Graphene Oxide Normal (GO + Mn2+) and Ultrapure: Short-Term Impact on Selected Antioxidant Stress Markers and Cytokines in NHDF and A549 Cell Lines
Abstract
1. Introduction
2. Materials and Methods
2.1. Graphene Oxide Synthesis
2.1.1. Graphene Oxide Manganese Ion Contaminated (GO + Mn2+, GOS)
2.1.2. Graphene Oxide Ultrapure (GO)
2.2. Cell Culture
2.3. GOS and GO Cytotoxic Activity
2.4. Oxidative Stress Markers Analysis
2.4.1. Oxidative Enzymes Analysis
2.4.2. Total Antioxidant Capacity (TAC)
2.4.3. Lipid Peroxidation
2.5. Analysis of Pro-Inflammatory Cytokines
2.6. Statistical Analysis
3. Results
3.1. Oxidative Stress Markers in Cells Exposed to Graphene Oxide Manganese Ion Contaminated (GO + Mn2+, GOS) and Graphene Oxide Ultrapure (GO) for 1, 24, and 48 h
3.1.1. NHDF Cell Line
3.1.2. A549 Cell Line
3.2. Pro-Inflammatory Cytokines in Cells Exposed to Graphene Oxide Manganese Ion Contaminated (GO + Mn2+, GOS) and Graphene Oxide Ultrapure (GO) for 1, 24, and 48 h
3.2.1. NHDF Cell Line
3.2.2. A549 Cell Line
4. Discussion
4.1. Oxidative Stress Markers’ Response to Graphene Oxide Exposure
4.2. Cytokines’ Response to Graphene Oxide Exposure
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- McCallion, C.; Burthem, J.; Rees-Unwin, K.; Golovanov, A.; Pluen, A. Graphene in therapeutics delivery: Problems, solutions and future opportunities. Eur. J. Pharm. Biopharm. 2016, 104, 235–250. [Google Scholar] [CrossRef]
- Xu, S.; Zhan, J.; Man, B.; Jiang, S.; Yue, W.; Gao, S.; Guo, C.; Liu, H.; Li, Z.; Wang, J.; et al. Real-time reliable determination of binding kinetics of DNA hybridization using a multi-channel graphene biosensor. Nat. Comm. 2017, 8, 14902. [Google Scholar] [CrossRef]
- Ge, Z.; Yang, L.; Xiao, F.; Wu, Y.; Yu, T.; Chen, J.; Lin, J.; Zhang, Y. Graphene Family Nanomaterials: Properties and Potential Applications in Dentistry. Int. J. Biomat. 2018, 2018, 1539678. [Google Scholar] [CrossRef] [PubMed]
- Yao, X.; Niu, X.; Ma, K.; Hunag, P.; Grothe, J.; Kaskel, S.; Zhu, Y. Graphene quantum dots-capped magnetic mesoporous silica nanoparticles as a multifunctional platform for controlled drug delivery, magnetic hyperthermia, and photothermal therapy. Small 2017, 13, 1602225. [Google Scholar] [CrossRef]
- Chang, Y.; Yang, S.-T.; Liu, J.H.; Dong, E.; Wang, Y.; Cao, A.; Liu, Y.; Wang, H. In vitro toxicity evaluation of graphene oxide on A549 cells. Toxicol. Lett. 2011, 200, 201–210. [Google Scholar] [CrossRef]
- Gurunathan, S.; Iqbal, M.A.; Qasim, M.; Park, C.H.; Yoo, H.; Hwang, J.H.; Uhm, S.J.; Song, H.; Park, C.; Do, J.T.; et al. Evaluation of graphene oxide induced cellular toxicity and transcriptome analysis in human embryonic kidney cells. Nanomaterials 2019, 9, 969. [Google Scholar] [CrossRef] [PubMed]
- Seabra, A.B.; Paula, A.J.; de Lima, R.; Alves, O.L.; Duran, N. Nanotoxicity of graphene and graphene oxide. Chem. Res. Toxicol. 2014, 27, 159–168. [Google Scholar] [CrossRef]
- Wang, Y.; Wu, S.; Zhao, X.; Su, Z.; Du, L.; Sui, A. In vitro toxicity evaluation of graphene oxide on human RPMI 8226 cells. Biomed. Mat. Eng. 2014, 24, 2007–2013. [Google Scholar] [CrossRef] [PubMed]
- Jarosz, A.; Skoda, M.; Dudek, I.; Szukiewicz, D. Oxidative stress and mitochondrial activation as the main mechanisms underlying graphene toxicity against human cancer cells. Oxid. Med. Cell. Longev. 2016, 2016, 5851035. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Jia, Z.; Trush, M.A. Defining ROS in biology and medicine. Reac. Oxyg. Species (Apex) 2016, 1, 9–21. [Google Scholar] [CrossRef] [PubMed]
- Montezano, C.; Touyz, R.M. Reactive oxygen species and endothelial function-role of nitric oxide synthase uncoupling and Nox family nicotinamide adenine dinucleotide phosphate oxidases. Basic Clin. Pharmacol. Toxicol. 2012, 110, 87–94. [Google Scholar] [CrossRef]
- Malarz, K.; Mrozek-Wilczkiewicz, A.; Serda, M.; Rejmund, M.; Polanski, J.; Musiol, R. The role of oxidative stress in activity of anticancer thiosemicarbazones. Oncotarget 2018, 9, 17689–17710. [Google Scholar] [CrossRef] [PubMed]
- Skrzep-Poloczek, B.; Poloczek, J.; Chełmecka, E.; Dulska, A.; Romuk, E.; Idzik, M.; Kazura, W.; Nabrdalik, K.; Gumprecht, J.; Jochem, J.; et al. The oxidative stress markers in the erythrocytes and heart muscle of obese rats relate to a high-fat diet but not to DJOS bariatric surgery. Antioxidants 2020, 9, 183. [Google Scholar] [CrossRef]
- Ghiselli, A.; Serafini, M.; Natella, F.; Scaccini, C. Total antioxidant capacity as a tool to assess redox status: Critical view and experimental data. Free Radic. Biol. Med. 2000, 29, 1106–1114. [Google Scholar] [CrossRef]
- Ciriza, J.; del Burgo, L.S.; Gurruchaga, H.; Borras, F.E.; Franquesa, M.; Orive, G.; Hernandez, R.M.; Pedraz, J.L. Graphene oxide enhances alginate encapsulated cells viability and functionality while not affecting the foreign body response. Drug Deliv. 2018, 25, 1147–1160. [Google Scholar] [CrossRef]
- Feito, M.J.; Vila, M.; Matesanz, M.C.; Linares, J.; Goncalves, G.; Marques, P.A.A.P.; Vallet-Regi, M.; Rojo, J.M.; Potoles, M.T. In vitro evaluation of graphene oxide nanosheets on immune fuction. J. Colloid Interface Sci. 2014, 432, 221–228. [Google Scholar] [CrossRef] [PubMed]
- Lim, M.-H.; Jeung, I.C.; Jeong, J.; Yoon, S.-J.; Lee, S.-H.; Park, J.; Kang, Y.-S.; Lee, H.; Park, Y.-J.; Lee, H.G.; et al. Graphene oxide induces apoptotic cell death in endothelial cells by activating autophagy via calcium-dependent phosphorylation of c-Jun-N-terminal kinases. Acta Biomater. 2016, 46, 191–203. [Google Scholar] [CrossRef] [PubMed]
- Marković, Z.; Jovanović, S.P.; Masković, P.Z.; Mojsin, M.M.; Stevanović, M.J.; Danko, M.; Micusik, M.; Jovanović, D.J.; Kleinova, A.; Spitalsky, Z.; et al. Graphene oxide siza and structure pro-oxidant and antioxidant activity and photoinduced cytotoxicity on three cancer cell lines. J. Photochem. Photobiol. B Biol. 2019, 200, 111647. [Google Scholar] [CrossRef]
- Halim, A.; Liu, L.; Ariyanti, A.D.; Ju, Y.; Luo, Q.; Song, G. Low-dose suspended graphene oxide nanosheets induce antioxidant response and osteogenic differentiation of bone marrow-derived mesenchymal stem cells via JNK-dependent FoxO1 activation. J. Mater. Chem. B 2019, 7, 5998–6009. [Google Scholar] [CrossRef]
- Zhao, G.; Li, J.; Ren, X.; Chen, C.; Wang, X. Few-layered graphene oxide nanosheets as superior sorbents for heavy metal ion pollution management. Environ. Sci. Technol. 2011, 45, 10454–10462. [Google Scholar] [CrossRef]
- Stengl, V. Preparation of graphene by using an intense cavitation field in a pressurized ultrasonic reactor. Chemistry 2012, 18, 14047–14054. [Google Scholar] [CrossRef]
- Ederer, J.; Janoš, P.; Ecorchard, P.; Tolasz, J.; Štengl, V.; Beneš, H.; Perchacz, M.; Pop-Georgievski, O. Determination of amino groups on functionalized graphene oxide for polyurethane nanomaterials: XPS quantitation vs. functional speciation. RSC Adv. 2017, 7, 12464–12473. [Google Scholar] [CrossRef]
- Ederer, J.; Janoš, P.; Ecorchard, P.; Štengl, V.; Bělčická, Z.; Šťastný, M.; Pop-Georgievski, O.; Dohnal, V. Quantitative determination of acidic groups in functionalized graphene by direct titration. React. Funct. Polym. 2016, 103, 44–53. [Google Scholar] [CrossRef]
- Ahlinder, L.; Henych, J.; Wiklund Lindström, S.; Ekstrand-Hammarström, B.; Stengl, V.; Österlund, L. Graphene oxide nanoparticle attachment and its toxicity on living lung epithelial cells. RSC Adv. 2015, 5, 59447–59457. [Google Scholar] [CrossRef]
- Carlberg, I.; Mannervik, B. Glutathione reductase. Meth. Enzymol. 1985, 113, 484–490. [Google Scholar]
- Aebi, H. Catalase in vitro. Meth. Enzymol. 1984, 105, 121–126. [Google Scholar]
- Erel, O. A novel automated direct measurement method for total antioxidant capacity using a new generation, more stable ABTS radical cation. Clin. Biochem. 2004, 37, 277–285. [Google Scholar] [CrossRef]
- Ohkawa, H.; Ohishi, N.; Yagi, K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal. Biochem. 1979, 95, 351–358. [Google Scholar] [CrossRef]
- Anjum, N.A.; Singh, N.; Singh, M.K.; Sayeed, I.; Duarte, A.C.; Pereira, E.; Ahmad, I. Single bilayer grapheme oxide sheet impacts and underlying potential mechanism assessment in germinating faba bean (Vicia faba L.). Sci. Total Environ. 2014, 472, 834–841. [Google Scholar] [CrossRef]
- Guo, X.; Mei, N. Assessment of the toxic potential of graphene family nanomaterials. J. Food Drug Anal. 2014, 22, 105–115. [Google Scholar] [CrossRef]
- Yu, B.P. Cellular defenses against damage from reactive oxygen species. Physiol. Rev. 1994, 74, 139–162. [Google Scholar] [CrossRef] [PubMed]
- Wei, X.-L.; Ge, Z.-Q. Effect of graphene oxide on conformation and activity of catalase. Carbon 2013, 60, 401–409. [Google Scholar] [CrossRef]
- Dreyer, D.R.; Park, S.; Bielawski, C.W.; Ruoff, R.S. The chemistry of graphene oxide. Chem. Soc. Rev. 2010, 39, 228–240. [Google Scholar] [CrossRef] [PubMed]
- Erel, O. A new automated colorimetric method for measuring total oxidant status. Clin. Biochem. 2005, 38, 1103–1111. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, L.B.; Gui, D.Y.; van der Heiden, M.G. Altered metabolite levels in cancer: Implications for tumour biology and cancer therapy. Nat. Rev. Cancer 2016, 16, 680–693. [Google Scholar] [CrossRef] [PubMed]
- Wullkopf, L.; West, A.V.; Leijnse, N.; Cox, T.R.; Madsen, C.D.; Oddershede, L.B.; Erler, J.T. Cancer cells’ ability to mechanically adjust to extracellular matrix stiffness correlates with their invasive potential. Mol. Biol. Cell 2018, 29, 2378–2385. [Google Scholar] [CrossRef] [PubMed]
- Gkretsi, V.; Stylianopoulos, T. Cell adhesion and matrix stiffness: Coordinating cancer cell invasion and metastasis. Front. Oncol. 2018, 8, 145. [Google Scholar] [CrossRef]
- Hu, W.; Peng, C.; Lv, M.; Li, X.; Zhang, Y.; Chen, N.; Fan, C.; Huang, Q. Protein corona-mediated mitigation of cytotoxicity of graphene oxide. ACS Nano 2011, 5, 3693–3700. [Google Scholar] [CrossRef]
- Liao, K.-H.; Lin, Y.-S.; Macosko, C.W.; Haynes, C.L. Cytotoxicity of graphene oxide and graphene in human erythrocytes and skin fibroblasts. ACS Appl. Mat. Interfaces 2011, 3, 2607–2615. [Google Scholar] [CrossRef] [PubMed]
- Aschner, M.; Guilarte, T.R.; Schneider, J.S.; Zheng, W. Manganese recent advances in understanding its transport and neurotoxicity. Toxicol. Appl. Pharmacol. 2007, 221, 131–147. [Google Scholar] [CrossRef]
- Sanchez, V.C.; Jachak, A.; Hurt, R.H.; Kane, A.B. Biological interactions of graphene-family nanomaterials: An interdisciplinary review. Chem. Res. Toxicol. 2012, 25, 15–34. [Google Scholar] [CrossRef] [PubMed]
- Neufeld, G.; Cohen, T.; Gengrinovitch, S.; Poltorak, Z. Vascular endothelial growth factor (VEGF) and its receptors. FASEB J. 1999, 13, 9–22. [Google Scholar] [CrossRef] [PubMed]
- Palmer, B.F.; Clegg, D.J. Oxygen sensing and metabolic homeostasis. Mol. Cell. Endocrinol. 2014, 397, 51–57. [Google Scholar] [CrossRef] [PubMed]
- Ferrara, N. Vascular endothelial growth factor: Basic science and clinical progress. Endocrine Rev. 2004, 25, 581–611. [Google Scholar] [CrossRef]
- Ou, L.; Song, B.; Liang, H.; Liu, J.; Feng, X.; Deng, B.; Sun, T.; Shao, L. Toxicity of graphene-family nanoparticles: A general review of the origins and mechanisms. Part. Fibre Toxicol. 2016, 13, 57. [Google Scholar] [CrossRef]
- Old, L.J. Tumor necrosis factor (TNF). Science 1985, 230, 630–632. [Google Scholar] [CrossRef]
- Oefner, C.; D’Arcy, A.; Winkler, F.K.; Eggimann, B.; Hosang, M. Crystal structure of human platelet-derived growth factor BB. EMBO J. 1992, 11, 3921–3926. [Google Scholar] [CrossRef]
- Braeuer, R.R.; Zigler, M.; Villares, G.J.; Dobroff, A.S.; Bar-Eli, M. Transcriptional control of melanoma metastasis: The importance of the tumor microenvironment. Seminars Cancer Biol. 2011, 21, 83–88. [Google Scholar] [CrossRef]
- Appelmann, I.; Liersch, R.; Kessler, T.; Mesters, R.M.; Berdel, W.E. Angiogenesis inhibition in cancer therapy: Platelet-derived growth factor (PDGF) and vascular endothelial growth factor (VEGF) and their receptors: Biological functions and role in malignancy. Recent Results Cancer Res. 2010, 180, 51–81. [Google Scholar]
- Gelfand, E.W.; Levy, N. Eosinophils in Human Disease. In Eosinophils in Health and Disease; Lee, J., Rosenberg, H., Eds.; Academic Press: Cambridge, MA, USA, 2012; pp. 431–536. [Google Scholar]
- Roberts, J.R.; Mercer, R.R.; Stefaniak, A.B.; Seehra, M.S.; Geddam, U.K.; Chaudhuri, I.S.; Kyrlidis, A.; Kodali, V.K.; Sager, T.; Kenyon, A.; et al. Evaluation of pulmonary and systemic toxicity following lung exposure to graphite nanoplates: A member of the graphene-based nanomaterial family. Particle Fibre Toxicol. 2015, 13, 34. [Google Scholar] [CrossRef] [PubMed]
- US EPA: Drinking Water Health Advisory for Manganese; Health and Ecological Criteria Division: Washington, DC, USA, 2004; EPA-822-R-04-003.
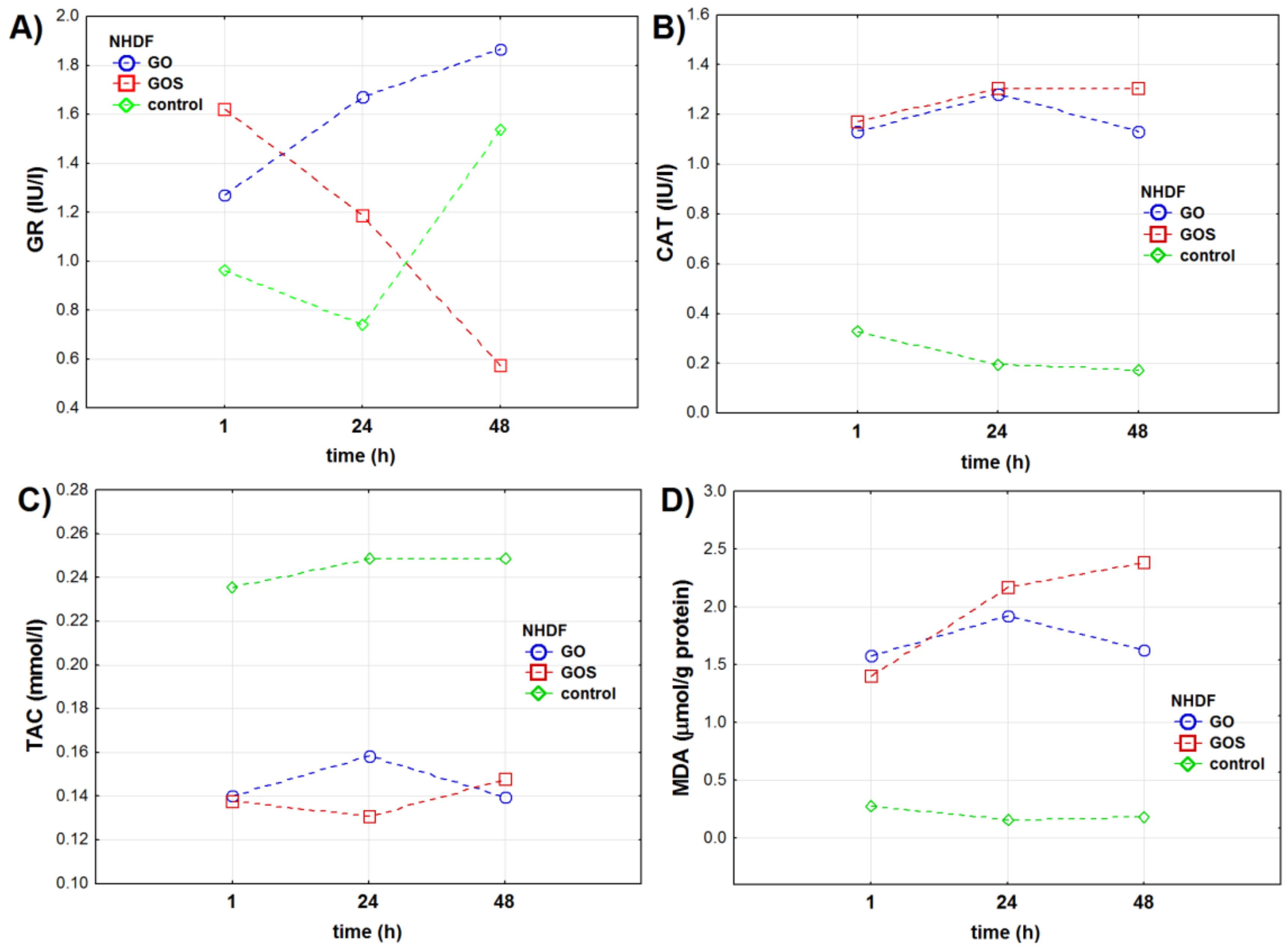
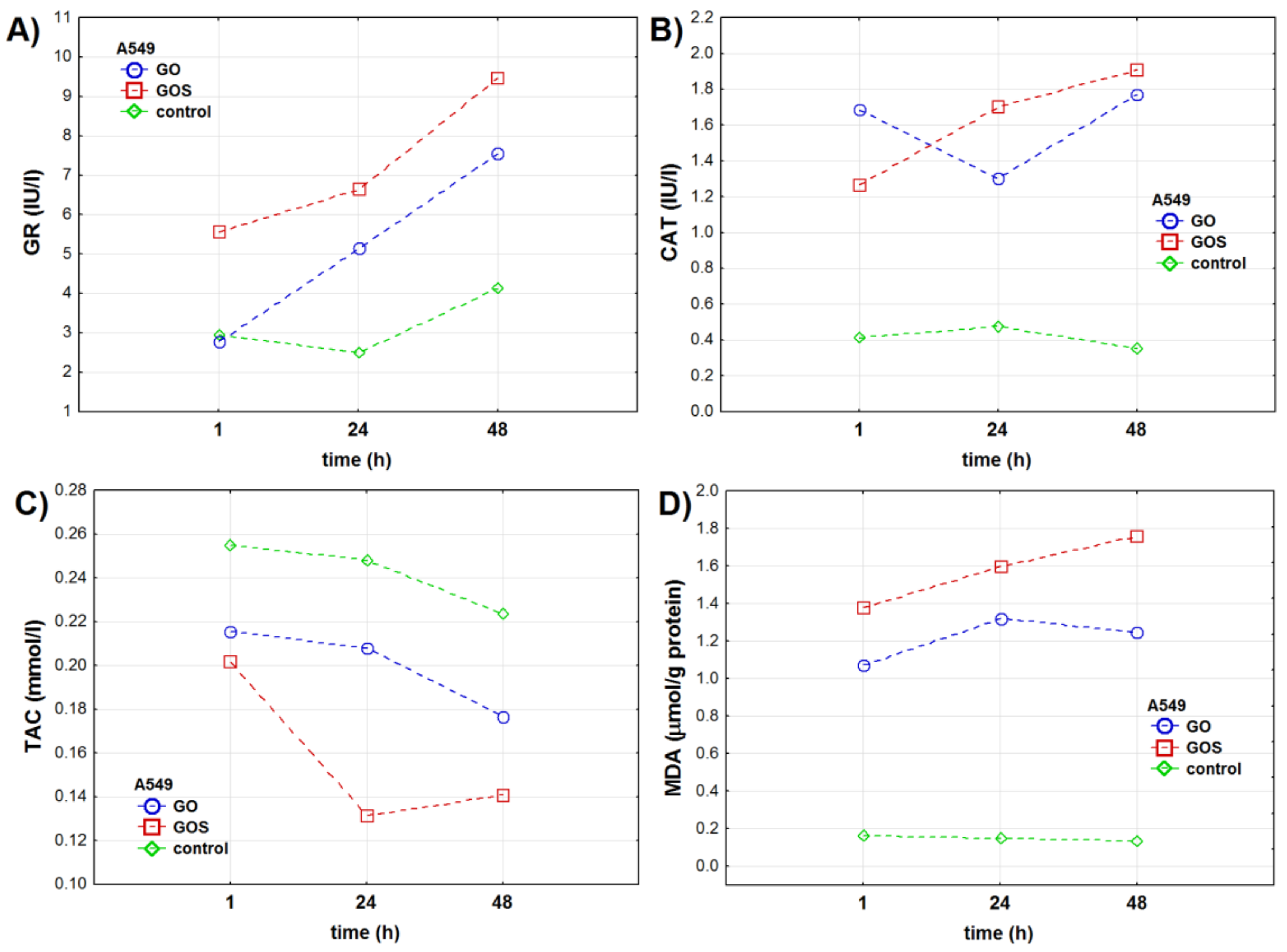
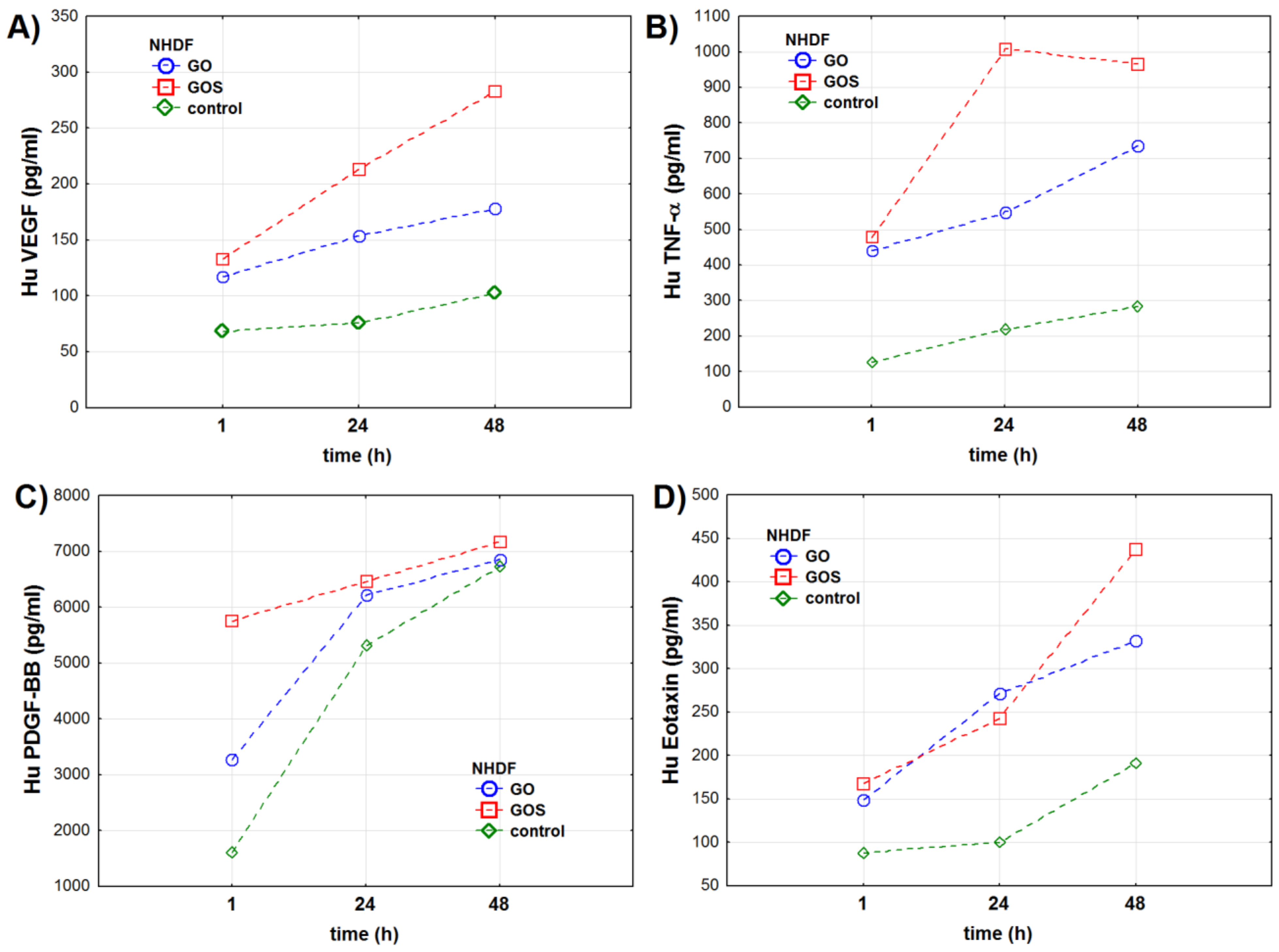
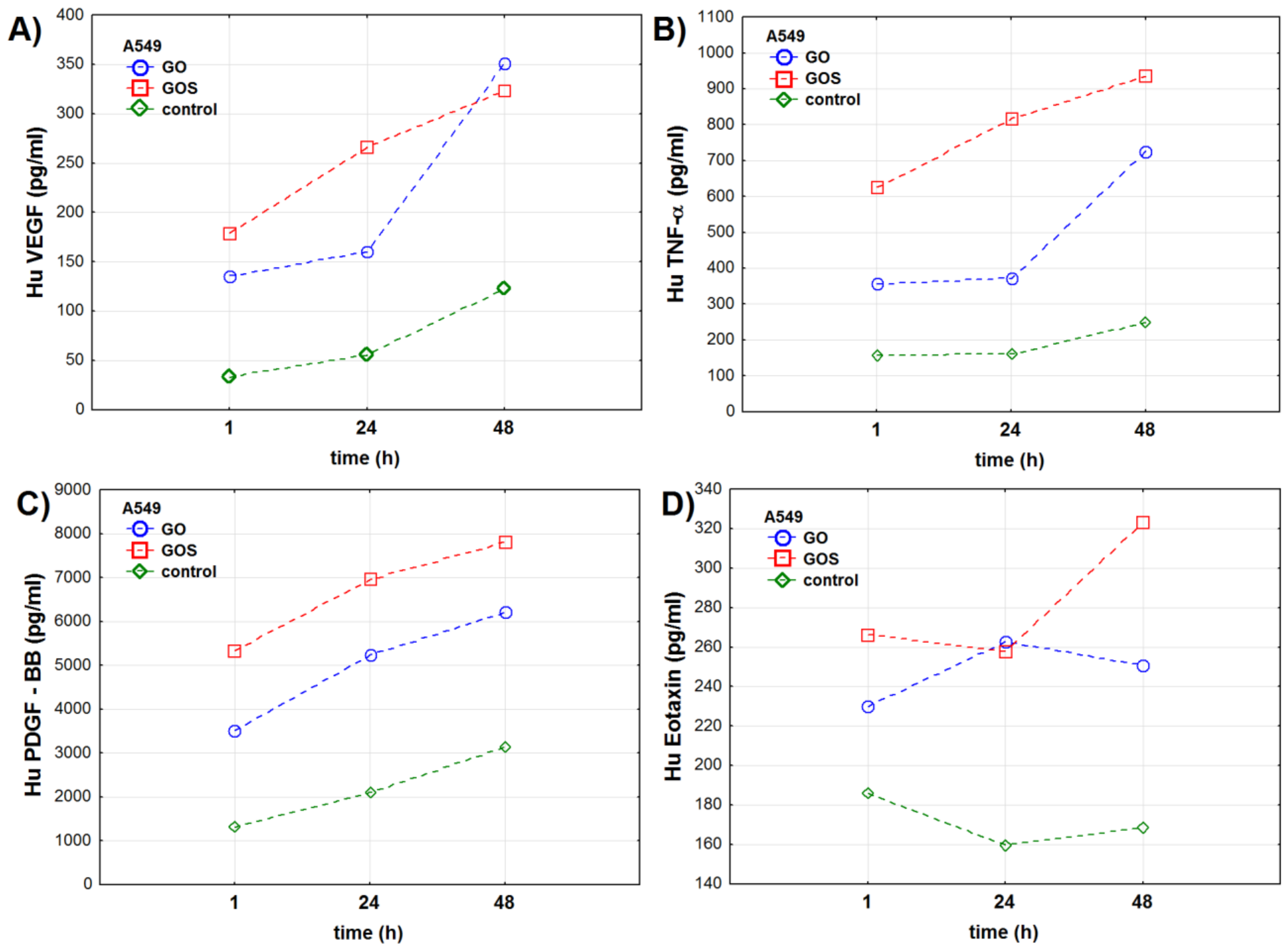
| Oxidative Stress Marker | Group | NHDF Cell Line | A549 Cell Line | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 h | 24 h | 48 h | ptime | pfactor | pint. | 1 h | 24 h | 48 h | ptime | pfactor | pint. | ||
| GR (IU/l) | control | 0.96 ± 0.04 | 0.74 ± 0.01 | 1.53 ± 0.01 | <0.001 | <0.001 | <0.001 | 2.95 ± 0.02 | 2.49 ± 0.01 | 4.12 ± 0.15 | <0.001 | – | <0.001 |
| GO | 1.27 ± 0.02 | 1.67 ± 0.01 | 1.87 ± 0.01 | 2.78 ± 0.01 | 5.14 ± 0.05 | 7.54 ± 0.03 | |||||||
| GOS | 1.62 ± 0.01 | 1.18 ± 0.04 | 0.57 ± 0.02 | 5.56 ± 0.04 | 6.64 ± 0.02 | 9.47 ± 0.02 | |||||||
| CAT (IU/l) | control | 0.32 ± 0.02 | 0.19 ± 0.02 | 0.17 ± 0.03 | <0.001 | – | <0.001 | 0.41 ± 0.11 | 0.48 ± 0.04 | 0.35 ± 0.03 | <0.001 | <0.001 | <0.001 |
| GO | 1.13 ± 0.02 | 1.28 ± 0.02 | 1.13 ± 0.02 | 1.68 ± 0.01 | 1.30 ± 0.03 | 1.77 ± 0.02 | |||||||
| GOS | 1.17 ± 0.02 | 1.31 ± 0.03 | 1.31 ± 0.04 | 1.27 ± 0.02 | 1.70 ± 0.05 | 1.91 ± 0.05 | |||||||
| TAC (mmol/l) | control | 0.24 ± 0.01 | 0.25 ± 0.03 | 0.25 ± 0.05 | 0.192 | <0.001 | 0.072 | 0.25 ± 0.02 | 0.25 ± 0.02 | 0.22 ± 0.01 | <0.001 | < 0.001 | <0.001 |
| GO | 0.14 ± 0.02 | 0.16 ± 0.01 | 0.14 ± 0.02 | 0.22 ± 0.03 | 0.21 ± 0.01 | 0.18 ± 0.01 | |||||||
| GOS | 0.14 ± 0.01 | 0.13 ± 0.01 | 0.15 ± 0.03 | 0.20 ± 0.02 | 0.13 ± 0.01 | 0.14 ± 0.01 | |||||||
| MDA (μmol/g protein) | control | 0.30 ± 0.08 | 0.16 ± 0.02 | 0.19 ± 0.02 | <0.001 | <0.001 | <0.001 | 0.16 ± 0.02 | 0.15 ± 0.02 | 0.14 ± 0.02 | <0.001 | – | <0.001 |
| GO | 1.60 ± 0.06 | 1.92 ± 0.02 | 1.63 ± 0.08 | 1.07 ± 0.03 | 1.32 ± 0.05 | 1.24 ± 0.08 | |||||||
| GOS | 1.40 ± 0.01 | 2.17 ± 0.02 | 2.39 ± 0.05 | 1.38 ± 0.04 | 1.60 ± 0.02 | 1.76 ± 0.08 | |||||||
| Cytokines Concentration [pg/mL] | Group | NHDF Cell Line | A549 Cell Line | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 h | 24 h | 48 h | ptime | pfactor | pint. | 1 h | 24 h | 48 h | ptime | pfactor | pint. | ||
| VEGF | Control | 68.0 ± 0.9 | 75.8 ± 0.7 | 102.1 ± 1.3 | <0.001 | <0.001 | <0.001 | 32.8 ± 0.6 | 55.3 ± 1.0 | 123.4 ± 1.3 | <0.001 | <0.001 | <0.001 |
| GO | 117.2 ± 0.6 | 153.7 ± 1.0 | 178.3 ± 2.0 | 135.9 ± 1.1 | 160.2 ± 0.5 | 351.0 ± 0.9 | |||||||
| GOS | 132.9 ± 1.8 | 213.7 ± 0.8 | 283.3 ± 1.4 | 178.8 ± 0.8 | 265.8 ± 0.9 | 323.9 ± 0.7 | |||||||
| TNF-α | Control | 125.5 ± 16.1 | 218.8 ± 26.1 | 283.5 ± 18.5 | <0.001 | <0.001 | <0.001 | 158.3 ± 11.8 | 161.3 ± 9.9 | 248.7 ± 29.8 | <0.001 | <0.001 | <0.001 |
| GO | 441.3 ± 37.0 | 550.2 ± 45.7 | 736.3 ± 28.7 | 357.5 ± 22.2 | 370.2 ± 38.2 | 726.3 ± 15.5 | |||||||
| GOS | 477.7 ± 47.7 | 1008.5 ± 21.1 | 966.8 ± 55.8 | 625.5 ± 11.6 | 817.0 ± 35.5 | 934.5 ± 20.9 | |||||||
| PDGF-BB | Control | 1606.7 ± 51.9 | 5306.3 ± 67.7 | 6725.0 ± 152.5 | <0.001 | <0.001 | <0.001 | 1304.8 ± 7.7 | 2117.8 ± 12.0 | 3134.8 ± 25.4 | <0.001 | <0.001 | <0.001 |
| GO | 3267.8 ± 84.1 | 6212.5 ± 101.6 | 6864.3 ± 43.6 | 3512.0 ± 9.2 | 5248.8 ± 19.8 | 6206.3 ± 142.3 | |||||||
| GOS | 5746.0 ± 75.1 | 6462.7 ± 124.7 | 7171.8 ± 96.9 | 5334.8 ± 103.1 | 6954.8 ± 93.8 | 7807.7 ± 74.3 | |||||||
| Eotaxin | Control | 88.0 ± 10.1 | 99.7 ± 16.2 | 191.0 ± 22.0 | <0.001 | <0.001 | <0.001 | 186.2 ± 10.8 | 159.8 ± 12.9 | 169.3 ± 19.4 | <0.01 | <0.001 | <0.001 |
| GO | 149.0 ± 41.2 | 271.3 ± 34.6 | 331.7 ± 36.0 | 229.7 ± 21.1 | 262.8 ± 22.8 | 251.2 ± 6.2 | |||||||
| GOS | 167.7 ± 18.0 | 242.3 ± 34.9 | 437.0 ± 39.2 | 266.3 ± 25.6 | 257.8 ± 28.2 | 323.0 ± 3.2 | |||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stygar, D.; Pogorzelska, A.; Chełmecka, E.; Skrzep-Poloczek, B.; Bażanów, B.; Gębarowski, T.; Jochem, J.; Henych, J. Graphene Oxide Normal (GO + Mn2+) and Ultrapure: Short-Term Impact on Selected Antioxidant Stress Markers and Cytokines in NHDF and A549 Cell Lines. Antioxidants 2021, 10, 765. https://doi.org/10.3390/antiox10050765
Stygar D, Pogorzelska A, Chełmecka E, Skrzep-Poloczek B, Bażanów B, Gębarowski T, Jochem J, Henych J. Graphene Oxide Normal (GO + Mn2+) and Ultrapure: Short-Term Impact on Selected Antioxidant Stress Markers and Cytokines in NHDF and A549 Cell Lines. Antioxidants. 2021; 10(5):765. https://doi.org/10.3390/antiox10050765
Chicago/Turabian StyleStygar, Dominika, Aleksandra Pogorzelska, Elżbieta Chełmecka, Bronisława Skrzep-Poloczek, Barbara Bażanów, Tomasz Gębarowski, Jerzy Jochem, and Jiří Henych. 2021. "Graphene Oxide Normal (GO + Mn2+) and Ultrapure: Short-Term Impact on Selected Antioxidant Stress Markers and Cytokines in NHDF and A549 Cell Lines" Antioxidants 10, no. 5: 765. https://doi.org/10.3390/antiox10050765
APA StyleStygar, D., Pogorzelska, A., Chełmecka, E., Skrzep-Poloczek, B., Bażanów, B., Gębarowski, T., Jochem, J., & Henych, J. (2021). Graphene Oxide Normal (GO + Mn2+) and Ultrapure: Short-Term Impact on Selected Antioxidant Stress Markers and Cytokines in NHDF and A549 Cell Lines. Antioxidants, 10(5), 765. https://doi.org/10.3390/antiox10050765







