Trends in H2S-Donors Chemistry and Their Effects in Cardiovascular Diseases
Abstract
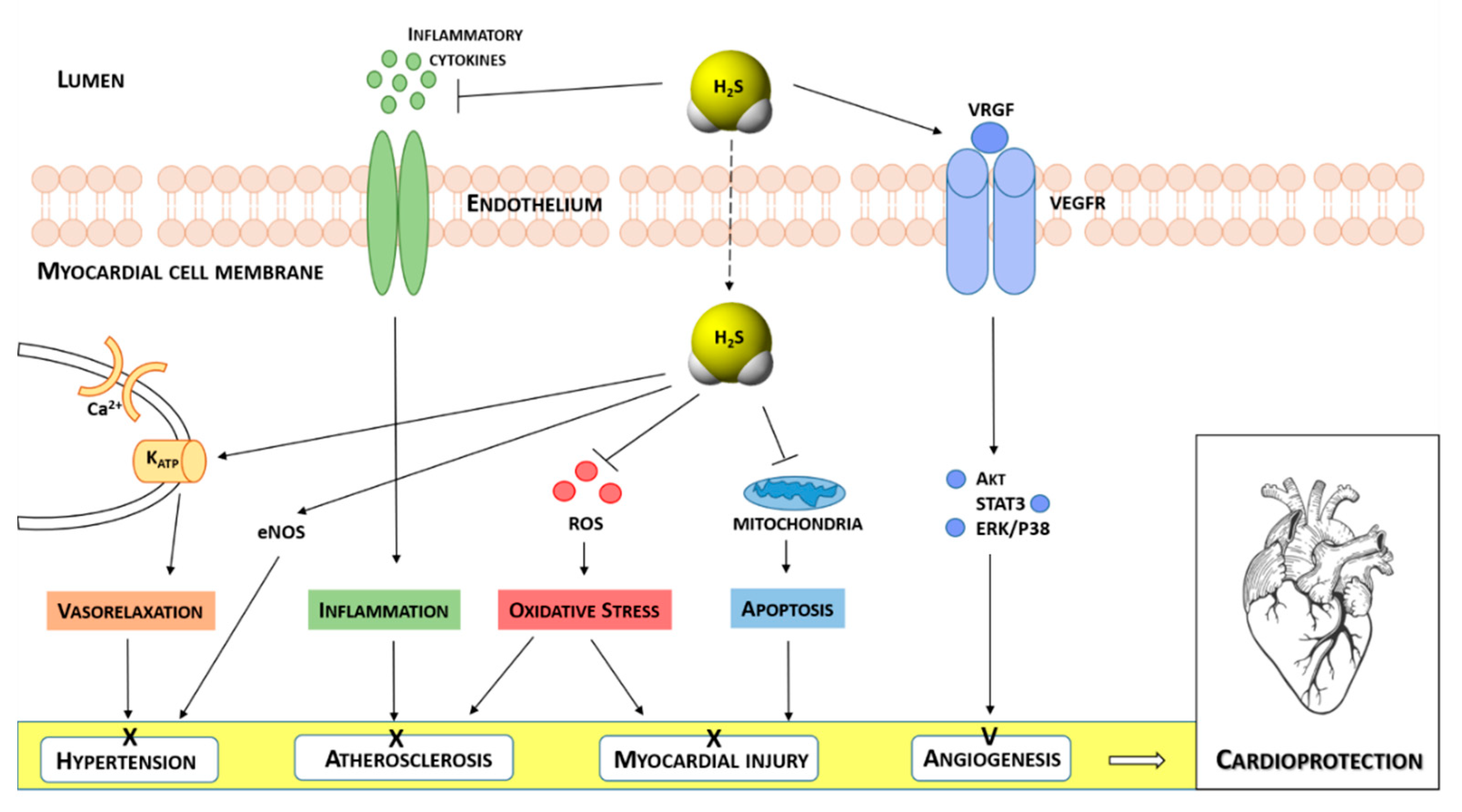
1. Introduction
2. H2S Donors
2.1. Sulfide Salts
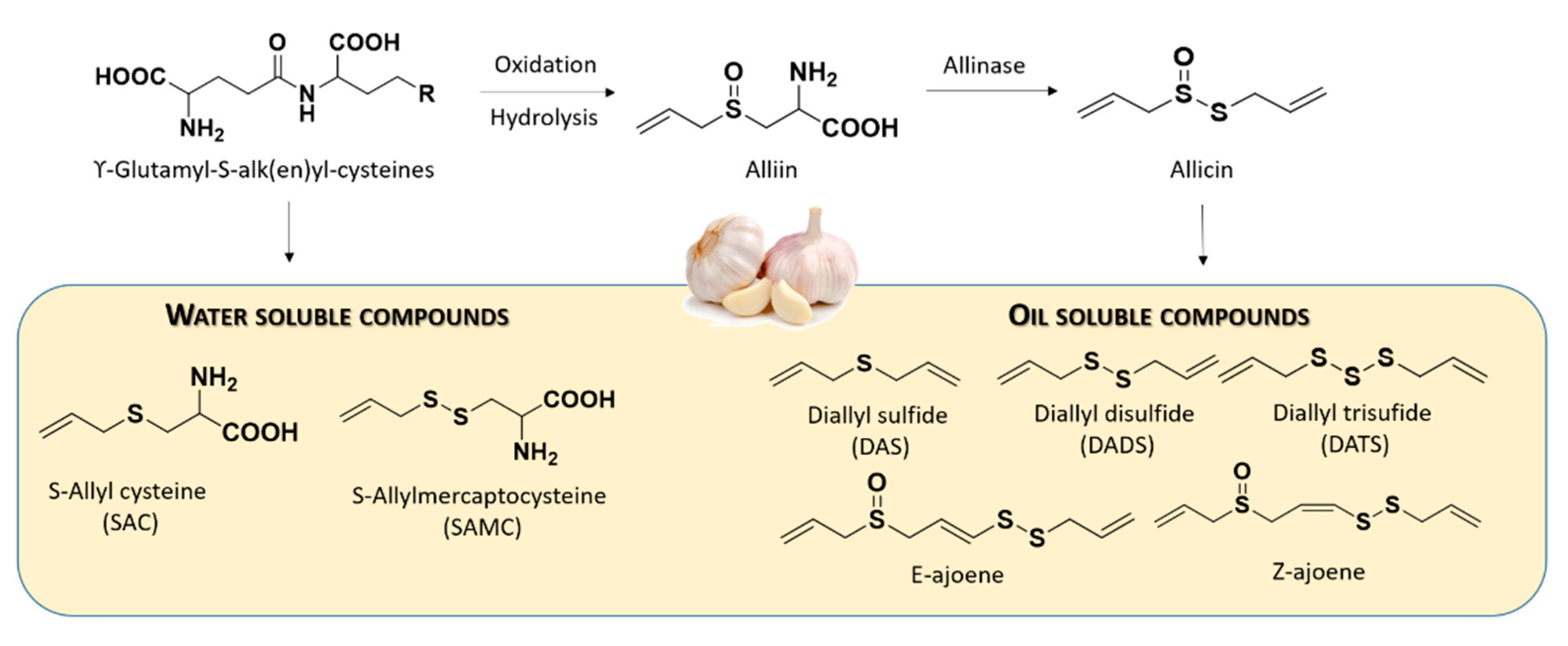
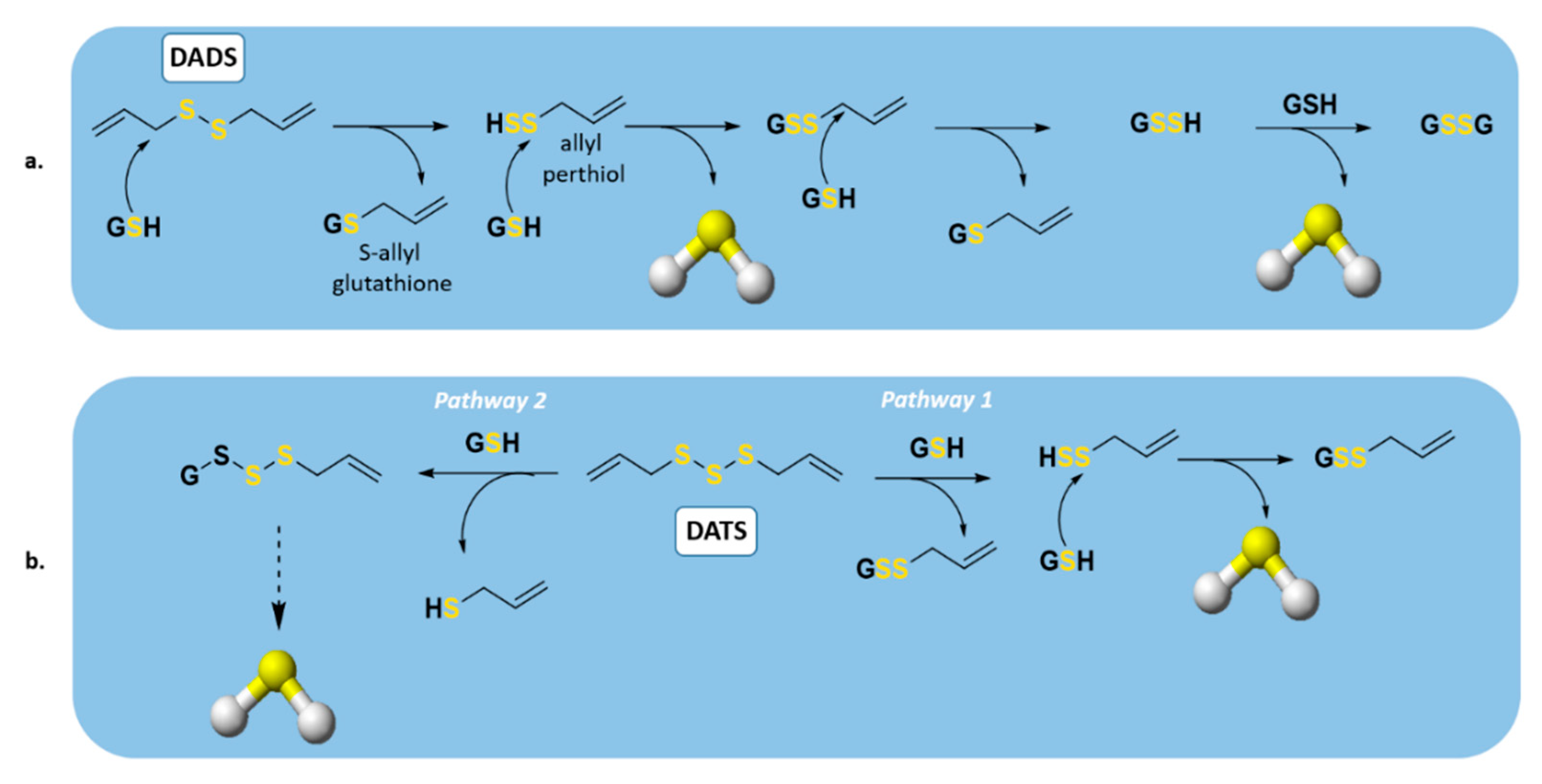
2.2. Naturally Occurring Donors
2.3. Synthetic Donors
2.3.1. Hydrolysis-Triggered Donors
2.3.2. pH-Controllable H2S Donors
2.3.3. Thiol-Triggered Donors
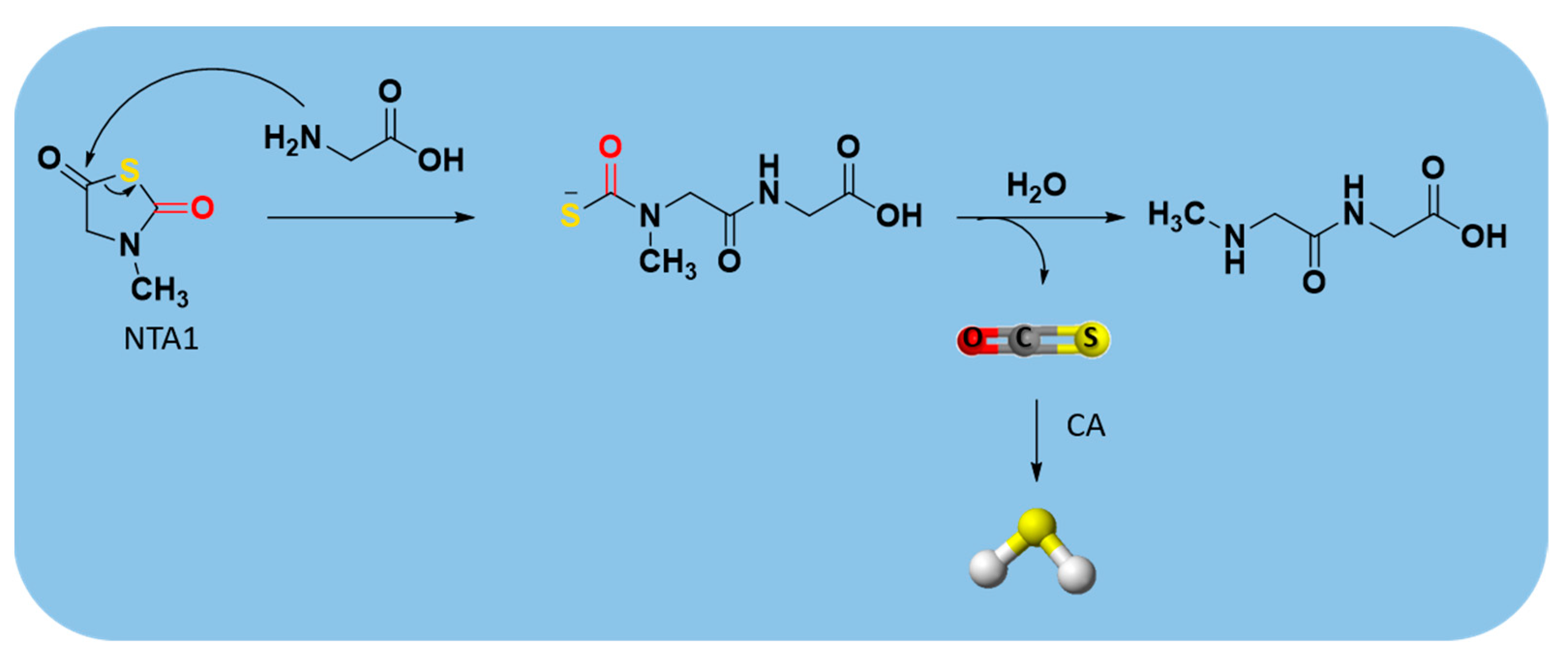
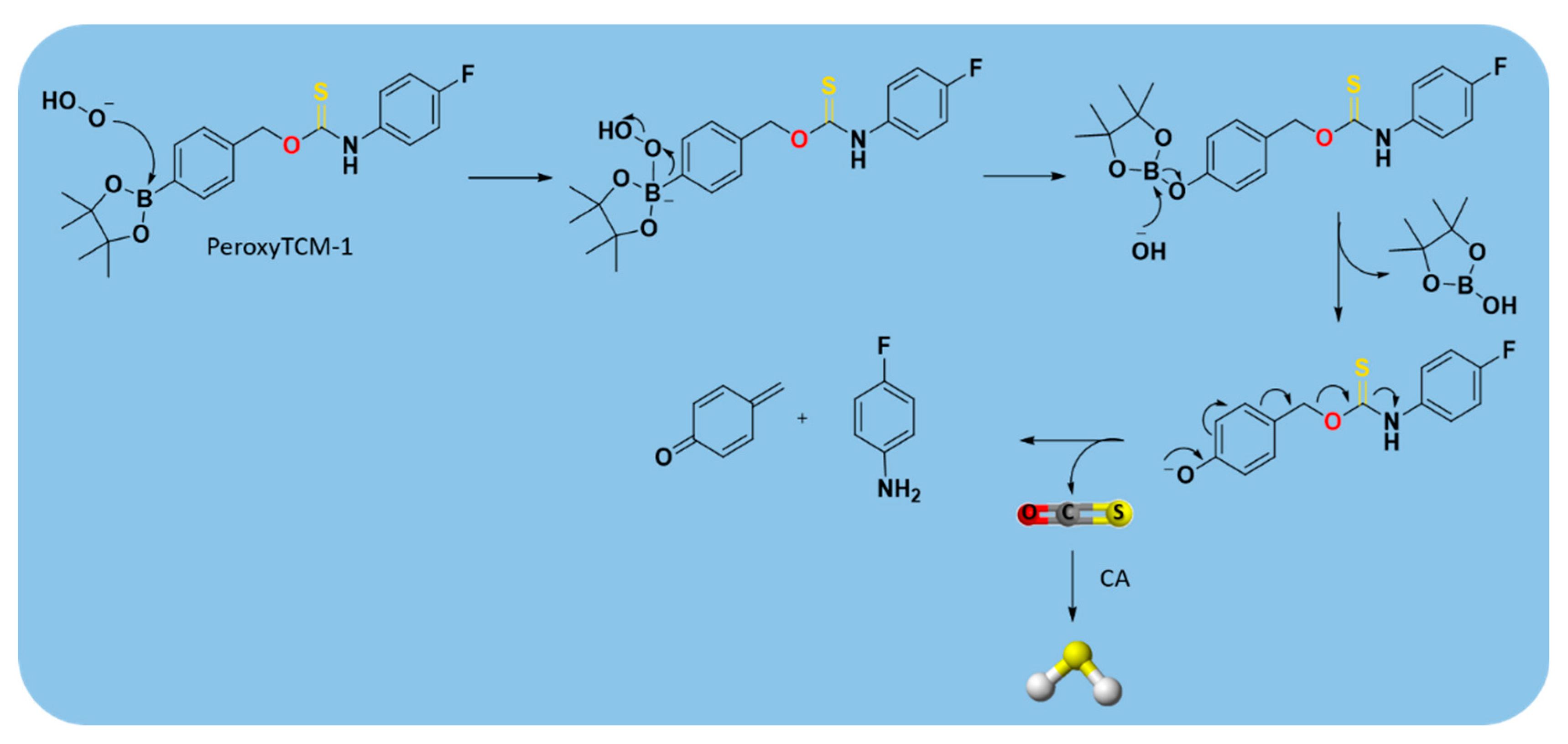
2.3.4. Enzyme-Triggered Donors
2.3.5. Others
3. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Wang, R. Two’s Company, Three’s a Crowd: Can H2S Be the Third Endogenous Gaseous Transmitter? FASEB J. 2002, 16, 1792–1798. [Google Scholar] [CrossRef]
- Ungerer, P.; Wender, A.; Demoulin, G.; Bourasseau, É.; Mougin, P. Application of Gibbs Ensemble and NPT Monte Carlo Sim-Ulation to the Development of Improved Processes for H2S-Rich Gases. Mol. Simul. 2004, 30, 631–648. [Google Scholar] [CrossRef]
- Kabil, O.; Banerjee, R. Redox Biochemistry of Hydrogen Sulfide. J. Biol. Chem. 2010, 285, 21903–21907. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Rose, P.; Moore, P.K. Hydrogen Sulfide and Cell Signaling. Annu. Rev. Pharmacol. Toxicol. 2011, 51, 169–187. [Google Scholar] [CrossRef]
- Gadalla, M.M.; Snyder, S.H. Hydrogen Sulfide as a Gasotransmitter. J. Neurochem. 2010, 113, 14–26. [Google Scholar] [CrossRef]
- Miles, E.W.; Kraus, J.P. Cystathionine β-Synthase: Structure, Function, Regulation, and Location of Homocystinuria-causing Mutations. J. Biol. Chem. 2004, 279, 29871–29874. [Google Scholar] [CrossRef]
- Pan, L.L.; Liu, X.H.; Gong, Q.H.; Yang, H.B.; Zhu, Y.Z. Role of Cystathionine C-Lyase/Hydrogen Sulfide Pathway in Cardio-Vascular Disease: A Novel Therapeutic Strategy? Antioxid. Redox Signal. 2011, 17, 106–118. [Google Scholar] [CrossRef]
- Shibuya, N.; Tanaka, M.; Yoshida, M.; Ogasawara, Y.; Togawa, T.; Ishii, K.; Kimura, H. 3-Mercaptopyruvate Sulfurtransferase Produces Hydrogen Sulfide and Bound Sulfane Sulfur in the Brain. Antioxidants Redox Signal. 2009, 11, 703–714. [Google Scholar] [CrossRef]
- Marcotte, P.; Walsh, C. Active Site-Directed Inactivation of Cystathionine Gamma-Synthetase and Glutamic Pyruvic Transami-Nase by Propargylglycine. Biochem. Biophys. Res. Commun. 1975, 62, 677–682. [Google Scholar] [CrossRef]
- Sun, Q.; Collins, R.; Huang, S.; Holmberg-Schiavone, L.; Anand, G.S.; Tan, C.H.; van-den-Berg, S.; Deng, L.W.; Moore, P.K.; Karlberg, T.; et al. Structural Basis for the Inhibition Mechanism of Human Cystathionine Gamma-Lyase, an Enzyme Responsible for the Production of H2S. J. Biol. Chem. 2009, 284, 3076–3085. [Google Scholar] [CrossRef]
- Pfeffer, M.; Ressler, C. β-Cyanoalanine, An Inhibitor of Rat Liver Cystathionase. Biochem. Pharmacol. 1967, 16, 2299–2308. [Google Scholar] [CrossRef]
- Corvino, A.; Severino, B.; Fiorino, F.; Frecentese, F.; Magli, E.; Perissutti, E.; Santagada, V.; Bucci, M.; Cirino, G.; Kelly, G.; et al. Fragment-Based de Novo Design of a Cystathionine γ-Lyase Selective Inhibitor Blocking Hydrogen Sulfide Production. Sci. Rep. 2016, 6, srep34398. [Google Scholar] [CrossRef]
- Asimakopoulou, A.; Panopoulos, P.; Chasapis, C.T.; Coletta, C.; Zhou, Z.; Cirino, G.; Giannis, A.; Szabo, C.; Spyroulias, G.A.; Papapetropoulos, A. Selectivity of Commonly Used Pharmacological Inhibitors for Cystathionine β Synthase (CBS) and Cystathi-Onine γ Lyase (CSE). Br. J. Pharm. 2013, 169, 922–932. [Google Scholar] [CrossRef]
- Moore, P.K.; Bhatia, M.; Moochhala, S. Hydrogen Sulfide: From the Smell of the past to the Mediator of the Future? Trends Pharmacol. Sci. 2003, 24, 609–611. [Google Scholar] [CrossRef]
- Wang, R. Hydrogen Sulfide: The Third Gasotransmitter in Biology and Medicine. Antioxid. Redox Signal. 2010, 12, 1061–1064. [Google Scholar] [CrossRef]
- Polhemus, D.J.; Lefer, D.J. Emergence of Hydrogen Sulfide as an Endogenous Gaseous Signaling Molecule in Cardiovascular Disease. Circ. Res. 2014, 114, 730–737. [Google Scholar] [CrossRef]
- Huang, S.; Li, H.; Ge, J. A Cardioprotective Insight of the Cystathionine γ-Lyase/Hydrogen Sulfide Pathway. IJC Hear. Vasc. 2015, 7, 51–57. [Google Scholar] [CrossRef]
- Meng, G.; Ma, Y.; Xie, L.; Ferro, A.; Ji, Y. Emerging Role of Hydrogen Sulfide in Hypertension and Related Cardiovascular Diseases. Br. J. Pharmacol. 2015, 172, 5501–5511. [Google Scholar] [CrossRef]
- Wang, X.-H.; Wang, F.; You, S.-J.; Cao, Y.-J.; Cao, L.-D.; Han, Q.; Liu, C.-F.; Hu, L.-F. Dysregulation of Cystathionine γ-Lyase (CSE)/Hydrogen Sulfide Pathway Contributes to Ox-LDL-Induced Inflammation in Macrophage. Cell. Signal. 2013, 25, 2255–2262. [Google Scholar] [CrossRef]
- Whiteman, M.; Moore, P.K. Hydrogen Sulfide and the Vasculature: A Novel Vasculoprotective Entity and Regulator of Nitric Oxide Bioavailability? J. Cell. Mol. Med. 2009, 13, 488–507. [Google Scholar] [CrossRef]
- Xu, Y.; Du, H.-P.; Li, J.; Xu, R.; Wang, Y.-L.; You, S.-J.; Liu, H.; Wang, F.; Cao, Y.-J.; Liu, C.-F.; et al. Statins Upregulate Cystathionine γ-Lyase Transcription and H2S Generation via Activating Akt Signaling in Macrophage. Pharmacol. Res. 2014, 87, 18–25. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.-H.; Cui, L.-B.; Wu, K.; Zheng, X.-L.; Cayabyab, F.S.; Chen, Z.-W.; Tang, C.-K. Hydrogen Sulfide as a Potent Cardiovascular Protective Agent. Clin. Chim. Acta 2014, 437, 78–87. [Google Scholar] [CrossRef]
- Zheng, Y.; Ji, X.; Ji, K.; Wang, B. Hydrogen Sulfide Prodrugs-A Review. Acta Pharm. Sin. B 2015, 5, 367–377. [Google Scholar] [CrossRef] [PubMed]
- Calvert, J.W.; Elston, M.; Nicholson, C.K.; Gundewar, S.; Jha, S.; Elrod, J.W.; Ramachandran, A.; Lefer, D.J. Genetic and Pharmacologic Hydrogen Sulfide Therapy Attenuates Ischemia-Induced Heart Failure in Mice. Circulation 2010, 122, 11–19. [Google Scholar] [CrossRef]
- Yang, G.; Wu, L.; Jiang, B.; Yang, W.; Qi, J.; Cao, K.; Meng, Q.; Mustafa, A.K.; Mu, W.; Zhang, S.; et al. H2S as a Physiologic Vasorelaxant: Hypertension in Mice with Deletion of Cystathionine Gamma-Lyase. Science 2008, 322, 587–590. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhao, X.; Jin, H.; Wei, H.; Li, W.; Bu, D.; Tang, X.; Ren, Y.; Tang, C.; Du, J. Role of Hydrogen Sulfide in the Devel-Opment of Atherosclerotic Lesions in Apolipoprotein E Knockout Mice. Arterioscler. Thromb. Vasc. Biol. 2009, 29, 173–179. [Google Scholar] [CrossRef]
- Van Den Born, J.C.; Mencke, R.; Conroy, S.; Zeebregts, C.J.; Van Goor, H.; Hillebrands, J.L. Cystathionine Gamma-Lyase Is Expressed in Human Atherosclerotic Plaque Microvessels and Is Involved in Micro-Angiogenesis. Sci. Rep. 2016, 6, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.; Zhang, J.; Lu, Y.; Wang, R. The Vasorelaxant Effect of H2S as a Novel Endogenous Gaseous KATP Channel Opener. EMBO J. 2001, 20, 6008–6016. [Google Scholar] [CrossRef] [PubMed]
- Dawe, G.S.; Han, S.P.; Bian, J.S.; Moore, P.K. Hydrogen Sulphide in the Hypothalamus Causes an ATP-Sensitive K Chan-Nel-Dependent Decrease in Blood Pressure in Freely Moving Rats. Neuroscience 2008, 152, 169–177. [Google Scholar] [CrossRef]
- Liu, W.Q.; Chai, C.; Li, X.Y.; Yuan, W.J.; Wang, W.Z.; Lu, Y. The Cardiovascular Effects of Central Hydrogen Sulfide Are Related to K(ATP) Channels Activation. Physiol. Res. 2011, 60, 729–738. [Google Scholar] [CrossRef]
- Wang, R. Physiological Implications of Hydrogen Sulfide: A Whiff Exploration That Blossomed. Physiol. Rev. 2012, 92, 791–896. [Google Scholar] [CrossRef]
- Xu, S.; Liu, Z.; Liu, P. Targeting Hydrogen Sulfide as a Promising Therapeutic Strategy for Atherosclerosis. Int. J. Cardiol. 2014, 172, 313–317. [Google Scholar] [CrossRef]
- Wang, R.; Szabo, C.; Ichinose, F.; Ahmed, A.; Whiteman, M.; Papapetropoulos, A. The Role of H2S Bioavailability in Endothelial Dysfunction. Trends Pharmacol. Sci. 2015, 36, 568–578. [Google Scholar] [CrossRef] [PubMed]
- Yuan, S.; Shen, X.; Kevil, C.G. Beyond a Gasotransmitter: Hydrogen Sulfide and Polysulfide in Cardiovascular Health and Im-Mune Response. Antioxid. Redox Signal. 2017, 27, 634–653. [Google Scholar] [CrossRef] [PubMed]
- Hosoki, R.; Matsuki, N.; Kimura, H. The Possible Role of Hydrogen Sulfide as an Endogenous Smooth Muscle Relaxant in Synergy with Nitric Oxide. Biochem. Biophys. Res. Commun. 1997, 237, 527–531. [Google Scholar] [CrossRef] [PubMed]
- Sanchez Aranguren, L.C.; Ahmad, S.; Dias, I.H.K.; Alzahrani, F.A.; Rezai, H.; Wang, K.; Ahmed, A. Bioenergetic Effects of Hydrogen Sulfde Suppress Soluble Flt 1 and Soluble Endoglin in Cystathionine Gamma Lyase Compromised Endothelial Cells. Sci. Rep. 2020, 10, 15810–15820. [Google Scholar] [CrossRef]
- Papapetropoulos, A.; Pyriochou, A.; Altaany, Z.; Yang, G.; Marazioti, A.; Zhou, Z.; Jeschke, M.G.; Branski, L.K.; Herndon, D.N.; Wang, R.; et al. Hydrogen Sulfide Is an Endogenous Stimulator of Angiogenesis. Proc. Natl. Acad. Sci. USA 2009, 106, 21972–21977. [Google Scholar] [CrossRef]
- Hughes, M.N.; Centelles, M.N.; Moore, K.P. Making and Working with Hydrogen Sulfide. Free. Radic. Biol. Med. 2009, 47, 1346–1353. [Google Scholar] [CrossRef] [PubMed]
- Ozturk, T.; Ertas, A.E.; Mert, O. Use of Lawesson’s Reagent in Organic Syntheses. Chem. Rev. 2007, 107, 5210–5278. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Whiteman, M.; Guan, Y.Y.; Neo, K.L.; Cheng, Y.; Lee, S.W.; Zhao, Y.; Baskar, R.; Tan, C.H.; Moore, P.K. Characterization of a Novel, Water-Soluble Hydrogen SulfiDe-releasing Molecule (GYY4137): New Insights into the Biology of Hydrogen Sulfide. Circulation 2008, 117, 2351–2360. [Google Scholar] [CrossRef]
- Rose, P.; Dymock, B.W.; Moore, P.K. GYY4137, a Novel Water-Soluble, H2S-Releasing Molecule. Methods Enzymol. 2015, 554, 143–167. [Google Scholar] [CrossRef]
- Park, C.; Zhao, Y.; Zhu, Z.; Pacheco, A.; Peng, B.; Devarie-Baez, N.O.; Bagdon, P.; Zhang, H.; Xian, M. Synthesis and Evaluation of Phosphorodithioate-Based Hydrogen Sulfide Donors. Mol. Biosyst. 2013, 9, 2430–2434. [Google Scholar] [CrossRef] [PubMed]
- Brodnitz, M.H.; Pascale, J.V.; Van Derslice, L. Flavor Components of Garlic Extract. J. Agric. Food Chem. 1971, 19, 273–275. [Google Scholar] [CrossRef]
- Rahman, M.S. Allicin and Other Functional Active Components in Garlic: Health Benefits and Bioavailability. Int. J. Food Prop. 2007, 10, 245–268. [Google Scholar] [CrossRef]
- Rana, S.V.; Pal, R.; Vaiphei, K.; Sharma, S.K.; Ola, R.P. Garlic in Health and Disease. Nutr. Res. Rev. 2011, 24, 60–71. [Google Scholar] [CrossRef] [PubMed]
- Butt, M.S.; Sultan, M.T.; Butt, M.S.; Iqbal, J. Garlic: Nature’s Protection against Physiological Threats. Crit. Rev. Food Sci. Nutr. 2009, 49, 538–551. [Google Scholar] [CrossRef]
- Citi, V.; Martelli, A.; Testai, L.; Marino, A.; Breschi, M.C.; Calderone, V. Hydrogen Sulfide Releasing Capacity of Natural Isothiocyanates: Is It a Reliable Explanation for the Multiple Biological Effects of Brassicaceae? Planta Medica 2014, 80, 610–613. [Google Scholar] [CrossRef]
- Zhou, Z.; Rekowski, M.V.W.; Coletta, C.; Szabo, C.; Bucci, M.; Cirino, G.; Topouzis, S.; Papapetropoulos, A.; Giannis, A. Thioglycine and L-Thiovaline: Biologically Active H2S-donors. Bioorganic Med. Chem. 2012, 20, 2675–2678. [Google Scholar] [CrossRef]
- Zhao, Y.; Wang, H.; Xian, M. Cysteine-Activated Hydrogen Sulfide (H2S) Donors. J. Am. Chem. Soc. 2010, 133, 15–17. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Bhushan, S.; Yang, C.; Otsuka, H.; Stein, J.D.; Pacheco, A.; Peng, B.; Devarie-Baez, N.O.; Aguilar, H.C.; Lefer, D.J.; et al. Controllable Hydrogen Sulfide Donors and Their Activity against Myocardial Ischemia-Reperfusion Injury. ACS Chem. Biol. 2013, 8, 1283–1290. [Google Scholar] [CrossRef]
- Roger, T.; Raynaud, F.; Bouillaud, F.; Ransy, C.; Simonet, S.; Crespo, C.; Bourguignon, M.-P.; Villeneuve, N.; Vilaine, J.-P.; Artaud, I.; et al. New Biologically Active Hydrogen Sulfide Donors. ChemBioChem 2013, 14, 2268–2271. [Google Scholar] [CrossRef] [PubMed]
- Martelli, A.; Testai, L.; Citi, V.; Marino, A.; Pugliesi, I.; Barresi, E.; Nesi, G.; Rapposelli, S.; Taliani, S.; Da Settimo, F.; et al. Arylthioamides as H2S Donors: L-Cysteine-Activated Releasing Properties and Vascular Effects in Vitro and in Vivo. ACS Med. Chem. Lett. 2013, 4, 904–908. [Google Scholar] [CrossRef]
- Severino, B.; Corvino, A.; Fiorino, F.; Luciano, P.; Frecentese, F.; Magli, E.; Saccone, I.; Di Vaio, P.; Citi, V.; Calderone, V.; et al. 1,2,4-Thiadiazolidin-3,5-diones as Novel Hydrogen Sulfide Donors. Eur. J. Med. Chem. 2018, 143, 1677–1686. [Google Scholar] [CrossRef] [PubMed]
- Barresi, E.; Nesi, G.; Citi, V.; Piragine, E.; Piano, I.; Taliani, S.; Da Settimo, F.; Rapposelli, S.; Testai, L.; Breschi, M.C.; et al. Iminothioethers as Hydrogen Sulfide Donors: From the Gasotransmitter Release to the Vascular Effects. J. Med. Chem. 2017, 60, 7512–7523. [Google Scholar] [CrossRef]
- Mitidieri, E.; Tramontano, T.; Gurgone, D.; Citi, V.; Calderone, V.; Brancaleone, V.; Katsouda, A.; Nagahara, N.; Pa-papetropoulos, A.; Cirino, G.; et al. Mercaptopyruvate Acts as Endogenous Vasodi-Lator Independently of 3-Mercaptopyruvate Sulfurtransferase Activity. Nitric Oxide 2018, 75, 53–59. [Google Scholar] [CrossRef] [PubMed]
- Ercolano, G.; De Cicco, P.; Frecentese, F.; Saccone, I.; Corvino, A.; Giordano, F.; Magli, E.; Fiorino, F.; Severino, B.; Calderone, V.; et al. Anti-metastatic Properties of Naproxen-HBTA in a Murine Model of Cutaneous Melanoma. Front. Pharmacol. 2019, 10, 66. [Google Scholar] [CrossRef]
- Martelli, A.; Testai, L.; Citi, V.; Marino, A.; Bellagambi, F.G.; Ghimenti, S.; Breschi, M.C.; Calderone, V. Pharmacological Characterization of the Vascular Effects of Aryl Isothiocyanates: Is Hydrogen Sulfide the Real Player? Vasc. Pharmacol. 2014, 60, 32–41. [Google Scholar] [CrossRef] [PubMed]
- Citi, V.; Corvino, A.; Fiorino, F.; Frecentese, F.; Magli, E.; Perissutti, E.; Santagada, V.; Brogi, S.; Flori, L.; Gorica, E.; et al. Structure-Activity Relationships Study of Isothiocyanates for H2S Releasing Properties: 3-Pyridyl-Isothiocyanate as a New Promising Cardioprotective Agent. J. Adv. Res. 2021, 27, 41–53. [Google Scholar] [CrossRef]
- Zhao, Y.; Steiger, A.K.; Pluth, M.D. Cyclic Sulfenyl Thiocarbamates Release Carbonyl Sulfide and Hydrogen Sulfide Inde-pendently in Thiol-Promoted Pathways. J. Am. Chem. Soc. 2019, 141, 13610–13618. [Google Scholar] [CrossRef] [PubMed]
- Esechie, A.; Kiss, L.; Olah, G.; Horváth, E.M.; Hawkins, H.; Szabo, C.; Traber, D.L. Protective Effect of Hydrogen Sulfide in a Murine Model of Acute Lung Injury Induced by Combined Burn and Smoke Inhalation. Clin. Sci. 2008, 115, 91–97. [Google Scholar] [CrossRef] [PubMed]
- Kloesch, B.; Liszt, M.; Steiner, G.; Bro¨ll, J. Inhibitors of p38 and ERK1/2 Mapkinase and Hydrogen Sulphide Block Constitutive and IL-1β-Induced IL-6 and IL-8 Expression in the Human Chondrocyte Cell Line C-28/I2. Rheumatol. Int. 2012, 32, 729. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Zhao, B.; Wang, C.; Wang, H.; Liu, Z.; Li, W.; Jin, H.; Tang, C.; Du, J. Regulatory Effects of Hydrogen Sulfide on IL-6, IL-8 and IL-10 Levels in the Plasma and Pulmonary Tissue of Rats with Acute Lung Injury. Exp. Biol. Med. 2008, 233, 1081–1087. [Google Scholar] [CrossRef] [PubMed]
- Ali, F.F.; Abdel-Hamid, H.A.; Toni, N.D. H2S Attenuates Acute Lung Inflammation Induced by Administration of Lipopolysac-Charide in Adult Male Rats. Gen. Physiol. Biophys. 2018, 37, 421–431. [Google Scholar] [CrossRef]
- Whiteman, M.; Cheung, N.S.; Zhu, Y.-Z.; Chu, S.H.; Siau, J.L.; Wong, B.S.; Armstrong, J.S.; Moore, P.K. Hydrogen Sulphide: A Novel Inhibitor of Hypochlorous Acid-Mediated Oxidative Damage in the Brain? Biochem. Biophys. Res. Commun. 2005, 326, 794–798. [Google Scholar] [CrossRef]
- Cao, L.; Cao, X.; Zhou, Y.; Nagpure, B.V.; Wu, Z.-Y.; Hu, L.F.; Yang, Y.; Sethi, G.; Moore, P.K.; Bian, J.-S. Hydrogen Sulfide Inhibits ATP-Induced Neuroinflammation and aβ1–42 Synthesis by Suppressing the Activation of STAT3 and Cathepsin S. Brain Behav. Immun. 2018, 73, 603–614. [Google Scholar] [CrossRef]
- Du, C.; Lin, X.; Xu, W.; Zheng, F.; Cai, J.; Yang, J.; Cui, Q.; Tang, C.; Cai, J.; Xu, G.; et al. Sulfhydrated Sirtuin-1 Increasing Its Deacetylation Activity Is an Essential Epigenetics Mechanism of Anti-Atherogenesis by Hydrogen Sulfide. Antioxid. Redox Signal. 2019, 30, 184–197. [Google Scholar] [CrossRef]
- Wallace, J.L.; Vong, L.; McKnight, W.; Dicay, M.; Martin, G.R. Endogenous and Exogenous Hydrogen Sulfide Promotes Resolu-Tion of Colitis in Rats. Gastroenterology 2009, 137, 569–578. [Google Scholar] [CrossRef] [PubMed]
- Wallace, J.L.; Dicay, M.; McKnight, W.; Martin, G.R. Hydrogen Sulfide Enhances Ulcer Healing in Rats. FASEB J. 2007, 21, 4070–4076. [Google Scholar] [CrossRef]
- Elrod, J.W.; Calvert, J.W.; Morrison, J.; Doeller, J.E.; Kraus, D.W.; Tao, L.; Jiao, X.; Scalia, R.; Kiss, L.; Szabo, C.; et al. Hydrogen Sulfide Attenuates Myocardial Ischemia-Reperfusion Injury by Preservation of Mitochondrial Function. Proc. Natl. Acad. Sci. USA 2007, 104, 15560–15565. [Google Scholar] [CrossRef]
- Calvert, J.W.; Jha, S.; Gundewar, S.; Elrod, J.W.; Ramachandran, A.; Pattillo, C.B.; Kevil, C.G.; Lefer, D.J. Hydrogen Sulfide Mediates Cardioprotection Through Nrf2 Signaling. Circ. Res. 2009, 105, 365–374. [Google Scholar] [CrossRef]
- Pan, T.-T.; Chen, Y.Q.; Bian, J.-S. All in the Timing: A Comparison between the Cardioprotection Induced by H2S Preconditioning and Post-infarction Treatment. Eur. J. Pharmacol. 2009, 616, 160–165. [Google Scholar] [CrossRef]
- Andreadou, I.; Iliodromitis, E.K.; Rassaf, T.; Schulz, R.; Papapetropoulos, A.; Ferdinandy, P. The Role of Gasotransmitters NO, H2S and CO in Myocardial Ischaemia/Reperfusion Injury and Cardioprotection by Preconditioning, Postconditioning and Remote Conditioning. Br. J. Pharmacol. 2015, 172, 1587–1606. [Google Scholar] [CrossRef]
- Andreadou, I.; Iliodromitis, E.K.; Szabo, C.; Papapetropoulos, A. Hydrogen Sulfide and PKG in Ischemia–Reperfusion Injury: Sources, Signaling, Accelerators and Brakes. Basic Res. Cardiol. 2015, 110, 1–6. [Google Scholar] [CrossRef]
- Mard, S.A.; Neisi, N.; Solgi, G.; Hassanpour, M.; Darbor, M.; Maleki, M. Gastroprotective Effect of NaHS against Mucosal Le-Sions Induced by Ischemia-Reperfusion Injury in Rat. Dig. Dis. Sci. 2012, 57, 1496–1503. [Google Scholar] [CrossRef]
- Li, N.; Wang, M.-J.; Jin, S.; Bai, Y.-D.; Hou, C.-L.; Ma, F.-F.; Li, X.-H.; Zhu, Y.-C. The H2S Donor NaHS Changes the Expression Pattern of H2S-Producing Enzymes after Myocardial Infarction. Oxidative Med. Cell. Longev. 2016, 2016, 1–11. [Google Scholar] [CrossRef]
- Gao, C.; Xu, D.Q.; Gao, C.J.; Ding, Q.; Yao, L.N.; Li, Z.C.; Chai, W. An Exogenous Hydrogen Sulphide Donor, NaHS, Inhibits the Nuclear Factor κB Inhibitor Kinase/Nuclear Factor κB Inhibitor/Nuclear Factor-κB Signaling Pathway and Exerts Cardioprotective Effects in a Rat Hemorrhagic Shock Model. Biol. Pharm. Bull. 2012, 35, 1029–1034. [Google Scholar] [CrossRef]
- Chen, K.Y.; Morris, J.C. Kinetics of Oxidation of Aqueous Sulfide by Oxygen. Environ. Sci. Technol. 1972, 6, 529–537. [Google Scholar] [CrossRef]
- Benavides, G.A.; Squadrito, G.L.; Mills, R.W.; Patel, H.D.; Isbell, T.S.; Patel, R.P.; Darley-Usmar, V.M.; Doeller, J.E.; Kraus, D.W. Hydrogen Sulfide Mediates the Vasoactivity of Garlic. Proc. Natl. Acad. Sci. USA 2007, 104, 17977–17982. [Google Scholar] [CrossRef] [PubMed]
- Qidwai, W.; Ashfaq, T. Role of Garlic Usage in Cardiovascular Disease Prevention: An Evidence-Based Approach. Evidence-Based Complement. Altern. Med. 2013, 2013, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Polhemus, D.J.; Calvert, J.W.; Butler, J.; Lefer, D.J. The Cardioprotective Actions of Hydrogen Sulfide in Acute Myocardial in-Farction and Heart Failure. Scientifica 2014, 768607, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Bahadoran, Z.; Mirmiran, P.; Momenan, A.A.; Azizi, F. Allium Vegetable Intakes and the Incidence of Cardiovascular Disease, Hypertension, Chronic Kidney Disease, and Type 2 Diabetes in Adults. J. Hypertens. 2017, 35, 1909–1916. [Google Scholar] [CrossRef] [PubMed]
- Chan, J.Y.-Y.; Yuen, A.C.-Y.; Chan, R.Y.-K.; Chan, S.-W. A Review of the Cardiovascular Benefits and Antioxidant Properties of Allicin. Phytother. Res. 2012, 27, 637–646. [Google Scholar] [CrossRef] [PubMed]
- Herrera-Mundo, M.N.; Silva-Adaya, D.; Maldonado, P.D.; Galván-Arzate, S.; Andrés-Martínez, L.; La Cruz, V.P.-D.; Pedraza-Chaverri, J.; Santamaría, A. S-Allylcysteine Prevents the Rat from 3-Nitropropionic Acid-Induced Hyperactivity, Early Markers of Oxidative Stress and Mitochondrial Dysfunction. Neurosci. Res. 2006, 56, 39–44. [Google Scholar] [CrossRef]
- Numagami, Y.; Ohnishi, S.T. S-Allylcysteine Inhibits Free Radical Production, Lipid Peroxidation and Neuronal Damage in Rat Brain Ischemia. J. Nutr. 2001, 131, 1100S–1105S. [Google Scholar] [CrossRef]
- Welch, C.; Wuarin, L.; Sidell, N. Anti-Proliferative Effect of the Garlic Compound S-Allylcysteine on Human Neuroblastoma Cell in Vitro. Cancer Lett. 1992, 63, 211–219. [Google Scholar] [CrossRef]
- Chu, Q.; Lee, D.T.; Tsao, S.W.; Wang, X.; Wong, Y.C. S-Allylcysteine, a Water-Soluble Garlic Derivative, Suppresses the Growth of a Human Androgen-Independent Prostate Cancer Xenograft, CWR22R, Under in Vivo Conditions. BJU Int. 2007, 99, 925–932. [Google Scholar] [CrossRef] [PubMed]
- Hsu, C.C.; Lin, C.C.; Liao, T.S.; Yin, M.C. Protective Effect of S-Allylcysteine and S-Propyl Cysteine on Acetaminophen-Induced Hepatotoxicity in Mice. Food Chem. Toxicol. 2006, 44, 393–397. [Google Scholar] [CrossRef] [PubMed]
- Nakagawa, S.; Kasuga, S.; Matsura, H. Prevention of Liver Damage by Aged Garlic Extract and Its Components in Mice. Phytother. Res. 1989, 3, 50–53. [Google Scholar] [CrossRef]
- Padmanabhan, M.; Prince, P.S.M. Preventive Effect of S-Allylcysteine on Lipid Peroxides and Antioxidants in Normal and Isoproterenol-Induced Cardiotoxicity in Rats: A Histopathological Study. Toxicology 2006, 224, 128–137. [Google Scholar] [CrossRef]
- Chuah, S.C.; Moore, P.K.; Zhu, Y.Z. S-Allylcysteine Mediates Cardioprotection in an Acute Myocardial Infarction Rat Model via a Hydrogen SulfiDe-mediated Pathway. Am. J. Physiol. Circ. Physiol. 2007, 293, H2693–H2701. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Liu, H.-R.; Mu, Q.; Rose, P.; Zhu, Y.Z. S-propargyl-cysteine Protects Both Adult Rat Hearts and Neonatal Cardiomyocytes From Ischemia/Hypoxia Injury: The Contribution of the Hydrogen Sulfide-Mediated Pathway. J. Cardiovasc. Pharmacol. 2009, 54, 139–146. [Google Scholar] [CrossRef]
- Wang, Q.; Wang, X.-L.; Liu, H.-R.; Rose, P.; Zhu, Y.-Z. Protective Effects of Cysteine Analogues on Acute Myocardial Ischemia: Novel Modulators of Endogenous H2S Production. Antioxidants Redox Signal. 2010, 12, 1155–1165. [Google Scholar] [CrossRef]
- Ried, K.; Frank, O.; Stocks, N.P. Aged Garlic Extract Reduces Blood Pressure in Hypertensives: A Dose–Response Trial. Eur. J. Clin. Nutr. 2012, 67, 64–70. [Google Scholar] [CrossRef]
- Jacob, C.; Anwar, A.; Burkholz, T. Perspective on Recent Developments on Sulfur-Containing Agents and Hydrogen Sulfide Signaling. Planta Medica 2008, 74, 1580–1592. [Google Scholar] [CrossRef]
- Kashfi, K.; Olson, K.R. Biology and Therapeutic Potential of Hydrogen Sulfide and Hydrogen SulfiDe-releasing Chimeras. Biochem. Pharmacol. 2013, 85, 689–703. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Hsu, A.; Moore, P.K. Actions and Interactions of Nitric Oxide, Carbon Monoxide and Hydrogen Sulphide in the Cardiovascular System and in Inflammation–a Tale of Three Gases! Pharmacol. Ther. 2009, 123, 386. [Google Scholar] [CrossRef]
- Nicholson, C.K.; Calvert, J.W. Hydrogen Sulfide and Ischemia-Reperfusion Injury. Pharmacol. Res. 2010, 62, 289. [Google Scholar] [CrossRef]
- Olson, K.R. The Therapeutic Potential of Hydrogen Sulfide: Separating Hype from Hope. Am. J. Physiol. Integr. Comp. Physiol. 2011, 301, R297–R312. [Google Scholar] [CrossRef]
- Predmore, B.L.; Lefer, D.J.; Gojon, G. Hydrogen Sulfide in Biochemistry and Medicine. Antioxid. Redox Signal. 2012, 17, 119–140. [Google Scholar] [CrossRef] [PubMed]
- Dinkova-Kostova, A.T.; Kostov, R.V. Glucosinolates and Isothiocyanates in Health and Disease. Trends Mol. Med. 2012, 18, 337–347. [Google Scholar] [CrossRef] [PubMed]
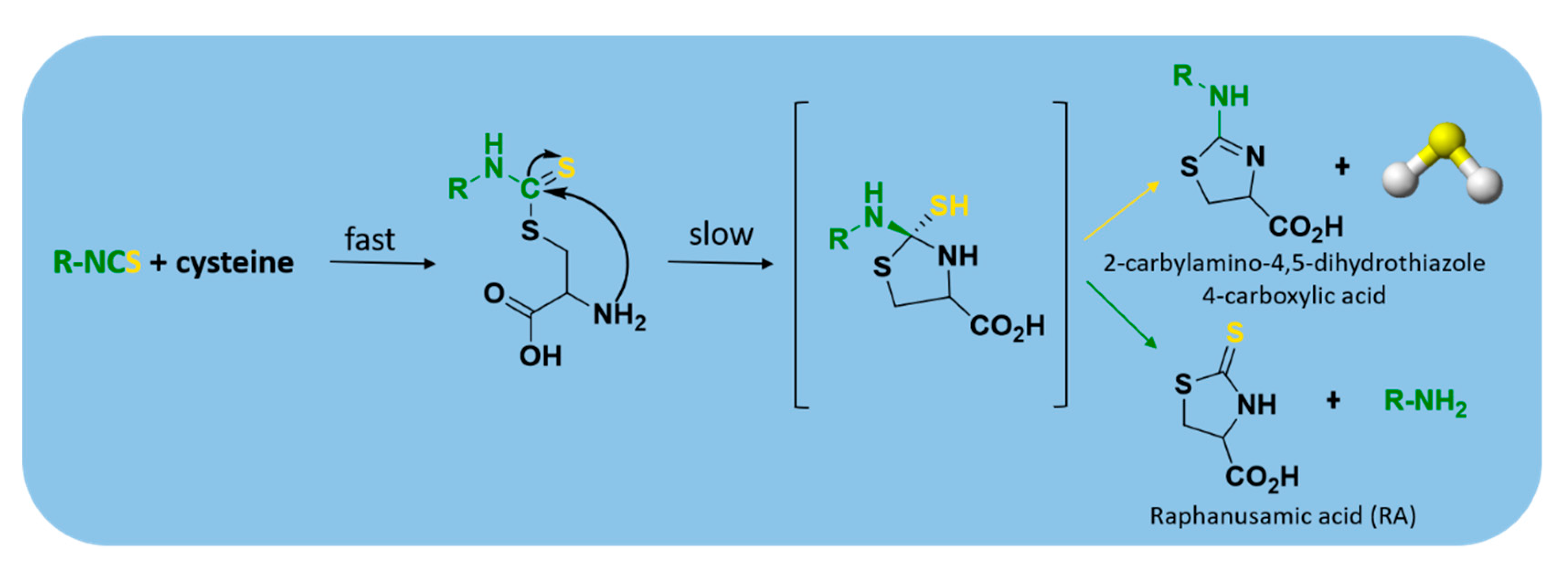
- Lin, Y.; Yang, X.; Lu, Y.; Liang, D.; Huang, D. Isothiocyanates as H2S Donors Triggered by Cysteine: Reaction Mechanism and Structure and Activity Relationship. Org. Lett. 2019, 21, 5977–5980. [Google Scholar] [CrossRef]
- Zhao, Y.; Biggs, T.D.; Xian, M. Hydrogen Sulfide (H2S) Releasing Agents: Chemistry and Biological Applications. Chem. Commun. 2014, 50, 11788–11805. [Google Scholar] [CrossRef] [PubMed]
- Lecher, H.Z.; Greenwood, R.A.; Whitehouse, K.C.; Chao, T.H. The Phosphonation of Aromatic Compounds with Phosphorus Pentasulfide. J. Am. Chem. Soc. 1956, 78, 5018–5022. [Google Scholar] [CrossRef]
- Thomsen, I.; Clausen, K.; Scheibye, S.; Lawesson, S.-O. Thiation with 2,4-Bis(4-Methoxyphenyl)-1,3,2,4- Dithiadiphosphetane 2,4-Disulfide:N-Methylthiopyrrolidone. Organic Syntheses 2003, 62, 158. [Google Scholar] [CrossRef]
- Karwi, Q.G.; Whiteman, M.; Wood, M.E.; Torregrossa, R.; Baxter, G.F. Pharmacological Postconditioning against Myocardial Infarction with a Slow-Releasing Hydrogen Sulfide Donor, GYY4137. Pharmacol. Res. 2016, 111, 442–451. [Google Scholar] [CrossRef] [PubMed]
- Qiu, Y.; Wu, Y.; Meng, M.; Luo, M.; Zhao, H.; Sun, H.; Gao, S. GYY4137 Protects against Myocardial Ischemia/Reperfusion Injury via Activation of the PHLPP-1/Akt/Nrf2 Signaling Pathway in Diabetic Mice. J. Surg. Res. 2018, 225, 29–39. [Google Scholar] [CrossRef]
- Alexander, B.E.; Coles, S.J.; Fox, B.C.; Khan, T.F.; Maliszewski, J.; Perry, A.; Pitak, M.B.; Whiteman, M.; Wood, M.E. Investigating the Generation of Hydrogen Sulfide from the Phosphonamidodithioate Slow-Release Donor GYY4137. MedChemComm 2015, 6, 1649–1655. [Google Scholar] [CrossRef]
- Moore, P.K.; Whiteman, M. Chemistry, Biochemistry and Pharmacology of Hydrogen Sulfide; Springer: Amsterdam, The Netherlands, 2015; pp. 344–354. [Google Scholar]
- Landis, P.S. The Chemistry of 1,2-Dithiole-3-thiones. Chem. Rev. 1965, 65, 237–245. [Google Scholar] [CrossRef]
- Curphey, T.J.; Libby, A.A. Dianions of 3-Oxo dithioic Acids: Preparation and Conversion to 3H-1,2-Dithiole-3-thiones. Tetrahedron Lett. 2000, 41, 6977. [Google Scholar] [CrossRef]
- Pedersen, B.S.; Lawesson, S.O. Syntheses of 3H-1,2-Dithiole-3-Thiones and 4H-1,3,2-Oxazaphosphorine Derivatives from the Dimer of P-Methoxyphenyl-Thionophosphine Sulfide and Der. Tetrahedron 1979, 35, 2433–2437. [Google Scholar] [CrossRef]
- Hamada, T.; Nakane, T.; Kimura, T.; Arisawa, K.; Yoneda, K.; Yamamoto, T.; Osaki, T. Treatment of Xerostomia with the Bile Secretion-Stimulating Drug Anethole Trithione: A Clinical Trial. Am. J. Med. Sci. 1999, 318, 146–151. [Google Scholar] [CrossRef]
- Perrino, E.; Cappelletti, G.; Tazzari, V.; Giavini, E.; Del Soldato, P.; Sparatore, A. New Sulfurated Derivatives of Valproic Acid with Enhanced Histone Deacetylase Inhibitory Activity. Bioorganic Med. Chem. Lett. 2008, 18, 1893–1897. [Google Scholar] [CrossRef]
- Tazzari, V.; Cappelletti, G.; Casagrande, M.; Perrino, E.; Renzi, L.; Del Soldato, P.; Sparatore, A. New Aryldithiolethione Derivatives as Potent Histone Deacetylase Inhibitors. Bioorganic Med. Chem. 2010, 18, 4187–4194. [Google Scholar] [CrossRef]
- Sen, C.K.; Traber, K.E.; Packer, L. Inhibition of NF-jB Activation in Human T-cell Lines by Anetholdithiolthione. Biochem. Biophys. Res. Commun. 1996, 218, 148–153. [Google Scholar] [CrossRef] [PubMed]
- Zanatta, S.D.; Jarrott, B.; Williams, S.J. Synthesis and Preliminary Pharmacological Evaluation of Aryl Dithiolethiones with Cyclooxygenase-2-Selective Inhibitory Activity and Hydrogen Sulfide-Releasing Properties. Aust. J. Chem. 2010, 63, 946–957. [Google Scholar] [CrossRef]
- Qandil, A.M. Prodrugs of Nonsteroidal Anti-Inflammatory Drugs (NSAIDs), More Than Meets the Eye: A Critical Review. Int. J. Mol. Sci. 2012, 13, 17244–17274. [Google Scholar] [CrossRef]
- Caliendo, G.; Cirino, G.; Santagada, V.; Wallace, J.L. Synthesis and Biological Effects of Hydrogen Sulfide (H2S): Development of H2S-Releasing Drugs as Pharmaceuticals. J. Med. Chem. 2010, 53, 6275–6286. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Rossoni, G.; Sparatore, A.; Lee, L.C.; Del Soldato, P.; Moore, P.K. Anti-inflammatory and gastrointestinal effects of a novel diclofenac derivative. Free. Radic. Biol. Med. 2007, 42, 706–719. [Google Scholar] [CrossRef] [PubMed]
- Wallace, J.L.; Caliendo, G.; Santagada, V.; Cirino, G.; Fiorucci, S. Gastrointestinal Safety and Anti-Inflammatory Effects of a Hydrogen Sulfide–Releasing Diclofenac Derivative in the Rat. Gastroenterology 2007, 132, 261–271. [Google Scholar] [CrossRef]
- Fiorucci, S.; Orlandi, S.; Mencarelli, A.; Caliendo, G.; Santagada, V.; Distrutti, E.; Santucci, L.; Cirino, G.; Wallace, J.L. Enhanced Activity of a Hydrogen SulphiDe-releasing Derivative of Mesalamine (ATB-429) in a Mouse Model of Colitis. Br. J. Pharmacol. 2007, 150, 996–1002. [Google Scholar] [CrossRef] [PubMed]
- Chattopadhyay, M.; Kodela, R.; Nath, N.; Dastagirzada, Y.M.; Velázquez-Martínez, C.A.; Boring, D.; Kashfi, K. Hydrogen SulfiDe-releasing NSAIDS Inhibit the Growth of Human Cancer Cells: A General Property and Evidence of a Tissue Type-Independent Effect. Biochem. Pharmacol. 2012, 83, 715–722. [Google Scholar] [CrossRef]
- Shukla, N.; Rossoni, G.; Hotston, M.; Sparatore, A.; Del Soldato, P.; Tazzari, V.; Persad, R.; Angelini, G.D.; Jeremy, J.Y. Effect of Hydrogen SulfiDe-donating Sildenafil (acs6) on Erectile Function and Oxidative Stress in Rabbit Isolated Corpus Cavernosum and in Hypertensive Rats. BJU Int. 2009, 103, 1522–1529. [Google Scholar] [CrossRef]
- Perrino, E.; Uliva, C.; Lanzi, C.; Del Soldato, P.; Masini, E.; Sparatore, A. New Prostaglandin Derivative for Glaucoma Treatment. Bioorg. Med. Chem. Lett. 2009, 19, 1639–1642. [Google Scholar] [CrossRef]
- Lee, M.; Tazzari, V.; Giustarini, D.; Rossi, R.; Sparatore, A.; Del Soldato, P.; McGeer, E.; McGeer, P.L. Effects of Hydrogen Sulfide-releasing l-DOPA Derivatives on Glial Activation. J. Biol. Chem. 2010, 285, 17318–17328. [Google Scholar] [CrossRef]
- Rossoni, G.; Manfredi, B.; Tazzari, V.; Sparatore, A.; Trivulzio, S.; Del Soldato, P.; Berti, F. Activity of a New Hydrogen SulfiDe-releasing Aspirin (ACS14) on Pathological Cardiovascular Alterations Induced by Glutathione Depletion in Rats. Eur. J. Pharmacol. 2010, 648, 139–145. [Google Scholar] [CrossRef] [PubMed]
- Kodela, R.; Chattopadhyay, M.; Kashfi, K. NOSH-Aspirin: A Novel Nitric Oxide–Hydrogen Sulfide-Releasing Hybrid: A New Class of Anti-inflammatory Pharmaceuticals. ACS Med. Chem. Lett. 2012, 3, 257–262. [Google Scholar] [CrossRef] [PubMed]
- Rossoni, G.; Berti, M.; Colonna, V.D.; Bernareggi, M.; Del Soldato, P.; Berti, F. Myocardial Protection by the Nitroderivative of Aspirin, NCX 4016: In Vitro and in Vivo Experiments in the Rabbit. Ital. Hear. J. Off. J. Ital. Fed. Cardiol. 2000, 1, 146–155. [Google Scholar]
- Szczesny, B.; Módis, K.; Yanagi, K.; Coletta, C.; Le Trionnaire, S.; Perry, A.; Wood, M.E.; Whiteman, M.; Szabo, C. AP39, A Novel Mitochondria-Targeted Hydrogen Sulfide Donor, Stimulates Cellular Bioenergetics, Exerts Cytoprotective Effects and Protects against the Loss of Mitochondrial DNA Integrity in Oxidatively Stressed Endothelial Cells in Vitro. Nitric Oxide 2014, 41, 120–130. [Google Scholar] [CrossRef] [PubMed]
- Ikeda, K.; Marutani, E.; Hirai, S.; Wood, M.E.; Whiteman, M.; Ichinose, F. Mitochondria-Targeted Hydrogen Sulfide Donor AP39 Improves Neurological Outcomes after Cardiac Arrest in Mice. Nitric Oxide 2015, 49, 90–96. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, A.; Olah, G.; Szczesny, B.; Wood, M.E.; Whiteman, M.; Szabo, C. AP39, A Mitochondrially Targeted Hydrogen Sulfide Donor, Exerts Protective Effects in Renal Epithelial Cells Subjected to Oxidative Stress in Vitro and in Acute Renal Injury in Vivo. Shock 2016, 45, 88–97. [Google Scholar] [CrossRef] [PubMed]
- Chatzianastasiou, A.; Bibli, S.-I.; Andreadou, I.; Efentakis, P.; Kaludercic, N.; Wood, M.E.; Whiteman, M.; Di Lisa, F.; Daiber, A.; Manolopoulos, V.G.; et al. Cardioprotection by H2S Donors: Nitric Oxide-Dependent and -Independent Mechanisms. J. Pharmacol. Exp. Ther. 2016, 358, 431–440. [Google Scholar] [CrossRef] [PubMed]
- Karwi, Q.G.; Bornbaum, J.; Boengler, K.; Torregrossa, R.; Whiteman, M.; Wood, M.E.; Schulz, R.; Baxter, G.F. AP39, A Mitochondria-Targeting Hydrogen Sulfide (H2S) Donor, Protects against Myocardial Reperfusion Injury Independently of Salvage Kinase Signalling. Br. J. Pharmacol. 2017, 174, 287–301. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.; Li, Z.; Organ, C.L.; Park, C.M.; Yang, C.T.; Pacheco, A.; Wang, D.; Lefer, D.J.; Xian, M. pH-Controlled Hydrogen Sulfide Release for Myocardial Ischemia-Reperfusion Injury. J. Am. Chem. Soc. 2016, 138, 6336–6339. [Google Scholar] [CrossRef]
- Wang, H.; Skeldon, P.; Thompson, G.; Wood, G. Synthesis and Characterization of Molybdenum Disulphide Formed from Ammonium Tetrathiomolybdate. J. Mater. Sci. 1997, 32, 497–502. [Google Scholar] [CrossRef]
- Xu, S.; Yang, C.-T.; Meng, F.-H.; Pacheco, A.; Chen, L.; Xian, M. Ammonium Tetrathiomolybdate as a Water-Soluble and Slow-Release Hydrogen Sulfide Donor. Bioorganic Med. Chem. Lett. 2016, 26, 1585–1588. [Google Scholar] [CrossRef]
- Dyson, A.; Dal-Pizzol, F.; Sabbatini, G.; Lach, A.B.; Galfo, F.; Cardoso, J.D.S.; Mendonça, B.P.; Hargreaves, I.; Pinto, B.B.; Bromage, D.I.; et al. Ammonium Tetrathiomolybdate Following Ischemia/Reperfusion Injury: Chemistry, Pharmacology, and Impact of a New Class of Sulfide Donor in Preclinical Injury Models. PLoS Med. 2017, 14, e1002310. [Google Scholar] [CrossRef]
- Zhang, H.; Zhang, A.; Guo, C.; Shi, C.; Zhang, Y.; Liu, Q.; Sparatore, A.; Wang, C. S-diclofenac Protects against Doxorubicin-Induced Cardiomyopathy in Mice via Ameliorating Cardiac Gap Junction Remodeling. PLoS ONE 2011, 6, e26441. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Guo, W.; Lin, S.-Z.; Wang, Z.-J.; Kan, J.-T.; Chen, S.-Y.; Zhu, Y.-Z. Gp130-Mediated STAT3 Activation by S-Propargyl-cysteine, an Endogenous Hydrogen Sulfide Initiator, Prevents Doxorubicin-Induced Cardiotoxicity. Cell Death Dis. 2016, 7, e2339. [Google Scholar] [CrossRef]
- Su, Y.-W.; Liang, C.; Jin, H.-F.; Tang, X.-Y.; Han, W.; Chai, L.-J.; Zhang, C.-Y.; Geng, B.; Tang, C.-S.; Du, J.-B. Hydrogen Sulfide Regulates Cardiac Function and Structure in Adriamycin-Induced Cardiomyopathy. Circ. J. 2009, 73, 741–749. [Google Scholar] [CrossRef] [PubMed]
- Chan, N.; Willis, A.; Kornhauser, N.; Ward, M.M.; Lee, S.B.; Nackos, E.; Seo, B.R.; Chuang, E.; Cigler, T.; Moore, A.; et al. Influencing the Tumor Microenvironment: A Phase II Study of Copper Depletion Using Tetrathiomolybdate in Patients with Breast Cancer at High Risk for Recurrence and in Preclinical Models of Lung Metastases. Clin. Cancer Res. 2017, 23, 666–676. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.-T.; Zhao, Y.; Xian, M.; Li, J.-H.; Dong, Q.; Bai, H.-B.; Xu, J.-D.; Zhang, M.-F. A Novel Controllable Hydrogen Sulfide-Releasing Molecule Protects Human Skin Keratinocytes Against Methylglyoxal-Induced Injury and Dysfunction. Cell. Physiol. Biochem. 2014, 34, 1304–1317. [Google Scholar] [CrossRef]
- Zhao, Y.; Yang, C.; Organ, C.; Li, Z.; Bhushan, S.; Otsuka, H.; Pacheco, A.; Kang, J.; Aguilar, H.C.; Lefer, D.J.; et al. Design, Synthesis, and Cardioprotective Effects of N-Mercapto-Based Hydrogen Sulfide Donors. J. Med. Chem. 2015, 58, 7501–7511. [Google Scholar] [CrossRef] [PubMed]
- Stipanuk, M.H.; Beck, P.W. Characterization of the Enzymic Capacity for Cysteine Desulphhydration in Liver and Kidney of the Rat. Biochem. J. 1982, 206, 267–277. [Google Scholar] [CrossRef] [PubMed]
- Frank, R.L.; Blegen, J.R. Benzoyl Disulfide. Organic Syntheses 2003, 28, 16. [Google Scholar] [CrossRef]
- Heimer, N.E.; Field, L.; Neal, R.A. Biologically Oriented Organic Sulfur Chemistry. 21. Hydrodisulfide of a Penicillamine Derivative and Related Compounds. J. Org. Chem. 1981, 46, 1374–1377. [Google Scholar] [CrossRef]
- Wallace, J.L.; Caliendo, G.; Santagada, V.; Cirino, G. Markedly Reduced Toxicity of a Hydrogen SulphiDe-releasing Derivative of Naproxen (ATB-346). Br. J. Pharmacol. 2010, 159, 1236–1246. [Google Scholar] [CrossRef]
- De Cicco, P.; Panza, E.; Ercolano, G.; Armogida, C.; Sessa, G.; Pirozzi, G.; Cirino, G.; Wallace, J.L.; Ianaro, A. ATB-346, A Novel Hydrogen SulfiDe-releasing Anti-inflammatory Drug, Induces Apoptosis of Human Melanoma Cells and Inhibits Melanoma Development in Vivo. Pharmacol. Res. 2016, 114, 67–73. [Google Scholar] [CrossRef]
- Elsheikh, W.; Blackler, R.W.; Flannigan, K.L.; Wallace, J.L. Enhanced Chemopreventive Effects of a Hydrogen SulfiDe-releasing Anti-inflammatory Drug (ATB-346) in Experimental Colorectal Cancer. Nitric Oxide 2014, 41, 131–137. [Google Scholar] [CrossRef]
- Wallace, J.L.; Vaughan, D.; Dicay, M.; Macnaughton, W.K.; De Nucci, G. Hydrogen Sulfide-Releasing Therapeutics: Translation to the Clinic. Antioxidants Redox Signal. 2018, 28, 1533–1540. [Google Scholar] [CrossRef]
- Testai, L.; Marino, A.; Piano, I.; Brancaleone, V.; Tomita, K.; Di Cesare Mannelli, L.; Martelli, A.; Citi, V.; Breschi, M.C.; Levi, R.; et al. The Novel H2S-donor 4-Carboxyphenyl Isothiocyanate Promotes Cardioprotective Effects against Ischemia/Reperfusion Injury through Activation of mitoK ATP Channels and Reduction of Oxidative Stress. Pharmacol. Res. 2016, 113, 290–299. [Google Scholar] [CrossRef]
- Nasim, S.; Crooks, P.A. N-Chlorosuccinimide Is a Convenient Oxidant for the Synthesis of 2,4-disubstituted 1,2,4-thiadiazolidine-3,5-diones. Tetrahedron Lett. 2009, 50, 257–259. [Google Scholar] [CrossRef]
- Kondo, K.; Bhushan, S.; King, A.L.; Prabhu, S.D.; Hamid, T.; Koenig, S.; Murohara, T.; Predmore, B.L.; Gojon, G., Sr.; Gojon, G., Jr.; et al. H2S Protects against Pressure Overload-Induced Heart Failure via Upregulation of Endothelial Nitric Oxide Synthase. Circulation 2013, 127, 1116–1127. [Google Scholar] [CrossRef]
- Gojon-Romanillos, G.; Gojon-Zorrilla, G. Preparation and Compositions of Highly Bioavailable Zerovalent Sulfur and Uses Thereof. U.S. Patent 8771755-B2, 8 July 2014. [Google Scholar]
- Rohwerder, T.; Sand, W. The Sulfane Sulfur of Persulfides Is the Actual Substrate of the Sulfur-Oxidizing Enzymes from Acidithiobacillus and Acidiphilium spp. Microbiology 2003, 149, 1699–1710. [Google Scholar] [CrossRef]
- Suzuki, I. Oxidation of Elemental Sulfur by an Enzyme System of Thiobacillus Thiooxidans. Biochim. Biophys. Acta (BBA) Gen. Subj. 1965, 104, 359–371. [Google Scholar] [CrossRef]
- Suzuki, I.; Werkman, C.H. Glutathione and Sulfur Oxidation by Thiobacillus Thiooxidans. Proc. Natl. Acad. Sci. USA 1959, 45, 239–244. [Google Scholar] [CrossRef] [PubMed]
- Barr, L.A.; Shimizu, Y.; Lambert, J.P.; Nicholson, C.K.; Calvert, J.W. Hydrogen Sulfide Attenuates High Fat Diet-Induced Cardiac Dysfunction via the Suppression of Endoplasmic Reticulum Stress. Nitric Oxide 2015, 46, 145–156. [Google Scholar] [CrossRef]
- Donnarumma, E.; Rushing, A.M.; Boisvert, S.F.; Scarborough, A.L.; Polhemus, D.J.; Trivedi, R.K.; Lefer, D.J.; Goodchild, T.T. The Novel H2S Pro-drug, SG1002, Preserves Coronary Artery Vascular Reactivity in the Setting of Critical Limb Ischemia in Swine. Circulation 2016, 134, A19028. [Google Scholar]
- ClinicalTrials.gov. Assessing the Safety and Ability of SG1002 to Overcome Deficits in Hydrogen Sulfide in Heart Failure Patients. Available online: https://clinicaltrials.gov/ct2/show/NCT01989208 (accessed on 18 January 2021).
- Polhemus, D.J.; Li, Z.; Pattillo, C.B.; Gojon, G.; Giordano, T.; Krum, H. A Novel Hydrogen Sulfide Prodrug, SG 1002, Promotes Hydrogen Sulfide and Nitric Oxide Bioavailability in Heart Failure Patients. Cardiovasc. Ther. 2015, 33, 216–226. [Google Scholar] [CrossRef]
- Borchardt, R.T.; Cohen, L.A. Stereopopulation Control. III. Facilitation of Intramolecular Conjugate Addition of the Carboxyl Group. J. Am. Chem. Soc. 1972, 94, 9175–9182. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Yueqin, Z.; Ji, K.; Pan, Z.; Chittavong, V.; Wang, B. Esterase-Sensitive Prodrugs with Tunable Release Rates and Direct Generation of Hydrogen Sulfide. Angew. Chem. Int. Ed. 2016, 55, 4514–4518. [Google Scholar] [CrossRef]
- Rao, Y.; Li, X.; Nagorny, P.; Hayashida, J.; Danishefsky, S.J. A Simple Method for the Conversion of Carboxylic Acids into Thioacids with Lawesson’s Reagent. Tetrahedron Lett. 2009, 50, 6684–6686. [Google Scholar] [CrossRef]
- Li, Z.; Organ, C.L.; Zheng, Y.; Wang, B.; Lefer, D.J. Abstract 17903: Novel Esterase Activated Hydrogen Sulfide Donors Attenuate Myocardial Ischemia/ Reperfusion Injury. Circulation 2016, 134, A17903. [Google Scholar]
- Chengelis, C.P.; Neal, R.A. Studies of Carbonyl Sulfide Toxicity: Metabolism by Carbonic Anhydrase. Toxicol. Appl. Pharmacol. 1980, 55, 198–202. [Google Scholar] [CrossRef]
- Chengelis, C.P.; Neal, R.A. Hepatic Carbonyl Sulfide Metabolism. Biochem. Biophys. Res. Commun. 1979, 90, 993–999. [Google Scholar] [CrossRef]
- Steiger, A.K.; Marcatti, M.; Szabo, C.; Szczesny, B.; Pluth, M.D. Inhibition of Mitochondrial Bioenergetics by Esterase-Triggered COS/H2S Donors. ACS Chem. Biol. 2017, 12, 2117–2123. [Google Scholar] [CrossRef] [PubMed]
- Steiger, A.K.; Pardue, S.; Kevil, C.G.; Pluth, M.D. Self-Immolative Thiocarbamates Provide Access to Triggered H2S Donors and Analyte Replacement Fluorescent Probes. J. Am. Chem. Soc. 2016, 138, 7256–7259. [Google Scholar] [CrossRef]
- Powell, C.R.; Foster, J.C.; Okyere, B.; Theus, M.H.; Matson, J.B. Therapeutic Delivery of H2S via COS: Small Molecule and Polymeric Donors with Benign Byproducts. J. Am. Chem. Soc. 2016, 138, 13477–13480. [Google Scholar] [CrossRef] [PubMed]
- Tao, X.; Deng, Y.; Shen, Z.; Ling, J. Controlled Polymerization of N-Substituted Glycine N-Thiocarboxyanhydrides Initiated by Rare Earth Borohydrides toward Hydrophilic and Hydrophobic Polypeptoids. Macromolecules 2014, 47, 6173–6180. [Google Scholar] [CrossRef]
- Zhao, Y.; Pluth, M.D. Hydrogen Sulfide Donors Activated by Reactive Oxygen Species. Angew. Chem. Int. Ed. 2016, 55, 14638–14642. [Google Scholar] [CrossRef]
- Dickinson, B.C.; Huynh, C.; Chang, C.J. A Palette of Fluorescent Probes with Varying Emission Colors for Imaging Hydrogen Peroxide Signaling in Living Cells. J. Am. Chem. Soc. 2010, 132, 5906–5915. [Google Scholar] [CrossRef]
- Miller, E.W.; Albers, A.E.; Pralle, A.; Isacoff, E.Y.; Chang, C.J. Boronate-Based Fluorescent Probes for Imaging Cellular Hydrogen Peroxide. J. Am. Chem. Soc. 2005, 127, 16652–16659. [Google Scholar] [CrossRef] [PubMed]
- Pan, J.; Devarie-Baez, N.O.; Xian, M. Facile Amide Formation viaS-Nitrosothioacids. Org. Lett. 2011, 13, 1092–1094. [Google Scholar] [CrossRef][Green Version]
- Rao, Y.; Li, X.; Danishefsky, S.J. Thio FCMA Intermediates as Strong Acyl Donors: A General Solution to the Formation of Complex Amide Bonds. J. Am. Chem. Soc. 2009, 131, 12924–12926. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Danishefshy, S.J. A Promising General Solution to the Problem of Ligating Peptides and Glycopeptides. J. Am. Chem. Soc. 2010, 132, 17045–17051. [Google Scholar] [CrossRef]
- Liu, R.; Orgel, L.E. Oxidative Acylation Using Thioacids. Nat. Cell Biol. 1997, 389, 52–54. [Google Scholar] [CrossRef] [PubMed]
- Dawson, E.P.; Muir, T.W.; Clark-Lewis, I.; Kent, S.B. Synthesis of Proteins by Native Chemical Ligation. Science 1994, 266, 776–779. [Google Scholar] [CrossRef]
- Crich, D.; Sharma, I. Epimerization-Free Block Synthesis of Peptides from Thioacids and Amines with the Sanger and Mukaiyama Reagents. Angew. Chem. Int. Ed. 2009, 48, 2355–2358. [Google Scholar] [CrossRef]
- Wu, D.; Hu, Q.; Ma, F.; Zhu, Y.Z. Vasorelaxant Effect of a New Hydrogen Sulfide-Nitric Oxide Conjugated Donor in Isolated Rat Aortic Rings through cGMP Pathway. Oxidative Med. Cell. Longev. 2016, 2016, 1–10. [Google Scholar] [CrossRef]
- Liu, C.; Guo, W.; Shi, X.; Kaium, M.; Gu, X.; Zhu, Y.Z. Leonurine-Cysteine Analog Conjugates as a New Class of Multifunctional Anti-myocardial Ischemia Agent. Eur. J. Med. Chem. 2011, 46, 3996–4009. [Google Scholar] [CrossRef]
- Ignarro, L.J.; Napoli, C.; Loscalzo, J. Nitric Oxide Donors and Cardiovascular Agents Modulating the Bioactivity of Nitric Oxide: An Overview. Circ. Res. 2002, 90, 21–28. [Google Scholar] [CrossRef]
- Xiong, Y.; Chang, L.-L.; Tran, B.; Dai, T.; Zhong, R.; Mao, Y.-C.; Zhu, Y.-Z. ZYZ-803, a Novel Hydrogen SulfiDe-nitric Oxide Conjugated Donor, Promotes Angiogenesis via Cross-Talk between STAT3 and CaMKII. Acta Pharmacol. Sin. 2020, 41, 218–228. [Google Scholar] [CrossRef]
- Hu, Q.; Wu, D.; Ma, F.; Yang, S.; Tan, B.; Xin, H.; Gu, X.; Chen, X.; Chen, S.; Mao, Y.; et al. Novel Angiogenic Activity and Molecular Mechanisms of ZYZ-803, a Slow-Releasing Hydrogen Sulfide–Nitric Oxide Hybrid Molecule. Antioxid. Redox Signal. 2016, 25, 498–514. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.; Hu, Q.; Xiong, Y.; Zhu, D.; Mao, Y.; Zhu, Y.Z. Novel H2S-NO Hybrid Molecule (ZYZ-803) Promoted Synergistic Effects Against Heart Failure. Redox Biol. 2018, 15, 243–252. [Google Scholar] [CrossRef]
- Bucci, M.; Vellecco, V.; Cantalupo, A.; Brancaleone, V.; Zhou, Z.; Evangelista, S.; Calderone, V.; Papapetropoulos, A.; Cirino, G. Hydrogen Sulfide Accounts for the Peripheral Vascular Effects of Zofenopril Independently of ACE Inhibition. Cardiovasc. Res. 2014, 102, 138–147. [Google Scholar] [CrossRef]
- Terzuoli, E.; Monti, M.; Vellecco, V.; Bucci, M.; Cirino, G.; Ziche, M.; Morbidelli, L. Characterization of Zofenoprilat as an Inducer of Functional Angiogenesis through Increased H2S Availability. Br. J. Pharmacol. 2015, 172, 2961–2973. [Google Scholar] [CrossRef]
- Monti, M.; Terzuoli, E.; Ziche, M.; Morbidelli, L. The Sulphydryl Containing ACE Inhibitor Zofenoprilat Protects Coronary Endothelium from Doxorubicin-Induced Apoptosis. Pharmacol. Res. 2013, 76, 171–181. [Google Scholar] [CrossRef] [PubMed]
- Monti, M.; Terzuoli, E.; Ziche, M.; Morbidelli, L. H2S Dependent and Independent Anti-inflammatory Activity of Zofenoprilat in Cells of the Vascular Wall. Pharmacol. Res. 2016, 113, 426–437. [Google Scholar] [CrossRef]
- Furuta, S.; Kiyosawa, K.; Higuchi, M.; Kasahara, H.; Saito, H.; Shioya, H.; Oguchi, H. Pharmacokinetics of Temocapril, an ACE Inhibitor with Preferential Biliary Excretion, in Patients with Impaired Liver Function. Eur. J. Clin. Pharmacol. 1993, 44, 383–385. [Google Scholar] [CrossRef] [PubMed]
- Donnarumma, E.; Ali, M.J.; Rushing, A.M.; Scarborough, A.L.; Bradley, J.M.; Organ, C.L.; Islam, K.N.; Polhemus, D.J.; Evangelista, S.; Cirino, G.; et al. Zofenopril Protects Against Myocardial Ischemia–Reperfusion Injury by Increasing Nitric Oxide and Hydrogen Sulfide Bioavailability. J. Am. Hear. Assoc. 2016, 5, 1–17. [Google Scholar] [CrossRef] [PubMed]
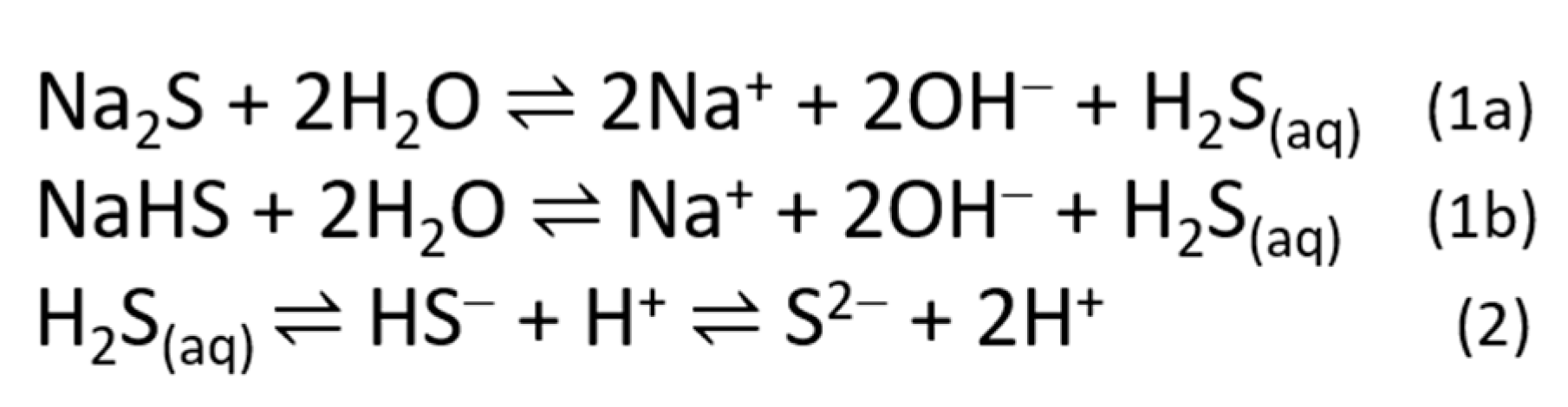





| H2S Donors | Chemical Structure | Mechanism of H2S Release | Drug Development Phases |
|---|---|---|---|
| Lawesson’s reagent |  | Hydrolysis-triggered | Pharmacological studies |
| GYY4137 | 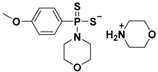 | Hydrolysis-triggered | Preclinical trials |
| Phosphorodithioates | 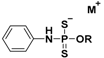 | Hydrolysis-triggered | Pharmacological studies |
| 1,2-dithiole-3-thiones (DTTs) | 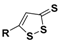 | Hydrolysis-triggered | Pharmacological studies |
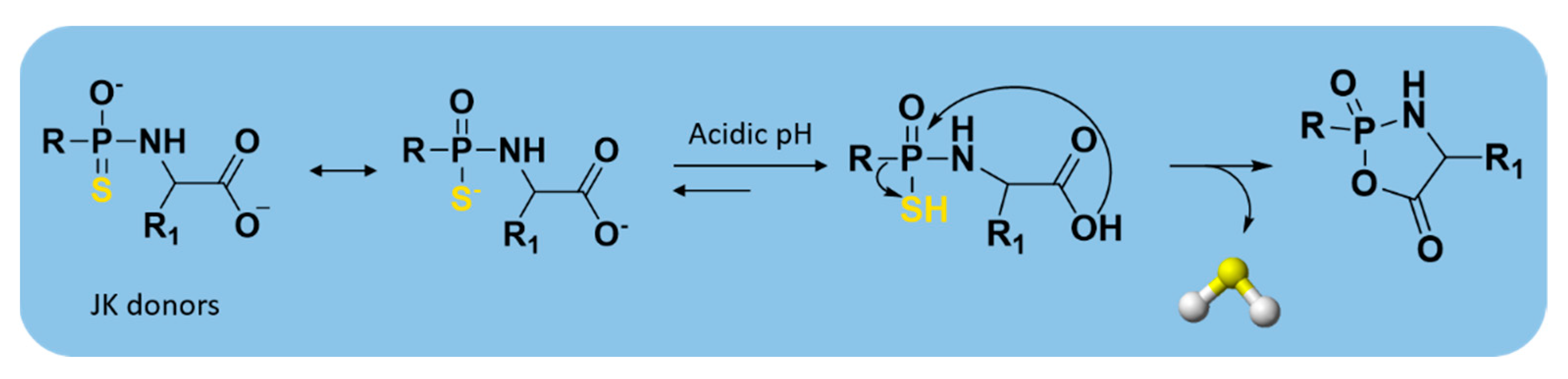
| JK donors | 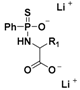 | pH-controlled | Preclinical trials |
| Ammonium tetrathiomolybdate (ATTM) | (NH4)2MoS4 | pH-controlled | Phase I and II clinical trials for breast cancer |
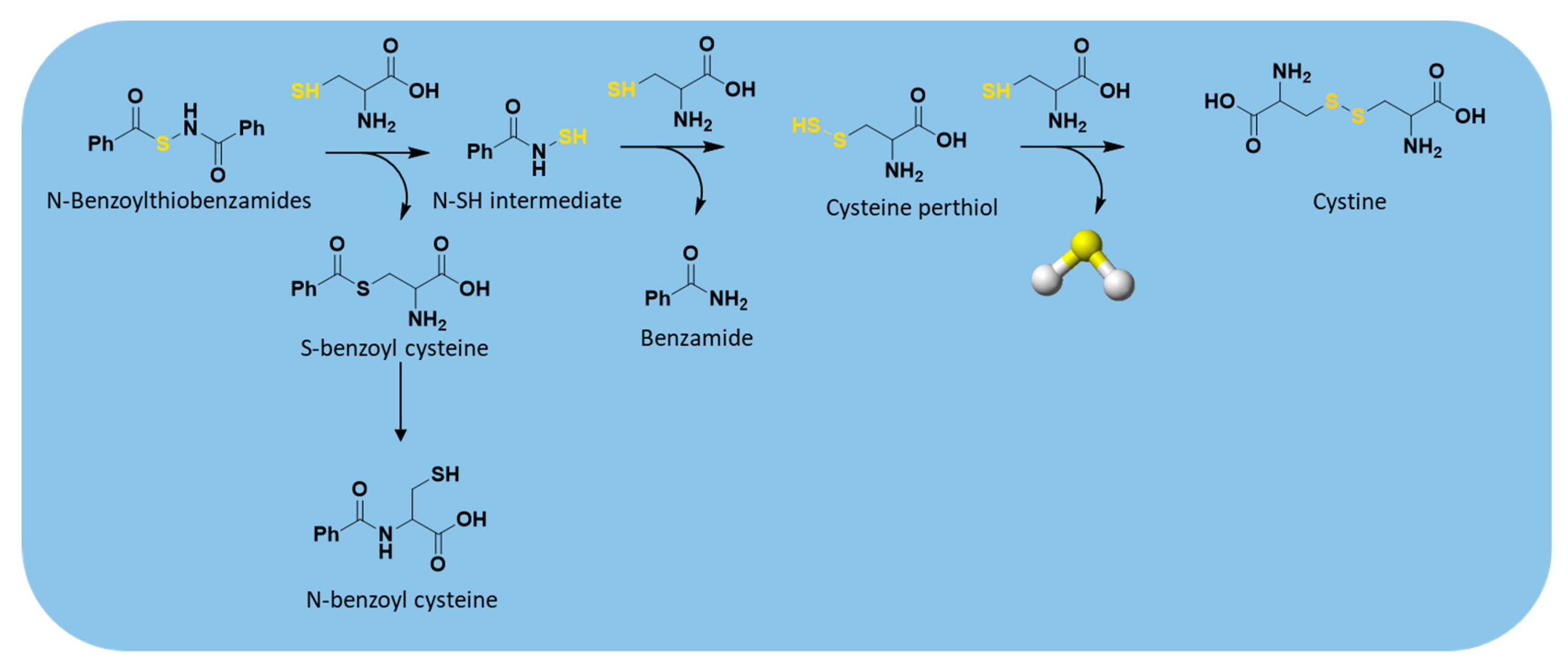
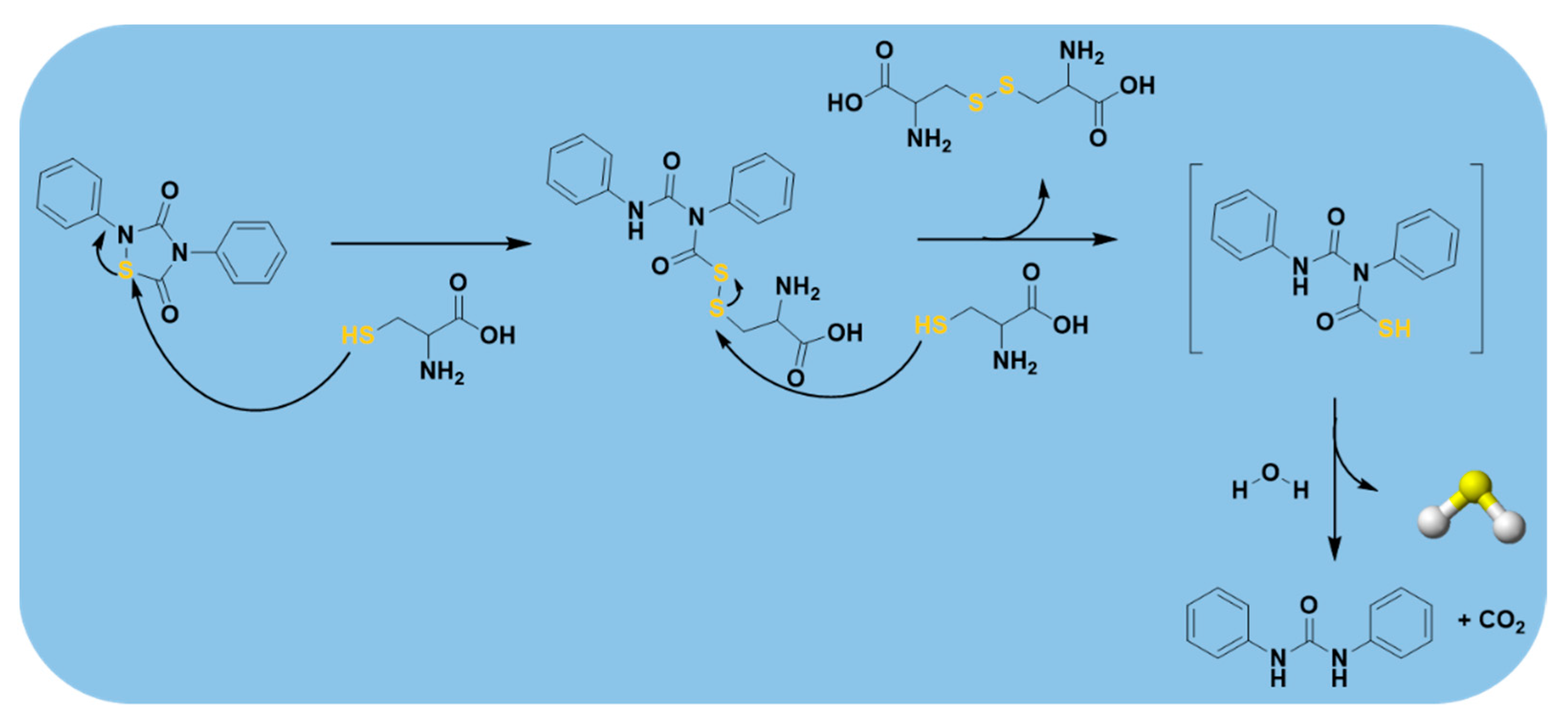
| N-Benzoylthiobenzamides | 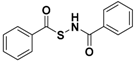 | Thiol-triggered | Pharmacological studies |
| Acyl perthiols | 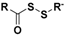 | Thiol-triggered | Preclinical trials |
| Dithioperoxyanhydrides | 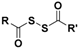 | Thiol-triggered | Pharmacological studies |
| Arylthioamides | 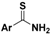 | Thiol-triggered | Preclinical trials |
| Arylisothiocyanates |  | Thiol-triggered | Pharmacological studies |
| 1,2,4-Thiadiazolidine-3,5-diones | 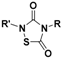 | Thiol-triggered | Pharmacological studies |
| SG-1002 |  | Thiol-triggered | Phase I clinical trial complete for heart failure |
| Trimethyl lock (TML) | 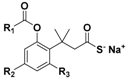 | Enzyme-triggered | Pharmacological studies |
| N-Thiocarboxyanhydrides (NTAs) |  | Enzyme-triggered | Preclinical trials |
| PeroxyTCM | 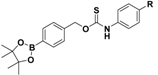 | Enzyme-triggered ROS-triggered | Preclinical trials |
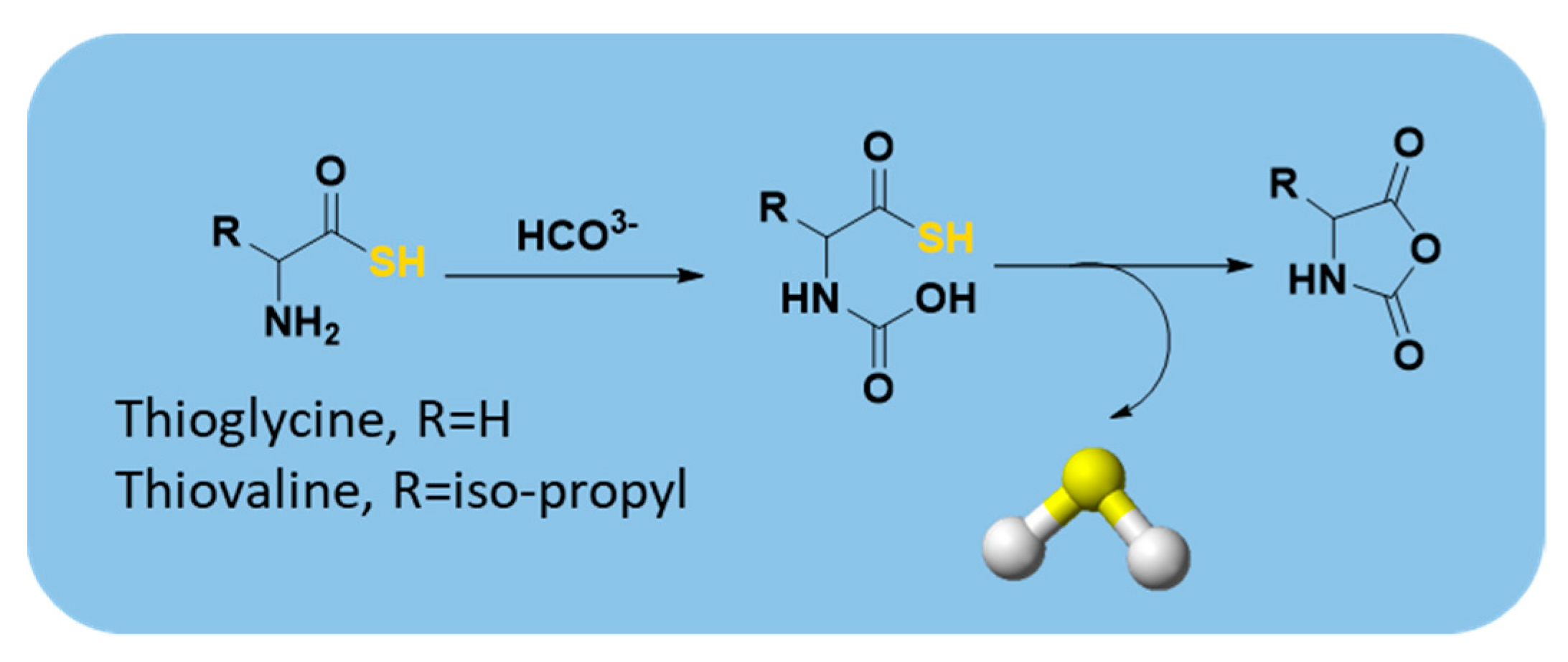
| Thioamino acids | 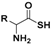 | Bicarbonate-triggered | Pharmacological studies |
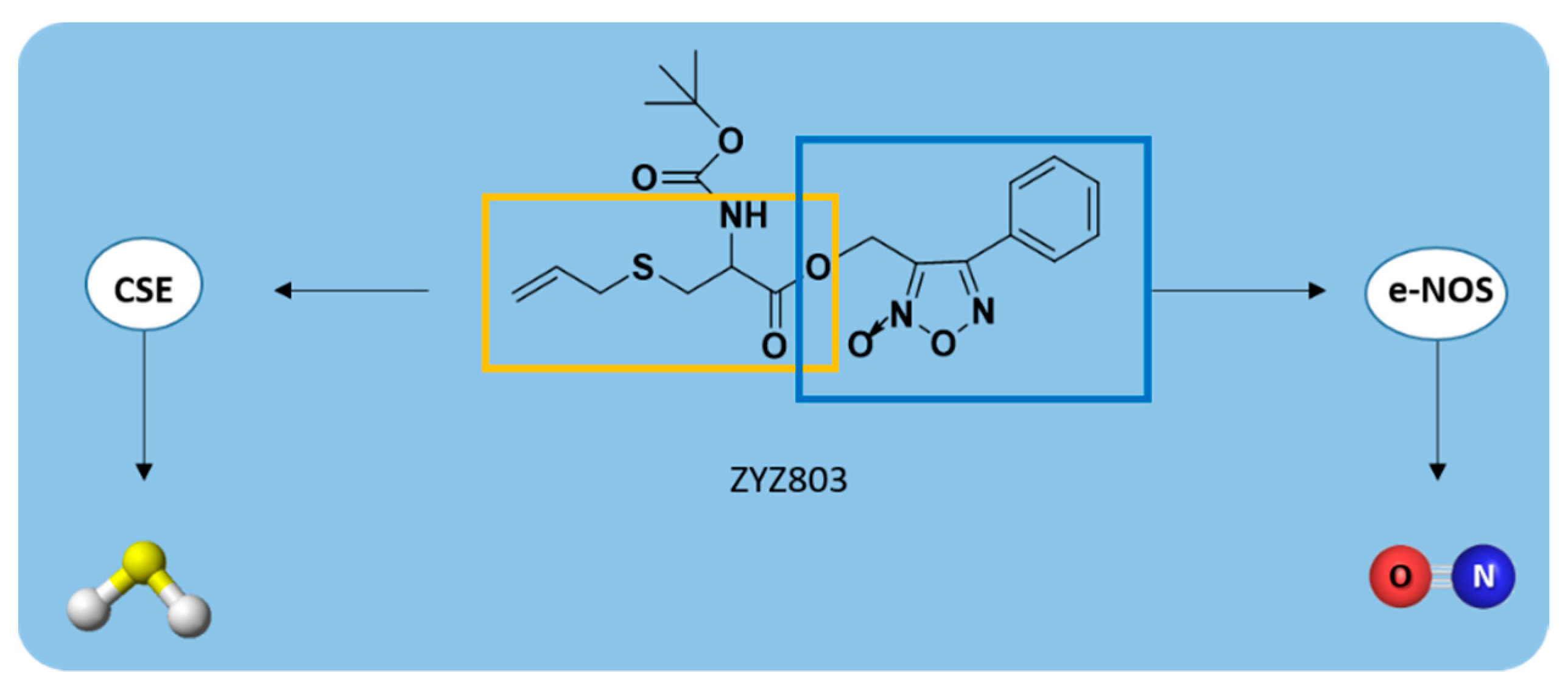
| ZYZ803 | 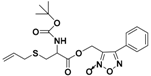 | CSE/eNOS-dependent | Preclinical trials |
| Zofenopril | 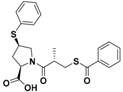 | ACE inhibitor | Clinical use for CVD |
| ACS 14 | 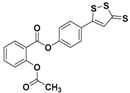 | HS–ASA hybrid | Preclinical trials |
| AP-39 | 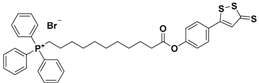 | Mithocondrial-targeted | Preclinical trials |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Corvino, A.; Frecentese, F.; Magli, E.; Perissutti, E.; Santagada, V.; Scognamiglio, A.; Caliendo, G.; Fiorino, F.; Severino, B. Trends in H2S-Donors Chemistry and Their Effects in Cardiovascular Diseases. Antioxidants 2021, 10, 429. https://doi.org/10.3390/antiox10030429
Corvino A, Frecentese F, Magli E, Perissutti E, Santagada V, Scognamiglio A, Caliendo G, Fiorino F, Severino B. Trends in H2S-Donors Chemistry and Their Effects in Cardiovascular Diseases. Antioxidants. 2021; 10(3):429. https://doi.org/10.3390/antiox10030429
Chicago/Turabian StyleCorvino, Angela, Francesco Frecentese, Elisa Magli, Elisa Perissutti, Vincenzo Santagada, Antonia Scognamiglio, Giuseppe Caliendo, Ferdinando Fiorino, and Beatrice Severino. 2021. "Trends in H2S-Donors Chemistry and Their Effects in Cardiovascular Diseases" Antioxidants 10, no. 3: 429. https://doi.org/10.3390/antiox10030429
APA StyleCorvino, A., Frecentese, F., Magli, E., Perissutti, E., Santagada, V., Scognamiglio, A., Caliendo, G., Fiorino, F., & Severino, B. (2021). Trends in H2S-Donors Chemistry and Their Effects in Cardiovascular Diseases. Antioxidants, 10(3), 429. https://doi.org/10.3390/antiox10030429












