Neuroprotective Effect of 4-Phenylbutyric Acid against Photo-Stress in the Retina
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Light-Induced Retinal Degeneration Model and Administration of 4-PBA
2.3. Electroretinogram (ERG)
2.4. Immunohistochemistry (IHC)
2.5. Terminal Deoxynucleotidyl Transferase 2′-Deoxyuridine, 5′-Triphosphate (dUTP) Nick End Labeling (TUNEL) Assay
2.6. Real-Time, Reverse-Transcription Polymerase Chain Reaction (RT-PCR)
2.7. Enzyme-Linked Immunosorbent Assay (ELISA)
2.8. Statistical Analysis
3. Results
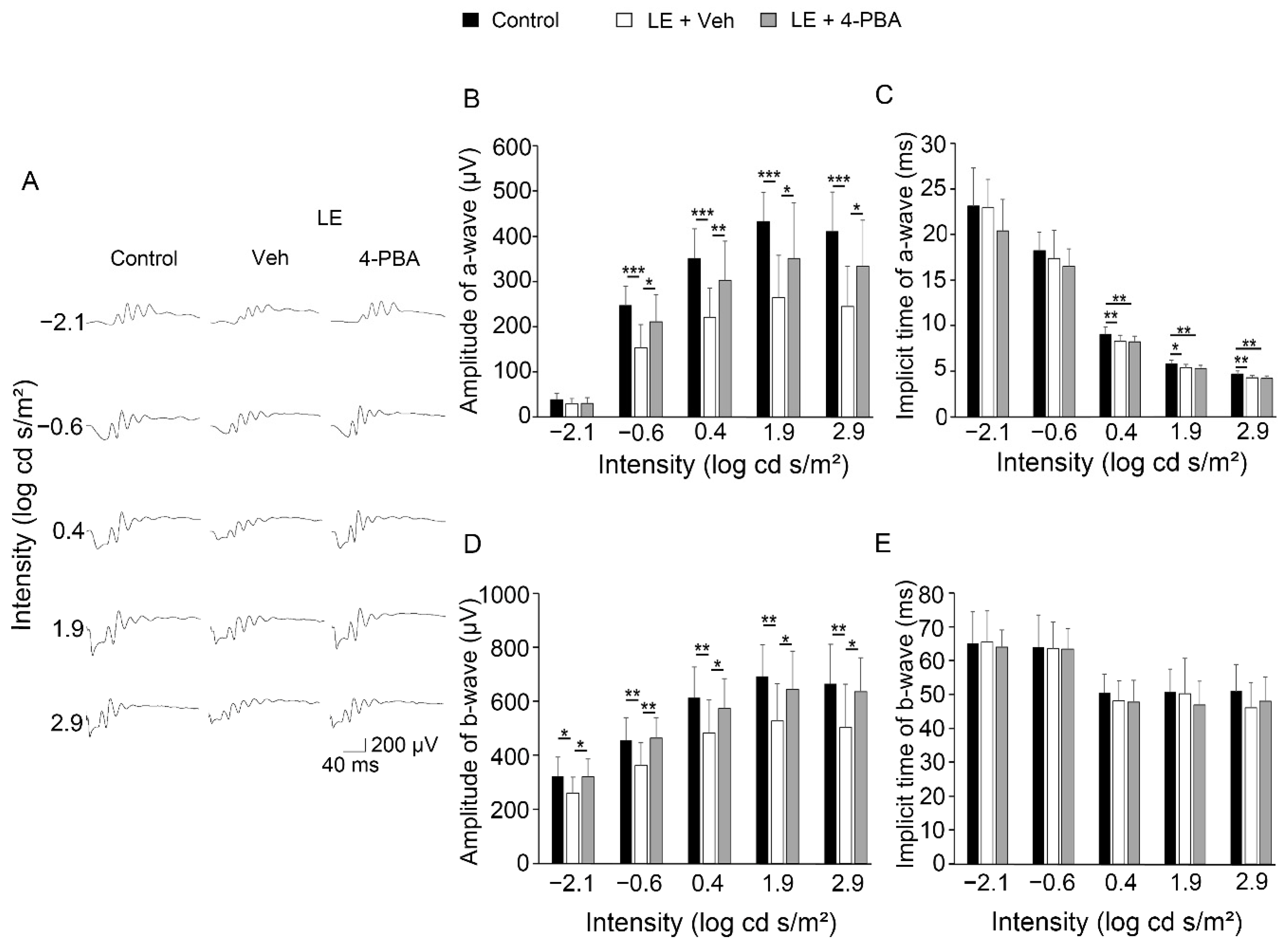
3.1. 4-PBA Protected Visual Function against Light Exposure
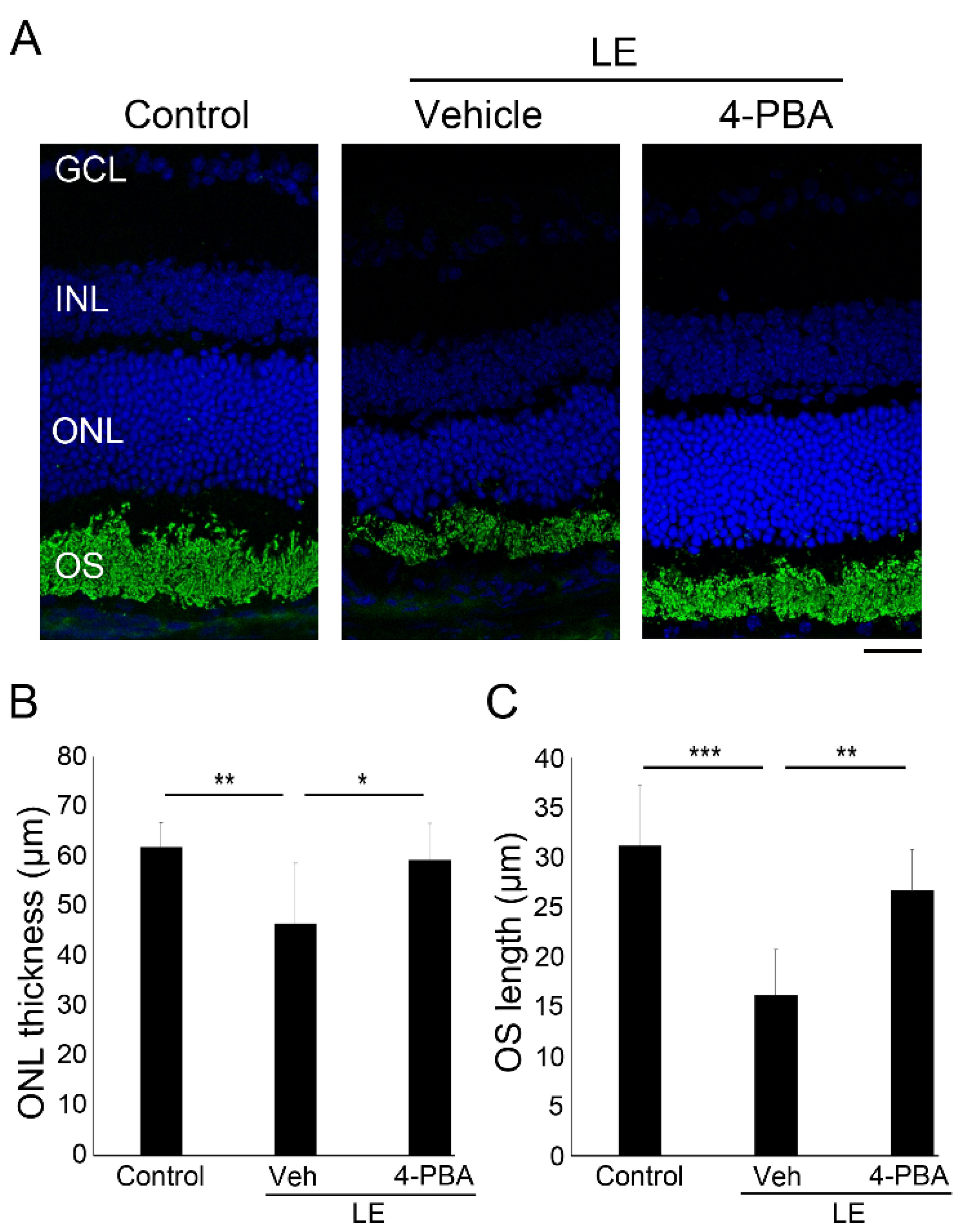
3.2. 4-PBA Suppressed Light-Induced Photoreceptor Histological Changes and Degeneration
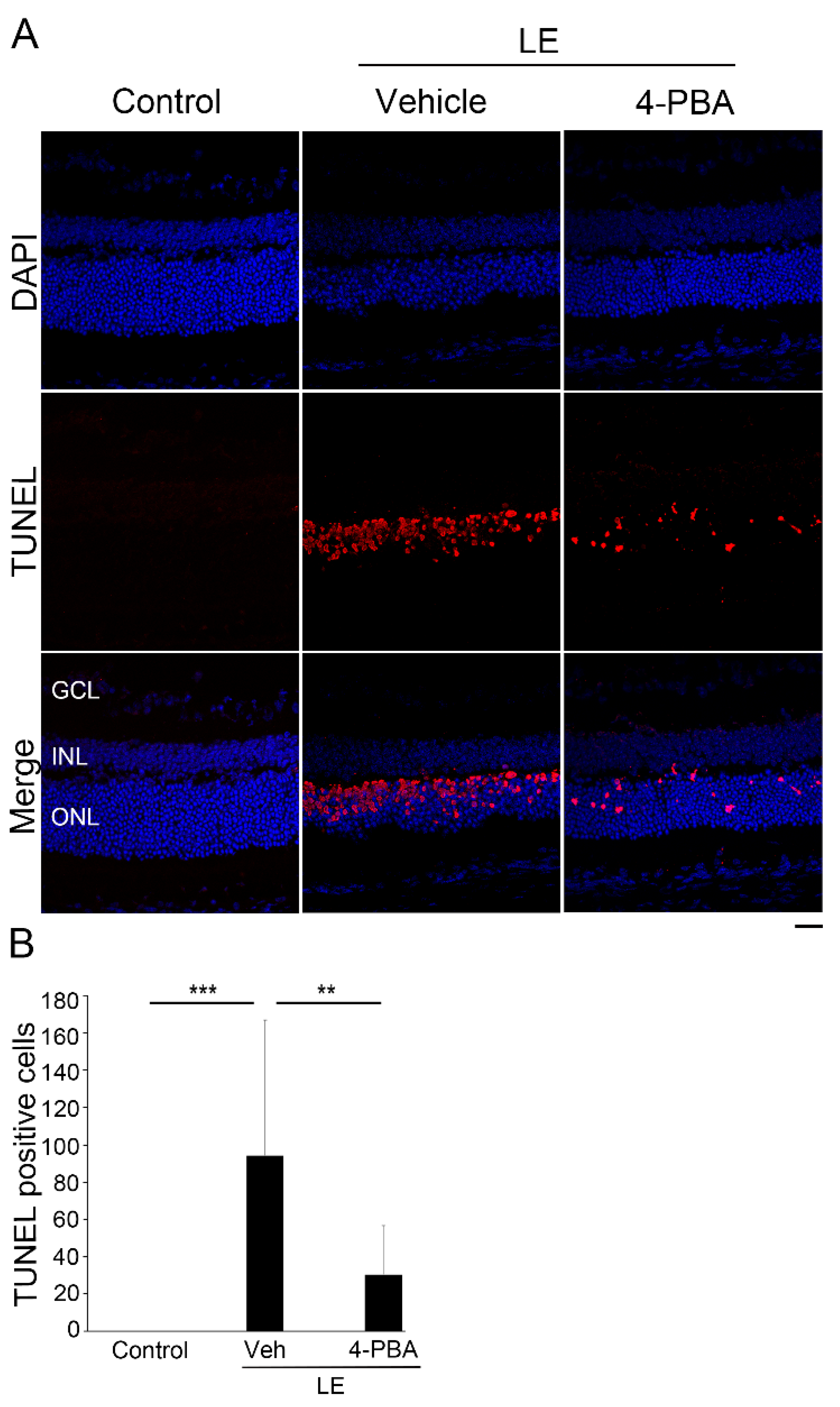
3.3. 4-PBA Attenuated Photoreceptor Death after Light Exposure
3.4. 4-PBA Suppressed ER and Oxidative Stress, and Apoptotic Markers
3.5. 4-PBA Attenuates Neuroinflammation
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kutsyr, O.; Sánchez-Sáez, X.; Gil, N.M.; De Juan, E.; Lax, P.; Maneu, V.; Cuenca, N. Gradual Increase in Environmental Light Intensity Induces Oxidative Stress and Inflammation and Accelerates Retinal Neurodegeneration. Investig. Opthalmology Vis. Sci. 2020, 61, 1. [Google Scholar] [CrossRef]
- Paskowitz, D.M.; LaVail, M.M.; Duncan, J.L. Light and inherited retinal degeneration. Br. J. Ophthalmol. 2006, 90, 1060–1066. [Google Scholar] [CrossRef] [Green Version]
- Orlans, H.O.; Merrill, J.; Barnard, A.R.; Issa, P.C.; Peirson, S.N.; MacLaren, R.E. Filtration of Short-Wavelength Light Provides Therapeutic Benefit in Retinitis Pigmentosa Caused by a Common Rhodopsin Mutation. Investig. Ophthalmol. Vis. Sci. 2019, 60, 2733–2742. [Google Scholar] [CrossRef]
- Otsuka, Y.; Oishi, A.; Miyata, M.; Uji, A.; Numa, S.; Tsujikawa, A. Investigation on light-induced retinal damage in retinitis pigmentosa. Investig. Ophthalmol. Vis. Sci. 2019, 60, 4518. [Google Scholar]
- Michalska-Malecka, K.; Kabiesz, A.; Nowak, M.; Śpiewak, D. Age related macular degeneration—challenge for future: Pathogenesis and new perspectives for the treatment. Eur. Geriatr. Med. 2015, 6, 69–75. [Google Scholar] [CrossRef] [Green Version]
- Bone, R.A.; Gibert, J.C.; Mukherjee, A. Light distributions on the retina: Relevance to macular pigment photoprotection. Acta Biochim. Pol. 2012, 59, 91–96. [Google Scholar] [CrossRef]
- Chalam, K.V.; Khetpal, V.; Rusovici, R.; Balaiya, S. A Review: Role of Ultraviolet Radiation in Age-Related Macular Degeneration. Eye Contact Lens: Sci. Clin. Pr. 2011, 37, 225–232. [Google Scholar] [CrossRef]
- Ozawa, Y. Oxidative stress in the light-exposed retina and its implication in age-related macular degeneration. Redox Biol. 2020, 37, 101779. [Google Scholar] [CrossRef]
- Garcia-Layana, A.; Cabrera-López, F.; García-Arumí, J.; Arias-Barquet, L.; Ruiz-Moreno, J.M. Early and intermediate age-related macular degeneration: Update and clinical review. Clin. Interv. Aging 2017, 12, 1579–1587. [Google Scholar] [CrossRef] [Green Version]
- Morizane, Y.; Morimoto, N.; Fujiwara, A.; Kawasaki, R.; Yamashita, H.; Ogura, Y.; Shiraga, F. Incidence and causes of visual impairment in Japan: The first nation-wide complete enumeration survey of newly certified visually impaired individuals. Jpn. J. Ophthalmol. 2019, 63, 26–33. [Google Scholar] [CrossRef]
- Wenzel, A.; Grimm, C.; Samardzija, M.; Remé, C.E. Molecular mechanisms of light-induced photoreceptor apoptosis and neuroprotection for retinal degeneration. Prog. Retin. Eye Res. 2005, 24, 275–306. [Google Scholar] [CrossRef]
- Osada, H.; Okamoto, T.; Kawashima, H.; Toda, E.; Miyake, S.; Nagai, N.; Ozawa, Y. Neuroprotective effect of bilberry extract in a murine model of photo-stressed retina. PLoS ONE 2017, 12, e0178627. [Google Scholar] [CrossRef]
- Nakanishi, T.; Shimazawa, M.; Sugitani, S.; Kudo, T.; Imai, S.; Inokuchi, Y.; Tsuruma, K.; Hara, H. Role of endoplasmic reticulum stress in light-induced photoreceptor degeneration in mice. J. Neurochem. 2013, 125, 111–124. [Google Scholar] [CrossRef]
- Yang, L.-P.; Wu, L.-M.; Guo, X.-J.; Li, Y.; Tso, M.O. Endoplasmic reticulum stress is activated in light-induced retinal degeneration. J. Neurosci. Res. 2008, 86, 910–919. [Google Scholar] [CrossRef]
- Sasaki, M.; Yuki, K.; Kurihara, T.; Miyake, S.; Noda, K.; Kobayashi, S.; Ishida, S.; Tsubota, K.; Ozawa, Y. Biological role of lutein in the light-induced retinal degeneration. J. Nutr. Biochem. 2012, 23, 423–429. [Google Scholar] [CrossRef]
- Ban, N.; Ozawa, Y.; Osada, H.; Lin, J.B.; Toda, E.; Watanabe, M.; Yuki, K.; Kubota, S.; Apte, R.S.; Tsubota, K. Neuroprotective role of retinal SIRT3 against acute photo-stress. npj Aging Mech. Dis. 2017, 3, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Oakes, S.A.; Papa, F.R. The Role of Endoplasmic Reticulum Stress in Human Pathology. Annu. Rev. Pathol. Mech. Dis. 2015, 10, 173–194. [Google Scholar] [CrossRef] [Green Version]
- Ghemrawi, R.; Khair, M. Endoplasmic Reticulum Stress and Unfolded Protein Response in Neurodegenerative Diseases. Int. J. Mol. Sci. 2020, 21, 6127. [Google Scholar] [CrossRef]
- Chan, P.; Stolz, J.; Kohl, S.; Chiang, W.-C.; Lin, J.H. Endoplasmic reticulum stress in human photoreceptor diseases. Brain Res. 2016, 1648, 538–541. [Google Scholar] [CrossRef] [Green Version]
- Hetz, C.; Saxena, S. ER stress and the unfolded protein response in neurodegeneration. Nat. Rev. Neurol. 2017, 13, 477–491. [Google Scholar] [CrossRef]
- Kaneko, M.; Imaizumi, K.; Saito, A.; Kanemoto, S.; Asada, R.; Matsuhisa, K.; Ohtake, Y. ER Stress and Disease: Toward Prevention and Treatment. Biol. Pharm. Bull. 2017, 40, 1337–1343. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, Y.; Jiang, M.; Chen, W.; Zhao, T.; Wei, Y. Cancer and ER stress: Mutual crosstalk between autophagy, oxidative stress and inflammatory response. Biomed. Pharm. 2019, 118, 109249. [Google Scholar] [CrossRef]
- Qi, Z.; Chen, L. Endoplasmic Reticulum Stress and Autophagy. Adv. Exp. Med. Biol. 2019, 1206, 167–177. [Google Scholar] [PubMed]
- Kolb, P.S.; Ayaub, E.A.; Zhou, W.; Yum, V.; Dickhout, J.G.; Ask, K. The therapeutic effects of 4-phenylbutyric acid in maintaining proteostasis. Int. J. Biochem. Cell Biol. 2015, 61, 45–52. [Google Scholar] [CrossRef]
- Basseri, S.; Lhoták, Š.; Sharma, A.M.; Austin, R.C. The chemical chaperone 4-phenylbutyrate inhibits adipogenesis by modulating the unfolded protein response. J. Lipid Res. 2009, 50, 2486–2501. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nissar, A.U.; Sharma, L.; Mudasir, M.A.; Nazir, L.A.; Umar, S.A.; Sharma, P.R.; Vishwakarma, R.A.; Tasduq, S.A. Chemical chaperone 4-phenyl butyric acid (4-PBA) reduces hepatocellular lipid accumulation and lipotoxicity through induction of autophagy. J. Lipid Res. 2017, 58, 1855–1868. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sharma, M.; Naura, A.S.; Singla, S.K. Modulatory effect of 4-phenyl butyric acid on hyperoxaluria-induced renal injury and inflammation. Mol. Cell Biochem. 2019, 451, 185–196. [Google Scholar] [CrossRef] [PubMed]
- Montane, J.; de Pablo, S.; Castaño, C.; Rodríguez-Comas, J.; Cadavez, L.; Obach, M.; Novials, A. Amyloid-induced β-cell dysfunction and islet inflammation are ameliorated by 4-phenylbutyrate (PBA) treatment. Faseb J. 2017, 31, 5296–5306. [Google Scholar] [CrossRef] [Green Version]
- Qiu, Y.; Yao, J.; Jia, L.; Thompson, D.; Zacks, D.N. Shifting the balance of autophagy and proteasome activation reduces proteotoxic cell death: A novel therapeutic approach for restoring photoreceptor homeostasis. Cell Death Dis. 2019, 10, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Kumar, V.; Mesentier-Louro, L.A.; Oh, A.J.; Heng, K.; Shariati, M.A.; Huang, H.; Hu, Y.; Liao, Y.J. Increased ER Stress After Experimental Ischemic Optic Neuropathy and Improved RGC and Oligodendrocyte Survival After Treatment With Chemical Chaperon. Investig. Opthalmology Vis. Sci. 2019, 60, 1953–1966. [Google Scholar] [CrossRef] [Green Version]
- Mesentier-Louro, L.A.; Shariati, M.A.; Dalal, R.; Camargo, A.; Kumar, V.; Shamskhou, E.A.; Perez, V.D.J.; Liao, Y.J. Systemic hypoxia led to little retinal neuronal loss and dramatic optic nerve glial response. Exp. Eye Res. 2020, 193, 107957. [Google Scholar] [CrossRef]
- Jeng, Y.Y.; Lin, N.T.; Chang, P.H.; Huang, Y.P.; Pang, V.F.; Liu, C.H.; Lin, C.T. Retinal ischemic injury rescued by sodium 4-phenylbutyrate in a rat model. Exp. Eye Res. 2007, 84, 486–492. [Google Scholar] [CrossRef]
- Adachi, T.; Yasuda, H.; Nakamura, S.; Kamiya, T.; Hara, H.; Hara, H.; Ikeda, T. Endoplasmic reticulum stress induces retinal endothelial permeability of extracellular-superoxide dismutase. Free. Radic. Res. 2011, 45, 1083–1092. [Google Scholar] [CrossRef]
- Li, S.; Samardzija, M.; Yang, Z.; Grimm, C.; Jin, M. Pharmacological Amelioration of Cone Survival and Vision in a Mouse Model for Leber Congenital Amaurosis. J. Neurosci. 2016, 36, 5808. [Google Scholar] [CrossRef] [Green Version]
- Kawashima, H.; Ozawa, Y.; Toda, E.; Homma, K.; Osada, H.; Narimatsu, T.; Tsubota, K. Neuroprotective and vision-protective effect of preserving ATP levels by AMPK activator. Faseb J. 2020, 34, 5016–5026. [Google Scholar] [CrossRef] [PubMed]
- Ozawa, Y.; Toda, E.; Kawashima, H.; Homma, K.; Osada, H.; Nagai, N.; Abe, Y.; Yasui, M.; Tsubota, K. Aquaporin 4 Suppresses Neural Hyperactivity and Synaptic Fatigue and Fine-Tunes Neurotransmission to Regulate Visual Function in the Mouse Retina. Mol. Neurobiol. 2019, 56, 8124–8135. [Google Scholar] [CrossRef] [Green Version]
- Osada, H.; Toda, E.; Homma, K.; Guzman, N.A.; Nagai, N.; Ogawa, M.; Negishi, K.; Arita, M.; Tsubota, K.; Ozawa, Y. ADIPOR1 deficiency-induced suppression of retinal ELOVL2 and docosahexaenoic acid levels during photoreceptor degeneration and visual loss. Cell Death Dis. 2021, 12, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Okamoto, T.; Kawashima, H.; Osada, H.; Toda, E.; Homma, K.; Nagai, N.; Ozawa, Y. Dietary Spirulina Supplementation Protects Visual Function From Photostress by Suppressing Retinal Neurodegeneration in Mice. Transl. Vis. Sci. Technol. 2019, 8, 20. [Google Scholar] [CrossRef] [Green Version]
- Narimatsu, T.; Ozawa, Y.; Miyake, S.; Kubota, S.; Yuki, K.; Nagai, N.; Tsubota, K.; Kubota, S. Biological effects of blocking blue and other visible light on the mouse retina. Clin. Exp. Ophthalmol. 2013, 42, 555–563. [Google Scholar] [CrossRef] [Green Version]
- Narimatsu, T.; Ozawa, Y.; Miyake, S.; Nagai, N.; Tsubota, K. Angiotensin II type 1 receptor blockade suppresses light-induced neural damage in the mouse retina. Free. Radic. Biol. Med. 2014, 71, 176–185. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wenzel, A.; Grimm, C.; Marti, A.; Kueng-Hitz, N.; Hafezi, F.; Niemeyer, G.; Reme, C.E. c-fos controls the “private pathway” of light-induced apoptosis of retinal photoreceptors. J. Neurosci. 2000, 20, 81–88. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ku, B.; Liang, C.; Jung, J.U.; Oh, B.-H. Evidence that inhibition of BAX activation by BCL-2 involves its tight and preferential interaction with the BH3 domain of BAX. Cell Res. 2010, 21, 627–641. [Google Scholar] [CrossRef] [PubMed]
- Clausen, B.H.; Lambertsen, K.L.; A Babcock, A.; Holm, T.H.; Dagnaes-Hansen, F.; Finsen, B. Interleukin-1beta and tumor necrosis factor-alpha are expressed by different subsets of microglia and macrophages after ischemic stroke in mice. J. Neuroinflamm. 2008, 5, 46. [Google Scholar] [CrossRef] [Green Version]
- Ben Haim, L.; Sauvage, M.-A.C.-D.; Ceyzériat, K.; Escartin, C. Elusive roles for reactive astrocytes in neurodegenerative diseases. Front. Cell. Neurosci. 2015, 9, 278. [Google Scholar] [CrossRef] [Green Version]
- Liddelow, S.A.; Guttenplan, K.A.; Clarke, L.E.; Bennett, F.C.; Bohlen, C.J.; Schirmer, L.; Bennett, M.L.; Münch, A.E.; Chung, W.-S.; Peterson, T.C.; et al. Neurotoxic reactive astrocytes are induced by activated microglia. Nature 2017, 541, 481–487. [Google Scholar] [CrossRef]
- De Raad, S.; Szczesny, P.J.; Munz, K.; Remé, C.E. Light damage in the rat retina: Glial fibrillary acidic protein accumulates in Müller cells in correlation with photoreceptor damage. Ophthalmic Res. 1996, 28, 99–107. [Google Scholar] [CrossRef] [PubMed]
- Lodish, H.F.; Kong, N.; Wikström, L. Calcium is required for folding of newly made subunits of the asialoglycoprotein receptor within the endoplasmic reticulum. J. Biol. Chem. 1992, 267, 12753–12760. [Google Scholar] [CrossRef]
- Yoshida, I.; Monji, A.; Tashiro, K.-I.; Nakamura, K.-I.; Inoue, R.; Kanba, S. Depletion of intracellular Ca2+ store itself may be a major factor in thapsigargin-induced ER stress and apoptosis in PC12 cells. Neurochem. Int. 2006, 48, 696–702. [Google Scholar] [CrossRef]
- Donovan, M.; Carmody, R.J.; Cotter, T. Light-induced Photoreceptor Apoptosis in Vivo Requires Neuronal Nitric-oxide Synthase and Guanylate Cyclase Activity and Is Caspase-3-independent. J. Biol. Chem. 2001, 276, 23000–23008. [Google Scholar] [CrossRef] [Green Version]
- Cao, S.S.; Kaufman, R.J. Endoplasmic Reticulum Stress and Oxidative Stress in Cell Fate Decision and Human Disease. Antioxid. Redox Signal. 2014, 21, 396–413. [Google Scholar] [CrossRef]
- Dandekar, A.; Mendez, R.; Zhang, K. Cross talk between ER Stress, oxidative stress, and inflammation in health and disease. In Stress Responses: Methods and Protocols; Oslowski, C.M., Ed.; Springer: New York, NY, USA, 2015; pp. 205–214. [Google Scholar]
- Zhang, Z.; Zhang, L.; Zhou, L.; Lei, Y.; Zhang, Y.; Huang, C. Redox signaling and unfolded protein response coordinate cell fate decisions under ER stress. Redox Biol. 2019, 25, 101047. [Google Scholar] [CrossRef]
- Seervi, M.; Sobhan, P.K.; Joseph, J.; Mathew, K.A.; Santhoshkumar, T.R. ERO1α-dependent endoplasmic reticulum-mitochondrial calcium flux contributes to ER stress and mitochondrial permeabilization by procaspase-activating compound-1 (PAC-1). Cell Death Dis. 2013, 4, e968. [Google Scholar] [CrossRef] [Green Version]
- Kutty, R.K.; Kutty, G.; Wiggert, B.; Chader, G.J.; Darrow, R.M.; Organisciak, D.T. Induction of heme oxygenase 1 in the retina by intense visible light: Suppression by the antioxidant dimethylthiourea. Proc. Natl. Acad. Sci. USA 1995, 92, 1177–1181. [Google Scholar] [CrossRef] [Green Version]
- Iurlaro, R.; Muñoz-Pinedo, C. Cell death induced by endoplasmic reticulum stress. Febs J. 2016, 283, 2640–2652. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chipuk, J.E.; Moldoveanu, T.; Llambi, F.; Parsons, M.J.; Green, D.R. The BCL-2 Family Reunion. Mol. Cell 2010, 37, 299–310. [Google Scholar] [CrossRef]
- Hardwick, J.M.; Soane, L. Multiple Functions of BCL-2 Family Proteins. Cold Spring Harb. Perspect. Biol. 2013, 5, a008722. [Google Scholar] [CrossRef] [Green Version]
- Cui, J.; Placzek, W.J. Post-Transcriptional Regulation of Anti-Apoptotic BCL2 Family Members. Int. J. Mol. Sci. 2018, 19, 308. [Google Scholar]
- Morón, E.B.; Abad-Jiménez, Z.; De Marañón, A.M.; Iannantuoni, F.; López, E.-; López-Domènech, S.; Salom, C.; Jover, A.; Mora, V.; Roldan, I.; et al. Relationship Between Oxidative Stress, ER Stress, and Inflammation in Type 2 Diabetes: The Battle Continues. J. Clin. Med. 2019, 8, 1385. [Google Scholar] [CrossRef] [PubMed] [Green Version]


| Gene | Forward Primer | Reverse Primer | GenBank Accession No. |
|---|---|---|---|
| Immunoglobulin heavy chain binding protein (BiP) | TGCAGCAGGACATCAAGTTC | TTTCTTCTGGGGCAAATGTC | NM_001163434 |
| C/EBP-Homologous Protein (CHOP) | CTGGAAGCCTGGTATGAGGA | GGACGCAGGGTCAAGAGTAG | NM_007837 |
| spliced- X-Box Binding Protein 1 (XBP1s) | CTGAGTCCGCAGCAGGTG | TGCCCAAAAGGATATCAGACT | NM_001271730 |
| Activating Transcription Factor 6 (ATF6) | GGACGAGGTGGTGTCAGAG | GACAGCTCTTCGCTTTGGAC | NM_001081304 |
| Activating Transcription Factor 4 (ATF4) | GAAACCTCATGGGTTCTCCA | TCCATTTTCTCCAACATCCA | NM_009716 |
| Nuclear factor erythroid 2–related factor 2 (Nrf2) | TGGCAGGAGCTATTTTCCATTC | TGCTGTCCATTTCTGTCAGTGTG | NM_010902 |
| Heme Oxygenase 1 (HO-1) | ACGCATATACCCGCTACCTG | CCAGAGTGTTCATTCGAGCA | NM_010442 |
| c-Fos | ATGGGCTCTCCTGTCAACAC | ACGGAGGAGACCAGAGTGG | NM_010234 |
| B-cell lymphoma 2 apoptosis regulator (Bcl-2)-associated death promoter (Bad) | GGAGCAACATTCATCAGCAG | GTACGAACTGTGGCGACTCC | NM_007522 |
| Caspase-1 | AGGAGGACATCCTTCATCCTC | CTTGAGGGTCCCAGTCAGTC | NM_009807 |
| Interleukin-1 beta (IL1-β) | AGCTCTCCACCTCAATGGAC | AGGCCACAGGTATTTTGTCG | NM_008361 |
| Interleukin-6 (IL-6) | GAGGATACCACTCCCAACAGACC | AAGTGCATCATCGTTGTTCATACA | NM_031168 |
| Tumor Necrosis Factor alpha (TNFα) | GCCACCACGCTCTTCTGTCTA | GATGAGAGGGAGGCCATTTG | NM_013693 |
| 18s | AGCATTTGCCAAGAATGTTTTCA | CCAGTCGGCATCGTTTATGG | NR_003278 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guzmán Mendoza, N.A.; Homma, K.; Osada, H.; Toda, E.; Ban, N.; Nagai, N.; Negishi, K.; Tsubota, K.; Ozawa, Y. Neuroprotective Effect of 4-Phenylbutyric Acid against Photo-Stress in the Retina. Antioxidants 2021, 10, 1147. https://doi.org/10.3390/antiox10071147
Guzmán Mendoza NA, Homma K, Osada H, Toda E, Ban N, Nagai N, Negishi K, Tsubota K, Ozawa Y. Neuroprotective Effect of 4-Phenylbutyric Acid against Photo-Stress in the Retina. Antioxidants. 2021; 10(7):1147. https://doi.org/10.3390/antiox10071147
Chicago/Turabian StyleGuzmán Mendoza, Naymel Alejandra, Kohei Homma, Hideto Osada, Eriko Toda, Norimitsu Ban, Norihiro Nagai, Kazuno Negishi, Kazuo Tsubota, and Yoko Ozawa. 2021. "Neuroprotective Effect of 4-Phenylbutyric Acid against Photo-Stress in the Retina" Antioxidants 10, no. 7: 1147. https://doi.org/10.3390/antiox10071147
APA StyleGuzmán Mendoza, N. A., Homma, K., Osada, H., Toda, E., Ban, N., Nagai, N., Negishi, K., Tsubota, K., & Ozawa, Y. (2021). Neuroprotective Effect of 4-Phenylbutyric Acid against Photo-Stress in the Retina. Antioxidants, 10(7), 1147. https://doi.org/10.3390/antiox10071147








