Impact of Fennel Essential Oil as an Antibiotic Alternative in Rabbit Diet on Antioxidant Enzymes Levels, Growth Performance, and Meat Quality
Abstract
1. Introduction
2. Materials and Methods
2.1. Fennel Essential Oil Preparation and Characterization
2.2. Biological System, Experimental Design, and Environmental Data
2.2.1. Growth Performance
2.2.2. Collection of Samples for Blood Hematological and Biochemical Parameters Evaluation
2.2.3. Analysis of Meat Quality Parameters
2.2.4. Antioxidant Activities
2.2.5. RNA Isolation and cDNA Synthesis
2.2.6. Quantitative Histomorphometric Analysis of Jejunum Segments
2.2.7. Villus Morphology and Morphometry
2.2.8. Statistical Analysis
3. Results
3.1. Chemical Composition of Fennel Oil
3.2. Growth Performance, Feed Intake Feed Conversion Ratio, and Carcass Traits
3.3. Physical Characteristics and Microbial Abundance of MLD Muscle
3.4. Blood Hematological and Biochemical
3.5. Antioxidant Enzyme Activity
3.6. Villus Morphology and Morphometry
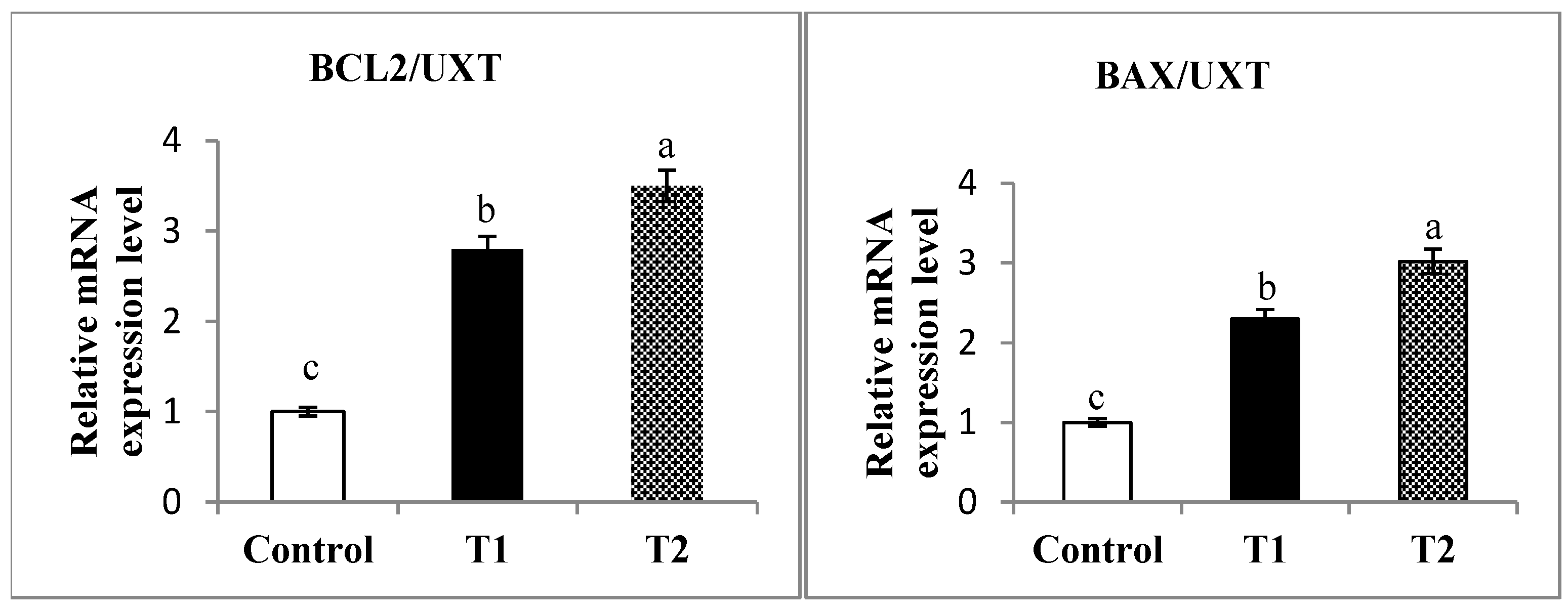
3.7. Antioxidant and Apoptosis Genes Expression
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bhatt, R.S.; Agrawal, A.R.; Sahoo, A. Effect of probiotic supplementation on growth performance, nutrient utilization and carcass characteristics of growing Chinchilla rabbits. J. Appl. Anim. Res. 2017, 45, 304–309. [Google Scholar] [CrossRef]
- Cesari, V.; Toschi, I.; Ferrazzi, V.; Cesari, N.; Grilli, G.; Lavazza, A. Effect of weaning age and diet on growth performance, caecal characteristics and potential pathogenetic microflora in rabbits. Ital. J. Anim. Sci. 2007, 6, 755–757. [Google Scholar] [CrossRef]
- Ali, B. Agents ameliorating or augmenting experimental gentamicin nephrotoxicity: Some recent research. Food Chem. Toxicol. 2003, 41, 1447–1452. [Google Scholar] [CrossRef]
- Khaki, A.; Novin, M.G.; Khaki, A.A.; Nouri, M.; Sanati, E.; Nikmanesh, M. Comparative Study of the Effects of Gentamicin, Neomycin, Streptomycin and Ofloxacin Antibiotics on Sperm Parameters and Testis Apoptosis in Rats. Pak. J. Biol. Sci. 2008, 11, 1683–1689. [Google Scholar] [CrossRef]
- Falcão-e-Cunha, L.; Castro-Solla, L.; Maertens, L.; Marounek, M.; Pinheiro, V.; Freire, J.; Mourão, J.L. Alternatives to antibiotic growth promoters in rabbit feeding: A review. World Rabbit. Sci. 2007, 15, 127–140. [Google Scholar] [CrossRef]
- Maertens, L.L.C. Strategies to reduce antibiotic use in rabbit production. J. Agric. Sci. Technol. 2011, 1, 783–792. [Google Scholar]
- Behrooz Lak, M.A.; Hassan Abadi, A.; Nasiri Moghadam, H.; Kermanshahi, H. Effect of different levels of Cinnamon Powder, with Antibiotic and Probiotic on Performance and Carcass characteristics of Broiler Chickens. Res. Anim. Prod. 2014, 5, 25–35. [Google Scholar]
- Khan, R.; Naz, S.; Nikousefat, Z.; Tufarelli, V.; Laudadio, V. Thymus vulgaris: Alternative to antibiotics in poultry feed. World’s Poult. Sci. J. 2012, 68, 401–408. [Google Scholar] [CrossRef]
- Ashour, E.A.; El-Hack, M.E.A.; Alagawany, M.; Swelum, A.A.; Osman, A.O.; Saadeldin, I.; Abdel-Hamid, M.; Hussein, E.-S.O. Use of Whey Protein Concentrates in Broiler Diets. J. Appl. Poult. Res. 2019, 28, 1078–1088. [Google Scholar] [CrossRef]
- Kishawy, A.T.Y.; Amer, S.A.; Osman, A.; Elsayed, S.A.M.; El-Hack, M.E.A.; Swelum, A.A.; Ba-Awadh, H.; Saadeldin, I.M. Impacts of supplementing growing rabbit diets with whey powder and citric acid on growth performance, nutrient digestibility, meat and bone analysis, and gut health. AMB Express 2018, 8, 86. [Google Scholar] [CrossRef]
- Omar, A.E.; Al-Khalaifah, H.S.; Mohamed, W.A.M.; Gharib, H.S.A.; Osman, A.; Al-Gabri, N.A.; Amer, S. Effects of Phenolic-Rich Onion (Allium cepa L.) Extract on the Growth Performance, Behavior, Intestinal Histology, Amino Acid Digestibility, Antioxidant Activity, and the Immune Status of Broiler Chickens. Front. Veter Sci. 2020, 7, 728. [Google Scholar] [CrossRef] [PubMed]
- Osman, A.; Bin-Jumah, M.; El-Hack, M.E.A.; Elaraby, G.; Swelum, A.A.; Taha, A.E.; Sitohy, M.; Allam, A.; Ashour, E.A. Dietary supplementation of soybean glycinin can alter the growth, carcass traits, blood biochemical indices, and meat quality of broilers. Poult. Sci. 2020, 99, 820–828. [Google Scholar] [CrossRef] [PubMed]
- Amer, S.; Ahmed, S.A.; Ibrahim, R.E.; Al-Gabri, N.A.; Osman, A.; Sitohy, M. Impact of partial substitution of fish meal by methylated soy protein isolates on the nutritional, immunological, and health aspects of Nile tilapia, Oreochromis niloticus fingerlings. Aquaculture 2020, 518, 734871. [Google Scholar] [CrossRef]
- El-Araby, D.A.; Amer, S.A.; Attia, G.A.; Osman, A.; Fahmy, E.M.; Altohamy, D.E.; Alkafafy, M.; Elakkad, H.A.; Tolba, S.A. Dietary Spirulina platensis phycocyanin improves growth, tissue histoarchitecture, and immune responses, with modulating immunoexpression of CD3 and CD20 in Nile tilapia, Oreochromis niloticus. Aquaculture 2021, 546, 737413. [Google Scholar] [CrossRef]
- Amer, S.A.; Osman, A.; Al-Gabri, N.A.; Elsayed, S.A.M.; El-Rahman, G.I.A.; Elabbasy, M.T.; Ahmed, S.A.A.; Ibrahim, R.E. The Effect of Dietary Replacement of Fish Meal with Whey Protein Concentrate on the Growth Performance, Fish Health, and Immune Status of Nile tilapia Fingerlings, Oreochromis niloticus. Animals 2019, 9, 1003. [Google Scholar] [CrossRef]
- Zeng, Z.; Zhang, S.; Wang, H.; Piao, X. Essential oil and aromatic plants as feed additives in non-ruminant nutrition: A review. J. Anim. Sci. Biotechnol. 2015, 6, 7. [Google Scholar] [CrossRef]
- Yang, C.; Chowdhury, M.A.K.; Huo, Y.; Gong, J. Phytogenic Compounds as Alternatives to In-Feed Antibiotics: Potentials and Challenges in Application. Pathogens 2015, 4, 137–156. [Google Scholar] [CrossRef] [PubMed]
- Stoni, A.; Zitterl-Egelseer, K.; Kroismayr, A.; Wetscherek, W.; Windisch, W. Tissue recovery of essential oils used as feed additive in piglet feeding and impact on nutrient digestibility. Proc. Soc. Nutr. Physiol. 2006, 15, 60. [Google Scholar]
- Stevanović, Z.D.; Bošnjak-Neumüller, J.; Pajić-Lijaković, I.; Raj, J.; Vasiljević, M. Essential Oils as Feed Additives—Future Perspectives. Molecules 2018, 23, 1717. [Google Scholar] [CrossRef]
- Calo, J.R.; Crandall, P.G.; O’Bryan, C.A.; Ricke, S.C. Essential oils as antimicrobials in food systems—A review. Food Control. 2015, 54, 111–119. [Google Scholar] [CrossRef]
- Hammouda, F.; Saleh, M.; Abdel-Azim, N.; Shams, K.; Ismail, S.; Shahat, A.; Saleh, I. Evaluation Of The Essential Oil Of Foeniculum Vulgare Mill (Fennel) Fruits Extracted By Three Different Extraction Methods By Gc/Ms. Afr. J. Tradit. Complement. Altern. Med. 2014, 11, 277–279. [Google Scholar] [CrossRef] [PubMed]
- Van Wyk, B.E.; Wink, M. Medicinal Plants of the World; CABI: Oxfordshire, UK, 2018. [Google Scholar]
- Roby, M.; Sarhan, M.A.; Selim, K.A.-H.; Khalel, K.I. Antioxidant and antimicrobial activities of essential oil and extracts of fennel (Foeniculum Vulgare L.) and chamomile (Matricaria chamomilla L.). Ind. Crop. Prod. 2013, 44, 437–445. [Google Scholar] [CrossRef]
- El Ouariachi, E.; Lahhit, N.; Bouyanzer, A.; Hammouti, B.; Paolini, J.; Majidi, L.; Desjobert, J.M.; Costa, J. Chemical composition and antioxidant activity of essential oils and solvent extracts of Foeniculum Vulgare Mill. from Morocco. J. Chem. Pharm. Res. 2014, 6, 743–748. [Google Scholar]
- Hassaan, M.S.; Soltan, M. Evaluation of Essential Oil of Fennel and Garlic Separately or Combined with Bacillus licheniformis on the Growth, Feeding Behaviour, Hemato-biochemical Indices of Oreochromis niloticus (L.) Fry. J. Aquac. Res. Dev. 2016, 7, 422–429. [Google Scholar] [CrossRef]
- Shahat, A.; Ibrahim, A.Y.; Hendawy, S.F.; Omer, E.; Hammouda, F.; Abdel-Rahman, F.H.; Saleh, M.A. Chemical Composition, Antimicrobial and Antioxidant Activities of Essential Oils from Organically Cultivated Fennel Cultivars. Molecules 2011, 16, 1366–1377. [Google Scholar] [CrossRef] [PubMed]
- Rather, M.A.; Dar, B.A.; Sofi, S.N.; Bhat, B.A.; Qurishi, M.A. Foeniculum Vulgare: A comprehensive review of its traditional use, phytochemistry, pharmacology, and safety. Arab. J. Chem. 2016, 9, S1574–S1583. [Google Scholar] [CrossRef]
- Omer, H.A.; El-Nomeary, Y.A.; El-Kady, R.I.; Badr, A.M.; Ali, F.A.; Ahmed, S.M.; El-Allawy, H.M.; Ibrahim, S.A. Improving the utilization of rabbit diets containing vegetable oil by using fennel (Foeniculum Vulgare) and oregano (Origanum vulgare L.) as feed additives. Life Sci. J. 2013, 10, 2625–2636. [Google Scholar]
- Mandras, N.; Roana, J.; Scalas, D.; Del Re, S.; Cavallo, L.; Ghisetti, V.; Tullio, V. The Inhibition of Non-albicans Candida Species and Uncommon Yeast Pathogens by Selected Essential Oils and Their Major Compounds. Molecules 2021, 26, 4937. [Google Scholar] [CrossRef]
- Iraqi, M.; García, M.; Khalil, M.; Baselga, M. Evaluation of milk yield and some related maternal traits in a crossbreeding project of Egyptian Gabali breed with Spanish V-line in rabbits. J. Anim. Breed. Genet. 2010, 127, 242–248. [Google Scholar] [CrossRef]
- Jain, N.C. Hematological techniques. In Schalm’s Veterinary Hematology; Lea & Febiger: Philadelphia, PA, USA, 1983; pp. 20–86. [Google Scholar]
- Morgenstern, S.; Oklander, M.; Auerbach, J.; Kaufman, J.; Klein, B. Automated Determination of Serum Glutamic Oxaloacetic Transaminase. Clin. Chem. 1966, 12, 95–111. [Google Scholar] [CrossRef]
- Elokil, A.A.; Imbabi, T.A.; Mohamed, H.I.; Abouelezz, K.F.M.; Ahmed-Farid, O.; Shishay, G.; Sabike, I.I.; Liu, H. Zinc and Copper with New Triazine Hydrazone Ligand: Two Novel Organic Complexes Enhanced Expression of Peptide Growth Factors and Cytokine Genes in Weaned V-Line Rabbit. Animals 2019, 9, 1134. [Google Scholar] [CrossRef]
- Horwitz, W. Official Methods of Analysis of AOAC International, 17th ed.; AOAC International: Gaithersburg, MD, USA, 2000. [Google Scholar]
- El-Bahr, S.; Shousha, S.; Shehab, A.; Khattab, W.; Ahmed-Farid, O.; Sabike, I.; El-Garhy, O.; Albokhadaim, I.; Albosadah, K. Effect of Dietary Microalgae on Growth Performance, Profiles of Amino and Fatty Acids, Antioxidant Status, and Meat Quality of Broiler Chickens. Animals 2020, 10, 761. [Google Scholar] [CrossRef]
- Osman, A.; Imbabi, T.; El-Hadary, A.; Sabeq, I.; Edris, S.; Merwad, A.-R.; Azab, E.; Gobouri, A.; Mohammadein, A.; Sitohy, M. Health Aspects, Growth Performance, and Meat Quality of Rabbits Receiving Diets Supplemented with Lettuce Fertilized with Whey Protein Hydrolysate Substituting Nitrate. Biomolecules 2021, 11, 835. [Google Scholar] [CrossRef]
- Sabike, I.; Fujikawa, H.; Edris, A.M. The Growth Kinetics of Salmonella Enteritidis in Raw Ground Beef. Biocontrol Sci. 2015, 20, 185–192. [Google Scholar] [CrossRef]
- Koracevic, D.; Harris, G.; Rayner, A.; Blair, J.; Watt, B. Method for the measurement of antioxidant activity in human fluids. J. Clin. Pathol. 2001, 54, 356–361. [Google Scholar] [CrossRef] [PubMed]
- Aebi, H. Catalase in vitro. In Methods in Enzymology; Elsevier: Amsterdam, The Netherlands, 1984; pp. 121–126. [Google Scholar]
- Ohkawa, H.; Ohishi, N.; Yagi, K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal. Biochem. 1979, 95, 351–358. [Google Scholar] [CrossRef]
- Nishikimi, M.; Rao, N.A.; Yagi, K. The occurrence of superoxide anion in the reaction of reduced phenazine methosulfate and molecular oxygen. Biochem. Biophys. Res. Commun. 1972, 46, 849–854. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Imbabi, T.A.; Ahmed-Farid, O.; Selim, D.A.; Sabeq, I.I. Antioxidant and anti-apoptotic potential of whole-pomegranate extract promoted growth performance, physiological homeostasis, and meat quality of V-line rabbits under hot summer conditions. Anim. Feed. Sci. Technol. 2021, 276, 114911. [Google Scholar] [CrossRef]
- SAS Institute. SAS/STAT Software: Changes and Enhancements for Release 6.12; SAS Institute: Cary, NC, USA, 1996. [Google Scholar]
- Anwar, F.; Hussain, A.I.; Sherazi, S.T.H.; Bhanger, M.I. Changes in Composition and Antioxidant and Antimicrobial Activities of Essential Oil of Fennel (Foeniculum Vulgare Mill.) Fruit at Different Stages of Maturity. J. Herbs Spices Med. Plants 2009, 15, 187–202. [Google Scholar] [CrossRef]
- Ertas, O.N.; Guler, T.; Çiftçi, M.; DalkIlIç, B.; Simsek, U.G. The effect of an essential oil mix derived from oregano, clove and anise on broiler performance. Int. J. Poult. Sci. 2005, 4, 879–884. [Google Scholar]
- Benlemlih, M.; Aarab, A.; Bakkali, M.; Arakrak, A.; Laglaoui, A. Effect of dietary fennel and thyme essential oil supplementation on zootechnical parameters and caecal microflora of growing rabbit. Rev. Microbiol. Ind. San Environ. 2014, 8, 25. [Google Scholar]
- Al-Kassie, G.A.M.; Abd-Al-Jaleel, R.A.; Mohseen, A.M. The effect of a mixture of anise and rosemary on broiler performance. Agric. Biol. J. North Am. 2011, 2, 1279–1282. [Google Scholar] [CrossRef]
- Schöne, F.; Vetter, A.; Hartung, H.; Bergmann, H.; Biertümpfel, A.; Richter, G.; Muller, S.; Breitschuh, G. Effects of essential oils from fennel (Foeniculi aetheroleum) and caraway (Carvi aetheroleum) in pigs. J. Anim. Physiol. Anim. Nutr. 2006, 90, 500–510. [Google Scholar] [CrossRef]
- Mohammed, A.A.; Abbas, R.J. The Effect of Using Fennel Seeds (Foeniculum Vulgare L.) on Productive Performance of Broiler Chickens. Int. J. Poult. Sci. 2009, 8, 642–644. [Google Scholar] [CrossRef]
- Singh, P.; Mishra, N.; Gupta, E. Phytochemistry and ethanopharmacology of Illicium verum (Staranise). In Interdisciplinary Approaches to Altering Neurodevelopmental Disorders; IGI Global: Hershey, PA, USA, 2020; pp. 93–105. [Google Scholar]
- Elghalid, O.A.; Kholif, A.; El-Ashry, G.; Matloup, O.; Olafadehan, O.; El-Raffa, A.; El-Hady, A.A. Oral supplementation of the diet of growing rabbits with a newly developed mixture of herbal plants and spices enriched with special extracts and essential oils affects their productive performance and immune status. Livest. Sci. 2020, 238, 104082. [Google Scholar] [CrossRef]
- Hernández, F.; Madrid, J.; García, V.; Orengo, J.; Megias, M. Influence of two plant extracts on broilers performance, digestibility, and digestive organ size. Poult. Sci. 2004, 83, 169–174. [Google Scholar] [CrossRef]
- Coelho-de-Souza, A.N.; Lahlou, S.; Barreto, J.E.; Yum, M.E.; Oliveira, A.C.; Oliveira, H.D.; Celedônio, N.R.; Feitosa, R.G.; Duarte, G.P.; Santos, C.F.; et al. Essential oil of Croton zehntneri and its major constituent anethole display gastroprotective effect by increasing the surface mucous layer. Fundam. Clin. Pharmacol. 2013, 27, 288–298. [Google Scholar] [CrossRef]
- De Oliveira Monteschio, J.; de Souza, K.A.; Vital, A.C.P.; Guerrero, A.; Valero, M.V.; Kempinski, E.M.B.C.; Barcelos, V.C.; Nascimento, K.F.; do Prado, I.N. Clove and rosemary essential oils and encapsuled active principles (eugenol, thymol and vanillin blend) on meat quality of feedlot-finished heifers. Meat Sci. 2017, 130, 50–57. [Google Scholar] [CrossRef]
- Ranucci, D.; Beghelli, D.; Trabalza-Marinucci, M.; Branciari, R.; Forte, C.; Olivieri, O.; Pazmay, G.B.; Cavallucci, C.; Acuti, G. Dietary effects of a mix derived from oregano (Origanum vulgare L.) essential oil and sweet chestnut (Castanea sativa Mill.) wood extract on pig performance, oxidative status and pork quality traits. Meat Sci. 2015, 100, 319–326. [Google Scholar] [CrossRef]
- Janz, J.; Morel, P.; Wilkinson, B.; Purchas, R. Preliminary investigation of the effects of low-level dietary inclusion of fragrant essential oils and oleoresins on pig performance and pork quality. Meat Sci. 2007, 75, 350–355. [Google Scholar] [CrossRef] [PubMed]
- Hunt, M.C.; King, A.; Barbut, S.; Clause, J.; Cornforth, D.; Hanson, D.; Lindahl, G.; Mancini, R.; Milkowski, A.; Mohan, A.; et al. AMSA Meat Color Measurement Guidelines; American Meat Science Association: Champaign, IL, USA, 2012; pp. 1–135. [Google Scholar]
- Rodríguez-Calleja, J.M.; García-López, M.-L.; Santos, J.A.; Otero, A. Development of the aerobic spoilage flora of chilled rabbit meat. Meat Sci. 2005, 70, 389–394. [Google Scholar] [CrossRef]
- Parejo, I.; Jauregui, O.; Sánchez-Rabaneda, F.; Viladomat, F.; Bastida, A.J.; Codina, C. Separation and Characterization of Phenolic Compounds in Fennel (Foeniculum Vulgare) Using Liquid Chromatography−Negative Electrospray Ionization Tandem Mass Spectrometry. J. Agric. Food Chem. 2004, 52, 3679–3687. [Google Scholar] [CrossRef]
- Hyldgaard, M.; Mygind, T.; Meyer, R.L. Essential Oils in Food Preservation: Mode of Action, Synergies, and Interactions with Food Matrix Components. Front. Microbiol. 2012, 3, 12. [Google Scholar] [CrossRef]
- Imbabi, T.; Hassan, A.; Ahmed-Farid, O.; El-Garhy, O.; Sabeq, I.; Moustafa, M.; Mohammadein, A.; Hassan, N.; Osman, A.; Sitohy, M. Supplementing rabbit diets with butylated hydroxyanisole affects oxidative stress, growth performance, and meat quality. Animal 2021, 15, 100339. [Google Scholar] [CrossRef]
- Valko, M.; Leibfritz, D.; Moncol, J.; Cronin, M.T.D.; Mazur, M.; Telser, J. Free radicals and antioxidants in normal physiological functions and human disease. Int. J. Biochem. Cell Biol. 2007, 39, 44–84. [Google Scholar] [CrossRef] [PubMed]
- Mohamad, R.H.; El-Bastawesy, A.M.; Abdel-Monem, M.G.; Noor, A.M.; Al-Mehdar, H.A.R.; Sharawy, S.M.; El-Merzabani, M.M. Antioxidant and anticarcinogenic effects of methanolic extract and volatile oil of fennel seeds (Foeniculum Vulgare). J. Med. Food 2011, 14, 986–1001. [Google Scholar] [CrossRef] [PubMed]
- Linseisen, J.; Wolfram, G. Odd-Numbered Medium-Chain Triglycerides (Trinonanoin) in Total Parenteral Nutrition: Effects on Parameters of Fat Metabolism in Rabbits. J. Parenter. Enter. Nutr. 1993, 17, 522–528. [Google Scholar] [CrossRef] [PubMed]
- Cunha, T.J.; Cheeke, P.R. Rabbit Feeding and Nutrition; Elsevier: Amsterdam, The Netherlands, 2012. [Google Scholar]
- Lee, K.-W. Essential Oils in Broiler Nutrition. Ph.D. Thesis, Utrecht University, Utrecht, The Netherlands, 2002. [Google Scholar]
- Case, G.L.; He, L.; Mo, H.; Elson, C.E. Induction of geranyl pyrophosphate pyrophosphatase activity by cholesterol-suppressive isoprenoids. Lipids 1995, 30, 357–359. [Google Scholar] [CrossRef]
- Sedláková, J.; Kocourková, B.; Lojková, L.; Kubáň, V. The essential oil content in caraway species (Carum carvi L.). Hortic. Sci. 2003, 30, 73–79. [Google Scholar] [CrossRef]
- Nazih, H.; Krempf, M.; Huvelin, J.M.; Mercier, S.; Bard, J.M. Butyrate stimulates ApoA-IV-containing lipoprotein secretion in differentiated Caco-2 cells: Role in cholesterol efflux. J. Cell. Biochem. 2001, 83, 230–238. [Google Scholar] [CrossRef] [PubMed]
- Podrez, E. Anti-oxidant properties of high-density lipoprotein and atherosclerosis. Clin. Exp. Pharmacol. Physiol. 2010, 37, 719–725. [Google Scholar] [CrossRef]
- Shahidullah, A.; Bhuiyan, M.; Hossain, I.; Islam, R.; Riaz, M. Effects of gentamicin on growth performance and hemato-biochemical parameters in mice. Int. J. Nat. Soc. Sci. 2016, 3, 43–51. [Google Scholar]
- Hong, J.-C.; Steiner, T.; Aufy, A.; Lien, T.-F. Effects of supplemental essential oil on growth performance, lipid metabolites and immunity, intestinal characteristics, microbiota and carcass traits in broilers. Livest. Sci. 2012, 144, 253–262. [Google Scholar] [CrossRef]
- Wetterling, T.; Veltrup, C.; Driessen, M.; John, U. Drinking pattern and alcohol-related medical disorders. Alcohol Alcohol. 1999, 34, 330–336. [Google Scholar] [CrossRef] [PubMed]
- Onyesom, I.; Onyesom, H.C.; Opajobi, A.O.; Esume, C.O. Effect of the permissive sociocultural consumption of alcohol on selected biochemical markers of liver function in the serum of some Nigerian drinkers. Adiktologie 2007, 4, 471–477. [Google Scholar]
- Abdel-Hamid, M.; Osman, A.; El-Hadary, A.; Romeih, E.; Sitohy, M.; Li, L. Hepatoprotective action of papain-hydrolyzed buffalo milk protein on carbon tetrachloride oxidative stressed albino rats. J. Dairy Sci. 2020, 103, 1884–1893. [Google Scholar] [CrossRef] [PubMed]
- Osman, A.; Abd-Elaziz, S.; Salama, A.; Eita, A.A.; Sitohy, M. Health protective actions of phycocyanin obtained from an Egyptian isolate of Spirulina platensis on albino rats. EurAsian J. Biosci. 2019, 13, 105–112. [Google Scholar]
- Kumar, A. A review on hepatoprotective herbal drugs. Int. J. Res. Pharm. Chem. 2012, 2, 96–102. [Google Scholar]
- Nazir, T.; Shakir, L.; Rahman, Z.-U.; Najam, K.; Choudhary, A.; Saeed, N.; Rasheed, H.-U.; Nazir, A.; Aslam, S.; Khanum, A.B. Hepatoprotective Activity of Foeniculum Vulgare Against Paracetamol Induced Hepatotoxicity in Rabbit. J. Appl. Pharm. 2020, 12. [Google Scholar] [CrossRef]
- Bovera, F.; Moniello, G.; de Riu, N.; Di Meo, C.; Pinna, W.; Nizza, A. Effect of diet on the metabolic profile of ostriches (Struthio camelus var. domesticus). Trop. Anim. Health Prod. 2007, 39, 265–270. [Google Scholar] [CrossRef] [PubMed]
- Moniello, G.; Bovera, F.; Solinas, I.; Piccolo, G.; Pinna, W.; Nizza, A. Effect of age and blood collection site on the metabolic profile of ostriches (Short communication). South Afr. J. Anim. Sci. 2005, 35, 268–272. [Google Scholar] [CrossRef][Green Version]
- Miguel, M.G. Antioxidant and Anti-Inflammatory Activities of Essential Oils: A Short Review. Molecules 2010, 15, 9252–9287. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Chen, X.; Devshilt, I.; Yun, Q.; Huang, C.; An, L.; Dorjbat, S.; He, X. Fennel main constituent, trans-anethole treatment against LPS-induced acute lung injury by regulation of Th17/Treg function. Mol. Med. Rep. 2018, 18, 1369–1376. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Ding, Y.; Ye, N.; Wild, C.; Chen, H.; Zhou, J. Direct Activation of Bax Protein for Cancer Therapy. Med. Res. Rev. 2016, 36, 313–341. [Google Scholar] [CrossRef] [PubMed]
- Naseri, M.H.; Mahdavi, M.; Davoodi, J.; Tackallou, S.H.; Goudarzvand, M.; Neishabouri, S.H. Up regulation of Bax and down regulation of Bcl2 during 3-NC mediated apoptosis in human cancer cells. Cancer Cell Int. 2015, 15, 55. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.-Q.; Jia, Y.; Chen, G. Possible involvement of cathepsin B/D and caspase-3 in deferoxamine-related neuroprotection of early brain injury after subarachnoid haemorrhage in rats. Neuropathol. Appl. Neurobiol. 2014, 40, 270–283. [Google Scholar] [CrossRef]
- Okuno, S.-I.; Shimizu, S.; Ito, T.; Nomura, M.; Hamada, E.; Tsujimoto, Y.; Matsuda, H. Bcl-2 Prevents Caspase-independent Cell Death. J. Biol. Chem. 1998, 273, 34272–34277. [Google Scholar] [CrossRef]
- Erşahin, M.; Özsavcı, D.; Şener, A.; Ozakpınar, O.B.; Toklu, H.Z.; Akakin, D.; Şener, G.; Yeğen, B.Ç. Obestatin alleviates subarachnoid haemorrhage-induced oxidative injury in rats via its anti-apoptotic and antioxidant effects. Brain Inj. 2013, 27, 1181–1189. [Google Scholar] [CrossRef]
- Tso, M.K.; Lass, E.; Ai, J.; Macdonald, R.L. Valproic Acid Treatment after Experimental Subarachnoid Hemorrhage; Springer: Berlin/Heidelberg, Germany, 2015; Volume 120, pp. 81–85. [Google Scholar]
- Topkoru, B.C.; Altay, O.; Duris, K.; Krafft, P.R.; Yan, J.; Zhang, J.H. Nasal Administration of Recombinant Osteopontin Attenuates Early Brain Injury after Subarachnoid Hemorrhage. Stroke 2013, 44, 3189–3194. [Google Scholar] [CrossRef]
- Sikiru, A.B.; Arangasamy, A.; Alemede, I.C.; Guvvala, P.R.; Egena, S.S.A.; Ippala, J.R.; Bhatta, R. Chlorella vulgaris supplementation effects on performances, oxidative stress and antioxidant genes expression in liver and ovaries of New Zealand White rabbits. Heliyon 2019, 5, e02470. [Google Scholar] [CrossRef] [PubMed]

| Gene | Accession Number | Primers Sequences (5′→3′) | Product Size (bp) |
|---|---|---|---|
| UXT | XM_008272555 | F: GCGGGACTTGCGAAAGGT | 100 |
| R: AGCTTCCTGGAGTCGTTCAATG | |||
| BAX | XM_008252361.2 | F: CCCGCGAGGTCTTTTTCC | 113 |
| R: CAGGGCCTTGAGTACCAGCTT | |||
| Bcl-2 | XM_008261439.2 | F: GGCTGGGATGCCTTCGT | 186 |
| R: TTTCGTGAACTGTTTGCATATCTG | |||
| CASP3 | NM_008261439.2 | F: GACAGTGGCATCGAGACAGACA | 110 |
| R: GAATAGTAACCAGGTGCTGTGGAA | |||
| GPX1 | NM_001085444.1 | F: CAGTTTGGGCATCAGGAGAAC | 94 |
| Retention Time (min) | Component | Concentration (%) |
|---|---|---|
| 5 | α-a pinene | 4 |
| 7.6 | limonene | 3.3 |
| 11 | Fenchone | 16 |
| 16.7 | Anethole | 75 |
| Growth Parameter | Control | Fennel | Gentamycin | SEM | p Value |
|---|---|---|---|---|---|
| Body weight (BW) (g) | |||||
| BW4 | 501.0 | 501.7 | 499.3 | 1.69 | 0.6262 |
| BW8 | 1007.3 b | 1124.0 a | 1110.0 a | 5.67 | 0.0001 |
| BW12 | 1443.0 b | 1684.7 a | 1700.6 a | 36.28 | 0.0040 |
| Average daily gain (ADG)(g/d) | |||||
| ADG8-4 | 18.07 b | 22.23 a | 21.77 a | 0.208 | 0.0001 |
| ADG12-8 | 15.53 b | 20.00 a | 21.10 a | 1.261 | 0.0445 |
| ADG12-4 | 16.83 b | 21.10 a | 21.47 a | 0.643 | 0.0039 |
| Feed intake (FI) (g) | |||||
| FI 4-12 | 70.60 | 71.00 | 71.47 | 0.345 | 0.3468 |
| Feed conversion ratio (g/g) | |||||
| FCR 4-12 | 2.75 a | 2.36 b | 2.35 b | 0.063 | 0.0067 |
| Parameters | Control | Fennel | Gentamycin | SEM | p Value |
|---|---|---|---|---|---|
| Carcass cuts | |||||
| Live body weight (g) | 1443.00 b | 1684.67 a | 1700.67 a | 36.28 | 0.0004 |
| Carcass (%) | 51.20 | 45.06 | 47.03 | 2.43 | 0.2656 |
| Head rate (%) | 7.29 a | 6.20 a,b | 5.52 b | 0.356 | 0.0336 |
| Hind legs rate (%) | 14.03 | 13.11 | 15.33 | 1.034 | 0.3730 |
| Saddle rate (%) | 10.33 | 9.57 | 10.69 | 0.414 | 0.2269 |
| Fore legs rate (%) | 10.20 | 9.73 | 10.25 | 0.239 | 0.3155 |
| Thoracical neck rate (%) | 12.42 | 9.65 | 13.57 | 1.254 | 0.1546 |
| Body organs (%) | |||||
| Liver (%) | 2.86 | 2.70 | 2.38 | 0.169 | 0.2125 |
| Kidney (%) | 0.672 | 0.584 | 0.559 | 0.047 | 0.2832 |
| Spleen (%) | 0.074 | 0.069 | 0.049 | 0.009 | 0.1949 |
| Lung (%) | 0.739 | 0.613 | 0.676 | 0.068 | 0.4748 |
| Heart (%) | 0.309 | 0.267 | 0.235 | 0.021 | 0.1252 |
| Meat Quality | Control | Treatment | SEM | p-Value | |
|---|---|---|---|---|---|
| Fennel | Gentamycin | ||||
| pH (24 h) | 6.00 a | 5.86 b | 5.82 b | 0.023 | 0.000 |
| WHC | 85.75 | 83.36 | 84.71 | 2.819 | 0.726 |
| Drip loss (48 h) % | 1.50 | 1.13 | 1.12 | 0.187 | 0.667 |
| Thawing loss | 7.26 | 6.45 | 10.01 | 1.365 | 0.431 |
| Cooking loss % | 16.89 | 20.59 | 17.46 | 1.256 | 0.284 |
| WBSF | 4.12 c | 5.44 b | 6.68 a | 0.25 | 0.0001 |
| L* | 54.59 | 54.64 | 51.89 | 0.69 | 0.24 |
| a* | 11.45 | 11.90 | 13.03 | 0.84 | 0.72 |
| b* | 6.23 | 5.68 | 5.77 | 0.19 | 0.08 |
| C | 13.04 b | 13.19 b | 14.25 a | 0.30 | 0.03 |
| h° | 28.46 | 25.57 | 23.88 | 0.84 | 0.05 |
| Moisture | 74.39 a | 71.17 b | 69.50 b | 0.336 | 0.009 |
| Keeping quality test | |||||
| APC (log CFU/g) | |||||
| Day 1 | 3.76 | 4.08 | 3.85 | 0.165 | 0.575 |
| Day 3 | 4.58 b | 4.71 a | 4.45 c | 0.014 | 0.003 |
| Day 5 | 5.24 | 4.90 | 5.06 | 0.08 | 0.050 |
| Day 7 | 5.67 a | 5.03 b | 5.57 a | 0.06 | 0.009 |
| Day 10 | 6.22 b | 6.94 a | 6.85 a | 0.18 | 0.018 |
| pH | |||||
| Day 1 | 5.98 a | 5.90 b | 5.83 c | 0.013 | 0.000 |
| Day 3 | 5.86 a | 5.77 b | 5.69 c | 0.014 | 0.000 |
| Day 5 | 6.06 a | 6.01 b | 5.84 d | 0.006 | 0.000 |
| Day 7 | 6.03 a | 6.04 a | 5.87 b | 0.006 | 0.000 |
| Day 10 | 6.10 a | 5.95 b | 5.91 b | 0.017 | 0.003 |
| Parameters | Control | Fennel | Gentamycin | SEM | p Value |
|---|---|---|---|---|---|
| Hematological variable of blood | |||||
| Hemoglobin (g/dL) | 20.00 | 18.60 | 17.97 | 0.620 | 0.1374 |
| RBCs (10^6/cmm) | 7.50 a | 6.80 a | 5.95 b | 0.214 | 0.0068 |
| HTC (vol%) | 46.00 a | 41.50 b | 43.00 a,b | 0.897 | 0.0313 |
| MCV (fl) | 61.60 b | 60.97 b | 72.30 a | 1.568 | 0.0036 |
| MCH (pg) | 26.70 | 27.50 | 30.17 | 1.107 | 0.1467 |
| MCHC (%) | 43.50 | 45.07 | 41.83 | 2.134 | 0.5914 |
| Platelets (10^3/cmm) | 644.5 | 710.0 | 683.5 | 63.09 | 0.7702 |
| WBC (10^3/cmm) | 10.10 | 9.97 | 10.20 | 0.731 | 0.9748 |
| Neutrophils % | 68.50 | 71.00 | 69.00 | 1.12 | 0.3170 |
| Lymphocytes % | 24.00 | 25.00 | 24.50 | 1.26 | 0.8574 |
| Monocytes% | 6.00 | 3.50 | 4.50 | 0.624 | 0.0764 |
| Eosinophils % | 1.50 a | 0.500 b | 2.00 a | 0.236 | 0.0110 |
| Basophils % | 0 | 0 | 0 | 0 | 0 |
| Parameter | Control | Fennel | Gentamycin | SEM | p Value |
|---|---|---|---|---|---|
| AST (U/I) | 49.76 a | 33.65 b | 26.19 b | 2.244 | 0.0008 |
| ALT (U/I) | 48.02 | 58.49 | 57.62 | 11.14 | 0.7707 |
| Creatinine (mg/dL) | 2.32 | 2.18 | 2.56 | 0.094 | 0.0762 |
| Total Cholesterol (mg/dL) | 80.59 a | 64.48 b | 54.11 c | 2.921 | 0.0020 |
| High-density lipoprotein (mg/dL) | 43.92 b | 57.11 a | 46.98 b | 2.222 | 0.0133 |
| Triglyceride (mg/dL) | 27.29 a | 23.87 b | 20.08 c | 0.576 | 0.0004 |
| Antioxidant Parameter | Control | Fennel Oil | Gentamycin | SEM | p Value |
|---|---|---|---|---|---|
| MDA (nM/g) | 265.42 a | 238.96 b | 217.20 c | 3.240 | 0.0001 |
| SOD (Ul/g) | 151.73 b | 165.63 b | 235.31 a | 5.452 | 0.0001 |
| CAT (u/mg) | 3.36 b | 3.90 a | 3.99 a | 0.101 | 0.0091 |
| TAC (mM/g) | 9.76 b | 13.37 a | 13.37 a | 0.229 | 0.0001 |
| Parameters | Control | Fennel Oil | Gentamycin | SEM | p Value |
|---|---|---|---|---|---|
| NVIS (100 μm) | 47.11 | 54.00 | 56.00 | 4.789 | 0.4016 |
| Villus width (100 μm) | 104.00 | 128.00 | 134.00 | 9.695 | 0.0890 |
| Villus length (100 μm) | 351.11 b | 477.00 a | 526.44 a | 19.14 | 0.0001 |
| MTh (100 μm) | 64.00 b | 77.00 a,b | 89.00 a | 5.123 | 0.0079 |
| G cell (100 μm) | 16.76 | 18.30 | 19.65 | 1.424 | 0.7434 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Imbabi, T.; Sabeq, I.; Osman, A.; Mahmoud, K.; Amer, S.A.; Hassan, A.M.; Kostomakhin, N.; Habashy, W.; Easa, A.A. Impact of Fennel Essential Oil as an Antibiotic Alternative in Rabbit Diet on Antioxidant Enzymes Levels, Growth Performance, and Meat Quality. Antioxidants 2021, 10, 1797. https://doi.org/10.3390/antiox10111797
Imbabi T, Sabeq I, Osman A, Mahmoud K, Amer SA, Hassan AM, Kostomakhin N, Habashy W, Easa AA. Impact of Fennel Essential Oil as an Antibiotic Alternative in Rabbit Diet on Antioxidant Enzymes Levels, Growth Performance, and Meat Quality. Antioxidants. 2021; 10(11):1797. https://doi.org/10.3390/antiox10111797
Chicago/Turabian StyleImbabi, Tharwat, Islam Sabeq, Ali Osman, Kamal Mahmoud, Shimaa A. Amer, Aziza M. Hassan, Nikolay Kostomakhin, Walid Habashy, and Ahmed A. Easa. 2021. "Impact of Fennel Essential Oil as an Antibiotic Alternative in Rabbit Diet on Antioxidant Enzymes Levels, Growth Performance, and Meat Quality" Antioxidants 10, no. 11: 1797. https://doi.org/10.3390/antiox10111797
APA StyleImbabi, T., Sabeq, I., Osman, A., Mahmoud, K., Amer, S. A., Hassan, A. M., Kostomakhin, N., Habashy, W., & Easa, A. A. (2021). Impact of Fennel Essential Oil as an Antibiotic Alternative in Rabbit Diet on Antioxidant Enzymes Levels, Growth Performance, and Meat Quality. Antioxidants, 10(11), 1797. https://doi.org/10.3390/antiox10111797







