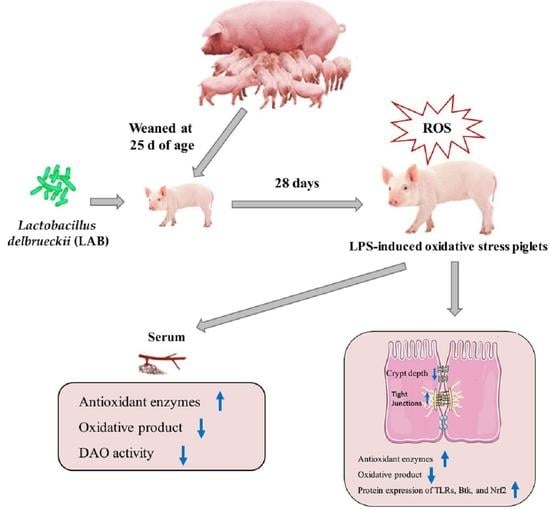
Lactobacillus delbrueckii Protected Intestinal Integrity, Alleviated Intestinal Oxidative Damage, and Activated Toll-Like Receptor–Bruton’s Tyrosine Kinase–Nuclear Factor Erythroid 2-Related Factor 2 Pathway in Weaned Piglets Challenged with Lipopolysaccharide
Abstract
1. Introduction
2. Materials and Methods
2.1. Bacterial Strain
2.2. Animals and Experimental Design
2.3. Sample Collection
2.4. Intestinal Morphology Analysis
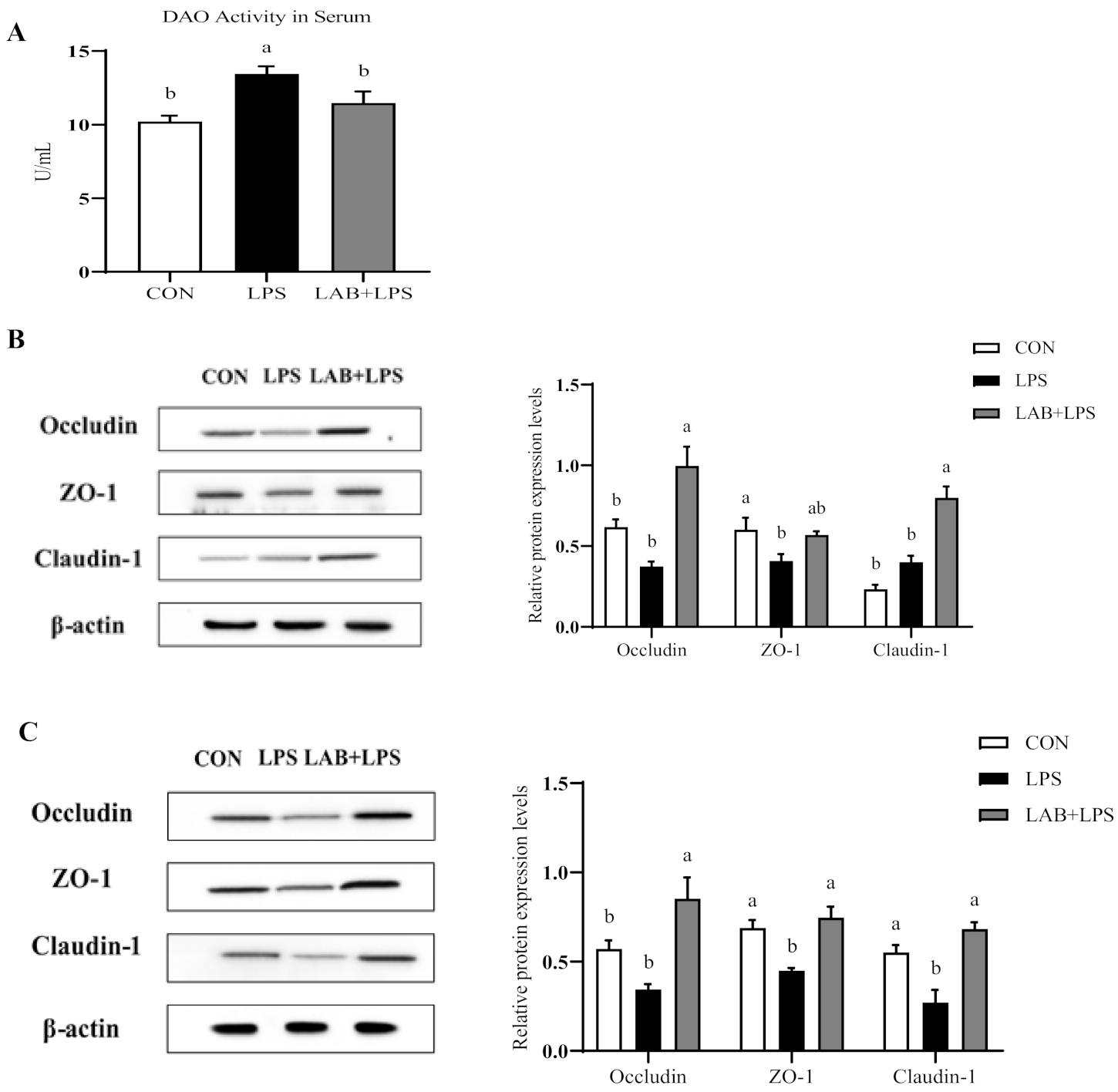
2.5. Measurement of Antioxidant Indices and Serum Diamine Oxidase Activity
2.6. Protein Expression Analysis by Western Blotting
2.7. Statistical Analysis
3. Results
3.1. Growth Performance
3.2. Intestinal Morphology
3.3. Intestinal Permeability and Tight Junction Protein Expression
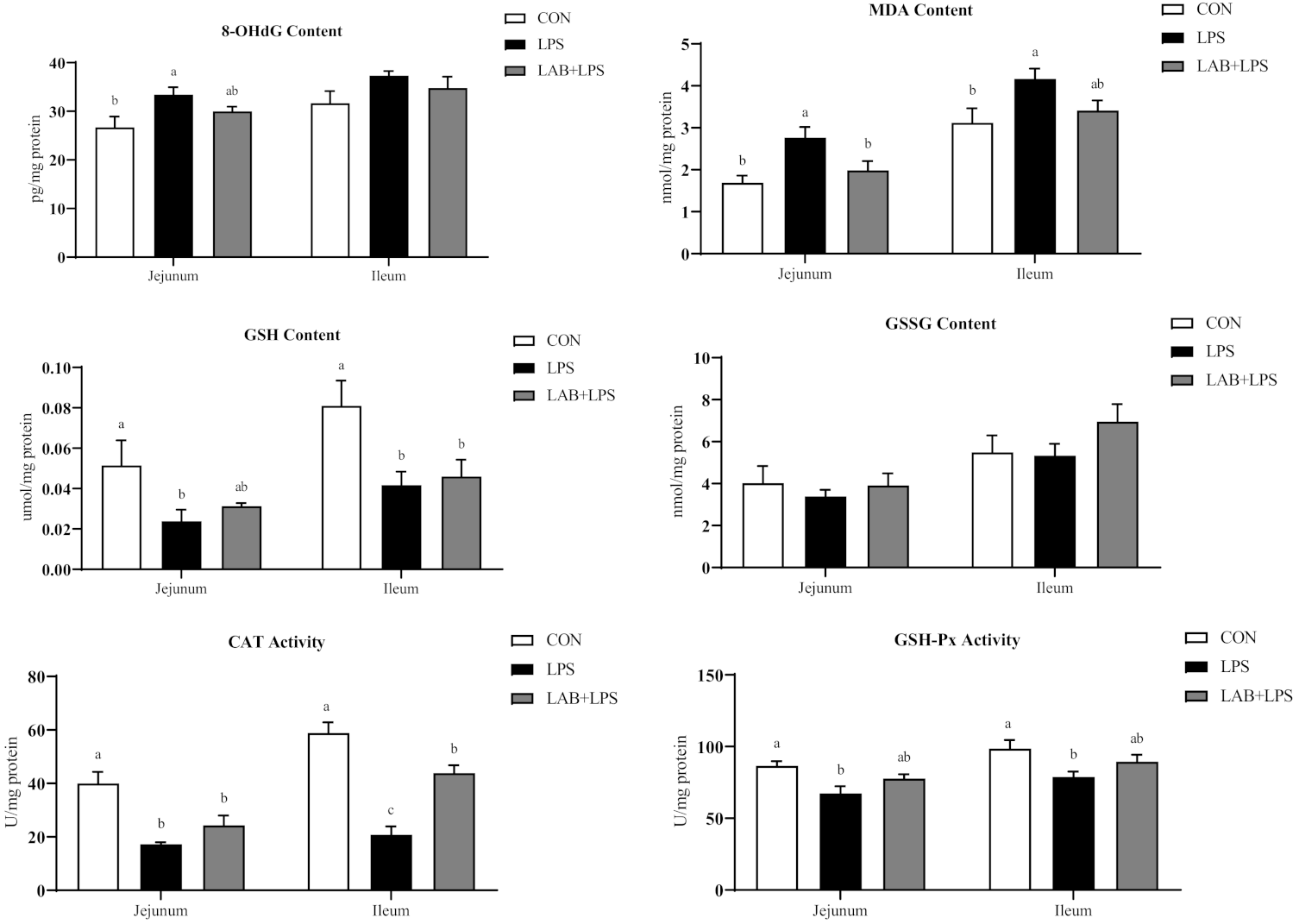
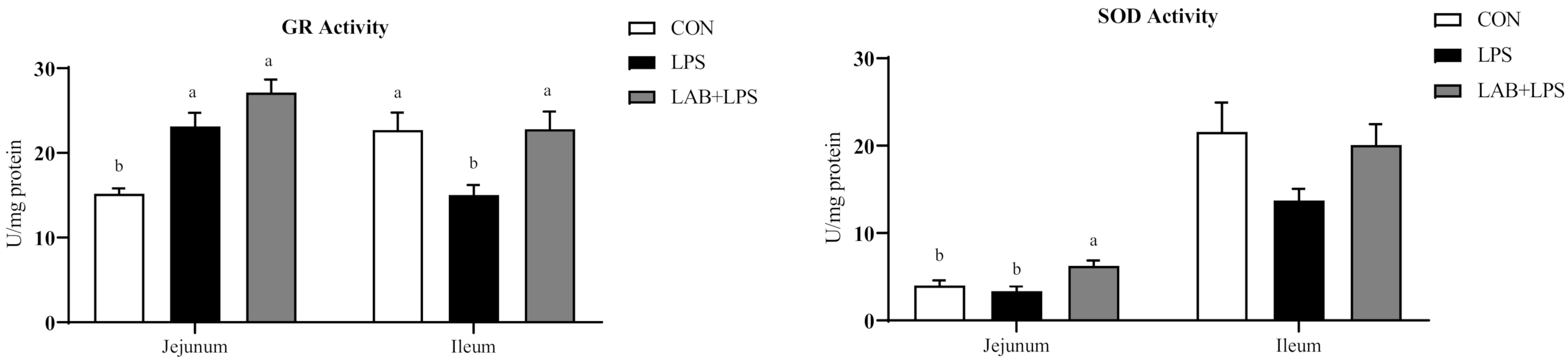
3.4. Intestinal Mucosal and Serum Antioxidative Indices
3.5. Protein Expression of TLRs, Btk, and Nrf2
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Whiting, T.L.; Pasma, T. Isolated weaning technology: Humane benefits and concerns in the production of pork. Can. Vet. J. 2008, 49, 293. [Google Scholar]
- Yin, J.; Wu, M.; Xiao, H.; Ren, W.; Duan, J.; Yang, G.; Li, T.; Yin, Y. Development of an antioxidant system after early weaning in piglets. J. Anim. Sci. 2014, 92, 612–619. [Google Scholar] [CrossRef]
- Bomba, L.; Minuti, A.; Moisá, S.; Trevisi, E.; Eufemi, E.; Lizier, M.; Chegdani, F.; Lucchini, F.; Rzepus, M.; Prandini, A.; et al. Gut response induced by weaning in piglet features marked changes in immune and inflammatory response. Funct. Integr. Genom. 2014, 14, 657–671. [Google Scholar] [CrossRef]
- Zheng, P.; Yu, B.; He, J.; Tian, G.; Luo, Y.; Mao, X.; Zhang, K.; Che, L.; Chen, D. Protective effects of dietary arginine supplementation against oxidative stress in weaned piglets. Br. J. Nutr. 2012, 109, 2253–2260. [Google Scholar] [CrossRef]
- Cao, S.; Wang, C.; Wu, H.; Zhang, Q.; Jiao, L.; Hu, C. Weaning disrupts intestinal antioxidant status, impairs intestinal barrier and mitochondrial function, and triggers mitophagy in piglets1. J. Anim. Sci. 2018, 96, 1073–1083. [Google Scholar] [CrossRef] [PubMed]
- Hall, D.; Buettner, G.; Oberley, L.; Xu, L.; Matthes, R.; Gisolfi, C. Mechanisms of circulatory and intestinal barrier dysfunction during whole body hyperthermia. Am. J. Physiol. Heart Circ. Physiol. 2001, 280, H509–H521. [Google Scholar] [CrossRef] [PubMed]
- Muccioli, G.; Naslain, D.; Bäckhed, F.; Reigstad, C.; Lambert, D.; Delzenne, N.; Cani, P. The endocannabinoid system links gut microbiota to adipogenesis. Mol. Syst. Biol. 2010, 6, 392. [Google Scholar] [CrossRef] [PubMed]
- Hill, C.; Guarner, F.; Reid, G.; Gibson, G.; Merenstein, D.; Pot, B.; Morelli, L.; Canani, R.; Flint, H.; Salminen, S.; et al. The International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat. Rev. Gastroenterol. Hepatol. 2014, 11, 506–514. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Tang, H.; Zhang, C.; Zhao, Y.; Derrien, M.; Rocher, E.; van-Hylckama Vlieg, J.; Strissel, K.; Zhao, L.; Obin, M.; et al. Modulation of gut microbiota during probiotic-mediated attenuation of metabolic syndrome in high fat diet-fed mice. Isme J. 2015, 9, 1–15. [Google Scholar] [CrossRef]
- Kong, Y.; Olejar, K.J.; On, S.; Chelikani, V. The Potential of Lactobacillus spp. for Modulating Oxidative Stress in the Gastrointestinal Tract. Antioxidants 2020, 9, 610. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wu, Y.; Wang, Y.; Xu, H.; Mei, X.; Yu, D.; Wang, Y.; Li, W. Antioxidant Properties of Probiotic Bacteria. Nutrients 2017, 9, 521. [Google Scholar] [CrossRef] [PubMed]
- Wang, A.; Yi, X.; Yu, H.; Dong, B.; Qiao, S. Free radical scavenging activity of Lactobacillus fermentum in vitro and its antioxidative effect on growing-finishing pigs. J. Appl. Microbiol. 2009, 107, 1140–1148. [Google Scholar] [CrossRef] [PubMed]
- Medzhitov, R. Recognition of microorganisms and activation of the immune response. Nature 2007, 449, 819–826. [Google Scholar] [CrossRef]
- Ma, Q. Role of Nrf2 in Oxidative Stress and Toxicity. Annu. Rev. Pharmacol. Toxicol. 2013, 53, 401–426. [Google Scholar] [CrossRef] [PubMed]
- Chauhan, R.; Sudhakaran Vasanthakumari, A.; Panwar, H.; Mallapa, R.; Duary, R.; Batish, V.; Grover, S. Amelioration of Colitis in Mouse Model by Exploring Antioxidative Potentials of an Indigenous Probiotic Strain of Lactobacillus fermentumLf1. Biomed. Res. Int. 2014, 2014, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Endo, H.; Niioka, M.; Kobayashi, N.; Tanaka, M.; Watanabe, T. Butyrate-Producing Probiotics Reduce Nonalcoholic Fatty Liver Disease Progression in Rats: New Insight into the Probiotics for the Gut-Liver Axis. PLoS ONE 2013, 8, e63388. [Google Scholar] [CrossRef]
- Jones, R.; Desai, C.; Darby, T.; Luo, L.; Wolfarth, A.; Scharer, C.; Ardita, C.; Reedy, A.; Keebaugh, E.; Neish, A. Lactobacilli Modulate Epithelial Cytoprotection through the Nrf2 Pathway. Cell Rep. 2015, 12, 1217–1225. [Google Scholar] [CrossRef]
- Vijayan, V.; Baumgart-Vogt, E.; Naidu, S.; Qian, G.; Immenschuh, S. Bruton’s Tyrosine Kinase Is Required for TLR-Dependent Heme Oxygenase-1 Gene Activation via Nrf2 in Macrophages. J. Immunol. 2011, 187, 817–827. [Google Scholar] [CrossRef]
- Li, Y.; Hou, S.; Peng, W.; Lin, Q.; Chen, F.; Yang, L.; Li, F.; Huang, X. Oral Administration of Lactobacillus delbrueckii during the Suckling Phase Improves Antioxidant Activities and Immune Responses after the Weaning Event in a Piglet Model. Oxidative Med. Cell. Longev. 2019, 2019, 1–10. [Google Scholar] [CrossRef]
- Chen, F.; Wang, H.; Chen, J.; Liu, Y.; Wen, W.; Li, Y.; Huang, X. Lactobacillus delbrueckii Ameliorates Intestinal Integrity and Antioxidant Ability in Weaned Piglets after a Lipopolysaccharide Challenge. Oxidative Med. Cell. Longev. 2020, 2020, 1–10. [Google Scholar] [CrossRef]
- NRC. Nutrient Requirements of Swine; National Academies Press: Washington, DC, USA, 2012. [Google Scholar]
- Song, Z.; Tong, G.; Xiao, K.; Jiao, L.; Ke, Y.; Hu, C. L-Cysteine protects intestinal integrity, attenuates intestinal inflammation and oxidant stress, and modulates NF-κB and Nrf2 pathways in weaned piglets after LPS challenge. Innate Immun. 2016, 22, 152–161. [Google Scholar] [CrossRef] [PubMed]
- Hewett, J.A.; Roth, R.A. Hepatic and extrahepatic pathobiology of bacterial lipopolysaccharides. Pharmacol. Rev. 1993, 45, 382–411. [Google Scholar] [PubMed]
- Yoshino, S.; Sasatomi, E.; Ohsawa, M. Bacterial lipopolysaccharide acts as an adjuvant to induce autoimmune arthritis in mice. Immunology 2000, 99, 607–614. [Google Scholar] [CrossRef]
- Chan, J.K.; Roth, J.; Oppenheim, J.J.; Tracey, K.J.; Vogl, T.; Feldmann, M.; Horwood, N.; Nanchahal, J. Alarmins: Awaiting a clinical response. J. Clin. Investig. 2012, 122, 2711–2719. [Google Scholar] [CrossRef]
- Sun, Z.; Li, H.; Li, Y.; Qiao, J. Lactobacillus salivarius, a Potential Probiotic to Improve the Health of LPS-Challenged Piglet Intestine by Alleviating Inflammation as Well as Oxidative Stress in a Dose-Dependent Manner During Weaning Transition. Front. Vet. Sci. 2020, 7. [Google Scholar] [CrossRef]
- Rolfe, R. The Role of Probiotic Cultures in the Control of Gastrointestinal Health. J. Nutr. 2000, 130, 396S–402S. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Hou, S.; Chen, J.; Peng, W.; Wen, W.; Chen, F.; Huang, X. Oral administration of Lactobacillus delbrueckii during the suckling period improves intestinal integrity after weaning in piglets. J. Funct. Foods 2019, 63, 103591. [Google Scholar] [CrossRef]
- Lan, R.; Lee, S.; Kim, I. Effects of multistrain probiotics on growth performance, nutrient digestibility, blood profiles, faecal microbial shedding, faecal score and noxious gas emission in weaning pigs. J. Anim. Physiol. Anim. Nutr. 2016, 100, 1130–1138. [Google Scholar] [CrossRef]
- Liu, C.; Zhu, Q.; Chang, J.; Yin, Q.; Song, A.; Li, Z.; Wang, E.; Lu, F. Effects of Lactobacillus caseiand Enterococcus faecalison growth performance, immune function and gut microbiota of suckling piglets. Arch. Anim. Nutr. 2017, 71, 120–133. [Google Scholar] [CrossRef]
- Ross, G.; Van Nieuwenhove, C.; González, S. Fatty Acid Profile of Pig Meat after Probiotic Administration. J. Agric. Food Chem. 2012, 60, 5974–5978. [Google Scholar] [CrossRef]
- Mair, C.; Plitzner, C.; Domig, K.; Schedle, K.; Windisch, W. Impact of inulin and a multispecies probiotic formulation on performance, microbial ecology and concomitant fermentation patterns in newly weaned piglets. J. Anim. Physiol. Anim. Nutr. 2010, 94, e164–e177. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Chen, F.; Odle, J.; Lin, X.; Jacobi, S.; Zhu, H.; Wu, Z.; Hou, Y. Fish Oil Enhances Intestinal Integrity and Inhibits TLR4 and NOD2 Signaling Pathways in Weaned Pigs after LPS Challenge. J. Nutr. 2012, 142, 2017–2024. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Hu, C.; Xia, M.; Zhan, X.; Wang, M. Effects of dietary fructooligosaccharide on digestive enzyme activities, intestinal microflora and morphology of male broilers. Poult. Sci. 2003, 82, 1030–1036. [Google Scholar] [CrossRef] [PubMed]
- Yang, K.; Jiang, Z.; Zheng, C.; Wang, L.; Yang, X. Effect of Lactobacillus plantarum on diarrhea and intestinal barrier function of young piglets challenged with enterotoxigenic Escherichia coli K881. J. Anim. Sci. 2014, 92, 1496–1503. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharyya, A.; Chattopadhyay, R.; Mitra, S.; Crowe, S. Oxidative Stress: An Essential Factor in the Pathogenesis of Gastrointestinal Mucosal Diseases. Physiol. Rev. 2014, 94, 329–354. [Google Scholar] [CrossRef]
- Celes, M.; Torres-Dueñas, D.; Prado, C.; Campos, E.; Moreira, J.; Cunha, F.; Rossi, M. Increased sarcolemmal permeability as an early event in experimental septic cardiomyopathy: A potential role for oxidative damage to lipids and proteins. Shock 2010, 33, 322–331. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Wang, G.; Hao, J.; Ma, J.; Wang, Y.; Jiang, X.; Jiang, H. Curcumin Ameliorates Hydrogen Peroxide-Induced Epithelial Barrier Disruption by Upregulating Heme Oxygenase-1 Expression in Human Intestinal Epithelial Cells. Dig. Dis. Sci. 2012, 57, 1792–1801. [Google Scholar] [CrossRef]
- Mukojima, K.; Mishima, S.; Oda, J.; Homma, H.; Sasaki, H.; Ohta, S.; Yukioka, T. Protective Effects of Free Radical Scavenger Edaravone Against Xanthine Oxidase–Mediated Permeability Increases in Human Intestinal Epithelial Cell Monolayer. J. Burn Care Res. 2009, 30, 335–340. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Liu, F.; Yin, P.; Zhao, H.; Luan, W.; Hou, X.; Zhong, Y.; Jia, D.; Zan, J.; Ma, W.; et al. Involvement of oxidative stress and mitogen-activated protein kinase signaling pathways in heat stress-induced injury in the rat small intestine. Stress 2012, 16, 99–113. [Google Scholar] [CrossRef]
- Gumbiner, B. Breaking through the tight junction barrier. J. Cell Biol. 1993, 123, 1631–1633. [Google Scholar] [CrossRef]
- Anderson, J.; Balda, M.; Fanning, A. The structure and regulation of tight junctions. Curr. Opin. Cell Biol. 1993, 5, 772–778. [Google Scholar] [CrossRef]
- Chen, J.; Zheng, P.; Zhang, C.; Yu, B.; He, J.; Yu, J.; Luo, J.; Mao, X.; Huang, Z.; Chen, D. Benzoic acid beneficially affects growth performance of weaned pigs which was associated with changes in gut bacterial populations, morphology indices and growth factor gene expression. J. Anim. Physiol. Anim. Nutr. 2016, 101, 1137–1146. [Google Scholar] [CrossRef]
- Yin, J.; Duan, J.; Cui, Z.; Ren, W.; Li, T.; Yin, Y. Hydrogen peroxide-induced oxidative stress activates NF-κB and Nrf2/Keap1 signals and triggers autophagy in piglets. Rsc Adv. 2015, 5, 15479–15486. [Google Scholar] [CrossRef]
- Gostner, J.; Becker, K.; Fuchs, D.; Sucher, R. Redox regulation of the immune response. Redox Rep. 2013, 18, 88–94. [Google Scholar] [CrossRef]
- Valko, M.; Leibfritz, D.; Moncol, J.; Cronin, M.; Mazur, M.; Telser, J. Free radicals and antioxidants in normal physiological functions and human disease. Int. J. Biochem. Cell Biol. 2007, 39, 44–84. [Google Scholar] [CrossRef]
- Yin, J.; Ren, W.; Wu, X.; Yang, G.; Wang, J.; Li, T.; Ding, J.; Cai, L.; Su, D. Oxidative stress-mediated signaling pathways: A review. J. Food Agric. Environ. 2013, 11, 132–139. [Google Scholar]
- Cataldi, A. Cell Responses to Oxidative Stressors. Curr. Pharm. Des. 2010, 16, 1387–1395. [Google Scholar] [CrossRef] [PubMed]
- Yin, J.; Liu, M.; Ren, W.; Duan, J.; Yang, G.; Zhao, Y.; Fang, R.; Chen, L.; Li, T.; Yin, Y. Effects of Dietary Supplementation with Glutamate and Aspartate on Diquat-Induced Oxidative Stress in Piglets. PLoS ONE 2015, 10, e0122893. [Google Scholar] [CrossRef] [PubMed]
- Zhao, D.; Wu, T.; Yi, D.; Wang, L.; Li, P.; Zhang, J.; Hou, Y.; Wu, G. Dietary Supplementation with Lactobacillus casei Alleviates Lipopolysaccharide-Induced Liver Injury in a Porcine Model. Int. J. Mol. Sci. 2017, 18, 2535. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Ji, H.; Wang, S.; Zhang, D.; Liu, H.; Shan, D.; Wang, Y. Lactobacillus plantarum ZLP001: In vitro Assessment of Antioxidant Capacity and Effect on Growth Performance and Antioxidant Status in Weaning Piglets. Asian-Australas. J. Anim. Sci. 2012, 25, 1153–1158. [Google Scholar] [CrossRef]
- Forsyth, C.; Farhadi, A.; Jakate, S.; Tang, Y.; Shaikh, M.; Keshavarzian, A. Lactobacillus GG treatment ameliorates alcohol-induced intestinal oxidative stress, gut leakiness, and liver injury in a rat model of alcoholic steatohepatitis. Alcohol 2009, 43, 163–172. [Google Scholar] [CrossRef]
- Asemi, Z.; Jazayeri, S.; Najafi, M.; Samimi, M.; Mofid, V.; Shidfar, F.; Shakeri, H.; Esmaillzadeh, A. Effect of Daily Consumption of Probiotic Yogurt on Oxidative Stress in Pregnant Women: A Randomized Controlled Clinical Trial. Ann. Nutr. Metab. 2012, 60, 62–68. [Google Scholar] [CrossRef] [PubMed]
- Anderson, R.; Ulluwishewa, D.; Young, W.; Ryan, L.; Henderson, G.; Meijerink, M.; Maier, E.; Wells, J.; Roy, N. Human oral isolate Lactobacillus fermentum AGR1487 induces a pro-inflammatory response in germ-free rat colons. Sci. Rep. 2016, 6. [Google Scholar] [CrossRef]
- Castillo, N.; Perdigón, G.; de Moreno de LeBlanc, A. Oral administration of a probiotic Lactobacillus modulates cytokine production and TLR expression improving the immune response against Salmonella enterica serovar Typhimurium infection in mice. BMC Microbiol. 2011, 11, 177. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Muhammad, I.; Li, W.; Sun, X.; Cheng, P.; Zhang, X. Sensitivity of Arbor Acres broilers and chemoprevention of aflatoxin B 1 -induced liver injury by curcumin, a natural potent inducer of phase-II enzymes and Nrf2. Environ. Toxicol. Pharmacol. 2018, 59, 94–104. [Google Scholar] [CrossRef] [PubMed]
- Kensler, T.; Wakabayashi, N.; Biswal, S. Cell Survival Responses to Environmental Stresses Via the Keap1-Nrf2-ARE Pathway. Annu. Rev. Pharmacol. Toxicol. 2007, 47, 89–116. [Google Scholar] [CrossRef]
- Banerjee, B.; Seth, V.; Bhattacharya, A.; Pasha, S.; Chakraborty, A. Biochemical effects of some pesticides on lipid peroxidation and free-radical scavengers. Toxicol. Lett. 1999, 107, 33–47. [Google Scholar] [CrossRef]
- Gozzelino, R.; Jeney, V.; Soares, M. Mechanisms of Cell Protection by Heme Oxygenase-1. Annu. Rev. Pharmacol. Toxicol. 2010, 50, 323–354. [Google Scholar] [CrossRef] [PubMed]
- You, X.; Liu, L.; Zeng, Y.; Li, R.; He, J.; Ma, X.; Jiang, C.; Zhu, C.; Chen, L.; Yu, M.; et al. Macrophage-Activating Lipopeptide-2 Requires Mal and PI3K for Efficient Induction of Heme Oxygenase-1. PLoS ONE 2014, 9, e103433. [Google Scholar] [CrossRef]
- Nadeem, A.; Siddiqui, N.; Al-Harbi, N.; Al-Harbi, M.; Ahmad, S. TLR-7 agonist attenuates airway reactivity and inflammation through Nrf2-mediated antioxidant protection in a murine model of allergic asthma. Int. J. Biochem. Cell Biol. 2016, 73, 53–62. [Google Scholar] [CrossRef]
- Yin, S.; Cao, W. Toll-Like Receptor Signaling Induces Nrf2 Pathway Activation through p62-Triggered Keap1 Degradation. Mol. Cell. Biol. 2015, 35, 2673–2683. [Google Scholar] [CrossRef]
- Xu, C.; Qiao, L.; Ma, L.; Guo, Y.; Dou, X.; Yan, S.; Zhang, B.; Román, A. Biogenic selenium nanoparticles synthesized by Lactobacillus casei ATCC 393 alleviate intestinal epithelial barrier dysfunction caused by oxidative stress via Nrf2 signaling-mediated mitochondrial pathway. Int. J. Nanomed. 2019, 14, 4491–4502. [Google Scholar] [CrossRef]
- Wang, Y.; Wu, Y.; Wang, B.; Cao, X.; Fu, A.; Li, Y.; Li, W. Effects of probiotic Bacillus as a substitute for antibiotics on antioxidant capacity and intestinal autophagy of piglets. Amb Express 2017, 7. [Google Scholar] [CrossRef]
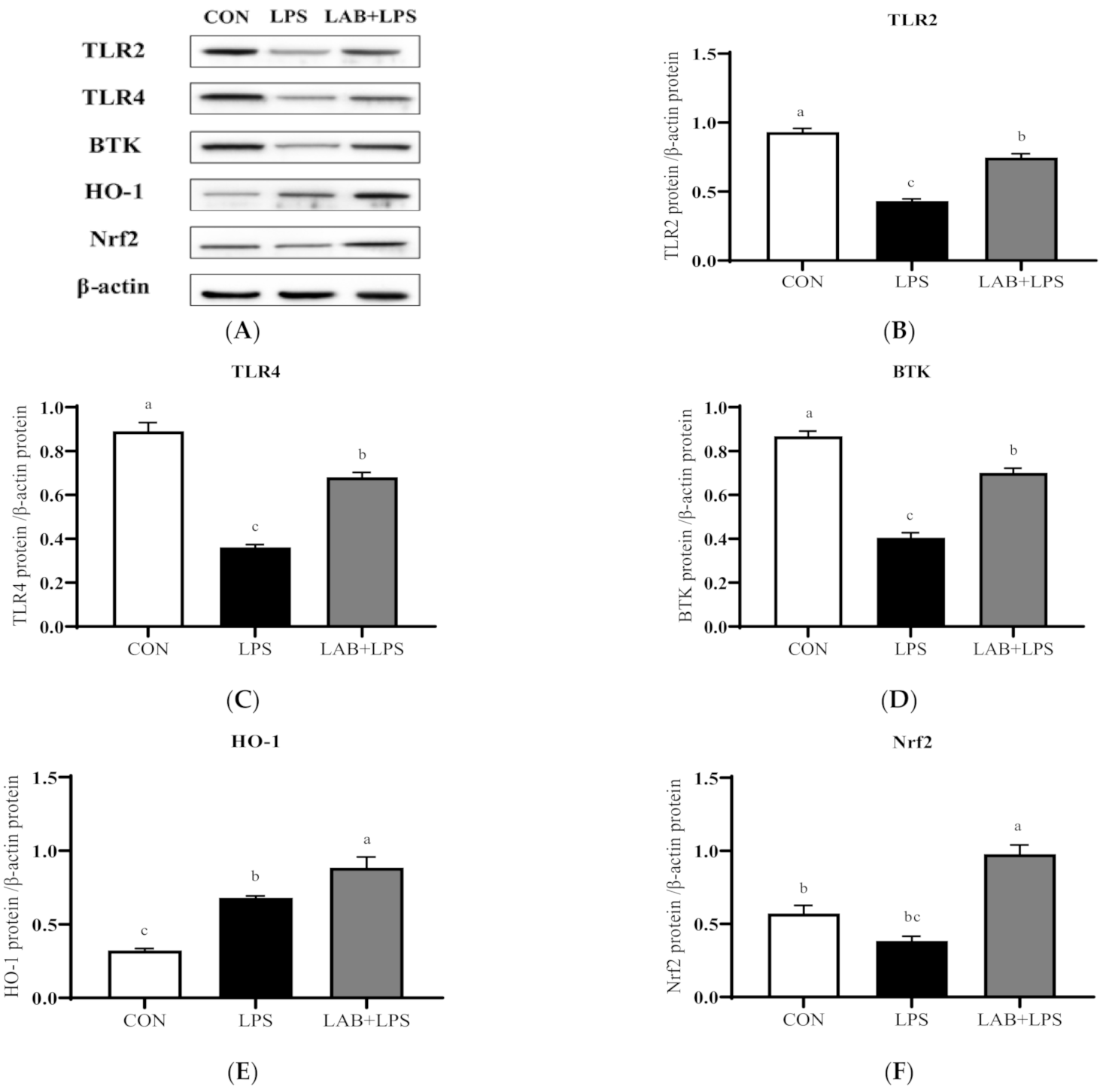
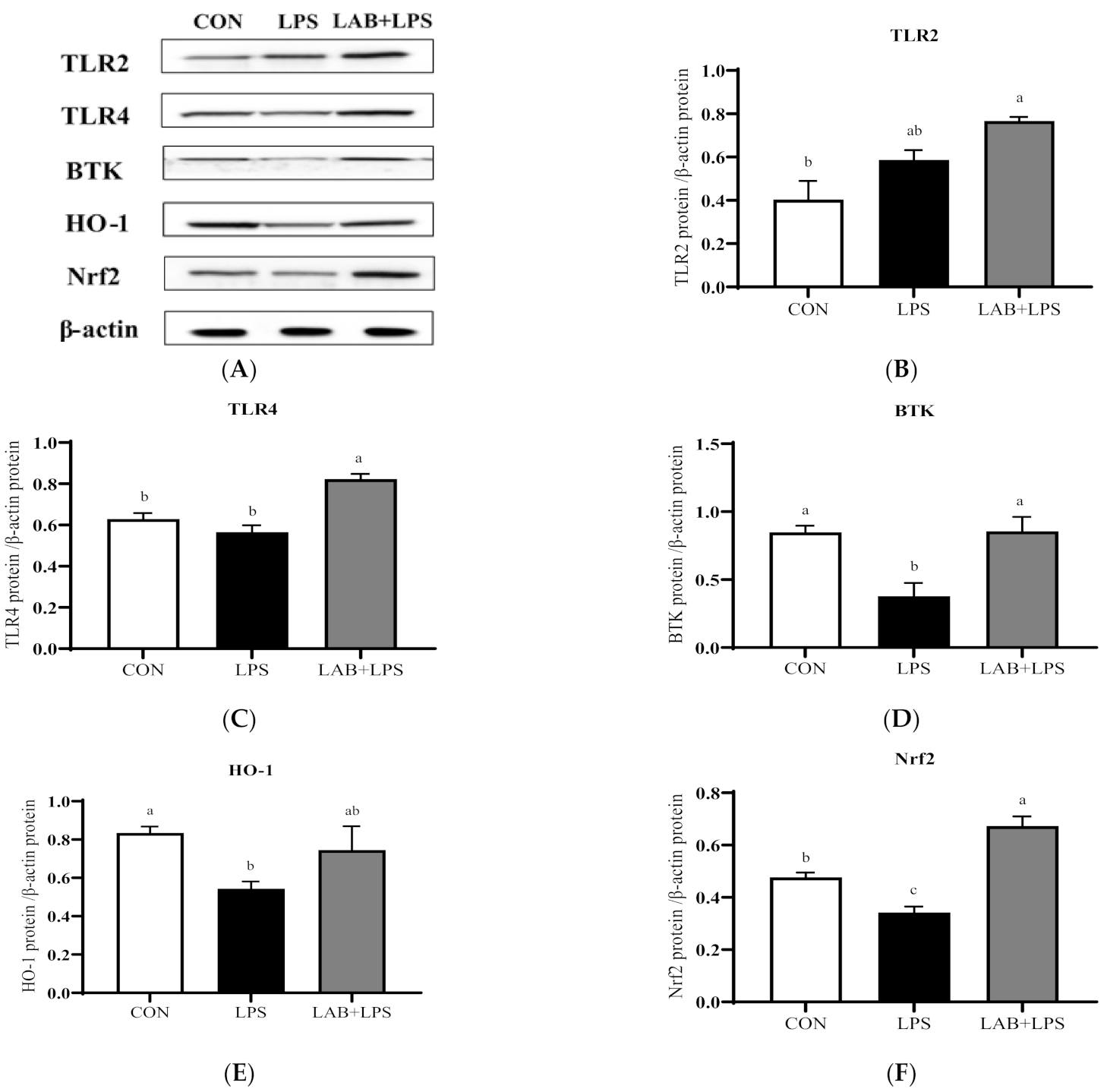
| Item | Content |
|---|---|
| Ingredient, % | |
| Extruded corn | 50.00 |
| Soybean meal, 43% crude protein | 19.20 |
| Extruded soybean | 13.30 |
| Fish meal | 2.50 |
| Whey powder | 10.00 |
| l-Lysine HCl, 78% | 0.28 |
| l-Methionine, 98% | 0.21 |
| l-Threonine, 98% | 0.10 |
| Dicalcium phosphate | 0.75 |
| Limestone | 0.76 |
| Vitamin and mineral Premix a | 2.00 |
| Nutrient composition b | |
| Digestible energy (MJ/kg) | 14.57 |
| Crude protein (%) | 19.12 |
| Calcium (%) | 0.77 |
| Available phosphorus (%) | 0.45 |
| Lysine (%) | 1.38 |
| Methionine (%) | 0.52 |
| Threonine (%) | 0.85 |
| Items | CON | LPS | LAB + LPS | p-Value |
|---|---|---|---|---|
| Initial BW (kg) | 7.13 ± 0.45 | 7.35 ± 0.18 | 6.94 ± 0.08 | 0.59 |
| Final BW (kg) | 14.55 ± 0.65 | 13.81 ± 0.64 | 13.9 ± 0.54 | 0.66 |
| ADFI (g) | 549.50 ± 48.33 | 561.50 ± 49.26 | 553.50 ± 23.80 | 0.97 |
| ADG (g) | 265.00 ± 9.26 | 230.75 ± 21.44 | 248.75 ± 20.02 | 0.43 |
| F/G | 2.07 ± 0.13 | 2.50 ± 0.32 | 2.29 ± 0.28 | 0.52 |
| Items | CON | LPS | LAB + LPS | p-Value |
|---|---|---|---|---|
| Jejunum | ||||
| Villus height (um) | 269.40 ± 21.66 | 257.59 ± 15.36 | 253.85 ± 15.26 | 0.81 |
| Crypt depth (mm) | 72.15 ± 2.59 a,b | 78.18 ± 5.59 a | 61.36 ± 4.55 b | 0.05 |
| VCR | 3.92 ± 0.27 | 3.46 ± 0.36 | 4.1 ± 0.24 | 0.32 |
| Ileum | ||||
| Villus height (um) | 284.21 ± 14.22 | 263.5 ± 22.84 | 250.08 ± 10.30 | 0.37 |
| Crypt depth (mm) | 82.43 ± 3.22 b | 102.75 ± 6.62 a | 73.58 ± 3.27 b | < 0.01 |
| VCR | 3.60 ± 0.32 a | 2.78 ± 0.24 b | 3.47 ± 0.17 a,b | 0.08 |
| Items | CON | LPS | LAB + LPS | p-Value |
|---|---|---|---|---|
| 8-OHdG (pg/mL) | 28.11 ± 3.07 | 36.38 ± 3.35 | 33.06 ± 2 | 0.17 |
| MDA (nmol/mL) | 11.93 ± 0.51 b | 15.07 ± 0.59 a | 12.07 ± 0.94 b | 0.01 |
| GSH (umol/mL) | 0.2 ± 0.04 | 0.13 ± 0.03 | 0.16 ± 0.03 | 0.48 |
| GSSG (nmol/mL) | 17.61 ± 1.54 | 17.22 ± 1.06 | 17.88 ± 0.72 | 0.95 |
| CAT (U/mL) | 228.06 ± 20.76 a | 136.8 ± 7.49 b | 164.94 ± 15.07 b | <0.01 |
| GSH-Px (U/mL) | 58.05 ± 4.68 a | 43.27 ± 4.09 b | 54.49 ± 3.63 a,b | 0.07 |
| GR (U/mL) | 149.7 ± 9.56 b | 115.49 ± 12.33 c | 206.46 ± 7.14 a | <0.01 |
| SOD (U/mL) | 4.19 ± 0.46 a | 2.88 ± 0.3 b | 2.54 ± 0.09 b | 0.01 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, F.; Chen, J.; Chen, Q.; Yang, L.; Yin, J.; Li, Y.; Huang, X. Lactobacillus delbrueckii Protected Intestinal Integrity, Alleviated Intestinal Oxidative Damage, and Activated Toll-Like Receptor–Bruton’s Tyrosine Kinase–Nuclear Factor Erythroid 2-Related Factor 2 Pathway in Weaned Piglets Challenged with Lipopolysaccharide. Antioxidants 2021, 10, 468. https://doi.org/10.3390/antiox10030468
Chen F, Chen J, Chen Q, Yang L, Yin J, Li Y, Huang X. Lactobacillus delbrueckii Protected Intestinal Integrity, Alleviated Intestinal Oxidative Damage, and Activated Toll-Like Receptor–Bruton’s Tyrosine Kinase–Nuclear Factor Erythroid 2-Related Factor 2 Pathway in Weaned Piglets Challenged with Lipopolysaccharide. Antioxidants. 2021; 10(3):468. https://doi.org/10.3390/antiox10030468
Chicago/Turabian StyleChen, Fengming, Jiayi Chen, Qinghua Chen, Lingyuan Yang, Jie Yin, Yinghui Li, and Xingguo Huang. 2021. "Lactobacillus delbrueckii Protected Intestinal Integrity, Alleviated Intestinal Oxidative Damage, and Activated Toll-Like Receptor–Bruton’s Tyrosine Kinase–Nuclear Factor Erythroid 2-Related Factor 2 Pathway in Weaned Piglets Challenged with Lipopolysaccharide" Antioxidants 10, no. 3: 468. https://doi.org/10.3390/antiox10030468
APA StyleChen, F., Chen, J., Chen, Q., Yang, L., Yin, J., Li, Y., & Huang, X. (2021). Lactobacillus delbrueckii Protected Intestinal Integrity, Alleviated Intestinal Oxidative Damage, and Activated Toll-Like Receptor–Bruton’s Tyrosine Kinase–Nuclear Factor Erythroid 2-Related Factor 2 Pathway in Weaned Piglets Challenged with Lipopolysaccharide. Antioxidants, 10(3), 468. https://doi.org/10.3390/antiox10030468