Oxidative Stress in the Pathogenesis of Antiphospholipid Syndrome: Implications for the Atherothrombotic Process
Abstract
:1. Introduction
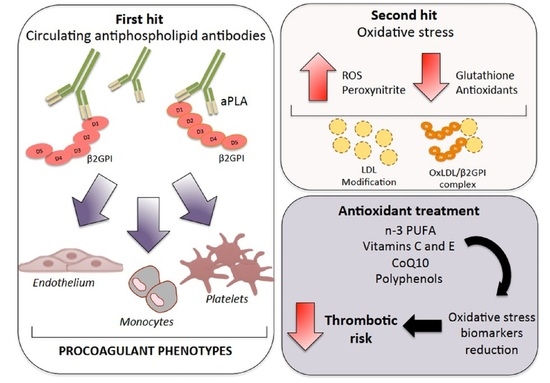
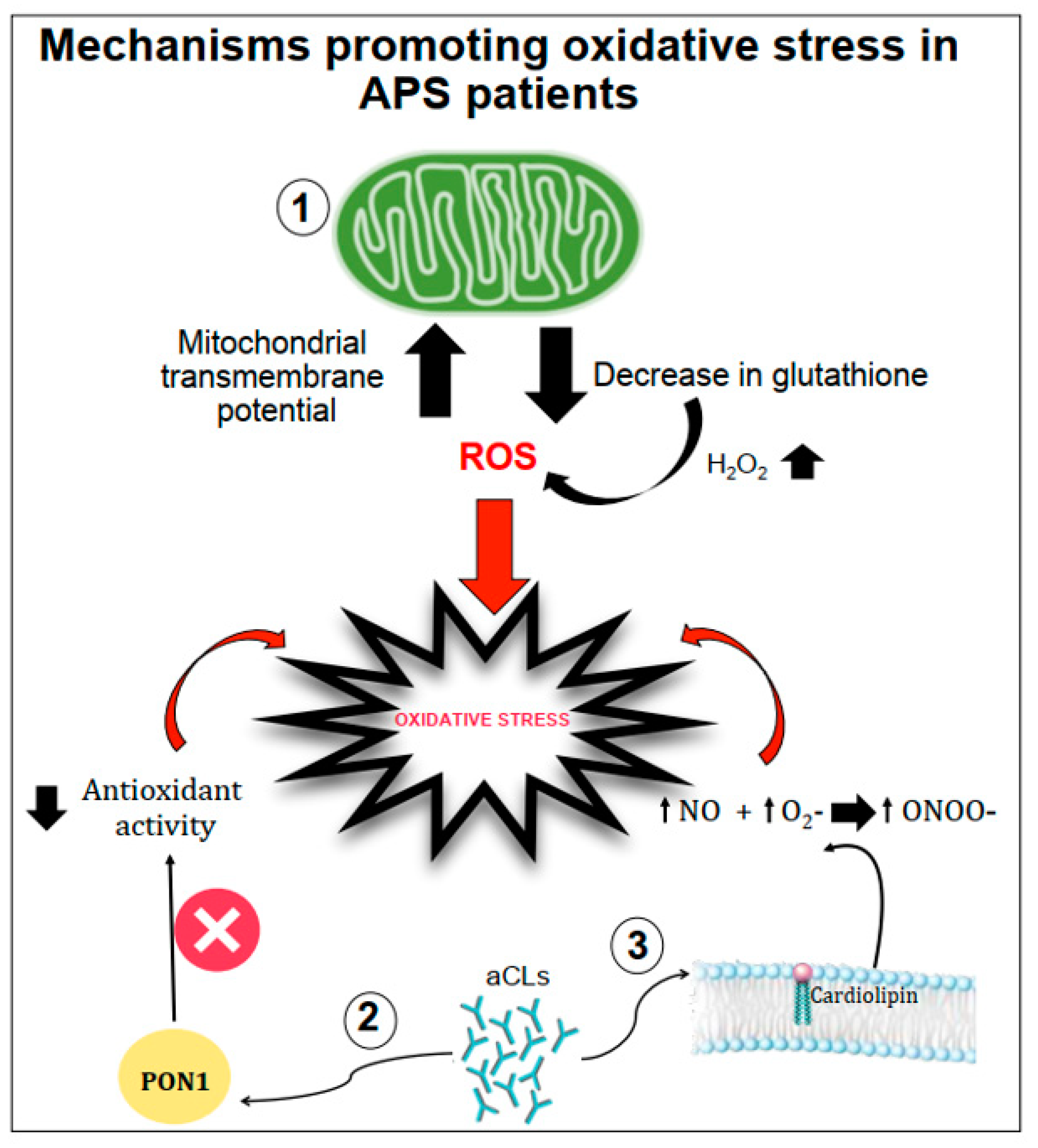
2. Mechanisms of Atherothrombosis in APS: The Role of Oxidative Stress
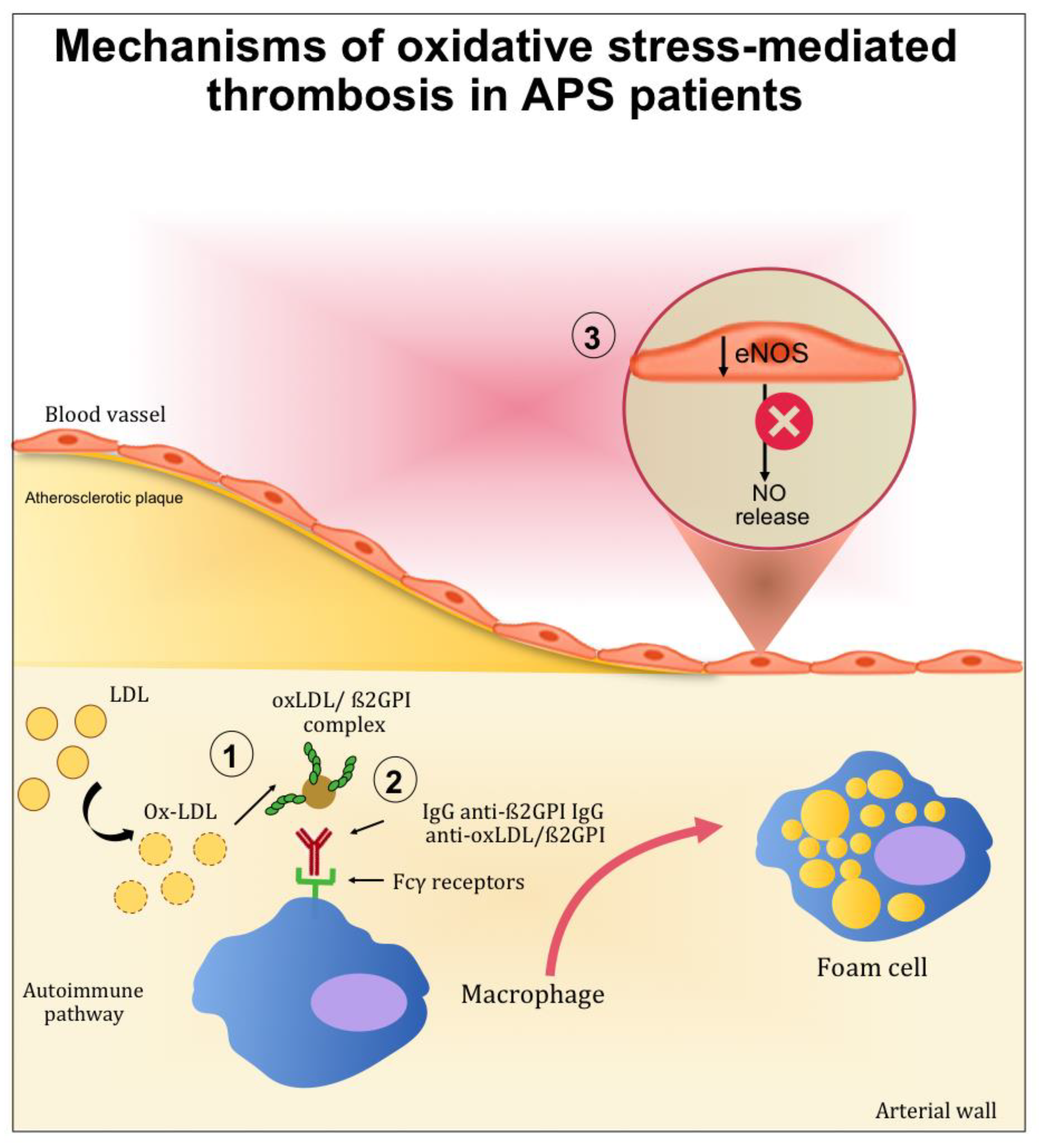
2.1. The Role of Oxidative-Mediated Modifications
2.2. Role of Oxidative Stress in Nitric Oxide Metabolism
3. Oxidative Stress in APS: Clinical and Experimental Studies
4. Antioxidant Treatment in APS Patients: The State of the Art
4.1. Human Studies
4.2. Experimental and In Vitro Studies
5. Conclusions and Future Perspective
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ruiz-Irastorza, G.; Crowther, M.; Branch, W.; Khamashta, M.A. Antiphospholipid syndrome. Lancet 2010, 376, 1498–1509. [Google Scholar] [CrossRef] [Green Version]
- Willis, R.; Pierangeli, S.S. Pathophysiology of the antiphospholipid antibody syndrome. Autoimmun. Highlights 2011, 2, 35–52. [Google Scholar] [CrossRef] [Green Version]
- Pastori, D.; Ames, P.R.J.; Triggiani, M.; Ciampa, A.; Cammisotto, V.; Carnevale, R.; Pignatelli, P.; Bucci, T. Antiphospholipid antibodies and heart failure with preserved ejection fraction. The multicenter athero-aps study. J. Clin. Med. 2021, 10, 3180. [Google Scholar] [CrossRef] [PubMed]
- Abreu, M.M.; Danowski, A.; Wahl, D.G.; Amigo, M.C.; Tektonidou, M.; Pacheco, M.S.; Fleming, N.; Domingues, V.; Sciascia, S.; Lyra, J.O.; et al. The relevance of “non-criteria” clinical manifestations of antiphospholipid syndrome: 14th International Congress on Antiphospholipid Antibodies Technical Task Force Report on Antiphospholipid Syndrome Clinical Features. Autoimmun. Rev. 2015, 14, 401–414. [Google Scholar] [CrossRef] [Green Version]
- Bucci, T.; Menichelli, D.; Pignatelli, P.; Triggiani, M.; Violi, F.; Pastori, D. Relationship of Antiphospholipid Antibodies to Risk of Dementia: A Systematic Review. J. Alzheimer’s Dis. 2019, 69, 561–576. [Google Scholar] [CrossRef] [PubMed]
- Meroni, P.L.; Borghi, M.O.; Raschi, E.; Tedesco, F. Pathogenesis of antiphospholipid syndrome: Understanding the antibodies. Nat. Rev. Rheumatol. 2011, 7, 330–339. [Google Scholar] [CrossRef] [PubMed]
- De Jesús, G.R.; Benson, A.E.; Chighizola, C.B.; Sciascia, S.; Branch, D.W. 16th International Congress on Antiphospholipid Antibodies Task Force Report on Obstetric Antiphospholipid Syndrome. Lupus 2020, 29, 1601–1615. [Google Scholar] [CrossRef]
- Pignatelli, P.; Ettorre, E.; Menichelli, D.; Pani, A.; Violi, F.; Pastori, D. Seronegative antiphospholipid syndrome: Refining the value of “non-criteria” antibodies for diagnosis and clinical management. Haematologica 2020, 105, 562–572. [Google Scholar] [CrossRef] [Green Version]
- El Hasbani, G.; Taher, A.T.; Sciascia, S.; Uthman, I. Antiphospholipid syndrome: The need for new international classification criteria. Expert Rev. Clin. Immunol. 2021, 17, 385–394. [Google Scholar] [CrossRef]
- Pastori, D.; Misasi, R.; Sorice, M.; Cribari, F.; Menichelli, D.; Violi, F.; Pignatelli, P. Multiple Arterial Thrombosis in Seronegative Antiphospholipid Syndrome: Need for New Diagnostic Criteria? Eur. J. Case Reports Intern. Med. 2019, 6, 1. [Google Scholar] [CrossRef]
- Cervera, R.; Serrano, R.; Pons-Estel, G.J.; Ceberio-Hualde, L.; Shoenfeld, Y.; De Ramón, E.; Buonaiuto, V.; Jacobsen, S.; Zeher, M.M.; Tarr, T.; et al. Morbidity and mortality in the antiphospholipid syndrome during a 10-year period: A multicentre prospective study of 1000 patients. Ann. Rheum. Dis. 2015, 74, 1011–1018. [Google Scholar] [CrossRef] [PubMed]
- Carnevale, R.; Pignatelli, P.; Lenti, L.; Buchetti, B.; Sanguigni, V.; Di Santo, S.; Violi, F. LDL are oxidatively modified by platelets via GP91(phox) and accumulate in human monocytes. FASEB J. 2007, 21, 927–934. [Google Scholar] [CrossRef] [Green Version]
- Carnevale, R.; Bartimoccia, S.; Nocella, C.; Di Santo, S.; Loffredo, L.; Illuminati, G.; Lombardi, E.; Boz, V.; Del Ben, M.; De Marco, L.; et al. LDL oxidation by platelets propagates platelet activation via an oxidative stress-mediated mechanism. Atherosclerosis 2014, 237, 108–116. [Google Scholar] [CrossRef]
- Violi, F.; Loffredo, L.; Carnevale, R.; Pignatelli, P.; Pastori, D. Atherothrombosis and Oxidative Stress: Mechanisms and Management in Elderly. Antioxid. Redox Signal. 2017, 27, 1083–1124. [Google Scholar] [CrossRef] [PubMed]
- Conti, V.; Forte, M.; Corbi, G.; Russomanno, G.; Formisano, L.; Landolfi, A.; Izzo, V.; Filippelli, A.; Vecchione, C.; Carrizzo, A. Sirtuins: Possible Clinical Implications in Cardio and Cerebrovascular Diseases. Curr. Drug Targets 2016, 18, 473–484. [Google Scholar] [CrossRef] [PubMed]
- Miceli, M.; Roma, E.; Rosa, P.; Feroci, M.; Loreto, M.A.; Tofani, D.; Gasperi, T. Synthesis of Benzofuran-2-one derivatives and evaluation of their antioxidant capacity by comparing DPPH assay and cyclic voltammetry. Molecules 2018, 23, 710. [Google Scholar] [CrossRef] [Green Version]
- Nocella, C.; Cammisotto, V.; Pigozzi, F.; Borrione, P.; Fossati, C.; D’amico, A.; Cangemi, R.; Peruzzi, M.; Gobbi, G.; Ettorre, E.; et al. Impairment between Oxidant and Antioxidant Systems: Short- and Long-term Implications for Athletes’ Health. Nutrients 2019, 11, 1353. [Google Scholar] [CrossRef] [Green Version]
- Gergely, P.; Grossman, C.; Niland, B.; Puskas, F.; Neupane, H.; Allam, F.; Banki, K.; Phillips, P.E.; Perl, A. Mitochondrial hyperpolarization and ATP depletion in patients with systemic lupus erythematosus. Arthritis Rheum. 2002, 46, 175–190. [Google Scholar] [CrossRef]
- Lambert, M.; Boullier, A.; Hachulla, E.; Fruchart, J.C.; Teissier, E.; Hatron, P.Y.; Duriez, P. Paraoxonase activity is dramatically decreased in patients positive for anticardiolipin antibodies. Lupus 2000, 9, 299–300. [Google Scholar] [CrossRef]
- Charakida, M.; Besler, C.; Batuca, J.R.; Sangle, S.; Marques, S.; Sousa, M.; Wang, G.; Tousoulis, D.; Delgado Alves, J.; Loukogeorgakis, S.P.; et al. Vascular abnormalities, paraoxonase activity, and dysfunctional HDL in primary antiphospholipid syndrome. JAMA—J. Am. Med. Assoc. 2009, 302, 1210–1217. [Google Scholar] [CrossRef] [Green Version]
- Alves, J.D.; Grima, B. Oxidative stress in systemic lupus erythematosus and antiphospholipid syndrome: A gateway to atherosclerosis. Curr. Rheumatol. Rep. 2003, 5, 383–390. [Google Scholar] [CrossRef] [PubMed]
- Sciascia, S.; Baldovino, S.; Schreiber, K.; Solfietti, L.; Radin, M.; Cuadrado, M.J.; Menegatti, E.; Erkan, D.; Roccatello, D. Thrombotic risk assessment in antiphospholipid syndrome: The role of new antibody specificities and thrombin generation assay. Clin. Mol. Allergy 2016, 14, 6. [Google Scholar] [CrossRef] [Green Version]
- Lozier, J.; Takahashi, N.; Putnam, F.W. Complete amino acid sequence of human plasma β2-glycoprotein I. Proc. Natl. Acad. Sci. USA 1984, 81, 3640–3644. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nakagawa, H.; Yasuda, S.; Miyazaki, T. Novel Function of Beta 2 Glycoprotein I in Angiogenesis. Curr. Angiogenes. 2015, 3, 132–138. [Google Scholar] [CrossRef]
- Hess, D.T.; Matsumoto, A.; Kim, S.O.; Marshall, H.E.; Stamler, J.S. Protein S-nitrosylation: Purview and parameters. Nat. Rev. Mol. Cell Biol. 2005, 6, 150–166. [Google Scholar] [CrossRef] [PubMed]
- Passam, F.H.; Giannakopoulos, B.; Mirarabshahi, P.; Krilis, S.A. Molecular pathophysiology of the antiphospholipid syndrome: The role of oxidative post-translational modification of beta 2 glycoprotein I. J. Thromb. Haemost. 2011, 9, 275–282. [Google Scholar] [CrossRef]
- Morgan, P.E.; Sturgess, A.D.; Davies, M.J. Increased levels of serum protein oxidation and correlation with disease activity in systemic lupus erythematosus. Arthritis Rheum. 2005, 52, 2069–2079. [Google Scholar] [CrossRef] [PubMed]
- Frostegård, J.; Svenungsson, E.; Wu, R.; Gunnarsson, I.; Lundberg, I.E.; Klareskog, L.; Hörkkö, S.; Witztum, J.L. Lipid peroxidation is enhanced in patients with systemic lupus erythematosus and is associated with arterial and renal disease manifestations. Arthritis Rheum. 2005, 52, 192–200. [Google Scholar] [CrossRef]
- Matsuura, E.; Lopez, L. Autoimmune-mediated atherothrombosis. Lupus 2008, 17, 879–888. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, K.; Kishi, M.; Atsumi, T.; Bertolaccini, M.L.; Makino, H.; Sakairi, N.; Yamamoto, I.; Yasuda, T.; Khamashta, M.A.; Hughes, G.R.V.; et al. Circulating oxidized LDL forms complexes with β 2-glycoprotein I: Implication as an atherogenic autoantigen. J. Lipid Res. 2003, 44, 716–726. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hasunuma, Y.; Matsuura, E.; Makita, Z.; Katahira, T.; Nishi, S.; Koike, T. Involvement of beta2-glycoprotein I and anticardiolipin antibodies in oxidatively modified low-density lipoprotein uptake by macrophages. Clin. Exp. Immunol. 1997, 107, 569–573. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, K.; Matsuura, E.; Liu, Q.; Furukawa, J.I.; Kaihara, K.; Inagaki, J.; Atsumi, T.; Sakairi, N.; Yasuda, T.; Voelker, D.R.; et al. A specific ligand for β2-glycoprotein I mediates autoantibody-dependent uptake of oxidized low density lipoprotein by macrophages. J. Lipid Res. 2001, 42, 697–709. [Google Scholar] [CrossRef]
- Förstermann, U.; Sessa, W.C. Nitric oxide synthases: Regulation and function. Eur. Heart J. 2012, 33, 829–837. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Loscalzo, J. Jin Vascular nitric oxide: Formation and function. J. Blood Med. 2010, 1, 147. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ames, P.R.J.; Tommasino, C.; Alves, J.; Morrow, J.D.; Iannaccone, L.; Fossati, G.; Caruso, S.; Caccavo, F.; Brancaccio, V. Antioxidant susceptibility of pathogenic pathways in subjects with antiphospholipid antibodies: A pilot study. Lupus 2000, 9, 688–695. [Google Scholar] [CrossRef] [PubMed]
- Ramesh, S.; Morrell, C.N.; Tarango, C.; Thomas, G.D.; Yuhanna, I.S.; Girardi, G.; Herz, J.; Urbanus, R.T.; De Groot, P.G.; Thorpe, P.E.; et al. Antiphospholipid antibodies promote leukocyte-endothelial cell adhesion and thrombosis in mice by antagonizing eNOS via β2GPI and apoER2. J. Clin. Investig. 2011, 121, 120–131. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Urbanus, R.T.; Pennings, M.T.T.; Derksen, R.H.W.M.; de Groot, P.G. Platelet activation by dimeric β2-glycoprotein I requires signaling via both glycoprotein Ibα and apolipoprotein E receptor 2′. J. Thromb. Haemost. 2008, 6, 1405–1412. [Google Scholar] [CrossRef]
- Lutters, B.C.H.; Derksen, R.H.W.M.; Tekelenburg, W.L.; Lenting, P.J.; Arnout, J.; De Groot, P.G. Dimers of β2-Glycoprotein I Increase Platelet Deposition to Collagen via Interaction with Phospholipids and the Apolipoprotein E Receptor 2′. J. Biol. Chem. 2003, 278, 33831–33838. [Google Scholar] [CrossRef] [Green Version]
- Ames, P.R.J.; Batuca, J.R.; Ciampa, A.; Iannaccone, L.; Alves, J.D. Clinical relevance of nitric oxide metabolites and nitrative stress in thrombotic primary antiphospholipid syndrome. J. Rheumatol. 2010, 37, 2523–2530. [Google Scholar] [CrossRef] [PubMed]
- Ramesh, S.; Morrell, C.N.; Tarango, C.; Thomas, G.D.; Yuhanna, I.S.; Girardi, G.; Herz, J.; Urbanus, R.T.; De Groot, P.G.; Thorpe, P.E.; et al. Antibodies to high-density lipoprotein and beta2-glycoprotein I are inversely correlated with paraoxonase activity in systemic lupus erythematosus and primary antiphospholipid syndrome. Arthritis Rheum. 2002, 46, 2686–2694. [Google Scholar] [CrossRef]
- Ferro, D.; Saliola, M.; Meroni, P.L.; Valesini, G.; Caroselli, C.; Praticó, D.; Fitzgerald, G.A.; Shoenfeld, Y.; Violi, F. Enhanced monocyte expression of tissue factor by oxidative stress in patients with antiphospholipid antibodies: Effect of antioxidant treatment. J. Thromb. Haemost. 2003, 1, 523–531. [Google Scholar] [CrossRef]
- Matsuura, E.; Kobayashi, K.; Hurley, B.L.; Lopez, L.R. Atherogenic oxidized low-density lipoprotein/β2- glycoprotein I (oxLDL/β2GPI) complexes in patients with systemic lupus erythematosus and antiphospholipid syndrome. Lupus 2006, 15, 478–483. [Google Scholar] [CrossRef]
- Sciascia, S.; Roccatello, D.; Bertero, M.T.; Di Simone, D.; Cosseddu, D.; Vaccarino, A.; Bazzan, M.; Rossi, D.; Garcia-Fernandez, C.; Ceberio, L.; et al. 8-Isoprostane, prostaglandin E2, C-reactive protein and serum amyloid A as markers of inflammation and oxidative stress in antiphospholipid syndrome: A pilot study. Inflamm. Res. 2012, 61, 809–816. [Google Scholar] [CrossRef] [PubMed]
- Perez-Sanchez, C.; Barbarroja, N.; Messineo, S.; Ruiz-Limon, P.; Rodriguez-Ariza, A.; Jimenez-Gomez, Y.; Khamashta, M.A.; Collantes-Estevez, E.; Cuadrado, M.J.; Aguirre, M.A.; et al. Gene profiling reveals specific molecular pathways in the pathogenesis of atherosclerosis and cardiovascular disease in antiphospholipid syndrome, systemic lupus erythematosus and antiphospholipid syndrome with lupus. Ann. Rheum. Dis. 2015, 74, 1441–1449. [Google Scholar] [CrossRef] [PubMed]
- Stanisavljevic, N.; Stojanovich, L.; Marisavljevic, D.; Djokovic, A.; Dopsaj, V.; Kotur-Stevuljevic, J.; Martinovic, J.; Memon, L.; Radovanovic, S.; Todic, B.; et al. Lipid peroxidation as risk factor for endothelial dysfunction in antiphospholipid syndrome patients. Clin. Rheumatol. 2016, 35, 2485–2493. [Google Scholar] [CrossRef] [PubMed]
- Lai, Z.-W.; Marchena-Mendez, I.; Perl, A. Oxidative stress and Treg depletion in lupus patients with anti-phospholipid syndrome. Clin. Immunol. 2015, 158, 148–152. [Google Scholar] [CrossRef] [Green Version]
- Ibrahim, A.A.; El-Lebedy, D.; Ashmawy, I.; Hady, M.A. Association between paraoxonase-1 gene Q192R and L55M polymorphisms in systemic lupus erythematosus (SLE) and anti-phospholipid syndrome (APS) in a population from Cairo of Egypt. Clin. Rheumatol. 2017, 36, 1305–1310. [Google Scholar] [CrossRef] [PubMed]
- Nojima, J.; Kaneshige, R.; Motoki, Y.; Ieko, M. Increased oxidative stress may be a risk factor for thromboembolic complications in patients with antiphospholipid syndrome. Thromb. Res. 2020, 196, 52–53. [Google Scholar] [CrossRef]
- Delgado Alves, J.; Mason, L.J.; Ames, P.R.J.; Chen, P.P.; Rauch, J.; Levine, J.S.; Subang, R.; Isenberg, D.A. Antiphospholipid antibodies are associated with enhanced oxidative stress, decreased plasma nitric oxide and paraoxonase activity in an experimental mouse model. Rheumatology 2005, 44, 1238–1244. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Benhamou, Y.; Miranda, S.; Armengol, G.; Harouki, N.; Drouot, L.; Zahr, N.; Thuillez, C.; Boyer, O.; Levesque, H.; Joannides, R.; et al. Infliximab improves endothelial dysfunction in a mouse model of antiphospholipid syndrome: Role of reduced oxidative stress. Vascul. Pharmacol. 2015, 71, 93–101. [Google Scholar] [CrossRef]
- Ding, X.; Yang, Z.; Han, Y.; Yu, H. Correlation of long-chain fatty acid oxidation with oxidative stress and inflammation in pre-eclampsia-like mouse models. Placenta 2015, 36, 1442–1449. [Google Scholar] [CrossRef]
- Simoncini, S. Role of reactive oxygen species and p38 MAPK in the induction of the pro-adhesive endothelial state mediated by IgG from patients with anti-phospholipid syndrome. Int. Immunol. 2005, 17, 489–500. [Google Scholar] [CrossRef] [Green Version]
- Milne, G.L.; Yin, H.; Hardy, K.D.; Davies, S.S.; Roberts, L.J. Isoprostane generation and function. Chem. Rev. 2011, 111, 5973–5996. [Google Scholar] [CrossRef] [Green Version]
- Pignatelli, P.; Pastori, D.; Carnevale, R.; Farcomeni, A.; Cangemi, R.; Nocella, C.; Bartimoccia, S.; Vicario, T.; Saliola, M.; Lip, G.Y.H.; et al. Serum NOX2 and urinary isoprostanes predict vascular events in patients with atrial fibrillation. Thromb. Haemost. 2015, 113, 617–624. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Amengual, O.; Atsumi, T.; Khamashta, M.A.; Hughes, G.R. The role of the tissue factor pathway in the hypercoagulable state in patients with the antiphospholipid syndrome. Thromb. Haemost. 1998, 79, 276–281. [Google Scholar] [CrossRef]
- Litvinov, D.; Mahini, H.; Garelnabi, M. Antioxidant and anti-inflammatory role of paraoxonase 1: Implication in arteriosclerosis diseases. N. Am. J. Med. Sci. 2012, 4, 523–532. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Manea, A. NADPH oxidase-derived reactive oxygen species: Involvement in vascular physiology and pathology. Cell Tissue Res. 2010, 342, 325–339. [Google Scholar] [CrossRef]
- Perez-Sanchez, C.; Ruiz-Limon, P.; Aguirre, M.A.; Bertolaccini, M.L.; Khamashta, M.A.; Rodriguez-Ariza, A.; Segui, P.; Collantes-Estevez, E.; Barbarroja, N.; Khraiwesh, H.; et al. Mitochondrial dysfunction in antiphospholipid syndrome: Implications in the pathogenesis of the disease and effects of coenzyme Q 10 treatment. Blood 2012, 119, 5859–5870. [Google Scholar] [CrossRef] [Green Version]
- Rossi, E.; Costa, M. Fish Oil Derivatives as a Prophylaxis of Recurrent Miscarriage Associated with Antiphospholipid Antibodies (APL): A Pilot Study. Lupus 1993, 2, 319–323. [Google Scholar] [CrossRef]
- Carta, G.; Iovenitti, P.; Falciglia, K. Recurrent miscarriage associated with antiphospholipid antibodies: Prophylactic treatment with low-dose aspirin and fish oil derivates. Clin. Exp. Obstet. Gynecol. 2005, 32, 49–51. [Google Scholar] [PubMed]
- Felau, S.M.; Sales, L.P.; Solis, M.Y.; Hayashi, A.P.; Roschel, H.; Sá-Pinto, A.L.; De Andrade, D.C.O.; Katayama, K.Y.; Irigoyen, M.C.; Consolim-Colombo, F.; et al. Omega-3 fatty acid supplementation improves endothelial function in primary antiphospholipid syndrome: A small-scale randomized double-blind placebo-controlled trial. Front. Immunol. 2018, 9, 336. [Google Scholar] [CrossRef] [Green Version]
- Stopa, J.D.; Neuberg, D.; Puligandla, M.; Furie, B.; Flaumenhaft, R.; Zwicker, J.I. Protein disulfide isomerase inhibition blocks thrombin generation in humans by interfering with platelet factor V activation. JCI Insight 2017, 2. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Sánchez, C.; Aguirre, M.Á.; Ruiz-Limón, P.; Ábalos-Aguilera, M.C.; Jiménez-Gómez, Y.; Arias-de la Rosa, I.; Rodriguez-Ariza, A.; Fernández-del Río, L.; González-Reyes, J.A.; Segui, P.; et al. Ubiquinol Effects on Antiphospholipid Syndrome Prothrombotic Profile. Arterioscler. Thromb. Vasc. Biol. 2017, 37, 1923–1932. [Google Scholar] [CrossRef] [Green Version]
- Maalouly, G.; Ward, C.; Smayra, V.; Saliba, Y.; Aftimos, G.; Haddad, F.; Farès, N. Fish oil attenuates neurologic severity of antiphospholipid syndrome in a mice experimental model. Nutr. Neurosci. 2017, 20, 563–570. [Google Scholar] [CrossRef] [PubMed]
- Ali, R.A.; Gandhi, A.A.; Dai, L.; Weiner, J.; Estes, S.K.; Yalavarthi, S.; Gockman, K.; Sun, D.; Knight, J.S. Antineutrophil properties of natural gingerols in models of lupus. JCI Insight 2021, 6. [Google Scholar] [CrossRef] [PubMed]
- Wei, A.; Xiao, H.; Xu, G.; Yu, X.; Guo, J.; Jing, Z.; Shi, S.; Song, Y. Hyperoside Protects Human Umbilical Vein Endothelial Cells Against Anticardiolipin Antibody-Induced Injury by Activating Autophagy. Front. Pharmacol. 2020, 11, 762. [Google Scholar] [CrossRef]
- Wang, T.; Zhou, H.; Xie, H.; Mu, Y.; Xu, Y.; Liu, J.; Zhang, X. Epigallocatechin-3-gallate inhibits TF and TNF-α expression induced by the anti-β2GPI/β2GPI complex in human THP-1 cells. Int. J. Mol. Med. 2014, 33, 994–1002. [Google Scholar] [CrossRef]
| HUMAN STUDIES | |||
|---|---|---|---|
| Author/(Year)/[Reference] | Study Type (Setting) | Markers of Oxidative Stress | Main Results vs. Controls |
| Lambert et al., (2000) [19] | n = 56 APS patients n = 71 HS | PON1 MDA-LDL | ↓ PON1 ↑ MDA-LDL |
| Delgado Alves et al., (2002) [40] | Cross-sectional study n = 32 SLE n = 36 with PAPS n = 20 controls | HDL cholesterol PON activity TAC | ↓ HDL ↑ anti-HDL antibodies ↓ PON activity ↓ TAC |
| Ferro et al., (2003) [41] | n = 13 APL patients n = 11 negative APL patients | Isoprostane | ↑ 8-isoprostane |
| Matsuura et al., (2006) [42] | n = 93 APS patients n = 161 HS | oxLDL/beta2GPI | ↑ oxLDL/beta2GPI |
| Sciascia et al., (2012) [43] | n = 45 APS patients n = 75 HS | Isoprostanes | ↑ 8-isoprostane ↑ Prostaglandin E2 (PGE) |
| Perez-Sanchez et al., (2015) [44] | n = 126 APS patients n = 61 HS | TAC MnSOD Catalase GPx | ↓ TAC ↑ MnSOD ↑ Catalase ↓ GPx |
| Stanisavljevic et al., (2016) [45] | Cross-sectional case–control n = 140 APS patients n = 40 HS | LOOH AOPP tSHG PON1 | ↔ LOOH ↑ AOPP ↓ tSHG ↓ PON1 |
| Lai et al., (2015) [46] | n = 12 APS patients n = 54 HS | mitochondrial mass O2− production mTOR and FoxP3 expression | ↑ mitochondrial mass ↑ O2− production ↔ mTOR expression ↓ FoxP3 expression |
| Ibrahim (2017) [47] | n = 75 APS patients n = 120 HS | polymorphisms of the PON1 | ↔ PON1 polymorphisms |
| Nojima et al., (2020) [48] | n = 58 APS patients n = 312 HS | OSI | ↑ OSI |
| EXPERIMENTAL STUDIES | |||
| Delgado Alves et al., (2005) [49] | mice with SCID+ aCL and anti-aβ2-GPI monoclonal antibodies | PON activity TAC | ↓ PON activity ↓ TAC |
| Benhamou et al., (2015) [50] | APS mice | gp91phox mRNA GSH/GSSH ratio | ↑ gp91phox mRNA ↑ left ventricular GSH/GSSH |
| Ding et al., (2015) [51] | APS mice wild-type mice | p47phox | ↑ p47phox mRNA ↑ p47phox phosphorylation |
| IN VITRO STUDIES | |||
| Ferro et al., (2003) [41] | Human monocytes from (HS) and anti-β2GP1 antibodies (50, 100, 200 µg/mL) | O2− production | ↑ O2− production |
| Simoncini et al., (2005) [52] | HUVEC and IgG (IgG-APS) from 12 APS patients | ROS production | ↑ ROS production MAP kinases pathway: ↑ p38 phosphorylation ↑ ATF-2 |
| INTERVENTION STUDIES | |||
|---|---|---|---|
| HUMAN STUDIES | |||
| Author/(Year)/[Reference] | Study Type (Setting) | Type of Intervention/Doses | Main Results |
| Rossi et al., (1993) [59] | Patients with PAPS associated with recurrent miscarriage n = 22 | EPA and DHA (5.1 g) | Fish oil prevents recurrent miscarriage in persistent APS |
| Carta et al., (2005) [60] | A prospective study Patients with positive antiphospholipid antibodies n = 30 | Fish oil derivates vs. low dose aspirin | No significant differences in adverse pregnancy outcome after fish oil derivates. |
| Felau et al., (2018) [61] | Randomized double-blind placebo-controlled trial Women with primary APS n = 22 | EPA (1.8 g) and DHA (1.3 g) 16 weeks | ↑ endothelial function ↓ circulating levels of interleukin-10 and TNF ↔ E- selectin, vascular adhesion molecule-1, and fibrinogen levels |
| Ferro et al., (2003) [41] | Randomized clinical trial APL positive patients n = 11 | Vitamin E (900 IU day) Vitamin C (2000 mg day) 6 weeks | ↓ Isoprostanes ↓ Monocyte TF antigen |
| Stopa et al., (2017) [62] | Clinical trial Patients with persistently elevated anti-phospholipid antibodies n = 6 | Isoquercetin (1000 mg) 4 h | ↓ thrombin generation (decrease of 63.6%) ↓ platelet factor Va generation |
| Perez-Sanchez et al., (2017) [63] | Prospective, randomized, crossover, placebo-controlled trial n = 36 | Qred (200 mg/d) vs. placebo 1 month | ↑ endothelial function ↓ monocyte expression of prothrombotic and proinflammatory mediators ↓ peroxides |
| EXPERIMENTAL STUDIES | |||
| Maalouly et al., (2017) [64] | Murine experimental models of antiphospholipid syndrome: BALB/c mice immunized with beta-2-glycoprotein I | Omega-3 fatty acids (0.5 g/kg) curcumin (200 mg/kg) 3 months in addition to the treatment with enoxaparin (1 mg/kg) | ↓ mortality |
| Ramadan et al., (2021) [65] | Murine experimental models of antiphospholipid syndrome: esiquimod-induced (R848-induced) lupus | 6-gingerol (20 mg/kg intraperitoneal injection) 3 times per week | ↓ NETs release ↓ Anti-dsDNA, anti-β2GPI, and total IgG ↓ thrombus length and weight |
| IN VITRO STUDIES | |||
| Author/(year)/[reference] | Types of cells | Type of antioxidant’s treatment | Main results |
| Ferro et al., (2003) [41] | Human healthy monocytes treated with polyclonal anti-b2GP1 antibodies | Vitamin E concentrations (50, 100 μM) | ↓ superoxide anion ↓ TF Ag and activity |
| Wei et al., (2020) [66] | HUVECs treated with anticardiolipin antibody (aCL) | Hyperoside (10, 20, 50 mM) | ↓ IL-1b, IL-8, TF, ICAM1, and VCAM1 ↑ autophagy ↓ mTOR/S6K ↓ TLR4/Myd88/NF-kB signalling transduction pathways |
| Perez-Sanchez et al., (2017) [63] | Human healthy monocytes treated with IgG-APS | CoQ10 | ↓ oxidative stress ↓ TF ↓ VEGF ↓ Flt1 receptor |
| Wang et al., (2014) [67] | Human acute monocytic leukaemia cell line treated with anti-β2 glycoprotein I (GPI)/β2GPI complex | Epigallocatechin-3-gallate (0–50 μg/mL) | ↓ TF expression ↓ TF activity |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nocella, C.; Bartimoccia, S.; Cammisotto, V.; D’Amico, A.; Pastori, D.; Frati, G.; Sciarretta, S.; Rosa, P.; Felici, C.; Riggio, O.; et al. Oxidative Stress in the Pathogenesis of Antiphospholipid Syndrome: Implications for the Atherothrombotic Process. Antioxidants 2021, 10, 1790. https://doi.org/10.3390/antiox10111790
Nocella C, Bartimoccia S, Cammisotto V, D’Amico A, Pastori D, Frati G, Sciarretta S, Rosa P, Felici C, Riggio O, et al. Oxidative Stress in the Pathogenesis of Antiphospholipid Syndrome: Implications for the Atherothrombotic Process. Antioxidants. 2021; 10(11):1790. https://doi.org/10.3390/antiox10111790
Chicago/Turabian StyleNocella, Cristina, Simona Bartimoccia, Vittoria Cammisotto, Alessandra D’Amico, Daniele Pastori, Giacomo Frati, Sebastiano Sciarretta, Paolo Rosa, Chiara Felici, Oliviero Riggio, and et al. 2021. "Oxidative Stress in the Pathogenesis of Antiphospholipid Syndrome: Implications for the Atherothrombotic Process" Antioxidants 10, no. 11: 1790. https://doi.org/10.3390/antiox10111790
APA StyleNocella, C., Bartimoccia, S., Cammisotto, V., D’Amico, A., Pastori, D., Frati, G., Sciarretta, S., Rosa, P., Felici, C., Riggio, O., Calogero, A., Carnevale, R., & SMiLe Group. (2021). Oxidative Stress in the Pathogenesis of Antiphospholipid Syndrome: Implications for the Atherothrombotic Process. Antioxidants, 10(11), 1790. https://doi.org/10.3390/antiox10111790