Circulating Superoxide Dismutase Concentrations in Obstructive Sleep Apnoea (OSA): A Systematic Review and Meta-Analysis
Abstract
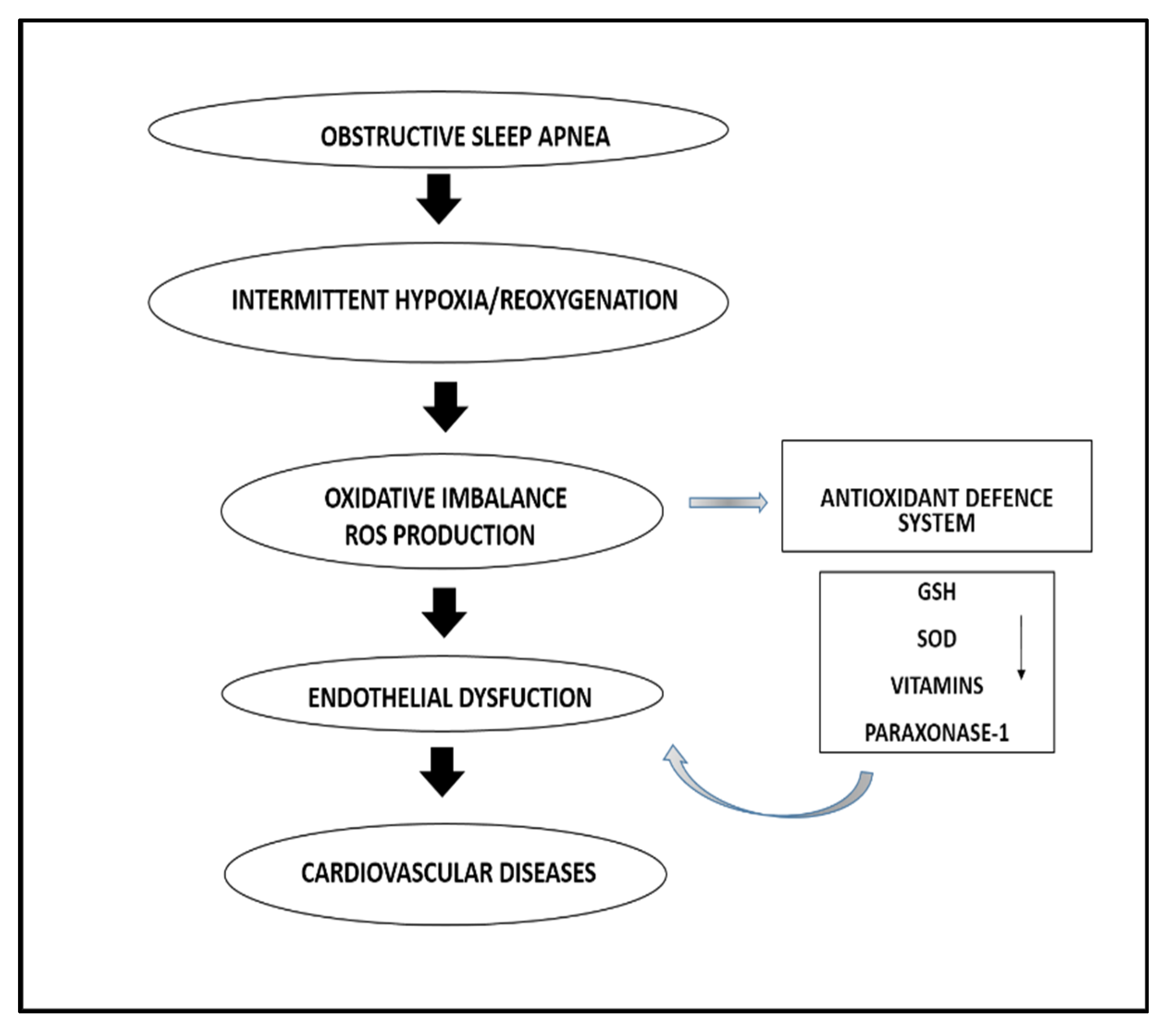
1. Introduction
2. Materials and Methods
2.1. Search Strategy, Eligibility Criteria, and Study Selection
2.2. Statistical Analysis
3. Results
3.1. Systematic Research
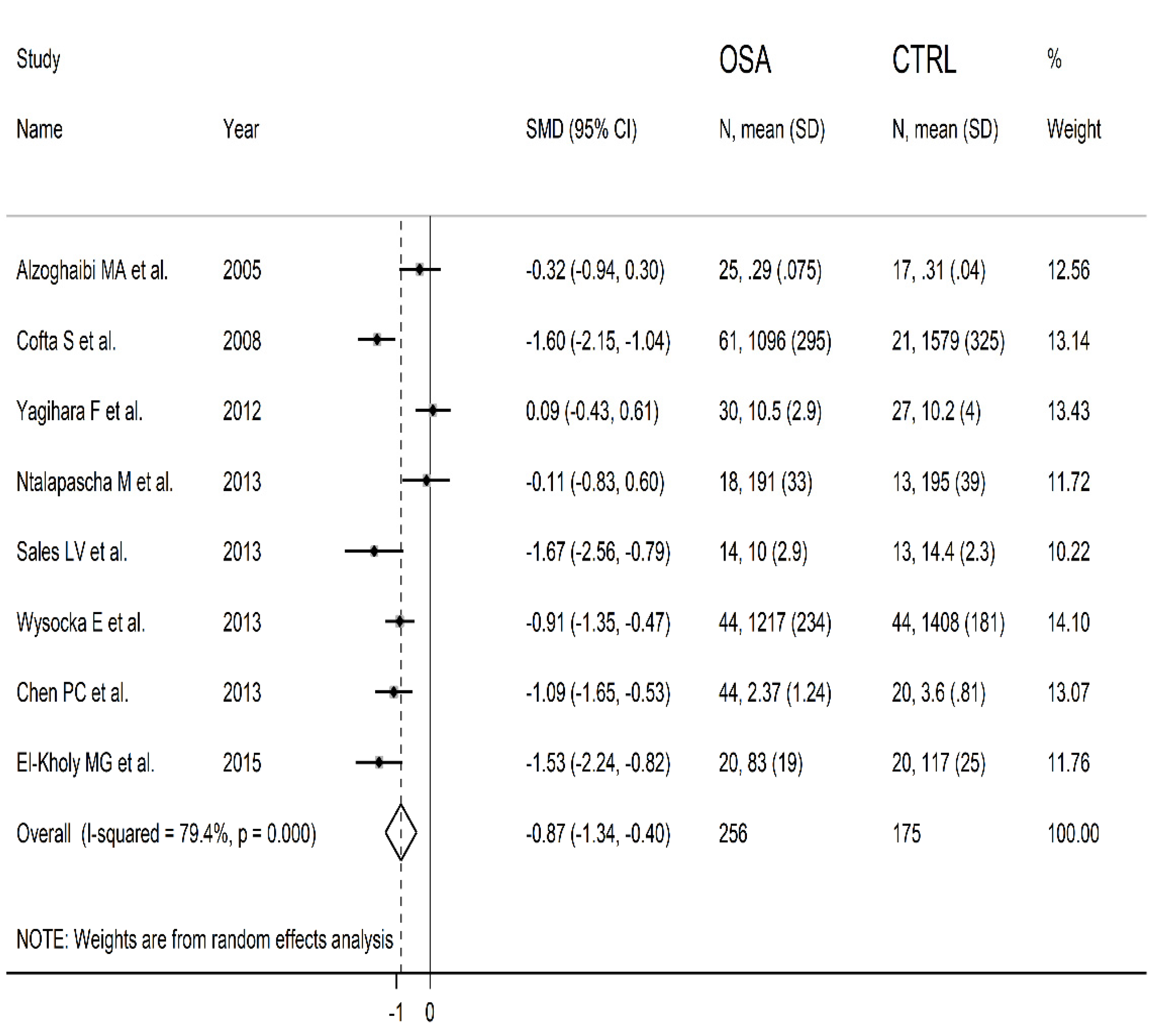
3.2. Meta-Analysis of Blood SOD Concentrations
3.2.1. Study Characteristics
3.2.2. Risk of Bias
3.2.3. Results of Individual Studies and Syntheses
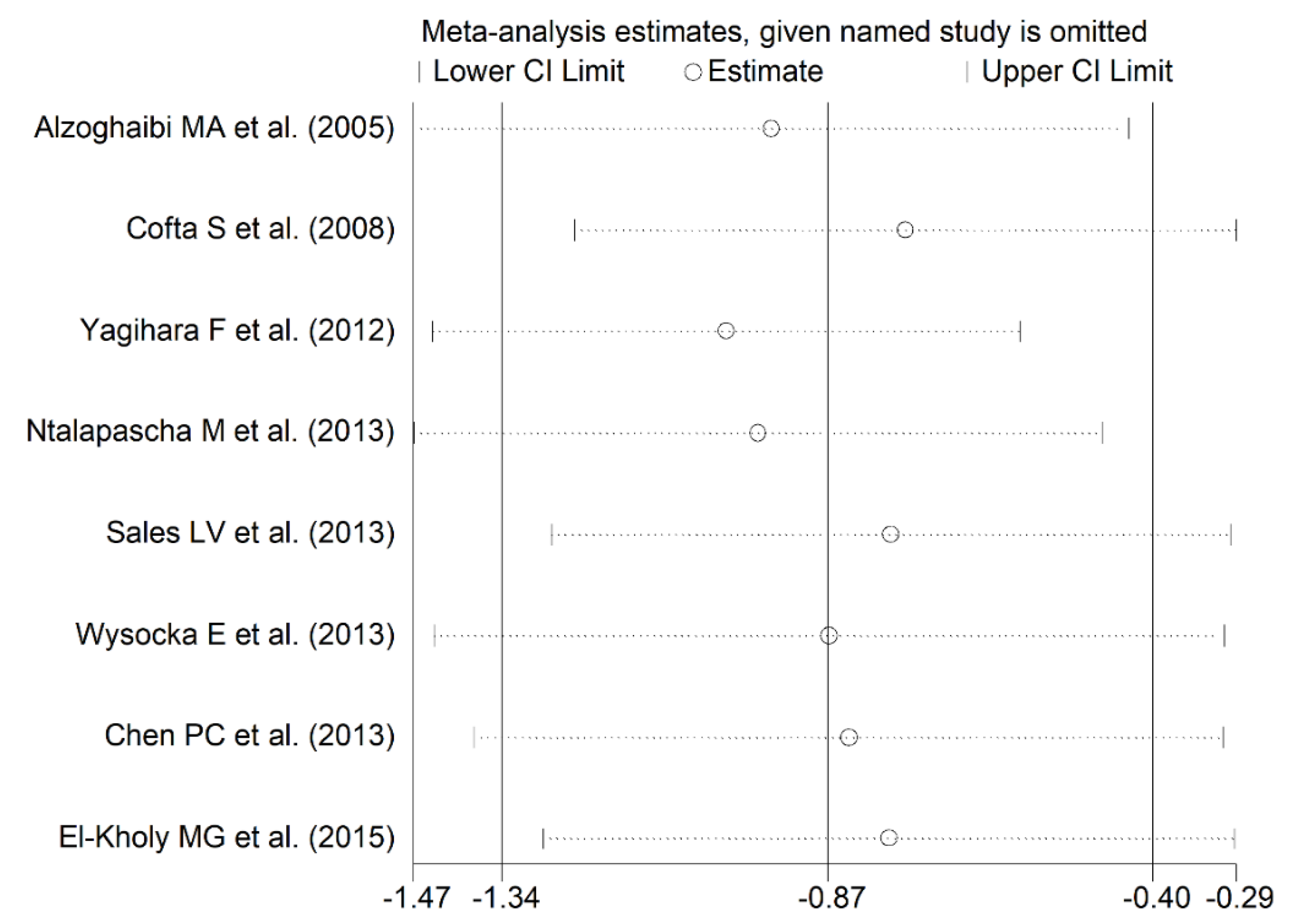
3.2.4. Publication Bias
3.2.5. Certainty of Evidence
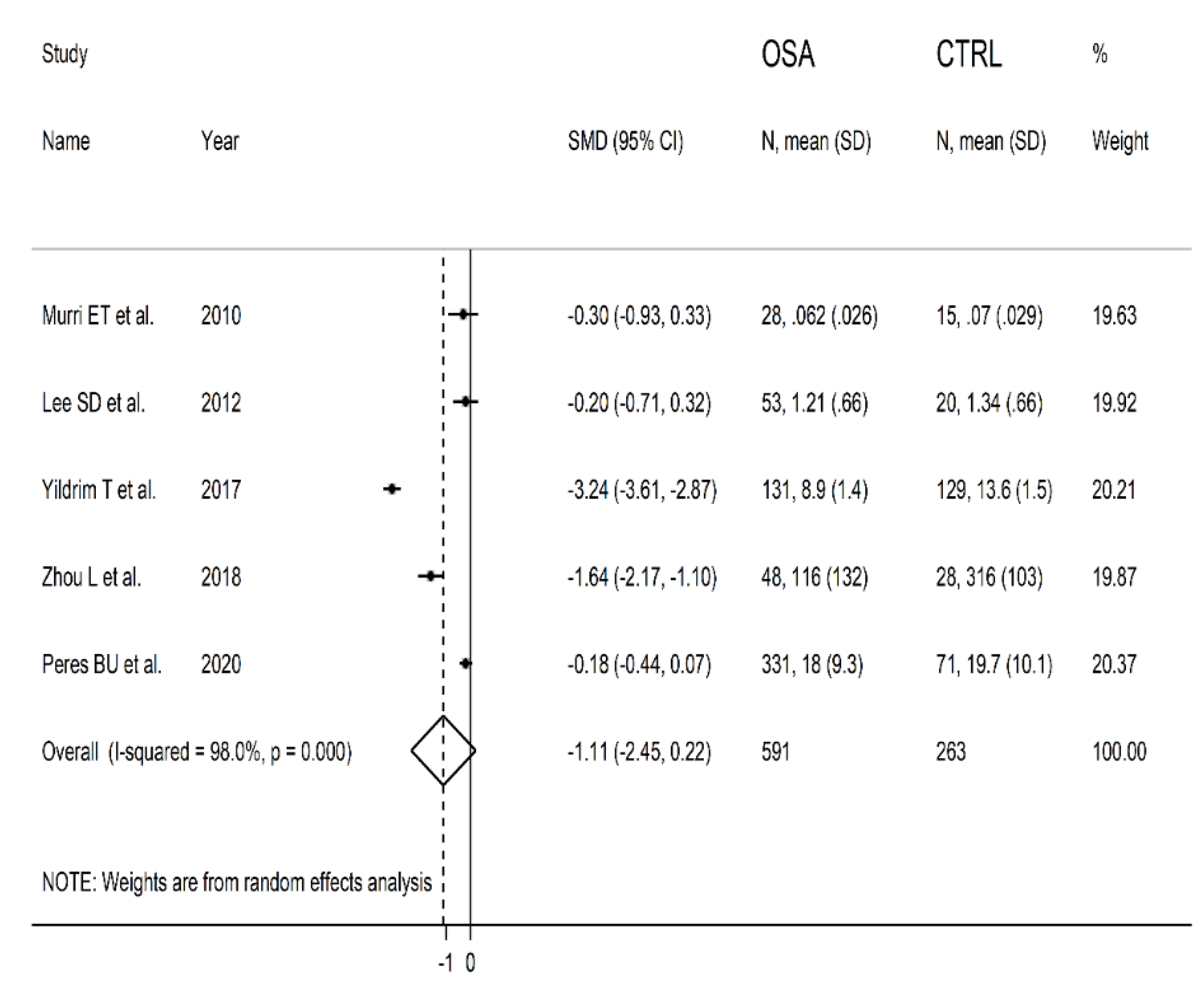
3.3. Meta-Analysis of Serum/Plasma SOD Concentrations
3.3.1. Study Characteristics
3.3.2. Risk of Bias
3.3.3. Results of Individual Studies and Syntheses
3.3.4. Publication Bias
3.3.5. Certainty of Evidence
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sforza, E.; Roche, F. Chronic intermittent hypoxia and obstructive sleep apnea: An experimental and clinical approach. Hypoxia 2016, 4, 99–108. [Google Scholar] [CrossRef] [PubMed]
- Yamauchi, M.; Nakano, H.; Maekawa, J.; Okamoto, Y.; Ohnishi, Y.; Suzuki, T.; Kimura, H. Oxidative stress in obstructive sleep apnea. Chest 2005, 127, 1674–1679. [Google Scholar] [CrossRef]
- Suzuki, Y.J.; Jain, V.; Park, A.M.; Day, R.M. Oxidative stress and oxidant signaling in obstructive sleep apnea and associated cardiovascular diseases. Free Radic. Biol. Med. 2006, 40, 1683–1692. [Google Scholar] [CrossRef]
- Dorasamy, P. Obstructive sleep apnea and cardiovascular risk. Ther. Clin. Risk Manag. 2007, 3, 1105–1111. [Google Scholar] [PubMed]
- Floras, J.S. Sleep Apnea and Cardiovascular Disease: An Enigmatic Risk Factor. Circ. Res. 2018, 122, 1741–1764. [Google Scholar] [CrossRef]
- Lavie, L. Oxidative stress in obstructive sleep apnea and intermittent hypoxia—Revisited—The bad ugly and good: Implications to the heart and brain. Sleep Med. Rev. 2015, 20, 27–45. [Google Scholar] [CrossRef] [PubMed]
- Eisele, H.J.; Markart, P.; Schulz, R. Obstructive Sleep Apnea, Oxidative Stress, and Cardiovascular Disease: Evidence from Human Studies. Oxidative Med. Cell. Longev. 2015, 2015, 608438. [Google Scholar] [CrossRef]
- Zorov, D.B.; Juhaszova, M.; Sollott, S.J. Mitochondrial Reactive Oxygen Species (ROS) and ROS-Induced ROS Release. Physiol. Rev. 2014, 94, 909–950. [Google Scholar] [CrossRef]
- Schieber, M.; Chandel, N.S. ROS Function in Redox Signaling and Oxidative Stress. Curr. Biol. 2014, 24, R453–R462. [Google Scholar] [CrossRef]
- Wang, Y.; Branicky, R.; Noë, A.; Hekimi, S. Superoxide dismutases: Dual roles incontrolling ROS damage and regulating ROS signaling. J. Cell. Biol. 2018, 217, 1915–1928. [Google Scholar] [CrossRef]
- Fiedorczuk, P.; Stróżyński, A.; Olszewska, E. Is the Oxidative Stress in Obstructive Sleep Apnea Associated With Cardiovascular Complications?—Systematic Review. J. Clin. Med. 2020, 9, 3734. [Google Scholar] [CrossRef]
- Wysocka, E.; Cofta, S.; Cymerys, M.; Gozdzik, J.; Torlinski, L.; Batura-Gabryel, H. The impact of the sleep apnea syndrome on oxidant-antioxidant balance in the blood of overweight and obese patients. J. Physiol Pharm. 2008, 59, 761–769. [Google Scholar]
- Liu, W.; Fang, Y.; Luo, M.; Liu, K. Correlation of serum 8-hydroxydeoxyguanylate level with disease severity and oxidative stress in osahs patients. Acta Med. Mediterr. 2020, 36, 3625. [Google Scholar]
- Murri, M.; Garcia-Delgado, R.; Alcázar-Ramirez, J.; Linde, F.; Fernández-Ramos, A.; Cardona, F.; Tinahones, F.J. Assessment of cellular and plasma oxidative stress in SAHS patients before and after continuous positive airway pressure treatment. Clin. Lab. 2010, 56, 397–406. [Google Scholar] [PubMed]
- Stanek, A.; Brożyna-Tkaczyk, K.; Myśliński, W. Oxidative Stress Markers among Obstructive Sleep Apnea Patients. Oxidative Med. Cell. Longev. 2021, 2021, 1–8. [Google Scholar] [CrossRef]
- Moola, S.; Munn, Z.; Tufanaru, C.; Aromataris, E.; Sears, K.; Sfetcu, R.; Currie, M.; Qureshi, R.; Mattis, P.; Lisy, K.; et al. Chapter 7: Systematic reviews of etiology and risk. In Joanna Briggs Institute Reviewer’s Manual for Evidence Synthesis; Aromataris, E., Munn, Z., Eds.; The Joanna Briggs Institute: Adelaide, Australia, 2020; Available online: https://reviewersmanual.joannabriggs.org/ (accessed on 31 October 2021).
- Wan, X.; Wang, W.; Liu, J.; Tong, T. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med. Res. Methodol. 2014, 14, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Hozo, S.P.; Djulbegovic, B.; Hozo, I. Estimating the mean and variance from the median, range, and the size of a sample. BMC Med. Res. Methodol. 2005, 5, 13. [Google Scholar] [CrossRef]
- Bowden, J.; Tierney, J.F.; Copas, A.J.; Burdett, S. Quantifying, displaying and accounting for heterogeneity in the meta-analysis of RCTs using standard and generalised Q statistics. BMC Med. Res. Methodol. 2011, 11, 41. [Google Scholar] [CrossRef]
- Higgins, J.P.T.; Thompson, S.G. Quantifying heterogeneity in a meta-analysis. Stat. Med. 2002, 21, 1539–1558. [Google Scholar] [CrossRef]
- Tobias, A. Assessing the influence of a single study in the meta-analysis estimate. Stata Tech. Bull. 1999, 47, 15–17. [Google Scholar]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed]
- Alzoghaibi, M.A.; BaHammam, A.S.O. Lipid peroxides, superoxide dismutase and circulating IL-8 and GCP-2 in patients with severe obstructive sleep apnea: A pilot study. Sleep Breath. 2005, 9, 119–126. [Google Scholar] [CrossRef] [PubMed]
- Cofta, S.; Wysocka, E.; Piorunek, T.; Rzymkowska, M.; Batura-Gabryel, H.; Torlinski, L. Oxidative stress markers in the blood of persons with different stages of obstructive sleep apnea syndrome. J. Physiol Pharm. 2008, 59, 183–190. [Google Scholar]
- Yagihara, F.; Lucchesi, L.M.; D’Almeida, V.; De Mello, M.T.; Tufik, S.; Bittencourt, L.R.A. Oxidative stress and quality of life in elderly patients with obstructive sleep apnea syndrome: Are there differences after six months of Continuous Positive Airway Pressure treatment? Clinics 2012, 67, 565–571. [Google Scholar] [CrossRef]
- Ntalapascha, M.; Makris, D.; Kyparos, A.; Tsilioni, I.; Kostikas, K.; Gourgoulianis, K.; Kouretas, D.; Zakynthinos, E. Oxidative stress in patients with obstructive sleep apnea syndrome. Sleep Breath. 2013, 17, 549–555. [Google Scholar] [CrossRef]
- Sales, L.V.; Bruin, V.M.; D’Almeida, V.; Pompéia, S.; Bueno, O.F.; Tufik, S.; Bittencourt, L. Cognition and biomarkers of oxidative stress in obstructive sleep apnea. Clinics 2013, 68, 449–455. [Google Scholar] [CrossRef]
- Wysocka, E.; Cofta, S.; Piorunek, T.; Dziegielewska-Gesiak, S.; Bryl, W.; Torlinski, L. Blood Antioxidant Status, Dysglycemia and Obstructive Sleep Apnea. Adv. Exp. Med. Biol. 2012, 756, 121–129. [Google Scholar]
- Chen, P.-C.; Guo, C.-H.; Tseng, C.-J.; Wang, K.-C.; Liu, P.-J. Blood trace minerals concentrations and oxidative stress in patients with obstructive sleep apnea. J. Nutr. Health Aging 2013, 17, 639–644. [Google Scholar] [CrossRef]
- El-Kholy, M.G.; El-Shafey, B.I.; Hantera, M.S.; Ganna, S.A.; El-Sorogy, H.A.; Faisl, A.E.-R.F. Effect of continuous positive airway pressure on oxidative stress accompanied by obstructive sleep apnea. Egypt. J. Bronchol. 2015, 9, 192–197. [Google Scholar] [CrossRef]
- Lee, S.D.; Ju, G.; Choi, J.-A.; Kim, J.-W.; Yoon, I.-Y. The association of oxidative stress with central obesity in obstructive sleep apnea. Sleep Breath. 2011, 16, 511–517. [Google Scholar] [CrossRef] [PubMed]
- Yildirim, T.; Alp, R. The role of oxidative stress in the relation between fibromyalgia and obstructive sleep apnea syndrome. Eur. Rev. Med. Pharmacol. Sci. 2017, 21, 20–29. [Google Scholar]
- Zhou, L.; Ouyang, R.; Luo, H.; Peng, Y.; Chen, P.; Ren, S.; Liu, G. Dysfunction of Nrf2-ARE Signaling Pathway: Potential Pathogenesis in the Development of Neurocognitive Impairment in Patients with Moderate to Severe Obstructive Sleep Apnea-Hypopnea Syndrome. Oxidative Med. Cell. Longev. 2018, 2018, 1–15. [Google Scholar] [CrossRef]
- Peres, B.U.; Allen, A.J.H.; Shah, A.; Fox, N.; Laher, I.; Almeida, F.; Jen, R.; Ayas, N. Obstructive Sleep Apnea and Circulating Biomarkers of Oxidative Stress: A Cross-Sectional Study. Antioxidants 2020, 9, 476. [Google Scholar] [CrossRef] [PubMed]
- Jean-Louis, G.; Zizi, F.; Brown, D.; Ogedegbe, G.; Borer, J.; McFarlane, S. Obstructive sleep apnea and cardiovascular disease: Evidence and underlying mechanisms. Minerva Pneumol. 2009, 48, 277–293. [Google Scholar]
- Schulz, R.; Mahmoudi, S.; Hattar, K.; Sibelius, U.; Olschewski, H.; Mayer, K.; Seeger, W.; Grimminger, F. Enhanced Release of Superoxide from Polymorphonuclear Neutrophils in Obstructive Sleep Apnea. Impact of continuous positive airway pressure therapy. Am. J. Respir. Crit. Care Med. 2000, 162, 566–570. [Google Scholar] [CrossRef]
- Ozturk, L.; Mansour, B.; Yuksel, M.; Yalcin, A.S.; Celicoglu, F.; Gokhan, N. Lipid peroxidation and osmotic fragility of red blood cells in sleep-apnea patients. Clin. Chim. Acta 2003, 332, 83–88. [Google Scholar] [CrossRef]
- Pau, M.C.; Zinellu, E.; Fois, S.S.; Piras, B.; Pintus, G.; Carru, C.; Mangoni, A.A.; Fois, A.G.; Zinellu, A.; Pirina, P. Circulating Malondialdehyde Concentrations in Obstructive Sleep Apnea (OSA): A Systematic Review and Meta-Analysis with Meta-Regression. Antioxidants 2021, 10, 1053. [Google Scholar] [CrossRef] [PubMed]
- Jordan, W.; Cohrs, S.; Degner, D.; Meier, A.; Rodenbeck, A.; Mayer, G.; Pilz, J.; Rüther, E.; Kornhuber, J.; Bleich, S. Evaluation of oxidative stress measurements in obstructive sleep apnea syndrome. J. Neural Transm. 2006, 113, 239–254. [Google Scholar] [CrossRef]
- Turan, H.; Demir, C. Investigation of Lipid Peroxidation and Antioxidant Enzyme Activity in Sleep Apnea Patients. Nat. Sci. Discov. 2016, 2, 36–40. [Google Scholar] [CrossRef][Green Version]
- Kolesnikova, L.I.; Madaeva, I.M.; Semenova, N.V.; Vlasov, B.Y.; Grebenkina, L.A.; Darenskaya, M.A.; Dolgikh, M.I. Antioxidant potential of the blood in men with obstructive sleep breathing disorders. Bull. Exp. Biol. Med. 2013, 154, 731–733. [Google Scholar] [CrossRef] [PubMed]
- Tukai, T.; Ushio-Fukai, M. Superoxide dismutases: Role in redox signalling, vascular function, and diseases. Antioxid. Redox Signal. 2011, 15, 1583–1606. [Google Scholar]
- Seyedsadjadi, N.; Grant, R. The Potential Benefit of Monitoring Oxidative Stress and Inflammation in the Prevention of Non-Communicable Diseases (NCDs). Antioxidants 2020, 10, 15. [Google Scholar] [CrossRef]
- Revin, V.V.; Gromova, N.V.; Revina, E.S.; Samonova, A.Y.; Tychkov, A.Y.; Bochkareva, S.S.; Moskovkin, A.A.; Kuzmenko, T.P. The Influence of Oxidative Stress and Natural Antioxidants on Morphometric Parameters of Red Blood Cells, the Hemoglobin Oxygen Binding Capacity, and the Activity of Antioxidant Enzymes. BioMed Res. Int. 2019, 2019, 2109269. [Google Scholar] [CrossRef] [PubMed]
- Tian, Z.; Sun, H.; Kang, J.; Mu, Z.; Liang, J.; Li, M. Association between the circulating superoxide dismutase and obstructive sleep apnea: A meta-analysis. Eur. Arch. Oto-Rhino-Laryngol. 2021. [Google Scholar] [CrossRef] [PubMed]


| Control | OSA | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| First Author Year, Country | n | Age Mean | Gender (M/F) | SOD Mean ± SD | n | Age Mean | Gender (M/F) | SOD Mean ± SD | AHI Events/h |
| BLOOD | |||||||||
| Alzoghaibi MA et al., 2005, Saudi Arabia | 17 | 50 | NR | 0.31 ± 0.04 U/L | 25 | 31 | NR | 0.29 ± 0.08 U/L | 73.5 |
| Cofta S et al., 2008, Poland | 21 | 53 | 11/10 | 1579 ± 325 U/gHb | 61 | 52 | 43/18 | 1096 ± 295 U/gHb | 24 |
| Yagihara F et al., 2012, Brasil | 27 | 66 | 27/0 | 10.2 ± 4.0 U/mgHb | 30 | 66 | 30/0 | 10.5 ± 2.9 U/mgHb | 38 |
| Ntalapascha M et al., 2013, Greece | 13 | 49 | 13/0 | 195 ± 39 ng/mL | 18 | 50 | 18/0 | 191 ± 33 ng/mL | 24 |
| Sales LV et al., 2013, Brasil | 13 | 37 | 13/0 | 14.4 ± 2.3 U/mgHb | 14 | 36 | 14/0 | 10.0 ± 2.9 U/mgHb | 36 |
| Wysocka E et al., 2013, Poland | 44 | 55 | 44/0 | 1408 ± 181 U/gHb | 44 | 53 | 44/0 | 1217 ± 234 U/gHb | 26 |
| Chen P et al., 2013, Taiwan | 20 | 42 | 15/5 | 3.60 ± 0.81 U/mgHb | 44 | 42 | 33/11 | 2.37 ± 1.24 U/mgHb | 15 |
| El-Kholy MG et al., 2015, Egypt | 20 | 49 | 10/10 | 117 ± 25 U/mL | 20 | 51 | 9/11 | 83 ± 19 U/mL | 30 |
| SERUM/PLASMA | |||||||||
| Murri ET et al., 2010, Spain | 15 | 54 | 15/0 | 0.070 ± 0.029 U/mL | 28 | 46 | NR | 0.062 ± 0.026 U/mL | 52 |
| Lee SD et al., 2012, South Korea | 20 | 44 | 20/0 | 1.34 ± 0.66 U/mL | 53 | 47 | 53/0 | 1.21 ± 0.66 U/mL | 32 |
| Yildrim T et al., 2017, Turkey | 129 | 49 | 78/51 | 13.6 ± 1.5 NR | 131 | 49 | 74/57 | 8.9 ± 1.4 NR | 34 |
| Zhou L et al., 2018, China | 28 | 45 | 20/8 | 316 ± 103 ng/mL | 48 | 41 | NR | 116 ± 132 ng/mL | 47 |
| Peres BU et al., 2020, Canada | 71 | 52 | 38/33 | 19.74 ± 10.05 % | 331 | 45 | 237/94 | 18.00 ± 9.30 % | 26 |
| Study | Were the Criteria for Inclusion in the Sample Clearly Defined? | Were the Study Subjects and the Setting Described in Detail? | Was the Exposure Measured in a Valid and Reliable Way? | Were Objective, Standard Criteria Used for Measurement of the Condition? | Were Confounding Factors Identified? | Were Strategies to Deal with Confounding Factors Stated? | Were the Outcomes Measured in a Valid and Reliable Way? | Was Appropriate Statistical Analysis Used? | Risk of Bias |
|---|---|---|---|---|---|---|---|---|---|
| Alzoghaibi | Unclear | Yes | Yes | Yes | No | No | Yes | No | Moderate |
| Cofta | Yes | Yes | Yes | Yes | No | No | Yes | No | Low |
| Yagihara | Yes | Yes | Yes | Yes | No | No | Yes | No | Low |
| Nptalapascha | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Low |
| Sales | Yes | Yes | Yes | Yes | No | No | Yes | No | Low |
| Wysocka | Yes | Yes | Yes | Yes | No | No | Yes | No | Low |
| Chen | Yes | Yes | Yes | Yes | No | No | Yes | No | Low |
| El-Kholy | Yes | Yes | Yes | Yes | No | No | Yes | No | Low |
| Murri | Yes | Yes | Yes | Yes | No | No | Yes | No | Low |
| Lee | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Low |
| Yildirim | Yes | Yes | Yes | Yes | No | No | Yes | No | Low |
| Zhou | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Low |
| Peres | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Low |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pau, M.C.; Mangoni, A.A.; Zinellu, E.; Pintus, G.; Carru, C.; Fois, A.G.; Pirina, P.; Zinellu, A. Circulating Superoxide Dismutase Concentrations in Obstructive Sleep Apnoea (OSA): A Systematic Review and Meta-Analysis. Antioxidants 2021, 10, 1764. https://doi.org/10.3390/antiox10111764
Pau MC, Mangoni AA, Zinellu E, Pintus G, Carru C, Fois AG, Pirina P, Zinellu A. Circulating Superoxide Dismutase Concentrations in Obstructive Sleep Apnoea (OSA): A Systematic Review and Meta-Analysis. Antioxidants. 2021; 10(11):1764. https://doi.org/10.3390/antiox10111764
Chicago/Turabian StylePau, Maria Carmina, Arduino Aleksander Mangoni, Elisabetta Zinellu, Gianfranco Pintus, Ciriaco Carru, Alessandro Giuseppe Fois, Pietro Pirina, and Angelo Zinellu. 2021. "Circulating Superoxide Dismutase Concentrations in Obstructive Sleep Apnoea (OSA): A Systematic Review and Meta-Analysis" Antioxidants 10, no. 11: 1764. https://doi.org/10.3390/antiox10111764
APA StylePau, M. C., Mangoni, A. A., Zinellu, E., Pintus, G., Carru, C., Fois, A. G., Pirina, P., & Zinellu, A. (2021). Circulating Superoxide Dismutase Concentrations in Obstructive Sleep Apnoea (OSA): A Systematic Review and Meta-Analysis. Antioxidants, 10(11), 1764. https://doi.org/10.3390/antiox10111764










