The Mechanisms and Management of Age-Related Oxidative Stress in Male Hypogonadism Associated with Non-communicable Chronic Disease
Abstract
1. Introduction
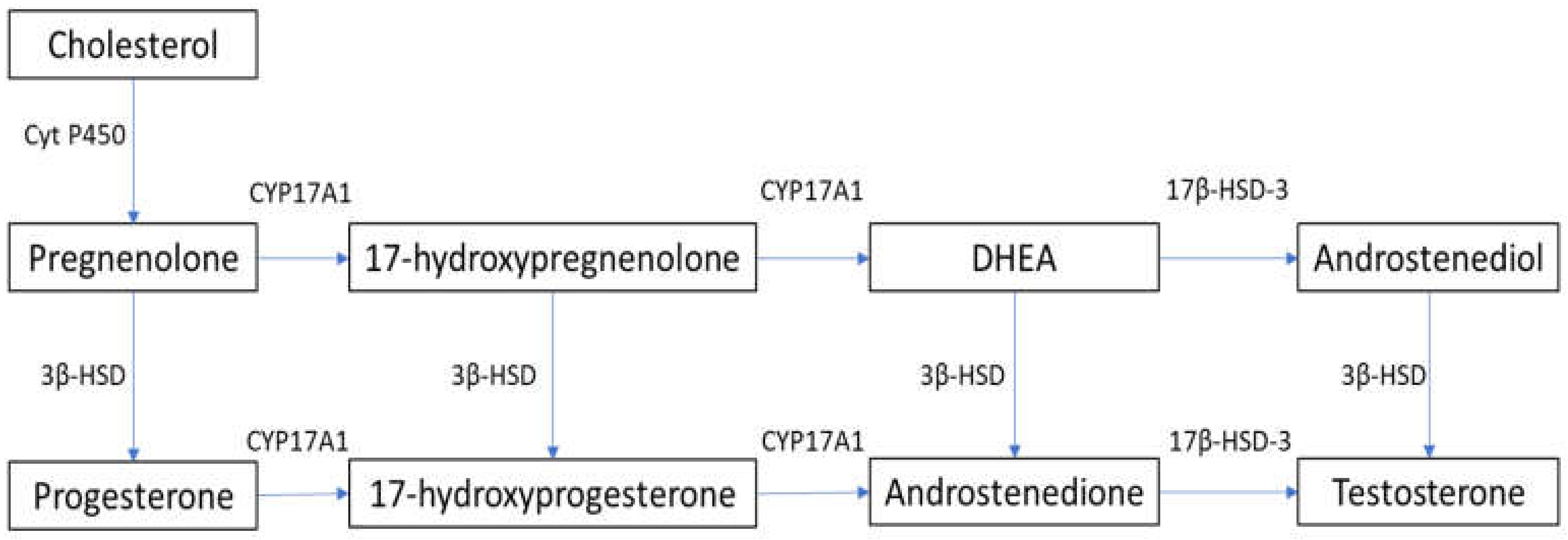
2. Steroidogenesis and Male Hypogonadism
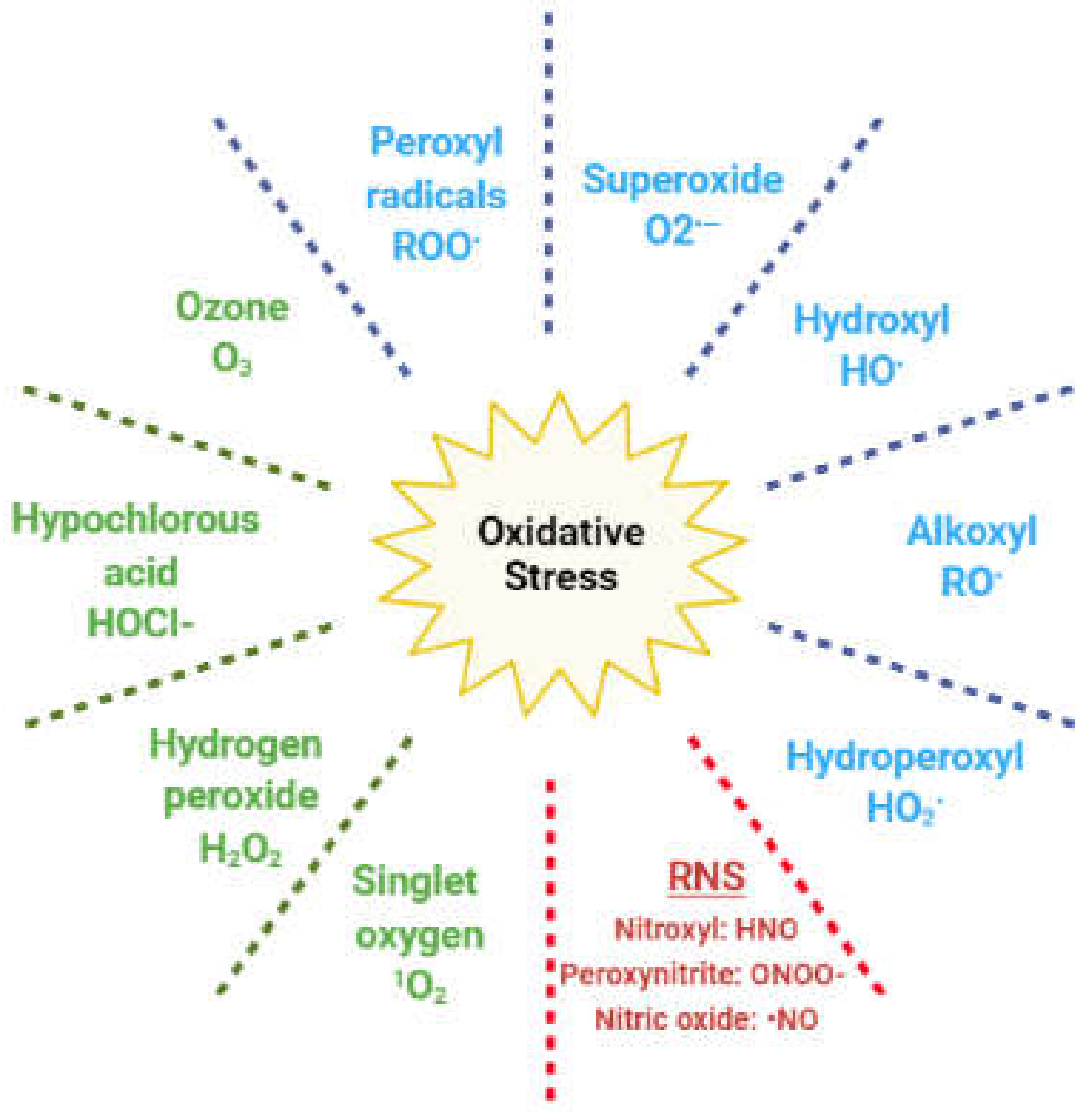
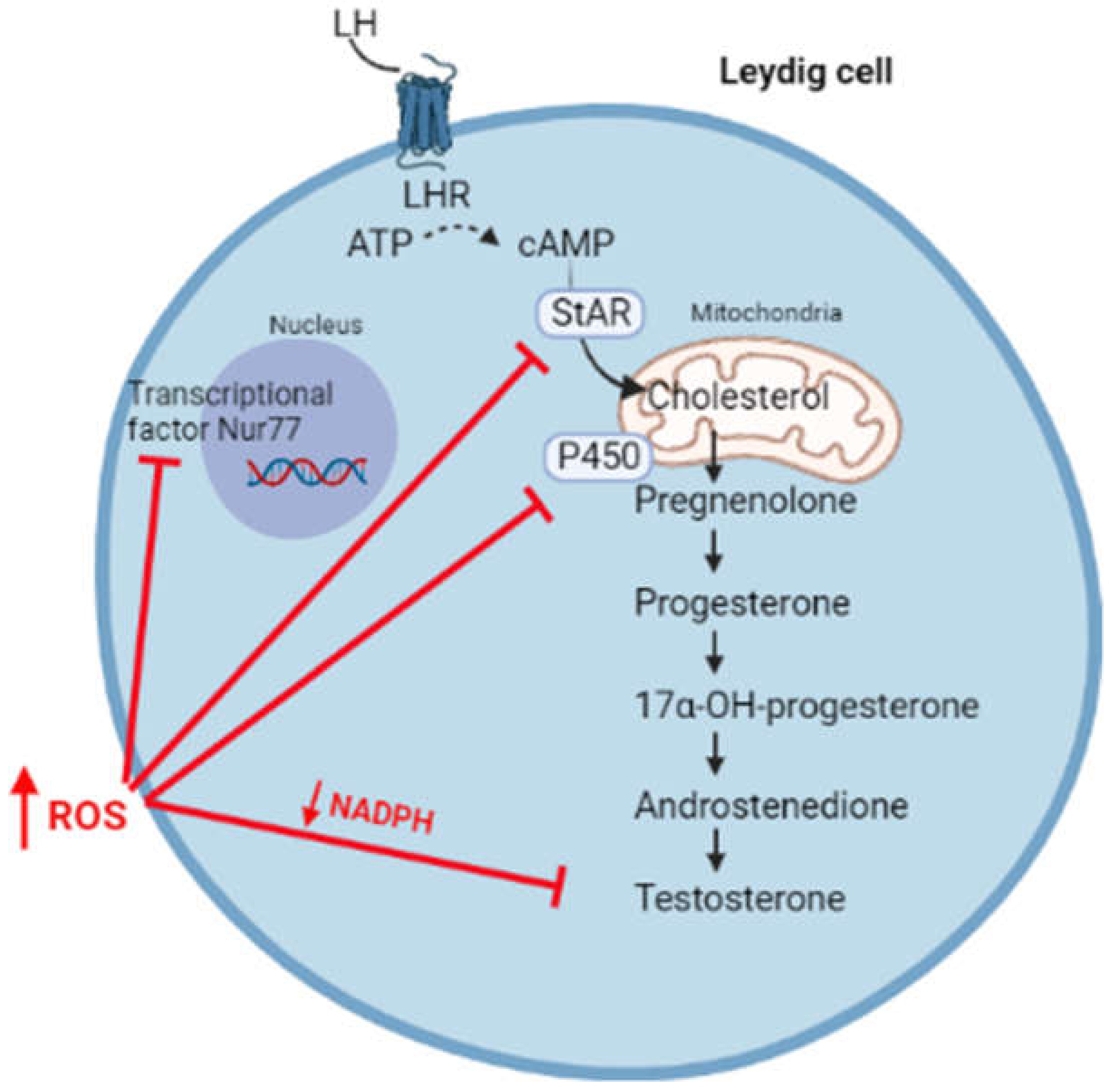
3. Oxidative Stress and Hypogonadism
4. The Complex Association between Ageing, Hypogonadism, Non-communicable Chronic Diseases, and Oxidative Stress
5. Management of Male Hypogonadism Associated with Non-communicable Chronic Diseases
5.1. Testosterone Replacement Therapy
5.2. Metformin
5.3. Nutrition and Weight Management
5.4. Antioxidants, Micronutrients, and Phytotherapy
6. Conclusions and Future Perspectives
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Pillerová, M.; Borbélyová, V.; Hodosy, J.; Riljak, V.; Renczés, E.; Frick, K.M.; Tóthová, Ľ. On the role of sex steroids in biological functions by classical and non-classical pathways. An update. Front. Neuroendocrinol. 2021, 62, 100926. [Google Scholar] [CrossRef]
- Traish, A.M. Negative impact of testosterone deficiency and 5α-reductase inhibitors therapy on metabolic and sexual function in men. Adv. Exp. Med. Biol. 2017, 1043, 473–526. [Google Scholar] [CrossRef]
- Dandona, P.; Rosenberg, M.T. A practical guide to male hypogonadism in the primary care setting. Int. J. Clin. Pract. 2010, 64, 682–696. [Google Scholar] [CrossRef] [PubMed]
- Araujo, A.B.; Wittert, G.A. Endocrinology of the aging male. Best Pract. Res. Clin. Endocrinol. Metab. 2011, 25, 303–319. [Google Scholar] [CrossRef]
- Morrell, C.N. Reactive oxygen species: Finding the right balance. Circ. Res. 2008, 103, 571–572. [Google Scholar] [CrossRef]
- Smetana, K.; Lacina, L.; Szabo, P.; Dvoánková, B.; Broẑ, P.; Ŝedo, A. Ageing as an important risk factor for cancer. Anticancer Res. 2016, 36, 5009–5017. [Google Scholar] [CrossRef]
- Luo, J.; Mills, K.; le Cessie, S.; Noordam, R.; van Heemst, D. Ageing, age-related diseases and oxidative stress: What to do next? Ageing Res. Rev. 2020, 57, 100982. [Google Scholar] [CrossRef] [PubMed]
- Höhn, A.; Weber, D.; Jung, T.; Ott, C.; Hugo, M.; Kochlik, B.; Kehm, R.; König, J.; Grune, T.; Castro, J.P. Happily (n)ever after: Aging in the context of oxidative stress, proteostasis loss and cellular senescence. Redox Biol. 2017, 11, 482–501. [Google Scholar] [CrossRef] [PubMed]
- Dohle, G.R.; Smit, M.; Weber, R.F.A. Androgens and male fertility. World J. Urol. 2003, 21, 341–345. [Google Scholar] [CrossRef] [PubMed]
- Miller, W.L.; Auchus, R.J. The molecular biology, biochemistry, and physiology of human steroidogenesis and its disorders. Endocr. Rev. 2011, 32, 81–151. [Google Scholar] [CrossRef] [PubMed]
- Payne, A.H.; Youngblood, G.L.; Sha, L.; Burgos-Trinidad, M.; Hammond, S.H. Hormonal regulation of steroidogenic enzyme gene expression in Leydig cells. J. Steroid Biochem. Mol. Biol. 1992, 43, 895–906. [Google Scholar] [CrossRef]
- Czub, M.P.; Venkataramany, B.S.; Majorek, K.A.; Handing, K.B.; Porebski, P.J.; Beeram, S.R.; Suh, K.; Woolfork, A.G.; Hage, D.S.; Shabalin, I.G.; et al. Testosterone meets albumin-the molecular mechanism of sex hormone transport by serum albumins. Chem. Sci. 2019, 10, 1607–1618. [Google Scholar] [CrossRef] [PubMed]
- Pivonello, R.; Menafra, D.; Riccio, E.; Garifalos, F.; Mazzella, M.; De Angelis, C.; Colao, A.A. Metabolic disorders and male hypogonadotropic hypogonadism. Front. Endocrinol. 2019, 10, 345. [Google Scholar] [CrossRef]
- Roychoudhury, S.; Chakraborty, S.; Choudhury, A.P.; Das, A.; Jha, N.K.; Slama, P.; Nath, M.; Massanyi, P.; Ruokolainen, J.; Kesari, K.K. Environmental factors-induced oxidative stress: Hormonal and molecular pathway disruptions in hypogonadism and erectile dysfunction. Antioxidants 2021, 10, 837. [Google Scholar] [CrossRef] [PubMed]
- Darby, E.; Anawalt, B.D. Male hypogonadism: An update on diagnosis and treatment. Treat. Endocrinol. 2005, 4, 293–309. [Google Scholar] [CrossRef]
- Baskaran, S.; Finelli, R.; Agarwal, A. Reactive oxygen species in male reproduction: A boon or a bane? Andrologia 2020, 53, e13577. [Google Scholar] [CrossRef]
- Rahal, A.; Kumar, A.; Singh, V.; Yadav, B.; Tiwari, R.; Chakraborty, S.; Dhama, K. Oxidative stress, prooxidants, and antioxidants: The interplay. Biomed. Res. Int. 2014, 2014, 761264. [Google Scholar] [CrossRef] [PubMed]
- Dröge, W. Free radicals in the physiological control of cell function. Physiol. Rev. 2002, 82, 47–95. [Google Scholar] [CrossRef]
- Martindale, J.L.; Holbrook, N.J. Cellular response to oxidative stress: Signaling for suicide and survival. J. Cell. Physiol. 2002, 192, 1–15. [Google Scholar] [CrossRef]
- Ray, P.; Huang, B.; Tsuji, Y. Reactive oxygen species (ROS) homeostasis and redox regulation in cellular signaling. Cell. Signal. 2012, 24, 981–990. [Google Scholar] [CrossRef] [PubMed]
- Beatty, S.; Koh, H.H.; Phil, M.; Henson, D.; Boulton, M. The role of oxidative stress in the pathogenesis of age-related macular degeneration. Surv. Ophthalmol. 2000, 45, 115–134. [Google Scholar] [CrossRef]
- Maritim, A.C.; Sanders, R.A.; Watkins, J.B. Diabetes, oxidative stress, and antioxidants: A review. J. Biochem. Mol. Toxicol. 2003, 17, 24–38. [Google Scholar] [CrossRef]
- Kattoor, A.J.; Pothineni, N.V.K.; Palagiri, D.; Mehta, J.L. Oxidative stress in atherosclerosis. Curr. Atheroscler. Rep. 2017, 19, 42. [Google Scholar] [CrossRef]
- Uttara, B.; Singh, A.; Zamboni, P.; Mahajan, R. Oxidative stress and neurodegenerative diseases: A review of upstream and downstream antioxidant therapeutic options. Curr. Neuropharmacol. 2009, 7, 65–74. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, A.; Parekh, N.; Panner Selvam, M.K.; Henkel, R.; Shah, R.; Homa, S.T.; Ramasamy, R.; Ko, E.; Tremellen, K.; Esteves, S.; et al. Male oxidative stress infertility (MOSI): Proposed terminology and clinical practice guidelines for management of idiopathic male infertility. World J. Men’s Health 2019, 37, 296. [Google Scholar] [CrossRef] [PubMed]
- Hanukoglu, I. Antioxidant protective mechanisms against reactive oxygen species (ROS) generated by mitochondrial P450 systems in steroidogenic cells. Drug Metab. Rev. 2006, 38, 171–196. [Google Scholar] [CrossRef] [PubMed]
- Ziegler, G.A.; Vonrhein, C.; Hanukoglu, I.; Schulz, G.E. The structure of adrenodoxin reductase of mitochondrial P450 systems: Electron transfer for steroid biosynthesis. J. Mol. Biol. 1999, 289, 981–990. [Google Scholar] [CrossRef]
- Quinn, P.G.; Payne, A.H. Steroid product-induced, oxygen-mediated damage of microsomal cytochrome P-450 enzymes in Leydig cell cultures. Relationship to desensitization. J. Biol. Chem. 1985, 260, 2092–2099. [Google Scholar] [CrossRef]
- Tai, P.; Ascoli, M. Reactive oxygen species (ROS) play a critical role in the cAMP-induced activation of RAS and the phosphorylation of ERK1/2 in leydig cells. Mol. Endocrinol. 2011, 25, 885–893. [Google Scholar] [CrossRef]
- Tai, P.; Shiraishi, K.; Ascoli, M. Activation of the lutropin/choriogonadotropin receptor inhibits apoptosis of immature Leydig cells in primary culture. Endocrinology 2009, 150, 3766–3773. [Google Scholar] [CrossRef]
- Martinelle, N.; Holst, M.; Söder, O.; Svechnikov, K. Extracellular signal-regulated kinases are involved in the acute activation of steroidogenesis in immature rat leydig cells by human chorionic gonadotropin. Endocrinology 2004, 145, 4629–4634. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.Y.; Gong, E.Y.; Hong, C.Y.; Kim, K.H.; Han, J.S.; Ryu, J.C.; Chae, H.Z.; Yun, C.H.; Lee, K. ROS inhibit the expression of testicular steroidogenic enzyme genes via the suppression of Nur77 transactivation. Free Radic. Biol. Med. 2009, 47, 1591–1600. [Google Scholar] [CrossRef]
- Lin, H.L.; Myshkin, E.; Waskell, L.; Hollenberg, P.F. Peroxynitrite inactivation of human cytochrome P450s 2B6 and 2E1: Heme modification and site-specific nitrotyrosine formation. Chem. Res. Toxicol. 2007, 20, 1612–1622. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Karuzina, I.I.; Archakov, A.I. The oxidative inactivation of cytochrome P450 in monooxygenase reactions. Free Radic. Biol. Med. 1994, 16, 73–97. [Google Scholar] [CrossRef]
- Chen, H.; Zhou, L.; Lin, C.; Beattie, M.; Liu, J.; Zirkin, B. Effect of glutathione redox state on Leydig cell susceptibility to acute oxidative stress. Mol. Cell. Endocrinol. 2010, 323, 147–154. [Google Scholar] [CrossRef] [PubMed]
- Abidi, P.; Zhang, H.; Zaidi, S.M.; Shen, W.J.; Leers-Sucheta, S.; Cortez, Y.; Han, J.; Azhar, S. Oxidative stress-induced inhibition of adrenal steroidogenesis requires participation of p38 mitogen-activated protein kinase signaling pathway. J. Endocrinol. 2008, 198, 193–207. [Google Scholar] [CrossRef] [PubMed]
- Zaidi, S.K.; Shen, W.J.; Bittner, S.; Bittner, A.; McLean, M.P.; Han, J.; Davis, R.J.; Kraemer, F.B.; Azhar, S. p38 MAPK regulates steroidogenesis through transcriptional repression of StAR gene. J. Mol. Endocrinol. 2014, 53, 1–16. [Google Scholar] [CrossRef]
- Wang, X.; Dyson, M.T.; Jo, Y.; Stocco, D.M. Inhibition of cyclooxygenase-2 activity enhances steroidogenesis and steroidogenic acute regulatory gene expression in MA-10 mouse Leydig cells. Endocrinology 2003, 144, 3368–3375. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.J.; Shen, C.L.; Dyson, M.T.; Eimerl, S.; Orly, J.; Hutson, J.C.; Stocco, D.M. Cyclooxygenase-2 regulation of the age-related decline in testosterone biosynthesis. Endocrinology 2005, 146, 4202–4208. [Google Scholar] [CrossRef]
- Diemer, T.; Allen, J.A.; Hales, H.K.; Hales, D.B. Reactive oxygen disrupts mitochondria in MA-10 tumor leydig cells and inhibits steroidogenic acute regulatory (STAR) protein and steroidogenesis. Endocrinology 2003, 144, 2882–2891. [Google Scholar] [CrossRef]
- Chen, H.; Jin, S.; Guo, J.; Kombairaju, P.; Biswal, S.; Zirkin, B.R. Knockout of the transcription factor Nrf2: Effects on testosterone production by aging mouse Leydig cells. Mol. Cell. Endocrinol. 2015, 409, 113–120. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Pechenino, A.S.; Liu, J.; Beattie, M.C.; Brown, T.R.; Zirkin, B.R. Effect of glutathione depletion on Leydig cell steroidogenesis in young and old Brown Norway rats. Endocrinology 2008, 149, 2612–2619. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Marcos, P.J.; Nóbrega-Pereira, S. NADPH: New oxygen for the ROS theory of aging. Oncotarget 2016, 7, 50814–50815. [Google Scholar] [CrossRef]
- Linford, N.J.; Schriner, S.E.; Rabinovitch, P.S. Oxidative damage and aging: Spotlight on mitochondria. Cancer Res. 2006, 66, 2497–2499. [Google Scholar] [CrossRef] [PubMed]
- Sharifi-Rad, M.; Anil Kumar, N.V.; Zucca, P.; Varoni, E.M.; Dini, L.; Panzarini, E.; Rajkovic, J.; Tsouh Fokou, P.V.; Azzini, E.; Peluso, I.; et al. Lifestyle, oxidative stress, and antioxidants: Back and forth in the pathophysiology of chronic diseases. Front. Physiol. 2020, 11, 694. [Google Scholar] [CrossRef]
- Rupérez, A.I.; Gil, A.; Aguilera, C.M. Genetics of oxidative stress in obesity. Int. J. Mol. Sci. 2014, 15, 3118–3144. [Google Scholar] [CrossRef]
- Chen, L.; Magliano, D.J.; Zimmet, P.Z. The worldwide epidemiology of type 2 diabetes mellitus—Present and future perspectives. Nat. Rev. Endocrinol. 2012, 8, 228–236. [Google Scholar] [CrossRef]
- Han, T.S.; Lean, M.E. A clinical perspective of obesity, metabolic syndrome and cardiovascular disease. JRSM Cardiovasc. Dis. 2016, 5, 204800401663337. [Google Scholar] [CrossRef]
- Lapik, I.A.; Galchenko, A.V.; Gapparova, K.M. Micronutrient status in obese patients: A narrative review. Obes. Med. 2020, 18, 100224. [Google Scholar] [CrossRef]
- Via, M. The Malnutrition of Obesity: Micronutrient Deficiencies That Promote Diabetes. Int. Sch. Res. Netw. Endocrinol. 2012, 2012, 103472. [Google Scholar] [CrossRef] [PubMed]
- Rolo, A.P.; Palmeira, C.M. Diabetes and mitochondrial function: Role of hyperglycemia and oxidative stress. Toxicol. Appl. Pharmacol. 2006, 212, 167–178. [Google Scholar] [CrossRef] [PubMed]
- Rovira-Llopis, S.; Bañuls, C.; de Marañon, A.M.; Diaz-Morales, N.; Jover, A.; Garzon, S.; Rocha, M.; Victor, V.M.; Hernandez-Mijares, A. Low testosterone levels are related to oxidative stress, mitochondrial dysfunction and altered subclinical atherosclerotic markers in type 2 diabetic male patients. Free Radic. Biol. Med. 2017, 108, 155–162. [Google Scholar] [CrossRef]
- Hernandez-Mijares, A.; Rocha, M.; Rovira-Llopis, S.; Bañuls, C.; Bellod, L.; De Pablo, C.; Alvarez, A.; Roldan-Torres, I.; Sola-Izquierdo, E.; Victor, V.M. Human leukocyte/endothelial cell interactions and mitochondrial dysfunction in type 2 diabetic patients and their association with silent myocardial ischemia. Diabetes Care 2013, 36, 1695–1702. [Google Scholar] [CrossRef] [PubMed]
- Giacco, F.; Brownlee, M. Oxidative stress and diabetic complications. Circ. Res. 2010, 107, 1058–1070. [Google Scholar] [CrossRef]
- Nishikawa, T.; Edelstein, D.; Du, X.L.; Yamagishi, S.I.; Matsumura, T.; Kaneda, Y.; Yorek, M.A.; Beebe, D.; Oates, P.J.; Hammes, H.P.; et al. Normalizing mitochondrial superoxide production blocks three pathways of hyperglycaemic damage. Nature 2000, 404, 787–790. [Google Scholar] [CrossRef] [PubMed]
- Yan, S.D.; Schmidt, A.M.; Anderson, G.M.; Zhang, J.; Brett, J.; Zou, Y.S.; Pinsky, D.; Stern, D. Enhanced cellular oxidant stress by the interaction of advanced glycation end products with their receptors/binding proteins. J. Biol. Chem. 1994, 269, 9889–9897. [Google Scholar] [CrossRef]
- Abbasihormozi, S.; Babapour, V.; Kouhkan, A.; Naslji, A.N.; Afraz, K.; Zolfaghary, Z.; Shahverdi, A. Stress hormone and oxidative stress biomarkers link obesity and diabetes with reduced fertility potential. Cell J. 2019, 21, 307–313. [Google Scholar]
- Tunc, O.; Bakos, H.W.; Tremellen, K. Impact of body mass index on seminal oxidative stress. Andrologia 2011, 43, 121–128. [Google Scholar] [CrossRef] [PubMed]
- Golan, R.; Scovell, J.M.; Ramasamy, R. Age-related testosterone decline is due to waning of both testicular and hypothalamic-pituitary function. Aging Male 2015, 18, 201–204. [Google Scholar] [CrossRef] [PubMed]
- Gruenewald, D.A.; Naai, M.A.; Marck, B.T.; Matsumoto, A.M. Age-related decrease in hypothalmic gonadotropin-releasing hormone (GnRH) gene expression, but not pituitary responsiveness to GnRH, in the male brown Norway rat. J. Androl. 2000, 21, 72–84. [Google Scholar] [PubMed]
- Elmlinger, M.W.; Kühnel, W.; Wormstall, H.; Döller, P.C. Reference intervals for testosterone, androstenedione and SHBG levels in healthy females and males from birth until old age. Clin. Lab. 2005, 51, 625–632. [Google Scholar] [PubMed]
- Beattie, M.C.; Adekola, L.; Papadopoulos, V.; Chen, H.; Zirkin, B.R. Leydig cell aging and hypogonadism. Exp. Gerontol. 2015, 68, 87–91. [Google Scholar] [CrossRef]
- Yoshii, S.R.; Kuma, A.; Akashi, T.; Hara, T.; Yamamoto, A.; Kurikawa, Y.; Itakura, E.; Tsukamoto, S.; Shitara, H.; Eishi, Y.; et al. Systemic analysis of Atg5-null mice rescued from neonatal lethality by transgenic ATG5 expression in neurons. Dev. Cell 2016, 39, 116–130. [Google Scholar] [CrossRef]
- Li, W.R.; Chen, L.; Chang, Z.J.; Xin, H.; Liu, T.; Zhang, Y.Q.; Li, G.Y.; Zhou, F.; Gong, Y.Q.; Gao, Z.Z.; et al. Autophagic deficiency is related to steroidogenic decline in aged rat Leydig cells. Asian J. Androl. 2011, 13, 881–888. [Google Scholar] [CrossRef] [PubMed]
- Gao, F.; Li, G.; Liu, C.; Gao, H.; Wang, H.; Liu, W.; Chen, M.; Shang, Y.; Wang, L.; Shi, J.; et al. Autophagy regulates testosterone synthesis by facilitating cholesterol uptake in Leydig cells. J. Cell Biol. 2018, 217, 2103–2119. [Google Scholar] [CrossRef] [PubMed]
- Hales, D.B.; Allen, J.A.; Shankara, T.; Janus, P.; Buck, S.; Diemer, T.; Hales, K.H. Mitochondrial function in Leydig cell steroidogenesis. Ann. New York Acad. Sci. 2005, 1061, 120–134. [Google Scholar] [CrossRef]
- Midzak, A.S.; Chen, H.; Aon, M.A.; Papadopoulos, V.; Zirkin, B.R. ATP synthesis, mitochondrial function, and steroid biosynthesis in rodent primary and tumor Leydig cells. Biol. Reprod. 2011, 84, 976–985. [Google Scholar] [CrossRef]
- Midzak, A.S.; Chen, H.; Papadopoulos, V.; Zirkin, B.R. Leydig cell aging and the mechanisms of reduced testosterone synthesis. Mol. Cell. Endocrinol. 2009, 299, 23–31. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, F.; Ye, L.; Zirkin, B.; Chen, H. Steroidogenesis in Leydig cells: Effects of aging and environmental factors. Reproduction 2017, 154, R111–R122. [Google Scholar] [CrossRef]
- Chua, M.L.K.; Bristow, R.G. Testosterone in androgen receptor signaling and DNA repair: Enemy or frenemy? Clin. Cancer Res. 2016, 22, 3124–3126. [Google Scholar] [CrossRef]
- Gautam, D.K.; Misro, M.M.; Chaki, S.P.; Sehgal, N. H2O2 at physiological concentrations modulates Leydig cell function inducing oxidative stress and apoptosis. Apoptosis 2006, 11, 39–46. [Google Scholar] [CrossRef] [PubMed]
- Michalakis, K.; Mintziori, G.; Kaprara, A.; Tarlatzis, B.C.; Goulis, D.G. The complex interaction between obesity, metabolic syndrome and reproductive axis: A narrative review. Metabolism 2013, 62, 457–478. [Google Scholar] [CrossRef] [PubMed]
- Baker, K.; McGill, J.; Sharma, R.; Agarwal, A.; Sabanegh, E. Pregnancy after varicocelectomy: Impact of postoperative motility and DFI. Urology 2013, 81, 760–766. [Google Scholar] [CrossRef]
- Biobaku, F.; Ghanim, H.; Batra, M.; Dandona, P. Macronutrient-mediated inflammation and oxidative stress: Relevance to insulin resistance, obesity, and atherogenesis. J. Clin. Endocrinol. Metab. 2019, 104, 6118–6128. [Google Scholar] [CrossRef]
- Tan, B.L.; Norhaizan, M.E.; Liew, W.P.P. Nutrients and oxidative stress: Friend or foe? Oxid. Med. Cell. Longev. 2018, 2018, 9719584. [Google Scholar] [CrossRef]
- Vincent, H.K.; Innes, K.E.; Vincent, K.R. Oxidative stress and potential interventions to reduce oxidative stress in overweight and obesity. Diabetes Obes. Metab. 2007, 9, 813–839. [Google Scholar] [CrossRef] [PubMed]
- Pitteloud, N.; Mootha, V.K.; Dwyer, A.A.; Hardin, M.; Lee, H.; Eriksson, K.F.; Tripathy, D.; Yialamas, M.; Groop, L.; Elahi, D.; et al. Relationship between testosterone levels, insulin sensitivity, and mitochondrial function in men. Diabetes Care 2005, 28, 1636–1642. [Google Scholar] [CrossRef] [PubMed]
- Bobjer, J.; Katrinaki, M.; Tsatsanis, C.; Lundberg Giwercman, Y.; Giwercman, A. Negative association between testosterone concentration and inflammatory markers in young men: A nested cross-sectional study. PLoS ONE 2013, 8, e61466. [Google Scholar]
- Kupelian, V.; Chiu, G.R.; Araujo, A.B.; Williams, R.E.; Clark, R.V.; McKinlay, J.B. Association of sex hormones and C-reactive protein levels in men. Clin. Endocrinol. 2010, 72, 527–533. [Google Scholar] [CrossRef] [PubMed]
- Dhindsa, S.; Ghanim, H.; Batra, M.; Kuhadiya, N.D.; Abuaysheh, S.; Sandhu, S.; Green, K.; Makdissi, A.; Hejna, J.; Chaudhuri, A.; et al. Insulin resistance and inflammation in hypogonadotropic hypogonadism and their reduction after testosterone replacement in men with type 2 diabetes. Diabetes Care 2016, 39, 82–91. [Google Scholar] [CrossRef] [PubMed]
- Reuter, S.; Gupta, S.C.; Chaturvedi, M.M.; Aggarwal, B.B. Oxidative stress, inflammation, and cancer: How are they linked? Free Radic. Biol. Med. 2010, 49, 1603–1616. [Google Scholar] [CrossRef]
- Hayes, J.D.; Dinkova-Kostova, A.T.; Tew, K.D. Oxidative stress in cancer. Cancer Cell 2020, 38, 167–197. [Google Scholar] [CrossRef] [PubMed]
- Fuoco, D.; Di Tomasso, J.; Boulos, C.; Kilgour, R.D.; Morais, J.A.; Borod, M.; Vigano, A. Identifying nutritional, functional, and quality of life correlates with male hypogonadism in advanced cancer patients. Ecancermedicalscience 2015, 9, 561. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Burney, B.O.; Garcia, J.M. Hypogonadism in male cancer patients. J. Cachexia Sarcopenia Muscle 2012, 3, 149–155. [Google Scholar] [CrossRef]
- Zarotsky, V.; Huang, M.-Y.; Carman, W.; Morgentaler, A.; Singhal, P.K.; Coffin, D.; Jones, T.H. Systematic literature review of the epidemiology of nongenetic forms of hypogonadism in adult males. J. Horm. 2014, 2014, 1–17. [Google Scholar] [CrossRef]
- Ng Tang Fui, M.; Hoermann, R.; Zajac, J.D.; Grossmann, M. The effects of testosterone on body composition in obese men are not sustained after cessation of testosterone treatment. Clin. Endocrinol. 2017, 87, 336–343. [Google Scholar] [CrossRef]
- Caliber, M.; Saad, F. Testosterone therapy for prevention and treatment of obesity in men. Androg. Clin. Res. Ther. 2020, 1, 40–61. [Google Scholar] [CrossRef]
- Cunningham, G.R. Testosterone and metabolic syndrome. Asian J. Androl. 2015, 17, 192–196. [Google Scholar] [CrossRef] [PubMed]
- Li, S.Y.; Zhao, Y.L.; Yang, Y.F.; Wang, X.; Nie, M.; Wu, X.Y.; Mao, J.F. Metabolic effects of testosterone replacement therapy in patients with type 2 diabetes mellitus or metabolic syndrome: A meta-analysis. Int. J. Endocrinol. 2020, 2020, 4732021. [Google Scholar] [CrossRef]
- Gianatti, E.J.; Grossmann, M. Testosterone deficiency in men with Type 2 diabetes: Pathophysiology and treatment. Diabet. Med. 2020, 37, 174–186. [Google Scholar] [CrossRef]
- Mancini, A.; Leone, E.; Festa, R.; Grande, G.; Silvestrini, A.; De Marinis, L.; Pontecorvi, A.; Maira, G.; Littarru, G.P.; Meucci, E. Effects of testosterone on antioxidant systems in male secondary hypogonadism. J. Androl. 2008, 29, 622–629. [Google Scholar] [CrossRef]
- Hwang, T.I.S.; Liao, T.L.; Lin, J.F.; Lin, Y.C.; Lee, S.Y.; Lai, Y.C.; Kao, S.H. Low-dose testosterone treatment decreases oxidative damage in TM3 Leydig cells. Asian J. Androl. 2011, 13, 432–437. [Google Scholar] [CrossRef] [PubMed]
- Choobineh, H.; Sadighi Gilani, M.A.; Pasalar, P.; Jahanzad, I.; Ghorbani, R.; Hassanzadeh, G. The effects of testosterone on oxidative stress markers in mice with spinal cord injuries. Int. J. Fertil. Steril. 2016, 10, 87–93. [Google Scholar] [PubMed]
- Makary, S.; Abdo, M.; Fekry, E. Oxidative stress burden inhibits spermatogenesis in adult male rats: Testosterone protective effect. Can. J. Physiol. Pharmacol. 2018, 96, 372–381. [Google Scholar] [CrossRef] [PubMed]
- Tóthová, L.; Celec, P.; Ostatníková, D.; Okuliarová, M.; Zeman, M.; Hodosy, J. Effect of exogenous testosterone on oxidative status of the testes in adult male rats. Andrologia 2013, 45, 417–423. [Google Scholar] [CrossRef]
- Bhasin, S.; Brito, J.P.; Cunningham, G.R.; Hayes, F.J.; Hodis, H.N.; Matsumoto, A.M.; Snyder, P.J.; Swerdloff, R.S.; Wu, F.C.; Yialamas, M.A. Testosterone therapy in men with hypogonadism: An endocrine society. J. Clin. Endocrinol. Metab. 2018, 103, 1715–1744. [Google Scholar] [CrossRef] [PubMed]
- Cui, Y.; Zong, H.; Yan, H.; Zhang, Y. The effect of testosterone replacement therapy on prostate cancer: A systematic review and meta-analysis. Prostate Cancer Prostatic Dis. 2014, 17, 132–143. [Google Scholar] [CrossRef]
- Kardoust Parizi, M.; Abufaraj, M.; Fajkovic, H.; Kimura, S.; Iwata, T.; D’Andrea, D.; Karakiewicz, P.I.; Shariat, S.F. Oncological safety of testosterone replacement therapy in prostate cancer survivors after definitive local therapy: A systematic literature review and meta-analysis. Urol. Oncol. Semin. Orig. Investig. 2019, 37, 637–646. [Google Scholar] [CrossRef] [PubMed]
- Patel, A.S.; Leong, J.Y.; Ramos, L.; Ramasamy, R. Testosterone is a contraceptive and should not be used in men who desire fertility. World J. Men’s Health 2019, 37, 45. [Google Scholar] [CrossRef]
- Ho, C.C.K.; Tan, H.M. Treatment of the hypogonadal infertile male—A review. Sex. Med. Rev. 2013, 1, 42–49. [Google Scholar] [CrossRef] [PubMed]
- Stokes, V.J.; Anderson, R.A.; George, J.T. How does obesity affect fertility in men—And what are the treatment options? Clin. Endocrinol. 2015, 82, 633–638. [Google Scholar] [CrossRef] [PubMed]
- Saisho, Y. Metformin and inflammation: Its potential beyond glucose-lowering effect. Endocr. Metab. Immune Disord. Targets 2015, 15, 196–205. [Google Scholar] [CrossRef]
- De Araújo, A.A.; Pereira, A.D.S.B.F.; De Medeiros, C.A.C.X.; Brito, G.A.D.C.; Leitão, R.F.D.C.; Araújo, L.D.S.; Guedes, P.M.M.; Hiyari, S.; Pirih, F.Q.; De Araújo, R.F. Effects of metformin on inflammation, oxidative stress, and bone loss in a rat model of periodontitis. PLoS ONE 2017, 12, e0183506. [Google Scholar] [CrossRef] [PubMed]
- Esteghamati, A.; Eskandari, D.; Mirmiranpour, H.; Noshad, S.; Mousavizadeh, M.; Hedayati, M.; Nakhjavani, M. Effects of metformin on markers of oxidative stress and antioxidant reserve in patients with newly diagnosed type 2 diabetes: A randomized clinical trial. Clin. Nutr. 2013, 32, 179–185. [Google Scholar] [CrossRef] [PubMed]
- Seifarth, C.; Schehler, B.; Schneider, H.J. Effectiveness of metformin on weight loss in non-diabetic individuals with obesity. Exp. Clin. Endocrinol. Diabetes 2013, 121, 27–31. [Google Scholar] [CrossRef] [PubMed]
- Faure, M.; Bertoldo, M.J.; Khoueiry, R.; Bongrani, A.; Brion, F.; Giulivi, C.; Dupont, J.; Froment, P. Metformin in reproductive biology. Front. Endocrinol. 2018, 9, 675. [Google Scholar] [CrossRef]
- Nasri, H.; Rafieian-Kopaei, M. Metformin: Current knowledge. J. Res. Med. Sci. 2014, 19, 658–664. [Google Scholar]
- Desilets, A.R.; Dhakal-Karki, S.; Dunican, K.C. Role of metformin for weight management in patients without type 2 diabetes. Ann. Pharmacother. 2008, 42, 817–826. [Google Scholar] [CrossRef]
- Morgante, G.; Tosti, C.; Orvieto, R.; Musacchio, M.C.; Piomboni, P.; De Leo, V. Metformin improves semen characteristics of oligo-terato-asthenozoospermic men with metabolic syndrome. Fertil. Steril. 2011, 95, 2150–2152. [Google Scholar] [CrossRef]
- Bertoldo, M.J.; Faure, M.; Dupont, J.; Froment, P. AMPK: A master energy regulator for gonadal function. Front. Neurosci. 2015, 9, 235. [Google Scholar] [CrossRef]
- Abdou, H.S.; Bergeron, F.; Tremblay, J.J. A cell-autonomous molecular cascade initiated by amp-activated protein kinase represses steroidogenesis. Mol. Cell. Biol. 2014, 34, 4257–4271. [Google Scholar] [CrossRef]
- Tartarin, P.; Moison, D.; Guibert, E.; Dupont, J.; Habert, R.; Rouiller-Fabre, V.; Frydman, N.; Pozzi, S.; Frydman, R.; Lecureuil, C.; et al. Metformin exposure affects human and mouse fetal testicular cells. Hum. Reprod. 2012, 27, 3304–3314. [Google Scholar] [CrossRef]
- Kim, C.; Barrett-Connor, E.; Aroda, V.R.; Mather, K.J.; Christophi, C.A.; Horton, E.S.; Pi-Sunyer, X.; Bray, G.A.; Labrie, F.; Golden, S.H. Testosterone and depressive symptoms among men in the Diabetes Prevention Program. Psychoneuroendocrinology 2016, 72, 63–71. [Google Scholar] [CrossRef]
- Hu, Y.; Ding, B.; Shen, Y.; Yan, R.N.; Li, F.F.; Sun, R.; Jing, T.; Lee, K.O.; Ma, J.H. Rapid changes in serum testosterone in men with newly diagnosed type 2 diabetes with intensive insulin and metformin. Diabetes Care 2021, 44, 1059–1061. [Google Scholar] [CrossRef] [PubMed]
- Al-Kuraishy, H.M.; Al-Gareeb, A.I. Erectile dysfunction and low sex drive in men with type 2 DM: The potential role of diabetic pharmacotherapy. J. Clin. Diagn. Res. 2016, 10, FC21–FC26. [Google Scholar] [CrossRef]
- Giahi, L.; Mohammadmoradi, S.; Javidan, A.; Sadeghi, M.R. Nutritional modifications in male infertility: A systematic review covering 2 decades. Nutr. Rev. 2016, 74, 118–130. [Google Scholar] [CrossRef] [PubMed]
- Salas-Huetos, A.; Bulló, M.; Salas-Salvadó, J. Dietary patterns, foods and nutrients in male fertility parameters and fecundability: A systematic review of observational studies. Hum. Reprod. Update 2017, 23, 371–389. [Google Scholar] [CrossRef] [PubMed]
- Karayiannis, D.; Kontogianni, M.D.; Mendorou, C.; Douka, L.; Mastrominas, M.; Yiannakouris, N. Association between adherence to the Mediterranean diet and semen quality parameters in male partners of couples attempting fertility. Hum. Reprod. 2017, 32, 215–222. [Google Scholar] [CrossRef] [PubMed]
- Di Daniele, N.; Noce, A.; Vidiri, M.F.; Moriconi, E.; Marrone, G.; Annicchiarico-Petruzzelli, M.; D’Urso, G.; Tesauro, M.; Rovella, V.; Lorenzo, A. Impact of Mediterranean diet on metabolic syndrome, cancer and longevity. Oncotarget 2017, 8, 8947. [Google Scholar] [CrossRef] [PubMed]
- Key, T.J.A.; Roe, L.; Thorogood, M.; Moore, J.W.; Clark, G.M.G.; Wang, D.Y. Testosterone, sex hormone-binding globulin, calculated free testosterone, and oestradiol in male vegans and omnivores. Br. J. Nutr. 1990, 64, 111–119. [Google Scholar] [CrossRef]
- Håkonsen, L.; Thulstrup, A.; Aggerholm, A.; Olsen, J.; Bonde, J.; Andersen, C.; Bungum, M.; Ernst, E.; Hansen, M.; Ernst, E.; et al. Does weight loss improve semen quality and reproductive hormones? Results from a cohort of severely obese men. Reprod. Health 2011, 8, 24. [Google Scholar] [CrossRef] [PubMed]
- Jaffar, M.; Ashraf, M. Does weight loss improve fertility with respect to semen parameters—Results from a large cohort study. Int. J. Infertil. Fetal Med. 2017, 8, 12–17. [Google Scholar] [CrossRef]
- Camacho, E.M.; Huhtaniemi, I.T.; O’Neill, T.W.; Finn, J.D.; Pye, S.R.; Lee, D.M.; Tajar, A.; Bartfai, G.; Boonen, S.; Casanueva, F.F.; et al. Age-associated changes in hypothalamic-pituitary-testicular function in middle-aged and older men are modified by weight change and lifestyle factors: Longitudinal results from the European Male Ageing Study. Eur. J. Endocrinol. 2013, 168, 445–455. [Google Scholar] [CrossRef] [PubMed]
- Kaukua, J.; Pekkarinen, T.; Sane, T.; Mustajoki, P. Sex hormones and sexual function in obese men losing weight. Obes. Res. 2003, 11, 689–694. [Google Scholar] [CrossRef]
- Grossmann, M. Hypogonadism and male obesity: Focus on unresolved questions. Clin. Endocrinol. 2018, 89, 11–21. [Google Scholar] [CrossRef] [PubMed]
- Niskanen, L.; Laaksonen, D.E.; Punnonen, K.; Mustajoki, P.; Kaukua, J.; Rissanen, A. Changes in sex hormone-binding globulin and testosterone during weight loss and weight maintenance in abdominally obese men with the metabolic syndrome. Diabetes Obes. Metab. 2004, 6, 208–215. [Google Scholar] [CrossRef]
- Kumagai, H.; Yoshikawa, T.; Zempo-Miyaki, A.; Myoenzono, K.; Tsujimoto, T.; Tanaka, K.; Maeda, S. Vigorous physical activity is associated with regular aerobic exercise-induced increased serum testosterone levels in overweight/obese men. Horm. Metab. Res. 2018, 50, 73–79. [Google Scholar] [CrossRef] [PubMed]
- Corona, G.; Rastrelli, G.; Monami, M.; Saad, F.; Luconi, M.; Lucchese, M.; Facchiano, E.; Sforza, A.; Forti, G.; Mannucci, E.; et al. Body weight loss reverts obesity-associated hypogonadotropic hypogonadism: A systematic review and meta-analysis. Eur. J. Endocrinol. 2013, 168, 829–843. [Google Scholar] [CrossRef]
- Moatt, J.P.; Nakagawa, S.; Lagisz, M.; Walling, C.A. The effect of dietary restriction on reproduction: A meta-analytic perspective. BMC Evol. Biol. 2016, 16, 199. [Google Scholar] [CrossRef]
- Adler, M.I.; Cassidy, E.J.; Fricke, C.; Bonduriansky, R. The lifespan-reproduction trade-off under dietary restriction is sex-specific and context-dependent. Exp. Gerontol. 2013, 48, 539–548. [Google Scholar] [CrossRef]
- Kowaltowski, A.J. Caloric restriction and redox state: Does this diet increase or decrease oxidant production? Redox Rep. 2011, 16, 237–241. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Ballantyne, C.M. Metabolic inflammation and insulin resistance in obesity. Circ. Res. 2020, 1549–1564. [Google Scholar] [CrossRef]
- Huang, C.J.; McAllister, M.J.; Slusher, A.L.; Webb, H.E.; Mock, J.T.; Acevedo, E.O. Obesity-related oxidative stress: The impact of physical activity and diet manipulation. Sports Med. Open 2015, 1, 32. [Google Scholar] [CrossRef] [PubMed]
- Sitzmann, B.D.; Brown, D.I.; Garyfallou, V.T.; Kohama, S.G.; Mattison, J.A.; Ingram, D.K.; Roth, G.S.; Ottinger, M.A.; Urbanski, H.F. Impact of moderate calorie restriction on testicular morphology and endocrine function in adult rhesus macaques (Macaca mulatta). Age 2014, 36, 183–197. [Google Scholar] [CrossRef][Green Version]
- Cangemi, R.; Friedmann, A.J.; Holloszy, J.O.; Fontana, L. Long-term effects of calorie restriction on serum sex-hormone concentrations in men. Aging Cell 2010, 9, 236–242. [Google Scholar] [CrossRef]
- Wong, H.K.; Hoermann, R.; Grossmann, M. Reversible male hypogonadotropic hypogonadism due to energy deficit. Clin. Endocrinol. 2019, 91, 3–9. [Google Scholar] [CrossRef] [PubMed]
- Schulte, D.M.; Hahn, M.; Oberhäuser, F.; Malchau, G.; Schubert, M.; Heppner, C.; Müller, N.; Güdelhöfer, H.; Faust, M.; Krone, W.; et al. Caloric restriction increases serum testosterone concentrations in obese male subjects by two distinct mechanisms. Horm. Metab. Res. 2014, 46, 283–286. [Google Scholar] [CrossRef]
- Calderón, B.; Galdón, A.; Calañas, A.; Peromingo, R.; Galindo, J.; García-Moreno, F.; Rodriguez-Velasco, G.; Martín-Hidalgo, A.; Vazquez, C.; Escobar-Morreale, H.F.; et al. Effects of bariatric surgery on male obesity-associated secondary hypogonadism: Comparison of laparoscopic gastric bypass with restrictive procedures. Obes. Surg. 2014, 24, 1686–1692. [Google Scholar] [CrossRef]
- Samavat, J.; Facchiano, E.; Lucchese, M.; Forti, G.; Mannucci, E.; Maggi, M.; Luconi, M. Hypogonadism as an additional indication for bariatric surgery in male morbid obesity? Eur. J. Endocrinol. 2014, 171, 555–560. [Google Scholar] [CrossRef]
- Escobar-Morreale, H.F.; Santacruz, E.; Luque-Ramírez, M.; Carretero, J.I.B. Prevalence of “obesity-associated gonadal dysfunction” in severely obese men and women and its resolution after bariatric surgery: A systematic review and meta-analysis. Hum. Reprod. Update 2017, 23, 390–408. [Google Scholar] [CrossRef] [PubMed]
- Pellitero, S.; Olaizola, I.; Alastrue, A.; Martínez, E.; Granada, M.L.; Balibrea, J.M.; Moreno, P.; Serra, A.; Navarro-Díaz, M.; Romero, R.; et al. Hypogonadotropic hypogonadism in morbidly obese males is reversed after bariatric surgery. Obes. Surg. 2012, 22, 1835–1842. [Google Scholar] [CrossRef] [PubMed]
- Rao, S.R.; Kini, S.; Tamler, R. Sex hormones and bariatric surgery in men. Gend. Med. 2011, 8, 300–311. [Google Scholar] [CrossRef]
- Xu, J.; Wu, Q.; Zhang, Y.; Pei, C. Effect of bariatric surgery on male sexual function: A meta-analysis and systematic review. Sex. Med. 2019, 7, 270–281. [Google Scholar] [CrossRef]
- Fariello, R.M.; de Carvalho, R.C.; Spaine, D.M.; Andretta, R.R.; Caetano, E.M.; Sá, G.P.D.; Cedenho, A.P.; Fraietta, R. Analysis of the functional aspects of sperm and testicular oxidative stress in individuals undergoing metabolic surgery. Obes. Surg. 2021, 31, 2887–2895. [Google Scholar] [CrossRef] [PubMed]
- Rigon, F.A.; Ronsoni, M.F.; Hohl, A.; van de Sande-Lee, S. Effects of bariatric surgery in male obesity-associated hypogonadism. Obes. Surg. 2019, 29, 2115–2125. [Google Scholar] [CrossRef] [PubMed]
- Schmatz, R.; Bitencourt, M.R.; Patias, L.D.; Beck, M.; Alvarez, G.D.C.; Zanini, D.; Gutierres, J.M.; Diehl, L.N.; Pereira, L.B.; Leal, C.A.; et al. Evaluation of the biochemical, inflammatory and oxidative profile of obese patients given clinical treatment and bariatric surgery. Clin. Chim. Acta 2017, 465, 72–79. [Google Scholar] [CrossRef] [PubMed]
- Choromańska, B.; Myśliwiec, P.; Łuba, M.; Wojskowicz, P.; Myśliwiec, H.; Choromańska, K.; Żendzian-Piotrowska, M.; Dadan, J.; Zalewska, A.; Maciejczyk, M. Impact of weight loss on the total antioxidant/oxidant potential in patients with morbid obesity—A longitudinal study. Antioxidants 2020, 9, 376. [Google Scholar] [CrossRef]
- Jiang, H.W.; Zhou, Y.; Zhou, P.Y.; Zhang, T.Y.; Hu, J.Y.; Bai, X.T. Protective effects of bariatric surgery on kidney functions by inhibiting oxidative stress responses through activating PPARα in rats with diabetes. Front. Physiol. 2021, 12, 662666. [Google Scholar] [CrossRef] [PubMed]
- Fejfer, K.; Buczko, P.; Niczyporuk, M.; Ładny, J.R.; Hady, H.R.; Knaś, M.; Waszkiel, D.; Klimiuk, A.; Zalewska, A.; Maciejczyk, M. Oxidative modification of biomolecules in the nonstimulated and stimulated saliva of patients with morbid obesity treated with bariatric surgery. BioMed Res. Int. 2017, 2017, 4923769. [Google Scholar] [CrossRef] [PubMed]
- Ferraz-Bannitz, R.; Welendorf, C.R.; Coelho, P.O.; Salgado, W.; Nonino, C.B.; Beraldo, R.A.; Foss-Freitas, M.C. Bariatric surgery can acutely modulate ER-stress and inflammation on subcutaneous adipose tissue in non-diabetic patients with obesity. Diabetol. Metab. Syndr. 2021, 13, 19. [Google Scholar] [CrossRef]
- Da Silva, V.R.G.; Moreira, E.A.M.; Wilhelm-Filho, D.; De Miranda, J.X.; Benincá, J.P.; Vigil, S.V.G.; Moratelli, A.M.B.; Garlet, T.R.; De Souza Meirelles, M.S.; Vannucchi, H.; et al. Proinflammatory and oxidative stress markers in patients submitted to Roux-en-Y gastric bypass after 1 year of follow-up. Eur. J. Clin. Nutr. 2012, 66, 891–899. [Google Scholar] [CrossRef] [PubMed]
- Peng, C.; Wang, X.; Chen, J.; Jiao, R.; Wang, L.; Li, Y.M.; Zuo, Y.; Liu, Y.; Lei, L.; Ma, K.Y.; et al. Biology of ageing and role of dietary antioxidants. BioMed Res. Int. 2014, 2014, 831841. [Google Scholar] [CrossRef] [PubMed]
- Akbari, M.; Ostadmohammadi, V.; Tabrizi, R.; Mobini, M.; Lankarani, K.B.; Moosazadeh, M.; Heydari, S.T.; Chamani, M.; Kolahdooz, F.; Asemi, Z. The effects of alpha-lipoic acid supplementation on inflammatory markers among patients with metabolic syndrome and related disorders: A systematic review and meta-analysis of randomized controlled trials. Nutr. Metab. 2018, 15, 39. [Google Scholar] [CrossRef]
- Fan, L.; Feng, Y.; Chen, G.C.; Qin, L.Q.; Fu, C.L.; Chen, L.H. Effects of coenzyme Q10 supplementation on inflammatory markers: A systematic review and meta-analysis of randomized controlled trials. Pharmacol. Res. 2017, 119, 128–136. [Google Scholar] [CrossRef] [PubMed]
- Brie, D.; Sahebkar, A.; Penson, P.E.; Dinca, M.; Ursoniu, S.; Serban, M.C.; Zanchetti, A.; Howard, G.; Ahmed, A.; Aronow, W.S.; et al. Effects of pentoxifylline on inflammatorymarkers and blood pressure: A systematic reviewandmeta-analysis of randomized controlled trials. J. Hypertens. 2016, 34, 2318–2329. [Google Scholar] [CrossRef]
- Martins Gregório, B.; Benchimol De Souza, D.; Amorim de Morais Nascimento, F.; Matta, L.; Fernandes-Santos, C. The potential role of antioxidants in metabolic syndrome. Curr. Pharm. Des. 2016, 22, 859–869. [Google Scholar] [CrossRef]
- Kooti, W.; Farokhipour, M.; Asadzadeh, Z.; Ashtary-Larky, D.; Asadi-Samani, M. The role of medicinal plants in the treatment of diabetes: A systematic review. Electron. Physician 2016, 8, 1832–1842. [Google Scholar] [CrossRef] [PubMed]
- Alizadeh, F.; Javadi, M.; Karami, A.A.; Gholaminejad, F.; Kavianpour, M.; Haghighian, H.K. Curcumin nanomicelle improves semen parameters, oxidative stress, inflammatory biomarkers, and reproductive hormones in infertile men: A randomized clinical trial. Phyther. Res. 2018, 32, 514–521. [Google Scholar] [CrossRef]
- Qin, F.; Shen, T.; Cao, H.; Qian, J.; Zou, D.; Ye, M.; Pei, H. CeO2NPs relieve radiofrequency radiation, improve testosterone synthesis, and clock gene expression in Leydig cells by enhancing antioxidation. Int. J. Nanomed. 2019, 14, 4601. [Google Scholar] [CrossRef]
- Yahyavy, S.; Valizadeh, A.; Saki, G.; Khorsandi, L. Taurine ameliorates cytotoxic effects of Di(2-ethylhexyl) phthalate on Leydig cells. Andrologia 2021, 53, e14146. [Google Scholar] [CrossRef]
- Opuwari, C.S.; Matshipi, M.N.; Phaahla, M.K.; Setumo, M.A.; Moraswi, R.T.; Zitha, A.A.; Offor, U.; Choma, S.S.R. Androgenic effect of aqueous leaf extract of Moringa oleifera on Leydig TM3 cells in vitro. Andrologia 2020, 52, e13825. [Google Scholar] [CrossRef] [PubMed]
- Jambor, T.; Arvay, J.; Ivanisova, E.; Tvrda, E.; Kovacik, A.; Greifova, H.; Lukac, N. Investigation of the properties and effects of Salvia Officinalis L. on the viability, steroidogenesis and reactive oxygen species (ROS) production in TM3 leydig cells in vitro. Physiol. Res. 2020, 69, 661–673. [Google Scholar] [CrossRef]
- Wang, J.Y.; Lee, Y.J.; Chou, M.C.; Chang, R.; Chiu, C.H.; Liang, Y.J.; Wu, L.S. Astaxanthin protects steroidogenesis from hydrogen peroxide-induced oxidative stress in mouse Leydig cells. Mar. Drugs 2015, 13, 1375–1388. [Google Scholar] [CrossRef]
- Deng, S.L.; Zhang, B.L.; Reiter, R.J.; Liu, Y.X. Melatonin ameliorates inflammation and oxidative stress by suppressing the P38MAPK signaling pathway in LPS-induced sheep orchitis. Antioxidants 2020, 9, 1277. [Google Scholar] [CrossRef]
- Greifová, H.; Jambor, T.; Tokárová, K.; Speváková, I.; Knížatová, N.; Lukáč, N. Resveratrol attenuates hydrogen peroxide-induced oxidative stress in TM3 Leydig cells in vitro. J. Environ. Sci. Health Part A Toxic Hazard. Subst. Environ. Eng. 2020, 55, 585–595. [Google Scholar] [CrossRef]
- Ma, J.; Yang, H.; Liu, L.; Wan, Y.; Wang, F. Melatonin alleviated oxidative stress induced by energy restriction on sheep Leydig cells through Sirt1/Sod2 pathway. Theriogenology 2021, 173, 83–92. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, B.; Chakraborty, S.; Chakraborty, P.; Ghosh, D.; Jana, K. Protective effect of resveratrol on benzo(a)pyrene induced dysfunctions of steroidogenesis and steroidogenic acute regulatory gene expression in Leydig cells. Front. Endocrinol. 2019, 10, 272. [Google Scholar] [CrossRef]
- Sun, J.; Wang, H.; Liu, B.; Shi, W.; Shi, J.; Zhang, Z.; Xing, J. Rutin attenuates H2O2-induced oxidation damage and apoptosis in Leydig cells by activating PI3K/Akt signal pathways. Biomed. Pharmacother. 2017, 88, 500–506. [Google Scholar] [CrossRef]
- Hu, J.; Yu, Q.; Zhao, F.; Ji, J.; Jiang, Z.; Chen, X.; Gao, P.; Ren, Y.; Shao, S.; Zhang, L.; et al. Protection of quercetin against triptolide-induced apoptosis by suppressing oxidative stress in rat Leydig cells. Chem. Biol. Interact. 2015, 240, 38–46. [Google Scholar] [CrossRef]
- Chang, M.S.; Kim, W.N.; Yang, W.M.; Kim, H.Y.; Oh, J.H.; Park, S.K. Cytoprotective effects of Morinda officinalis against hydrogen peroxide-induced oxidative stress in Leydig TM3 cells. Asian J. Androl. 2008, 10, 667–674. [Google Scholar] [CrossRef] [PubMed]
- Murugesan, P.; Muthusamy, T.; Balasubramanian, K.; Arunakaran, J. Studies on the protective role of vitamin C and E against polychlorinated biphenyl (Aroclor 1254)-Induced oxidative damage in Leydig cells. Free Radic. Res. 2005, 39, 1259–1272. [Google Scholar] [CrossRef]
- Bashandy, S.A.E.M.; Omara, E.A.A.; Ebaid, H.; Amin, M.M.; Soliman, M.S. Role of zinc as an antioxidant and anti-inflammatory to relieve cadmium oxidative stress induced testicular damage in rats. Asian Pac. J. Trop. Biomed. 2016, 6, 1056–1064. [Google Scholar] [CrossRef]
- Aggarwal, A.; Misro, M.M.; Maheshwari, A.; Sehgal, N. Differential modulation of apoptotic gene expression by N-acetyl-l-cysteine in Leydig cells stimulated persistently with hCG in vivo. Mol. Cell. Endocrinol. 2012, 348, 155–164. [Google Scholar] [CrossRef]
- Gao, T.; Lin, M.; Shao, B.; Zhou, Q.; Wang, Y.; Chen, X.; Zhao, D.; Dai, X.; Shen, C.; Cheng, H.; et al. BMI1 promotes steroidogenesis through maintaining redox homeostasis in mouse MLTC-1 and primary Leydig cells. Cell Cycle 2020, 19, 1884–1898. [Google Scholar] [CrossRef]
- Altındağ, F.; Meydan, İ. Evaluation of protective effects of gallic acid on cisplatin-induced testicular and epididymal damage. Andrologia 2021, 53, e14189. [Google Scholar] [CrossRef] [PubMed]
- Elumalai, P.; Krishnamoorthy, G.; Selvakumar, K.; Arunkumar, R.; Venkataraman, P.; Arunakaran, J. Studies on the protective role of lycopene against polychlorinated biphenyls (Aroclor 1254)-induced changes in StAR protein and cytochrome P450 scc enzyme expression on Leydig cells of adult rats. Reprod. Toxicol. 2009, 27, 41–45. [Google Scholar] [CrossRef] [PubMed]
- Girish, B.P.; Reddy, P.S. Forskolin ameliorates mancozeb-induced testicular and epididymal toxicity in Wistar rats by reducing oxidative toxicity and by stimulating steroidogenesis. J. Biochem. Mol. Toxicol. 2018, 32, e22026. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.J.; Wang, Q.; Lv, Z.M.; Wang, C.L.; Li, C.P.; Rong, Y.L. Resveratrol appears to protect against oxidative stress and steroidogenesis collapse in mice fed high-calorie and high-cholesterol diet. Andrologia 2015, 47, 59–65. [Google Scholar] [CrossRef] [PubMed]
- Mosbah, R.; Yousef, M.I.; Maranghi, F.; Mantovani, A. Protective role of Nigella sativa oil against reproductive toxicity, hormonal alterations, and oxidative damage induced by chlorpyrifos in male rats. Toxicol. Ind. Health 2016, 32, 1266–1277. [Google Scholar] [CrossRef]
- Jeong, H.C.; Jeon, S.H.; Guan Qun, Z.; Bashraheel, F.; Choi, S.W.; Kim, S.J.; Bae, W.J.; Cho, H.J.; Ha, U.S.; Hong, S.H.; et al. Lycium chinense Mill improves hypogonadism via anti-oxidative stress and anti-apoptotic effect in old aged rat model. Aging Male 2020, 23, 287–296. [Google Scholar] [CrossRef]
- Alotaibi, B.; El-Masry, T.A.; Tousson, E.; Alarfaj, S.J.; Saleh, A. Therapeutic effects of rocket seeds (Eruca sativa L.) against testicular toxicity and oxidative stress caused by silver nanoparticles injection in rats. Environ. Toxicol. 2020, 35, 952–960. [Google Scholar]
- Abd, H.H.; Ahmed, H.A.; Mutar, T.F. Moringa oleifera leaves extract modulates toxicity, sperms alterations, oxidative stress, and testicular damage induced by tramadol in male rats. Toxicol. Res. 2020, 9, 101–106. [Google Scholar] [CrossRef] [PubMed]
- Karna, K.K.; Choi, B.R.; Kim, M.J.; Kim, H.K.; Park, J.K. The effect of Schisandra chinensis baillon on crosstalk between oxidative stress, endoplasmic reticulum stress, and mitochondrial signaling pathway in testes of varicocele-induced SD rat. Int. J. Mol. Sci. 2019, 20, 5785. [Google Scholar] [CrossRef]
- Choi, S.W.; Jeon, S.H.; Kwon, E.B.; Zhu, G.Q.; Lee, K.W.; Choi, J.B.; Jeong, H.C.; Kim, K.S.; Bae, S.R.; Bae, W.J.; et al. Effect of Korean herbal formula (modified Ojayeonjonghwan) on androgen receptor expression in an aging rat model of late onset hypogonadism. World J. Men’s Health 2019, 37, 105–112. [Google Scholar] [CrossRef] [PubMed]
- Bae, W.J.; Zhu, G.Q.; Choi, S.W.; Jeong, H.C.; Bashraheel, F.; Kim, K.S.; Kim, S.J.; Cho, H.J.; Ha, U.S.; Hong, S.H.; et al. Antioxidant and antifibrotic effect of a herbal formulation in vitro and in the experimental andropause via nrf2/ho-1 signaling pathway. Oxid. Med. Cell. Longev. 2017, 2017, 6024839. [Google Scholar] [CrossRef]
- Zhang, K.; Fu, L.; An, Q.; Hu, W.; Liu, J.; Tang, X.; Ding, Y.; Lu, W.; Liang, X.; Shang, X.; et al. Effects of Qilin pills on spermatogenesis, reproductive hormones, oxidative stress, and the TSSK2 gene in a rat model of oligoasthenospermia. BMC Complement. Med. Ther. 2020, 20, 42. [Google Scholar] [CrossRef]
- Zhu, Y.; Tchkonia, T.; Pirtskhalava, T.; Gower, A.C.; Ding, H.; Giorgadze, N.; Palmer, A.K.; Ikeno, Y.; Hubbard, G.B.; Lenburg, M.; et al. The achilles’ heel of senescent cells: From transcriptome to senolytic drugs. Aging Cell 2015, 14, 644–658. [Google Scholar] [CrossRef]
- Dookun, E.; Passos, J.F.; Arthur, H.M.; Richardson, G.D. Therapeutic potential of senolytics in cardiovascular disease. Cardiovasc. Drugs Ther. 2020, 1–10. [Google Scholar] [CrossRef]
- Kaur, A.; Macip, S.; Stover, C.M. An appraisal on the value of using nutraceutical based senolytics and senostatics in aging. Front. Cell Dev. Biol. 2020, 8, 218. [Google Scholar] [CrossRef]
- Li, W.; Qin, L.; Feng, R.; Hu, G.; Sun, H.; He, Y.; Zhang, R. Emerging senolytic agents derived from natural products. Mech. Ageing Dev. 2019, 181, 1–6. [Google Scholar] [CrossRef]
- Zhang, C.; Xie, Y.; Chen, H.; Lv, L.; Yao, J.; Zhang, M.; Xia, K.; Feng, X.; Li, Y.; Liang, X.; et al. FOXO4-DRI alleviates age-related testosterone secretion insufficiency by targeting senescent Leydig cells in aged mice. Aging 2020, 12, 1272–1284. [Google Scholar] [CrossRef]
- Leisegang, K. Herbal pharmacognosy: An introduction. In Herbal Medicine in Andrology; Academic Press: Cambridge, MA, USA, 2021; pp. 17–26. [Google Scholar]
- Pérez-Torres, I.; Castrejón-Téllez, V.; Soto, M.E.; Rubio-Ruiz, M.E.; Manzano-Pech, L.; Guarner-Lans, V. Oxidative stress, plant natural antioxidants, and obesity. Int. J. Mol. Sci. 2021, 22, 1786. [Google Scholar] [CrossRef]
- Mitjavila, M.T.; Moreno, J.J. The effects of polyphenols on oxidative stress and the arachidonic acid cascade. Implications for the prevention/treatment of high prevalence diseases. Biochem. Pharmacol. 2012, 84, 1113–1122. [Google Scholar] [CrossRef] [PubMed]
- Nazarian-Samani, Z.; Sewell, R.D.E.; Lorigooini, Z.; Rafieian-Kopaei, M. Medicinal plants with multiple effects on diabetes mellitus and its complications: A systematic review. Curr. Diab. Rep. 2018, 18, 72. [Google Scholar] [CrossRef]
- Karunakaran, U.; Park, K.G. A systematic review of oxidative stress and safety of antioxidants in diabetes: Focus on islets and their defense. Diabetes Metab. J. 2013, 37, 106–112. [Google Scholar] [CrossRef]
- Alahmadi, B. Effect of herbal medicine on fertility potential in experimental animals—An update review. Mater. Socio-Med. 2020, 32, 140. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, A.; Majzoub, A.; Baskaran, S.; Selvam, M.K.P.; Cho, C.L.C.L.; Henkel, R.; Finelli, R.; Leisegang, K.; Sengupta, P.; Barbarosie, C.; et al. Sperm DNA fragmentation: A new guideline for clinicians. World J. Men’s Health 2020, 38, 412–471. [Google Scholar] [CrossRef]
- Smith, S.J.; Lopresti, A.L.; Teo, S.Y.M.; Fairchild, T.J. Examining the effects of herbs on testosterone concentrations in men: A systematic review. Adv. Nutr. 2021, 12, 744–765. [Google Scholar] [CrossRef]
- Leisegang, K.; Finelli, R. Alternative medicine and herbal remedies in the treatment of erectile dysfunction: A systematic review. Arab J. Urol. 2021, 19, 323–339. [Google Scholar] [CrossRef]
- Martin, L.J.; Touaibia, M. Improvement of testicular steroidogenesis using flavonoids and isoflavonoids for prevention of late-onset male hypogonadism. Antioxidants 2020, 9, 237. [Google Scholar] [CrossRef] [PubMed]
- Halliwell, B. The antioxidant paradox: Less paradoxical now? Br. J. Clin. Pharmacol. 2013, 75, 637–644. [Google Scholar] [CrossRef] [PubMed]
- Kurutas, E.B. The importance of antioxidants which play the role in cellular response against oxidative/nitrosative stress: Current state. Nutr. J. 2016, 15, 71. [Google Scholar] [CrossRef] [PubMed]
- Henkel, R.; Sandhu, I.S.; Agarwal, A. The excessive use of antioxidant therapy: A possible cause of male infertility? Andrologia 2019, 51, e13162. [Google Scholar] [CrossRef]
- Mentor, S.; Fisher, D. Aggressive Antioxidant Reductive Stress Impairs Brain Endothelial Cell Angiogenesis and Blood Brain Barrier Function. Curr. Neurovasc. Res. 2016, 14, 71–81. [Google Scholar] [CrossRef] [PubMed]
- Singh, F.; Charles, A.L.; Schlagowski, A.I.; Bouitbir, J.; Bonifacio, A.; Piquard, F.; Krähenbühl, S.; Geny, B.; Zoll, J. Reductive stress impairs myoblasts mitochondrial function and triggers mitochondrial hormesis. Biochim. Biophys. Acta Mol. Cell Res. 2015, 1853, 1574–1585. [Google Scholar] [CrossRef]
| Enzymatic | Nonenzymatic |
|---|---|
| Superoxide dismutase (SOD) | Vitamin C, vitamin E, vitamin B9 |
| Catalase (CAT) | Selenium, Zinc, Mn2+ |
| Glutathione peroxidase (GPx) | Carotenoids, flavonoids, lycopene |
| Glutathione reductase (GR) | Taurine, hypotaurine |
| Glutathione-S-transferase (GST) | Glutathione, inositol, cysteine, coenzyme Q10 |
| Thioredoxin |
| Management Strategy | Obesity | Metabolic Syndrome | Diabetes | Hypo-gonadism | Oxidative Stress |
|---|---|---|---|---|---|
| TRT | Improves [86,87] | Improves [88,89] | Improves [88,89] | Improves [85] | Unclear |
| Metformin | Improves [102,103] | Improves [102,103] | Improves [102,103] | Unclear | Improves [102,103] |
| Caloric restriction | Improves [74,132,133] | Improves [74,132,133] | Improves [74,132,133] | Unclear | Improves [129,130] |
| Bariatric surgery | Improves [138,139,140,141] | Improves [138,139,140,141] | Improves [138,139,140,141] | Improves [138] | Improves [146,147] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Leisegang, K.; Roychoudhury, S.; Slama, P.; Finelli, R. The Mechanisms and Management of Age-Related Oxidative Stress in Male Hypogonadism Associated with Non-communicable Chronic Disease. Antioxidants 2021, 10, 1834. https://doi.org/10.3390/antiox10111834
Leisegang K, Roychoudhury S, Slama P, Finelli R. The Mechanisms and Management of Age-Related Oxidative Stress in Male Hypogonadism Associated with Non-communicable Chronic Disease. Antioxidants. 2021; 10(11):1834. https://doi.org/10.3390/antiox10111834
Chicago/Turabian StyleLeisegang, Kristian, Shubhadeep Roychoudhury, Petr Slama, and Renata Finelli. 2021. "The Mechanisms and Management of Age-Related Oxidative Stress in Male Hypogonadism Associated with Non-communicable Chronic Disease" Antioxidants 10, no. 11: 1834. https://doi.org/10.3390/antiox10111834
APA StyleLeisegang, K., Roychoudhury, S., Slama, P., & Finelli, R. (2021). The Mechanisms and Management of Age-Related Oxidative Stress in Male Hypogonadism Associated with Non-communicable Chronic Disease. Antioxidants, 10(11), 1834. https://doi.org/10.3390/antiox10111834








