The Relationship between Myoglobin, Aerobic Capacity, Nitric Oxide Synthase Activity and Mitochondrial Function in Fish Hearts
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Animals
2.2. Mitochondrial Isolation
2.3. Mitochondrial Physiology
2.4. Nitric Oxide Synthase Activity
2.5. Citrate Synthase Activity
2.6. Myoglobin Content
2.7. Chemicals
2.8. Statistical Analyses
3. Results
3.1. Ventricular Characteristics
3.2. Mitochondrial Respiration and Coupling
3.3. Mitochondrial Reactive Oxygen Species (ROS) Production/Release Rate
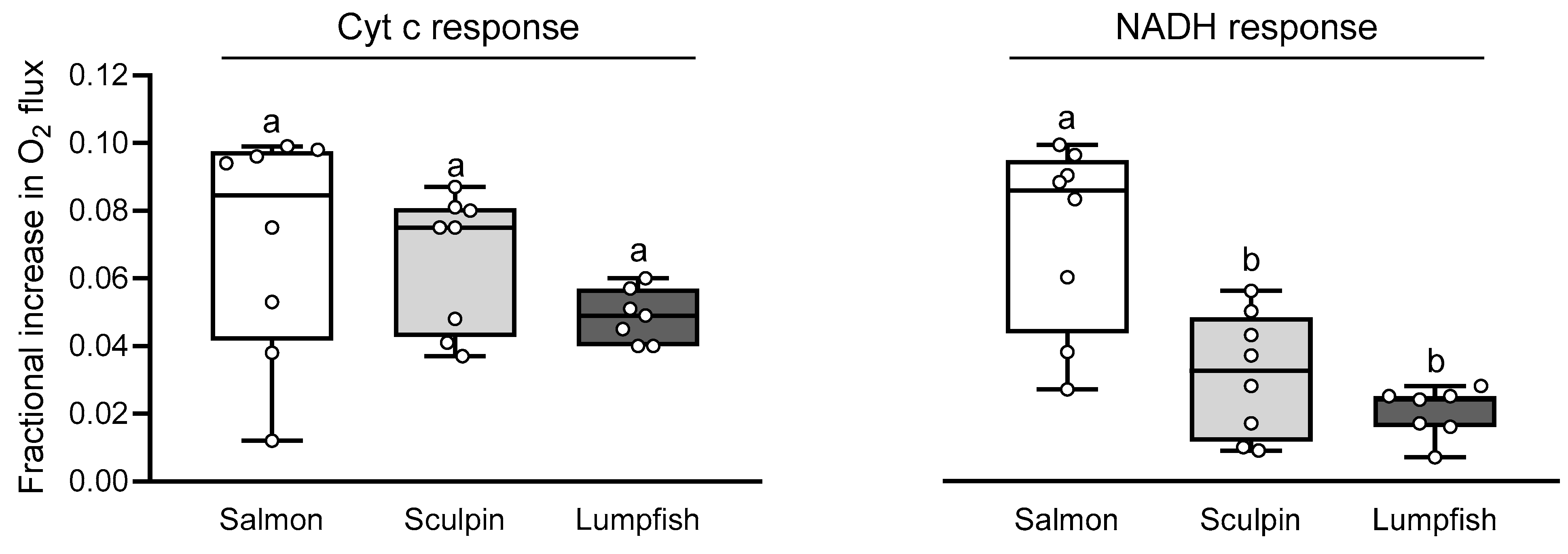
3.4. Mitochondrial Integrity and Permeability
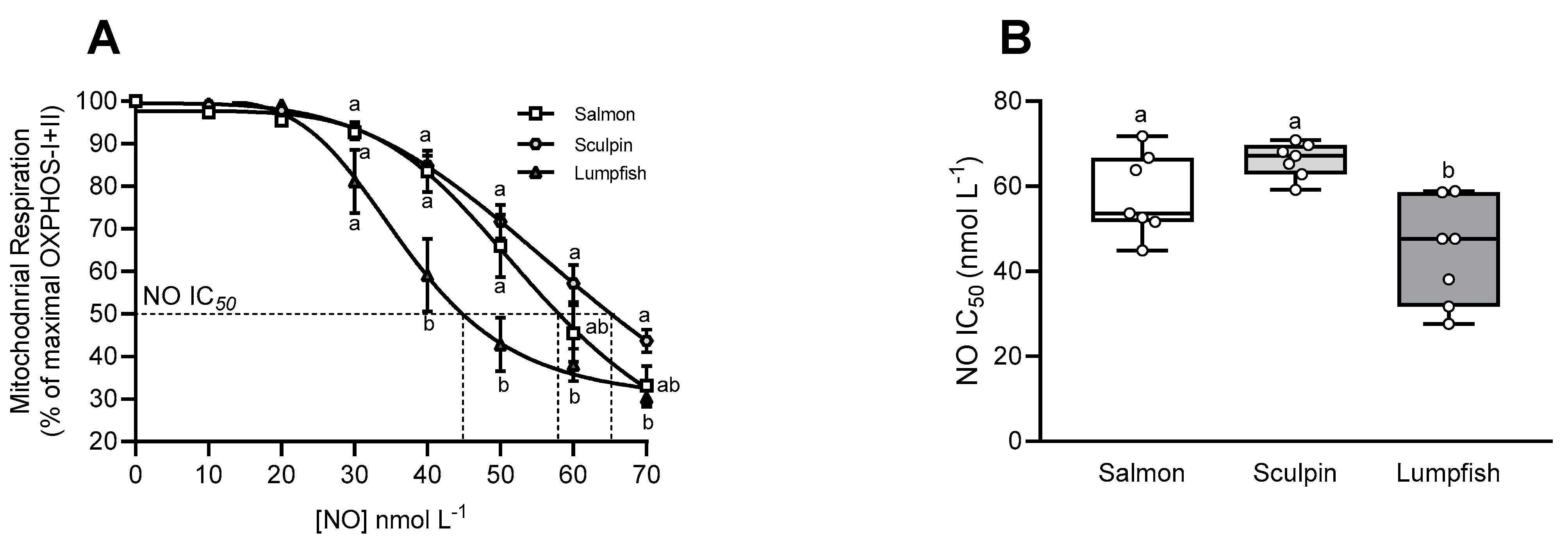
3.5. Mitochondrial Sensitivity to NO
3.6. Ventricular and Mitochondrial NOS Activity
3.7. Relationships between Mb Content, Aerobic Metabolism and the NOS/NO System
4. Discussion
4.1. Myoglobin Content
4.2. Relative Ventricular Mass
4.3. Citrate Synthase Activity
4.4. Relationship between Mb Content and Mitochondrial Function
4.5. Relationship between Myoglobin Content and the NOS/NO System
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Moncada, S.; Higgs, A. The L-arginine–Nitric oxide pathway. N. Engl. J. Med. 1993, 329, 2002–2012. [Google Scholar] [CrossRef]
- Park, J.W.; Piknova, B.; Dey, S.; Noguchi, C.T.; Schechter, A.N. Compensatory mechanisms in myoglobin deficient mice preserve NO homeostasis. Nitric Oxide 2019, 90, 10–14. [Google Scholar] [CrossRef] [PubMed]
- Brunori, M. Nitric oxide, cytochrome c oxidase and myoglobin: Competition and reaction pathways. Trends Biochem. Sci. 2001, 26, 21–23. [Google Scholar] [CrossRef]
- Flögel, U.; Fago, A.; Rassaf, T. Keeping the heart in balance: The functional interactions of myoglobin with nitrogen oxides. J. Exp. Biol. 2010, 213, 2726–2733. [Google Scholar] [CrossRef]
- Kamga, C.; Krishnamurthy, S.; Shiva, S. Myoglobin and mitochondria: A relationship bound by oxygen and nitric oxide. Nitric Oxide 2012, 26, 251–258. [Google Scholar] [CrossRef]
- Shiva, S.; Huang, Z.; Grubina, R.; Sun, J.; Ringwood, L.A.; MacArthur, P.H.; Xu, X.; Murphy, E.; Darley-Usmar, V.M.; Gladwin, M.T. Deoxymyoglobin is a nitrite reductase that generates nitric oxide and regulates mitochondrial respiration. Circ. Res. 2007, 100, 654–661. [Google Scholar] [CrossRef]
- Brown, G.C. Regulation of mitochondrial respiration by nitric oxide inhibition of cytochrome c oxidase. Biochim. Biophys. Acta 2001, 1504, 46–57. [Google Scholar] [CrossRef]
- Bundgaard, A.; James, A.M.; Joyce, W.; Murphy, M.P.; Fago, A. Suppression of reactive oxygen species generation in heart mitochondria from anoxic turtles: The role of complex I S-nitrosation. J. Exp. Biol. 2018, 221, jeb174391. [Google Scholar] [CrossRef] [PubMed]
- Gödecke, A. On the impact of NO-globin interactions in the cardiovascular system. Cardiovasc. Res. 2006, 69, 309–317. [Google Scholar] [CrossRef][Green Version]
- Massion, P.B.; Feron, O.; Dessy, C.; Balligand, J.L. Nitric oxide and cardiac function: Ten years after, and continuing. Circ. Res. 2003, 93, 388–398. [Google Scholar] [CrossRef]
- Hendgen-Cotta, U.B.; Kelm, M.; Rassaf, T. Myoglobin functions in the heart. Free Radic. Biol. Med. 2014, 73, 252–259. [Google Scholar] [CrossRef]
- Sidell, B.D.; Driedzic, W.R.; Stowe, D.B.; Johnston, I.A. Biochemical correlations of power development and metabolic fuel preferenda in fish hearts. Physiol. Zool. 1987, 60, 221–232. [Google Scholar] [CrossRef]
- Cerra, M.C.; Angelone, T.; Parisella, M.L.; Pellegrino, D.; Tota, B. Nitrite modulates contractility of teleost (Anguilla anguilla and Chionodraco hamatus, i.e. the Antarctic hemoglobinless icefish) and frog (Rana esculenta) hearts. Biochim. Biophys. Acta Bioenerg. 2009, 1787, 849–855. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Pedersen, C.L.; Faggiano, S.; Helbo, S.; Gesser, H.; Fago, A. Roles of nitric oxide, nitrite and myoglobin on myocardial efficiency in trout (Oncorhynchus mykiss) and goldfish (Carassius auratus): Implications for hypoxia tolerance. J. Exp. Biol. 2010, 213, 2755–2762. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Pellegrino, D.; Palmerini, C.A.; Tota, B. No hemoglobin but NO: The icefish (Chionodraco hamatus) heart as a paradigm. J. Exp. Biol. 2004, 207, 3855–3864. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Driedzic, W.R. The fish heart as a model system for the study of myoglobin. Comp. Biochem. Physiol. 1983, 76, 487–493. [Google Scholar] [CrossRef]
- Sidell, B.D.; O’Brien, K.M. When bad things happen to good fish: The loss of hemoglobin and myoglobin expression in Antarctic icefishes. J. Exp. Biol. 2006, 209, 1791–1802. [Google Scholar] [CrossRef]
- Macqueen, D.J.; De La Serrana, D.G.; Johnston, I.A. Cardiac myoglobin deficit has evolved repeatedly in teleost fishes. Biol. Lett. 2014, 10, 20140225. [Google Scholar] [CrossRef]
- O’Brien, K.M.; Sidell, B.D. The interplay among cardiac ultrastructure, metabolism and the expression of oxygen-binding proteins in Antarctic fishes. J. Exp. Biol. 2000, 203, 1287–1297. [Google Scholar] [CrossRef] [PubMed]
- Bailey, J.R.; Sephton, D.H.; Driedzic, W.R. Oxygen uptake by isolated perfused fish hearts with differing myoglobin concentrations under hypoxic conditions. J. Mol. Cell. Cardiol. 1990, 22, 1125–1134. [Google Scholar] [CrossRef]
- Driedzic, W.R.; Stewart, J.M. Myoglobin content and the activities of enzymes of energy metabolism in red and white fish hearts. J. Comp. Physiol. Part B 1982, 149, 67–73. [Google Scholar] [CrossRef]
- Legate, N.J.N.; Bailey, J.R.; Driedzic, W.R. Oxygen consumption in myoglobin-rich and myoglobin-poor isolated fish cardiomyocytes. J. Exp. Zool. 1998, 280, 269–276. [Google Scholar] [CrossRef]
- Beers, J.M.; Borley, K.A.; Sidell, B.D. Relationship among circulating hemoglobin, nitric oxide synthase activities and angiogenic poise in red- and white-blooded Antarctic notothenioid fishes. Comp. Biochem. Physiol. Part A 2010, 156, 422–429. [Google Scholar] [CrossRef]
- Garofalo, F.; Pellegrino, D.; Amelio, D.; Tota, B. The Antarctic hemoglobinless icefish, fifty five years later: A unique cardiocirculatory interplay of disaptation and phenotypic plasticity. Comp. Biochem. Physiol. Part A 2009, 154, 10–28. [Google Scholar] [CrossRef] [PubMed]
- Morlá, M.; Agusti, A.G.N.; Rahman, I.; Motterlini, R.; Saus, C.; Morales-Nin, B.; Company, J.B.; Busquets, X. Nitric oxide synthase type I (nNOS), vascular endothelial growth factor (VEGF) and myoglobin-like expression in skeletal muscle of Antarctic icefishes (Notothenioidei: Channichthyidae). Polar Biol. 2003, 26, 458–462. [Google Scholar] [CrossRef]
- Grove, T.J.; Sidell, B.D. Myoglobin deficiency in the hearts of phylogenetically diverse temperate-zone fish species. Can. J. Zool. 2002, 80, 893–901. [Google Scholar] [CrossRef]
- Turner, J.D.; Driedzic, W.R. Mechanical and metabolic response of the perfused isolated fish heart to anoxia and acidosis. Can. J. Zool. 1980, 58, 886–889. [Google Scholar] [CrossRef]
- Gerber, L.; Clow, K.A.; Katan, T.; Emam, M.; Leeuwis, R.H.J.; Parrish, C.C.; Gamperl, A.K. Cardiac mitochondrial function, nitric oxide sensitivity and lipid composition following hypoxia acclimation in sablefish. J. Exp. Biol. 2019. [Google Scholar] [CrossRef] [PubMed]
- Gerber, L.; Clow, K.A.; Gamperl, A.K. Acclimation to warm temperatures has important implications for mitochondrial function in Atlantic salmon (Salmo salar). J. Exp. Biol. 2021, 224, jeb.236257. [Google Scholar] [CrossRef]
- Fasching, M.; Gnaiger, E. O2k Quality Control 2: Instrumental oxygen background correction and accuracy of oxygen flux. Mitochondrial Physiol. Netw. 2018, 14, 1–14. [Google Scholar]
- Gnaiger, E. O2k Quality Control 1: Polarographic oxygen sensors and accuracy of calibration. Mitochondrial Physiol. Netw. 2018, 6, 1–20. [Google Scholar]
- Krumschnabel, G.; Fontana-Ayoub, M.; Fasching, M.; Gnaiger, E. O2k-FluoRespirometry: HRR and simultaneous determination of H2O2 production with Amplex UltraRed. Mitochondrial Physiol. Netw. 2019, 20, 1–6. [Google Scholar]
- Makrecka-Kuka, M.; Krumschnabel, G.; Gnaiger, E. High-resolution respirometry for simultaneous measurement of oxygen and hydrogen peroxide fluxes in permeabilized cells, tissue homogenate and isolated mitochondria. Biomolecules 2015, 5, 1319–1338. [Google Scholar] [CrossRef] [PubMed]
- Fasching, M.; Gnaiger, E. O2k-MultiSensor system with amperometric sensors: NO, H2S, H2O2. Mitochondrial Physiol. Netw. 2016, 15, 1–11. [Google Scholar]
- Hevel, J.M.; Marletta, M.A. Nitric oxide synthase assays. Methods Enzymol. 1994, 233, 250–258. [Google Scholar] [CrossRef]
- Gross, S.S. Microtiter plate assay for determining kinetics of nitric oxide synthesis. Methods Enzymol. 1996, 268, 159–168. [Google Scholar] [CrossRef] [PubMed]
- Murphy, M.E.; Noack, E. Nitric oxide assay using hemoglobin method. Methods Enzymol. 1994, 233, 240–250. [Google Scholar] [CrossRef]
- Antonini, E.; Brunori, M. Hemoglobin and Myoglobin in Their Reactions with Ligands; American Elsevier: New York, NY, USA, 1971; p. 436. [Google Scholar]
- Treberg, J.R.; MacCormack, T.J.; Lewis, J.M.; Almeida-Val, V.M.F.; Val, A.L.; Driedzic, W.R. Intracellular glucose and binding of hexokinase and phosphofructokinase to particulate fractions increase under hypoxia in heart of the Amazonian armored catfish (Liposarcus pardalis). Physiol. Biochem. Zool. 2007, 80, 542–550. [Google Scholar] [CrossRef] [PubMed]
- Sidell, B.D. Responses of goldfish (Carassius auratus, L.) muscle to acclimation temperature: Alterations in biochemistry and proportions of different fiber. Physiol. Zool. 1980, 53, 98–107. [Google Scholar] [CrossRef]
- Ewart, H.S.; Driedzic, W.R. Enzymes of energy metabolism in salmonid hearts: Spongy versus cortical myocardia. Can. J. Zool. 1987, 65, 623–627. [Google Scholar] [CrossRef]
- Jones, D.R.; Randall, D.J. The respiratory and circulatory systems during exercise. In Fish physiology; Hoar, W.S., Randall, D.J., Eds.; Academic Press: New York, NY, USA, 1978; pp. 425–501. [Google Scholar]
- Poupa, O.; Gesser, H.; Jonsson, S.; Sullivan, L. Coronary-supplied compct shell of ventricular myocardium in salmonids: Growth and enzyme pattern. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 1974, 48, 85–95. [Google Scholar] [CrossRef]
- Mandic, M.; Todgham, A.E.; Richards, J.G. Mechanisms and evolution of hypoxia tolerance in fish. Proc. R. Soc. B Biol. Sci. 2009, 276, 735–744. [Google Scholar] [CrossRef]
- Farrell, A.P.; Smith, F. Cardiac form, function and physiology. In The Cardiovascular System: Morphology, Control and Function; Gamperl, A.K., Gillis, T.E., Farrell, A.P., Brauner, C.J., Eds.; Elsevier Inc.: Cambridge, MA, USA, 2017; Volume 36, pp. 155–264. [Google Scholar]
- Santer, R.M. Morphology and innervation of the fish heart. In Advances in Anatomy Embryology and Cell Biology; Springer: Berlin, Germany, 1985; pp. 1–97. [Google Scholar]
- Farrell, A.P.; Altimiras, J.; Franklin, C.E.; Axelsson, M. Niche expansion of the shorthorn sculpin (Myoxocephalus scorpius) to arctic waters is supported by a thermal independence of cardiac performance at low temperature. Can. J. Zool. 2013, 91, 573–580. [Google Scholar] [CrossRef]
- Penney, C.M.; Nash, G.W.; Kurt Gamperl, A. Cardiorespiratory responses of seawater-acclimated adult Arctic char (Salvelinus alpinus) and Atlantic salmon (Salmo salar) to an acute temperature increase. Can. J. Fish. Aquat. Sci. 2014, 71, 1096–1105. [Google Scholar] [CrossRef]
- Hvas, M.; Folkedal, O.; Imsland, A.; Oppedal, F. Metabolic rates, swimming capabilities, thermal niche and stress response of the lumpfish, Cyclopterus lumpus. Biol. Open 2018, 7, 1–9. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, K.M.; Crockett, E.L.; Philip, J.; Oldham, C.A.; Hoffman, M.; Kuhn, D.E.; Barry, R.; McLaughlin, J. The loss of hemoglobin and myoglobin does not minimize oxidative stress in Antarctic icefishes. J. Exp. Biol. 2018, 221, jeb.162503. [Google Scholar] [CrossRef] [PubMed]
- Lau, G.Y.; Arndt, M.; Murphy, M.P.; Richards, J.G. Species and tissue specific differences in ROS metabolism to hypoxia- and hyperoxia-recovery exposure in marine sculpins. J. Exp. Biol. 2019, 222, jeb.206896. [Google Scholar] [CrossRef] [PubMed]
- Gerber, L.; Clow, K.A.; Mark, F.C.; Gamperl, A.K. Improved mitochondrial function in salmon (Salmo salar) following high temperature acclimation suggests that there are cracks in the proverbial “ceiling”. Sci. Rep. 2020, 10, 21636. [Google Scholar] [CrossRef]
- Flögel, U.; Gödecke, A.; Klotz, L.; Schrader, J. Role of myoglobin in the antioxidant defense of the heart. FASEB J. 2004, 18, 1156–1158. [Google Scholar] [CrossRef]
- Merx, M.W.; Gödecke, A.; Flögel, U.; Schrader, J. Oxygen supply and nitric oxide scavenging by myoglobin contribute to exercise endurance and cardiac function. FASEB J. 2005, 2, 1–15. [Google Scholar] [CrossRef]
- Gnaiger, E.; Lassnig, B.; Kusnetsov, A.; Rieger, G.; Margreiter, R. Mitochondrial oxygen affinity, respiratory flux control and excess capacity of cytochrome c oxidase. J. Exp. Biol. 1998, 201, 1129–1139. [Google Scholar] [CrossRef] [PubMed]
- Hinkle, P.C. P/O ratios of mitochondrial oxidative phosphorylation. Biochim. Biophys. Acta 2005, 1706, 1–11. [Google Scholar] [CrossRef]
- Wilson, D.F.; Owen, C.; Mela, L.; Weiner, L. Control of mitochondrial respiration by the phosphate potential. Biochem. Biophys. Res. Commun. 1973, 53, 326–333. [Google Scholar] [CrossRef]
- Yamada, T.; Takakura, H.; Jue, T.; Hashimoto, T.; Ishizawa, R.; Furuichi, Y.; Kato, Y.; Iwanaka, N.; Masuda, K. Myoglobin and the regulation of mitochondrial respiratory chain complex IV. J. Physiol. 2016, 594, 483–495. [Google Scholar] [CrossRef] [PubMed]
- Carnevale, C.; Roberts, J.C.; Syme, D.A.; Gamperl, A.K. Hypoxic acclimation negatively impacts the contractility of steelhead trout (Oncorhynchus mykiss) spongy myocardium. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2020, 318, R214–R226. [Google Scholar] [CrossRef]
- Kleinbongard, P.; Dejam, A.; Lauer, T.; Rassaf, T.; Schindler, A.; Picker, O.; Scheeren, T.; Gödecke, A.; Schrader, J.; Schulz, R.; et al. Plasma nitrite reflects constitutive nitric oxide synthase activity in mammals. Free Radic. Biol. Med. 2003, 35, 790–796. [Google Scholar] [CrossRef]
- Jensen, F.B. Nitrite disrupts multiple physiological functions in aquatic animals. Comp. Biochem. Physiol. A. Mol. Integr. Physiol. 2003, 135, 9–24. [Google Scholar] [CrossRef]
- Jensen, F.B.; Gerber, L.; Hansen, M.N.; Madsen, S.S. Metabolic fates and effects of nitrite in brown trout under normoxic and hypoxic conditions: Blood and tissue nitrite metabolism and interactions with branchial NOS, Na+/K+-ATPase and hsp70 expression. J. Exp. Biol. 2015, 218, 2015–2022. [Google Scholar] [CrossRef]
- Flögel, U.; Merx, M.W.; Gödecke, A.; Decking, U.K.M.; Schrader, J. Myoglobin: A scavenger of bioactive NO. Proc. Natl. Acad. Sci. USA 2001, 98, 735–740. [Google Scholar] [CrossRef]
- Grange, R.W.; Isotani, E.; Lau, K.S.; Kamm, K.E.; Huang, P.L.; Stull, J.T. Nitric oxide contributes to vascular smooth muscle relaxation in contracting fast-twitch muscles. Physiol. Genom. 2001, 5, 35–44. [Google Scholar] [CrossRef] [PubMed]
- Jørgensen, S.M.; Castro, V.; Krasnov, A.; Torgersen, J.; Timmerhaus, G.; Hevrøy, E.M.; Hansen, T.J.; Susort, S.; Breck, O.; Takle, H. Cardiac responses to elevated seawater temperature in Atlantic salmon. BMC Physiol. 2014, 14, 2. [Google Scholar] [CrossRef]
- Rochon, E.R.; Corti, P. Globins and nitric oxide homeostasis in fish embryonic development. Mar. Genom. 2020, 49, 100721. [Google Scholar] [CrossRef] [PubMed]
- Barnes, R.K.; King, H.; Carter, C.G. Hypoxia tolerance and oxygen regulation in Atlantic salmon, Salmo salar from a Tasmanian population. Aquaculture 2011, 318, 397–401. [Google Scholar] [CrossRef]
- Stevens, E.D.; Sutterlin, A.; Cook, T. Respiratory metabolism and swimming performance in growth hormone transgenic Atlantic salmon. Can. J. Fish. Aquat. Sci. 1998, 55, 2028–2035. [Google Scholar] [CrossRef]
- Remen, M.; Oppedal, F.; Imsland, A.K.; Olsen, R.E.; Torgersen, T. Hypoxia tolerance thresholds for post-smolt Atlantic salmon: Dependency of temperature and hypoxia acclimation. Aquaculture 2013, 416–417, 41–47. [Google Scholar] [CrossRef]
- Ern, R.; Norin, T.; Gamperl, A.K.; Esbaugh, A.J. Oxygen dependence of upper thermal limits in fishes. J. Exp. Biol. 2016, 219, 3376–3383. [Google Scholar] [CrossRef]
- Leeuwis, R.H.J.; Nash, G.W.; Sandrelli, R.M.; Zanuzzo, F.S.; Gamperl, A.K. The environmental tolerances and metabolic physiology of sablefish (Anoplopoma fimbria). Comp. Biochem. Physiol. Part A 2019, 231, 140–148. [Google Scholar] [CrossRef]
- Gattuso, A.; Garofalo, F.; Cerra, M.C.; Imbrogno, S. Hypoxia tolerance in teleosts: Implications of cardiac nitrosative signals. Front. Physiol. 2018, 9, 1–13. [Google Scholar] [CrossRef]
- Filice, M.; Mazza, R.; Leo, S.; Gattuso, A.; Cerra, M.C.; Imbrogno, S. The hypoxia tolerance of the goldfish (Carassius auratus) heart: The NOS/NO system and beyond. Antioxidants 2020, 9, 555. [Google Scholar] [CrossRef]
- Giulivi, C.; Jose, J.; Boveris, A. Production of nitric oxide by mitochondria. J. Biol. Chem. 1998, 273, 11038–11043. [Google Scholar] [CrossRef]
- Haynes, V.; Elfering, S.; Traaseth, N.; Giulivi, C. Mitochondrial nitric-oxide synthase: Enzyme expression, characterization, and regulation. J. Bioenerg. Biomembr. 2004, 36, 341–346. [Google Scholar] [CrossRef]
- Aguirre, E.; López-Bernardo, E.; Cadenas, S. Functional evidence for nitric oxide production by skeletal-muscle mitochondria from lipopolysaccharide-treated mice. Mitochondrion 2012, 12, 126–131. [Google Scholar] [CrossRef]
- Sakamuri, S.S.V.P.; Sperling, J.A.; Evans, W.R.; Dholakia, M.H.; Albuck, A.L.; Sure, V.N.; Satou, R.; Mostany, R.; Katakam, P.V.G. Nitric oxide synthase inhibitors negatively regulate respiration in isolated rodent cardiac and brain mitochondria. Am. J. Physiol. Heart Circ. Physiol. 2020, 318, H295–H300. [Google Scholar] [CrossRef]
- French, S.; Giulivi, C.; Balaban, R.S. Nitric oxide synthase in porcine heart mitochondria: Evidence for low physiological activity. Am. J. Physiol. Heart Circ. Physiol. 2001, 280, H2863–H2867. [Google Scholar] [CrossRef]



| S. salar | M. scorpius | C. lumpus | |
|---|---|---|---|
| Relative ventricular mass (%) | 0.075 ± 0.004 a | 0.149 ± 0.010 b | 0.079 ± 0.004 a |
| Myoglobin content (nmol g tissue−1) | 26.49 ± 4.6 a | 20.05 ± 2.1 a | ND b |
| Citrate synthase activity (µmol g tissue−1 min−1) | 6.48 ± 0.34 a | 3.81 ± 0.23 b | 5.08 ± 0.23 c |
| S. salar | M. scorpius | C. lumpus | |
|---|---|---|---|
| Ventricular NOS activity (nmol g tissue−1 min−1) | 3.30 ± 0.24 a | 3.90 ± 0.27 a | 2.17 ± 0.15 b |
| Mitochondrial NOS activity (nmol mg protein−1 min−1) | 0.15 ± 0.010 a | 0.10 ± 0.017 b | 0.04 ± 0.004 c |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gerber, L.; Clow, K.A.; Driedzic, W.R.; Gamperl, A.K. The Relationship between Myoglobin, Aerobic Capacity, Nitric Oxide Synthase Activity and Mitochondrial Function in Fish Hearts. Antioxidants 2021, 10, 1072. https://doi.org/10.3390/antiox10071072
Gerber L, Clow KA, Driedzic WR, Gamperl AK. The Relationship between Myoglobin, Aerobic Capacity, Nitric Oxide Synthase Activity and Mitochondrial Function in Fish Hearts. Antioxidants. 2021; 10(7):1072. https://doi.org/10.3390/antiox10071072
Chicago/Turabian StyleGerber, Lucie, Kathy A. Clow, William R. Driedzic, and Anthony K. Gamperl. 2021. "The Relationship between Myoglobin, Aerobic Capacity, Nitric Oxide Synthase Activity and Mitochondrial Function in Fish Hearts" Antioxidants 10, no. 7: 1072. https://doi.org/10.3390/antiox10071072
APA StyleGerber, L., Clow, K. A., Driedzic, W. R., & Gamperl, A. K. (2021). The Relationship between Myoglobin, Aerobic Capacity, Nitric Oxide Synthase Activity and Mitochondrial Function in Fish Hearts. Antioxidants, 10(7), 1072. https://doi.org/10.3390/antiox10071072







