TCA Cycle and Fatty Acids Oxidation Reflect Early Cardiorenal Damage in Normoalbuminuric Subjects with Controlled Hypertension
Abstract
:1. Introduction
2. Methods
2.1. Patients Selection and Urine Samples Collection
2.2. Metabolic Analysis by Targeted Mass Spectrometry
2.3. Analysis of β-oxidation Targets: Urinary Free Fatty Acids, Liver Fatty Acid Binding Protein and Nephrin
2.4. Statistical Analysis
3. Results
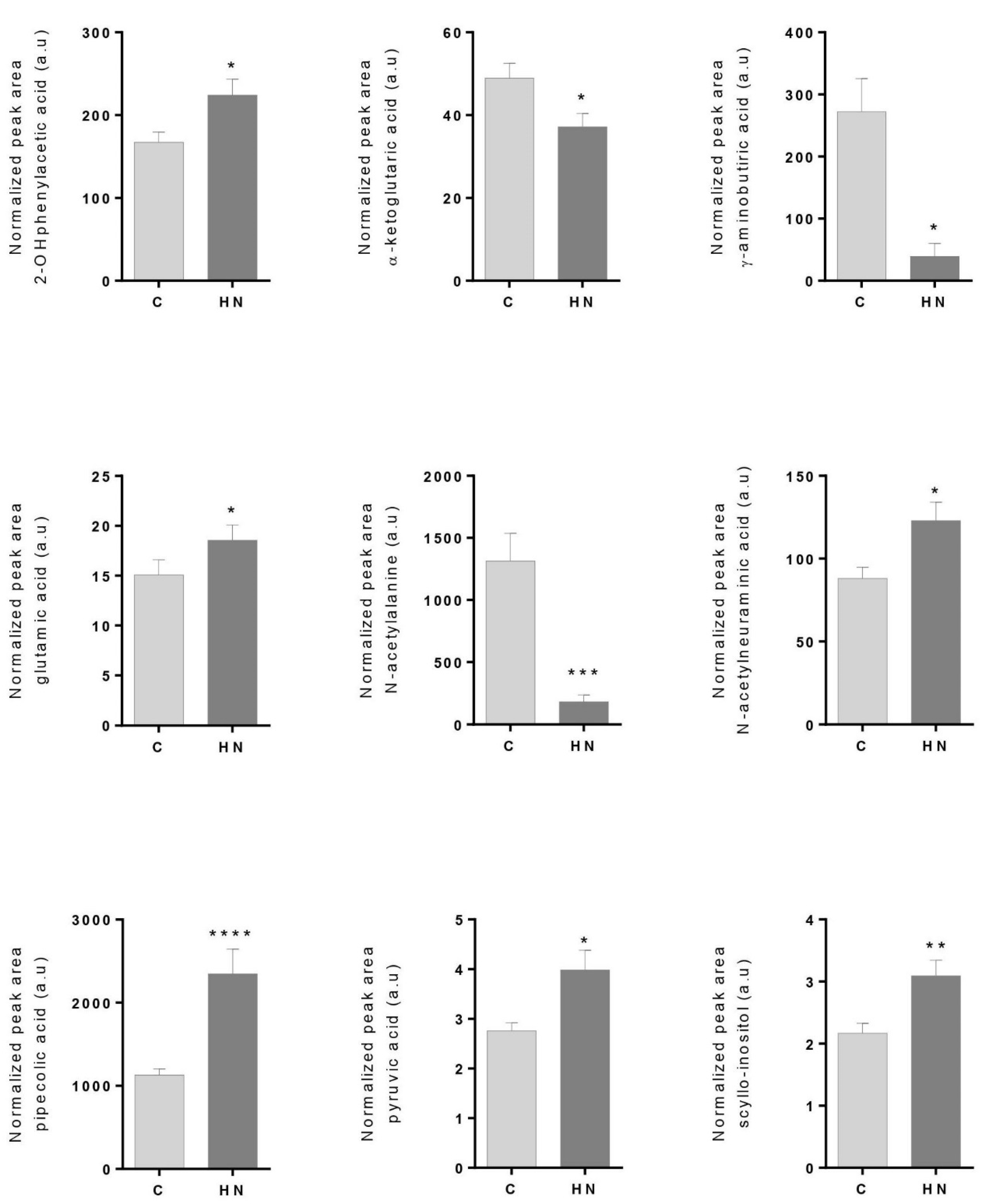
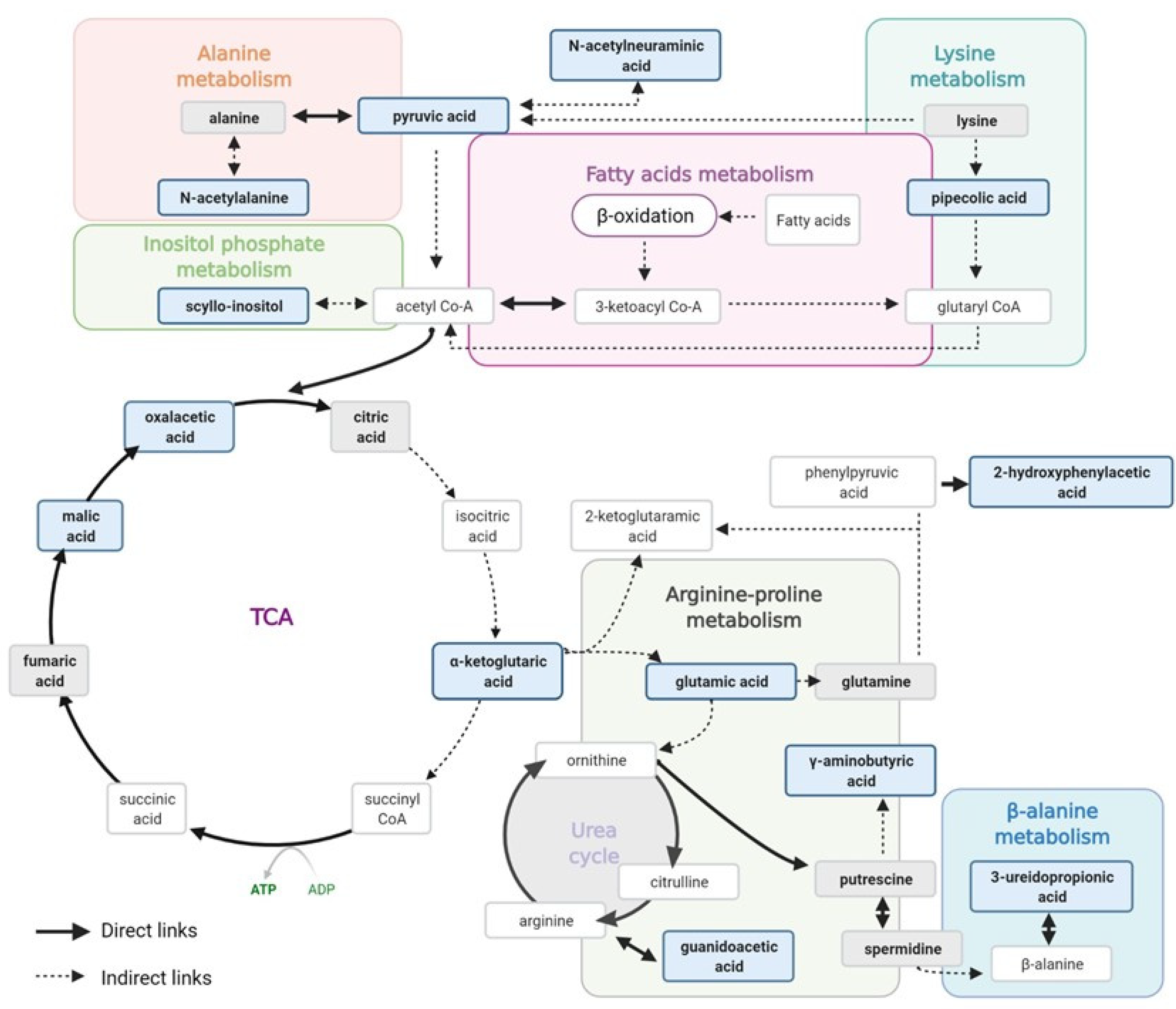
3.1. Cardiorenal Metabolites Show an Altered Profile in the High-Normal Range
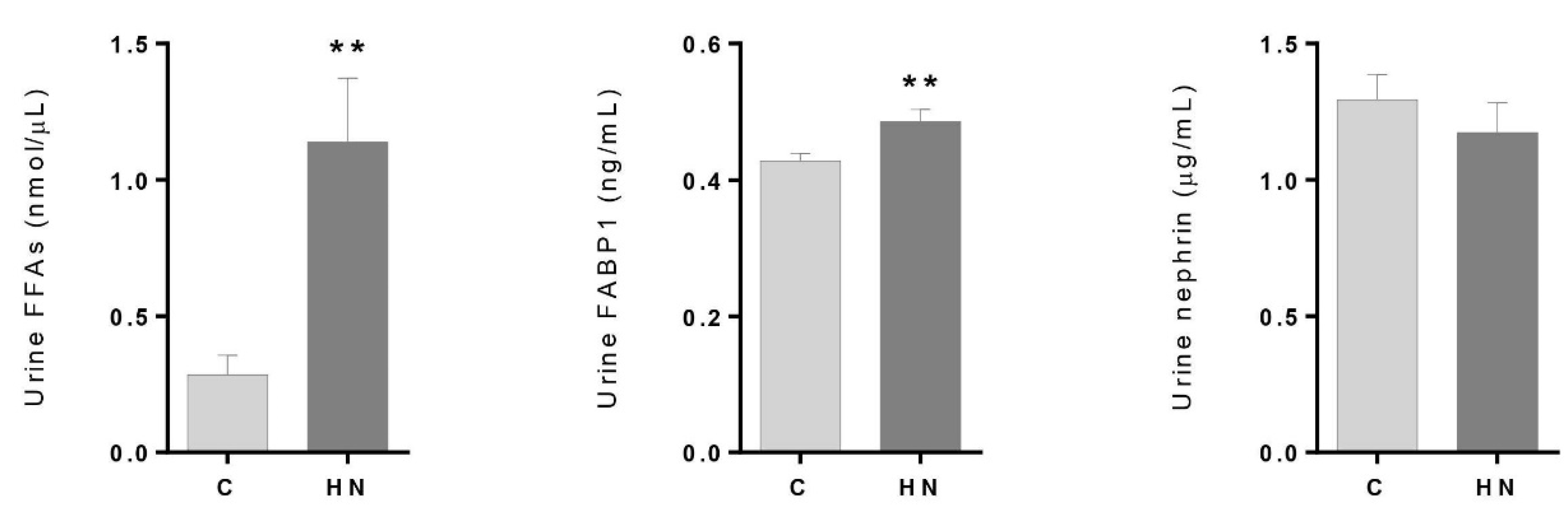
3.2. Renal Damage Evaluation: Altered Levels of FFAs and FABP1 in the High–Normal Range
4. Discussion
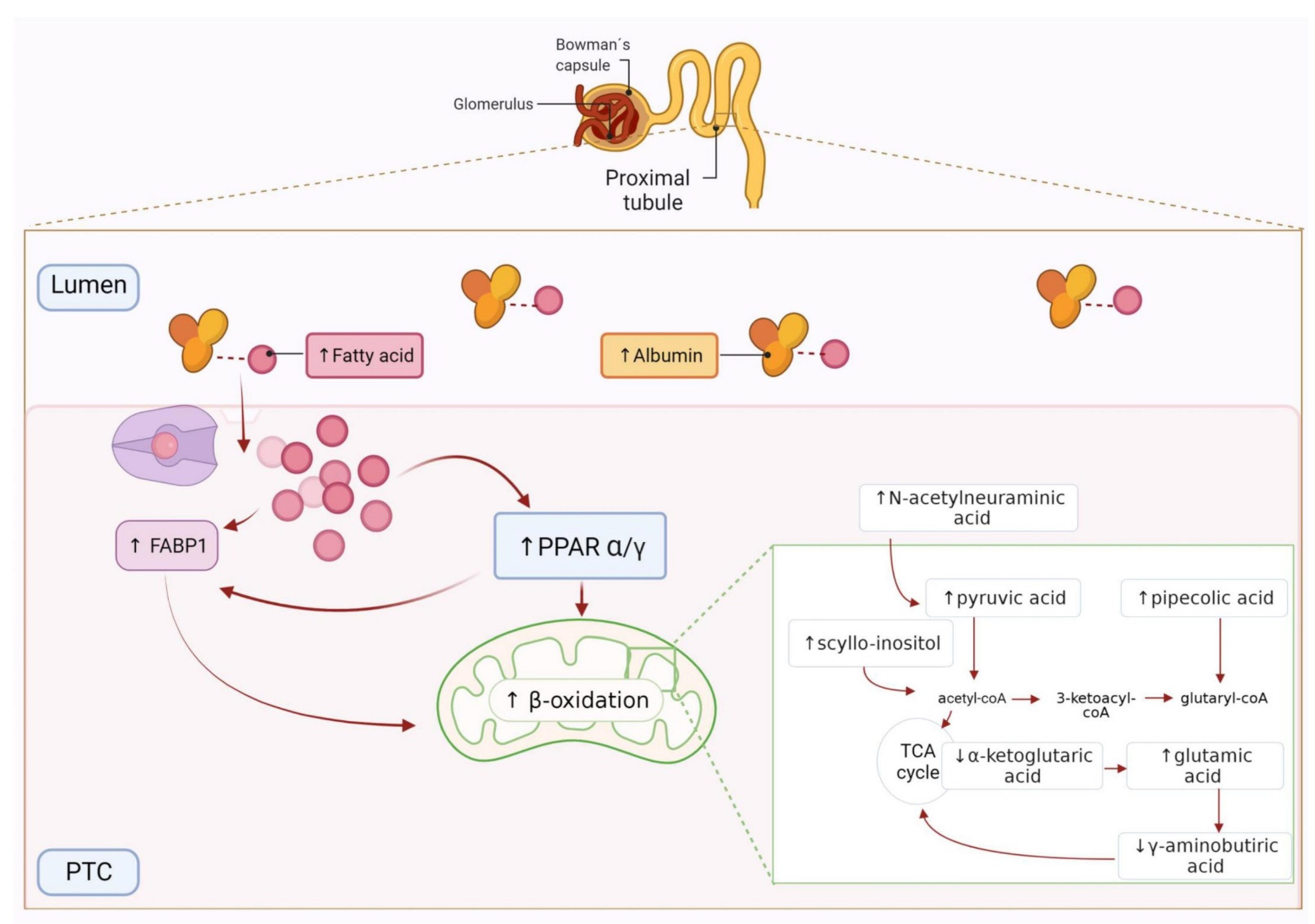
4.1. Free Fatty Acids Overload, β-oxidation and Tubular Injury in the High–Normal Range
4.2. TCA Cycle Alteration and Increased ROS in the HN Range
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kong, X.; Jia, X.; Wei, Y.; Cui, M.; Wang, Z.; Tang, L.; Li, W.; Zhu, Z.; Chen, P.; Xu, D. Association between microalbuminuria and subclinical atherosclerosis evaluated by carotid artery intima-media in elderly patients with normal renal function. BMC Nephrol. 2012, 13, 37. [Google Scholar] [CrossRef] [Green Version]
- Arnlov, J.; Evans, J.C.; Meigs, J.B.; Wang, T.J.; Fox, C.S.; Levy, D.; Benjamin, E.J.; D’Agostino, R.B.; Vasan, R.S. Low-grade albuminuria and incidence of cardiovascular disease events in nonhypertensive and nondiabetic individuals: The Framingham Heart Study. Circulation 2005, 112, 969–975. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sehestedt, T.; Jeppesen, J.; Hansen, T.W.; Wachtell, K.; Ibsen, H.; Torp-Pedersen, C.; Hildebrandt, P.; Olsen, M.H. Risk prediction is improved by adding markers of subclinical organ damage to SCORE. Eur. Heart J. 2010, 31, 883–891. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Volpe, M.; Battistoni, A.; Tocci, G.; Rosei, E.A.; Catapano, A.L.; Coppo, R.; del Prato, S.; Gentile, S.; Mannarino, E.; Novo, S.; et al. Cardiovascular risk assessment beyond Systemic Coronary Risk Estimation: A role for organ damage markers. J. Hypertens. 2012, 30, 1056–1064. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ruggenenti, P.; Porrini, E.; Motterlini, N.; Perna, A.; Ilieva, A.P.; Iliev, I.P.; Dodesini, A.R.; Trevisan, R.; Bossi, A.; Sampietro, G.; et al. Measurable urinary albumin predicts cardiovascular risk among normoalbuminuric patients with type 2 diabetes. J. Am. Soc. Nephrol. 2012, 23, 1717–1724. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blecker, S.; Matsushita, K.; Kottgen, A.; Loehr, L.R.; Bertoni, A.G.; Boulware, L.E.; Coresh, J. High-normal albuminuria and risk of heart failure in the community. Am. J. Kidney Dis. 2011, 58, 47–55. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Okubo, A.; Nakashima, A.; Doi, S.; Doi, T.; Ueno, T.; Maeda, K.; Tamura, R.; Yamane, K.; Masaki, T. High-normal albuminuria is strongly associated with incident chronic kidney disease in a nondiabetic population with normal range of albuminuria and normal kidney function. Clin. Exp. Nephrol. 2020, 24, 435–443. [Google Scholar] [CrossRef]
- Melsom, T.; Solbu, M.D.; Schei, J.; Stefansson, V.T.N.; Norvik, J.V.; Jenssen, T.G.; Wilsgaard, T.; Eriksen, B.O. Mild Albuminuria Is a Risk Factor for Faster GFR Decline in the Nondiabetic Population. Kidney Int. Rep. 2018, 3, 817–824. [Google Scholar] [CrossRef] [Green Version]
- Chen, C.; Wang, C.; Hu, C.; Han, Y.; Zhao, L.; Zhu, X.; Xiao, L.; Sun, L. Normoalbuminuric diabetic kidney disease. Front. Med. 2017, 11, 310–318. [Google Scholar] [CrossRef]
- Cerezo, C.; Ruilope, L.M.; Segura, J.; Garcia-Donaire, J.A.; de la Cruz, J.J.; Banegas, J.R.; Waeber, B.; Rabelink, T.J.; Messerli, F.H. Microalbuminuria breakthrough under chronic renin-angiotensin-aldosterone system suppression. J. Hypertens. 2012, 30, 204–209. [Google Scholar] [CrossRef]
- Gonzalez-Calero, L.; Martin-Lorenzo, M.; Martínez, P.J.; Baldan-Martin, M.; Ruiz-Hurtado, G.; Segura, J.; de la Cuesta, F.; Barderas, M.G.; Ruilope, L.M.; Vivanco, F.; et al. Hypertensive patients exhibit an altered metabolism. A specific metabolite signature in urine is able to predict albuminuria progression. Transl. Res. 2016, 178, 25–37. [Google Scholar] [CrossRef]
- Santiago-Hernandez, A.; Martinez, P.J.; Martin-Lorenzo, M.; Ruiz-Hurtado, G.; María, G.B.; Segura, J.; Ruilope, L.M.; Alvarez-Llamas, G. Differential metabolic profile associated with the condition of normoalbuminuria in the hypertensive population. Nefrologia (Engl. Ed.) 2020, 40, 440–445. [Google Scholar] [CrossRef] [PubMed]
- Haukka, J.K.; Sandholm, N.; Forsblom, C.; Cobb, J.E.; Groop, P.H.; Ferrannini, E. Metabolomic Profile Predicts Development of Microalbuminuria in Individuals with Type 1 Diabetes. Sci. Rep. 2018, 8, 13853. [Google Scholar] [CrossRef] [PubMed]
- Winther, S.A.; Ollgaard, J.C.; Hansen, T.W.; von Scholten, B.J.; Reinhard, H.; Ahluwalia, T.S.; Wang, Z.; Gaede, P.; Parving, H.H.; Hazen, S.; et al. Plasma trimethylamine N-oxide and its metabolic precursors and risk of mortality, cardiovascular and renal disease in individuals with type 2-diabetes and albuminuria. PLoS ONE 2021, 16, e0244402. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.J.; Liu, S.; Gurung, R.L.; Ching, J.; Kovalik, J.P.; Tan, T.Y.; Lim, S.C. Urine Tricarboxylic Acid Cycle Metabolites Predict Progressive Chronic Kidney Disease in Type 2 Diabetes. J. Clin. Endocrinol. Metab. 2018, 103, 4357–4364. [Google Scholar] [CrossRef] [PubMed]
- Martin-Lorenzo, M.; Martinez, P.J.; Baldan-Martin, M.; Ruiz-Hurtado, G.; Prado, J.C.; Segura, J.; de la Cuesta, F.; Barderas, M.G.; Vivanco, F.; Ruilope, L.M.; et al. Citric Acid Metabolism in Resistant Hypertension: Underlying Mechanisms and Metabolic Prediction of Treatment Response. Hypertension 2017, 70, 1049–1056. [Google Scholar] [CrossRef]
- Martinez, P.J.; Agudiez, M.; Molero, D.; Martin-Lorenzo, M.; Baldan-Martin, M.; Santiago-Hernandez, A.; Garcia-Segura, J.M.; Madruga, F.; Cabrera, M.; Calvo, E.; et al. Urinary metabolic signatures reflect cardiovascular risk in the young, middle-aged, and elderly populations. J. Mol. Med. (Berl.) 2020, 98, 1603–1613. [Google Scholar] [CrossRef] [PubMed]
- Martin-Lorenzo, M.; Zubiri, I.; Maroto, A.S.; Gonzalez-Calero, L.; Posada-Ayala, M.; de la Cuesta, F.; Mourino-Alvarez, L.; Lopez-Almodovar, L.F.; Calvo-Bonacho, E.; Ruilope, L.M.; et al. KLK1 and ZG16B proteins and arginine-proline metabolism identified as novel targets to monitor atherosclerosis, acute coronary syndrome and recovery. Metabolomics 2015, 11, 1056–1067. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martin-Lorenzo, M.; Gonzalez-Calero, L.; Maroto, A.S.; Martinez, P.J.; Zubiri, I.; de la Cuesta, F.; Mourino-Alvarez, L.; Barderas, M.G.; Heredero, A.; Aldamiz-Echevarría, G.; et al. Cytoskeleton deregulation and impairment in amino acids and energy metabolism in early atherosclerosis at aortic tissue with reflection in plasma. Biochim. Biophys. Acta 2016, 1862, 725–732. [Google Scholar] [CrossRef]
- Posada-Ayala, M.; Zubiri, I.; Martin-Lorenzo, M.; Sanz-Maroto, A.; Molero, D.; Gonzalez-Calero, L.; Fernandez-Fernandez, B.; de la Cuesta, F.; Laborde, C.M.; Barderas, M.G.; et al. Identification of a urine metabolomic signature in patients with advanced-stage chronic kidney disease. Kidney Int. 2014, 85, 103–111. [Google Scholar] [CrossRef] [Green Version]
- Chong, J.; Soufan, O.; Li, C.; Caraus, I.; Li, S.; Bourque, G.; Wishart, D.S.; Xia, J. MetaboAnalyst 4.0: Towards more transparent and integrative metabolomics analysis. Nucleic Acids Res. 2018, 46, W486–W494. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jang, H.S.; Noh, M.R.; Kim, J.; Padanilam, B.J. Defective Mitochondrial Fatty Acid Oxidation and Lipotoxicity in Kidney Diseases. Front. Med. (Lausanne) 2020, 7, 65. [Google Scholar] [CrossRef] [Green Version]
- Dickson, L.E.; Wagner, M.C.; Sandoval, R.M.; Molitoris, B.A. The proximal tubule and albuminuria: Really! J. Am. Soc. Nephrol. 2014, 25, 443–453. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Takaori, K.; Nakamura, J.; Yamamoto, S.; Nakata, H.; Sato, Y.; Takase, M.; Nameta, M.; Yamamoto, T.; Economides, A.N.; Kohno, K.; et al. Severity and Frequency of Proximal Tubule Injury Determines Renal Prognosis. J. Am. Soc. Nephrol. 2016, 27, 2393–2406. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Long, K.R.; Rbaibi, Y.; Gliozzi, M.L.; Ren, Q.; Weisz, O.A. Differential kidney proximal tubule cell responses to protein overload by albumin and its ligands. Am. J. Physiol. Renal Physiol. 2020, 318, F851–F859. [Google Scholar] [CrossRef]
- Kamijo, A.; Kimura, K.; Sugaya, T.; Yamanouchi, M.; Hase, H.; Kaneko, T.; Hirata, Y.; Goto, A.; Fujita, T.; Omata, M. Urinary free fatty acids bound to albumin aggravate tubulointerstitial damage. Kidney Int. 2002, 62, 1628–1637. [Google Scholar] [CrossRef] [Green Version]
- Moorhead, J.F.; Chan, M.K.; El-Nahas, M.; Varghese, Z. Lipid nephrotoxicity in chronic progressive glomerular and tubulo-interstitial disease. Lancet 1982, 2, 1309–1311. [Google Scholar] [CrossRef]
- Ge, M.; Fontanesi, F.; Merscher, S.; Fornoni, A. The Vicious Cycle of Renal Lipotoxicity and Mitochondrial Dysfunction. Front. Physiol. 2020, 11, 732. [Google Scholar] [CrossRef]
- Su, H.; Wan, C.; Lei, C.T.; Zhang, C.Y.; Ye, C.; Tang, H.; Qiu, Y.; Zhang, C. Lipid Deposition in Kidney Diseases: Interplay Among Redox, Lipid Mediators, and Renal Impairment. Antioxid. Redox Signal. 2018, 28, 1027–1043. [Google Scholar] [CrossRef]
- Bobulescu, I.A. Renal lipid metabolism and lipotoxicity. Curr. Opin. Nephrol. Hypertens. 2010, 19, 393–402. [Google Scholar] [CrossRef] [Green Version]
- Opazo-Rios, L.; Mas, S.; Marin-Royo, G.; Mezzano, S.; Gomez-Guerrero, C.; Moreno, J.A.; Egido, J. Lipotoxicity and Diabetic Nephropathy: Novel Mechanistic Insights and Therapeutic Opportunities. Int. J. Mol. Sci. 2020, 21, 2632. [Google Scholar] [CrossRef] [Green Version]
- Kamijo, A.; Sugaya, T.; Hikawa, A.; Okada, M.; Okumura, F.; Yamanouchi, M.; Honda, A.; Okabe, M.; Fujino, T.; Hirata, Y.; et al. Urinary excretion of fatty acid-binding protein reflects stress overload on the proximal tubules. Am. J. Pathol. 2004, 165, 1243–1255. [Google Scholar] [CrossRef] [Green Version]
- Sasaki, H.; Kamijo-Ikemori, A.; Sugaya, T.; Yamashita, K.; Yokoyama, T.; Koike, J.; Sato, T.; Yasuda, T.; Kimura, K. Urinary fatty acids and liver-type fatty acid binding protein in diabetic nephropathy. Nephron Clin. Pract. 2009, 112, c148–c156. [Google Scholar] [CrossRef]
- Kume, S.; Maegawa, H. Lipotoxicity, Nutrient-Sensing Signals, and Autophagy in Diabetic Nephropathy. JMA J. 2020, 3, 87–94. [Google Scholar] [CrossRef]
- Wang, G.; Bonkovsky, H.L.; de Lemos, A.; Burczynski, F.J. Recent insights into the biological functions of liver fatty acid binding protein 1. J. Lipid Res. 2015, 56, 2238–2247. [Google Scholar] [CrossRef] [Green Version]
- Afshinnia, F.; Nair, V.; Lin, J.; Rajendiran, T.M.; Soni, T.; Byun, J.; Sharma, K.; Fort, P.E.; Gardner, T.W.; Looker, H.C.; et al. Increased lipogenesis and impaired beta-oxidation predict type 2 diabetic kidney disease progression in American Indians. JCI Insight 2019, 4, e130317. [Google Scholar] [CrossRef] [PubMed]
- Matsui, K.; Kamijo-Ikemori, A.; Imai, N.; Sugaya, T.; Yasuda, T.; Tatsunami, S.; Toyama, T.; Shimizu, M.; Furuichi, K.; Wada, T.; et al. Clinical significance of urinary liver-type fatty acid-binding protein as a predictor of ESRD and CVD in patients with CKD. Clin. Exp. Nephrol. 2016, 20, 195–203. [Google Scholar] [CrossRef]
- Xu, Y.; Xie, Y.; Shao, X.; Ni, Z.; Mou, S. L-FABP: A novel biomarker of kidney disease. Clin. Chim. Acta 2015, 445, 85–90. [Google Scholar] [CrossRef]
- Arici, M.; Chana, R.; Lewington, A.; Brown, J.; Brunskill, N.J. Stimulation of proximal tubular cell apoptosis by albumin-bound fatty acids mediated by peroxisome proliferator activated receptor-gamma. J. Am. Soc. Nephrol. 2003, 14, 17–27. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Patterson, A.D.; Bonzo, J.A.; Li, F.; Krausz, K.W.; Eichler, G.S.; Aslam, S.; Tigno, X.; Weinstein, J.N.; Hansen, B.C.; Idle, J.R.; et al. Metabolomics reveals attenuation of the SLC6A20 kidney transporter in nonhuman primate and mouse models of type 2 diabetes mellitus. J. Biol. Chem. 2011, 286, 19511–19522. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ohta, T.; Masutomi, N.; Tsutsui, N.; Sakairi, T.; Mitchell, M.; Milburn, M.V.; Ryals, J.A.; Beebe, K.D.; Guo, L. Untargeted metabolomic profiling as an evaluative tool of fenofibrate-induced toxicology in Fischer 344 male rats. Toxicol. Pathol. 2009, 37, 521–535. [Google Scholar] [CrossRef] [PubMed]
- Hou, P.; Hu, S.; Wang, J.; Yang, Z.; Yin, J.; Zhou, G.; Guo, S. Exogenous supplement of N-acetylneuraminic acid improves macrophage reverse cholesterol transport in apolipoprotein E-deficient mice. Lipids Health Dis. 2019, 18, 24. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smith, S.A. Peroxisome proliferator-activated receptors and the regulation of mammalian lipid metabolism. Biochem. Soc. Trans. 2002, 30, 1086–1090. [Google Scholar] [CrossRef]
- Rosca, M.G.; Vazquez, E.J.; Chen, Q.; Kerner, J.; Kern, T.S.; Hoppel, C.L. Oxidation of fatty acids is the source of increased mitochondrial reactive oxygen species production in kidney cortical tubules in early diabetes. Diabetes 2012, 61, 2074–2083. [Google Scholar] [CrossRef] [Green Version]
- Liu, S.; He, L.; Yao, K. The Antioxidative Function of Alpha-Ketoglutarate and Its Applications. BioMed Res. Int. 2018, 2018, 3408467. [Google Scholar] [CrossRef]
- Del Puerto-Nevado, L.; Santiago-Hernandez, A.; Solanes-Casado, S.; Gonzalez, N.; Ricote, M.; Corton, M.; Prieto, I.; Mas, S.; Sanz, A.B.; Aguilera, O.; et al. Diabetes-mediated promotion of colon mucosa carcinogenesis is associated with mitochondrial dysfunction. Mol. Oncol. 2019, 13, 1887–1897. [Google Scholar] [CrossRef] [Green Version]
- Hallan, S.; Afkarian, M.; Zelnick, L.R.; Kestenbaum, B.; Sharma, S.; Saito, R.; Darshi, M.; Barding, G.; Raftery, D.; Ju, W.; et al. Metabolomics and Gene Expression Analysis Reveal Down-regulation of the Citric Acid (TCA) Cycle in Non-diabetic CKD Patients. EBioMedicine 2017, 26, 68–77. [Google Scholar] [CrossRef] [Green Version]
- Sen, S.; Roy, S.; Bandyopadhyay, G.; Scott, B.; Xiao, D.; Ramadoss, S.; Mahata, S.K.; Chaudhuri, G. gamma-Aminobutyric Acid Is Synthesized and Released by the Endothelium: Potential Implications. Circ. Res. 2016, 119, 621–634. [Google Scholar] [CrossRef] [PubMed]
| Characteristics | Control (C) (n = 21) | High–Normal (HN) (n = 16) | p-Value |
|---|---|---|---|
| Age (years) | 56 ± 9 | 64 ± 5 | 0.0053 |
| Sex (% male) | 62 | 76 | 0.4912 |
| BMI (Kg/m2) | 31 ± 5 | 30 ± 5 | 0.5274 |
| Cholesterol (mg/dL) | 181 ± 38 | 166 ± 29 | 0.2904 |
| Triglycerides (mg/dL) | 111 ± 34 | 125 ± 59 | 0.2494 |
| Cholesterol HDL (mg/dL) | 54 ± 16 | 53 ± 16 | 0.7232 |
| Cholesterol LDL (mg/dL) | 103 ± 31 | 88 ± 30 | 0.3452 |
| Glycemia (mg/dL) | 102 ± 10 | 107 ± 19 | 0.6657 |
| Uric acid (mg/dL) | 6 ± 1 | 6 ± 2 | 0.1923 |
| eGFR (mL/min/1.73 m2) | 86 ± 16 | 85 ± 20 | 0.9577 |
| ACR (mg/g) | 5 ± 2 | 22 ± 7 | <0.0001 |
| Diabetes mellitus type 2 (%) | 14 | 24 | 0.6745 |
| SBP (mmHg) | 140 ± 14 | 144 ± 14 | 0.4989 |
| DBP (mmHg) | 85 ± 8 | 83 ± 8 | 0.4707 |
| Antihypertensive Treatment (%) | |||
| iECAs | 19 | 18 | >0.9999 |
| ARA | 76 | 71 | 0.7165 |
| Diuretic | 48 | 53 | 0.7463 |
| Calcium channel blocker | 43 | 76 | 0.0453 |
| α-blocker | 19 | 0 | 0.1182 |
| β-blocker | 33 | 29 | 0.7228 |
| Other Treatments (%) | |||
| Anticoagulant | 5 | 6 | >0.9999 |
| Lipid lowering | 71 | 59 | 0.4891 |
| Antidiabetic | 10 | 12 | >0.9999 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Santiago-Hernandez, A.; Martin-Lorenzo, M.; Martin-Blazquez, A.; Ruiz-Hurtado, G.; Barderas, M.G.; Segura, J.; Ruilope, L.M.; Alvarez-Llamas, G. TCA Cycle and Fatty Acids Oxidation Reflect Early Cardiorenal Damage in Normoalbuminuric Subjects with Controlled Hypertension. Antioxidants 2021, 10, 1100. https://doi.org/10.3390/antiox10071100
Santiago-Hernandez A, Martin-Lorenzo M, Martin-Blazquez A, Ruiz-Hurtado G, Barderas MG, Segura J, Ruilope LM, Alvarez-Llamas G. TCA Cycle and Fatty Acids Oxidation Reflect Early Cardiorenal Damage in Normoalbuminuric Subjects with Controlled Hypertension. Antioxidants. 2021; 10(7):1100. https://doi.org/10.3390/antiox10071100
Chicago/Turabian StyleSantiago-Hernandez, Aranzazu, Marta Martin-Lorenzo, Ariadna Martin-Blazquez, Gema Ruiz-Hurtado, Maria G Barderas, Julian Segura, Luis M Ruilope, and Gloria Alvarez-Llamas. 2021. "TCA Cycle and Fatty Acids Oxidation Reflect Early Cardiorenal Damage in Normoalbuminuric Subjects with Controlled Hypertension" Antioxidants 10, no. 7: 1100. https://doi.org/10.3390/antiox10071100
APA StyleSantiago-Hernandez, A., Martin-Lorenzo, M., Martin-Blazquez, A., Ruiz-Hurtado, G., Barderas, M. G., Segura, J., Ruilope, L. M., & Alvarez-Llamas, G. (2021). TCA Cycle and Fatty Acids Oxidation Reflect Early Cardiorenal Damage in Normoalbuminuric Subjects with Controlled Hypertension. Antioxidants, 10(7), 1100. https://doi.org/10.3390/antiox10071100









