The Phosphofructokinase Isoform AtPFK5 Is a Novel Target of Plastidic Thioredoxin-f-Dependent Redox Regulation
Abstract
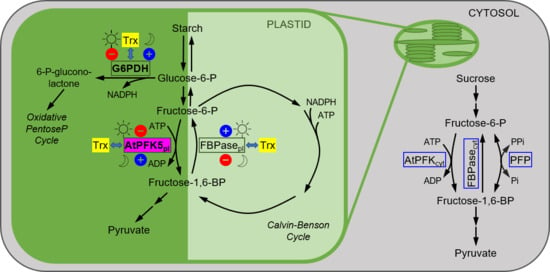
1. Introduction
2. Materials and Methods
2.1. Plant Material, Growth Conditions and Treatment
2.2. Creation of CRISPR/Cas9 Knockout Lines for AtPFK5
2.3. Cultivation of Trypanosoma Brucei Brucei
2.4. Agrobacterium tumefaciens-Mediated Transient Gene Expression in Nicotiana benthamiana Plants
2.5. Cloning and Site-Directed Mutagenesis of PFKs for Transient Overexpression in Nicotiana benthamiana
2.6. Preparation of Chloroplast Extracts and Treatment
2.7. Enzyme Analysis
2.8. Sequence Alignments and Statistics
3. Results
3.1. Arabidopsis Contains Six Active PFK Isoforms
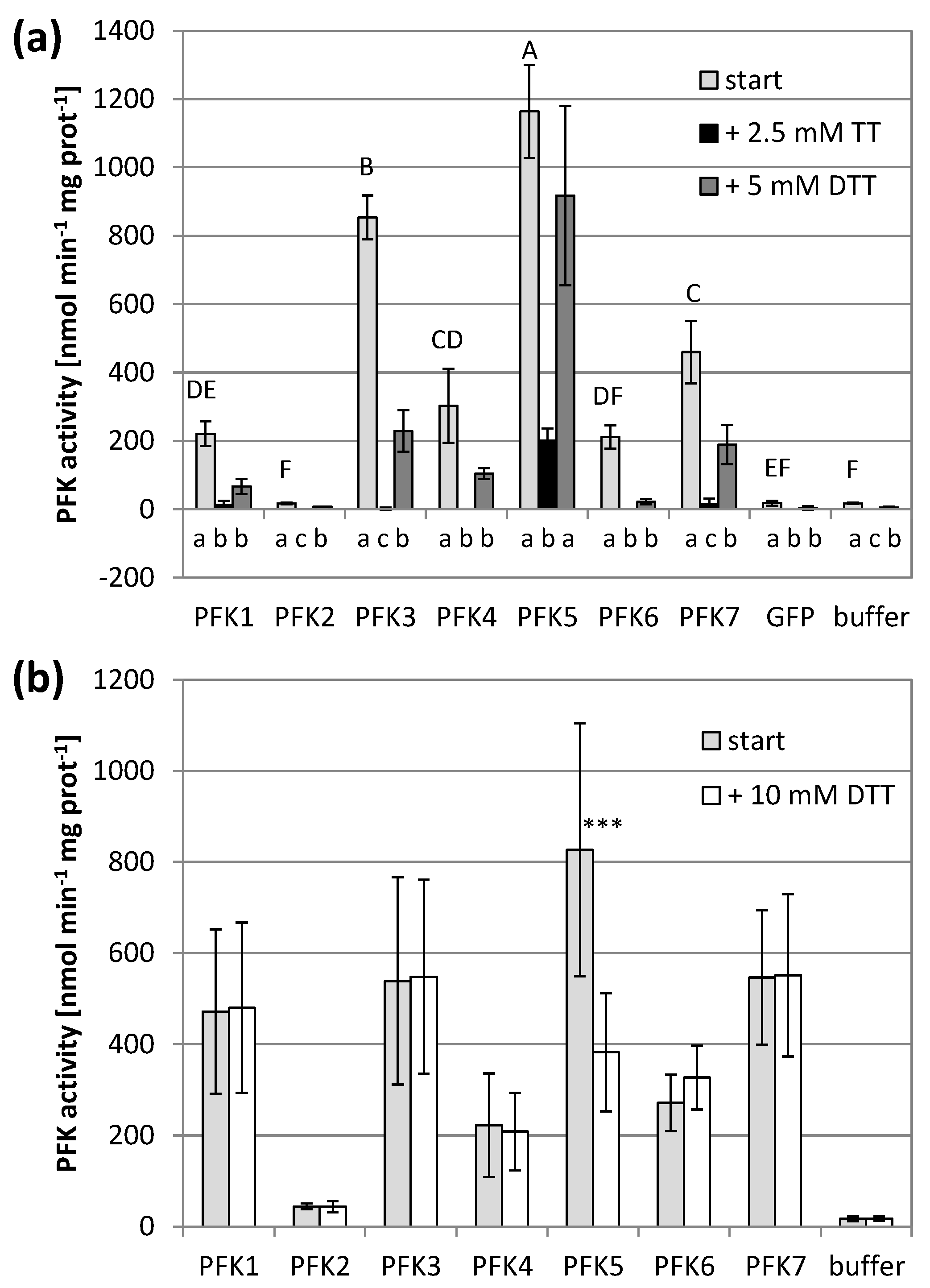
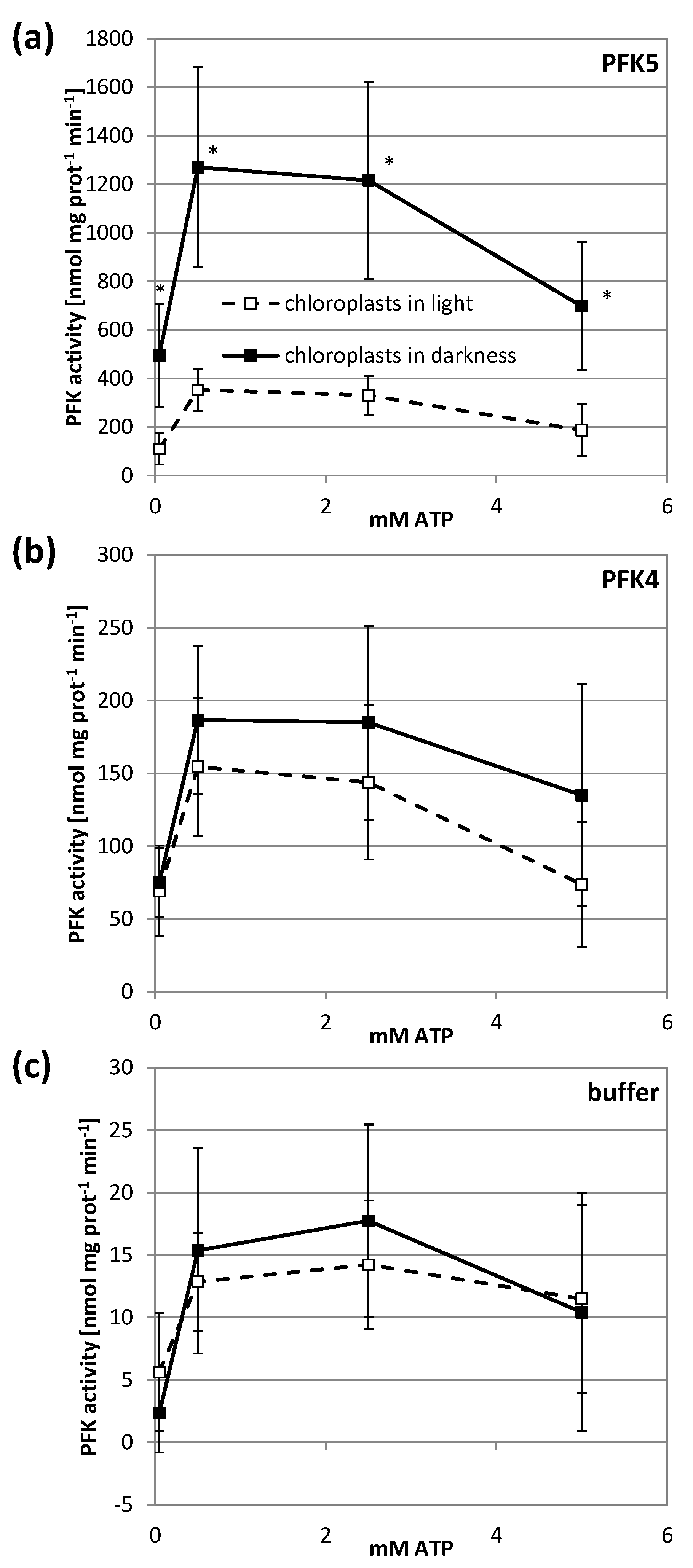
3.2. Arabidopsis PFKs Are Sensitive to Redox Modifications
3.3. PFK Isoforms Contain Multiple Conserved Cys
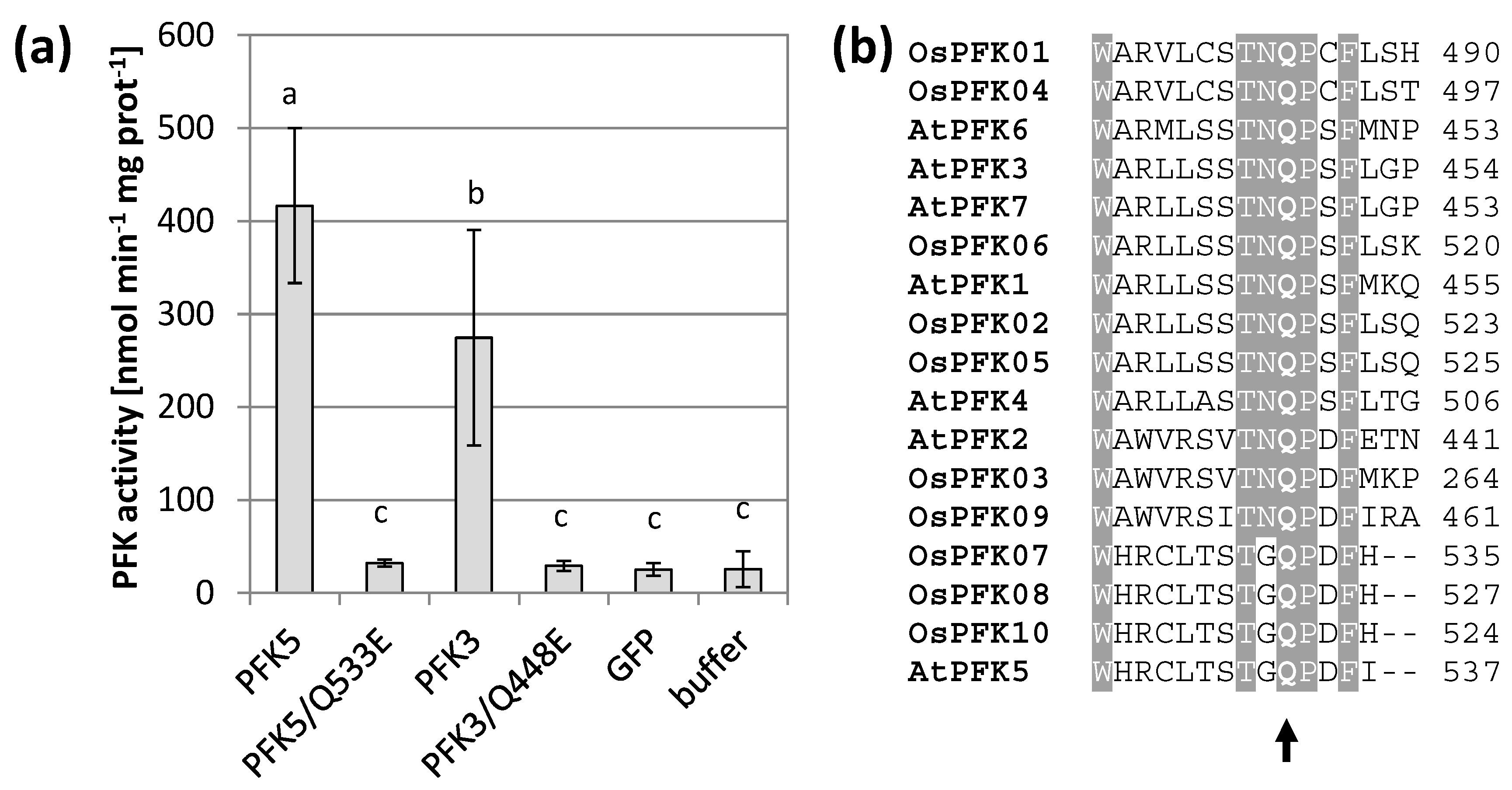
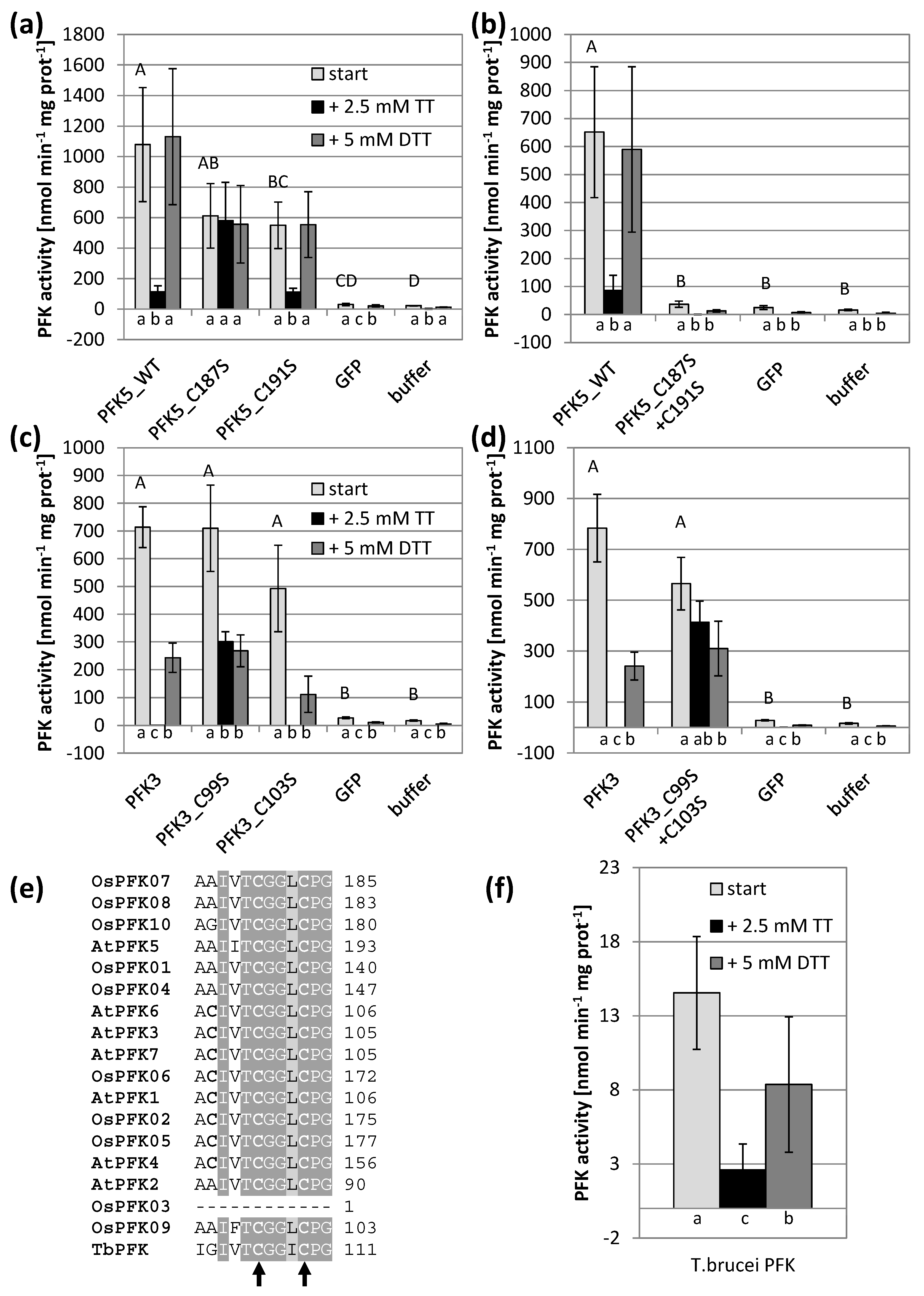
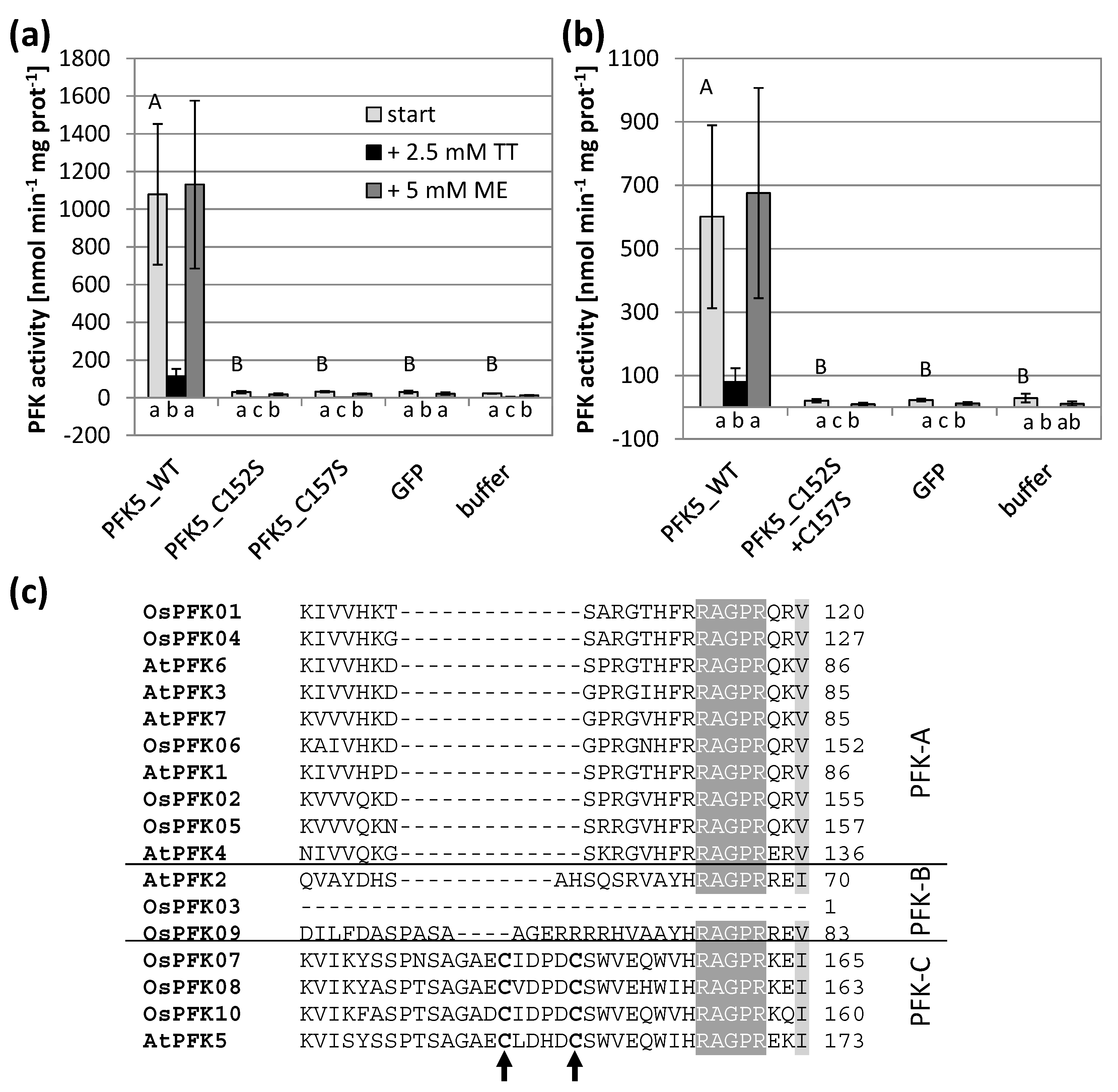
3.4. Mutations of Conserved Cys Modify Enzyme Activity
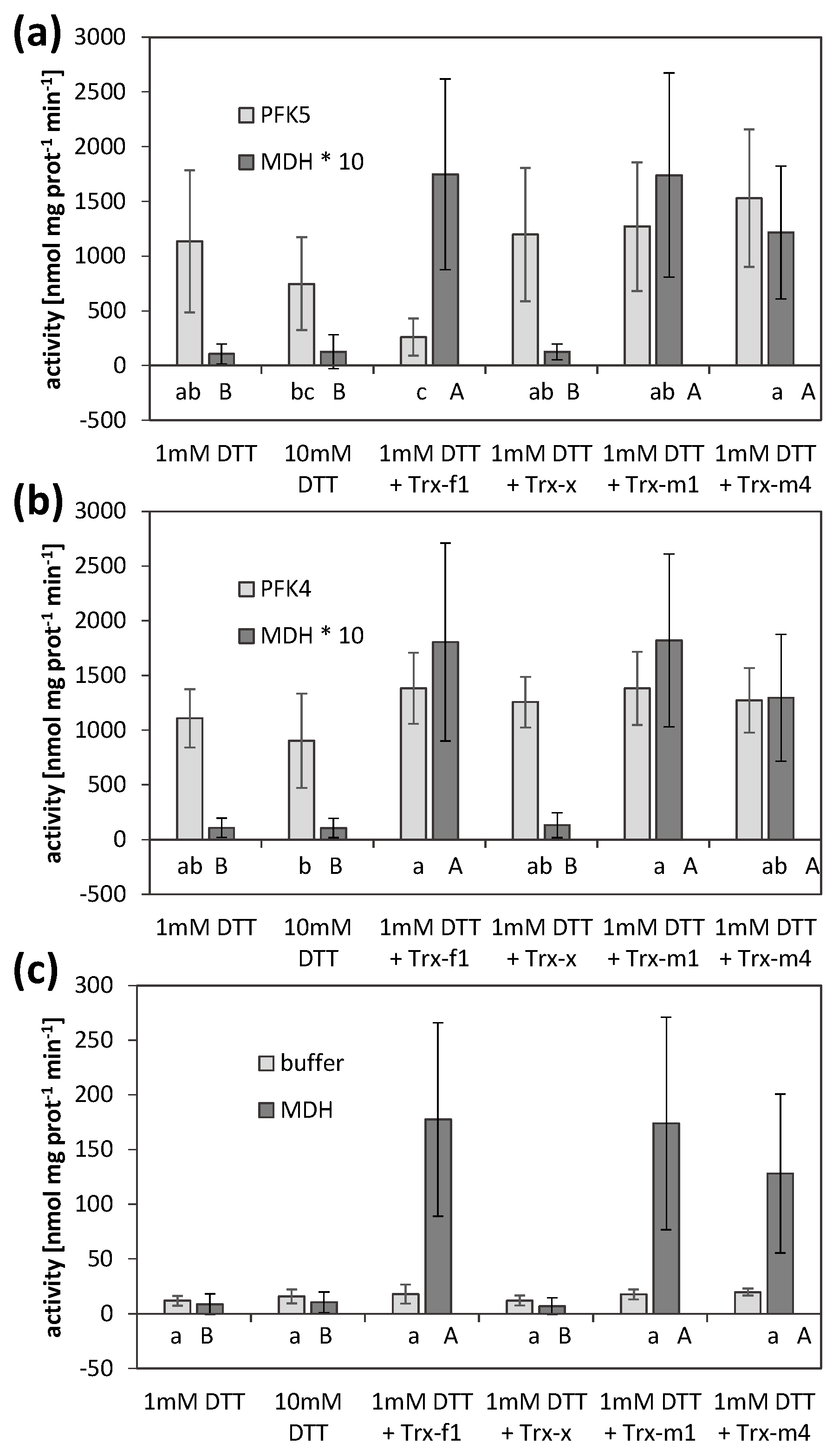
3.5. AtPFK5 Is a Target of Thioredoxin-f1
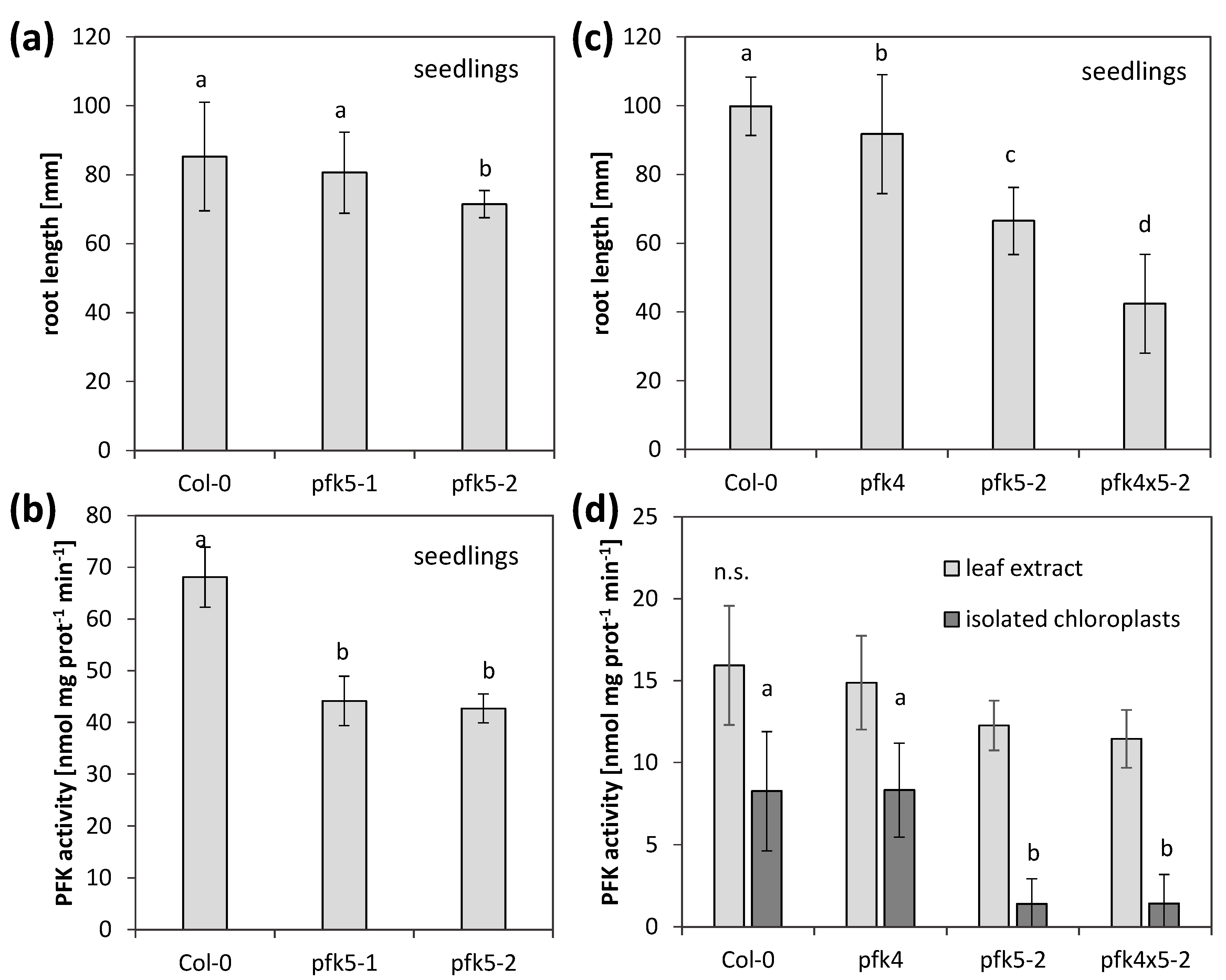
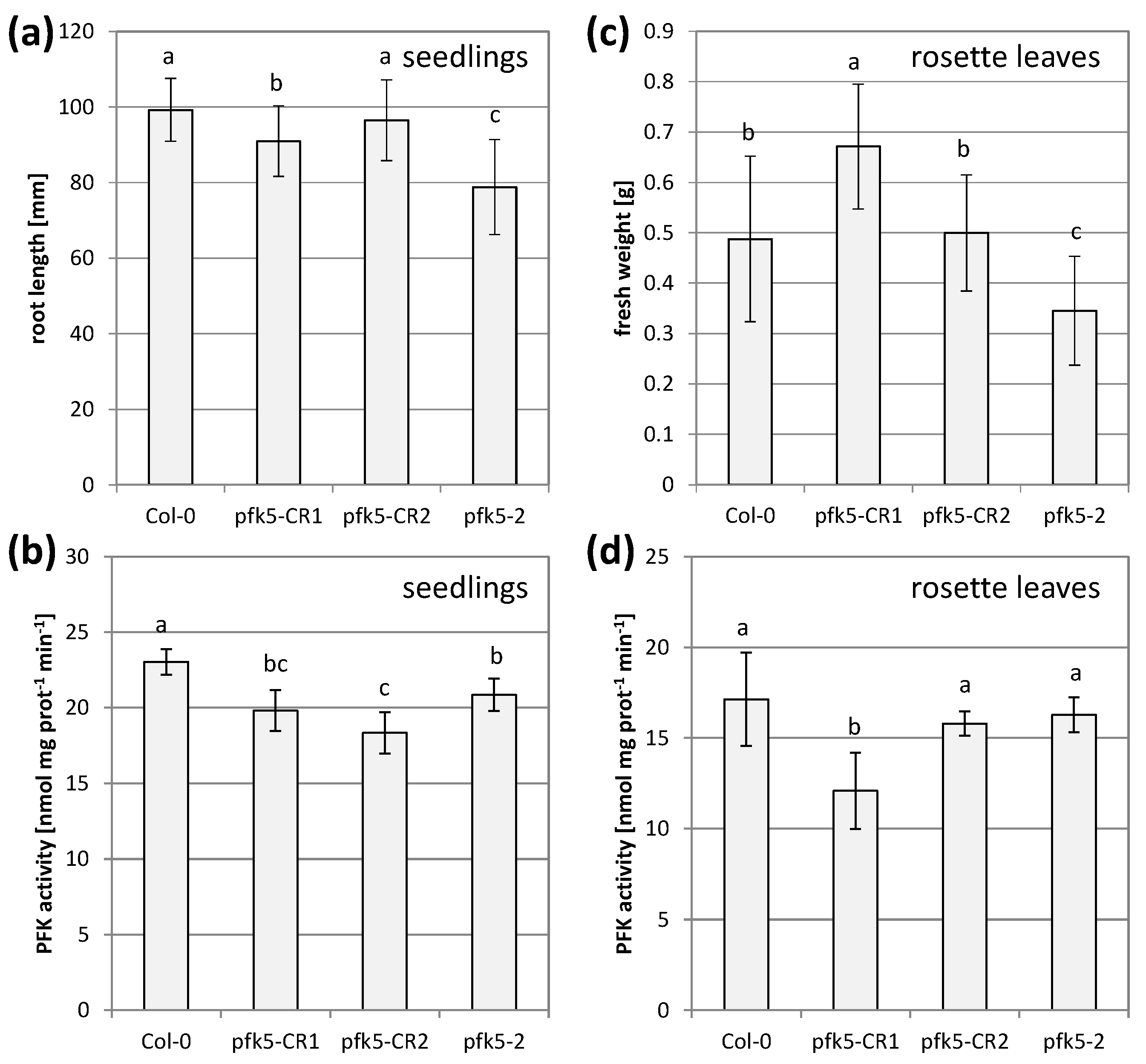
3.6. A Knockout of AtPFK5 Has Little Effects in Arabidopsis Plants
4. Discussion
4.1. Redox Sensitivity of Plant and Trypanosoma PFKs
4.2. Plastidic PFKs Are Redox Regulated through the CXDXXC Motif
4.3. What Could Be the Function of Redox-Regulated AtPFK5?
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gütle, D.D.; Roret, T.; Hecker, A.; Reski, R.; Jacquot, J.-P. Dithiol Disulphide Exchange in Redox Regulation of Chloroplast Enzymes in Response to Evolutionary and Structural Constraints. Plant Sci. 2017, 255, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Geigenberger, P.; Thormählen, I.; Daloso, D.M.; Fernie, A.R. The Unprecedented Versatility of the Plant Thioredoxin System. Trends Plant Sci. 2017, 22, 249–262. [Google Scholar] [CrossRef]
- Vogelsang, L.; Dietz, K.-J. Regulatory Thiol Oxidation in Chloroplast Metabolism, Oxidative Stress Response and Environmental Signaling in Plants. Biochem. J. 2020, 477, 1865–1878. [Google Scholar] [CrossRef] [PubMed]
- Chibani, K.; Couturier, J.; Selles, B.; Jacquot, J.-P.; Rouhier, N. The Chloroplastic Thiol Reducing Systems: Dual Functions in the Regulation of Carbohydrate Metabolism and Regeneration of Antioxidant Enzymes, Emphasis on the Poplar Redoxin Equipment. Photosynth. Res. 2010, 104, 75–99. [Google Scholar] [CrossRef]
- Meyer, Y.; Buchanan, B.B.; Vignols, F.; Reichheld, J.-P. Thioredoxins and Glutaredoxins: Unifying Elements in Redox Biology. Annu. Rev. Genet. 2009, 43, 335–367. [Google Scholar] [CrossRef]
- Meyer, Y.; Belin, C.; Delorme-Hinoux, V.; Reichheld, J.-P.; Riondet, C. Thioredoxin and Glutaredoxin Systems in Plants: Molecular Mechanisms, Crosstalks, and Functional Significance. Antioxid. Redox Signal. 2012, 17, 1124–1160. [Google Scholar] [CrossRef]
- Balmer, Y.; Vensel, W.H.; Cai, N.; Manieri, W.; Schurmann, P.; Hurkman, W.J.; Buchanan, B.B. A Complete Ferredoxin/Thioredoxin System Regulates Fundamental Processes in Amyloplasts. Proc. Natl. Acad. Sci. USA 2006, 103, 2988–2993. [Google Scholar] [CrossRef] [PubMed]
- Balmer, Y.; Koller, A.; del Val, G.; Manieri, W.; Schürmann, P.; Buchanan, B.B. Proteomics Gives Insight into the Regulatory Function of Chloroplast Thioredoxins. Proc. Natl. Acad. Sci. USA 2003, 100, 370–375. [Google Scholar] [CrossRef] [PubMed]
- Lendzian, K.J. Modulation of Glucose-6-Phosphate Dehydrogenase by NADPH, NADP (+) and Dithiothreitol at Variable NADPH/NADP (+) Ratios in an Illuminated Reconstituted Spinach (Spinacia oleracea L.) Chloroplast System. Planta 1980, 148, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Scheibe, R.; Anderson, L.E. Dark Modulation of NADP-Dependent Malate Dehydrogenase and Glucose-6-Phosphate Dehydrogenase in the Chloroplast. Biochim. Biophys. Acta Bioenerg. 1981, 636, 58–64. [Google Scholar] [CrossRef]
- Scheibe, R.; Geissler, A.; Fickenscher, K. Chloroplast Glucose-6-Phosphate Dehydrogenase: Km Shift upon Light Modulation and Reduction. Arch. Biochem. Biophys. 1989, 274, 290–297. [Google Scholar] [CrossRef]
- Selinski, J.; Scheibe, R. Malate Valves: Old Shuttles with New Perspectives. Plant Biol. J. 2019, 21, 21–30. [Google Scholar] [CrossRef]
- Lemaire, S.D.; Michelet, L.; Zaffagnini, M.; Massot, V.; Issakidis-Bourguet, E. Thioredoxins in Chloroplasts. Curr. Genet. 2007, 51, 343–365. [Google Scholar] [CrossRef]
- Tiessen, A.; Hendriks, J.H.M.; Stitt, M.; Branscheid, A.; Gibon, Y.; Farré, E.M.; Geigenberger, P. Starch Synthesis in Potato Tubers Is Regulated by Post-Translational Redox Modification of ADP-Glucose Pyrophosphorylase: A Novel Regulatory Mechanism Linking Starch Synthesis to the Sucrose Supply. Plant Cell 2002, 14, 2191–2213. [Google Scholar] [CrossRef] [PubMed]
- Hendriks, J.H.M.; Kolbe, A.; Gibon, Y.; Stitt, M.; Geigenberger, P. ADP-Glucose Pyrophosphorylase Is Activated by Posttranslational Redox-Modification in Response to Light and to Sugars in Leaves of Arabidopsis and Other Plant Species. Plant Physiol. 2003, 133, 838–849. [Google Scholar] [CrossRef] [PubMed]
- Michalska, J.; Zauber, H.; Buchanan, B.B.; Cejudo, F.J.; Geigenberger, P. NTRC Links Built-in Thioredoxin to Light and Sucrose in Regulating Starch Synthesis in Chloroplasts and Amyloplasts. Proc. Natl. Acad. Sci. USA 2009, 106, 9908–9913. [Google Scholar] [CrossRef] [PubMed]
- Ojeda, V.; Pérez-Ruiz, J.M.; Cejudo, F.J. 2-Cys Peroxiredoxins Participate in the Oxidation of Chloroplast Enzymes in the Dark. Mol. Plant 2018, 11, 1377–1388. [Google Scholar] [CrossRef] [PubMed]
- Vaseghi, M.-J.; Chibani, K.; Telman, W.; Liebthal, M.F.; Gerken, M.; Schnitzer, H.; Mueller, S.M.; Dietz, K.-J. The Chloroplast 2-Cysteine Peroxiredoxin Functions as Thioredoxin Oxidase in Redox Regulation of Chloroplast Metabolism. Elife 2018, 7. [Google Scholar] [CrossRef]
- Yoshida, K.; Hara, A.; Sugiura, K.; Fukaya, Y.; Hisabori, T. Thioredoxin-Like2/2-Cys Peroxiredoxin Redox Cascade Supports Oxidative Thiol Modulation in Chloroplasts. Proc. Natl. Acad. Sci. USA 2018, 115, E8296–E8304. [Google Scholar] [CrossRef]
- Balmer, Y.; Koller, A.; del Val, G.; Schürmann, P.; Buchanan, B.B. Proteomics Uncovers Proteins Interacting Electrostatically with Thioredoxin in Chloroplasts. Photosynth. Res. 2004, 79, 275–280. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.; Lee, J.; Kim, Y.; Bae, D.; Kang, K.Y.; Yoon, S.C.; Lim, D. Defining the Plant Disulfide Proteome. ELECTROPHORESIS 2004, 25, 532–541. [Google Scholar] [CrossRef]
- Marchand, C.; Maréchal, P.L.; Meyer, Y.; Miginiac-Maslow, M.; Issakidis-Bourguet, E.; Decottignies, P. New Targets of Arabidopsis Thioredoxins Revealed by Proteomic Analysis. PROTEOMICS 2004, 4, 2696–2706. [Google Scholar] [CrossRef] [PubMed]
- Michelet, L.; Zaffagnini, M.; Morisse, S.; Sparla, F.; Pérez-Pérez, M.E.; Francia, F.; Danon, A.; Marchand, C.H.; Fermani, S.; Trost, P.; et al. Redox Regulation of the Calvin-Benson Cycle: Something Old, Something New. Front. Plant Sci. 2013, 4, 470. [Google Scholar] [CrossRef] [PubMed]
- Van der Linde, K.; Gutsche, N.; Leffers, H.-M.; Lindermayr, C.; Müller, B.; Holtgrefe, S.; Scheibe, R. Regulation of Plant Cytosolic Aldolase Functions by Redox-Modifications. Plant Physiol. Biochem. 2011, 49, 946–957. [Google Scholar] [CrossRef]
- Kachru, R.B.; Anderson, L.E. Inactivation of Pea Leaf Phosphofructokinase by Light and Dithiothreitol. Plant Physiol. 1975, 55, 199–202. [Google Scholar] [CrossRef][Green Version]
- Cséke, C.; Nishizawa, A.N.; Buchanan, B.B. Modulation of Chloroplast Phosphofructokinase by NADPH. Plant Physiol. 1982, 70, 658–661. [Google Scholar] [CrossRef]
- Heuer, B.; Hansen, M.J.; Anderson, L.E. Light Modulation of Phosphofructokinase in Pea Leaf Chloroplasts. Plant Physiol. 1982, 69, 1404–1406. [Google Scholar] [CrossRef] [PubMed]
- Mustroph, A.; Sonnewald, U.; Biemelt, S. Characterisation of the ATP-Dependent Phosphofructokinase Gene Family from Arabidopsis thaliana. FEBS Lett. 2007, 581, 2401–2410. [Google Scholar] [CrossRef]
- Winkler, C.; Delvos, B.; Martin, W.; Henze, K. Purification, Microsequencing and Cloning of Spinach ATP-Dependent Phosphofructokinase Link Sequence and Function for the Plant Enzyme. FEBS J. 2007, 274, 429–438. [Google Scholar] [CrossRef]
- Mustroph, A.; Stock, J.; Hess, N.; Aldous, S.; Dreilich, A.; Grimm, B. Characterization of the Phosphofructokinase Gene Family in Rice and Its Expression under Oxygen Deficiency Stress. Front. Plant Sci. 2013, 4, 125. [Google Scholar] [CrossRef]
- Plaxton, W.C. The Organization and Regulation of Plant Glycolysis. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1996, 47, 185–214. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, K.; Hisabori, T. Biochemical Basis for Redox Regulation of Chloroplast-Localized Phosphofructokinase from Arabidopsis thaliana. Plant Cell Physiol. 2021. [Google Scholar] [CrossRef] [PubMed]
- Alonso, J.M.; Stepanova, A.N.; Leisse, T.J.; Kim, C.J.; Chen, H.; Shinn, P.; Stevenson, D.K.; Zimmerman, J.; Barajas, P.; Cheuk, R.; et al. Genome-Wide Insertional Mutagenesis of Arabidopsis thaliana. Science 2003, 301, 653–657. [Google Scholar] [CrossRef]
- Klecker, M.; Gasch, P.; Peisker, H.; Dörmann, P.; Schlicke, H.; Grimm, B.; Mustroph, A. A Shoot-Specific Hypoxic Response of Arabidopsis Sheds Light on the Role of the Phosphate-Responsive Transcription Factor PHOSPHATE STARVATION RESPONSE1. Plant Physiol. 2014, 165, 774–790. [Google Scholar] [CrossRef]
- Kühnlenz, T.; Schmidt, H.; Uraguchi, S.; Clemens, S. Arabidopsis thaliana Phytochelatin Synthase 2 Is Constitutively Active in Vivo and Can Rescue the Growth Defect of the PCS1-Deficient Cad1-3 Mutant on Cd-Contaminated Soil. J. Exp. Bot. 2014, 65, 4241–4253. [Google Scholar] [CrossRef] [PubMed]
- Xing, H.-L.; Dong, L.; Wang, Z.-P.; Zhang, H.-Y.; Han, C.-Y.; Liu, B.; Wang, X.-C.; Chen, Q.-J. A CRISPR/Cas9 Toolkit for Multiplex Genome Editing in Plants. BMC Plant Biol. 2014, 14, 327. [Google Scholar] [CrossRef]
- Bendahmane, A.; Querci, M.; Kanyuka, K.; Baulcombe, D.C. Agrobacterium Transient Expression System as a Tool for the Isolation of Disease Resistance Genes: Application to the Rx2 Locus in Potato: Isolation of Rx2 by Transient Expression. Plant J. 2000, 21, 73–81. [Google Scholar] [CrossRef]
- Zheng, L. An Efficient One-Step Site-Directed and Site-Saturation Mutagenesis Protocol. Nucleic Acids Res. 2004, 32, e115. [Google Scholar] [CrossRef] [PubMed]
- Scheibe, R.; Fickenscher, K.; Ashton, A.R. Studies on the Mechanism of the Reductive Activation of NADP-Malate Dehydrogenase by Thioredoxin m and Low-Molecular-Weight Thiols. Biochim. Biophys. Acta Protein Struct. Mol. Enzymol. 1986, 870, 191–197. [Google Scholar] [CrossRef]
- Bradford, M.M. A Rapid and Sensitive Method for the Quantitation of Microgram Quantities of Protein Utilizing the Principle of Protein-Dye Binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Goodstein, D.M.; Shu, S.; Howson, R.; Neupane, R.; Hayes, R.D.; Fazo, J.; Mitros, T.; Dirks, W.; Hellsten, U.; Putnam, N.; et al. Phytozome: A Comparative Platform for Green Plant Genomics. Nucleic Acids Res. 2012, 40, D1178–D1186. [Google Scholar] [CrossRef]
- Larkin, M.A.; Blackshields, G.; Brown, N.P.; Chenna, R.; McGettigan, P.A.; McWilliam, H.; Valentin, F.; Wallace, I.M.; Wilm, A.; Lopez, R.; et al. Clustal W and Clustal X Version 2.0. Bioinformatics 2007, 23, 2947–2948. [Google Scholar] [CrossRef] [PubMed]
- Kelley, L.A.; Sternberg, M.J.E. Protein Structure Prediction on the Web: A Case Study Using the Phyre Server. Nat. Protoc. 2009, 4, 363–371. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Oyanedel, J.; McNae, I.W.; Nowicki, M.W.; Keillor, J.W.; Michels, P.A.M.; Fothergill-Gilmore, L.A.; Walkinshaw, M.D. The First Crystal Structure of Phosphofructokinase from a Eukaryote: Trypanosoma Brucei. J. Mol. Biol. 2007, 366, 1185–1198. [Google Scholar] [CrossRef] [PubMed]
- Issakidis, E.; Miginiac-Maslow, M.; Decottignies, P.; Jacquot, J.P.; Crétin, C.; Gadal, P. Site-Directed Mutagenesis Reveals the Involvement of an Additional Thioredoxin-Dependent Regulatory Site in the Activation of Recombinant Sorghum Leaf NADP-Malate Dehydrogenase. J. Biol. Chem. 1992, 267, 21577–21583. [Google Scholar] [CrossRef]
- McNae, I.W.; Martinez-Oyanedel, J.; Keillor, J.W.; Michels, P.A.M.; Fothergill-Gilmore, L.A.; Walkinshaw, M.D. The Crystal Structure of ATP-Bound Phosphofructokinase from Trypanosoma brucei Reveals Conformational Transitions Different from Those of Other Phosphofructokinases. J. Mol. Biol. 2009, 385, 1519–1533. [Google Scholar] [CrossRef]
- Meinke, D.W. Genome-Wide Identification of EMBRYO-DEFECTIVE (EMB) Genes Required for Growth and Development in Arabidopsis. New Phytol. 2020, 226, 306–325. [Google Scholar] [CrossRef] [PubMed]
- Walters, D.W.; Gilbert, H.F. Thiol/Disulfide Exchange between Rabbit Muscle Phosphofructokinase and Glutathione. Kinetics and Thermodynamics of Enzyme Oxidation. J. Biol. Chem. 1986, 261, 15372–15377. [Google Scholar] [CrossRef]
- Brooks, D.J.; Fresco, J.R. Increased Frequency of Cysteine, Tyrosine, and Phenylalanine Residues Since the Last Universal Ancestor. Mol. Cell. Proteom. 2002, 1, 125–131. [Google Scholar] [CrossRef]
- Wong, J.W.H.; Ho, S.Y.W.; Hogg, P.J. Disulfide Bond Acquisition through Eukaryotic Protein Evolution. Mol. Biol. Evol. 2011, 28, 327–334. [Google Scholar] [CrossRef]
- Balsera, M.; Uberegui, E.; Susanti, D.; Schmitz, R.A.; Mukhopadhyay, B.; Schürmann, P.; Buchanan, B.B. Ferredoxin:Thioredoxin Reductase (FTR) Links the Regulation of Oxygenic Photosynthesis to Deeply Rooted Bacteria. Planta 2013, 237, 619–635. [Google Scholar] [CrossRef]
- Nishihira, J.; Ishibashi, T.; Sakai, M.; Nishi, S.; Kumazaki, T.; Hatanaka, Y.; Tsuda, S.; Hikichi, K. Characterization of Cysteine Residues of Glutathione S-Transferase P: Evidence for Steric Hindrance of Substrate Binding by a Bulky Adduct to Cysteine 47. Biochem. Biophys. Res. Commun. 1992, 188, 424–432. [Google Scholar] [CrossRef]
- Liu, M.; Huang, Y.; Wu, J.; Wang, E.; Wang, Y. Effect of Cysteine Residues on the Activity of Arginyl-TRNA Synthetase from Escherichia coli. Biochemistry 1999, 38, 11006–11011. [Google Scholar] [CrossRef]
- Tremblay, J.M.; Li, H.; Yarbrough, L.R.; Helmkamp, G.M. Modifications of Cysteine Residues in the Solution and Membrane-Associated Conformations of Phosphatidylinositol Transfer Protein Have Differential Effects on Lipid Transfer Activity. Biochemistry 2001, 40, 9151–9158. [Google Scholar] [CrossRef]
- Lemaire, M.; Issakidis, E.; Ruelland, E.; Decottignies, P.; Miginiac-Maslow, M. An Active-Site Cysteine of Sorghum Leaf NADP-Malate Dehydrogenase Studied by Site-Directed Mutagenesis. FEBS Lett. 1996, 382, 137–140. [Google Scholar] [CrossRef]
- Parker, D.J.; Allison, W.S. The Mechanism of Inactivation of Glyceraldehyde 3-Phosphate Dehydrogenase by Tetrathionate, o-Iodosobenzoate, and Iodine Monochloride. J. Biol. Chem. 1969, 244, 180–189. [Google Scholar] [CrossRef]
- Shirakihara, Y.; Evans, P.R. Crystal Structure of the Complex of Phosphofructokinase from Escherichia coli with Its Reaction Products. J. Mol. Biol. 1988, 204, 973–994. [Google Scholar] [CrossRef]
- Richardson, J.S. The Anatomy and Taxonomy of Protein Structure. In Advances in Protein Chemistry; Elsevier: Amsterdam, The Netherlands, 1981; Volume 34, pp. 167–339. ISBN 978-0-12-034234-1. [Google Scholar]
- Stumpp, M.T.; Motohashi, K.; Hisabori, T. Chloroplast Thioredoxin Mutants without Active-Site Cysteines Facilitate the Reduction of the Regulatory Disulphide Bridge on the Gamma-Subunit of Chloroplast ATP Synthase. Biochem. J. 1999, 341, 157–163. [Google Scholar] [CrossRef]
- Hisabori, T.; Sunamura, E.-I.; Kim, Y.; Konno, H. The Chloroplast ATP Synthase Features the Characteristic Redox Regulation Machinery. Antioxid. Redox Signal. 2013, 19, 1846–1854. [Google Scholar] [CrossRef]
- Wenderoth, I.; Scheibe, R.; von Schaewen, A. Identification of the Cysteine Residues Involved in Redox Modification of Plant Plastidic Glucose-6-Phosphate Dehydrogenase. J. Biol. Chem. 1997, 272, 26985–26990. [Google Scholar] [CrossRef]
- Née, G.; Zaffagnini, M.; Trost, P.; Issakidis-Bourguet, E. Redox Regulation of Chloroplastic Glucose-6-Phosphate Dehydrogenase: A New Role for f-Type Thioredoxin. FEBS Lett. 2009, 583, 2827–2832. [Google Scholar] [CrossRef]
- Née, G.; Aumont-Nicaise, M.; Zaffagnini, M.; Nessler, S.; Valerio-Lepiniec, M.; Issakidis-Bourguet, E. Redox Regulation of Chloroplastic G6PDH Activity by Thioredoxin Occurs through Structural Changes Modifying Substrate Accessibility and Cofactor Binding. Biochem. J. 2014, 457, 117–125. [Google Scholar] [CrossRef] [PubMed]
- König, J.; Galliardt, H.; Jütte, P.; Schäper, S.; Dittmann, L.; Dietz, K.-J. The Conformational Bases for the Two Functionalities of 2-Cysteine Peroxiredoxins as Peroxidase and Chaperone. J. Exp. Bot. 2013, 64, 3483–3497. [Google Scholar] [CrossRef]
- Liebthal, M.; Schuetze, J.; Dreyer, A.; Mock, H.-P.; Dietz, K.-J. Redox Conformation-Specific Protein–Protein Interactions of the 2-Cysteine Peroxiredoxin in Arabidopsis. Antioxidants 2020, 9, 515. [Google Scholar] [CrossRef]
- Winter, D.; Vinegar, B.; Nahal, H.; Ammar, R.; Wilson, G.V.; Provart, N.J. An “Electronic Fluorescent Pictograph” Browser for Exploring and Analyzing Large-Scale Biological Data Sets. PLoS ONE 2007, 2, e718. [Google Scholar] [CrossRef] [PubMed]
- Mustroph, A.; Zanetti, M.E.; Jang, C.J.H.; Holtan, H.E.; Repetti, P.P.; Galbraith, D.W.; Girke, T.; Bailey-Serres, J. Profiling Translatomes of Discrete Cell Populations Resolves Altered Cellular Priorities during Hypoxia in Arabidopsis. Proc. Natl. Acad. Sci. USA 2009, 106, 18843–18848. [Google Scholar] [CrossRef] [PubMed]
- Anderson, L.E.; Duggan, J.X. Light Modulation of Glucose-6-Phosphate Dehydrogenase: Partial Characterization of the Light Inactivation System and Its Effects on the Properties of the Chloroplastic and Cytoplasmic Forms of the Enzyme. Plant Physiol. 1976, 58, 135–139. [Google Scholar] [CrossRef]
- Baier, D.; Latzko, E. Properties and Regulation of C-1-Fructose-1,6-Diphosphatase from Spinach Chloroplasts. Biochim. Biophys. Acta Bioenerg. 1975, 396, 141–147. [Google Scholar] [CrossRef]
- Baret, P.; Cadet, F. Regulation of Photosynthetic Enzymes via Redox Systems. Biochem. Educ. 1997, 25, 24–26. [Google Scholar] [CrossRef]
- Weise, S.E.; Weber, A.P.M.; Sharkey, T.D. Maltose Is the Major Form of Carbon Exported from the Chloroplast at Night. Planta 2004, 218, 474–482. [Google Scholar] [CrossRef]
- Niittylä, T.; Messerli, G.; Trevisan, M.; Chen, J.; Smith, A.M.; Zeeman, S.C. A Previously Unknown Maltose Transporter Essential for Starch Degradation in Leaves. Science 2004, 303, 87–89. [Google Scholar] [CrossRef]
- Yanovsky, M.J.; Kay, S.A. Molecular Basis of Seasonal Time Measurement in Arabidopsis. Nature 2002, 419, 308–312. [Google Scholar] [CrossRef]
- Mockler, T.C.; Michael, T.P.; Priest, H.D.; Shen, R.; Sullivan, C.M.; Givan, S.A.; McEntee, C.; Kay, S.A.; Chory, J. The DIURNAL Project: DIURNAL and Circadian Expression Profiling, Model-Based Pattern Matching, and Promoter Analysis. Cold Spring Harb. Symp. Quant. Biol. 2007, 72, 353–363. [Google Scholar] [CrossRef] [PubMed]
- Endo, M.; Shimizu, H.; Nohales, M.A.; Araki, T.; Kay, S.A. Tissue-Specific Clocks in Arabidopsis Show Asymmetric Coupling. Nature 2014, 515, 419–422. [Google Scholar] [CrossRef] [PubMed]
- Hebbelmann, I.; Selinski, J.; Wehmeyer, C.; Goss, T.; Voss, I.; Mulo, P.; Kangasjärvi, S.; Aro, E.-M.; Oelze, M.-L.; Dietz, K.-J.; et al. Multiple Strategies to Prevent Oxidative Stress in Arabidopsis Plants Lacking the Malate Valve Enzyme NADP-Malate Dehydrogenase. J. Exp. Bot. 2012, 63, 1445–1459. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hess, N.; Richter, S.; Liebthal, M.; Dietz, K.-J.; Mustroph, A. The Phosphofructokinase Isoform AtPFK5 Is a Novel Target of Plastidic Thioredoxin-f-Dependent Redox Regulation. Antioxidants 2021, 10, 401. https://doi.org/10.3390/antiox10030401
Hess N, Richter S, Liebthal M, Dietz K-J, Mustroph A. The Phosphofructokinase Isoform AtPFK5 Is a Novel Target of Plastidic Thioredoxin-f-Dependent Redox Regulation. Antioxidants. 2021; 10(3):401. https://doi.org/10.3390/antiox10030401
Chicago/Turabian StyleHess, Natalia, Simon Richter, Michael Liebthal, Karl-Josef Dietz, and Angelika Mustroph. 2021. "The Phosphofructokinase Isoform AtPFK5 Is a Novel Target of Plastidic Thioredoxin-f-Dependent Redox Regulation" Antioxidants 10, no. 3: 401. https://doi.org/10.3390/antiox10030401
APA StyleHess, N., Richter, S., Liebthal, M., Dietz, K.-J., & Mustroph, A. (2021). The Phosphofructokinase Isoform AtPFK5 Is a Novel Target of Plastidic Thioredoxin-f-Dependent Redox Regulation. Antioxidants, 10(3), 401. https://doi.org/10.3390/antiox10030401