CUPRAC-Reactive Advanced Glycation End Products as Prognostic Markers of Human Acute Myocardial Infarction
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents
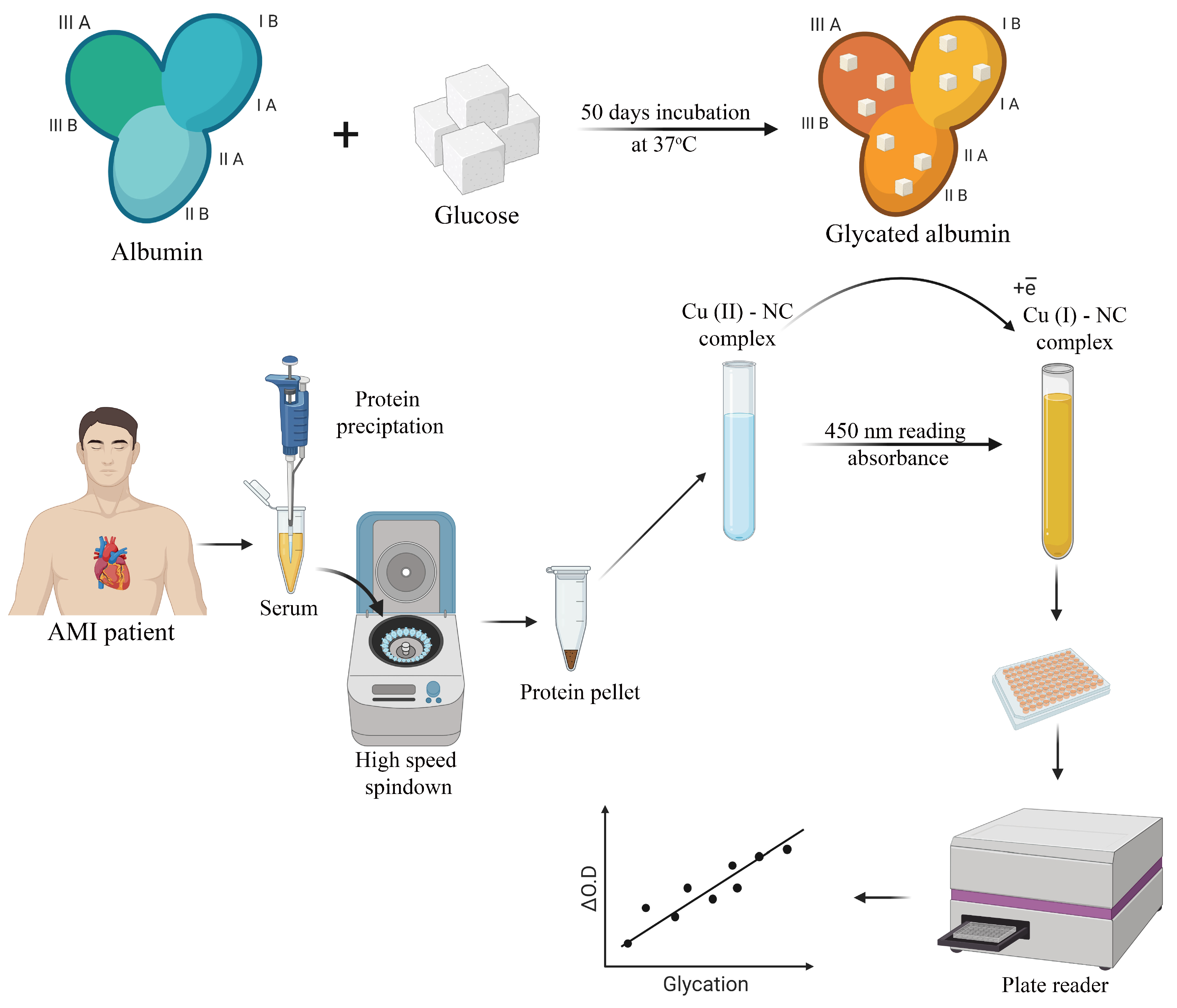
2.2. Albumin Glycation In Vitro
2.3. Human Serum Sampling and Preparation
2.4. CUPRAC Measurement of AGEs in Protein Samples
2.5. Statistical Analyses
3. Results and Discussion
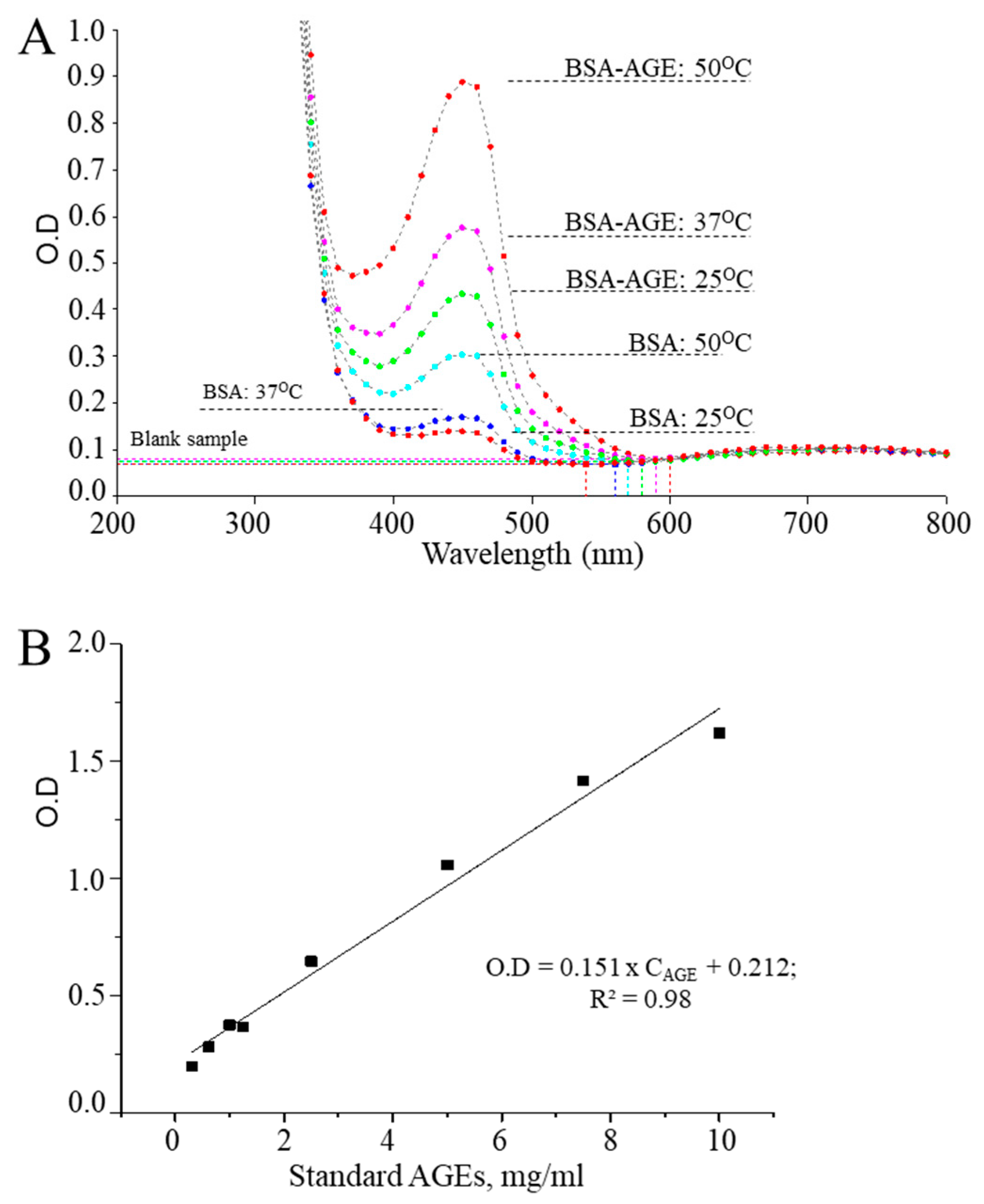
3.1. Optimization of CUPRAC Assays for Detecting AGEs
3.2. CUPRAC Measurement Results of AGEs
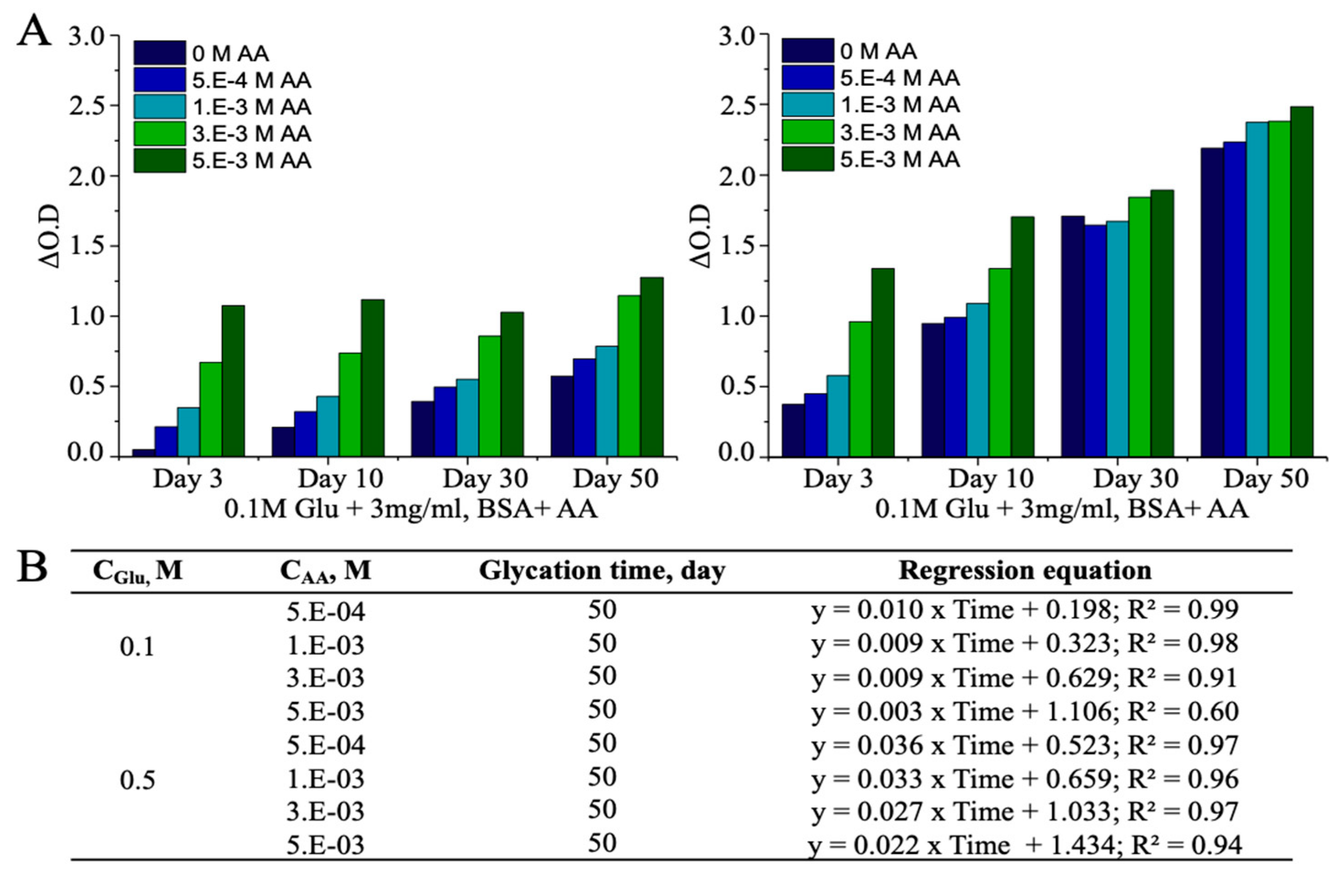
3.3. Ascorbic Acid (AA)-Induced Alterations in AGEs
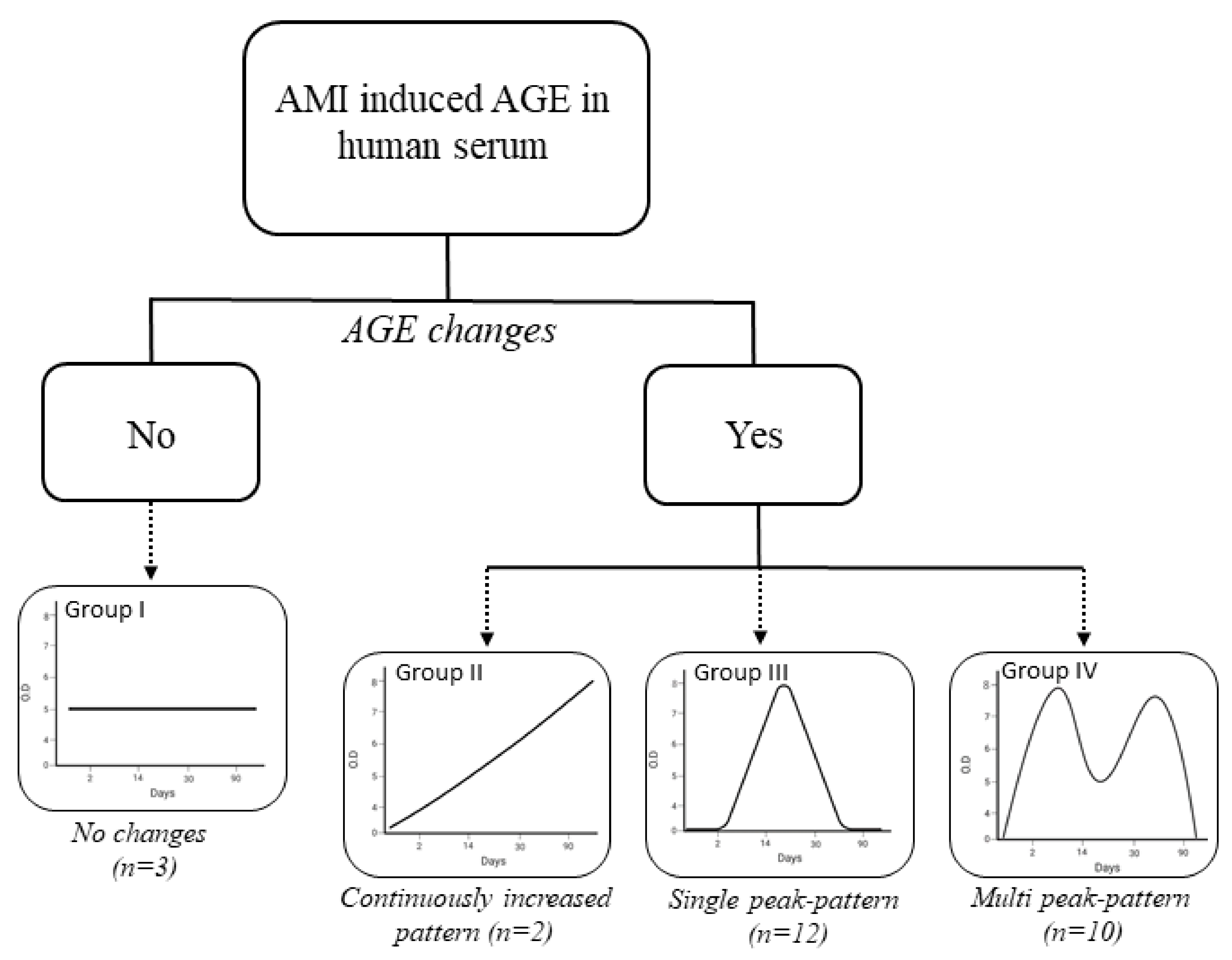
3.4. Detection of AMI-Induced AGEs in Human Serum
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Erdmann, J.; Linsel-Nitschke, P.; Schunkert, H. Genetic causes of myocardial infarction: New insights from genome-wide association studies. Dtsch. Arztebl. Int. 2010, 107, 694–699. [Google Scholar]
- Jensen, G.; Nyboe, J.; Appleyard, M.; Schnohr, P. Risk factors for acute myocardial infarction in Copenhagen, II: Smoking, alcohol intake, physical activity, obesity, oral contraception, diabetes, lipids, and blood pressure. Eur. Heart J. 1991, 12, 298–308. [Google Scholar] [CrossRef]
- Selvin, E.; Rawlings, A.M.; Lutsey, P.L.; Maruthur, N.; Pankow, J.S.; Steffes, M.; Coresh, J. Fructosamine and Glycated Albumin and the Risk of Cardiovascular Outcomes and Death. Circulation 2015, 132, 269–277. [Google Scholar] [CrossRef]
- Chen, Y.; Tao, Y.; Zhang, L.; Xu, W.; Zhou, X. Diagnostic and prognostic value of biomarkers in acute myocardial infarction. Postgrad. Med. J. 2019, 95, 210–216. [Google Scholar] [CrossRef] [PubMed]
- Kristono, G.A.; Holley, A.S.; Hally, K.E.; Brunton-O’Sullivan, M.M.; Shi, B.; Harding, S.A.; Larsen, P.D. An IL-6-IL-8 score derived from principal component analysis is predictive of adverse outcome in acute myocardial infarction. Cytokine X 2020, 2, 100037. [Google Scholar] [CrossRef] [PubMed]
- Aleshin, A.; Ananthakrishnan, R.; Li, Q.; Rosario, R.; Lu, Y.; Qu, W.; Song, F.; Bakr, S.; Szabolcs, M.; D’Agati, V.; et al. RAGE modulates myocardial injury consequent to LAD infarction via impact on JNK and STAT signaling in a murine model. Am. J. Physiol. Heart Circ. Physiol. 2008, 294, H1823–H1832. [Google Scholar] [CrossRef] [PubMed]
- Ramya, R.; Coral, K.; Bharathidevi, S.R. RAGE silencing deters CML-AGE induced inflammation and TLR4 expression in endothelial cells. Exp. Eye Res. 2021, 108519. [Google Scholar] [CrossRef] [PubMed]
- Blackburn, N.J.R.; Vulesevic, B.; McNeill, B.; Cimenci, C.E.; Ahmadi, A.; Gonzalez-Gomez, M.; Ostojic, A.; Zhong, Z.; Brownlee, M.; Beisswenger, P.J.; et al. Methylglyoxal-derived advanced glycation end products contribute to negative cardiac remodeling and dysfunction post-myocardial infarction. Basic Res. Cardiol. 2017, 112, 57. [Google Scholar] [CrossRef]
- Bayarsaikhan, D.; Bayarsaikhan, G.; Lee, B. AGE-RAGE System and Its Application in Stem Cell Therapy. J. Stem Cell Res. Dev. Ther. 2021, 7, 060. [Google Scholar]
- Thornalley, P.J.; Langborg, A.; Minkhas, H.S. Formation of glyoxal, methylglyoxal and 3-deoxyglucosone in the glycation of proteins by glucose. Biochem. J. 1999, 344, 109–116. [Google Scholar] [CrossRef]
- Chen, J.-H.; Lin, X.; Bu, C.; Zhang, X. Role of advanced glycation end products in mobility and considerations in possible dietary and nutritional intervention strategies. Nutr. Metab. (Lond.) 2018, 15, 72. [Google Scholar] [CrossRef] [PubMed]
- Berlett, B.S.; Stadtman, E.R. Protein oxidation in aging, disease, and oxidative stress. J. Biol. Chem. 1997, 272, 20313–20316. [Google Scholar] [CrossRef] [PubMed]
- Chao, P.-C.; Huang, C.-N.; Hsu, C.-C.; Yin, M.-C.; Guo, Y.-R. Association of dietary AGEs with circulating AGEs, glycated LDL, Il-1alpha and MCP-1 levels in type 2 diabetic patients. Eur. J. Nutr. 2010, 49, 429–434. [Google Scholar] [CrossRef]
- Pamplona, R.; Dalfó, E.; Ayala, V.; Bellmunt, M.J.; Prat, J.; Ferrer, I.; Portero-Otín, M. Proteins in human brain cortex are modified by oxidation, glycoxidation, and lipoxidation. Effects of Alzheimer disease and identification of lipoxidation targets. J. Biol. Chem. 2005, 280, 21522–21530. [Google Scholar] [CrossRef]
- Lyons, T.J.; Jenkins, A.J. Glycation, oxidation, and lipoxidation in the development of the complications of diabetes: A carbonyl stress hypothesis. Diabetes Rev. (Alex.) 1997, 5, 365–391. [Google Scholar]
- Barnaby, O.S.; Wa, C.; Cerny, R.L.; Clarke, W.; Hage, D.S. Quantitative analysis of glycation sites on human serum albumin using (16)O/(18)O-labeling and matrix-assisted laser desorption/ionization time-of-flight mass spectrometry. Clin. Chim. Acta 2010, 411, 1102–1110. [Google Scholar] [CrossRef]
- Stefanowicz, P.; Kijewska, M.; Kluczyk, A.; Szewczuk, Z. Detection of glycation sites in proteins by high-resolution mass spectrometry combined with isotopic labeling. Anal. Biochem. 2010, 400, 237–243. [Google Scholar] [CrossRef]
- Miyamoto, H.; Kohzuma, T.; Ohnishi, A. Changes in the albumin glycation site, plasma pentosidine and esRAGE concentrations before and after intensive diabetic treatment in patients with abnormally high glycated albumin levels. Ann. Clin. Biochem. 2018, 55, 84–91. [Google Scholar] [CrossRef] [PubMed]
- Inoue, Y.; Inoue, M.; Saito, M.; Yoshikawa, H.; Tamiya, E. Sensitive Detection of Glycated Albumin in Human Serum Albumin Using Electrochemiluminescence. Anal. Chem. 2017, 89, 5909–5915. [Google Scholar] [CrossRef]
- Niwa, T. Mass spectrometry for the study of protein glycation in disease. Mass Spectrom. Rev. 2006, 25, 713–723. [Google Scholar] [CrossRef]
- Apak, R.; Güçlü, K.; Ozyürek, M.; Karademir, S.E. Novel total antioxidant capacity index for dietary polyphenols and vitamins C and E, using their cupric ion reducing capability in the presence of neocuproine: CUPRAC method. J. Agric. Food Chem. 2004, 52, 7970–7981. [Google Scholar] [CrossRef] [PubMed]
- Cekiç, S.D.; Başkan, K.S.; Tütem, E.; Apak, R. Modified cupric reducing antioxidant capacity (CUPRAC) assay for measuring the antioxidant capacities of thiol-containing proteins in admixture with polyphenols. Talanta 2009, 79, 344–351. [Google Scholar] [CrossRef] [PubMed]
- Uzunboy, S.; Çekiç, S.D.; Eksin, E.; Erdem, A.; Apak, R. CUPRAC colorimetric and electroanalytical methods determining antioxidant activity based on prevention of oxidative DNA damage. Anal. Biochem. 2017, 518, 69–77. [Google Scholar] [CrossRef]
- Bayarsaikhan, G.; Avan, A.N.; Çekiç, S.D.; Apak, R. Use of modified CUPRAC and dinitrophenylhydrazine colorimetric methods for simultaneous measurement of oxidative protein damage and antioxidant defense against oxidation. Talanta 2019, 204, 613–625. [Google Scholar] [CrossRef]
- Çekiç, S.D.; Çetinkaya, A.; Avan, A.N.; Apak, R. Correlation of total antioxidant capacity with reactive oxygen species (ROS) consumption measured by oxidative conversion. J. Agric. Food Chem. 2013, 61, 5260–5270. [Google Scholar] [CrossRef] [PubMed]
- Abdelkader, H.; Longman, M.; Alany, R.G.; Pierscionek, B. On the Anticataractogenic Effects of L-Carnosine: Is It Best Described as an Antioxidant, Metal-Chelating Agent or Glycation Inhibitor? Oxid. Med. Cell. Longev. 2016, 3240261. [Google Scholar] [CrossRef]
- Genuth, S.; Sun, W.; Cleary, P.; Gao, X.; Sell, D.R.; Lachin, J. DCCT/EDIC Research Group; Monnier, V.M. Skin advanced glycation end products glucosepane and methylglyoxal hydroimidazolone are independently associated with long-term microvascular complication progression of type 1 diabetes. Diabetes 2015, 64, 266–278. [Google Scholar] [CrossRef] [PubMed]
- Poulsen, M.W.; Hedegaard, R.V.; Andersen, J.M.; de Courten, B.; Bügel, S.; Nielsen, J.; Skibsted, L.H.; Dragsted, L.O. Advanced glycation endproducts in food and their effects on health. Food Chem. Toxicol. 2013, 60, 10–37. [Google Scholar] [CrossRef] [PubMed]
- Wilker, S.C.; Chellan, P.; Arnold, B.M.; Nagaraj, R.H. Chromatographic quantification of argpyrimidine, a methylglyoxal-derived product in tissue proteins: Comparison with pentosidine. Anal. Biochem. 2001, 290, 353–358. [Google Scholar] [CrossRef] [PubMed]
- Sadowska-Bartosz, I.; Stefaniuk, I.; Galiniak, S.; Bartosz, G. Glycation of bovine serum albumin by ascorbate in vitro: Possible contribution of the ascorbyl radical. Redox Biol. 2015, 6, 93–99. [Google Scholar] [CrossRef] [PubMed]
- BioRender. Available online: https://app.biorender.com (accessed on 11 January 2021).
- Miller, J.N.; Miller, J.C. Statistics and Chemometrics for Analytical Chemistry, 6th ed.; Pearson: London, UK, 2010; pp. 171–172. [Google Scholar]
- Avan, A.N.; Çekiç, S.D.; Uzunboy, S.; Apak, R. Spectrophotometric Determination of Phenolic Antioxidants in the Presence of Thiols and Proteins. Int. J. Mol. Sci. 2016, 17, 1325. [Google Scholar] [CrossRef] [PubMed]
- Apak, R.; Güçlü, K.; Demirata, B.; Ozyürek, M.; Celik, S.E.; Bektaşoğlu, B.; Berker, K.I.; Ozyurt, D. Comparative evaluation of various total antioxidant capacity assays applied to phenolic compounds with the CUPRAC assay. Molecules 2007, 12, 1496–1547. [Google Scholar] [CrossRef] [PubMed]
- Jun, J.E.; Lee, S.-E.; Lee, Y.-B.; Jee, J.H.; Bae, J.C.; Jin, S.-M.; Hur, K.Y.; Lee, M.-K.; Kim, J.H. Increase in serum albumin concentration is associated with prediabetes development and progression to overt diabetes independently of metabolic syndrome. PLoS ONE 2017, 12, e0176209. [Google Scholar] [CrossRef]
- Eriguchi, R.; Obi, Y.; Rhee, C.M.; Chou, J.A.; Tortorici, A.R.; Mathew, A.T.; Kim, T.; Soohoo, M.; Streja, E.; Kovesdy, C.P.; et al. Changes in urine volume and serum albumin in incident hemodialysis patients. Hemodial. Int. 2017, 21, 507–518. [Google Scholar] [CrossRef] [PubMed]
- Peterson, E.J.; Ng, T.M.H.; Patel, K.A.; Lou, M.; Elkayam, U. Association of admission vs. nadir serum albumin concentration with short-term treatment outcomes in patients with acute heart failure. J. Int. Med. Res. 2018, 46, 3665–3674. [Google Scholar] [CrossRef] [PubMed]
- Gotsman, I.; Shauer, A.; Zwas, D.R.; Tahiroglu, I.; Lotan, C.; Keren, A. Low serum albumin: A significant predictor of reduced survival in patients with chronic heart failure. Clin. Cardiol. 2019, 42, 365–372. [Google Scholar] [CrossRef] [PubMed]
- Furukawa, M.; Kinoshita, K.; Yamaguchi, J.; Hori, S.; Sakurai, A. Sepsis patients with complication of hypoglycemia and hypoalbuminemia are an early and easy identification of high mortality risk. Intern. Emerg. Med. 2019, 14, 539–548. [Google Scholar] [CrossRef]
- Chaudhuri, J.; Bains, Y.; Guha, S.; Kahn, A.; Hall, D.; Bose, N.; Gugliucci, A.; Kapahi, P. The Role of Advanced Glycation End Products in Aging and Metabolic Diseases: Bridging Association and Causality. Cell Metab. 2018, 28, 337–352. [Google Scholar] [CrossRef]
- Frolov, A.; Hoffmann, R. Identification and relative quantification of specific glycation sites in human serum albumin. Anal. Bioanal. Chem. 2010, 397, 2349–2356. [Google Scholar] [CrossRef]
- Spiller, S.; Li, Y.; Blüher, M.; Welch, L.; Hoffmann, R. Diagnostic Accuracy of Protein Glycation Sites in Long-Term Controlled Patients with Type 2 Diabetes Mellitus and Their Prognostic Potential for Early Diagnosis. Pharmaceuticals 2018, 11, 38. [Google Scholar] [CrossRef]
- Soboleva, A.; Schmidt, R.; Vikhnina, M.; Grishina, T.; Frolov, A. Maillard Proteomics: Opening New Pages. Int. J. Mol. Sci. 2017, 18, 2677. [Google Scholar] [CrossRef]
- Assefa, A.D.; Ko, E.Y.; Moon, S.H.; Keum, Y.S. Antioxidant and antiplatelet activities of flavonoid-rich fractions of three citrus fruits from Korea. 3 Biotech 2016, 6, 109. [Google Scholar] [CrossRef] [PubMed]
- Nemet, I.; Monnier, V.M. Vitamin C degradation products and pathways in the human lens. J. Biol. Chem. 2011, 286, 37128–37136. [Google Scholar] [CrossRef]
- Zhu, Y.; Zhao, Y.; Wang, P.; Ahmedna, M.; Ho, C.-T.; Sang, S. Tea Flavanols Block Advanced Glycation of Lens Crystallins Induced by Dehydroascorbic Acid. Chem. Res. Toxicol. 2015, 28, 135–143. [Google Scholar] [CrossRef]
- Sadowska-Bartosz, I.; Bartosz, G. Ascorbic acid and protein glycation in vitro. Chem. Biol. Interact. 2015, 240, 154–162. [Google Scholar] [CrossRef] [PubMed]
- Simpson, G.L.; Ortwerth, B.J. The non-oxidative degradation of ascorbic acid at physiological conditions. Biochim. Biophys. Acta 2000, 1501, 12–24. [Google Scholar] [CrossRef]
- Sharma, A.; Kaur, S.; Sarkar, M.; Sarin, B.C.; Changotra, H. The AGE-RAGE Axis and RAGE Genetics in Chronic Obstructive Pulmonary Disease. Clin. Rev. Allergy Immunol. 2020, 1–15. [Google Scholar] [CrossRef]
- Nowotny, K.; Schröter, D.; Schreiner, M.; Grune, T. Dietary advanced glycation end products and their relevance for human health. Ageing Res. Rev. 2018, 47, 55–66. [Google Scholar] [CrossRef] [PubMed]
- Yamagishi, S.-I.; Nakamura, N.; Matsui, T. Glycation and cardiovascular disease in diabetes: A perspective on the concept of metabolic memory. J. Diabetes 2017, 9, 141–148. [Google Scholar] [CrossRef] [PubMed]
- Son, M.; Kang, W.C.; Oh, S.; Bayarsaikhan, D.; Ahn, H.; Lee, J.; Park, H.; Lee, S.; Choi, J.; Lee, H.S.; et al. Advanced glycation end-product (AGE)-albumin from activated macrophage is critical in human mesenchymal stem cells survival and post-ischemic reperfusion injury. Sci. Rep. 2017, 7, 11593. [Google Scholar] [CrossRef]
- Byun, K.; Yoo, Y.; Son, M.; Lee, J.; Jeong, G.-B.; Park, Y.M.; Salekdeh, G.H.; Lee, B. Advanced glycation end-products produced systemically and by macrophages: A common contributor to inflammation and degenerative diseases. Pharmacol. Ther. 2017, 177, 44–55. [Google Scholar] [CrossRef]
- Mulder, D.J.; van Haelst, P.L.; Graaff, R.; Gans, R.O.; Zijlstra, F.; Smit, A.J. Skin autofluorescence is elevated in acute myocardial infarction and is associated with the one-year incidence of major adverse cardiac events. Neth. Heart J. 2009, 17, 162–168. [Google Scholar] [CrossRef][Green Version]
- Costa, T.N.; Cassaro- Strunz, C.M.; Nicolau, J.C.; Gutierrez, P.S. Comparison of MB fraction of creatine kinase mass and troponin I serum levels with necropsy findings in acute myocardial infarction. Am. J. Cardiol. 2008, 101, 311–314. [Google Scholar] [CrossRef] [PubMed]
- Brunetti, N.D.; Quagliara, D.; Di Biase, M. Troponin ratio and risk stratification in subjects with acute coronary syndrome undergoing percutaneous coronary intervention. Eur. J. Intern. Med. 2008, 19, 435–442. [Google Scholar] [CrossRef] [PubMed]
- Rocha, A.S.; Dassa, N.P.; Pittella, F.J.; Barbosa, O.N.; Brito, J.O.; Tura, B.; Silva, P.R. High mortality associated with precluded coronary artery bypass surgery caused by severe distal coronary artery disease. Circulation 2005, 112, I328–I331. [Google Scholar]
- Gruberg, L.; Sudarsky, D.; Kerner, A.; Hammerman, H.; Kapeliovich, M.; Beyar, R. Troponin-positive, CK-MB-negative acute myocardial infarction: Clinical, electrocardiographic and angiographic characteristics. J. Invasive Cardiol. 2008, 20, 125–128. [Google Scholar] [PubMed]
- Rao, S.V.; Ohman, E.M.; Granger, C.B.; Armstrong, P.W.; Gibler, W.B.; Christenson, R.H.; Hasselblad, V.; Stebbins, A.; McNulty, S.; Newby, L.K. Prognostic value of isolated troponin elevation across the spectrum of chest pain syndromes. Am. J. Cardiol. 2003, 91, 936–940. [Google Scholar] [CrossRef]
- Chien, S.-C.; Chen, C.-Y.; Lin, C.-F.; Yeh, H.-I. Critical appraisal of the role of serum albumin in cardiovascular disease. Biomark. Res. 2017, 5, 31. [Google Scholar] [CrossRef] [PubMed]


| Reaction Mixture | CUPRAC Reactive AGEs, mg/mL | AGEs/BSA | ||||||
|---|---|---|---|---|---|---|---|---|
| D-5 | D-15 | D-30 | D-50 | D-5 | D-15 | D-30 | D-50 | |
| 3 mg/mL BSA + 0.1M Glu | 0.48 | 0.13 | 2.64 | 3.33 | 0.16 | 0.04 | 0.88 | 1.11 |
| 3 mg/mL BSA + 0.2M Glu | 1.08 | 2.06 | 5.87 | 7.28 | 0.36 | 0.69 | 1.96 | 2.43 |
| 3 mg/mL BSA + 0.3M Glu | 2.10 | 3.89 | 8.80 | 10.59 | 0.70 | 1.30 | 2.93 | 3.53 |
| 3 mg/mL BSA + 0.4M Glu | 2.32 | 5.23 | 10.66 | 12.20 | 0.77 | 1.74 | 3.55 | 4.07 |
| 3 mg/mL BSA + 0.5M Glu | 3.10 | 6.43 | 12.89 | 15.12 | 1.03 | 2.14 | 4.30 | 5.04 |
| Reaction Mixture | CUPRAC-Reactive AGEs, mg/mL | |||
|---|---|---|---|---|
| Day-3 | Day-10 | Day-30 | Day-50 | |
| BSA+ 0.5M Glu + 2.5E-04M AA | 1.07 | 4.86 | 9.90 | 13.08 |
| BSA+ 0.5M Glu + 5.0E-04M AA | 1.57 | 5.15 | 9.48 | 13.37 |
| BSA+ 0.5M Glu + 1.0E-03M AA | 2.42 | 5.80 | 9.65 | 14.29 |
| BSA+ 0.5M Glu + 2.5E-03M AA | 4.95 | 7.44 | 10.79 | 14.33 |
| BSA+ 0.5M Glu + 5.0E-03M AA | 7.44 | 9.87 | 11.12 | 15.02 |
| BSA+ 2.5E-04M AA | N.A | N.A | N.A | N.A |
| BSA+ 5.0E-04M AA | N.A | N.A | N.A | 0.35 |
| BSA+ 1.0E-03M AA | N/A | N.A | N.A | 1.20 |
| BSA+ 2.5E-03M AA | 1.73 | 1.59 | 0.88 | 1.51 |
| BSA+ 5.0E-03M AA | 4.19 | 4.52 | 3.77 | 2.23 |
| AGE/total Serum Protein | ||||||
|---|---|---|---|---|---|---|
| Patient No. | Group I (10% ≤ RSD) | |||||
| Day 0 | Day 2 | Day 14 | Day 30 | Day 90 | RSD, % | |
| 1 | 1.1 | 1.6 | 1.6 | 1.9 | 1.7 | 12.7 |
| 3 | 1.1 | 1.4 | 1.5 | 1.4 | 1.7 | 10.3 |
| 4 | 1.1 | 1.6 | 1.7 | 1.8 | 1.7 | 12.3 |
| 5 | 1.0 | 1.5 | 0.0 | 1.7 | 1.3 | 13.2 |
| 13 | 1.2 | 1.6 | 1.3 | 1.0 | 1.4 | 11.1 |
| 16 | 0.9 | 0.9 | 1.0 | 1.0 | 2.1 | 26.3 |
| 17 | 1.0 | 1.1 | 1.5 | 1.6 | 1.4 | 11.9 |
| 22 | 1.0 | 1.2 | 1.3 | 1.4 | 1.6 | 12.1 |
| Patient No. | Group II (5% < RSD < 10%) | |||||
| Day 0 | Day 2 | Day 14 | Day 30 | Day 90 | RSD, % | |
| 6 | 1.3 | 1.6 | 1.6 | 1.5 | 1.6 | 5.4 |
| 9 | 1.2 | 1.6 | 1.5 | 1.5 | 1.7 | 9.2 |
| 10 | 1.5 | 1.8 | 1.6 | 1.5 | 1.4 | 6.9 |
| 11 | 1.2 | 1.4 | 1.5 | 1.7 | 1.5 | 8.9 |
| 12 | 1.1 | 1.4 | 1.6 | 1.6 | 1.5 | 9.2 |
| 14 | 1.3 | 1.6 | 1.5 | 1.5 | 1.9 | 9.7 |
| 15 | 1.0 | 1.5 | 1.5 | 1.5 | 1.4 | 9.6 |
| 18 | 1.3 | 1.7 | 1.7 | 1.6 | 1.3 | 8.9 |
| 19 | 1.2 | 1.3 | 1.1 | 1.5 | 1.5 | 7.8 |
| 20 | 1.2 | 1.4 | 1.6 | 1.4 | 1.5 | 6.2 |
| 21 | 1.1 | 1.0 | 1.3 | 1.3 | 1.2 | 5.9 |
| 23 | 1.2 | 1.5 | 1.6 | 1.5 | 1.4 | 6.0 |
| 25 | 1.0 | 0.9 | 1.3 | 1.1 | 1.3 | 8.9 |
| 26 | 1.1 | 1.1 | 1.4 | 1.3 | 1.2 | 7.0 |
| 27 | 1.3 | 1.6 | 1.5 | 1.1 | 0.9 | 14.6 |
| 28 | 1.0 | 1.2 | 1.5 | 1.1 | 1.2 | 10.1 |
| Patient No. | Group III (5% ≥ RSD) | |||||
| Day 0 | Day 2 | Day 14 | Day 30 | Day 90 | RSD, % | |
| 7 | 1.4 | 1.5 | 1.6 | 1.5 | 1.6 | 4.0 |
| 8 | 1.3 | 1.4 | 1.5 | 1.3 | 1.4 | 3.7 |
| 24 | 1.4 | 1.3 | 1.3 | 1.3 | 1.4 | 3.2 |
| Prognostic Factors | Group I | Group II | Group III | Group IV |
|---|---|---|---|---|
| Number of patients | 3 | 2 | 12 | 10 |
| Baseline Troponin I | 2323.5 ± 3940.0 | 3505.1 ± 4340.7 | 200.6 ± 287.4 | 3659 ± 8334.3 |
| Peak Troponin I | 25,000 | 25,000 | 21,465 ± 7454.8 | 24,874.7 ± 434.0 |
| Peak/Baseline value of Troponin I | 10.8 | 7.1 | 107.0 | 6.8 |
| Baseline CKMB | 52.6 ± 85.6 | 49.2 ± 64.1 | 21.7 ± 44.9 | 3.2 ± 3.2 |
| Peak CKMB | 300 | 248 ± 73.6 | 209.8 ± 111.6 | 221.2 ± 90.1 |
| Peak/Baseline value of CKMB | 5.7 | 3.4 | 9.7 | 69.1 |
| Extent of CAD | ||||
| 1 vessel | 2 (66.7%) | 0 (0.0%) | 4 (33.3%) | 3 (30.0%) |
| 2 vessel | 1 (33.3%) | 2 (100%) | 4 (33.3%) | 3 (30.0%) |
| 3 vessel | 0 (0.0%) | 0 (0.0%) | 4 (33.3%) | 4 (40.0%) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bayarsaikhan, G.; Bayarsaikhan, D.; Oh, P.C.; Kang, W.C.; Lee, B. CUPRAC-Reactive Advanced Glycation End Products as Prognostic Markers of Human Acute Myocardial Infarction. Antioxidants 2021, 10, 434. https://doi.org/10.3390/antiox10030434
Bayarsaikhan G, Bayarsaikhan D, Oh PC, Kang WC, Lee B. CUPRAC-Reactive Advanced Glycation End Products as Prognostic Markers of Human Acute Myocardial Infarction. Antioxidants. 2021; 10(3):434. https://doi.org/10.3390/antiox10030434
Chicago/Turabian StyleBayarsaikhan, Govigerel, Delger Bayarsaikhan, Pyung Chun Oh, Woong Chol Kang, and Bonghee Lee. 2021. "CUPRAC-Reactive Advanced Glycation End Products as Prognostic Markers of Human Acute Myocardial Infarction" Antioxidants 10, no. 3: 434. https://doi.org/10.3390/antiox10030434
APA StyleBayarsaikhan, G., Bayarsaikhan, D., Oh, P. C., Kang, W. C., & Lee, B. (2021). CUPRAC-Reactive Advanced Glycation End Products as Prognostic Markers of Human Acute Myocardial Infarction. Antioxidants, 10(3), 434. https://doi.org/10.3390/antiox10030434







