Protective Effects of Cannabidiol on the Membrane Proteome of UVB-Irradiated Keratinocytes
Abstract
1. Introduction
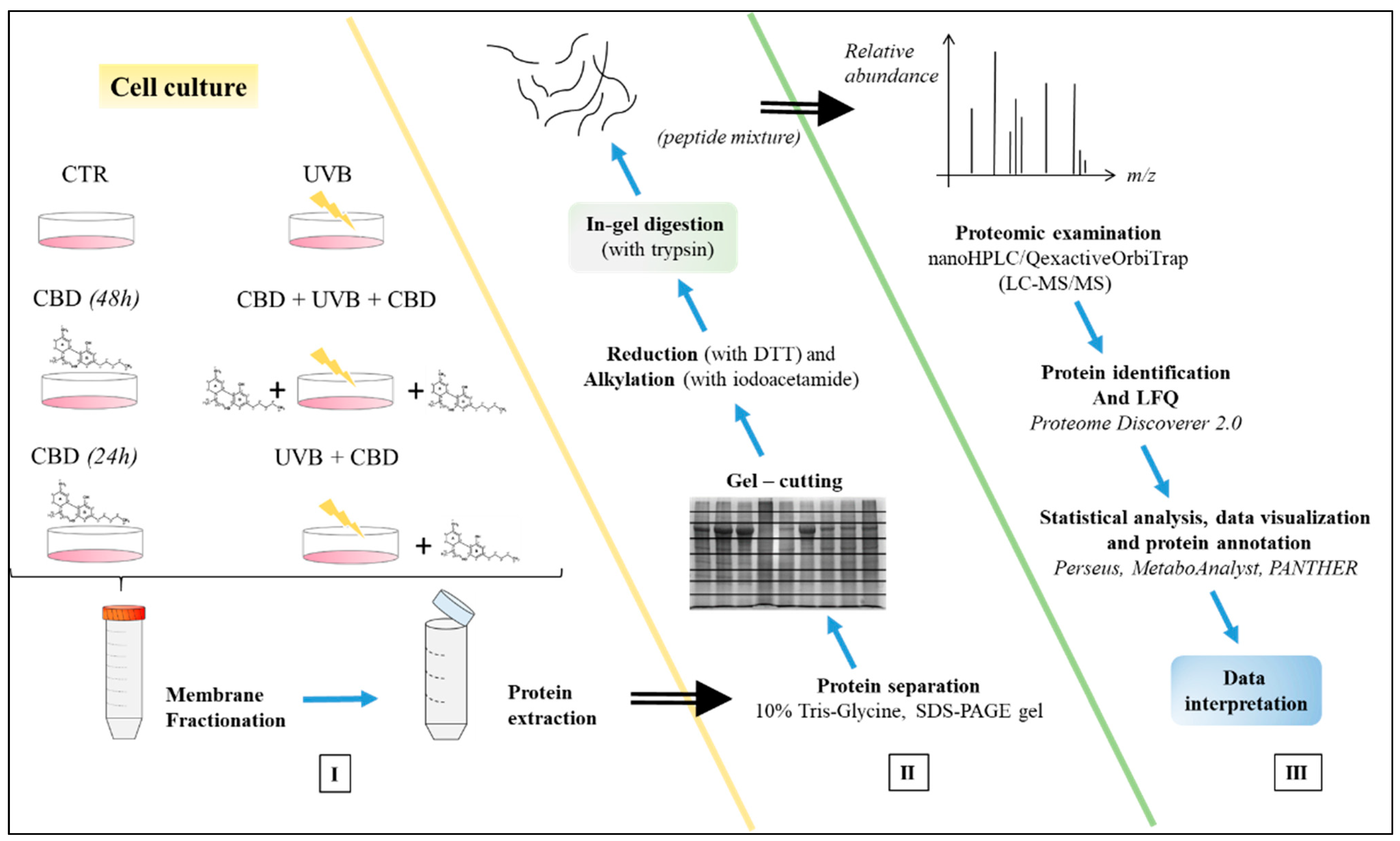
2. Materials and Methods
2.1. Cell Culture and Experimental Cell Groups
- Control cells cultured with standard medium (CTR).
- Cells cultured in medium containing 4 µM CBD (Sigma-Aldrich, MO, USA—98.5% pure) in 0.2% ethanol for 48 h (CBD(48 h))
- Cells cultured in medium containing 4 µM CBD in 0.2% ethanol for 24 h (CBD(24 h)).
- IV.
- Cells irradiated by UVB (312 nm) at 60 mJ/cm2 (Bio-Link Crosslinker BLX 312; Vilber Lourmat, Germany; six lamps at a distance of 15 cm) (UVB).
- V.
- Cells cultured in medium containing 4 μM CBD in 0.2% ethanol for 24 h before and 24 h after UVB irradiation (pretreatment + treatment) (CBD + UVB + CBD)
- VI.
- Cells cultured in medium containing 4 μM CBD in 0.2% ethanol for 24 h after UVB irradiation (treatment only) (UVB + CBD).
2.2. Cell Membrane Fractionation
2.3. Proteomic Analysis
2.4. Identification and Quantification of Proteins
2.5. Statistical Analysis
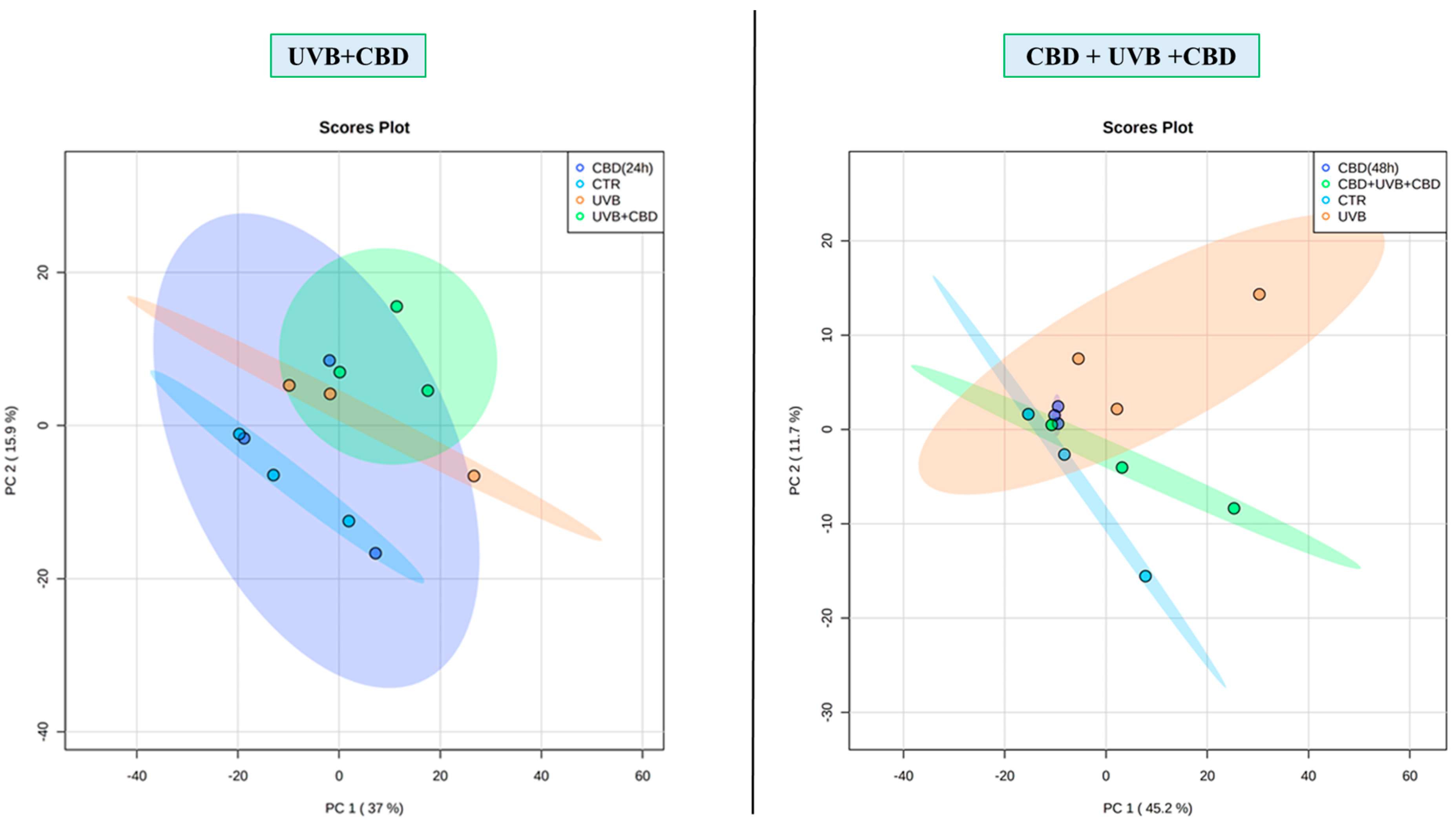
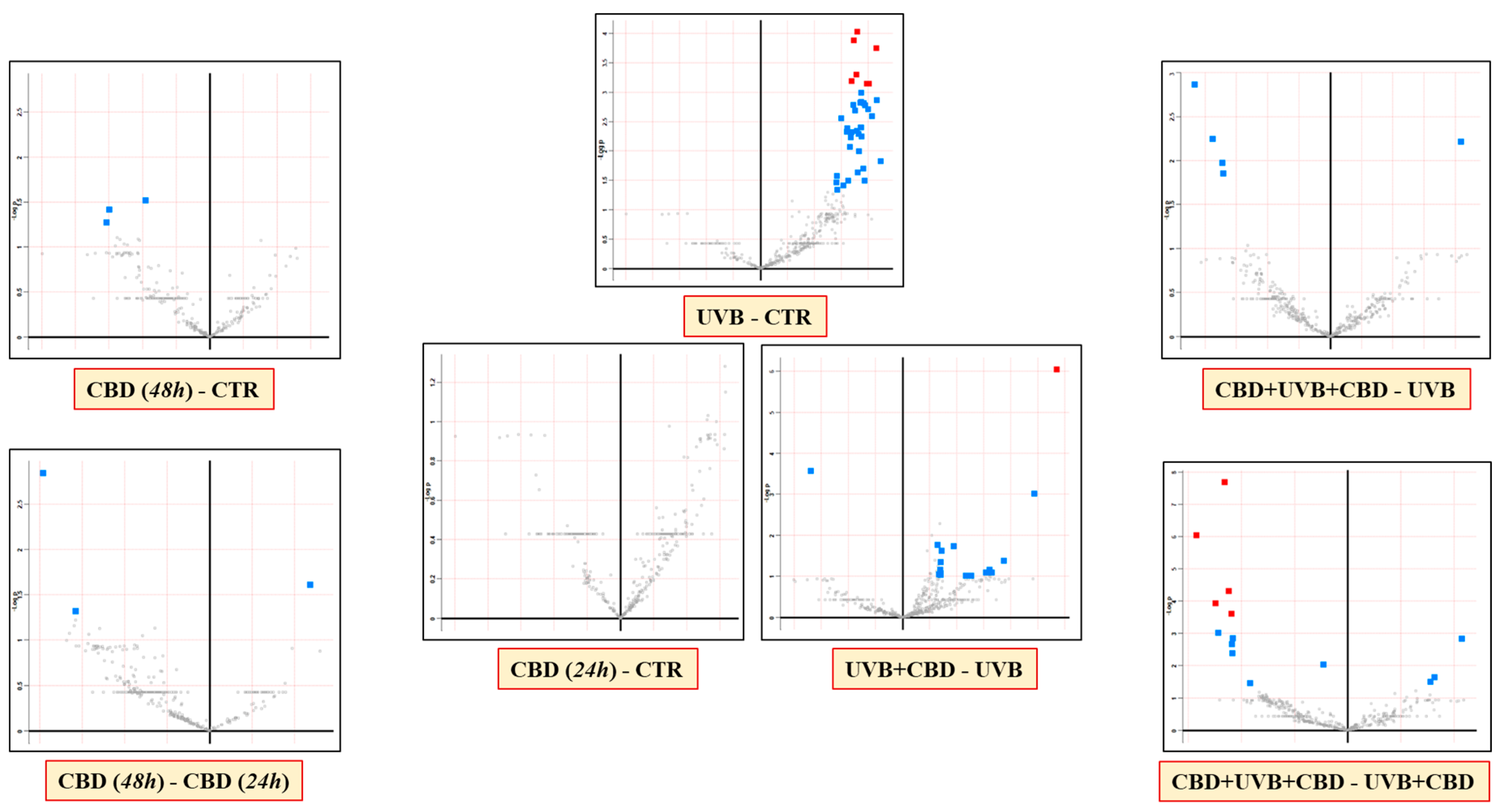
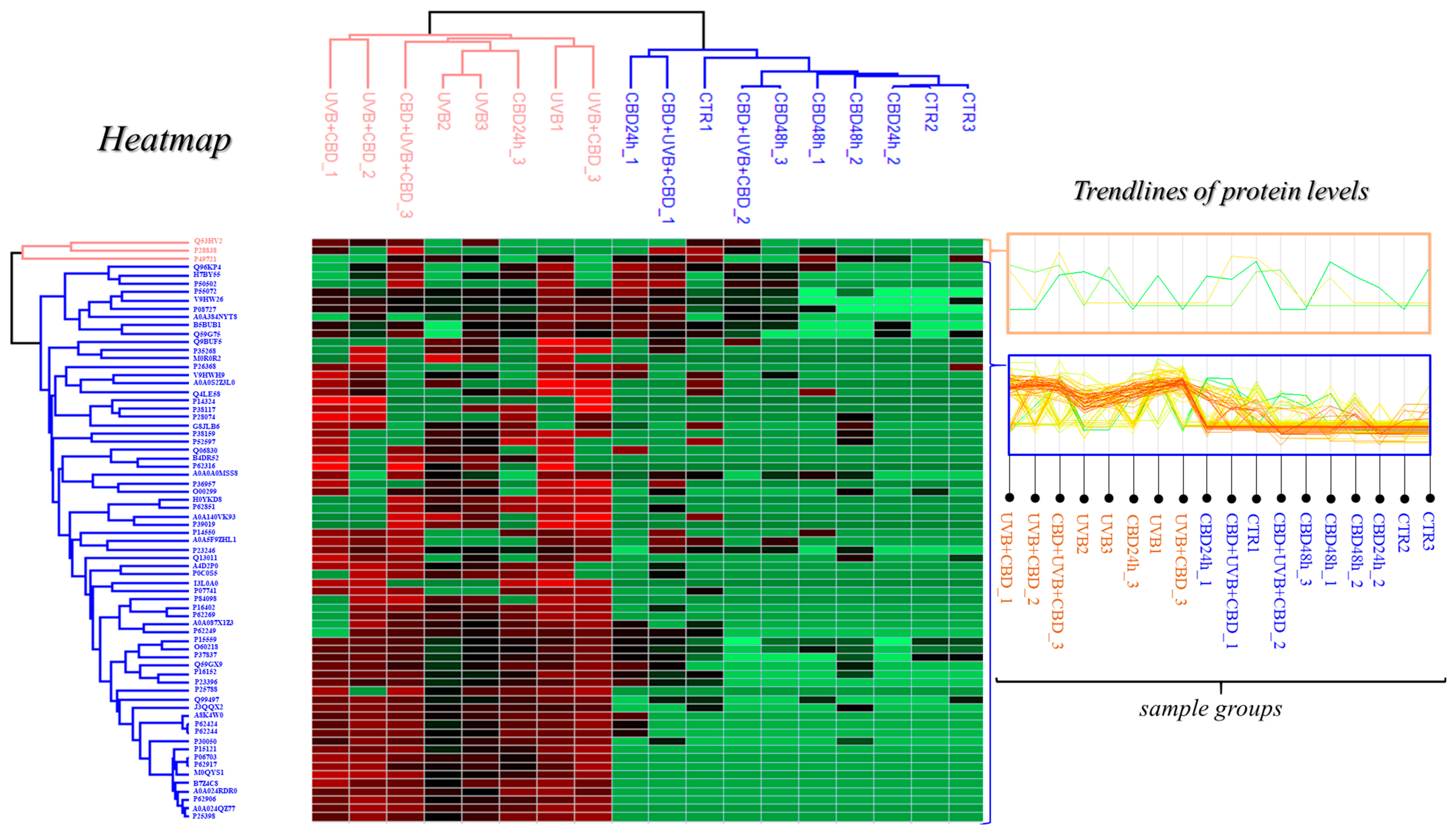
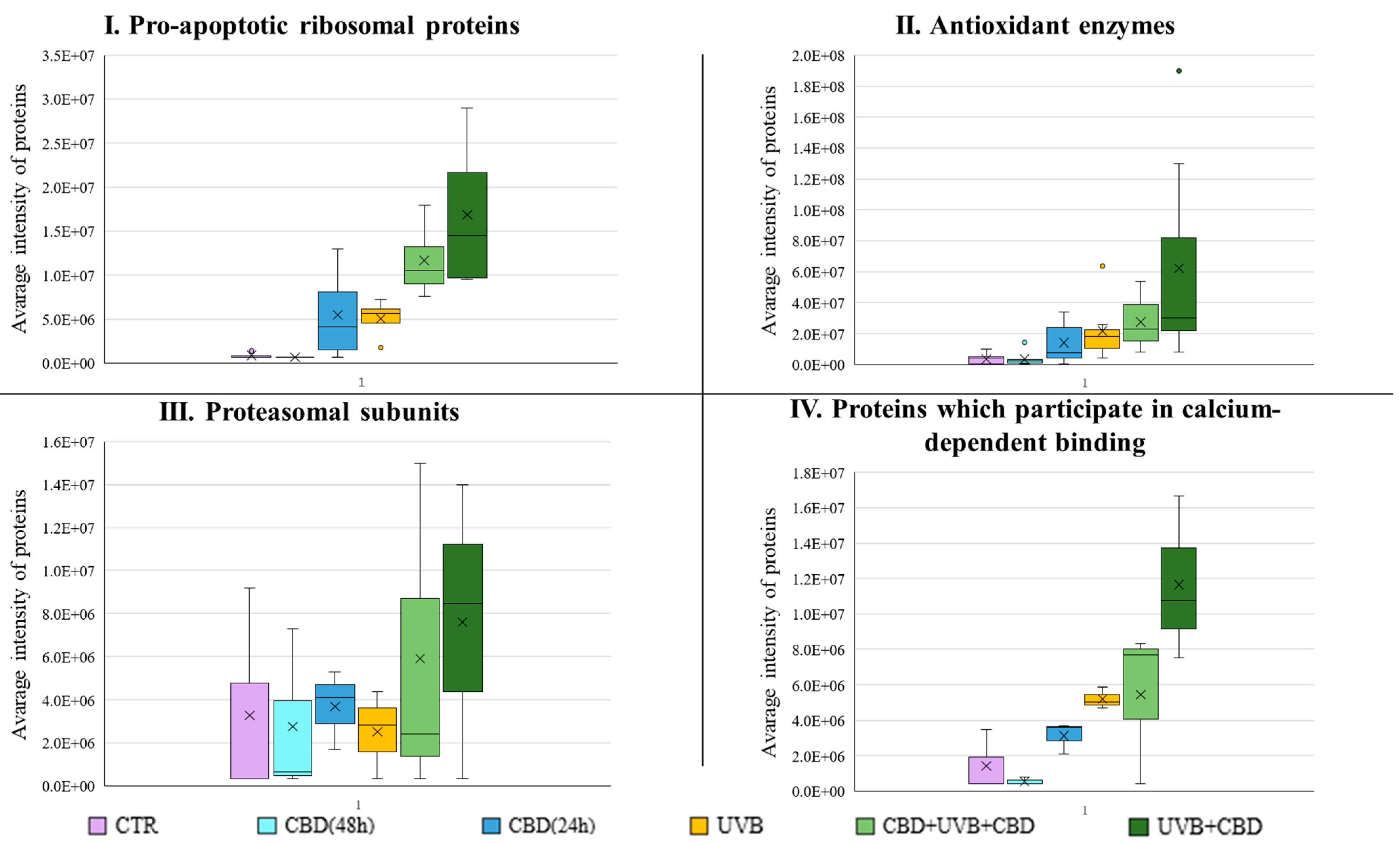
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Matsumura, Y.; Ananthaswamy, H.N. Toxic effects of ultraviolet radiation on the skin. Toxicol. Appl. Pharmacol. 2004, 195, 298–308. [Google Scholar] [CrossRef] [PubMed]
- Orazio, D.J.; Jarrett, S.; Ortiz, A.A.; Scott, T. UV Radiation and the Skin. Int. J. Mol. Sci. 2013, 14, 12222–12248. [Google Scholar] [CrossRef] [PubMed]
- Schuch, A.P.; Moreno, N.C.; Schuch, N.J.; Menck, C.F.M.; Garcia, C.C.M. Sunlight damage to cellular DNA: Focus on oxidatively generated lesions. Free Radic. Biol. Med. 2017, 107, 110–124. [Google Scholar] [CrossRef]
- Zhu, X.; Li, N.; Wang, Y.; Ding, L.; Chen, H.; Yu, Y.; Shi, X. Protective effects of quercetin on UVB irradiation-induced cytotoxicity through ROS clearance in keratinocyte cells. Oncol. Rep. 2016, 37, 209–218. [Google Scholar] [CrossRef] [PubMed]
- Ortiz, A.A.; Yan, B.; Orazio, O.J.A. Ultraviolet Radiation, Aging and the Skin: Prevention of Damage by Topical cAMP Manipulation. Molecules 2014, 19, 6202–6219. [Google Scholar] [CrossRef] [PubMed]
- Rastogi, R.P.; Kumar, A.; Tyagi, M.B.; Sinha, R.P. Molecular mechanisms of ultraviolet radiation-induced DNA damage and repair. J. Nucleic Acids 2010, 2010, 2980. [Google Scholar] [CrossRef]
- Qin, J.Z.; Chaturvedi, V.; Denning, M.F.; Bacon, P.; Panella, J.; Choubey, D.; Nickoloff, B.J. Regulation of apoptosis by p53 in UV-irradiated human epidermis, psoriatic plaques and senescent keratinocytes. Oncogene 2002, 21, 2991–3002. [Google Scholar] [CrossRef]
- Coelho, S.G.; Choi, W.; Brenner, M.; Miyamura, Y.; Yamaguchi, Y.; Wolber, R.; Smuda, C.; Batzer, J.; Kolbe, L.; Ito, S.; et al. Short- and Long-Term Effects of UV Radiation on the Pigmentation of Human Skin. J. Investig. Dermatol. Symp. Proc. 2009, 14, 32–35. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, M.M.; Ratti, B.A.; Daré, R.G.; Silva, S.O.; Truiti, M.D.C.T.; Nakamura, U.T.; Velty, A.R.; Nakamura, C.V. Dihydrocaffeic Acid Prevents UVB-Induced Oxidative Stress Leading to the Inhibition of Apoptosis and MMP-1 Expression via p38 Signaling Pathway. Oxidative Med. Cell. Longev. 2019, 2019, 2419096. [Google Scholar] [CrossRef]
- Kremslehner, C.; Miller, A.; Nica, R.; Nagelreiter, I.M.; Narzt, M.S.; Golabi, B.; Vorstandlechner, V.; Mildner, M.; Lachner, J.; Tschachler, E.; et al. Imaging of metabolic activity adaptations to UV stress, drugs and differentiation at cellular resolution in skin and skin equivalents–Implications for oxidative UV damage. Redox Biol. 2020, 37, 1583. [Google Scholar] [CrossRef]
- Martínez, V.; De Hond, A.I.; Borrelli, F.; Capasso, R.; Del Castillo, M.D.; Abalo, R. Cannabidiol and Other Non-Psychoactive Cannabinoids for Prevention and Treatment of Gastrointestinal Disorders: Useful Nutraceuticals? Int. J. Mol. Sci. 2020, 21, 3067. [Google Scholar] [CrossRef]
- Atalay, S.; Karpowicz, J.I.; Skrzydlewska, E. Antioxidative and Anti-Inflammatory Properties of Cannabidiol. Antioxidants 2019, 9, 21. [Google Scholar] [CrossRef]
- Di Giacomo, V.; Recinella, L.; Chiavaroli, A.; Orlando, G.; Cataldi, A.; Rapino, M.; Di Valerio, V.; Politi, M.; Antolini, M.D.; Acquaviva, A.; et al. Metabolomic profile and antioxidant/anti-inflammatory effects of industrial hemp water extract in fi-broblasts, keratinocytes and isolated mouse skin specimens. Antioxidants 2021, 10, 44. [Google Scholar] [CrossRef]
- Jastrząb, A.; Gęgotek, A.; Skrzydlewska, E. Cannabidiol Regulates the Expression of Keratinocyte Proteins Involved in the Inflammation Process through Transcriptional Regulation. Cells 2019, 8, 827. [Google Scholar] [CrossRef] [PubMed]
- Atalay, S.; Dobrzyńska, I.; Gęgotek, A.; Skrzydlewska, E. Cannabidiol protects keratinocyte cell membranes following exposure to UVB and hydrogen peroxide. Redox Biol. 2020, 36, 1613. [Google Scholar] [CrossRef] [PubMed]
- Bradford, M. A Rapid and Sensitive Method for the Quantitation of Microgram Quantities of Protein Utilizing the Principle of Protein-Dye Binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Gęgotek, A.; Domingues, P.; Wroński, A.; Wójcik, P.; Skrzydlewska, E. Proteomic plasma profile of psoriatic patients. J. Pharm. Biomed. Anal. 2018, 155, 185–193. [Google Scholar] [CrossRef] [PubMed]
- Tyanova, S.; Temu, T.; Sinitcyn, P.; Carlson, A.; Hein, M.Y.; Geiger, T.; Mann, M.; Cox, J. The Perseus computational platform for comprehensive analysis of (prote)omics data. Nat. Methods 2016, 13, 731–740. [Google Scholar] [CrossRef] [PubMed]
- Chong, J.; Soufan, O.; Li, C.; Caraus, I.; Li, S.; Bourque, G.; Wishart, D.S.; Xia, J. MetaboAnalyst 4.0: Towards more trans-parent and integrative metabolomics analysis. Nucleic Acids Res. 2018, 46, W486–W494. [Google Scholar] [CrossRef]
- Thomas, P.D.; Campbell, M.J.; Kejariwal, A.; Mi, H.; Karlak, B.; Daverman, R.; Diemer, K.; Muruganujan, A.; Narechania, A. PANTHER: A Library of Protein Families and Subfamilies Indexed by Function. Genome Res. 2003, 13, 2129–2141. [Google Scholar] [CrossRef]
- Szklarczyk, D.; Gable, A.L.; Lyon, D.; Junge, A.; Wyder, S.; Cepas, H.J.; Simonovic, M.; Doncheva, N.T.; Morris, J.H.; Bork, P.; et al. STRING v11: Protein–protein association networks with increased coverage, supporting functional discovery in genome-wide experimental datasets. Nucleic Acids Res. 2019, 47, D607–D613. [Google Scholar] [CrossRef]
- Parrado, C.; Saenz, M.S.; Davo, P.A.; Gilaberte, Y.; Gonzalez, S.; Juarranz, Á. Environmental Stressors on Skin Aging. Mechanistic Insights. Front. Pharmacol. 2019, 10, 759. [Google Scholar] [CrossRef] [PubMed]
- Umar, S.A.; Tasduq, S.A. Integrating DNA damage response and autophagy signalling axis in ultraviolet-B induced skin photo-damage: A positive association in protecting cells against genotoxic stress. RSC Adv. 2020, 10, 36317–36336. [Google Scholar] [CrossRef]
- Watson, H. Biological membranes. Essays Biochem. 2015, 59, 43–69. [Google Scholar] [CrossRef] [PubMed]
- Atalay, S.; Gęgotek, A.; Wroński, A.; Domigues, P.; Skrzydlewska, E. Therapeutic application of cannabidiol on UVA and UVB irradiated rat skin. A proteomic study. J. Pharm. Biomed. Anal. 2021, 192, 3656. [Google Scholar] [CrossRef]
- Khalil, C.; Shebaby, W. UVB damage onset and progression 24 h post exposure in human-derived skin cells. Toxicol. Rep. 2017, 4, 441–449. [Google Scholar] [CrossRef] [PubMed]
- Karpowicz, J.I.; Biernacki, M.; Wroński, A.; Gęgotek, A.; Skrzydlewska, E. Cannabidiol Effects on Phospholipid Metabolism in Keratinocytes from Patients with Psoriasis Vulgaris. Biomolecules 2020, 10, 367. [Google Scholar] [CrossRef]
- Łuczaj, W.; Domingues, M.D.R.; Domingues, P.; Skrzydlewska, E. Changes in Lipid Profile of Keratinocytes from Rat Skin Exposed to Chronic UVA or UVB Radiation and Topical Application of Cannabidiol. Antioxidants 2020, 9, 1178. [Google Scholar] [CrossRef]
- Dobrzyńska, I.; Petelska, S.B.; Wroński, A.; Karpowicz, J.I.; Skrzydlewska, E. Changes in the physico-chemical properties of blood and skin cell membranes as a result of psoriasis vulgaris and psoriatic arthritis development. Int. J. Mol. Sci. 2020, 21, 9129. [Google Scholar] [CrossRef]
- Gęgotek, A.; Ambrożewicz, E.; Jastrząb, A.; Karpowicz, J.I.; Skrzydlewska, E. Rutin and ascorbic acid cooperation in antioxidant and antiapoptotic effect on human skin keratinocytes and fibroblasts exposed to UVA and UVB radiation. Arch. Dermatol. Res. 2019, 311, 203–219. [Google Scholar] [CrossRef]
- Yuki, T.; Hachiya, A.; Kusaka, A.; Sriwiriyanont, P.; Visscher, M.O.; Morita, K.; Muto, M.; Miyachi, Y.; Sugiyama, Y.; Inoue, S. Characterization of Tight Junctions and Their Disruption by UVB in Human Epidermis and Cultured Keratinocytes. J. Investig. Dermatol. 2011, 131, 744–752. [Google Scholar] [CrossRef]
- Wang, P.W.; Hung, Y.C.; Lin, T.Y.; Fang, J.Y.; Yang, P.M.; Chen, M.H.; Pan, T.L. Comparison of the Biological Impact of UVA and UVB upon the Skin with Functional Proteomics and Immunohistochemistry. Antioxidants 2019, 8, 569. [Google Scholar] [CrossRef]
- Gęgotek, A.; Atalay, S.; Domingues, P.; Skrzydlewska, E. The Differences in the Proteome Profile of Cannabidiol-Treated Skin Fibroblasts following UVA or UVB Irradiation in 2D and 3D Cell Cultures. Cells 2019, 8, 995. [Google Scholar] [CrossRef]
- Jugé, R.; Breugnot, J.; Da Silva, C.; Bordes, S.; Closs, B.; Aouacheria, A. Quantification and Characterization of UVB-Induced Mitochondrial Fragmentation in Normal Primary Human Keratinocytes. Sci. Rep. 2016, 6, 35065. [Google Scholar] [CrossRef]
- Gęgotek, A.; Karpowicz, J.I.; Skrzydlewska, E. Cytoprotective Effect of Ascorbic Acid and Rutin against Oxidative Changes in the Proteome of Skin Fibroblasts Cultured in a Three-Dimensional System. Nutrients 2020, 12, 1074. [Google Scholar] [CrossRef] [PubMed]
- Perluigi, M.; Di Domenico, F.; Blarzino, C.; Foppoli, C.; Cini, C.; Giorgi, A.; Grillo, C.; De Marco, F.; Butterfield, D.A.; Schininà, M.E.; et al. Effects of UVB-induced oxidative stress on protein expression and specific protein oxidation in normal human epithelial keratinocytes: A proteomic approach. Proteome Sci. 2010, 8, 13. [Google Scholar] [CrossRef]
- Pichler, H.; Augustin, E.A. Modification of membrane lipid compositions in single-celled organisms—From basics to applications. Methods 2018, 147, 50–65. [Google Scholar] [CrossRef]
- Casares, D.; Escribá, P.V.; Rosselló, C.A. Membrane lipid composition: Effect on membrane and organelle structure, function and compartmentalization and therapeutic avenues. Int. J. Mol. Sci. 2019, 20, 2167. [Google Scholar] [CrossRef] [PubMed]
- Penning, T.M. Aldo-Keto Reductase Regulation by the Nrf2 System: Implications for Stress Response, Chemotherapy Drug Resistance, and Carcinogenesis. Chem. Res. Toxicol. 2017, 30, 162–176. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Li, W.; Su, Z.Y.; Kong, A.N.T. The complexity of the Nrf2 pathway: Beyond the antioxidant response. J. Nutr. Biochem. 2015, 26, 1401–1413. [Google Scholar] [CrossRef] [PubMed]
- Ross, D.; Siegel, D. Functions of NQO1 in Cellular Protection and CoQ10 Metabolism and its Potential Role as a Redox Sensitive Molecular Switch. Front. Physiol. 2017, 8, 595. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.; Jin, B.; Wu, C.; Yang, J.; Zhan, X.; Wang, L.; Shen, X.; Chen, J.; Chen, H.; Mao, Z. NQO1 Stabilizes p53 in Response to Oncogene-Induced Senescence. Int. J. Biol. Sci. 2015, 11, 762–771. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Han, K.; Yuan, D.H.; Meng, C.Y. Overexpression of NAD(P)H: Quinone Oxidoreductase 1 Inhibits Hepatocel-lular Carcinoma Cell Proliferation and Induced Apoptosis by Activating AMPK/PGC-1α Pathway. DNA Cell Biol. 2017, 36, 256–263. [Google Scholar] [CrossRef] [PubMed]
- Shrivastava, A.; Kuzontkoski, P.M.; Groopman, J.E.; Prasad, A. Cannabidiol Induces Programmed Cell Death in Breast Cancer Cells by Coordinating the Cross-talk between Apoptosis and Autophagy. Mol. Cancer Ther. 2011, 10, 1161–1172. [Google Scholar] [CrossRef]
- Lukhele, S.T.; Motadi, L.R. Cannabidiol rather than Cannabis sativa extracts inhibit cell growth and induce apoptosis in cervical cancer cells. BMC Complement. Altern. Med. 2016, 16, 335. [Google Scholar] [CrossRef]
- Csala, M.; Kardon, T.; Legeza, B.; Lizák, B.; Mandl, J.; Margittai, É.; Puskás, F.; Száraz, P.; Szelényi, P.; Bánhegyi, G. On the role of 4-hydroxynonenal in health and disease. Biochim. Biophys. Acta (BBA) Mol. Basis Dis. 2015, 1852, 826–838. [Google Scholar] [CrossRef]
- Feng, H.; Li, Z.; Du, J.; Sun, J.; Feng, W.; Li, D.; Liu, S.; Wang, W.; Liu, H.; Amizuka, N.; et al. Dual function of peroxiredoxin I in lipopolysaccharide-induced osteoblast apoptosis via reactive oxygen species and the apoptosis signal-regulating kinase 1 signaling pathway. Cell Death Discov. 2018, 4, 1–11. [Google Scholar] [CrossRef]
- Kim, Y.N.; Jung, H.Y.; Eum, W.S.; Kim, D.W.; Shin, M.J.; Ahn, E.H.; Kim, S.J.; Lee, C.H.; Yong, J.I.; Ryu, E.J.; et al. Neuro-protective effects of PEP-1-carbonyl reductase 1 against oxidative-stress-induced ischemic neuronal cell damage. Free Radic. Biol. Med. 2014, 69, 181–196. [Google Scholar] [CrossRef]
- Han, B.; Shin, H.J.; Bak, I.S.; Bak, Y.; Jeong, Y.L.; Kwon, T.; Park, Y.H.; Sun, H.N.; Kim, C.H.; Yu, D.Y. Peroxiredoxin I is important for cancer-cell survival in Ras-induced hepatic tumorigenesis. Oncotarget 2016, 7, 68044–68056. [Google Scholar] [CrossRef]
- Nassour, H.; Wang, Z.; Saad, A.; Papaluca, A.; Brosseau, N.; Affar, E.B.; Jamali, A.M.A.; Ramotar, D. Peroxiredoxin 1 interacts with and blocks the redox factor APE1 from activating interleukin-8 expression. Sci. Rep. 2016, 6, 9389. [Google Scholar] [CrossRef]
- Tebay, L.E.; Robertson, H.; Durant, S.T.; Vitale, S.R.; Penning, T.M.; Kostova, D.A.T.; Hayes, J.D. Mechanisms of activation of the transcription factor Nrf2 by redox stressors, nutrient cues, and energy status and the pathways through which it attenuates degenerative disease. Free Radic. Biol. Med. 2015, 88, 108–146. [Google Scholar] [CrossRef]
- Wardyn, J.D.; Ponsford, A.H.; Sanderson, C.M. Dissecting molecular cross-talk between Nrf2 and NF-κB response pathways. Biochem. Soc. Trans. 2015, 43, 621–626. [Google Scholar] [CrossRef]
- Maier, C.S.; Chavez, J.; Wang, J.; Wu, J. Protein Adducts of Aldehydic Lipid Peroxidation Products: Identification and Characterization of Protein Adducts Using an Aldehyde/Keto-Reactive Probe in Combination with Mass Spectrometry. In Methods in Enzymology; Academic Press Inc.: Cambridge, MA, USA, 2010; Volume 473, pp. 305–330. [Google Scholar]
- Hyvärinen, S.; Uchida, K.; Varjosalo, M.; Jokela, R.; Jokiranta, T.S. Recognition of Malondialdehyde-modified Proteins by the C Terminus of Complement Factor H Is Mediated via the Polyanion Binding Site and Impaired by Mutations Found in Atypical Hemolytic Uremic Syndrome. J. Biol. Chem. 2014, 289, 4295–4306. [Google Scholar] [CrossRef]
- Dalleau, S.; Baradat, M.; Guéraud, F.; Huc, L. Cell death and diseases related to oxidative stress:4-hydroxynonenal (HNE) in the balance. Cell Death Differ. 2013, 20, 1615–1630. [Google Scholar] [CrossRef] [PubMed]
- Castro, J.P.; Jung, T.; Grune, T.; Siems, W. 4-Hydroxynonenal (HNE) modified proteins in metabolic diseases. Free Radic. Biol. Med. 2017, 111, 309–315. [Google Scholar] [CrossRef] [PubMed]
- Dash, R.; Ali, C.; Jahan, I.; Munni, Y.A.; Mitra, S.; Hannan, A.; Timalsina, B.; Oktaviani, D.F.; Choi, H.J.; Moon, I.S. Emerging potential of cannabidiol in reversing proteinopathies. Ageing Res. Rev. 2021, 65, 1209. [Google Scholar] [CrossRef]
- Yu, Y.; Shen, S.M.; Zhang, F.F.; Wu, Z.X.; Han, B.; Wang, L.S. Acidic leucine-rich nuclear phosphoprotein 32 family member B (ANP32B) contributes to retinoic acid-induced differentiation of leukemic cells. Biochem. Biophys. Res. Commun. 2012, 423, 721–725. [Google Scholar] [CrossRef]
- Zhou, X.; Liao, W.J.; Liao, J.M.; Liao, P.; Lu, H. Ribosomal proteins: Functions beyond the ribosome. J. Mol. Cell Biol. 2015, 7, 92–104. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.W.; Ioannou, Y.A. Ribosomal Proteins in Cell Proliferation and Apoptosis. Int. Rev. Immunol. 1999, 18, 429–448. [Google Scholar] [CrossRef]
- Xu, X.; Xiong, X.; Sun, Y. The role of ribosomal proteins in the regulation of cell proliferation, tumorigenesis, and genomic integrity. Sci. China Life Sci. 2016, 59, 656–672. [Google Scholar] [CrossRef]
- Kale, J.; Osterlund, E.J.; Andrews, D.W. BCL-2 family proteins: Changing partners in the dance towards death. Cell Death Differ. 2018, 25, 65–80. [Google Scholar] [CrossRef] [PubMed]
- Wan, F.; Anderson, D.E.; Barnitz, R.A.; Snow, A.; Bidere, N.; Zheng, L.; Hegde, V.; Lam, L.T.; Staudt, L.M.; Levens, D.; et al. Ribosomal Protein S3: A KH Domain Subunit in NF-κB Complexes that Mediates Selective Gene Regulation. Cell 2007, 131, 927–939. [Google Scholar] [CrossRef]
- Stanborough, T.; Niederhauser, J.; Koch, B.; Bergler, H.; Pertschy, B. Ribosomal protein S3 interacts with the NF-κB inhibitor IκBα. FEBS Lett. 2014, 588, 659–664. [Google Scholar] [CrossRef] [PubMed]
- Varghese, E.; Samuel, S.M.; Sadiq, Z.; Kubatka, P.; Liskova, A.; Benacka, J.; Pazinka, P.; Kruzliak, P.; Büsselberg, D. Anti-cancer agents in proliferation and cell death: The calcium connection. Int. J. Mol. Sci. 2019, 20, 3017. [Google Scholar] [CrossRef] [PubMed]
- Rizzuto, R.; Pinton, P.; Ferrari, D.; Chami, M.; Gy, O.; Szabadkai, R.; Magalhães, P.J. Calcium and apoptosis: Facts and hypotheses. Oncogene 2003, 22, 8619–8627. [Google Scholar] [CrossRef]
- Gronski, M.A.; Kinchen, J.M.; Juncadella, I.J.; Franc, N.C.; Ravichandran, K.S. An essential role for calcium flux in phag-ocytes for apoptotic cell engulfment and the anti-inflammatory response. Cell Death Differ. 2009, 16, 1323–1331. [Google Scholar] [CrossRef] [PubMed]
- Donato, R.; Sorci, G.; Giambanco, I. S100A6 protein: Functional roles. Cell. Mol. Life Sci. 2017, 74, 2749–2760. [Google Scholar] [CrossRef]
- Allgöwer, C.; Kretz, A.L.; Von Karstedt, S.; Wittau, M.; Bruns, H.D.; Lemke, J. Friend or Foe: S100 Proteins in Cancer. Cancers 2020, 12, 2037. [Google Scholar] [CrossRef] [PubMed]
- Joung, H.J.; Sun, Y.Y.; Joo, H.K.; Paik, S.G.; Sung, R.M.; Lim, J.S.; In, S.C.; Choi, I.; Jae, W.K. S100A6 (calcyclin) enhances the sensitivity to apoptosis via the upregulation of caspase-3 activity in Hep3B cells. J. Cell. Biochem. 2008, 103, 1183–1197. [Google Scholar]
- Słomnicki, Ł.P.; Nawrot, B.; Leśniak, W. S100A6 binds p53 and affects its activity. Int. J. Biochem. Cell Biol. 2009, 41, 784–790. [Google Scholar] [CrossRef]
- Leclerc, E.; Fritz, G.; Weibel, M.; Heizmann, C.W.; Galichet, A. S100B and S100A6 Differentially Modulate Cell Survival by Interacting with Distinct RAGE (Receptor for Advanced Glycation End Products) Immunoglobulin Domains. J. Biol. Chem. 2007, 282, 31317–31331. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Atalay, S.; Gęgotek, A.; Skrzydlewska, E. Protective Effects of Cannabidiol on the Membrane Proteome of UVB-Irradiated Keratinocytes. Antioxidants 2021, 10, 402. https://doi.org/10.3390/antiox10030402
Atalay S, Gęgotek A, Skrzydlewska E. Protective Effects of Cannabidiol on the Membrane Proteome of UVB-Irradiated Keratinocytes. Antioxidants. 2021; 10(3):402. https://doi.org/10.3390/antiox10030402
Chicago/Turabian StyleAtalay, Sinemyiz, Agnieszka Gęgotek, and Elżbieta Skrzydlewska. 2021. "Protective Effects of Cannabidiol on the Membrane Proteome of UVB-Irradiated Keratinocytes" Antioxidants 10, no. 3: 402. https://doi.org/10.3390/antiox10030402
APA StyleAtalay, S., Gęgotek, A., & Skrzydlewska, E. (2021). Protective Effects of Cannabidiol on the Membrane Proteome of UVB-Irradiated Keratinocytes. Antioxidants, 10(3), 402. https://doi.org/10.3390/antiox10030402







