Antioxidant Therapy in Inflammatory Bowel Diseases
Abstract
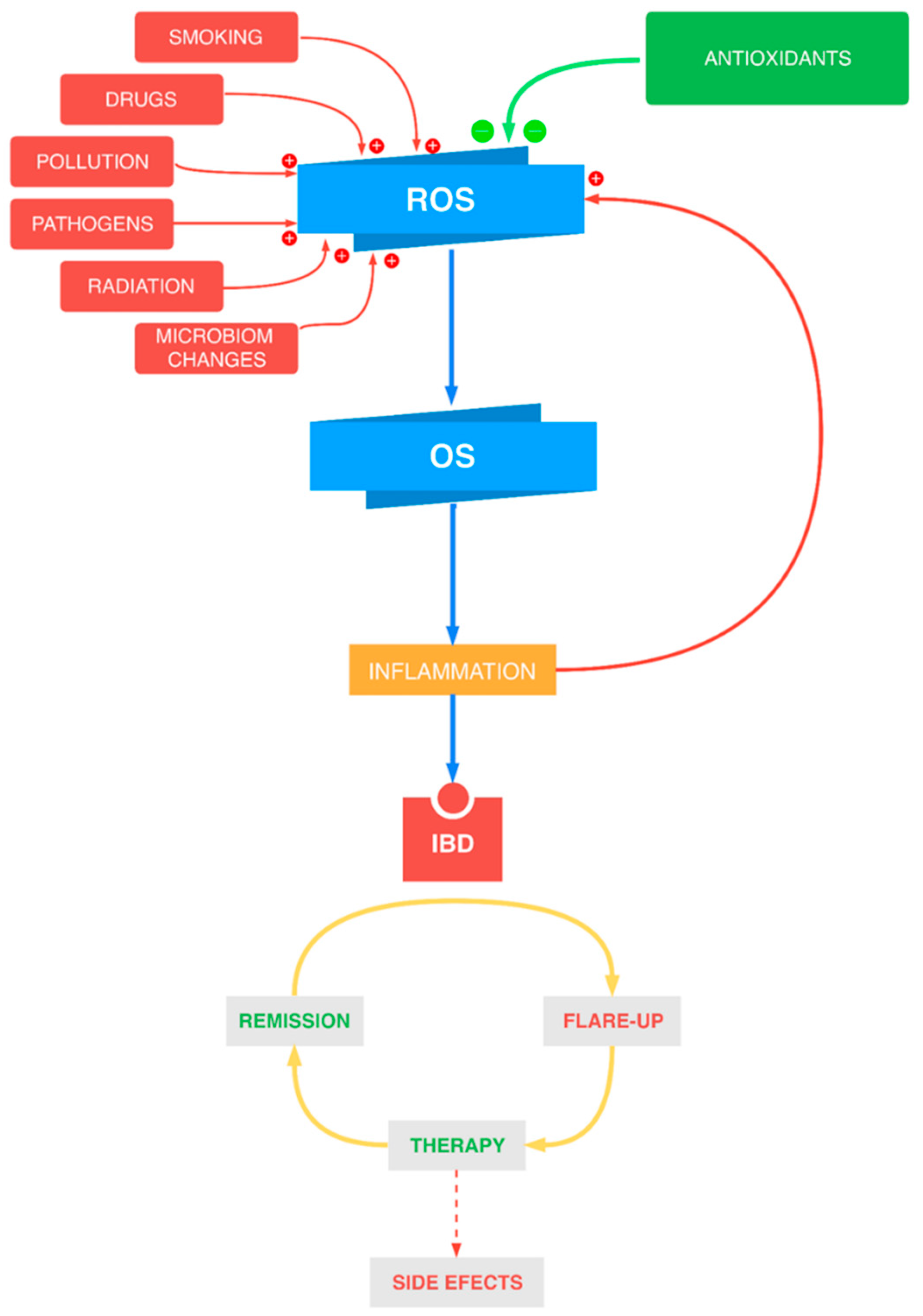
1. Introduction
2. Oxidative/Nitrosative Stress in IBD
3. Antioxidant Therapy in IBD
3.1. Synthetic Antioxidants
3.1.1. Inhibitors of 3-Hydroxy-3-Methylglutaryl Coenzyme A Reductase
3.1.2. Angiotensin-Converting-Enzyme Inhibitors
3.1.3. Melatonin
3.1.4. N-Acetylcysteine
3.1.5. Modified Superoxide Dismutase
3.1.6. Propionyl-L-Carnitine
3.2. Natural Antioxidants
3.2.1. Resveratrol
3.2.2. Curcumin
3.2.3. Quercetin
3.2.4. Catechins
3.2.5. Other Substances Derived from Plants
3.3. Micronutrient Antioxidants
3.4. Probiotics
4. Conclusions and Prospects for Future Research
Author Contributions
Funding
Conflicts of Interest
References
- De Lange, K.M.; Barrett, J.C. Understanding inflammatory bowel disease via immunogenetics. J. Autoimmun. 2015, 64, 91–100. [Google Scholar] [CrossRef]
- Molodecky, N.A.; Soon, I.S.; Rabi, D.M.; Ghali, W.A.; Ferris, M.; Chernoff, G.; Benchimol, E.I.; Panaccione, R.; Ghosh, S.; Barkema, H.W.; et al. Increasing incidence and prevalence of the inflammatory bowel diseases with time, based on sys-tematic review. Gastroenterology 2012, 142, 46–54. [Google Scholar] [CrossRef]
- Zhang, Y.-Z.; Li, Y.-Y. Inflammatory bowel disease: Pathogenesis. World J. Gastroenterol. 2014, 20, 91–99. [Google Scholar] [CrossRef]
- Beaugerie, L.; Brousse, N.; Bouvier, A.M.; Colombel, J.F.; Lemann, M.; Cosnes, J.; Hébuterne, X.; Cortot, A.; Bouhnik, Y.; Gendre, J.P.; et al. CESAME Study Group. Lymphoproliferative disorders in patients receiving thiopurines for inflammatory bowel disease: A prospective observational cohort study. Lancet 2009, 374, 1617–1625. [Google Scholar] [CrossRef]
- Connor, V. Anti-TNF therapies: A comprehensive analysis of adverse effects associated with immunosuppression. Rheumatol. Int. 2011, 31, 327–337. [Google Scholar] [CrossRef]
- Ransford, R.A.J.; Langman, M.J.S. Sulphasalazine and mesalazine: Serious adverse reactions re-evaluated on the basis of suspected adverse reaction reports to the Committee on Safety of Medicines. Gut 2002, 51, 536–539. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharyya, A.; Chattopadhyay, R.; Mitra, S.; Crowe, S.E. Oxidative stress: An essential factor in the pathogenesis of gastrointestinal mucosal diseases. Physiol. Rev. 2014, 94, 329–354. [Google Scholar] [CrossRef]
- Federico, A.; Morgillo, F.; Tuccillo, C.; Ciardiello, F.; Loguercio, C. Chronic inflammation and oxidative stress in human carcinogenesis. Int. J. Cancer 2007, 121, 2381–2386. [Google Scholar] [CrossRef]
- Sies, H. Oxidative stress: A concept in redox biology and medicine. Redox Biol. 2015, 4, 180–183. [Google Scholar] [CrossRef] [PubMed]
- Piechota-Polanczyk, A.; Fichna, J. Review article: The role of oxidative stress in pathogenesis and treatment of inflammatory bowel diseases. Naunyn Schmiedebergs Arch. Pharmacol. 2014, 387, 605–620. [Google Scholar] [CrossRef] [PubMed]
- Zuo, L.; Zhou, T.; Pannell, B.K.; Ziegler, A.C.; Best, T.M. Biological and physiological role of reactive oxygen species—The good, the bad and the ugly. Acta Physiol. 2015, 214, 329–348. [Google Scholar] [CrossRef]
- Alzoghaibi, M.A. Concepts of oxidative stress and antioxidant defense in Crohn’s disease. World J. Gastroenterol. 2013, 19, 6540–6547. [Google Scholar] [CrossRef] [PubMed]
- Balmus, I.M.; Ciobica, A.; Trifan, A.; Stanciu, C. The implications of oxidative stress and antioxidant therapies in Inflam-matory Bowel Disease: Clinical aspects and animal models. Saudi J. Gastroenterol. 2016, 22, 3–17. [Google Scholar] [CrossRef]
- Rezaie, A.; Parker, R.D.; Abdollahi, M. Oxidative stress and pathogenesis of inflammatory bowel disease: An epiphenomenon or the cause? Dig. Dis. Sci. 2007, 52, 2015–2021. [Google Scholar] [CrossRef] [PubMed]
- Spirlandeli, A.L.; Deminice, R.; Jordao, A.A. Plasma malondialdehyde as biomarker of lipid peroxidation: Effects of acute exercise. Int. J. Sports Med. 2014, 35, 14–18. [Google Scholar] [CrossRef]
- Ananthakrishnan, A.N.; Cagan, A.; Cai, T.; Gainer, V.S.; Shaw, S.Y.; Churchill, S.; Karlson, E.W.; Murphy, S.N.; Liao, K.P.; Kohane, I. Statin Use Is Associated With Reduced Risk of Colorectal Cancer in Patients With Inflammatory Bowel Diseases. Clin. Gastroenterol. Hepatol. 2016, 14, 973–979. [Google Scholar] [CrossRef]
- Ungaro, R.; Chang, H.L.; Cote-Daigneault, J.; Mehandru, S.; Atreja, A.; Colombel, J.-F. Statins Associated With Decreased Risk of New Onset Inflammatory Bowel Disease. Am. J. Gastroenterol. 2016, 111, 1416–1423. [Google Scholar] [CrossRef] [PubMed]
- Maheshwari, R.A.; Balaraman, R.; Sailor, G.U.; Sen, D.B. Protective effect of simvastatin and rosuvastatin on trinitroben-zene sulfonic acid-induced colitis in rats. Indian J. Pharmacol. 2015, 47, 17–21. [Google Scholar] [CrossRef]
- Shin, S.K.; Cho, J.H.; Kim, E.J.; Kim, E.-K.; Park, D.K.; Kwon, K.A.; Chung, J.-W.; Kim, K.O.; Kim, Y.J. Anti-inflammatory and anti-apoptotic effects of rosuvastatin by regulation of oxidative stress in a dextran sulfate sodium-induced colitis model. World J. Gastroenterol. 2017, 23, 4559–4568. [Google Scholar] [CrossRef] [PubMed]
- Lei, A.; Yang, Q.; Li, X.; Chen, H.; Shi, M.; Xiao, Q.; Cao, Y.; He, Y.; Zhou, J. Atorvastatin promotes the expansion of mye-loid-derived suppressor cells and attenuates murine colitis. Immunology 2016, 149, 432–446. [Google Scholar] [CrossRef]
- Arab, H.H.; Al-Shorbagy, M.Y.; Abdallah, D.M.; Nassar, N.N. Telmisartan attenuates colon inflammation, oxidative per-turbations and apoptosis in a rat model of experimental inflammatory bowel disease. PLoS ONE 2014, 9, e97193. [Google Scholar] [CrossRef]
- Arumugam, S.; Sreedhar, R.; Thandavarayan, R.A.; Giridharan, V.V.; Karuppagounder, V.; Pitchaimani, V.; Afrin, M.R.; Miyashita, S.; Nomoto, M.; Harima, M.; et al. Telmisartan treatment targets inflammatory cytokines to suppress the pathogenesis of acute colitis induced by dextran sulphate sodium. Cytokine 2015, 74, 305–312. [Google Scholar] [CrossRef]
- Guerra, G.C.B.; Araujo, A.A.; Lira, G.A.; Melo, M.N.; Souto, K.K.O.; Fernandes, D.; Silva, A.L.; Araújo Júnior, R.F. Telmisartan decreases inflammation by modulating TNF-alpha, IL-10, and RANK/RANKL in a rat model of ulcerative colitis. Pharmacol. Rep. 2015, 67, 520–526. [Google Scholar] [CrossRef] [PubMed]
- El-Medany, A.H.; Guemei, A.A.; Hagar, H.; El-Medany, J.H.; Baraka, A.M. Comparative study between effect of angiotensin converting enzyme inhibitors and angiotensin receptor blockers on acetic acid-induced ulcerative colitis in rats. Int. Res. J. Pharm. Pharmacol. 2011, 1, 100–108. [Google Scholar]
- Chojnacki, C.; Wisniewska-Jarosinska, M.; Walecka-Kapica, E.; Klupinska, G.; Jaworek, J.; Chojnacki, J. Evaluation of melatonin effectiveness in the adjuvant treatment of ulcerative colitis. J. Physiol. Pharmacol. 2011, 62, 327–334. [Google Scholar]
- Esiringu, F.; Tugcu-Demiroz, F.; Acarturk, F.; Coskun Cevher, S.; Bircan, F.; Sari Kilicaslan, S.M. Investigation of the effect of intracolonic melatonin gel formulation on acetic acid-induced colitis. Drug Deliv. 2016, 23, 2318–2326. [Google Scholar] [CrossRef]
- Park, Y.-S.; Chung, S.-H.; Lee, S.-K.; Kim, J.-H.; Kim, J.-B.; Kim, T.-K.; Kim, D.-S.; Baik, H.-W. Melatonin improves experimental colitis with sleep deprivation. Int. J. Mol. Med. 2015, 35, 979–986. [Google Scholar] [CrossRef] [PubMed]
- Zielinska, M.; Jarmuz, A.; Salaga, M.; Kordek, R.; Laudon, M.; Storr, M.; Fichna, J. Melatonin, but not melatonin receptor agonists Neu-P11 and Neu-P67, attenuates TNBS-induced colitis in mice. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2016, 389, 511–519. [Google Scholar] [CrossRef]
- Ancha, H.R.; Kurella, R.R.; McKimmey, C.C.; Lightfoot, S.; Harty, R.F. Effects of N-acetylcysteine plus mesalamine on prostaglandin synthesis and nitric oxide generation in TNBS-induced colitis in rats. Dig. Dis. Sci. 2009, 54, 758–766. [Google Scholar] [CrossRef]
- Hou, C.L.; Zhang, J.; Liu, X.T.; Liu, H.; Zeng, X.F.; Qiao, S.Y. Superoxide dismutase recombinant Lactobacillus fermentum ameliorates intestinal oxidative stress through inhibiting NF-kappaB activation in a trinitrobenzene sulphonic ac-id-induced colitis mouse model. J. Appl. Microbiol. 2014, 116, 1621–1631. [Google Scholar] [CrossRef]
- Suzuki, Y.; Matsumoto, T.; Okamoto, S.; Hibi, T. A lecithinized superoxide dismutase (PC-SOD) improves ulcerative colitis. Colorectal Dis. 2008, 10, 931–934. [Google Scholar] [CrossRef]
- Ishihara, T.; Tanaka, K.-I.; Tasaka, Y.; Namba, T.; Suzuki, J.; Ishihara, T.; Okamoto, S.; Hibi, T.; Takenaga, M.; Igarashi, R.; et al. Therapeutic effect of lecithinized superoxide dismutase against colitis. J. Pharmacol. Exp. Ther. 2009, 328, 152–164. [Google Scholar] [CrossRef] [PubMed]
- Scioli, M.G.; Stasi, M.A.; Passeri, D.; Doldo, E.; Costanza, G.; Camerini, R.; Fociani, P.; Arcuri, G.; Lombardo, K.; Pace, S.; et al. Propionyl-L-Carnitine is Efficacious in Ulcerative Colitis Through its Action on the Immune Function and Microvasculature. Clin. Transl. Gastroenterol. 2014, 5, e55. [Google Scholar] [CrossRef]
- Merra, G.; Gasbarrini, G.; Laterza, L.; Pizzoferrato, M.; Poscia, A.; Scaldaferri, F.; Arena, V.; Fiore, F.; Cittadini, A.; Sgam-bato, A.; et al. Propionyl-L-carnitine hydrochloride for treatment of mild to moderate colonic inflammatory bowel diseases. World J. Gastroenterol. 2012, 18, 5065–5071. [Google Scholar] [CrossRef] [PubMed]
- Mikhailova, T.L.; Sishkova, E.; Poniewierka, E.; Zhidkov, K.P.; Bakulin, I.G.; Kupcinskas, L.; Lesniakowski, K.; Grinevich, V.B.; Malecka-Panas, E.; Ardizzone, S.; et al. Randomised clinical trial: The efficacy and safety of propionyl-L-carnitine therapy in patients with ulcerative colitis receiving stable oral treatment. Aliment. Pharmacol. Ther. 2011, 34, 1088–1097. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Yang, T.; Wang, C. Are statins beneficial for the treatment of pulmonary hypertension? Chronic Dis. Transl. Med. 2017, 3, 213–220. [Google Scholar] [CrossRef]
- Esposito, E.; Cuzzocrea, S. Antiinflammatory activity of melatonin in central nervous system. Curr. Neuropharmacol. 2010, 8, 228–242. [Google Scholar] [CrossRef]
- Sanchez, A.; Calpena, A.C.; Clares, B. Evaluating the Oxidative Stress in Inflammation: Role of Melatonin. Int. J. Mol. Sci. 2015, 16, 16981–17004. [Google Scholar] [CrossRef]
- Reiter, R.J.; Mayo, J.C.; Tan, D.-X.; Sainz, R.M.; Alatorre-Jimenez, M.; Qin, L. Melatonin as an antioxidant: Under promises but over delivers. J. Pineal Res. 2016, 61, 253–278. [Google Scholar] [CrossRef]
- Jaworek, J.; Leja-Szpak, A.; Nawrot-Porabka, K.; Szklarczyk, J.; Kot, M.; Pierzchalski, P.; Góralska, M.; Ceranowicz, P.; Warzecha, Z.; Dembinski, A.; et al. Effects of Melatonin and Its Analogues on Pancreatic Inflammation, Enzyme Secretion, and Tumorigenesis. Int. J. Mol. Sci. 2017, 18, 1014. [Google Scholar] [CrossRef]
- Favero, G.; Franceschetti, L.; Bonomini, F.; Rodella, L.F.; Rezzani, R. Melatonin as an Anti-Inflammatory Agent Modulating Inflammasome Activation. Int. J. Endocrinol. 2017, 2017, 1835195. [Google Scholar] [CrossRef] [PubMed]
- Morgan, X.C.; Tickle, T.L.; Sokol, H.; Gevers, D.; Devaney, K.L.; Ward, D.V.; Reyes, J.A.; Shah, S.A.; LeLeiko, N.; Snapper, S.B.; et al. Dysfunction of the intestinal microbiome in inflammatory bowel disease and treatment. Genome Biol. 2012, 13, R79. [Google Scholar] [CrossRef] [PubMed]
- Zhu, D.; Ma, Y.; Ding, S.; Jiang, H.; Fang, J. Effects of Melatonin on Intestinal Microbiota and Oxidative Stress in Colitis Mice. BioMed Res. Int. 2018, 2018, 2607679. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, X.; Hu, C.-A.A. Therapeutic Potential of Amino Acids in Inflammatory Bowel Disease. Nutrients 2017, 9, 920. [Google Scholar] [CrossRef]
- Hou, Y.; Wang, L.; Yi, D.; Wu, G. N-acetylcysteine and intestinal health: A focus on its mechanism of action. Front Biosci. 2015, 20, 872–891. [Google Scholar] [CrossRef]
- Kamio, K.; Azuma, A.; Ohta, K.; Sugiyama, Y.; Nukiwa, T.; Kudoh, S.; Mizushima, T. Double-blind controlled trial of lecithinized superoxide dismutase in patients with idiopathic interstitial pneumonia—Short term evaluation of safety and tolerability. BMC Pulm. Med. 2014, 14, 86. [Google Scholar] [CrossRef]
- Scioli, M.G.; Lo Giudice, P.; Bielli, A.; Tarallo, V.; de Rosa, A.; de Falco, S.; Orlandi, A. Propionyl-L-Carnitine Enhances Wound Healing and Counteracts Microvascular Endothelial Cell Dysfunction. PLoS ONE 2015, 10, e0140697. [Google Scholar] [CrossRef]
- Lapi, D.; Sabatino, L.; Altobelli, G.G.; Mondola, P.; Cimini, V.; Colantuoni, A. Effects of propionyl-L-carnitine on ische-mia-reperfusion injury in hamster cheek pouch microcirculation. Front. Physiol. 2010, 1, 132. [Google Scholar] [CrossRef] [PubMed]
- Biasi, F.; Astegiano, M.; Maina, M.; Leonarduzzi, G.; Poli, G. Polyphenol supplementation as a complementary medicinal approach to treating inflammatory bowel disease. Curr. Med. Chem. 2011, 18, 4851–4865. [Google Scholar] [CrossRef]
- Mattera, R.; Benvenuto, M.; Giganti, M.G.; Tresoldi, I.; Pluchinotta, F.R.; Bergante, S.; Tettamanti, G.; Masuelli, L.; Manzari, V.; Modesti, A.; et al. Effects of Polyphenols on Oxidative Stress-Mediated Injury in Cardiomyocytes. Nutrients 2017, 9, 523. [Google Scholar] [CrossRef]
- Yildiz, G.; Yildiz, Y.; Ulutas, P.A.; Yaylali, A.; Ural, M. Resveratrol Pretreatment Ameliorates TNBS Colitis in Rats. Recent Pat. Endocr. Metab. Immune Drug Discov. 2015, 9, 134–140. [Google Scholar] [CrossRef] [PubMed]
- Rahal, K.; Schmiedlin-Ren, P.; Adler, J.; Dhanani, M.; Sultani, V.; Rittershaus, A.C.; Reingold, L.; Zhu, J.; McKenna, B.J.; Christman, G.M.; et al. Resveratrol has antiinflammatory and antifibrotic effects in the peptidoglycan-polysaccharide rat model of Crohn’s disease. Inflamm. Bowel Dis. 2012, 18, 613–623. [Google Scholar] [CrossRef] [PubMed]
- Abdallah, D.M.; Ismael, N.R. Resveratrol abrogates adhesion molecules and protects against TNBS-induced ulcerative colitis in rats. Can. J. Physiol. Pharmacol. 2011, 89, 811–818. [Google Scholar] [CrossRef]
- Samsamikor, M.; Daryani, N.E.; Asl, P.R.; Hekmatdoost, A. Resveratrol Supplementation and Oxidative/Anti-Oxidative Status in Patients with Ulcerative Colitis: A Randomized, Double-Blind, Placebo-controlled Pilot Study. Arch. Med. Res. 2016, 47, 304–309. [Google Scholar] [CrossRef]
- Samsami-Kor, M.; Daryani, N.E.; Asl, P.R.; Hekmatdoost, A. Anti-Inflammatory Effects of Resveratrol in Patients with Ulcerative Colitis: A Randomized, Double-Blind, Placebo-controlled Pilot Study. Arch. Med. Res. 2015, 46, 280–285. [Google Scholar] [CrossRef]
- Topcu-Tarladacalisir, Y.; Akpolat, M.; Uz, Y.H.; Kizilay, G.; Sapmaz-Metin, M.; Cerkezkayabekir, A.; Omurlu, I.K. Effects of curcumin on apoptosis and oxidoinflammatory regulation in a rat model of acetic acid-induced colitis: The roles of c-Jun N-terminal kinase and p38 mitogen-activated protein kinase. J. Med. Food 2013, 16, 296–305. [Google Scholar] [CrossRef]
- Yang, J.-Y.; Zhong, X.; Kim, S.-J.; Kim, D.-H.; Kim, H.S.; Lee, J.-S.; Yum, H.-W.; Lee, J.; Na, H.-K.; and Surh, Y.-J. Comparative Effects of Curcumin and Tetrahydrocurcumin on Dextran Sulfate Sodium-induced Colitis and Inflammatory Signaling in Mice. J. Cancer Prev. 2018, 23, 18–24. [Google Scholar] [CrossRef]
- Zeng, Z.; Zhan, L.; Liao, H.; Chen, L.; Lv, X. Curcumin improves TNBS-induced colitis in rats by inhibiting IL-27 expression via the TLR4/NF-kappaB signaling pathway. Planta Med. 2013, 79, 102–109. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.-M.; Xu, R.; Huang, X.-Y.; Cheng, S.-M.; Huang, M.-F.; Yue, H.-Y.; Wang, X.; Zou, Y.; Lu, A.-P.; Liu, D.-Y. Curcumin Suppressed Activation of Dendritic Cells via JAK/STAT/SOCS Signal in Mice with Experimental Colitis. Front. Pharmacol. 2016, 7, 455. [Google Scholar] [CrossRef]
- Lang, A.; Salomon, N.; Wu, J.C.Y.; Kopylov, U.; Lahat, A.; Har-Noy, O.; Ching, J.Y.L.; Cheong, P.K.; Avidan, B.; Gamus, D.; et al. Curcumin in Combination With Mesalamine Induces Remission in Patients With Mild-to-Moderate Ulcerative Colitis in a Randomized Controlled Trial. Clin. Gastroenterol. Hepatol. 2015, 13, 1444–1449. [Google Scholar] [CrossRef] [PubMed]
- Singla, V.; Pratap Mouli, V.; Garg, S.K.; Rai, T.; Choudhury, B.N.; Verma, P.; Deb, R.; Tiwari, V.; Rohatgi, S.; Dhingra, R.; et al. Induction with NCB-02 (curcumin) enema for mild-to-moderate distal ulcerative colitis—A randomized, place-bo-controlled, pilot study. J. Crohn’s Colitis 2014, 8, 208–214. [Google Scholar] [CrossRef]
- Dodda, D.; Chhajed, R.; Mishra, J.; Padhy, M. Targeting oxidative stress attenuates trinitrobenzene sulphonic acid induced inflammatory bowel disease like symptoms in rats: Role of quercetin. Indian J. Pharmacol. 2014, 46, 286–291. [Google Scholar] [CrossRef]
- Joo, M.; Kim, H.S.; Kwon, T.H.; Palikhe, A.; Zaw, T.S.; Jeong, J.H.; Sohn, U.D. Anti-inflammatory Effects of Flavonoids on TNBS-induced Colitis of Rats. Korean J. Physiol. Pharmacol. 2015, 19, 43–50. [Google Scholar] [CrossRef] [PubMed]
- Dryden, G.W.; Lam, A.; Beatty, K.; Qazzaz, H.H.; McClain, C.J. A pilot study to evaluate the safety and efficacy of an oral dose of (-)-epigallocatechin-3-gallate-rich polyphenon E in patients with mild to moderate ulcerative colitis. Inflamm. Bowel Dis. 2013, 19, 1904–1912. [Google Scholar] [CrossRef] [PubMed]
- Byun, E.-B.; Kim, W.S.; Sung, N.-Y.; Byun, E.-H. Epigallocatechin-3-Gallate Regulates Anti-Inflammatory Action Through 67-kDa Laminin Receptor-Mediated Tollip Signaling Induction in Lipopolysaccharide-Stimulated Human Intestinal Epi-thelial Cells. Cell Physiol. Biochem. 2018, 46, 2072–2081. [Google Scholar] [CrossRef]
- Bitzer, Z.T.; Elias, R.J.; Vijay-Kumar, M.; Lambert, J.D. (-)-Epigallocatechin-3-gallate decreases colonic inflammation and permeability in a mouse model of colitis, but reduces macronutrient digestion and exacerbates weight loss. Mol. Nutr. Food Res. 2016, 60, 2267–2274. [Google Scholar] [CrossRef]
- Bruckner, M.; Westphal, S.; Domschke, W.; Kucharzik, T.; Lugering, A. Green tea polyphenol epigallocatechin-3-gallate shows therapeutic antioxidative effects in a murine model of colitis. J. Crohn’s Colitis 2012, 6, 226–235. [Google Scholar] [CrossRef] [PubMed]
- Yeoh, B.S.; Aguilera Olvera, R.; Singh, V.; Xiao, X.; Kennett, M.J.; Joe, B.; Lambert, J.D.; Vijay-Kumar, M. Epigallocate-chin-3-Gallate Inhibition of Myeloperoxidase and Its Counter-Regulation by Dietary Iron and Lipocalin 2 in Murine Model of Gut Inflammation. Am. J. Pathol. 2016, 186, 912–926. [Google Scholar] [CrossRef]
- Gerges Geagea, A.; Rizzo, M.; Eid, A.; Hajj Hussein, I.; Zgheib, Z.; Zeenny, M.N.; Jurjus, R.; Uzzo, M.L.; Spatola, G.F.; Bonaventura, G.; et al. Tea catechins induce crosstalk between signaling pathways and stabilize mast cells in ulcerative colitis. J. Biol. Regul. Homeost. Agents 2017, 31, 865–877. [Google Scholar]
- Biedermann, L.; Mwinyi, J.; Scharl, M.; Frei, P.; Zeitz, J.; Kullak-Ublick, G.A.; Vavricka, S.R.; Fried, M.; Weber, A.; Humpf, H.-U.; et al. Bilberry ingestion improves disease activity in mild to moderate ulcerative colitis—An open pilot study. J. Crohn’s Colitis 2013, 7, 271–279. [Google Scholar] [CrossRef]
- Rastegarpanah, M.; Malekzadeh, R.; Vahedi, H.; Mohammadi, M.; Elahi, E.; Chaharmahali, M.; Safarnavadeh, T.; Abdollahi, M. A randomized, double blinded, placebo-controlled clinical trial of silymarin in ulcerative colitis. Chin. J. Intergr. Med. 2015, 21, 902–906. [Google Scholar] [CrossRef] [PubMed]
- Kolacek, M.; Muchova, J.; Dvorakova, M.; Paduchova, Z.; Zitnanova, I.; Cierna, I.; Orszaghova, Z.; Szekyova, D.; Jajcaio-va-Zednickova, N.; Kovacs, L.; et al. Effect of natural polyphenols (Pycnogenol) on oxidative stress markers in children suffering from Crohn’s disease—A pilot study. Free Rad. Res. 2013, 47, 624–634. [Google Scholar] [CrossRef]
- Vecchi Brumatti, L.; Marcuzzi, A.; Tricarico, P.M.; Zanin, V.; Girardelli, M.; Bianco, A.M. Curcumin and inflammatory bowel disease: Potential and limits of innovative treatments. Molecules 2014, 19, 21127–21153. [Google Scholar] [CrossRef] [PubMed]
- Pulido-Moran, M.; Moreno-Fernandez, J.; Ramirez-Tortosa, C.; Ramirez-Tortosa, M. Curcumin and Health. Molecules 2016, 21, 264. [Google Scholar] [CrossRef] [PubMed]
- Kadri, C.J.; Pereira, J.A.; Campos, F.G.; Ortega, M.M.; Bragion, C.B.; Martinez, C.A.R. Anti-inflammatory effects of enemas containing an oily extract of curcumin in an experimental model of diversion colitis. Histol. Histopathol. 2017, 32, 161–169. [Google Scholar] [CrossRef]
- Cunha Neto, F.; Marton, L.T.; de Marqui, S.V.; Lima, T.A.; Barbalho, S.M. Curcuminoids from Curcuma Longa: New ad-juvants for the treatment of Crohn’s disease and ulcerative colitis? Crit. Rev. Food Sci. Nutr. 2019, 59, 2136–2143. [Google Scholar] [CrossRef]
- Fadus, M.C.; Lau, C.; Bikhchandani, J.; Lynch, H.T. Curcumin: An age-old anti-inflammatory and anti-neoplastic agent. J. Tradit. Complement. Med. 2016, 7, 339–346. [Google Scholar] [CrossRef]
- Simadibrata, M.; Halimkesuma, C.C.; Suwita, B.M. Efficacy of Curcumin as Adjuvant Therapy to Induce or Maintain Re-mission in Ulcerative Colitis Patients: An Evidence-based Clinical Review. Acta Med. Indones. 2017, 49, 363–368. [Google Scholar]
- Ju, S.; Ge, Y.; Li, P.; Tian, X.; Wang, H.; Zheng, X.; Ju, S. Dietary quercetin ameliorates experimental colitis in mouse by remodeling the function of colonic macrophages via a heme oxygenase-1-dependent pathway. Cell Cycle 2018, 17, 53–63. [Google Scholar] [CrossRef]
- Kamishikiryo, J.; Matsumura, R.; Takamori, T.; Sugihara, N. Effect of quercetin on the transport of N-acetyl 5-aminosalicylic acid. J. Pharm. Pharmacol. 2013, 65, 1037–1043. [Google Scholar] [CrossRef]
- Fan, F.-Y.; Sang, L.-X.; Jiang, M. Catechins and Their Therapeutic Benefits to Inflammatory Bowel Disease. Molecules 2017, 22, 484. [Google Scholar] [CrossRef] [PubMed]
- Pan, M.-H.; Chiou, Y.-S.; Wang, Y.-J.; Ho, C.-T.; Lin, J.-K. Multistage carcinogenesis process as molecular targets in cancer chemoprevention by epicatechin-3-gallate. Food Funct. 2011, 2, 101–110. [Google Scholar] [CrossRef] [PubMed]
- Contreras, T.C.; Ricciardi, E.; Cremonini, E.; Oteiza, P.I. (-)-Epicatechin in the prevention of tumor necrosis alpha-induced loss of Caco-2 cell barrier integrity. Arch. Biochem. Biophys. 2015, 573, 84–91. [Google Scholar] [CrossRef] [PubMed]
- Martin, D.A.; Bolling, B.W. A review of the efficacy of dietary polyphenols in experimental models of inflammatory bowel diseases. Food Funct. 2015, 6, 1773–1786. [Google Scholar] [CrossRef]
- Salaritabar, A.; Darvishi, B.; Hadjiakhoondi, F.; Manayi, A.; Sureda, A.; Nabavi, S.F.; Fitzpatrick, L.R.; Nabavi, S.M.; Bishayee, A. Therapeutic potential of flavonoids in inflammatory bowel disease: A comprehensive review. World J. Gas-troenterol. 2017, 23, 5097–5114. [Google Scholar] [CrossRef]
- Mirbagheri, S.-A.; Nezami, B.-G.; Assa, S.; Hajimahmoodi, M. Rectal administration of d-alpha tocopherol for active ulcerative colitis: A preliminary report. World J. Gastroenterol. 2008, 14, 5990–5995. [Google Scholar] [CrossRef]
- Tahan, G.; Aytac, E.; Aytekin, H.; Gunduz, F.; Dogusoy, G.; Aydin, S.; Tahan, V.; Uzun, H. Vitamin E has a dual effect of anti-inflammatory and antioxidant activities in acetic acid-induced ulcerative colitis in rats. Can. J. Surg. 2011, 54, 333–338. [Google Scholar] [CrossRef] [PubMed]
- Minaiyan, M.; Mostaghel, E.; Mahzouni, P. Preventive Therapy of Experimental Colitis with Selected iron Chelators and Anti-oxidants. Int. J. Prev. Med. 2012, 3, S162–S169. [Google Scholar]
- Jeon, H.-J.; Yeom, Y.; Kim, Y.-S.; Kim, E.; Shin, J.-H.; Seok, P.R.; Woo, M.J.; Kim, Y. Effect of vitamin C on azoxymethane (AOM)/dextran sulfate sodium (DSS)-induced colitis-associated early colon cancer in mice. Nutr. Res. Pract. 2018, 12, 101–109. [Google Scholar] [CrossRef]
- Yan, H.; Wang, H.; Zhang, X.; Li, X.; Yu, J. Ascorbic acid ameliorates oxidative stress and inflammation in dextran sulfate sodium-induced ulcerative colitis in mice. Int. J. Clin. Exp. Med. 2015, 8, 20245–20253. [Google Scholar]
- Hiller, F.; Oldorff, L.; Besselt, K.; Kipp, A.P. Differential acute effects of selenomethionine and sodium selenite on the se-verity of colitis. Nutrients 2015, 7, 2687–2706. [Google Scholar] [CrossRef] [PubMed]
- Kaushal, N.; Kudva, A.K.; Patterson, A.D.; Chiaro, C.; Kennett, M.J.; Desai, D.; Amin, S.; Carlson, B.A.; Cantorna, M.T.; Prabhu, K.S. Crucial role of macrophage selenoproteins in experimental colitis. J. Immunol. 2014, 193, 3683–3692. [Google Scholar] [CrossRef] [PubMed]
- Xue, H.; Ren, H.; Zhang, L.; Sun, X.; Wang, W.; Zhang, S.; Zhao, J.; Ming, L. Alpha-tocopherol ameliorates experimental autoimmune encephalomyelitis through the regulation of Th1 cells. Iran. J. Basic Med. Sci. 2016, 19, 561–566. [Google Scholar] [PubMed]
- Ioannidis, O.; Varnalidis, I.; Paraskevas, G.; Botsios, D. Nutritional modulation of the inflammatory bowel response. Digestion 2011, 84, 89–101. [Google Scholar] [CrossRef] [PubMed]
- Oudemans-van Straaten, H.M.; Spoelstra-de Man, A.M.; de Waard, M.C. Vitamin C revisited. Crit. Care 2014, 18, 460. [Google Scholar] [CrossRef]
- Sorice, A.; Guerriero, E.; Capone, F.; Colonna, G.; Castello, G.; Costantini, S. Ascorbic acid: Its role in immune system and chronic inflammation diseases. Mini Rev. Med. Chem. 2014, 14, 444–452. [Google Scholar] [CrossRef]
- Seidner, D.L.; Lashner, B.A.; Brzezinski, A.; Banks, P.L.C.; Goldblum, J.; Fiocchi, C.; Katz, J.; Lichtenstein, G.R.; Anton, P.A.; Kam, L.Y.; et al. An oral supplement enriched with fish oil, soluble fiber, and antioxidants for corticosteroid sparing in ulcerative colitis: A randomized, controlled trial. Clin. Gastroenterol. Hepatol. 2005, 3, 358–369. [Google Scholar] [CrossRef]
- Hakuna, L.; Doughan, B.; Escobedo, J.O.; Strongin, R.M. A simple assay for glutathione in whole blood. Analyst 2015, 140, 3339–3342. [Google Scholar] [CrossRef]
- Lu, S.C. Glutathione synthesis. Biochim. Biophys. Acta 2013, 1830, 3143–3153. [Google Scholar] [CrossRef] [PubMed]
- Qian, J.; Song, Z.; Lv, Y.; Huang, X.; Mao, B. Glutathione S-Transferase T1 Null Genotype is Associated with Susceptibility to Inflammatory Bowel Disease. Cell Physiol. Biochem. 2017, 41, 2545–2552. [Google Scholar] [CrossRef] [PubMed]
- Dominiak, A.; Wilkaniec, A.; Wroczyński, P.; Adamczyk, A. Selenium in the Therapy of Neurological Diseases. Where is it Going? Curr. Neuropharmacol. 2016, 14, 282–299. [Google Scholar] [CrossRef] [PubMed]
- Kudva, A.K.; Shay, A.E.; Prabhu, K.S. Selenium and inflammatory bowel disease. Am. J. Physiol. Gastrointest. Liver Physiol. 2015, 309, G71–G77. [Google Scholar] [CrossRef] [PubMed]
- Kostic, A.D.; Xavier, R.J.; Gevers, D. The microbiome in inflammatory bowel disease: Current status and the future ahead. Gastroenterology 2014, 146, 1489–1499. [Google Scholar] [CrossRef] [PubMed]
- Prosberg, M.; Bendtsen, F.; Vind, I.; Petersen, A.M.; Gluud, L.L. The association between the gut microbiota and the in-flammatory bowel disease activity: A systematic review and meta-analysis. Scand. J. Gastroenterol. 2016, 51, 1407–1415. [Google Scholar] [CrossRef]
- Tamaki, H.; Nakase, H.; Inoue, S.; Kawanami, C.; Itani, T.; Ohana, M.; Kusaka, T.; Uose, S.; Hisatsune, H.; Tojo, M.; et al. Efficacy of probiotic treatment with Bifidobacterium longum 536 for induction of remission in active ulcerative colitis: A randomized, double-blinded, placebo-controlled multicenter trial. Dig. Endosc. 2016, 28, 67–74. [Google Scholar] [CrossRef] [PubMed]
- Miele, E.; Pascarella, F.; Giannetti, E.; Quaglietta, L.; Baldassano, R.N.; Staiano, A. Effect of a probiotic preparation (VSL#3) on induction and maintenance of remission in children with ulcerative colitis. Am. J. Gastroenterol. 2009, 104, 437–443. [Google Scholar] [CrossRef] [PubMed]
- Magro, F.; Gionchetti, P.; Eliakim, R.; Ardizzone, S.; Armuzzi, A.; Barreiro-de Acosta, M.; Burisch, J.; Gecse, K.B.; Hart, A.L.; Hindryckx, P.; et al. Third European Evidence-based Consensus on Diagnosis and Management of Ulcerative Colitis. Part 1: Definitions, Diagnosis, Extra-intestinal Manifestations, Pregnancy, Cancer Surveillance, Surgery, and Ileo-anal Pouch Disorders. J. Crohn’s Colitis 2017, 11, 649–670. [Google Scholar] [CrossRef]
- Harbord, M.; Eliakim, R.; Bettenworth, D.; Karmiris, K.; Katsanos, K.; Kopylov, U.; Kucharzik, T.; Molnár, T.; Raine, T.; Sebastian, S.; et al. Third European Evidence-based Consensus on Diagnosis and Management of Ulcerative Colitis. Part 2: Current Management. J. Crohn’s Colitis 2017, 11, 769–784. [Google Scholar] [CrossRef]
- Gomollon, F.; Dignass, A.; Annese, V.; Tilg, H.; van Assche, G.; Lindsay, J.O.; Peyrin-Biroulet, L.; Cullen, G.J.; Daperno, M.; Kucharzik, T.; et al. 3rd European Evidence-based Consensus on the Diagnosis and Management of Crohn’s Disease 2016: Part 1: Diagnosis and Medical Management. J. Crohn’s Colitis 2017, 11, 3–25. [Google Scholar] [CrossRef]
- Shen, J.; Zuo, Z.-X.; Mao, A.-P. Effect of probiotics on inducing remission and maintaining therapy in ulcerative colitis, Crohn’s disease, and pouchitis: Meta-analysis of randomized controlled trials. Inflamm. Bowel Dis. 2014, 20, 21–35. [Google Scholar] [CrossRef]
- Jonkers, D.; Penders, J.; Masclee, A.; Pierik, M. Probiotics in the management of inflammatory bowel disease: A systematic review of intervention studies in adult patients. Drugs 2012, 72, 803–823. [Google Scholar] [CrossRef] [PubMed]
- Derwa, Y.; Gracie, D.J.; Hamlin, P.J.; Ford, A.C. Systematic review with meta-analysis: The efficacy of probiotics in in-flammatory bowel disease. Aliment. Pharmacol. Ther. 2017, 46, 389–400. [Google Scholar] [CrossRef] [PubMed]
- Losurdo, G.; Iannone, A.; Contaldo, A.; Ierardi, E.; Di Leo, A.; Principi, M. Escherichia coli Nissle 1917 in Ulcerative Colitis Treatment: Systematic Review and Meta-analysis. J. Gastrointestin. Liver Dis. 2015, 24, 499–505. [Google Scholar] [CrossRef] [PubMed]
| Antioxidant | Clinical Studies/Animal Model | Reference |
|---|---|---|
| 3-hydroxy-3-methylglutaryl coenzyme A (HMG-CoA) reductase inhibitors | patients with UC or CD TNBS-induced ulcerative colitis in rats DSS-induced colitis in mice | [16,17,18,19,20] |
| Angiotensin-converting-enzyme (ACE) inhibitors | TNBS-induced colitis in rats DSS-induced colitis in mice AA-induced ulcerative colitis in rats | [21,22,23,24] |
| Melatonin (MEL) | patients with UC in remission AA-induced colitis in rats DSS-induced colitis in mice TNBS-induced colitis in mice | [25,26,27,28] |
| N-acetylcysteine (NAC) | TNBS-induced colitis in rats | [29] |
| Modified superoxide dismutase (SOD) | TNBS-induced colitis in mice patients with UC DSS-induced colitis in mice | [30,31,32] |
| Propionyl-L-carnitine (PLC) | patients with mild-to-moderate UC or CD TNBS-induced colitis in rat TNF-α-stimulated human intestinal microvascular endothelial cells | [33,34,35] |
| Antioxidant | Clinical Studies/Animal Model | Reference |
|---|---|---|
| Resveratrol (RSV) | TNBS-induced colitis in rats PG-PS model of Crohn’s disease in rats TNBS-induced ulcerative-colitis in rats patients with mild-to-moderate UC | [51,52,53,54,55] |
| Curcumin | AA-induced colitis in rats DSS-induced colitis in mice TNBS-induced colitis in rats TNBS-induced colitis in mice patients with mild-to-moderate UC | [56,57,58,59,60,61] |
| Quercetin (QCT) | TNBS-induced colitis in rats | [62,63] |
| Catechines | patients with mild-to-moderate UC human intestinal epithelial cells DSS-induced colitis in mice TNBS-induced colitis in rats | [64,65,66,67,68,69] |
| Anthocyanins | patients with mild-to-moderate UC | [70] |
| Silymarin | patients with UC in remission pediatric CD patients in remission | [71,72] |
| Antioxidant | Clinical Studies/Animal Model | Reference |
|---|---|---|
| Vitamin E (alpha-tocopherol) | patients with mild-to-moderate UC AA-induced ulcerative colitis in rats TNBS-induced colitis in rats | [86,87,88] |
| Vitamin C (ascorbic acid) | DSS-induced ulcerative colitis in mice | [89,90] |
| Selenium | DSS-induced colitis in mice | [91,92] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dziąbowska-Grabias, K.; Sztanke, M.; Zając, P.; Celejewski, M.; Kurek, K.; Szkutnicki, S.; Korga, P.; Bulikowski, W.; Sztanke, K. Antioxidant Therapy in Inflammatory Bowel Diseases. Antioxidants 2021, 10, 412. https://doi.org/10.3390/antiox10030412
Dziąbowska-Grabias K, Sztanke M, Zając P, Celejewski M, Kurek K, Szkutnicki S, Korga P, Bulikowski W, Sztanke K. Antioxidant Therapy in Inflammatory Bowel Diseases. Antioxidants. 2021; 10(3):412. https://doi.org/10.3390/antiox10030412
Chicago/Turabian StyleDziąbowska-Grabias, Katarzyna, Małgorzata Sztanke, Przemysław Zając, Michał Celejewski, Katarzyna Kurek, Stanisław Szkutnicki, Patryk Korga, Włodzimierz Bulikowski, and Krzysztof Sztanke. 2021. "Antioxidant Therapy in Inflammatory Bowel Diseases" Antioxidants 10, no. 3: 412. https://doi.org/10.3390/antiox10030412
APA StyleDziąbowska-Grabias, K., Sztanke, M., Zając, P., Celejewski, M., Kurek, K., Szkutnicki, S., Korga, P., Bulikowski, W., & Sztanke, K. (2021). Antioxidant Therapy in Inflammatory Bowel Diseases. Antioxidants, 10(3), 412. https://doi.org/10.3390/antiox10030412






