MicroRNA Sequencing Analysis in Obstructive Sleep Apnea and Depression: Anti-Oxidant and MAOA-Inhibiting Effects of miR-15b-5p and miR-92b-3p through Targeting PTGS1-NF-κB-SP1 Signaling
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Polysomnography and CPAP Titration Study
2.3. Blood Collection and RNA Isolation
2.4. Whole Genome microRNA Profiles and Analysis by NGS
2.5. Prediction of microRNA Target and Pathway Enrichment
2.6. Analysis of miRNAs by Quantitative Reverse-Transcriptase Polymerase Chain Reaction (RT-PCR) in an Independent Validation Cohort
2.7. Determination of Target Gene mRNA Expressions of Isolated PBMCs Using Quantitative RT-PCR
2.8. In Vitro IHR Stimuli in Cell Culture Models
2.9. Transfection with miRNA-15b-5p Mimic/miR-92b-3p Mimic
2.10. Reporter Constructs, Mutagenesis and Luciferase Reporter Assay
2.11. Measurement of Cell Apoptosis by Flow Cytometry Analysis
2.12. Measurement of Intracellular Reactive Oxygen Species (ROS)
2.13. Measurement of MAO Catalytic Activity
2.14. Measurement of Cell Viability by WST-1
2.15. Immunofluorescence Stain
2.16. Statistical Analysis
3. Results
3.1. 22 Differentially Expressed miRs in OSA Patients Versus Healthy Non-Snorers in the NGS Discovery Experiment
3.2. Down-Regulated miR-15b-5p/miR-92b-3p in Treatment-Naïve OSA Patients Versus either PS Subjects or OSA Patient on CPAP Treatment in the Validation Cohort
3.3. Up-Regulated PTGS1 in Treatment-Naive OSA Patients and Depression in the Validation Cohort
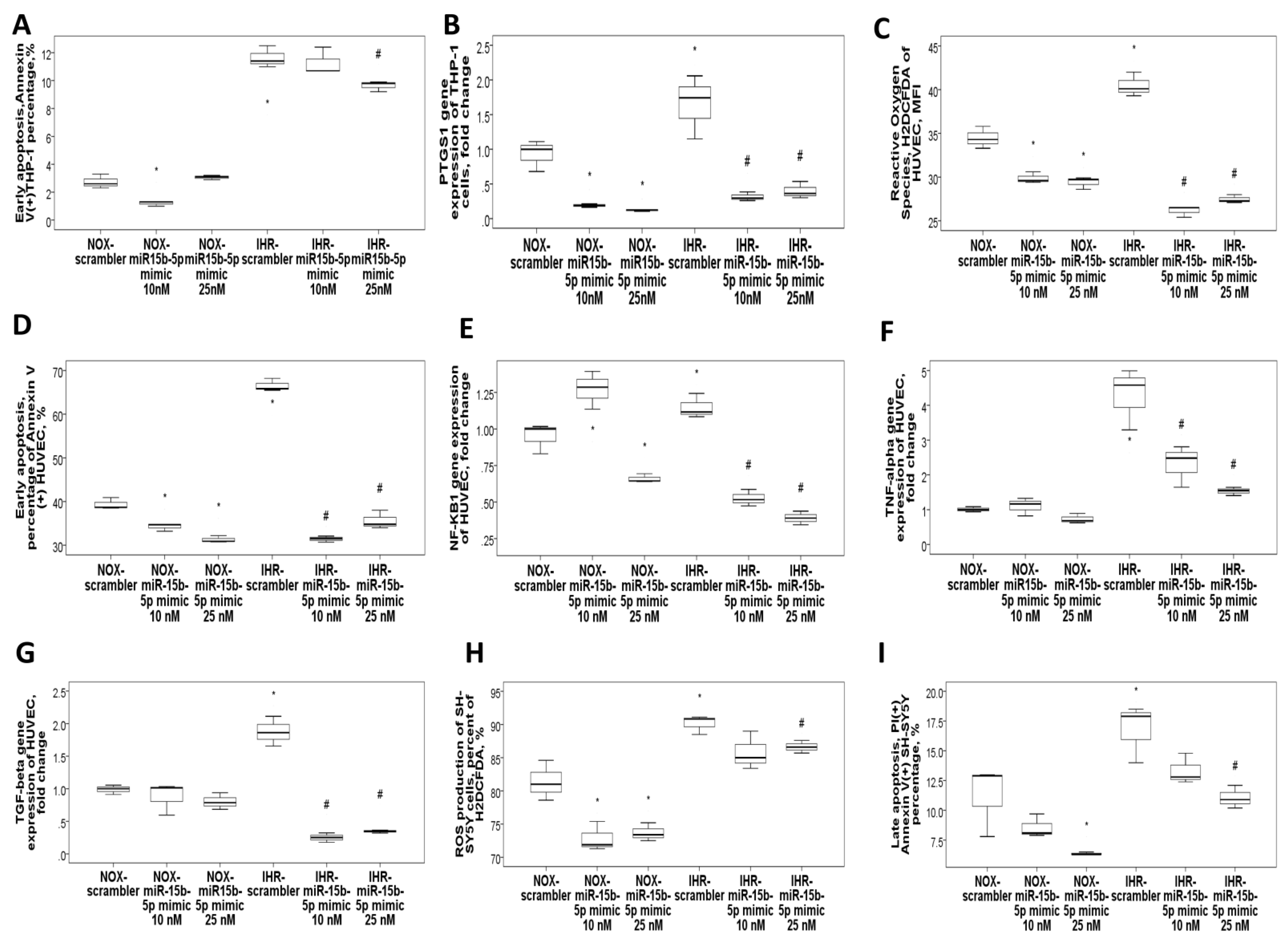
3.4. MiR-15b-5p Over-Expression Reversed IHR-Induced Apoptosis, ROS Production, and Target Gene Up-Regulations
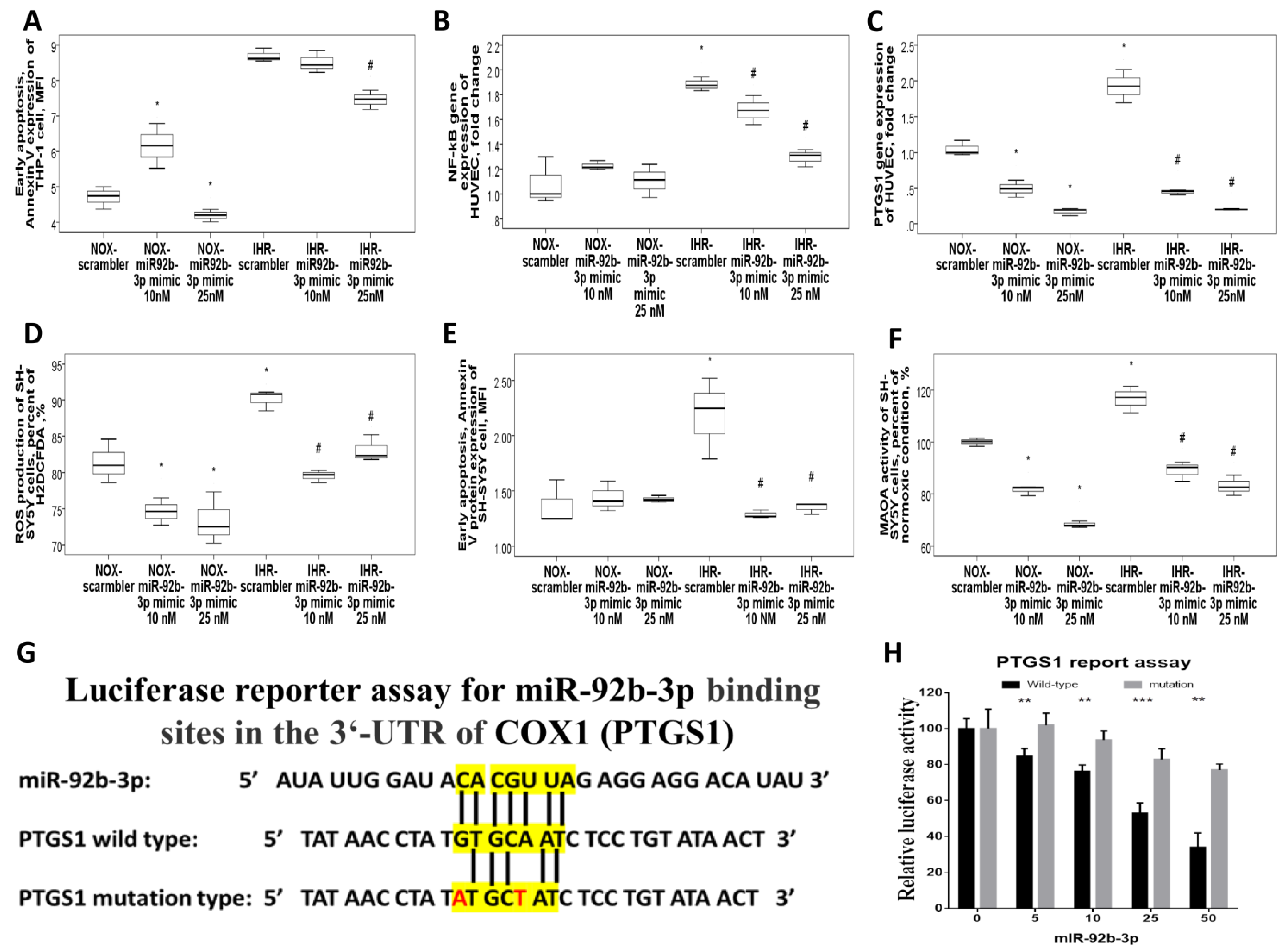
3.5. MiR-92b-3p Over-Expression Reversed IHR-Induced Apoptosis, ROS Production, and Target Gene Up-Regulations
3.6. MiR-92b-3p Negatively Regulated PTGS1 in a Direct Manner
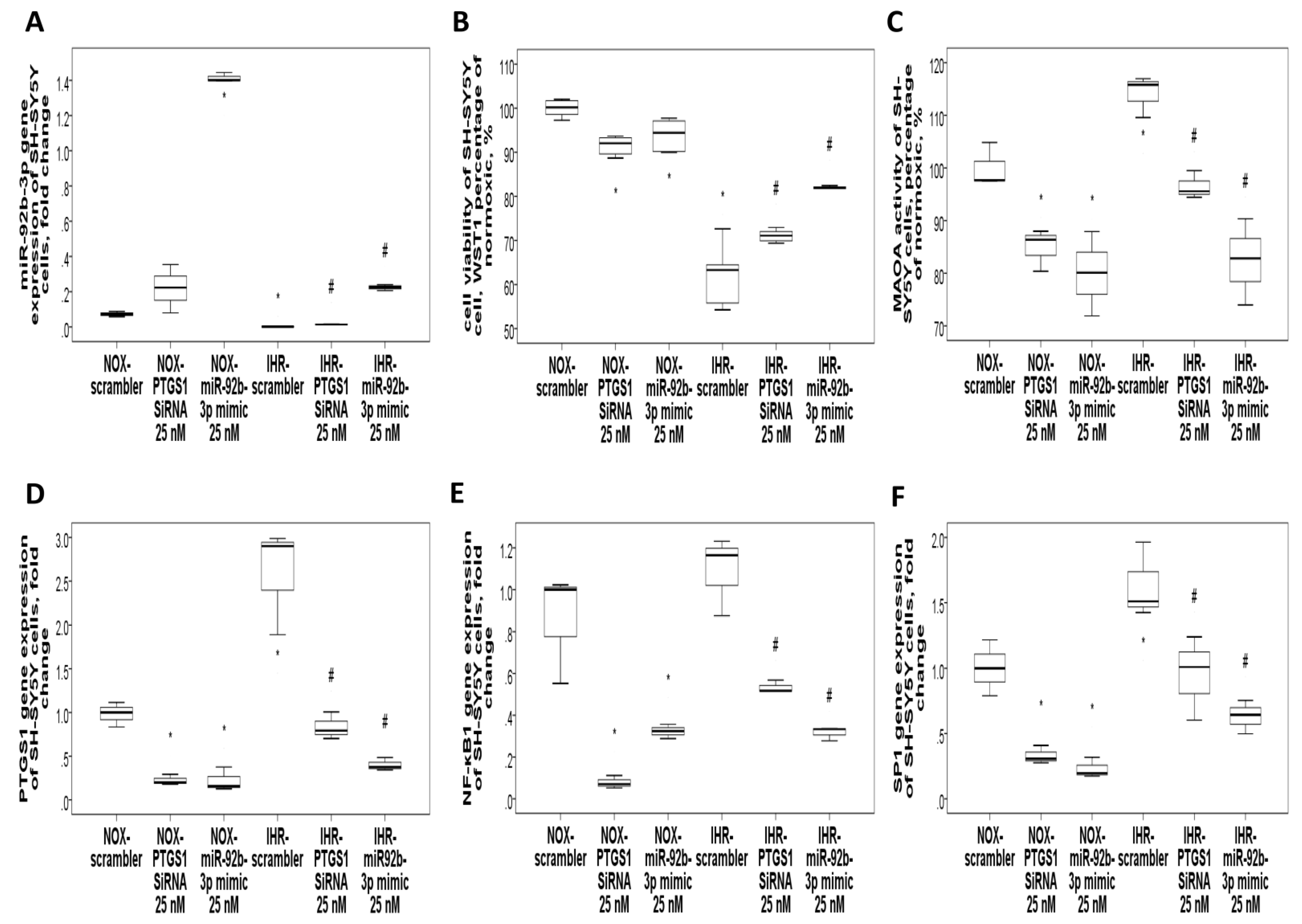
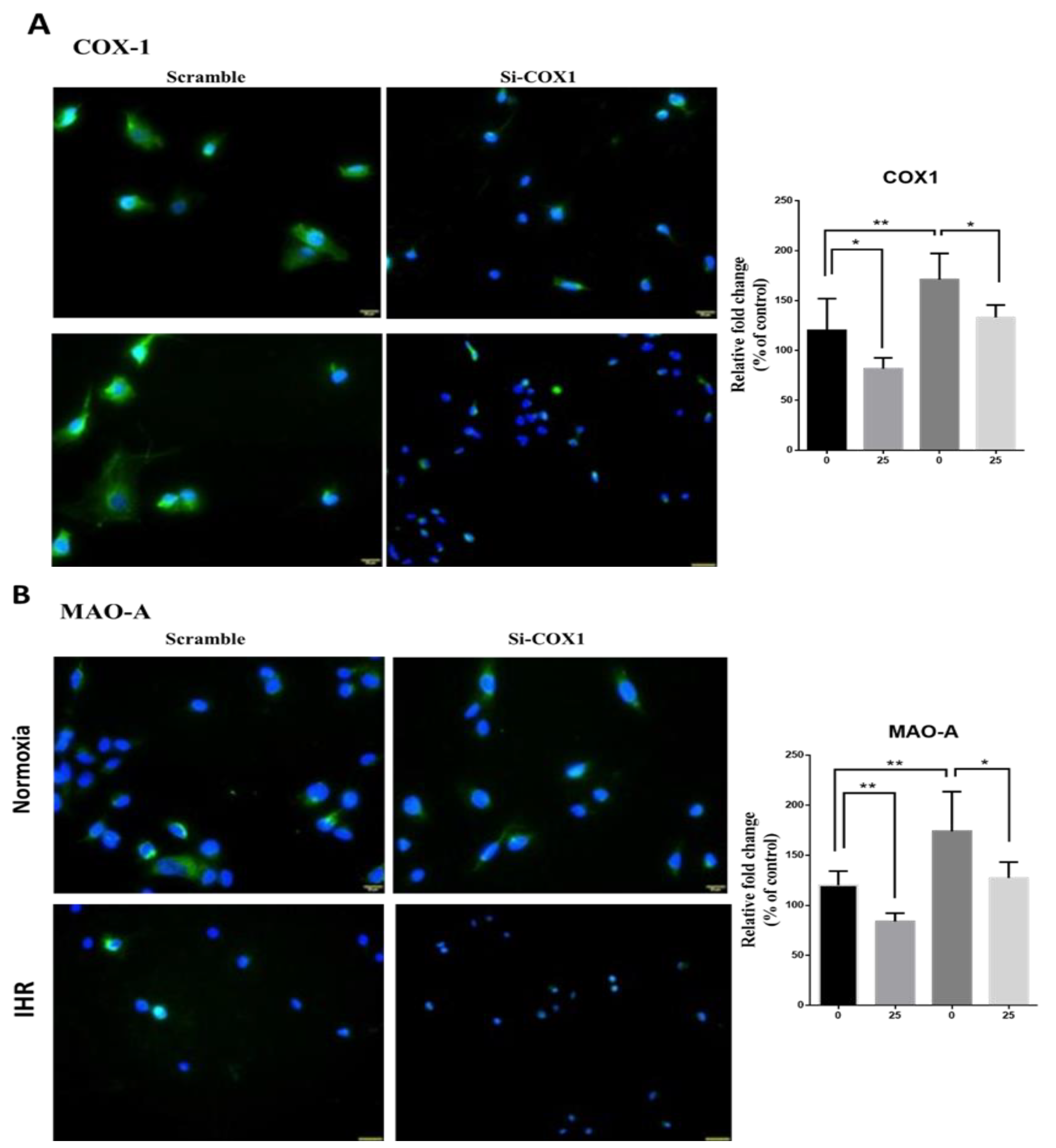
3.7. Knockdown of PTGS1 or Overexpression of miR-15b-5p/miR-92b-3p Alleviates IHR-Induced Neuron Cell Injury, Oxidative Stress, and MAOA Hyperactivity via Mediating NF-κB1-SP1 Signaling
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Franklin, K.A.; Lindberg, E. Obstructive sleep apnea is a common disorder in the population-a review on the epidemiology of sleep apnea. J. Thorac. Dis. 2015, 7, 1311–1322. [Google Scholar]
- Xie, W.; Zheng, F.; Song, X. Obstructive sleep apnea and serious adverse outcomes in patients with cardiovascular or cerebrovascular disease: A PRISMA-compliant systematic review and meta-analysis. Medicine 2014, 93, e336. [Google Scholar] [CrossRef] [PubMed]
- Bjornsdottir, E.; Keenan, B.T.; Eysteinsdottir, B.; Arnardottir, E.S.; Janson, C.; Gislason, T.; Sigurdsson, J.F.; Kuna, S.T.; Pack, A.I.; Benediktsdottir, B. Quality of life among untreated sleep apnea patients compared with the general population and changes after treatment with positive airway pressure. J. Sleep Res. 2015, 24, 328–338. [Google Scholar] [CrossRef] [PubMed]
- Labarca, G.; Dreyse, J.; Drake, L.; Jorquera, J.; Barbe, F. Efficacy of continuous positive airway pressure (CPAP) in the prevention of cardiovascular events in patients with obstructive sleep apnea: Systematic review and meta-analysis. Sleep Med. Rev. 2020, 52, 101312. [Google Scholar] [CrossRef] [PubMed]
- Nanduri, J.; Peng, Y.J.; Yuan, G.; Kumar, G.K.; Prabhakar, N.R. Hypoxia-inducible factors and hypertension: Lessons from sleep apnea syndrome. J. Mol. Med. 2015, 93, 473–480. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.C.; Hsu, P.Y.; Hsiao, C.C.; Lin, M.C. Epigenetics: A Potential Mechanism Involved in the Pathogenesis of Various Adverse Consequences of Obstructive Sleep Apnea. Int. J. Mol. Sci. 2019, 20, 2937. [Google Scholar] [CrossRef]
- Wu, J.G.; Xun, N.; Zeng, L.J.; Li, Z.Y.; Liang, Y.B.; Tang, H.; Ma, Z.F. Effects of small interfering RNA targeting TLR4 on expressions of adipocytokines in obstructive sleep apnea hyponea syndrome with hypertension in a rat model. J. Cell Physiol. 2018, 233, 6613–6620. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y.; Liu, K.; Pan, Y.; Ren, J.; Shang, J.; Chen, L.; Liu, H. TLR2 antagonism attenuates the hippocampal neuronal damage in a murine model of sleep apnea via inhibiting neuroinflammation and oxidative stress. Sleep Breath 2020, 24, 1613–1621. [Google Scholar] [CrossRef]
- Caputo, M.; Saif, J.; Rajakaruna, C.; Brooks, M.; Angelini, G.D.; Emanueli, C. MicroRNAs in vascular tissue engineering and post-ischemic neovascularization. Adv. Drug Deliv. Rev. 2015, 88, 78–91. [Google Scholar] [CrossRef]
- SoRelle, J.A.; Wachsmann, M.; Cantarel, B.L. Assembling and Validating Bioinformatic Pipelines for Next-Generation Sequencing Clinical Assays. Arch. Pathol. Lab. Med. 2020, 144, 1118–1130. [Google Scholar] [CrossRef]
- Gupta, V.; Khan, A.A.; Sasi, B.K.; Mahapatra, N.R. Molecular mechanism of monoamine oxidase A gene regulation under inflammation and ischemia-like conditions: Key roles of the transcription factors GATA2, Sp1 and TBP. J. Neurochem. 2015, 134, 21–38. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Liu, Y.; Zhang, M.; Lv, L.; Zhang, X.; Zhang, P.; Zhou, Y. Inhibition of PTGS1 promotes osteogenic differentiation of adipose-derived stem cells by suppressing NF-kB signaling. Stem Cell Res. Ther. 2019, 10, 57. [Google Scholar] [CrossRef] [PubMed]
- Berry, R.B.; Budhiraja, R.; Gottlieb, D.J.; Gozal, D.; Iber, C.; Kapur, V.K.; Marcus, C.L.; Mehra, R.; Parthasarathy, S.; Quan, S.F.; et al. Rules for scoring respiratory events in sleep: Update of the 2007 AASM Manual for the Scoring of Sleep and Associated Events. Deliberations of the Sleep Apnea Definitions Task Force of the American Academy of Sleep Medicine. J. Clin. Sleep Med. JCSM Off. Publ. Am. Acad. Sleep Med. 2012, 8, 597–619. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.C.; Su, M.C.; Liou, C.W.; Liu, S.F.; Chen, C.J.; Lin, H.C.; Hsiao, C.C.; Wang, T.Y.; Wang, C.C.; Chin, C.H.; et al. Co-upregulation of Toll-like receptors 2 and 6 on peripheral blood cells in patients with obstructive sleep apnea. Sleep Breath. Schlaf Atm. 2015, 19, 873–882. [Google Scholar] [CrossRef]
- Johns, M.W. A new method for measuring daytime sleepiness: The Epworth sleepiness scale. Sleep 1991, 14, 540–545. [Google Scholar] [CrossRef]
- Wu, X.; Kim, T.K.; Baxter, D.; Scherler, K.; Gordon, A.; Fong, O.; Etheridge, A.; Galas, D.J.; Wang, K. sRNAnalyzer-a flexible and customizable small RNA sequencing data analysis pipeline. Nucleic Acids Res. 2017, 45, 12140–12151. [Google Scholar] [CrossRef] [PubMed]
- Xiao, F.; Zuo, Z.; Cai, G.; Kang, S.; Gao, X.; Li, T. miRecords: An integrated resource for microRNA-target interactions. Nucleic Acids Res. 2009, 37, D105–D110. [Google Scholar] [CrossRef] [PubMed]
- Hsu, S.D.; Lin, F.M.; Wu, W.Y.; Liang, C.; Huang, W.C.; Chan, W.L.; Tsai, W.T.; Chen, G.Z.; Lee, C.J.; Chiu, C.M.; et al. miRTarBase: A database curates experimentally validated microRNA-target interactions. Nucleic Acids Res. 2011, 39, D163–D169. [Google Scholar] [CrossRef] [PubMed]
- Karagkouni, D.; Paraskevopoulou, M.D.; Chatzopoulos, S.; Vlachos, I.S.; Tastsoglou, S.; Kanellos, I.; Papadimitriou, D.; Kavakiotis, I.; Maniou, S.; Skoufos, G.; et al. DIANA-TarBase v8: A decade-long collection of experimentally supported miRNA-gene interactions. Nucleic Acids Res. 2018, 46, D239–D245. [Google Scholar] [CrossRef] [PubMed]
- Ru, Y.; Kechris, K.J.; Tabakoff, B.; Hoffman, P.; Radcliffe, R.A.; Bowler, R.; Mahaffey, S.; Rossi, S.; Calin, G.A.; Bemis, L.; et al. The multiMiR R package and database: Integration of microRNA-target interactions along with their disease and drug associations. Nucleic Acids Res. 2014, 42, e133. [Google Scholar] [CrossRef] [PubMed]
- Kramer, A.; Green, J.; Pollard, J., Jr.; Tugendreich, S. Causal analysis approaches in Ingenuity Pathway Analysis. Bioinformatics 2014, 30, 523–530. [Google Scholar] [CrossRef] [PubMed]
- Ashburner, M.; Ball, C.A.; Blake, J.A.; Botstein, D.; Butler, H.; Cherry, J.M.; Davis, A.P.; Dolinski, K.; Dwight, S.S.; Eppig, J.T.; et al. Gene ontology: Tool for the unification of biology. The Gene Ontology Consortium. Nat. Genet. 2000, 25, 25–29. [Google Scholar] [CrossRef] [PubMed]
- Gene Ontology, C. The Gene Ontology resource: Enriching a GOld mine. Nucleic Acids Res. 2021, 49, D325–D334. [Google Scholar] [CrossRef]
- Kanehisa, M.; Goto, S. KEGG: Kyoto encyclopedia of genes and genomes. Nucleic Acids Res. 2000, 28, 27–30. [Google Scholar] [CrossRef] [PubMed]
- Kanehisa, M. Toward understanding the origin and evolution of cellular organisms. Protein Sci. 2019, 28, 1947–1951. [Google Scholar] [CrossRef]
- Kanehisa, M.; Furumichi, M.; Sato, Y.; Ishiguro-Watanabe, M.; Tanabe, M. KEGG: Integrating viruses and cellular organisms. Nucleic Acids Res. 2021, 49, D545–D551. [Google Scholar] [CrossRef] [PubMed]
- Yoav Benjamini, Y.H. Controlling the False Discovery Rate: A Practical and Powerful Approach to Multiple Testing. J. R. Stat. Soc. 1995, 57, 289–300. [Google Scholar] [CrossRef]
- Yu, G.; Wang, L.G.; Han, Y.; He, Q.Y. clusterProfiler: An R package for comparing biological themes among gene clusters. OMICS 2012, 16, 284–287. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, M.S.; Singh, P.; Wolk, R.; Narkiewicz, K.; Somers, V.K. Obstructive sleep apnea and intermittent hypoxia increase expression of dual specificity phosphatase 1. Atherosclerosis 2013, 231, 378–383. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Ma, Z.; Wang, W.; Liu, R.; Zhang, Y.; Yuan, M.; Li, G. Effect of doxycycline on chronic intermittent hypoxia-induced atrial remodeling in rats. Herz 2020, 45, 668–675. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.L.; Zhao, L.L.; He, K.; Liu, Q.; Luo, J.; Zhang, D.M.; Liang, J.; Liao, L.; Ma, J.D.; Yang, S. MicroRNA regulation in hypoxic environments: Differential expression of microRNAs in the liver of largemouth bass (Micropterus salmoides). Fish Physiol. Biochem. 2020, 46, 2227–2242. [Google Scholar] [CrossRef] [PubMed]
- Gao, H.; Han, Z.; Huang, S.; Bai, R.; Ge, X.; Chen, F.; Lei, P. Intermittent hypoxia caused cognitive dysfunction relate to miRNAs dysregulation in hippocampus. Behav. Brain Res. 2017, 335, 80–87. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Niu, X.; Xiao, Y.; Lin, K.; Chen, X. MiRNA expression profiles in healthy OSAHS and OSAHS with arterial hypertension: Potential diagnostic and early warning markers. Respir. Res. 2018, 19, 194. [Google Scholar] [CrossRef]
- Yu, C.; Liu, Y.; Sun, L.; Wang, D.; Wang, Y.; Zhao, S.; Dai, H.; Zhao, J.; Zhang, S.; Li, M.; et al. Chronic obstructive sleep apnea promotes aortic remodeling in canines through miR-145/Smad3 signaling pathway. Oncotarget 2017, 8, 37705–37716. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Polonis, K.; Becari, C.; Chahal, C.A.A.; Zhang, Y.; Allen, A.M.; Kellogg, T.A.; Somers, V.K.; Singh, P. Chronic Intermittent Hypoxia Triggers a Senescence-like Phenotype in Human White Preadipocytes. Sci. Rep. 2020, 10, 6846. [Google Scholar] [CrossRef]
- Khalyfa, A.; Marin, J.M.; Qiao, Z.; Rubio, D.S.; Kheirandish-Gozal, L.; Gozal, D. Plasma exosomes in OSA patients promote endothelial senescence: Effect of long-term adherent continuous positive airway pressure. Sleep 2020, 43, zsz217. [Google Scholar] [CrossRef]
- Lin, C.C.; Wang, H.Y.; Liaw, S.F.; Chiu, C.H.; Lin, M.W. Effect of oral appliance on circulating leukocyte telomere length and SIRT1 in obstructive sleep apnea. Clin. Oral. Investig. 2019, 23, 1397–1405. [Google Scholar] [CrossRef]
- Salihu, H.M.; King, L.; Patel, P.; Paothong, A.; Pradhan, A.; Louis, J.; Naik, E.; Marty, P.J.; Whiteman, V. Association between maternal symptoms of sleep disordered breathing and fetal telomere length. Sleep 2015, 38, 559–566. [Google Scholar] [CrossRef]
- Marrone, O.; Bonsignore, M.R. Obstructive sleep apnea and cancer: A complex relationship. Curr. Opin. Pulm. Med. 2020, 26, 657–667. [Google Scholar] [CrossRef]
- Xu, H.; Wang, Y.; Guan, J.; Yi, H.; Yin, S. Effect of CPAP on Endothelial Function in Subjects With Obstructive Sleep Apnea: A Meta-Analysis. Respir. Care 2015, 60, 749–755. [Google Scholar] [CrossRef]
- Chen, J.; Lin, S.; Zeng, Y. An Update on Obstructive Sleep Apnea for Atherosclerosis: Mechanism, Diagnosis, and Treatment. Front. Cardiovasc. Med. 2021, 8, 647071. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Liu, Y.; Li, Y.; Chen, Z.; Xiong, Y.; Zhou, T.; Tao, W.; Xu, F.; Yang, H.; Yla-Herttuala, S.; et al. MicroRNA-15b Targets VEGF and Inhibits Angiogenesis in Proliferative Diabetic Retinopathy. J. Clin. Endocrinol. Metab. 2020, 105, 3404–3415. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Gao, Y.; Song, T.; Yu, C.; Nie, Z.; Wang, X. MicroRNA-15b participates in the development of peripheral arterial disease by modulating the growth of vascular smooth muscle cells. Exp. Ther. Med. 2019, 18, 77–84. [Google Scholar] [CrossRef]
- Liu, Y.; Yang, L.; Yin, J.; Su, D.; Pan, Z.; Li, P.; Wang, X. MicroRNA-15b deteriorates hypoxia/reoxygenation-induced cardiomyocyte apoptosis by downregulating Bcl-2 and MAPK3. J. Investig. Med. 2018, 66, 39–45. [Google Scholar] [CrossRef] [PubMed]
- Lang, A.; Grether-Beck, S.; Singh, M.; Kuck, F.; Jakob, S.; Kefalas, A.; Altinoluk-Hambuchen, S.; Graffmann, N.; Schneider, M.; Lindecke, A.; et al. MicroRNA-15b regulates mitochondrial ROS production and the senescence-associated secretory phenotype through sirtuin 4/SIRT4. Aging 2016, 8, 484–505. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Xu, X.; Liang, Y.; Zhu, R. Downregulation of microRNA-15b-5p Targeting the Akt3-Mediated GSK-3beta/beta-Catenin Signaling Pathway Inhibits Cell Apoptosis in Parkinson’s Disease. Biomed. Res. Int. 2021, 2021, 8814862. [Google Scholar] [CrossRef]
- Guo, L.; Zhu, Z.; Wang, G.; Cui, S.; Shen, M.; Song, Z.; Wang, J.H. microRNA-15b contributes to depression-like behavior in mice by affecting synaptic protein levels and function in the nucleus accumbens. J. Biol. Chem. 2020, 295, 6831–6848. [Google Scholar] [CrossRef]
- Liu, R.; Ma, X.; Chen, L.; Yang, Y.; Zeng, Y.; Gao, J.; Jiang, W.; Zhang, F.; Li, D.; Han, B.; et al. MicroRNA-15b Suppresses Th17 Differentiation and Is Associated with Pathogenesis of Multiple Sclerosis by Targeting O-GlcNAc Transferase. J. Immunol. 2017, 198, 2626–2639. [Google Scholar] [CrossRef]
- Kang, L.; Yang, C.; Yin, H.; Zhao, K.; Liu, W.; Hua, W.; Wang, K.; Song, Y.; Tu, J.; Li, S.; et al. MicroRNA-15b silencing inhibits IL-1beta-induced extracellular matrix degradation by targeting SMAD3 in human nucleus pulposus cells. Biotechnol. Lett. 2017, 39, 623–632. [Google Scholar] [CrossRef]
- Yu, X.J.; Huang, Y.Q.; Shan, Z.X.; Zhu, J.N.; Hu, Z.Q.; Huang, L.; Feng, Y.Q.; Geng, Q.S. MicroRNA-92b-3p suppresses angiotensin II-induced cardiomyocyte hypertrophy via targeting HAND2. Life Sci. 2019, 232, 116635. [Google Scholar] [CrossRef]
- Xu, L.; Cao, H.; Xie, Y.; Zhang, Y.; Du, M.; Xu, X.; Ye, R.; Liu, X. Exosome-shuttled miR-92b-3p from ischemic preconditioned astrocytes protects neurons against oxygen and glucose deprivation. Brain Res. 2019, 1717, 66–73. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Li, Z.; Jiang, C.; Jiang, X.; Zhang, J. MiR-92b-3p promotes neurite growth and functional recovery via the PTEN/AKT pathway in acute spinal cord injury. J. Cell Physiol. 2019, 234, 23043–23052. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.; Ji, R.; Ge, S.; Zhou, D.; Liu, Z.; Sun, Y.; Huang, W.; Lu, C. MicroRNA-92b-3p promotes the progression of liver fibrosis by targeting CREB3L2 through the JAK/STAT signaling pathway. Pathol. Res. Pract. 2021, 219, 153367. [Google Scholar] [CrossRef] [PubMed]
- Nowakowski, T.J.; Fotaki, V.; Pollock, A.; Sun, T.; Pratt, T.; Price, D.J. MicroRNA-92b regulates the development of intermediate cortical progenitors in embryonic mouse brain. Proc. Natl. Acad. Sci. USA 2013, 110, 7056–7061. [Google Scholar] [CrossRef] [PubMed]
- Garbarino, S.; Bardwell, W.A.; Guglielmi, O.; Chiorri, C.; Bonanni, E.; Magnavita, N. Association of Anxiety and Depression in Obstructive Sleep Apnea Patients: A Systematic Review and Meta-Analysis. Behav. Sleep Med. 2020, 18, 35–57. [Google Scholar] [CrossRef]
- Edwards, C.; Almeida, O.P.; Ford, A.H. Obstructive sleep apnea and depression: A systematic review and meta-analysis. Maturitas 2020, 142, 45–54. [Google Scholar] [CrossRef] [PubMed]
- Sacher, J.; Rekkas, P.V.; Wilson, A.A.; Houle, S.; Romano, L.; Hamidi, J.; Rusjan, P.; Fan, I.; Stewart, D.E.; Meyer, J.H. Relationship of monoamine oxidase-A distribution volume to postpartum depression and postpartum crying. Neuropsychopharmacology 2015, 40, 429–435. [Google Scholar] [CrossRef]
- Corbineau, S.; Breton, M.; Mialet-Perez, J.; Costemale-Lacoste, J.F. Major depression and heart failure: Interest of monoamine oxidase inhibitors. Int. J. Cardiol. 2017, 247, 1–6. [Google Scholar] [CrossRef]
- Li, X.; Qin, X.M.; Tian, J.S.; Gao, X.X.; Du, G.H.; Zhou, Y.Z. Integrated network pharmacology and metabolomics to dissect the combination mechanisms of Bupleurum chinense DC-Paeonia lactiflora Pall herb pair for treating depression. J. Ethnopharmacol. 2021, 264, 113281. [Google Scholar] [CrossRef]
- Miao, C.; Chang, J. The important roles of microRNAs in depression: New research progress and future prospects. J. Mol. Med. 2021, 99, 619–636. [Google Scholar] [CrossRef]
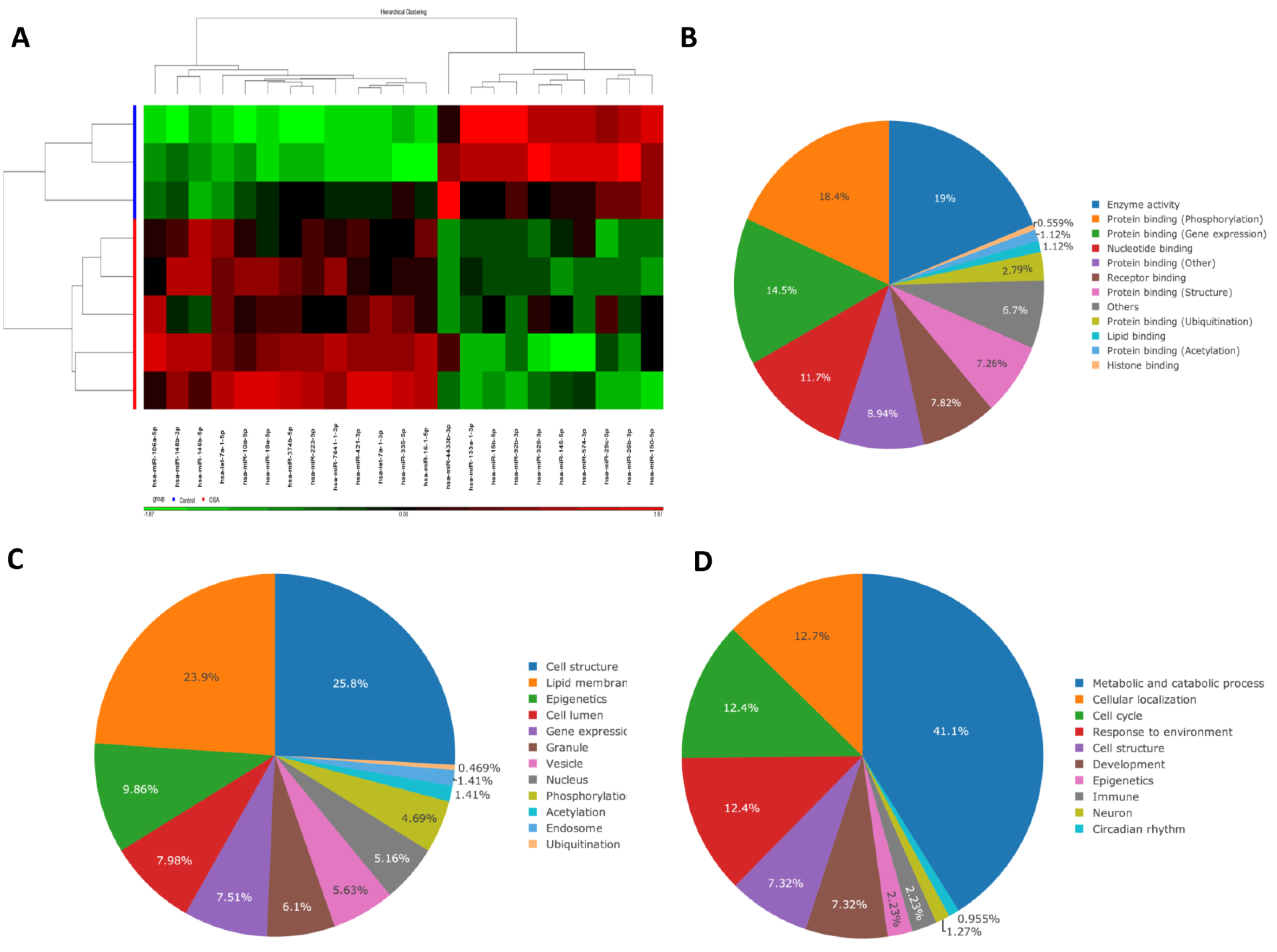
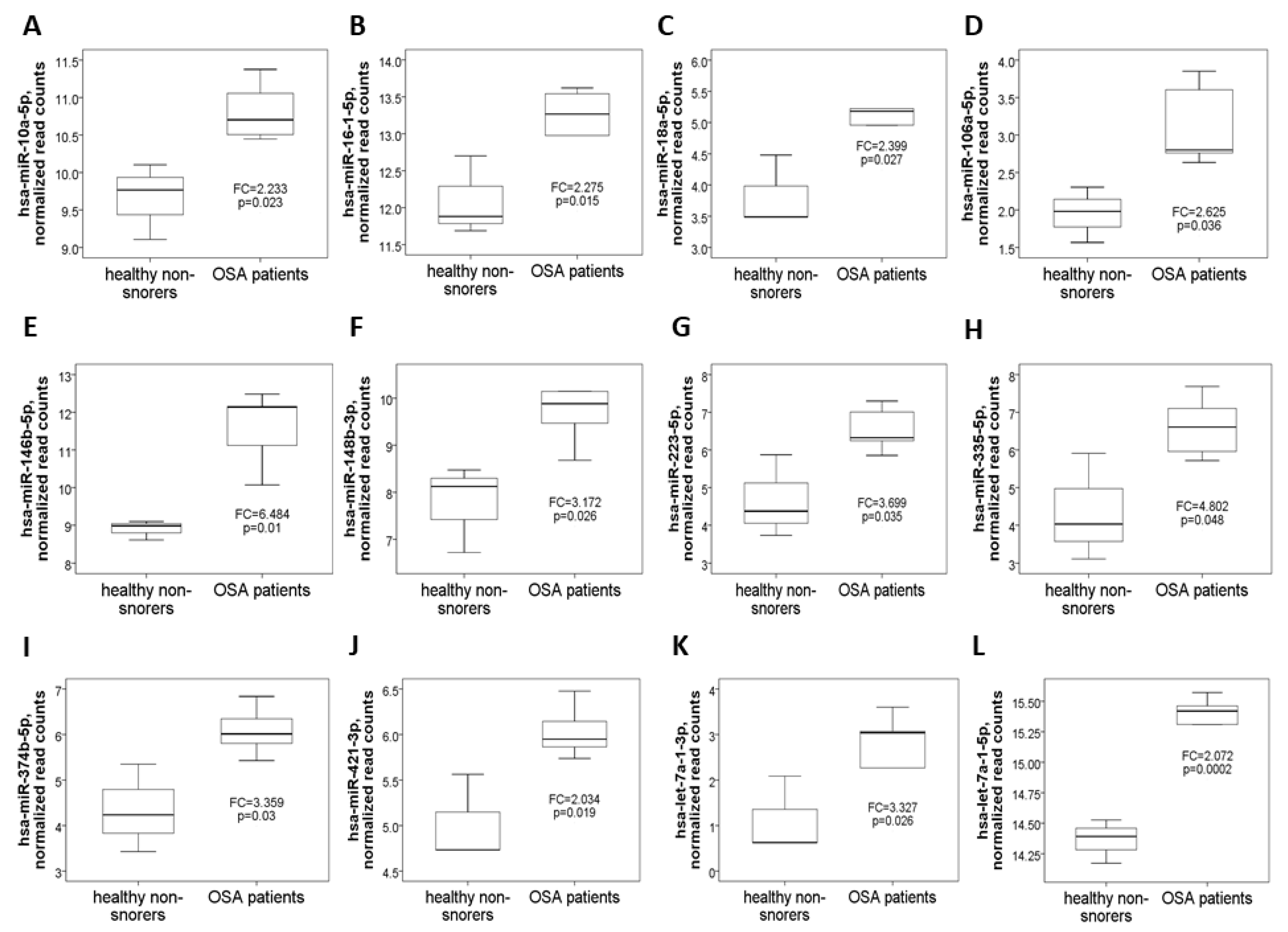

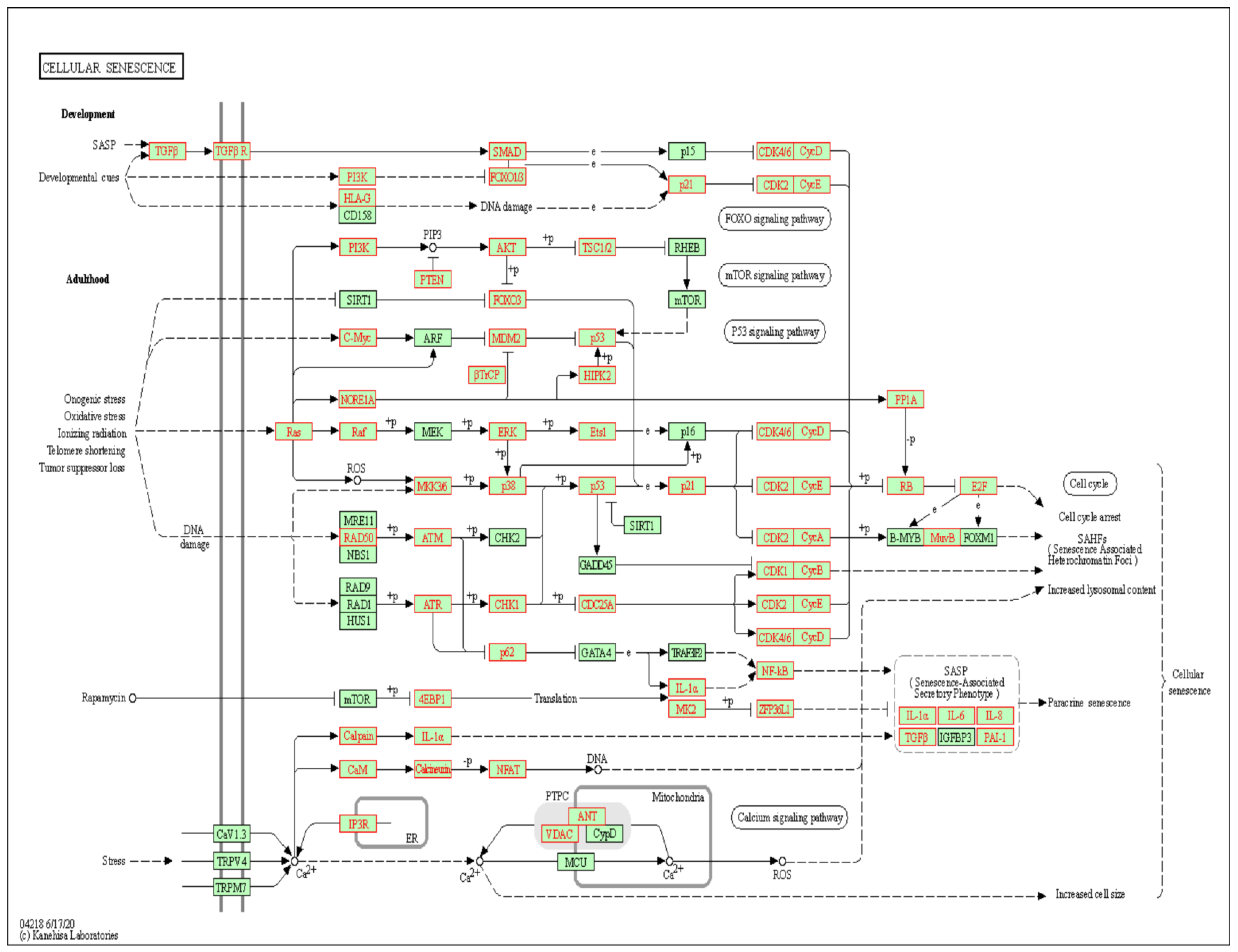
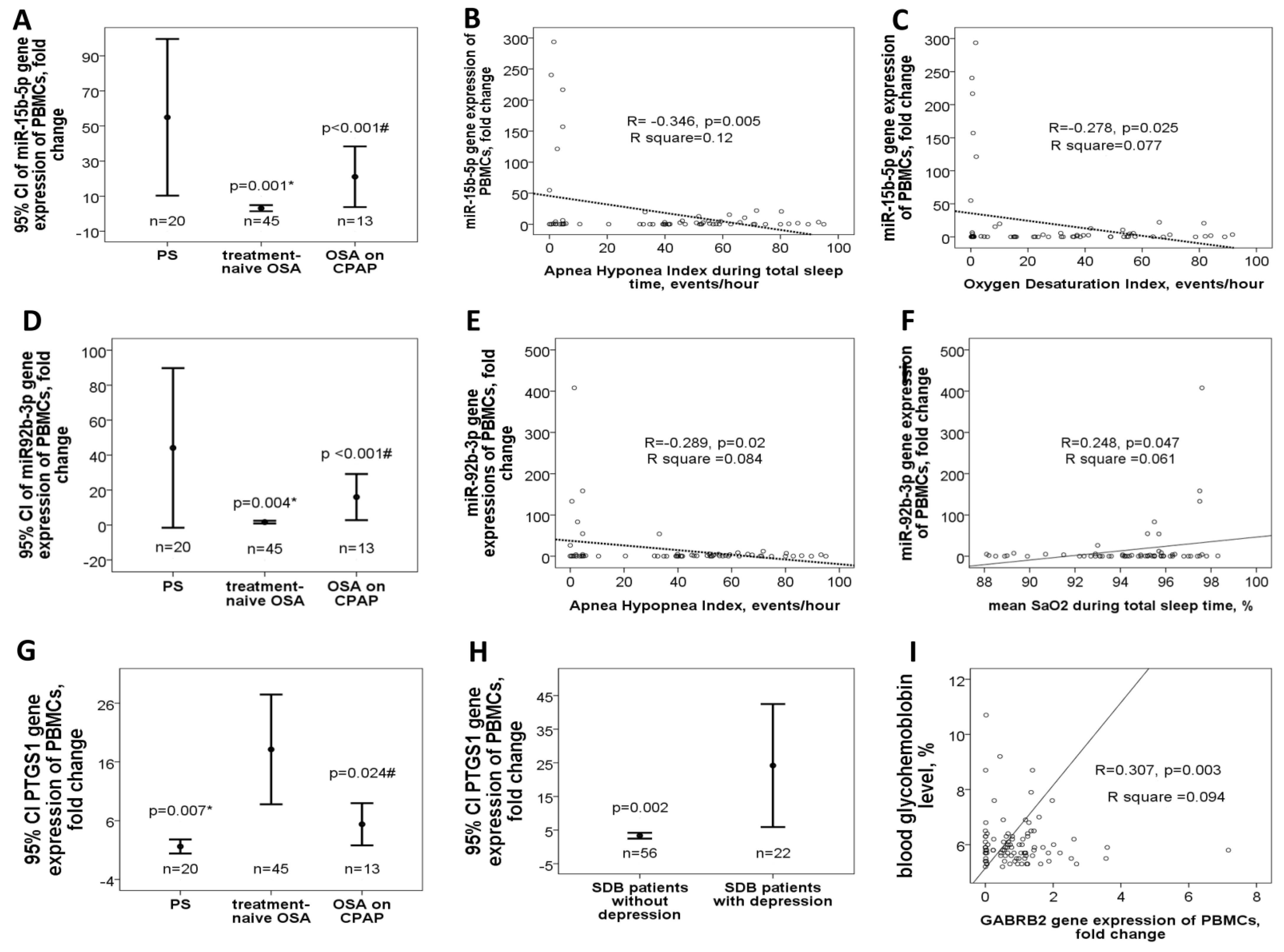


| Discovery Cohort | Validation Cohort | ||||||
|---|---|---|---|---|---|---|---|
| OSA Patients N = 16 | Healthy Subjects N = 8 | p Value | Primary Snoring N = 20 | Treatment-Naïve OSA Patients N = 45 | OSA Patients on CPAP N = 13 | p Value | |
| Age, years | 55.2 ± 14.8 | 47.9 ± 13.6 | 0.242 | 44.6 ± 12.6 | 48.5 ± 120.9 | 50.6 ± 7.5 | 0.248 |
| Male Sex, n (%) | 12 (75) | 8 (100) | 0.121 | 12 (60) | 34 (75.6) | 11 (84.6) | 0.252 |
| BMI, kg/m2 | 27.3 ± 3.7 | 25.0 ± 3.2 | 0.144 | 25.1 ± 3.6 | 26.4 ± 3.4 | 27.3 ± 2.9 | 0.173 |
| AHI, events/hour | 44.5 ± 24.5 | NA | 2.7 ± 1.8 | 54.8 ± 19.2 | 62.5 ± 23.3 | <0.001 | |
| ODI, events/hour | 25.8 ± 27.5 | NA | 0.8 ± 0.2 | 41.9 ± 24.7 | 44.9 ± 29.4 | <0.001 | |
| Mean SaO2, % | 95.0 ± 2.4 | NA | 96.1 ± 1.6 | 93.7 ± 2.5 | 93.7 ± 2.5 | 0.001 | |
| Nadir SaO2, % | 78.5 ± 13.2 | NA | 89.1 ± 3.7 | 70.4 ± 15.8 | 72.5 ± 13 | <0.001 | |
| Snoring index, counts/hour | 359.3 ± 278.4 | NA | 129.7 ± 147.6 | 374.7 ± 200.1 | 315.2 ± 189.5 | <0.001 | |
| Smoking, n (%) | 7 (43.8) | 3 (37.5) | 0.77 | 4 (20) | 11 (24.4) | 4 (30.8) | 0.78 |
| Alcoholism, n (%) | 0 (0) | 0 (0) | 1.0 | 0 (0) | 0 (0) | 1 (7.7) | 0.138 |
| Cholesterol, mg/dl | 212.1 ± 58.6 | 183.2 ± 41.5 | 0.346 | 186.7 ± 41.8 | 200.1 ± 38.6 | 188.4 ± 38.6 | 0.375 |
| Triglycerides, mg/dl | 158 ± 89.8 | 75.6 ± 29.1 | 0.071 | 118.7 ± 71.4 | 170.1 ± 82.6 | 143.8 ± 66.6 | 0.063 |
| Hypertension, n (%) | 4 (25) | 2 (25) | 1.0 | 3 (15) | 17 (37.8) | 8 (61.5) | 0.023 |
| Diabetes mellitus, n (%) | 1 (6.3) | 1 (12.5) | 0.602 | 1 (5) | 7 (15.6) | 1 (7.7) | 0.419 |
| Heart disease, n (%) | 2 (12.5) | 0 (0) | 0.296 | 2 (10) | 2 (4.4) | 1 (7.7) | 0.686 |
| Stroke, n (%) | 1 (6.3) | 1 (12.5) | 0.602 | 1 (5) | 0 (0) | 0 (0) | 0.23 |
| COPD, n (%) | 2 (12.5) | 1 (12.5) | 1.0 | 2 (10) | 2 (4.4) | 2 (15.4) | 0.386 |
| CKD, n (%) | 0 (0) | 0 (0) | 1.0 | 1 (5) | 0 (0) | 0 (0) | 0.23 |
| Depression, n (%) | 6 (37.5) | 1 (12.5) | 0.204 | 8 (40) | 12 (26.7) | 2 (15.4) | 0.298 |
| ID | Description | Gene Ratio * | Bg Ratio # | Adjusted p Value | q Value | Excluded miRNA |
|---|---|---|---|---|---|---|
| hsa04218 | Cellular senescence | 85/1996 | 156/8105 | 1.19 × 10−13 | 7.06 × 10−14 | hsa-miR-29c-5p |
| hsa04390 | Hippo signaling pathway | 83/1996 | 157/8105 | 1.77 × 10−12 | 1.05 × 10−12 | hsa-miR-29c-5p |
| hsa04110 | Cell cycle | 69/1996 | 124/8105 | 6.62 × 10−12 | 3.94 × 10−12 | 22 miRs involved |
| hsa04520 | Adherens junction | 44/1996 | 71/8105 | 1.27 × 10−9 | 7.53 × 10−10 | hsa-miR-29c-5p |
| hsa04933 | AGE-RAGE signaling pathway in diabetic complications | 54/1996 | 100/8105 | 1.06 × 10−8 | 6.28 × 10−9 | 22 miRs involved |
| hsa04150 | mTOR signaling pathway | 74/1996 | 155/8105 | 1.06 × 10−8 | 6.28 × 10−9 | hsa-miR-29c-5p |
| hsa04350 | TGF-beta signaling pathway | 51/1996 | 94/8105 | 1.6 × 10−8 | 9.49 × 10−9 | 22 miRs involved |
| hsa04141 | Protein processing in endoplasmic reticulum | 78/1996 | 171/8105 | 2.97 × 10−8 | 1.76 × 10−8 | hsa-miR-29c-5p |
| hsa04115 | p53 signaling pathway | 42/1996 | 73/8105 | 3.89 × 10−8 | 2.31 × 10−8 | hsa-miR-29c-5p |
| hsa04510 | Focal adhesion | 87/1996 | 201/8105 | 6.84 × 10−8 | 4.06 × 10−8 | hsa-miR-29c-5p |
| hsa04068 | FoxO signaling pathway | 63/1996 | 131/8105 | 7.04 × 10−8 | 4.18 × 10−8 | hsa-miR-29c-5p |
| hsa04550 | Signaling pathways regulating pluripotency of stem cells | 67/1996 | 143/8105 | 8.35 × 10−8 | 4.96 × 10−8 | 22 miRs involved |
| hsa04120 | Ubiquitin mediated proteolysis | 64/1996 | 140/8105 | 4.24 × 10−7 | 2.52 × 10−7 | hsa-miR-29c-5p |
| hsa01522 | Endocrine resistance | 49/1996 | 98/8105 | 4.95 × 10−7 | 2.94 × 10−7 | hsa-miR-29c-5p |
| hsa04010 | MAPK signaling pathway | 112/1996 | 294/8105 | 1.37 × 10−6 | 8.13 × 10−7 | 22 miRs involved |
| hsa04668 | TNF signaling pathway | 53/1996 | 112/8105 | 1.38 × 10−6 | 8.17 × 10−7 | hsa-miR-29c-5p, has-miR-4433b-3p, hsa-miR-574-3p |
| hsa04152 | AMPK signaling pathway | 55/1996 | 120/8105 | 2.85 × 10−6 | 1.69 × 10−6 | hsa-miR-574-3p |
| hsa04810 | Regulation of actin cytoskeleton | 87/1996 | 218/8105 | 3.01 × 10−6 | 1.79 × 10−6 | 22 miRs involved |
| hsa04211 | Longevity regulating pathway | 44/1996 | 89/8105 | 3.01 × 10−6 | 1.79 × 10−6 | hsa-miR-29c-5p, has-miR-4433b-3p, hsa-miR-574-3p |
| hsa04151 | PI3K-Akt signaling pathway | 128/1996 | 354/8105 | 4.15 × 10−6 | 2.47 × 10−6 | 22 miRs involved |
| hsa05202 | Transcriptional mis-regulation in cancer | 78/1996 | 192/8105 | 4.62 × 10−6 | 2.74 × 10−6 | hsa-miR-29c-5p, hsa-miR-574-3p |
| hsa04722 | Neurotrophin signaling pathway | 53/1996 | 119/8105 | 1.01 × 10−5 | 6.01 × 10−6 | 22 miRs involved |
| hsa05417 | Lipid and atherosclerosis | 83/1996 | 215/8105 | 2.01 × 10−5 | 1.19 × 10−5 | hsa-miR-29c-5p |
| hsa04071 | Sphingolipid signaling pathway | 52/1996 | 119/8105 | 2.38 × 10−5 | 1.42 × 10−5 | hsa-miR-4433b-3p |
| hsa04530 | Tight junction | 68/1996 | 169/8105 | 2.9 × 10−5 | 1.72 × 10−5 | hsa-miR-29c-5p |
| hsa04919 | Thyroid hormone signaling pathway | 52/1996 | 121/8105 | 4.14 × 10−5 | 2.46 × 10−5 | 22 miRs involved |
| hsa04144 | Endocytosis | 92/1996 | 252/8105 | 7.94 × 10−5 | 4.72 × 10−5 | 22 miRs involved |
| hsa04710 | Circadian rhythm | 19/1996 | 31/8105 | 8.95 × 10−5 | 5.32 × 10−5 | hsa-miR-223-5p, hsa-miR-29c-5p, hsa-miR-574-3p |
| hsa04310 | Wnt signaling pathway | 65/1996 | 166/8105 | 1.13 × 10−4 | 6.69 × 10−5 | hsa-miR-29c-5p |
| hsa04910 | Insulin signaling pathway | 55/1996 | 137/8105 | 2.02 × 10−4 | 1.2 × 10−4 | hsa-miR-29c-5p, hsa-miR-574-3p |
| hsa05010 | Alzheimer disease | 124/1996 | 369/8105 | 2.32 × 10−4 | 1.38 × 10−4 | 22 miRs involved |
| hsa04210 | Apoptosis | 54/1996 | 136/8105 | 3.08 × 10−4 | 1.83 × 10−4 | hsa-miR-29c-5p, hsa-miR-574-3p |
| hsa04066 | HIF-1 signaling pathway | 45/1996 | 109/8105 | 4.05 × 10−4 | 2.4 × 10−4 | 22 miRs involved |
| hsa04340 | Hedgehog signaling pathway | 27/1996 | 56/8105 | 4.85 × 10−4 | 2.88 × 10−4 | hsa-miR-29c-5p, hsa-miR-574-3p |
| hsa04015 | Rap1 signaling pathway | 75/1996 | 210/8105 | 8.14 × 10−4 | 4.84 × 10−4 | hsa-miR-4433b-3p |
| hsa04935 | Growth hormone synthesis, secretion and action | 47/1996 | 119/8105 | 9.53 × 10−4 | 5.66 × 10−4 | 22 miRs involved |
| hsa04657 | IL-17 signaling pathway | 39/1996 | 94/8105 | 9.53 × 10−4 | 5.66 × 10−4 | hsa-miR-223-5p, hsa-miR-29c-5p, hsa-miR-574-3p |
| hsa04962 | Vasopressin-regulated water reabsorption | 21/1996 | 44/8105 | 2.797 × 10−3 | 1.662 × 10−3 | hsa-miR-223-5p, hsa-miR-4433b-3p |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Y.-C.; Hsu, P.-Y.; Su, M.-C.; Chen, T.-W.; Hsiao, C.-C.; Chin, C.-H.; Liou, C.-W.; Wang, P.-W.; Wang, T.-Y.; Lin, Y.-Y.; et al. MicroRNA Sequencing Analysis in Obstructive Sleep Apnea and Depression: Anti-Oxidant and MAOA-Inhibiting Effects of miR-15b-5p and miR-92b-3p through Targeting PTGS1-NF-κB-SP1 Signaling. Antioxidants 2021, 10, 1854. https://doi.org/10.3390/antiox10111854
Chen Y-C, Hsu P-Y, Su M-C, Chen T-W, Hsiao C-C, Chin C-H, Liou C-W, Wang P-W, Wang T-Y, Lin Y-Y, et al. MicroRNA Sequencing Analysis in Obstructive Sleep Apnea and Depression: Anti-Oxidant and MAOA-Inhibiting Effects of miR-15b-5p and miR-92b-3p through Targeting PTGS1-NF-κB-SP1 Signaling. Antioxidants. 2021; 10(11):1854. https://doi.org/10.3390/antiox10111854
Chicago/Turabian StyleChen, Yung-Che, Po-Yuan Hsu, Mao-Chang Su, Ting-Wen Chen, Chang-Chun Hsiao, Chien-Hung Chin, Chia-Wei Liou, Po-Wen Wang, Ting-Ya Wang, Yong-Yong Lin, and et al. 2021. "MicroRNA Sequencing Analysis in Obstructive Sleep Apnea and Depression: Anti-Oxidant and MAOA-Inhibiting Effects of miR-15b-5p and miR-92b-3p through Targeting PTGS1-NF-κB-SP1 Signaling" Antioxidants 10, no. 11: 1854. https://doi.org/10.3390/antiox10111854
APA StyleChen, Y.-C., Hsu, P.-Y., Su, M.-C., Chen, T.-W., Hsiao, C.-C., Chin, C.-H., Liou, C.-W., Wang, P.-W., Wang, T.-Y., Lin, Y.-Y., Lee, C.-P., & Lin, M.-C. (2021). MicroRNA Sequencing Analysis in Obstructive Sleep Apnea and Depression: Anti-Oxidant and MAOA-Inhibiting Effects of miR-15b-5p and miR-92b-3p through Targeting PTGS1-NF-κB-SP1 Signaling. Antioxidants, 10(11), 1854. https://doi.org/10.3390/antiox10111854






