriol. 1999, 181, 3307–3309. [Google Scholar] [CrossRef] [PubMed]
- Dahl, J.U.; Radon, C.; Bühning, M.; Nimtz, M.; Leichert, L.I.; Denis, Y.; Jourlin-Castelli, C.; Iobbi-Nivol, C.; Méjean, V.; Leimkühler, S. The Sulfur Carrier Protein tusA has a Pleiotropic Role in Escherichia coli that also Affects Molybdenum Cofactor Biosynthesis. J. Biol. Chem. 2013, 288, 5426–5442. [Google Scholar] [CrossRef]
- Mueller, E.G.; Buck, C.J.; Palenchar, P.M.; Barnhart, L.E.; Paulson, J.L. Identification of a Gene Involved in the Generation of 4-thiouridine in tRNA. Nucleic Acids Res. 1998, 26, 2606–2610. [Google Scholar] [CrossRef] [PubMed]
- Ikeuchi, Y.; Shigi, N.; Kato, J.I.; Nishimura, A.; Suzuki, T. Mechanistic Insights into Sulfur Relay by Multiple Sulfur Mediators Involved in Thiouridine Biosynthesis at tRNA Wobble Positions. Mol. Cell 2006, 21, 97–108. [Google Scholar] [CrossRef]
- Rajakovich, L.J.; Tomlinson, J.; Santos, P.C.D. Functional Analysis of Bacillus subtilis Genes Involved in the Biosynthesis of 4-thiouridine in tRNA. J. Bacteriol. 2012, 194, 4933–4940. [Google Scholar] [CrossRef] [PubMed]
- Yuvaniyama, P.; Agar, J.N.; Cash, V.L.; Johnson, M.K.; Dean, D.R. NifS-directed Assembly of a Transient [2Fe-2S] Cluster within the NifU Protein. Proc. Natl. Acad. Sci. USA 2000, 97, 599. [Google Scholar] [CrossRef] [PubMed]
- Outten, F.W.; Wood, M.J.; Muñoz, F.M.; Storz, G. The SufE Protein and the SufBCD Complex Enhance SufS Cysteine Desulfurase Activity as Part of a Sulfur Transfer Pathway for Fe-S Cluster Assembly in Escherichia coli. J. Biol. Chem. 2003, 278, 45713–45719. [Google Scholar] [CrossRef] [PubMed]
- Selbach, B.; Earles, E.; Santos, P.C.D. Kinetic Analysis of the Bisubstrate Cysteine Desulfurase SufS from Bacillus subtilis. Biochemistry 2010, 49, 8794–8802. [Google Scholar] [CrossRef] [PubMed]
- Loiseau, L.; Ollagnier-de-Choudens, S.; Lascoux, D.; Forest, E.; Fontecave, M.; Barras, F. Analysis of the Heteromeric CsdA-CsdE Cysteine Desulfurase, Assisting Fe-S Cluster Biogenesis in Escherichia coli. J. Biol. Chem. 2005, 280, 26760–26769. [Google Scholar] [CrossRef]
- Bouvier, D.; Labessan, N.; Clémancey, M.; Latour, J.M.; Ravanat, J.L.; Fontecave, M.; Atta, M. TtcA a New tRNA-thioltransferase with an Fe-S Cluster. Nucleic Acids Res. 2014, 42, 7960–7970. [Google Scholar] [CrossRef]
- Clausen, T.; Kaiser, J.T.; Steegborn, C.; Huber, R.; Kessler, D. Crystal Structure of the Cystine C-S lyase from Synechocystis: Stabilization of Cysteine Persulfide for Fe-S Cluster Biosynthesis. Proc. Natl. Acad. Sci. USA 2000, 97, 3856–3861. [Google Scholar] [CrossRef]
- Leibrecht, I.; Kessler, D. A Novel L-Cysteine/Cystine C-S-lyase Directing [2Fe-2S] Cluster Formation of Synechocystis Ferredoxin. J. Biol. Chem. 1997, 272, 10442–10447. [Google Scholar] [CrossRef]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular Evolutionary Genetics Analysis across Computing Platforms. Mol. Biol. Evol. 2018, 35, 1547–1549. [Google Scholar] [CrossRef] [PubMed]
- Py, B.; Barras, F. Building Fe-S Proteins: Bacterial Strategies. Nat. Rev. Microbiol. 2010, 8, 436–446. [Google Scholar] [CrossRef] [PubMed]
- Lill, R. Function and Biogenesis of Iron-Sulphur Proteins. Nature 2009, 460, 831–838. [Google Scholar] [CrossRef] [PubMed]
- Roche, B.; Aussel, L.; Ezraty, B.; Mandin, P.; Py, B.; Barras, F. Iron/sulfur Proteins Biogenesis in Prokaryotes: Formation, Regulation and Diversity. Biochim. Biophys. Acta Bioenerg. 2013, 1827, 455–469. [Google Scholar] [CrossRef]
- Outten, F.W. Recent Advances in the Suf Fe-S Cluster Biogenesis Pathway: Beyond the Proteobacteria. Biochim. Biophys. Acta Mol. Cell Res. 2015, 1853, 1464–1469. [Google Scholar] [CrossRef] [PubMed]
- Willemse, D.; Weber, B.; Masino, L.; Warren, R.M.; Adinolfi, S.; Pastore, A.; Williams, M.J. Rv1460, a SufR Homologue, is a Repressor of the suf Operon in Mycobacterium tuberculosis. PLoS ONE 2018, 13, e0200145. [Google Scholar] [CrossRef]
- Pandey, M.; Talwar, S.; Bose, S.; Pandey, A.K. Iron Homeostasis in Mycobacterium tuberculosis is Essential for Persistence. Sci. Rep. 2018, 8, 17359. [Google Scholar] [CrossRef]
- Mashruwala, A.A.; Bhatt, S.; Poudel, S.; Boyd, E.S.; Boyd, J.M. The DUF59 Containing Protein SufT Is Involved in the Maturation of Iron-Sulfur (Fe-S) Proteins during Conditions of High Fe-S Cofactor Demand in Staphylococcus aureus. PLoS Genet. 2016, 12, e1006233. [Google Scholar] [CrossRef]
- Tamuhla, T.; Joubert, L.; Willemse, D.; Williams, M.J. SufT is Required for growth of Mycobacterium smegmatis under Iron Limiting Conditions. Microbiology 2020, 166, 296–305. [Google Scholar] [CrossRef]
- Rybniker, J.; Pojer, F.; Marienhagen, J.; Kolly, G.S.; Chen, J.M.; Gumpel, E.V.; Hartmann, P.; Cole, S.T. The Cysteine Desulfurase IscS of Mycobacterium tuberculosis is Involved in Iron-sulfur Cluster Biogenesis and Oxidative Stress Defence. Biochem. J. 2014, 459, 467–478. [Google Scholar] [CrossRef]
- Huet, G.; Daffé, M.; Saves, I. Identification of the Mycobacterium tuberculosis SUF Machinery as the Exclusive Mycobacterial System of [Fe-S] Cluster Assembly: Evidence for its Implication in the Pathogen’s Survival. J. Bacteriol. 2005, 187, 6137–6146. [Google Scholar] [CrossRef]
- Kaiser, J.T.; Clausen, T.; Bourenkow, G.P.; Bartunik, H.D.; Steinbacher, S.; Huber, R. Crystal Structure of a NifS-like Protein from Thermotoga maritima: Implications for Iron-sulfur Cluster Assembly. J. Mol. Biol. 2000, 297, 451–464. [Google Scholar] [CrossRef] [PubMed]
- Cupp-Vickery, J.R.; Urbina, H.; Vickery, L.E. Crystal Structure of IscS, a Cysteine Desulfurase from Escherichia coli. J. Mol. Biol. 2003, 330, 1049–1059. [Google Scholar] [CrossRef]
- Fujii, T.; Maeda, M.; Mihara, H.; Kurihara, T.; Esaki, N.; Hata, Y. Structure of a NifS Homologue: X-ray Structure Analysis of CsdB, an Escherichia coli Counterpart of Mammalian Selenocysteine Lyase. Biochemistry 2000, 39, 1263–1273. [Google Scholar] [CrossRef] [PubMed]
- Mihara, H.; Fujii, T.; Kato, S.; Kurihara, T.; Hata, Y.; Esaki, N. Structure of External Aldimine of Escherichia coli CsdB, an IscS/NifS Homolog: Implications for its Specificity Toward Selenocysteine. J. Biochem. 2002, 131, 679–685. [Google Scholar] [CrossRef] [PubMed]
- Tirupati, B.; Vey, J.L.; Drennan, C.L.; Bollinger, J.M. Kinetic and Structural Characterization of Slr0077/SufS, the Essential Cysteine Desulfurase from Synechocystis sp. PCC 6803. Biochemistry 2004, 43, 12210–12219. [Google Scholar] [CrossRef] [PubMed]
- Grishin, N.V.; Phillips, M.A.; Goldsmith, E.J. Modeling of the Spatial Structure of Eukaryotic Ornithine Decarboxylases. Protein Sci. 1995, 4, 1291–1304. [Google Scholar] [CrossRef] [PubMed]
- Schneider, G.; Käck, H.; Lindqvist, Y. The Manifold of Vitamin B6 Dependent Enzymes. Structure 2000, 8, 1–6. [Google Scholar] [CrossRef]
- Nakamura, R.; Hikita, M.; Ogawa, S.; Takahashi, Y.; Fujishiro, T. Snapshots of PLP-substrate and PLP-product External Aldimines as Intermediates in Two Types of Cysteine Desulfurase Enzymes. FEBS J. 2020, 287, 1138–1154. [Google Scholar] [CrossRef]
- Nilsson, K.; Lundgren, H.K.; Hagervall, T.G.; Björk, G.R. The Cysteine Desulfurase IscS is Required for Synthesis of all Five Thiolated Nucleosides Present in tRNA from Salmonella enterica Serovar Typhimurium. J. Bacteriol. 2002, 184, 6830–6835. [Google Scholar] [CrossRef]
- Dunkle, J.A.; Bruno, M.R.; Outten, F.W.; Frantom, P.A. Structural Evidence for Dimer-interface-driven Regulation of the Type II Cysteine Desulfurase, SufS. Biochemistry 2019, 58, 687–696. [Google Scholar] [CrossRef]
- Eliot, A.C.; Kirsch, J.F. Pyridoxal Phosphate Enzymes: Mechanistic, Structural, and Evolutionary Considerations. Annu. Rev. Biochem. 2004, 73, 383–415. [Google Scholar] [CrossRef] [PubMed]
- Behshad, E.; Bollinger, J.M. Kinetic Analysis of Cysteine Desulfurase CD0387 from Synechocystis sp. PCC 6803: Formation of the Persulfide Intermediate. Biochemistry 2009, 48, 12014–12023. [Google Scholar] [CrossRef] [PubMed]
- Mueller, E.G. Trafficking in Persulfides: Delivering Sulfur in Biosynthetic Pathways. Nat. Chem. Biol. 2006, 2, 185–194. [Google Scholar] [CrossRef] [PubMed]
- Santos, P.C.D. B. subtilis as a Model for Studying the Assembly of Fe–S Clusters in Gram-Positive Bacteria. In Methods in Enzymology; Academic Press Inc.: Cambridge, MA, USA, 2017; Volume 595, pp. 185–212. [Google Scholar]
- Giles, G.I.; Tasker, K.M.; Jacob, C. Hypothesis: The Role of Reactive Sulfur Species in Oxidative Stress. Free Radic. Biol. Med. 2001, 31, 1279–1283. [Google Scholar] [CrossRef]
- Giles, G.I.; Jacob, C. Reactive Sulfur Species: An Emerging Concept in Oxidative Stress. Biol. Chem. 2002, 383, 375–388. [Google Scholar] [CrossRef]
- Jacob, C.; Giles, G.I.; Giles, N.M.; Sies, H. Sulfur and Selenium: The Role of Oxidation State in Protein Structure and Function. Angew. Chem. Int. Ed. 2003, 42, 4742–4758. [Google Scholar] [CrossRef] [PubMed]
- Selbach, B.P.; Pradhan, P.K.; Santos, P.C.D. Protected Sulfur Transfer Reactions by the Escherichia coli Suf System. Biochemistry 2013, 52, 4089–4096. [Google Scholar] [CrossRef]
- Singh, A.; Guidry, L.; Narasimhulu, K.V.; Mai, D.; Trombley, J.; Redding, K.E.; Giles, G.I.; Lancaster, J.R., Jr.; Steyn, A.J. Mycobacterium tuberculosis WhiB3 Responds to O2 and Nitric oxide via its [4Fe-4S] Cluster and is Essential for Nutrient Starvation Survival. Proc. Natl. Acad. Sci. USA 2007, 104, 11562–11567. [Google Scholar] [CrossRef]
- Imlay, J.A. Iron-sulfur Clusters and the Problem with Oxygen. Mol. Microbiol. 2006, 59, 1073–1082. [Google Scholar] [CrossRef]
- Johnson, D.C.; Dean, D.R.; Smith, A.D.; Johnson, M.K. Structure, Function, and Formation of Biological Iron-sulfur Clusters. Annu. Rev. Biochem. 2005, 74, 247–281. [Google Scholar] [CrossRef] [PubMed]
- Cardenas-Rodriguez, M.; Chatzi, A.; Tokatlidis, K. Iron–sulfur Clusters: From Metals through Mitochondria Biogenesis to Disease. J. Biol. Inorg. Chem. 2018, 23, 509–520. [Google Scholar] [CrossRef]
- Shimomura, Y.; Takahashi, Y.; Kakuta, Y.; Fukuyama, K. Crystal Structure of Escherichia coli YfhJ Protein, a Member of the ISC Machinery Involved in Assembly of Iron-sulfur Clusters. Proteins 2005, 60, 566–569. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, C.J.; Djaman, O.; Imlay, J.A.; Kiley, P.J. The Cysteine Desulfurase, IscS, has a Major Role in in vivo Fe-S Cluster Formation in Escherichia coli. Proc. Natl. Acad. Sci. USA 2000, 97, 9009–9014. [Google Scholar] [CrossRef]
- Mettert, E.L.; Kiley, P.J. Fe-S Proteins that Regulate Gene Expression. BBA Mol. Cell Res. 2015, 1853, 1284–1293. [Google Scholar] [CrossRef] [PubMed]
- Saini, V.; Farhana, A.; Glasgow, J.N.; Steyn, A.J.C. Iron-sulfur Cluster Proteins and Microbial Regulation: Implications for Understanding Tuberculosis. Curr. Opin. Chem. Biol. 2012, 16, 45–53. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, Y.; Tokumoto, U. A Third Bacterial System for the Assembly of Iron-sulfur Clusters with Homologs in Archaea and Plastids. J. Biol. Chem. 2002, 277, 28380–28383. [Google Scholar] [CrossRef]
- Zheng, C.; Black, K.A.; Santos, P.C.D. Diverse Mechanisms of Sulfur Decoration in Bacterial tRNA and their Cellular Functions. Biomolecules 2017, 7, 33. [Google Scholar] [CrossRef]
- Čavužić, M.; Liu, Y. Biosynthesis of Sulfur-Containing tRNA Modifications: A Comparison of Bacterial, Archaeal, and Eukaryotic Pathways. Biomolecules 2017, 7, 27. [Google Scholar] [CrossRef]
- Lauhon, C.T. Requirement for IscS in Biosynthesis of All Thionucleosides in Escherichia coli. J. Bacteriol. 2002, 184, 6820. [Google Scholar] [CrossRef]
- Landgraf, B.J.; Arcinas, A.J.; Lee, K.H.; Booker, S.J. Identification of an Intermediate Methyl Carrier in the Radical S-Adenosylmethionine Methylthiotransferases RimO and MiaB. J. Am. Chem. Soc. 2013, 135, 15404–15416. [Google Scholar] [CrossRef] [PubMed]
- Maiocco, S.J.; Arcinas, A.J.; Landgraf, B.J.; Lee, K.H.; Booker, S.J.; Elliott, S.J. Transformations of the FeS Clusters of the Methylthiotransferases MiaB and RimO, Detected by Direct Electrochemistry. Biochemistry 2016, 55, 5531–5536. [Google Scholar] [CrossRef] [PubMed]
- Hernández, H.L.; Pierrel, F.; Elleingand, E.; García-Serres, R.; Huynh, B.H.; Johnson, M.K.; Fontecave, M.; Atta, M. MiaB, a Bifunctional Radical-S-Adenosylmethionine Enzyme Involved in the Thiolation and Methylation of tRNA, Contains Two Essential [4Fe-4S] Clusters. Biochemistry 2007, 46, 5140–5147. [Google Scholar] [CrossRef]
- Chionh, Y.H.; McBee, M.; Babu, I.R.; Hia, F.; Lin, W.; Zhao, W.; Cao, J.; Dziergowska, A.; Malkiewicz, A.; Begley, T.J.; et al. tRNA-mediated Codon-biased Translation in Mycobacterial Hypoxic Persistence. Nat. Commun. 2016, 7, 13302. [Google Scholar] [CrossRef] [PubMed]
- Black, K.A.; Santos, P.C.D. Abbreviated Pathway for Biosynthesis of 2-Thiouridine in Bacillus subtilis. J. Bacteriol. 2015, 197, 1952–1962. [Google Scholar] [CrossRef] [PubMed]
- Kambampati, R.; Lauhon, C.T. Evidence for the Transfer of Sulfane Sulfur from IscS to ThiI during the in Vitro Biosynthesis of 4-Thiouridine in Escherichia coli tRNA. J. Biol. Chem. 2000, 275, 10727–10730. [Google Scholar] [CrossRef] [PubMed]
- Numata, T.; Ikeuchi, Y.; Fukai, S.; Suzuki, T.; Nureki, O. Snapshots of tRNA Sulphuration via an Adenylated Intermediate. Nature 2006, 442, 419–424. [Google Scholar] [CrossRef]
- Palenchar, P.M.; Buck, C.J.; Cheng, H.; Larson, T.J.; Mueller, E.G. Evidence that ThiI, an Enzyme Shared Between Thiamin and 4-Thiouridine Biosynthesis, may be a Sulfurtransferase that Proceeds through a Persulfide Intermediate. J. Biol. Chem. 2000, 275, 8283–8286. [Google Scholar] [CrossRef]
- Mueller, E.G.; Palenchar, P.M.; Buck, C.J. The Role of the Cysteine Residues of ThiI in the Generation of 4-Thiouridine in tRNA. J. Biol. Chem. 2001, 276, 33588–33595. [Google Scholar] [CrossRef] [PubMed]
- Jäger, G.; Leipuviene, R.; Pollard, M.G.; Qian, Q.; Björk, G.R. The Conserved Cys-X1-X2-Cys Motif Present in the TtcA Protein Is Required for the Thiolation of Cytidine in Position 32 of tRNA from Salmonella enterica Serovar Typhimurium. J. Bacteriol. 2004, 186, 750. [Google Scholar] [CrossRef]
- Leipuviene, R.; Qian, Q.; Björk, G.R. Formation of Thiolated Nucleosides Present in tRNA from Salmonella enterica Serovar Typhimurium Occurs in Two Principally Distinct Pathways. J. Bacteriol. 2004, 186, 758–766. [Google Scholar] [CrossRef] [PubMed]
- Mihara, H.; Hidese, R.; Yamane, M.; Kurihara, T.; Esaki, N. The iscS Gene Deficiency Affects the Expression of Pyrimidine Metabolism Genes. Biochem. Biophys. Res. 2008, 372, 407–411. [Google Scholar] [CrossRef] [PubMed]
- Lacourciere, G.M. Selenium Is Mobilized In Vivo from Free Selenocysteine and is Incorporated Specifically into Formate Dehydrogenase H and tRNA Nucleosides. J. Bacteriol. 2002, 184, 1940–1946. [Google Scholar] [CrossRef] [PubMed]
- Mihara, H.; Kato, S.; Lacourciere, G.M.; Stadtman, T.C.; Kennedy, R.A.; Kurihara, T.; Tokumoto, U.; Takahashi, Y.; Esaki, N. The iscS Gene is Essential for the Biosynthesis of 2-Selenouridine in tRNA and the Selenocysteine-containing Formate Dehydrogenase H. Proc. Natl. Acad. Sci. USA 2002, 99, 6679–6683. [Google Scholar] [CrossRef] [PubMed]
- Cronan, J.E. Assembly of Lipoic Acid on its Cognate Enzymes: An Extraordinary and Essential Biosynthetic Pathway. Microbiol. Mol. Biol. Rev. 2016, 80, 429. [Google Scholar] [CrossRef]
- Spalding, M.D.; Prigge, S.T. Lipoic Acid Metabolism in Microbial Pathogens. Microbiol. Mol. Biol. Rev. 2010, 74, 200–228. [Google Scholar] [CrossRef]
- Solmonson, A.; DeBerardinis, R.J. Lipoic Acid Metabolism and Mitochondrial Redox Regulation. J. Biol. Chem. 2018, 293, 7522–7530. [Google Scholar] [CrossRef]
- Cronan, J.E. Biotin and Lipoic Acid: Synthesis, Attachment, and Regulation. EcoSal Plus 2013, 6. [Google Scholar] [CrossRef]
- Cicchillo, R.M.; Booker, S.J. Mechanistic Investigations of Lipoic Acid Biosynthesis in Escherichia coli: Both Sulfur Atoms in Lipoic Acid are Contributed by the same Lipoyl Synthase Polypeptide. J. Am. Chem. Soc. 2005, 127, 2860–2861. [Google Scholar] [CrossRef]
- McCarthy, E.L.; Booker, S.J. Destruction and Reformation of an Iron-Sulfur Cluster during Catalysis by Lipoyl Synthase. Science 2017, 358, 373–377. [Google Scholar] [CrossRef]
- Kriek, M.; Peters, L.; Takahashi, Y.; Roach, P.L. Effect of Iron-Sulfur Cluster Assembly Proteins on the Expression of Escherichia coli Lipoic Acid Synthase. Protein Expr. Purif. 2003, 28, 241–245. [Google Scholar] [CrossRef]
- Reed, L.J. From Lipoic Acid to Multi-enzyme Complexes. Protein Sci. 1998, 7, 220–224. [Google Scholar] [CrossRef] [PubMed]
- Ma, Q.; Zhao, X.; Eddine, A.N.; Geerlof, A.; Li, X.; Cronan, J.E.; Kaufmann, S.H.; Wilmanns, M. The Mycobacterium tuberculosis LipB Enzyme Functions as a Cysteine/Lysine Dyad Acyltransferase. Proc. Natl. Acad. Sci. USA 2006, 103, 8662–8667. [Google Scholar] [CrossRef]
- Shi, S.; Ehrt, S. Dihydrolipoamide Acyltransferase is Critical for Mycobacterium tuberculosis Pathogenesis. Infect. Immun. 2006, 74, 56–63. [Google Scholar] [CrossRef] [PubMed]
- Bryk, R.; Gold, B.; Venugopal, A.; Singh, J.; Samy, R.; Pupek, K.; Cao, H.; Popescu, C.; Gurney, M.; Hotha, S.; et al. Selective Killing of Nonreplicating Mycobacteria. Cell Host Microbe 2008, 3, 137–145. [Google Scholar] [CrossRef]
- Venugopal, A.; Bryk, R.; Shi, S.; Rhee, K.; Rath, P.; Schnappinger, D.; Ehrt, S.; Nathan, C. Virulence of Mycobacterium tuberculosis Depends on Lipoamide Dehydrogenase, a Member of Three Multienzyme Complexes. Cell Host Microbe 2011, 9, 21–31. [Google Scholar] [CrossRef]
- Salaemae, W.; Booker, G.W.; Polyak, S.W. The Role of Biotin in Bacterial Physiology and Virulence: A Novel Antibiotic Target for Mycobacterium tuberculosis. Microbiol. Spectr. 2016, 4, 1–20. [Google Scholar] [CrossRef]
- Gande, R.; Gibson, K.J.; Brown, A.K.; Krumbach, K.; Dover, L.G.; Sahm, H.; Shioyama, S.; Oikawa, T.; Besra, G.S.; Eggeling, L. Acyl-CoA Carboxylases (accD2 and accD3), Together with a Unique Polyketide Synthase (Cg-pks), are Key to Mycolic Acid Biosynthesis in Corynebacterianeae such as Corynebacterium glutamicum and Mycobacterium tuberculosis. J. Biol. Chem. 2004, 279, 44847–44857. [Google Scholar] [CrossRef]
- Ehrt, S.; Schnappinger, D. Mycobacterium tuberculosis Virulence: Lipids Inside and Out. Nat. Med. 2007, 13, 284–285. [Google Scholar] [CrossRef]
- Marquet, A.; Bui, B.T.S.; Florentin, D. Biosynthesis of Biotin and Lipoic Acid. In Vitamins and Hormones; Academic Press Inc.: Cambridge, MA, USA, 2001; Volume 61, pp. 51–101. [Google Scholar] [CrossRef]
- Lotierzo, M.; Bui, B.T.S.; Florentin, D.; Escalettes, F.; Marquet, A. Biotin Synthase Mechanism: An overview. Biochem. Soc. Trans. 2005, 33, 820–823. [Google Scholar] [CrossRef]
- Dey, S.; Lane, J.M.; Lee, R.E.; Rubin, E.J.; Sacchettini, J.C. Structural Characterization of the Mycobacterium tuberculosis Biotin Biosynthesis Enzymes 7,8-diaminopelargonic acid Synthase and Dethiobiotin Synthetase. Biochemistry 2010, 49, 6746–6760. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.; Cronan, J.E. Closing in on Complete Pathways of Biotin Biosynthesis. Mol. Biosyst. 2011, 7, 1811–1821. [Google Scholar] [CrossRef] [PubMed]
- Begley, T.P.; Xi, J.; Kinsland, C.; Taylor, S.; McLafferty, F. The Enzymology of Sulfur Activation during Thiamin and Biotin Biosynthesis. Curr. Opin. Chem. Biol. 1999, 3, 623–629. [Google Scholar] [CrossRef]
- Ollagnier-de-Choudens, S.; Mulliez, E.; Hewitson, K.S.; Fontecave, M. Biotin Synthase is a Pyridoxal Phosphate-Dependent Cysteine Desulfurase. Biochemistry 2002, 41, 9145–9152. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Hamid, A.M.; Cronan, J.E. In Vivo Resolution of Conflicting in Vitro Results: Synthesis of Biotin from Dethiobiotin does not Require Pyridoxal Phosphate. Chem. Biol. 2007, 14, 1215–1220. [Google Scholar] [CrossRef][Green Version]
- Kiyasu, T.; Asakura, A.; Nagahashi, Y.; Hoshino, T. Contribution of Cysteine Desulfurase (NifS protein) to the Biotin Synthase Reaction of Escherichia coli. J. Bacteriol. 2000, 182, 2879–2885. [Google Scholar] [CrossRef][Green Version]
- Tiwari, D.; Park, S.W.; Essawy, M.M.; Dawadi, S.; Mason, A.; Nandakumar, M.; Zimmerman, M.; Mina, M.; Ho, H.P.; Engelhart, C.A.; et al. Targeting Protein Biotinylation Enhances Tuberculosis Chemotherapy. Sci. Transl. Med. 2018, 10. [Google Scholar] [CrossRef]
- Lonsdale, D. A Review of the Biochemistry, Metabolism and Clinical Benefits of Thiamin(e) and its Derivatives. Evid. Based Complement. Alternat. Med. 2006, 3, 49–59. [Google Scholar] [CrossRef]
- Begley, T.P.; Downs, D.M.; Ealick, S.E.; McLafferty, F.W.; Van Loon, A.P.; Taylor, S.; Campobasso, N.; Chiu, H.J.; Kinsland, C.; Reddick, J.J.; et al. Thiamin Biosynthesis in Prokaryotes. Arch. Microbiol. 1999, 171, 293–300. [Google Scholar] [CrossRef]
- Kriek, M.; Martins, F.; Leonardi, R.; Fairhurst, S.A.; Lowe, D.J.; Roach, P.L. Thiazole Synthase from Escherichia coli: An Investigation of the Substrates and Purified Proteins Required for Activity In Vitro. J. Biol. Chem. 2007, 282, 17413–17423. [Google Scholar] [CrossRef]
- Ealick, S.E.; Jurgenson, C.T.; Begley, T.P. Biosynthesis of Thiamin Pyrophosphate. EcoSal Plus 2009, 3, 1–16. [Google Scholar] [CrossRef]
- Taylor, S.V.; Kelleher, N.L.; Kinsland, C.; Chiu, H.J.; Costello, C.A.; Backstrom, A.D.; McLafferty, F.W.; Begley, T.P. Thiamin Biosynthesis in Escherichia coli: Identification of this Thiocarboxylate as the Immediate Sulfur Donor in the Thiazole Formation. J. Biol. Chem. 1998, 273, 16555–16560. [Google Scholar] [CrossRef] [PubMed]
- Tokumoto, U.; Takahashi, Y. Genetic Analysis of the isc Operon in Escherichia coli Involved in the Biogenesis of Cellular Iron-Sulfur Proteins1. J. Biochem. 2001, 130, 63–71. [Google Scholar] [CrossRef] [PubMed]
- Skovran, E.; Downs, D.M. Metabolic Defects Caused by Mutations in the Isc Gene Cluster in Salmonella enterica Serovar Typhimurium: Implications for Thiamine Synthesis. J. Bacteriol. 2000, 182, 3896–3903. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Lauhon, C.T.; Kambampati, R. The IscS Gene in Escherichia coli is Required for the Biosynthesis of 4- Thiouridine, Thiamin, and NAD. J. Biol. Chem. 2000, 275, 20096–20103. [Google Scholar] [CrossRef]
- Leimkühler, S.; Wuebbens, M.M.; Rajagopalan, K.V. The History of the Discovery of the Molybdenum Cofactor and Novel Aspects of its Biosynthesis in Bacteria. Coord. Chem. Rev. 2011, 255, 1129–1144. [Google Scholar] [CrossRef]
- Hille, R.; Hall, J.; Basu, P. The Mononuclear Molybdenum Enzymes. Chem. Rev. 2014, 114, 3963–4038. [Google Scholar] [CrossRef]
- Romão, M.J.; Knäblein, J.; Huber, R.; Moura, J.J. Structure and Function of Molybdopterin Containing Enzymes. Prog. Biophys. Mol. Biol. 1997, 68, 121–144. [Google Scholar] [CrossRef]
- Yokoyama, K.; Leimkühler, S. The Role of FeS Clusters for Molybdenum Cofactor Biosynthesis and Molybdoenzymes in Bacteria. Biochim. Biophys. Acta Mol. Cell Res. 2015, 1853, 1335–1349. [Google Scholar] [CrossRef]
- Zhang, W.; Urban, A.; Mihara, H.; Leimkühler, S.; Kurihara, T.; Esaki, N. IscS Functions as a Primary Sulfur-donating Enzyme by Interacting Specifically with MoeB and MoaD in the Biosynthesis of Molybdopterin in Escherichia coli. J. Biol. Chem. 2010, 285, 2302–2308. [Google Scholar] [CrossRef]
- Kabil, O.; Banerjee, R. Redox Biochemistry of Hydrogen Sulfide. J. Biol. Chem. 2010, 285, 21903–21907. [Google Scholar] [CrossRef] [PubMed]
- Kabil, O.; Banerjee, R. Enzymology of H2S Biogenesis, Decay and Signaling. Antioxid. Redox Signal. 2014, 20, 770–782. [Google Scholar] [CrossRef] [PubMed]
- Kimura, H. Production and Physiological Effects of Hydrogen Sulfide. Antioxid. Redox Signal. 2014, 20, 783–793. [Google Scholar] [CrossRef] [PubMed]
- Pal, V.K.; Bandyopadhyay, P.; Singh, A. Hydrogen Sulfide in Physiology and Pathogenesis of Bacteria and Viruses. IUBMB Life 2018, 70, 393–410. [Google Scholar] [CrossRef]
- Wang, J.; Guo, X.; Li, H.; Qi, H.; Qian, J.; Yan, S.; Shi, J.; Niu, W. Hydrogen Sulfide from Cysteine Desulfurase, not 3-Mercaptopyruvate Sulfurtransferase, Contributes to Sustaining Cell Growth and Bioenergetics in E. coli under Anaerobic Conditions. Front. Microbiol. 2019, 10, 2357. [Google Scholar] [CrossRef]
- Großhennig, S.; Ischebeck, T.; Gibhardt, J.; Busse, J. Hydrogen Sulfide is a Novel Potential Virulence Factor of Mycoplasma pneumoniae: Characterization of the Unusual Cysteine Desulfurase/Desulfhydrase HapE. Mol. Microbiol. 2016, 100, 42–54. [Google Scholar] [CrossRef]
- Wang, L.; Jiang, S.; Deng, Z.; Dedon, P.C.; Chen, S. DNA Phosphorothioate Modification-A New Multi-Functional Epigenetic System in Bacteria. FEMS Microbiol. Rev. 2019, 43, 109–122. [Google Scholar] [CrossRef]
- Liu, L.; Jiang, S.; Xing, M.; Chen, C.; Lai, C.; Li, N.; Liu, G.; Wu, D.; Gao, H.; Hong, L.; et al. Structural Analysis of an l-Cysteine Desulfurase from an Ssp DNA Phosphorothioation System. mBio 2020, 11. [Google Scholar] [CrossRef]
- Tong, T.; Chen, S.; Wang, L.; Tang, Y.; Ryu, J.Y.; Jiang, S.; Wu, X.; Chen, C.; Luo, J.; Deng, Z.; et al. Occurrence, Evolution, and Functions of DNA Phosphorothioate Epigenetics in Bacteria. Proc. Natl. Acad. Sci. USA 2018, 115, E2988–E2996. [Google Scholar] [CrossRef]
- Dai, D.; Du, A.; Xiong, K.; Pu, T.; Zhou, X.; Deng, Z.; Liang, J.; He, X.; Wang, Z. DNA Phosphorothioate Modification Plays a role in Peroxides Resistance in Streptomyces lividans. Front. Microbiol. 2016, 7, 1380. [Google Scholar] [CrossRef]
- Yang, Y.; Xu, G.; Liang, J.; He, Y.; Xiong, L.; Li, H.; Bartlett, D.; Deng, Z.; Wang, Z.; Xiao, X. DNA Backbone Sulfur-Modification Expands Microbial Growth Range under Multiple Stresses by its Anti-oxidation Function. Sci. Rep. 2017, 7, 3516. [Google Scholar] [CrossRef] [PubMed]
- Outten, F.W.; Djaman, O.; Storz, G. A suf Operon Requirement for Fe-S Cluster Assembly during Iron Starvation in Escherichia coli. Mol. Microbiol. 2004, 52, 861–872. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.C.; Yeo, W.S.; Roe, J.H. Oxidant-responsive Induction of the suf Operon, Encoding a Fe-S Assembly System, through Fur and IscR in Escherichia coli. J. Bacteriol. 2008, 190, 8244–8247. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Yeo, W.S.; Roe, J.H. Induction of the sufA Operon Encoding Fe-S Assembly Proteins by Superoxide Generators and Hydrogen Peroxide: Involvement of OxyR, IHF and an Unidentified Oxidant-responsive Factor. Mol. Microbiol. 2004, 51, 1745–1755. [Google Scholar] [CrossRef] [PubMed]
- Miller, H.K.; Kwuan, L.; Schwiesow, L.; Bernick, D.L.; Mettert, E.; Ramirez, H.A.; Ragle, J.M.; Chan, P.P.; Kiley, P.J.; Lowe, T.M.; et al. IscR Is Essential for Yersinia pseudotuberculosis Type III Secretion and Virulence. PLoS Pathog. 2014, 10, e1004194. [Google Scholar] [CrossRef]
- Lim, J.G.; Choi, S.H. IscR is a Global Regulator Essential for Pathogenesis of Vibrio vulnificus and Induced by Host Cells. Infect. Immun. 2014, 82, 569–578. [Google Scholar] [CrossRef]
- Santos, J.A.; Pereira, P.J.B.; Macedo-Ribeiro, S. What a Difference a Cluster Makes: The Multifaceted Roles of IscR in Gene Regulation and DNA Recognition. Biochim. Biophys. Acta Proteins Proteom. 2015, 1854, 1101–1112. [Google Scholar] [CrossRef]
- Schwartz, C.J.; Giel, J.L.; Patschkowski, T.; Luther, C.; Ruzicka, F.J.; Beinert, H.; Kiley, P.J. IscR, An Fe-S Cluster-containing Transcription Factor, Represses Expression of Escherichia coli Genes Encoding Fe-S Cluster Assembly Proteins. Proc. Natl. Acad. Sci. USA 2001, 98, 14895–14900. [Google Scholar] [CrossRef]
- Giel, J.L.; Rodionov, D.; Liu, M.; Blattner, F.R.; Kiley, P.J. IscR-dependent Gene Expression links Iron-Sulphur Cluster Assembly to the Control of O2-regulated Genes in Escherichia coli. Mol. Microbiol. 2006, 60, 1058–1075. [Google Scholar] [CrossRef]
- Yeo, W.S.; Lee, J.H.; Lee, K.C.; Roe, J.H. IscR Acts as an Activator in Response to Oxidative Stress for the suf Operon Encoding Fe-S Assembly Proteins. Mol. Microbiol. 2006, 61, 206–218. [Google Scholar] [CrossRef]
- Wang, T.; Shen, G.; Balasubramanian, R.; McIntosh, L.; Bryant, D.A.; Golbeck, J.H. The sufR Gene (sll0088 in Synechocystis sp. Strain PCC 6803) Functions as a Repressor of the sufBCDS Operon in Iron-Sulfur Cluster Biogenesis in Cyanobacteria. J. Bacteriol. 2004, 186, 956–967. [Google Scholar] [CrossRef] [PubMed]
- Balasubramanian, R.; Shen, G.; Bryant, D.A.; Golbeck, J.H. Regulatory Roles for IscA and SufA in Iron Homeostasis and Redox Stress Responses in the Cyanobacterium Synechococcus sp. Strain PCC 7002. J. Bacteriol. 2006, 188, 3182–3191. [Google Scholar] [CrossRef] [PubMed]
- Shen, G.; Balasubramanian, R.; Wang, T.; Wu, Y.; Hoffart, L.M.; Krebs, C.; Bryant, D.A.; Golbeck, J.H. SufR Coordinates Two [4Fe-4S]2+, 1+ Clusters and Functions as a Transcriptional Repressor of the sufBCDS Operon and an Autoregulator of sufR in Cyanobacteria. J. Biol. Chem. 2007, 282, 31909–31919. [Google Scholar] [CrossRef]
- Cortes, T.; Schubert, O.T.; Rose, G.; Arnvig, K.B.; Comas, I.; Aebersold, R.; Young, D.B. Genome-wide Mapping of Transcriptional Start Sites Defines an Extensive Leaderless Transcriptome in Mycobacterium tuberculosis. Cell Rep. 2013, 5, 1121–1131. [Google Scholar] [CrossRef] [PubMed]
- Massé, E.; Vanderpool, C.K.; Gottesman, S. Effect of RyhB small RNA on Global Iron use in Escherichia coli. J. Bacteriol. 2005, 187, 6962–6971. [Google Scholar] [CrossRef]
- Desnoyers, G.; Morissette, A.; Prévost, K.; Massé, E. Small RNA-induced Differential Degradation of the Polycistronic mRNA iscRSUA. EMBO J. 2009, 28, 1551–1561. [Google Scholar] [CrossRef]
- Riboldi, G.P.; de Oliveira, J.S.; Frazzon, J. Enterococcus faecalis SufU Scaffold Protein Enhances SufS Desulfurase Activity by Acquiring Sulfur from its Cysteine-153. Biochim. Biophys. Acta Proteins Proteom. 2011, 1814, 1910–1918. [Google Scholar] [CrossRef]
- Albrecht, A.G.; Netz, D.J.; Miethke, M.; Pierik, A.J.; Burghaus, O.; Peuckert, F.; Lill, R.; Marahiel, M.A. SufU is an Essential Iron-Sulfur Cluster Scaffold Protein in Bacillus subtilis. J. Bacteriol. 2010, 192, 1643–1651. [Google Scholar] [CrossRef]
- Shan, Y.; Napoli, E.; Cortopassi, G. Mitochondrial Frataxin Interacts with ISD11 of the NFS1/ISCU Complex and Multiple Mitochondrial Chaperones. Hum. Mol. Genet. 2007, 16, 929–941. [Google Scholar] [CrossRef]
- Adinolfi, S.; Iannuzzi, C.; Prischi, F.; Pastore, C.; Iametti, S.; Martin, S.R.; Bonomi, F.; Pastore, A. Bacterial Frataxin CyaY is the Gatekeeper of Iron-Sulfur Cluster Formation Catalyzed by IscS. Nat. Struct. Mol. Biol. 2009, 16, 390–396. [Google Scholar] [CrossRef]
- Yoon, T.; Cowan, J.A. Iron-Sulfur Cluster Biosynthesis. Characterization of Frataxin as an Iron Donor for Assembly of [2Fe-2S] Clusters in ISU-type Proteins. J. Am. Chem. Soc. 2003, 125, 20, 6078–84. [Google Scholar] [CrossRef]
- Layer, G.; Ollagnier-de-Choudens, S.; Sanakis, Y.; Fontecave, M. Iron-Sulfur Cluster Biosynthesis: Characterization of Escherichia coli CyaY as an Iron Donor for the Assembly of [2Fe-2S] Clusters in the Scaffold IscU. J. Biol. Chem. 2003, 281, 16256–16263. [Google Scholar] [CrossRef]
- Li, D.S.; Ohshima, K.; Jiralerspong, S.; Bojanowski, M.W.; Pandolfo, M. Knock-out of the cyaY Gene in Escherichia coli does not Affect Cellular Iron Content and Sensitivity to Oxidants. FEBS Lett. 1999, 456, 13–16. [Google Scholar] [CrossRef]
- Vivas, E.; Skovran, E.; Downs, D.M. Salmonella enterica Strains Lacking the Frataxin Homolog CyaY Show Defects in Fe-S Cluster Metabolism in Vivo. J. Bacteriol. 2006, 188, 1175–1179. [Google Scholar] [CrossRef]
- Velayudhan, J.; Karlinsey, J.E.; Frawley, E.R.; Becker, L.A.; Nartea, M.; Fang, F.C. Distinct Roles of the Salmonella enterica Serovar Typhimurium CyaY and YggX Proteins in the Biosynthesis and Repair of Iron-Sulfur Clusters. Infect. Immun. 2014, 82, 1390–1401. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Frederick, R.O.; Reinen, N.M.; Troupis, A.T.; Markley, J.L. [2Fe-2S]-Ferredoxin Binds Directly to Cysteine Desulfurase and Supplies an Electron for Iron-Sulfur Cluster Assembly but is Displaced by the Scaffold Protein or Bacterial Frataxin. J. Am. Chem. Soc. 2013, 135, 8117–8120. [Google Scholar] [CrossRef] [PubMed]
- Zheng, C.; Guo, S.; Tennant, W.G.; Pradhan, P.K.; Black, K.A.; Santos, P.C.D. The Thioredoxin System Reduces Protein Persulfide Intermediates Formed during the Synthesis of Thio-Cofactors in Bacillus subtilis. Biochemistry 2019, 58, 1892–1904. [Google Scholar] [CrossRef] [PubMed]
- Py, B.; Moreau, P.L.; Barras, F. Fe-S Clusters, Fragile Sentinels of the Cell. Curr. Opin. Microbiol. 2011, 14, 218–223. [Google Scholar] [CrossRef]
- Touati, D. Iron and Oxidative Stress in Bacteria. Arch. Biochem. Biophys. 2000, 373, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Ezraty, B.; Vergnes, A.; Banzhaf, M.; Duverger, Y.; Huguenot, A.; Brochado, A.R.; Su, S.Y.; Espinosa, L.; Loiseau, L.; Py, B.; et al. Fe-S cluster Biosynthesis Controls Uptake of Aminoglycosides in a ROS-less Death Pathway. Science 2013, 340, 1583–1587. [Google Scholar] [CrossRef]
- Patel, R.; Rinker, L.; Peng, J.; Rinker, L.; Peng, J.; Chilian, M. Reactive Oxygen Species: The Good and the Bad. Intech Open 2018. [Google Scholar] [CrossRef]
- Lanciano, P.; Khalfaoui-Hassani, B.; Selamoglu, N.; Ghelli, A.; Rugolo, M.; Daldal, F. Molecular Mechanisms of Superoxide Production by Complex III: A Bacterial versus Human Mitochondrial Comparative Case Study. Biochim. Biophys. Acta 2014, 1827, 1332–1339. [Google Scholar] [CrossRef] [PubMed]
- Imlay, J.A. The Molecular Mechanisms and Physiological Consequences of Oxidative Stress: Lessons from a Model Bacterium. Nat. Rev. Microbiol. 2013, 11, 443–454. [Google Scholar] [CrossRef] [PubMed]
- Moini, H.; Packer, L.; Saris, N.L. Antioxidant and Prooxidant Activities of α-Lipoic Acid and Dihydrolipoic Acid. Toxicol. Appl. Pharmacol. 2002, 182, 84–90. [Google Scholar] [CrossRef] [PubMed]
- Packer, L.; Witt, E.H.; Tritschler, H.J. Alpha-Lipoic Acid as a Biological Antioxidant. Free Radic. Biol. Med. 1995, 19, 227–250. [Google Scholar] [CrossRef]
- Vertuani, S.; Angusti, A.; Manfredini, S. The Antioxidants and Pro-Antioxidants Network: An Overview. Curr. Pharm. Des. 2005, 10, 1677–1694. [Google Scholar] [CrossRef]
- Ehrt, S.; Schnappinger, D. Mycobacterial Survival Strategies in the Phagosome: Defence Against Host Stresses. Cell Microbiol. 2009, 11, 1170–1178. [Google Scholar] [CrossRef]
- Mehta, M.; Singh, A. Mycobacterium tuberculosis WhiB3 Maintains Redox Homeostasis and Survival in Response to Reactive Oxygen and Nitrogen species. Free Radic. Biol. Med. 2019, 131, 50–58. [Google Scholar] [CrossRef]
- Rivera-Chávez, F.; Zhang, L.F.; Faber, F.; Lopez, C.A.; Byndloss, M.X.; Olsan, E.E.; Xu, G.; Velazquez, E.M.; Lebrilla, C.B.; Winter, S.E.; et al. Depletion of Butyrate-Producing Clostridia from the Gut Microbiota Drives an Aerobic Luminal Expansion of Salmonella. Cell Host Microbe 2016, 19, 443–454. [Google Scholar] [CrossRef]
- Alamuri, P.; Mehta, N.; Burk, A.; Maier, R.J. Regulation of the Helicobacter pylori Fe-S Cluster Synthesis Protein NifS by Iron, Oxidative Stress Conditions, and Fur. J. Bacteriol. 2006, 188, 5325–5330. [Google Scholar] [CrossRef]
- Dai, Y.; Outten, F.W. The E. coli SufS-SufE Sulfur Transfer System is more Resistant to Oxidative Stress than IscS-IscU. FEBS Lett. 2012, 586, 4016–4022. [Google Scholar] [CrossRef] [PubMed]
- Shukla, P.; Khodade, V.S.; Chandra, M.S.; Chauhan, P.; Mishra, S.; Siddaramappa, S.; Pradeep, B.E.; Singh, A.; Chakrapani, H. ‘On Demand’ Redox Buffering by H2S Contributes to Antibiotic Resistance Revealed by a Bacteria-specific H2S Donor. Chem. Sci. 2017, 8, 4967–4972. [Google Scholar] [CrossRef] [PubMed]
- Mishra, R.; Kohli, S.; Malhotra, N.; Bandyopadhyay, P.; Mehta, M.; Munshi, M.; Adiga, V.; Ahuja, V.K.; Shandil, R.K.; Rajmani, R.S.; et al. Targeting Redox Heterogeneity to Counteract Drug Tolerance in Replicating Mycobacterium tuberculosis. Sci. Transl. Med. 2019, 11. [Google Scholar] [CrossRef] [PubMed]
- Fuentes, D.E.; Fuentes, E.L.; Castro, M.E.; Pérez, J.M.; Araya, M.A.; Chasteen, T.G.; Pichuantes, S.E.; Vásquez, C.C. Cysteine Metabolism-Related Genes and Bacterial Resistance to Potassium Tellurite. J. Bacteriol. 2007, 189, 8953–8960. [Google Scholar] [CrossRef][Green Version]
- Rojas, D.M.; Vásquez, C.C. Sensitivity to Potassium Tellurite of Escherichia coli Cells Deficient in CSD, CsdB and IscS Cysteine Desulfurases. Res. Microbiol. 2005, 156, 465–471. [Google Scholar] [CrossRef]
- Lithgow, J.K.; Hayhurst, E.J.; Cohen, G.; Aharonowitz, Y.; Foster, S.J. Role of a Cysteine Synthase in Staphylococcus aureus. J. Bacteriol. 2004, 186, 1579–1590. [Google Scholar] [CrossRef]
- Vernis, L.; el Banna, N.; Baïlle, D.; Hatem, E.; Heneman, A.; Huang, M. Fe-S Clusters Emerging as Targets of Therapeutic Drugs. Oxidative Med. Cell. Longev. 2017. [Google Scholar] [CrossRef]
- Ling, J.; Cho, C.; Guo, L.T.; Aerni, H.R.; Rinehart, J.; Söll, D. Protein Aggregation Caused by Aminoglycoside Action Is Prevented by a Hydrogen Peroxide Scavenger. Mol. Cell. 2012, 48, 713–722. [Google Scholar] [CrossRef]
- Wu, A.; Zhang, Y.; Zheng, C.; Dai, Y.; Liu, Y.; Zeng, J.; Gu, G.; Liu, J. Purification and Enzymatic Characteristics of Cysteine Desulfurase, IscS, in Acidithiobacillus ferrooxidans ATCC 23270. Trans. Nonferrous Met. Soc. China 2008, 18, 1450–1457. [Google Scholar] [CrossRef]
- Charan, M.; Singh, N.; Kumar, B.; Srivastava, K.; Siddiqi, I. Sulfur Mobilization for Fe-S Cluster Assembly by the Essential SUF Pathway in the Plasmodium falciparum Apicoplast and Its Inhibition. Antimicrob. Agents Chemother. 2014, 58, 3389–3398. [Google Scholar] [CrossRef]
- Li, Y.; Wang, F.; Wu, L.; Zhu, M.; He, G.; Chen, X.; Sun, F.; Liu, Q.; Wang, X.; Zhang, W. Cycloserine for Treatment of Multidrug-resistant Tuberculosis: A Retrospective Cohort Study in China. Infect. Drug Resist. 2019, 12, 721–731. [Google Scholar] [CrossRef]
- Evangelopoulos, D.; Prosser, G.A.; Rodgers, A.; Dagg, B.M.; Khatri, B.; Ho, M.M.; Gutierrez, M.G.; Cortes, T.; de Carvalho, L.P.S. Comparative Fitness Analysis of D-Cycloserine Resistant Mutants Reveals both Fitness-neutral and High-fitness Cost Genotypes. Nat. Commun. 2019, 10, 4177. [Google Scholar] [CrossRef]
- Pérard, J.; Ollagnier-de-Choudens, S. Iron–sulfur Clusters Biogenesis by the SUF Machinery: Close to the Molecular Mechanism Understanding. J. Biol. Inorg. Chem. 2018, 23, 581–596. [Google Scholar] [CrossRef] [PubMed]
- Choby, J.E.; Mike, L.A.; Mashruwala, A.A.; Dutter, B.F.; Dunman, P.M.; Sulikowski, G.A.; Boyd, J.M.; Skaar, E.P. A Small-Molecule Inhibitor of Iron-Sulfur Cluster Assembly Uncovers a Link between Virulence Regulation and Metabolism in Staphylococcus aureus. Cell Chem. Biol. 2017, 23, 1351–1361. [Google Scholar] [CrossRef]
- Mike, L.A.; Dutter, B.F.; Stauff, D.L.; Moore, J.L.; Vitko, N.P.; Aranmolate, O.; Kehl-Fie, T.E.; Sullivan, S.; Reid, P.R.; DuBois, J.L.; et al. Activation of Heme Biosynthesis by a Small Molecule that is Toxic to Fermenting Staphylococcus aureus. Proc. Natl. Acad. Sci. USA 2013. [Google Scholar] [CrossRef] [PubMed]
- Ezraty, B.; Barras, F. The ‘Liaisons Dangereuses’ Between Iron and Antibiotics. FEMS Microbiol. Rev. 2016, 40, 418–435. [Google Scholar] [CrossRef] [PubMed]

| Enzyme | Organism | Class | Identifier I a | Identifier II b | Identifier III c | |
|---|---|---|---|---|---|---|
| NifS | Azotobacter vinelandii | I | SSGSACTS | Insertion near conserved cysteine | Dimer | |
| IscS | Azotobacter vinelandii | I | SSGSACTS | Insertion near conserved cysteine | ||
| IscS | Helicobacter pylori | I | STGSACAS | Insertion near conserved cysteine | ||
| IscS | Escherichia coli | I | SSGSACTS | Insertion near conserved cysteine | ||
| SufS/CsdB | Escherichia coli | II | RTGHHCA | Insertion near conserved lysine | ||
| CsdA | Escherichia coli | II | RAGQHCA | Insertion near conserved lysine | ||
| SufS | Bacillus subtilis | II | RAGHHCA | Insertion near conserved lysine | ||
| SufS/CSD | Mycobacterium tuberculosis | II | RVGHHCA | Insertion near conserved lysine | ||
| IscS | Mycobacterium tuberculosis | I | STGSACTA | Insertion near conserved cysteine | ||
| CSD | Thermotoga maritima | II | RSGHHCA | Insertion near conserved lysine | ||
| C-DES * | Synechocystis PCC 6714 | Monomer |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Das, M.; Dewan, A.; Shee, S.; Singh, A. The Multifaceted Bacterial Cysteine Desulfurases: From Metabolism to Pathogenesis. Antioxidants 2021, 10, 997. https://doi.org/10.3390/antiox10070997
Das M, Dewan A, Shee S, Singh A. The Multifaceted Bacterial Cysteine Desulfurases: From Metabolism to Pathogenesis. Antioxidants. 2021; 10(7):997. https://doi.org/10.3390/antiox10070997
Chicago/Turabian StyleDas, Mayashree, Arshiya Dewan, Somnath Shee, and Amit Singh. 2021. "The Multifaceted Bacterial Cysteine Desulfurases: From Metabolism to Pathogenesis" Antioxidants 10, no. 7: 997. https://doi.org/10.3390/antiox10070997
APA StyleDas, M., Dewan, A., Shee, S., & Singh, A. (2021). The Multifaceted Bacterial Cysteine Desulfurases: From Metabolism to Pathogenesis. Antioxidants, 10(7), 997. https://doi.org/10.3390/antiox10070997







