The Effect of Cold Plasma Pretreatment on Water-Suspended Herbs Measured in the Content of Bioactive Compounds, Antioxidant Activity, Volatile Compounds and Microbial Count of Final Extracts
Abstract
:1. Introduction
2. Materials and Methods
2.1. Extraction Process
2.2. pH Determination
2.3. Color Analysis
2.4. Total Phenolic Content Analysis
2.5. Total Anthocyanin Analysis
2.6. Total Flavonoid Analysis
2.7. DPPH Analysis
2.8. FRAP Analysis
2.9. ABTS Radical Cation Scavenging Activity
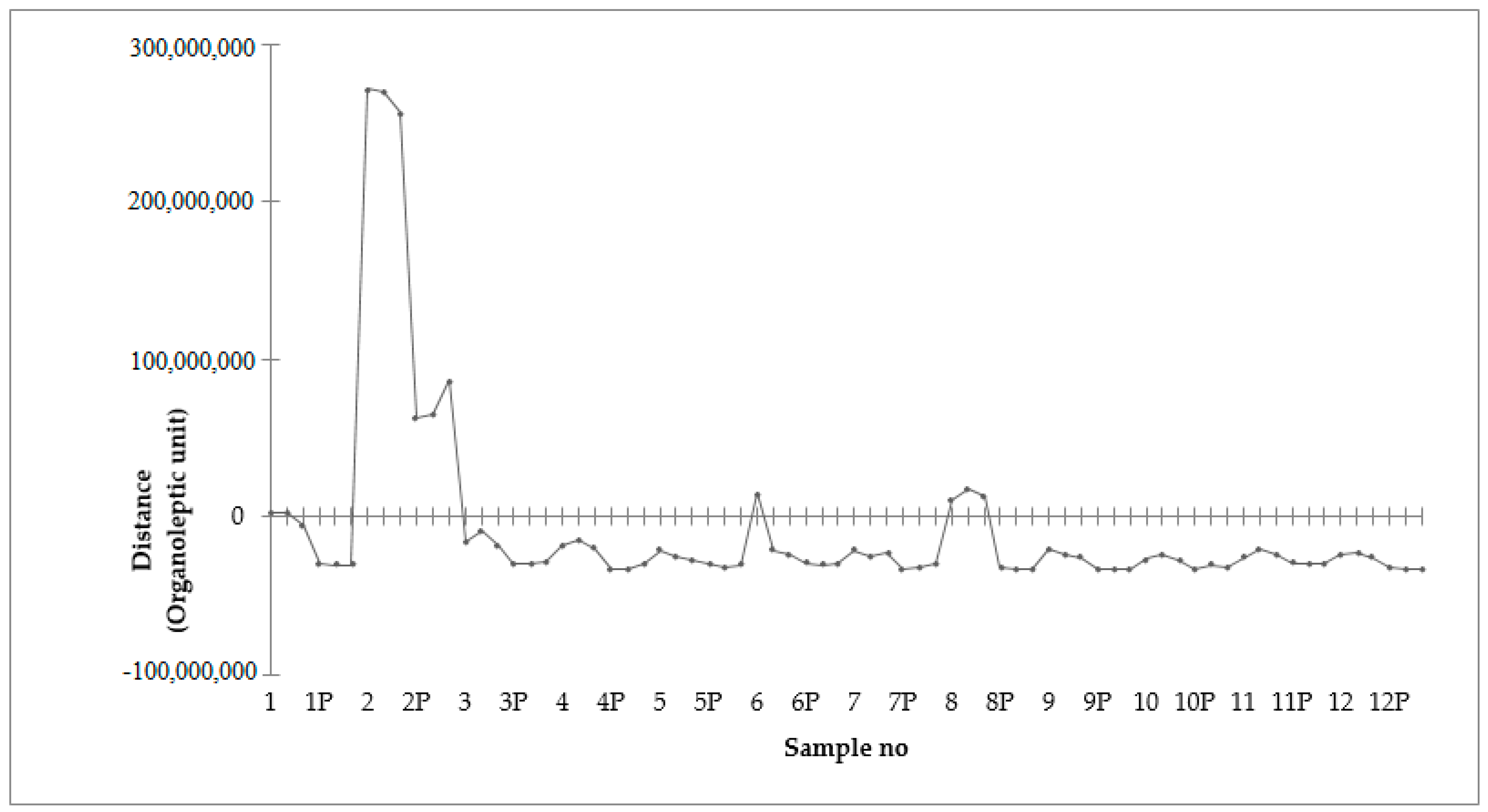
2.10. Electronic Nose Analysis
2.11. Microbiological Analysis—Total Aerobic Bacteria Count
2.12. Statistical Analysis
3. Results and Discussion
3.1. Color and pH
3.2. Total Phenolic, Anthocyanins and Flavonoids Content
3.3. Antioxidant Activity
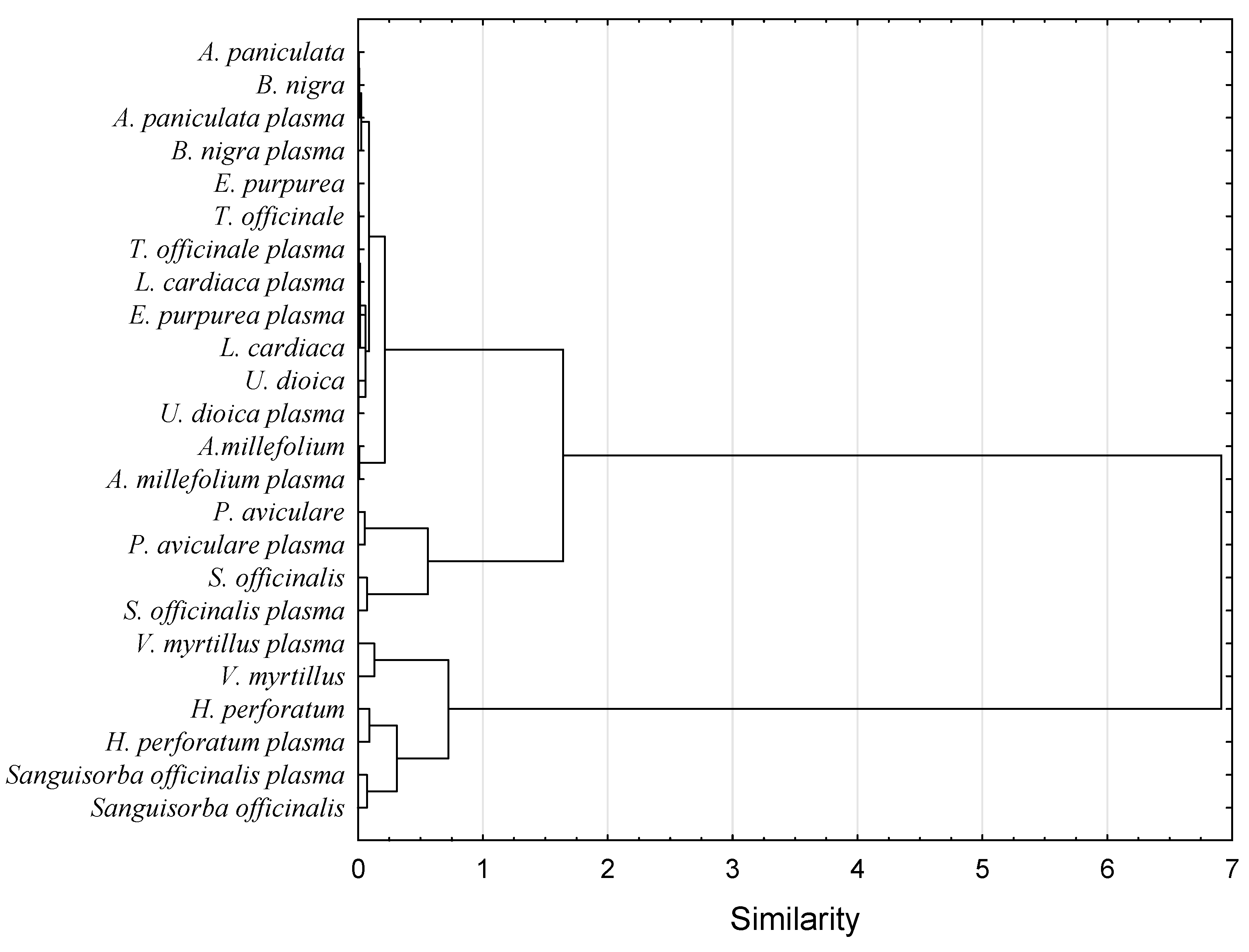
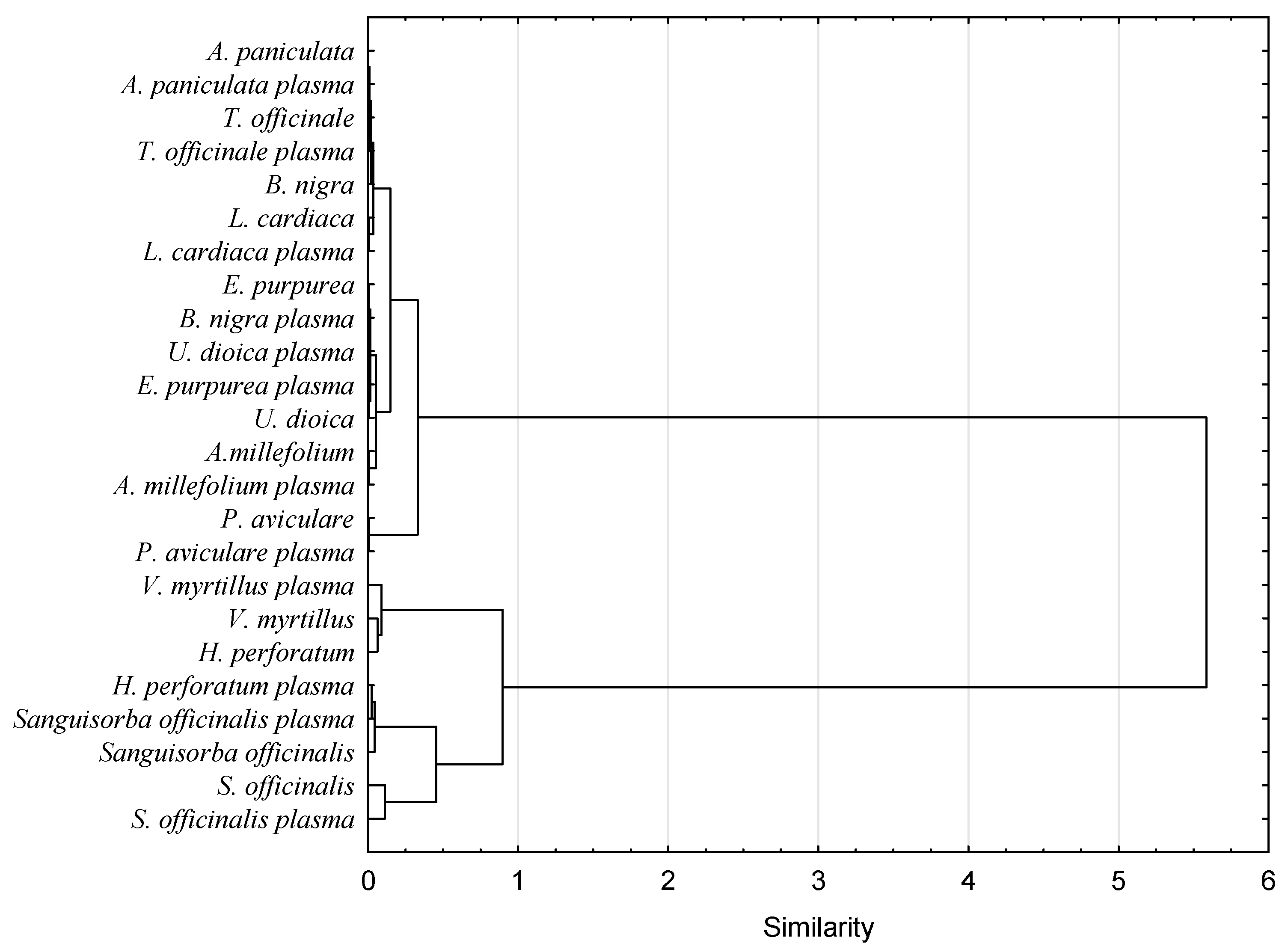
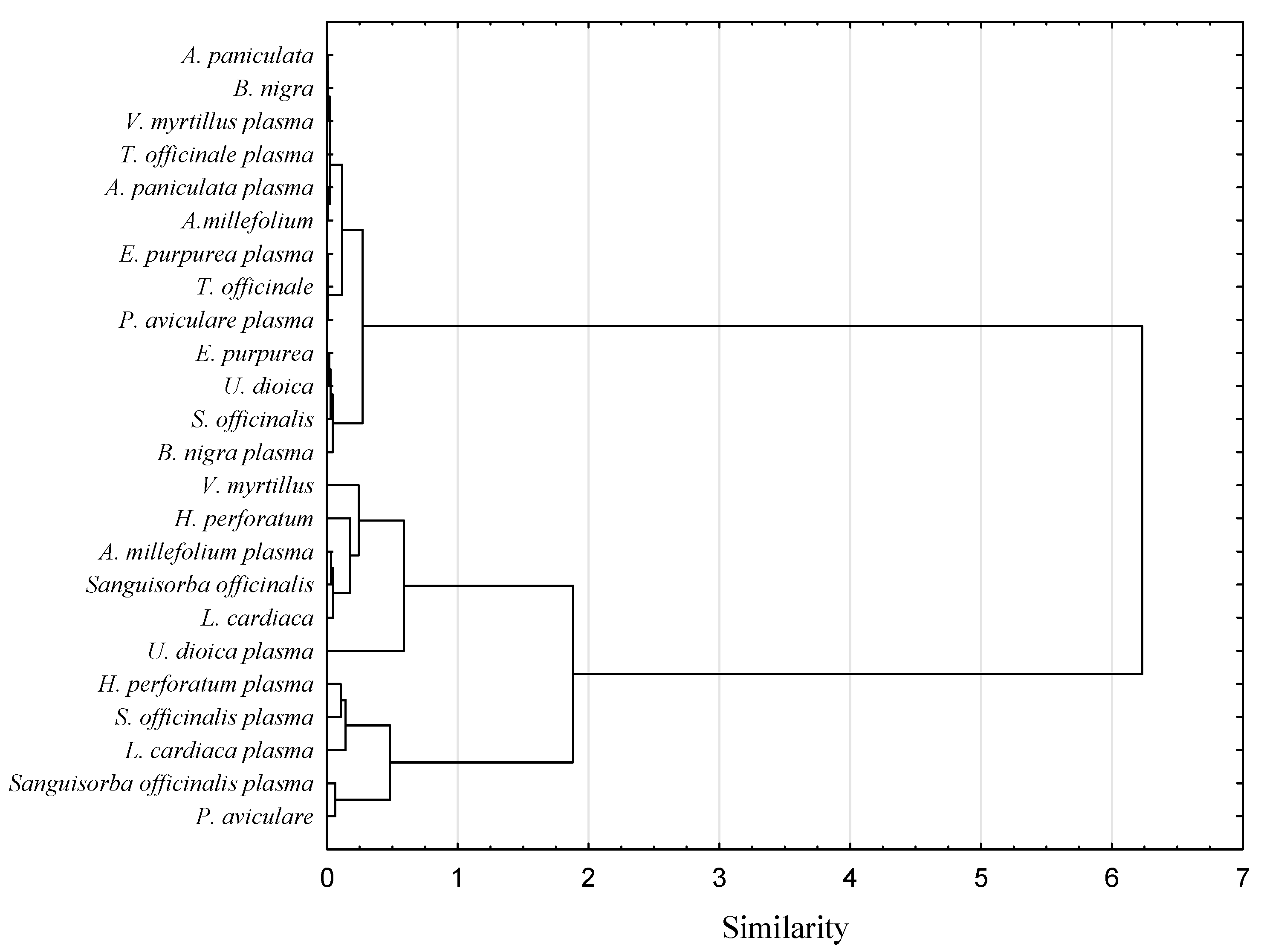
3.4. Volatile Compounds Profile
3.5. Total Aerobic Bacteria Count
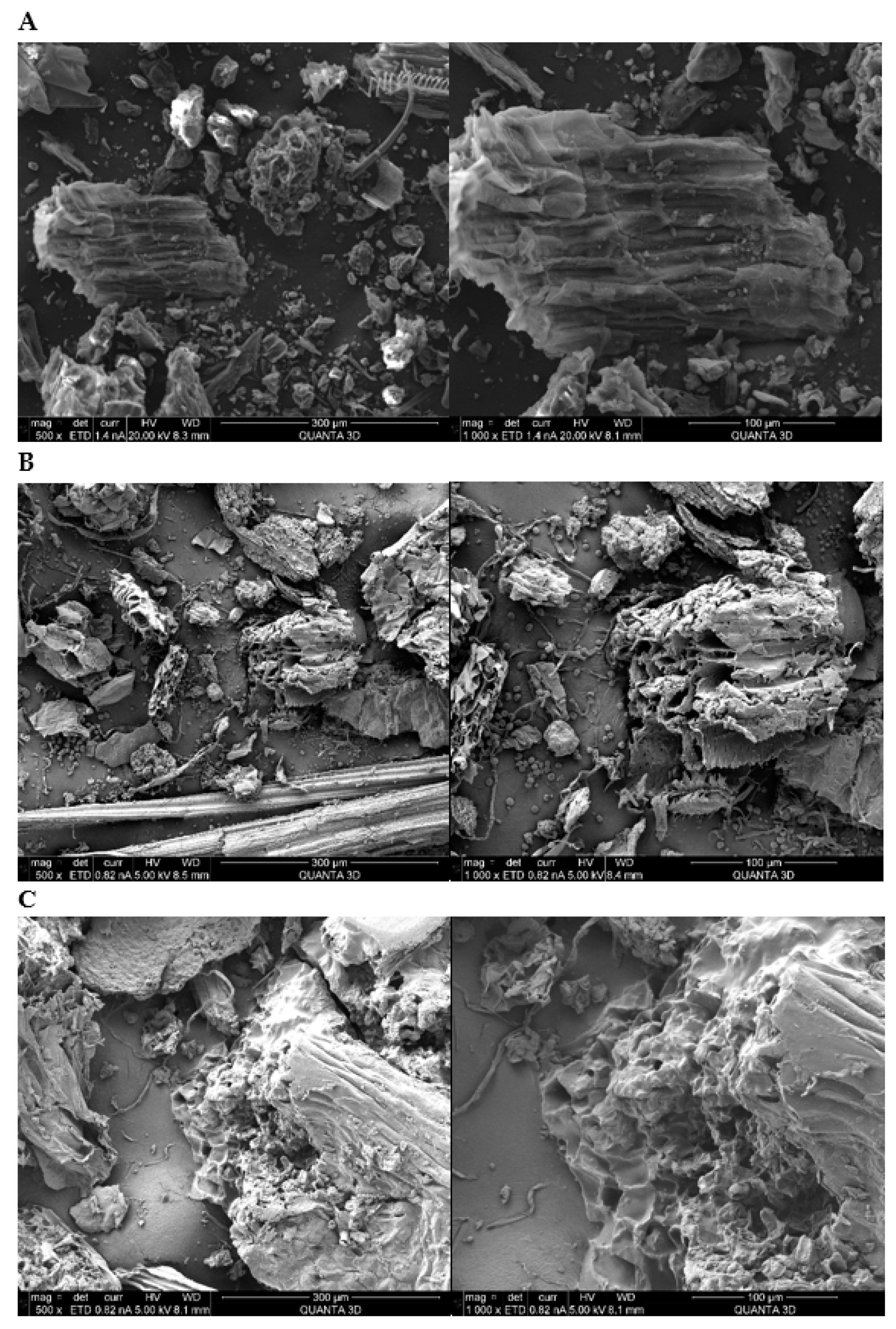
3.6. Surface Morphology Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Shirazi, O.U.; Khattak, M.; Shukri, N.A.M.; Nasyriq, M.N. Determination of total phenolic, flavonoid content and free radical scavenging activities of common herbs and spices. J. Pharmacogn. Phytochem. 2014, 3, 104–108. [Google Scholar]
- Durazzo, A.; Lucarini, M.; Souto, E.B.; Cicala, C.; Caiazzo, E.; Izzo, A.A.; Novellino, E.; Santini, A. Polyphenols: A concise overview on the chemistry, occurrence, and human health. Phytother. Res. 2019, 33, 2221–2243. [Google Scholar] [CrossRef] [Green Version]
- Chemat, F.; Vian, M.A.; Fabiano-Tixier, A.-S.; Nutrizio, M.; Jambrak, A.R.; Munekata, P.E.S.; Lorenzo, J.M.; Barba, F.J.; Binello, A.; Cravotto, G. A review of sustainable and intensified techniques for extraction of food and natural products. Green Chem. 2020, 22, 2325–2353. [Google Scholar] [CrossRef] [Green Version]
- Hanula, M.; Wyrwisz, J.; Moczkowska, M.; Horbańczuk, O.K.; Pogorzelska-Nowicka, E.; Wierzbicka, A. Optimization of microwave and ultrasound extraction methods of açai berries in terms of highest content of phenolic compounds and anti-oxidant activity. Appl. Sci. 2020, 10, 8325. [Google Scholar] [CrossRef]
- Ramazzina, I.; Berardinelli, A.; Rizzi, F.; Tappi, S.; Ragni, L.; Sacchetti, G.; Rocculi, P. Effect of cold plasma treatment on physico-chemical parameters and antioxidant activity of minimally processed kiwifruit. Postharvest Biol. Technol. 2015, 107, 55–65. [Google Scholar] [CrossRef]
- Hemmati, V.; Garavand, F.; Goudarzi, M.; Sarlak, Z.; Cacciotti, I.; Tiwari, B.K. Cold atmospheric-pressure plasma treatment of turmeric powder: Microbial load, essential oil profile, bioactivity and microstructure analyses. Int. J Food Sci. Technol. 2020, 56, 2224–2232. [Google Scholar] [CrossRef]
- Akyoo, A.; Lazaro, E. Institutional Capacity for Standards Conformity Assessment: A Case Study on Spices in Tanzania; DIIS Working Paper: Copenhagen, Danish, 2008. [Google Scholar]
- Gil, M.I.; Selma, M.V.; López-Gálvez, F.; Allende, A. Fresh-cut product sanitation and wash water disinfection: Problems and solutions. Int. J. Food Microbiol. 2009, 134, 37–45. [Google Scholar] [CrossRef] [PubMed]
- Keshavarzi, M.; Najafi, G.; Gavlighi, H.A.; Seyfi, P.; Ghomi, H. Enhancement of polyphenolic content extraction rate with maximal antioxidant activity from green tea leaves by cold plasma. J. Food Sci. 2020, 85, 3415–3422. [Google Scholar] [CrossRef] [PubMed]
- Surowsky, B.; Bußler, S.; Schlüter, O. Cold Plasma Interactions With Food Constituents in Liquid and Solid Food Matrices. In Cold Plasma in Food and Agriculture; Elsevier B: Amsterdam, The Netherlands, 2016; pp. 179–203. [Google Scholar]
- Singleton, V.L.; Rossi, J.A. Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar]
- Belwal, T.; Dhyani, P.; Bhatt, I.D.; Rawal, R.S.; Pande, V. Optimization extraction conditions for improving phenolic content and antioxidant activity in Berberis asiatica fruits using response surface methodology (RSM). Food Chem. 2016, 207, 115–124. [Google Scholar] [CrossRef]
- Chang, C.C.; Yang, M.H.; Wen, H.M.; Chern, J.C. Estimation of total flavonoid content in propolis by two complementary colorimetric methods. J. Food Drug Anal. 2002, 10, 178–182. [Google Scholar]
- Shimada, K.; Fujikawa, K.; Yahara, K.; Nakamura, T. Antioxidative properties of xanthan on the autoxidation of soybean oil in cyclodextrin emulsion. J. Agric. Food Chem. 1992, 40, 945–948. [Google Scholar] [CrossRef]
- Paixão, L.M.N.; Fonteles, T.V.; Oliveira, V.S.; Fabiano, A.N.F.; Rodrigues, S. Cold plasma effect on functional com-pounds of Siriguela Juice. Food Bioprocess Technol. 2019, 12, 110–121. [Google Scholar] [CrossRef]
- Chen, Y.-Q.; Cheng, J.-H.; Sun, D.-W. Chemical, physical and physiological quality attributes of fruit and vegetables induced by cold plasma treatment: Mechanisms and application advances. Crit. Rev. Food Sci. Nutr. 2019, 60, 2676–2690. [Google Scholar] [CrossRef]
- Segat, A.; Misra, N.; Cullen, P.; Innocente, N. Atmospheric pressure cold plasma (ACP) treatment of whey protein isolate model solution. Innov. Food Sci. Emerg. Technol. 2015, 29, 247–254. [Google Scholar] [CrossRef]
- Pankaj, S.K.; Wan, Z.; Colonna, W.; Keener, K.M. Effect of high voltage atmospheric cold plasma on white grape juice quality. J. Sci. Food Agric. 2017, 97, 4016–4021. [Google Scholar] [CrossRef]
- Pandey, K.B.; Rizvi, S.I. Plant Polyphenols as Dietary Antioxidants in Human Health and Disease. Oxidative Med. Cell. Longev. 2009, 2, 270–278. [Google Scholar] [CrossRef] [Green Version]
- Dasan, B.G.; Boyaci, I.H. Effect of Cold Atmospheric Plasma on Inactivation of Escherichia coli and Physicochemical Properties of Apple, Orange, Tomato Juices, and Sour Cherry Nectar. Food Bioprocess Technol. 2017, 11, 334–343. [Google Scholar] [CrossRef]
- Sarangapani, C.; Thirumdas, R.; Devi, Y.; Trimukhe, A.; Deshmukh, R.; Annapure, U.S. Effect of low-pressure plasma on physico–chemical and functional properties of parboiled rice flour. LWT 2016, 69, 482–489. [Google Scholar] [CrossRef]
- Mehta, D.; Sharma, N.; Bansal, V.; Sangwan, R.S.; Yadav, S.K. Impact of ultrasonication, ultraviolet and atmospheric cold plasma processing on quality parameters of tomato-based beverage in comparison with thermal processing. Innov. Food Sci. Emerg. Technol. 2019, 52, 343–349. [Google Scholar] [CrossRef]
- Garofulić, I.E.; Jambrak, A.R.; Milošević, S.; Dragović-Uzelac, V.; Zorić, Z.; Herceg, Z. The effect of gas phase plasma treatment on the anthocyanin and phenolic acid content of sour cherry Marasca (Prunus cerasus var. Marasca) juice. LWT Food Sci. Technol. 2015, 62, 894–900. [Google Scholar] [CrossRef]
- Misra, N.N.; Pankaj, S.K.; Segat, A.; Ishikawa, K. Cold plasma interactions with enzymes in foods and model systems. Trends Food Sci. Technol. 2016, 55, 39–47. [Google Scholar] [CrossRef]
- Paz, M.; Gúllon, P.; Barroso, M.F.; Carvalho, A.P.; Domingues, V.; Gomes, A.M.; Becker, H.; Longhinotti, E.; Delerue-Matos, C. Brazilian fruit pulps as functional foods and additives: Evaluation of bioactive compounds. Food Chem. 2015, 172, 462–468. [Google Scholar] [CrossRef] [Green Version]
- Chen, C.; Liu, C.; Jiang, A.; Guan, Q.; Sun, X.; Liu, S.; Hao, K.; Hu, W. The Effects of Cold Plasma-Activated Water Treatment on the Microbial Growth and Antioxidant Properties of Fresh-Cut Pears. Food Bioprocess Technol. 2019, 12, 1842–1851. [Google Scholar] [CrossRef]
- Rodríguez, Ó.; Gomes, W.F.; Rodrigues, S.; Fernandes, F.A.N. Effect of indirect cold plasma treatment on cashew apple juice (Anacardium occidentale L.). LWT-Food Sci. Technol. 2017, 84, 457–463. [Google Scholar] [CrossRef]
- Zhang, X.-L.; Zhong, C.-S.; Mujumdar, A.S.; Yang, X.-H.; Deng, L.-Z.; Wang, J.; Xiao, H.-W. Cold plasma pretreatment enhances drying kinetics and quality attributes of chili pepper (Capsicum annuum L.). J. Food Eng. 2018, 241, 51–57. [Google Scholar] [CrossRef]
- Sharopov, F.S.; Wink, M.; Setzer, W.N. Radical scavenging and antioxidant activities of essential oil components--an experi-mental and computational investigation. Nat. Prod. Commun. 2015, 10, 153–156. [Google Scholar]
- Ebadi, M.; Abbasi, S.; Harouni, A.; Sefidkon, F. Effect of cold plasma on essential oil content and composition of lemon verbena. Food Sci. Nutr. 2019, 7, 1166–1171. [Google Scholar] [CrossRef] [Green Version]
- Kodama, S.; Thawatchaipracha, B.; Sekiguchi, H. Enhancement of Essential Oil Extraction for Steam Distillation by DBD Surface Treatment. Plasma Process. Polym. 2014, 11, 126–132. [Google Scholar] [CrossRef]
- Mir, S.A.; Shah, M.A.; Mir, M.M. Understanding the Role of Plasma Technology in Food Industry. Food Bioprocess Technol. 2016, 9, 734–750. [Google Scholar] [CrossRef]
- Dghaim, R.; Al Sabbah, H.; Al Zarooni, A.H.; Khan, M.A. Antibacterial effects and microbial quality of commonly consumed herbs in Dubai, United Arab Emirates. Int. Food Res. J. 2017, 24, 2677–2684. [Google Scholar]
- Nur, F.; Libra, U.K.; Rowsan, P.; Azad, A.K.; Begum, K. Assessment of Bacterial Contamination of Dried Herbs and Spices Collected from Street Markets in Dhaka. Bangladesh Pharm. J. 2018, 21, 96–100. [Google Scholar] [CrossRef]
- Kim, J.E.; Kim, I.-H.; Min, S.C. Microbial Decontamination of Vegetables and Spices Using Cold Plasma Treatments. Korean J. Food Sci. Technol. 2013, 45, 735–741. [Google Scholar] [CrossRef] [Green Version]
- Lee, H.S.; Park, H.H.; Min, S.C. Microbial decontamination of red pepper powder using pulsed light plasma. J. Food Eng. 2020, 284, 110075. [Google Scholar] [CrossRef]
- Zhang, Q.; Liang, Y.; Feng, H.; Ma, R.; Tian, Y.; Zhang, J.; Fang, J. A study of oxidative stress induced by non-thermal plasma-activated water for bacterial damage. Appl. Phys. Lett. 2013, 102, 203701. [Google Scholar] [CrossRef]
- Attri, P.; Choi, E.H. Influence of Reactive Oxygen Species on the Enzyme Stability and Activity in the Presence of Ionic Liquids. PLoS ONE 2013, 8, e75096. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Attri, P.; Sarinont, T.; Kim, M.; Amano, T.; Koga, K.; Cho, A.E.; Choi, E.H.; Shirtani, M. Influence of ionic liquid and ionic salt on protein against the reactive species generated using dielectric barrier discharge plasma. Sci. Rep. 2015, 5, 1–13. [Google Scholar] [CrossRef]
- Grzegorzewski, F.; Ehlbeck, J.; Schlüter, O.; Kroh, L.W.; Rohn, S. Treating lamb’s lettuce with a cold plasma—Influence of atmospheric pressure Ar plasma immanent species on the phenolic profile of Valerianella locusta. LWT Food Sci. Technol. 2011, 44, 2285–2289. [Google Scholar] [CrossRef]
- Moritz, S.; Schmidt, A.; Sann, J.; Thoma, M.H. Surface modifications caused by cold atmospheric plasma sterilization treatment. J. Phys. D Appl. Phys. 2020, 53, 325203. [Google Scholar] [CrossRef] [Green Version]
| Herb Species | L* | a* | b* | |||
|---|---|---|---|---|---|---|
| Control | P-Treated | Control | P-Treated | Control | P-Treated | |
| Echinacea purpurea | 23.05 ± 0.56 | 24 ± 0.53 | 4.2 ± 0.3 a | 7.88 ± 0.37 b | 4.95 ± 0.32 a | 7.88 ± 0.76 b |
| Salvia officinalis | 31.79 ± 0.37 | 32.23 ± 0.55 | 17.06 ± 0.39 | 17.05 ± 0.33 | 20.62 ± 0.48 | 21.2 ± 0.76 |
| Urtica dioica | 21.03 ± 0.4 | 20.89 ± 0.49 | -0.05 ± 0.1 | 0.07 ± 0.12 | 2.05 ± 0.11 | 2.35 ± 0.13 |
| Polygonum aviculare | 61.4 ± 1.33 | 60.42 ± 0.9 | 1.09 ± 0.08 | 1.42 ± 0.1 | 21.98 ± 0.76 a | 24.16 ± 0.44 b |
| Vaccinium myrtillus | 61.23 ± 2.16 | 58.49 ± 1.47 | 0 ± 0.07 a | 2.08 ± 0.42 b | 27.74 ± 2.39 a | 34.05 ± 0.24 b |
| Taraxacum officinale | 26.13 ± 0.65 | 25.64 ± 0.67 | 14.28 ± 0.56 a | 15.15 ± 0.86 b | 9.76 ± 0.34 | 10.41 ± 0.67 |
| Hypericum perforatum | 47.28 ± 0.3 | 47.74 ± 0.56 | 8.93 ± 0.18 | 8.8 ± 0.22 | 21.55 ± 0.48 a | 25.87 ± 0.19 b |
| Achillea millefolium | 33.35 ± 0.55 | 32.63 ± 0.48 | 15.19 ± 0.29 a | 16.55 ± 0.32 b | 22.44 ± 0.5 | 21.93 ± 0.75 |
| Sanguisorba officinalis | 49.77 ± 0.72 a | 51.17 ± 0.45 b | 8.94 ± 0.38 | 8.52 ± 0.24 | 35.33 ± 0.65 | 36.67 ± 0.47 |
| Leonurus cardiaca | 36.28 ± 0.43 a | 37.57 ± 0.43 b | 16.82 ± 0.63 | 16.52 ± 0.48 | 26.2 ± 1 a | 28.17 ± 1.42 b |
| Ballota nigra | 31.16 ± 0.45 a | 32.41 ± 0.38 b | 17.41 ± 0.88 a | 18.57 ± 0.16 b | 17.95 ± 1.15 a | 21.74 ± 0.49 b |
| Andrographis paniculata | 35.21 ± 0.43 a | 33.13 ± 0.74 b | -0.49 ± 0.11 a | 2.02 ± 0.68 b | 18.2 ± 2.39 | 19.6 ± 1.01 |
| Herb Species | Total Polyphenols [mg Gallic Acid/g DW] | Anthocyanins [mg Gallic Acid/g DW] | Flavonoids [mg Gallic Acid/g DW] | |||
|---|---|---|---|---|---|---|
| Control | P-Treated | Control | P-Treated | Control | P-Treated | |
| Echinacea purpurea | 17.26 ± 1.57 a | 19.34 ± 0.76 b | 9.8 ± 1.43 a | 13.36 ± 1.98 b | 3.04 ± 0.08 | 3.15 ± 0.24 |
| Salvia officinalis | 40.35 ± 1.22 a | 43.64 ± 1.05 b | 20.39 ± 1.51 a | 30.31 ± 2.52 b | 8.81 ± 1.09 a | 10.28 ± 0.25 b |
| Urtica dioica | 18.01 ± 0.78 | 17.33 ± 0.51 | 89.47 ± 10.26 a | 0 ± 0 b | 4.04 ± 0.25 a | 4.54 ± 0.09 b |
| Polygonum aviculare | 19.96 ± 1.41 a | 21.88 ± 0.74 b | 4.42 ± 0.3 a | 7.85 ± 0.88 b | 2.86 ± 0.07 | 2.8 ± 0.03 |
| Vaccinium myrtillus | 74.39 ± 1.05 a | 79.46 ± 0.79 b | 0 ± 0 | 0 ± 0 | 5.47 ± 0.84 a | 6.49 ± 0.07 b |
| Taraxacum officinale | 14.43 ± 0.35 a | 16.02 ± 0.8 b | 11.48 ± 0.93 | 11.55 ± 3.21 | 3.24 ± 0.25 | 3.45 ± 0.05 |
| Hypericum perforatum | 56.95 ± 1.81 a | 61 ± 0.69 b | 21.33 ± 2.99 | 21.96 ± 1.55 | 12.05 ± 0.13 a | 11.53 ± 0.16 b |
| Achillea millefolium | 19.11 ± 0.57 a | 21.26 ± 0.35 b | 13.81 ± 1.38 | 12.44 ± 3.14 | 4.66 ± 0.08 | 4.72 ± 0.11 |
| Sanguisorba officinalis | 42.87 ± 2.15 a | 47.75 ± 0.77 b | 17.5 ± 1.47 | 15.95 ± 3.04 | 4.77 ± 0.11 a | 5.8 ± 0.12 b |
| Leonurus cardiaca | 9.96 ± 0.44 a | 11 ± 0.7 b | 0.22 ± 0.27 | 1.11 ± 1.22 | 1.86 ± 0.06 | 1.84 ± 0.02 |
| Ballota nigra | 12.99 ± 0.64 a | 15.05 ± 0.33 b | 0 ± 0 | 0 ± 0 | 2.35 ± 0.04 | 2.28 ± 0.03 |
| Andrographis paniculata | 10.63 ± 0.42 a | 11.7 ± 0.37 b | 10.51 ± 1.23 a | 14.15 ± 0.81 b | 2.18 ± 0.05 | 2.14 ± 0.03 |
| Compound | DB5 | DB1701 | 1 | 1P | 2 | 2P | 3 | 3P | 4 | P | 5 | 5P | 6 | 6P | 7 | 7P | 8 | 8P | 9 | 9P | 10 | 10P | 11 | 11P | 12 | 12P |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Methyl/ethyl formate | 389 | 471 | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | ||
| Acetaldehyde | 418 | 497 | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | ||||
| Ethanol | 443 | 536 554 | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | |
| Diethyl eter | 477 | 535 | + | + | ||||||||||||||||||||||
| Pentane | 500 | 500 | + | |||||||||||||||||||||||
| Propanal | 504 | 607 | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | ||
| 2-methylpropanal | 515 | 617 | + | + | ||||||||||||||||||||||
| 2-mercaptoethanol | 546 | 890 | ||||||||||||||||||||||||
| 1-propanol | 548 | 649 | + | |||||||||||||||||||||||
| Butanal | 565 | 663 | + | + | ||||||||||||||||||||||
| Formic acid | 586 | 777 | + | + | + | |||||||||||||||||||||
| 1-propanol, 2-methyl- | 615 | 749 | + | + | ||||||||||||||||||||||
| Ethyl acetate | 616 | 695 | + | + | + | + | + | + | + | + | + | + | + | + | + | + | ||||||||||
| Acetic acid | 623 | 760 | ||||||||||||||||||||||||
| 2-methylbutanal | 685 | 728 | + | + | + | + | + | + | ||||||||||||||||||
| Isopropyl acetate | 650 | 729 | ||||||||||||||||||||||||
| Pentanal | 686 | 779 | + | + | + | + | ||||||||||||||||||||
| Acetoin | 687 | 868 | + | + | ||||||||||||||||||||||
| Pentan-2-one | 689 | 763 | + | + | + | |||||||||||||||||||||
| Ethyl propanoate | 716 | 762 | + | + | + | + | + | + | + | + | + | + | + | + | ||||||||||||
| Propyl acetate | 717 | 804 | + | + | ||||||||||||||||||||||
| 2- ethyl furan | 718 | 729 | + | + | + | + | + | |||||||||||||||||||
| Butanethiol | 745 | 750 | + | + | + | + | + | + | + | + | + | + | + | + | + | + | ||||||||||
| Ethyl isobutyrate | 746 | 824 | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | ||||||||
| Hexenal | 784 | 890 | + | + | + | + | + | + | + | + | + | + | + | + | ||||||||||||
| Cyclopentanone | 794 | 907 917 | + | + | ||||||||||||||||||||||
| Butanoic acid | 803 | 970 | + | + | + | + | + | + | ||||||||||||||||||
| Ethyl 2-methylbutyrate 3-hexanol | 823 | 929 | + | + | ||||||||||||||||||||||
| Furfural | 824 | 949 | + | + | ||||||||||||||||||||||
| Ethyl isovalerate | 854 | 927 | + | + | + | + | ||||||||||||||||||||
| 3-methylbutanoic acid | 855 | 1023 | + | + | ||||||||||||||||||||||
| Pentanoic acid | 889 | 1065 | + | + | + | + | ||||||||||||||||||||
| 2-heptanone | 891 | 1011 | + | + | ||||||||||||||||||||||
| 3-heptanone | 897 | 961 | + | + | ||||||||||||||||||||||
| Nonan | 900 | 900 | + | + | + | + | + | + | + | + | + | + | + | + | + | |||||||||||
| Heptanal | 901 | 979 | + | + | + | + | + | + | ||||||||||||||||||
| Alpha-pinene | 935 | 963 | + | + | + | + | + | + | + | + | + | + | + | + | + | + | ||||||||||
| Benzaldehyde | 965 | 1057 | + | + | + | |||||||||||||||||||||
| Beta-pinene | 956 | 979 | + | + | + | + | + | |||||||||||||||||||
| Sabinene = Thujene | 959 | 1000 | + | + | + | + | + | + | + | |||||||||||||||||
| 1-octen-3-one | 979 | 1061 | + | + | + | |||||||||||||||||||||
| Butyl butanoate | 981 | 1062 | + | + | + | + | + | + | ||||||||||||||||||
| Myrcene | 991 | 1025 | + | + | + | + | ||||||||||||||||||||
| Z-3-hexen-1-ol acetate | 1001 | 1084 | + | + | + | + | + | + | + | + | + | + | + | + | ||||||||||||
| Beta-pinene | 1004 | 991 | + | + | + | + | + | + | + | + | + | |||||||||||||||
| Alpha-terpinene | 1022 | 1081 | + | + | + | + | + | + | + | + | + | + | + | |||||||||||||
| Formic acid | 1023 | 1122 | + | + | ||||||||||||||||||||||
| E-3-octen-2-one | 1026 | 1124 | + | + | + | + | ||||||||||||||||||||
| Alpha-phellandrene | 1027 | 1009 | + | + | + | + | + | + | + | + | ||||||||||||||||
| 2-octenal | 1036 | 1135 | + | + | + | + | + | + | + | + | ||||||||||||||||
| L-limonene Limonene | 1056 | 1060 | + | + | + | + | + | |||||||||||||||||||
| 1-octenal | 1071 | 1176 | + | + | + | + | + | + | + | |||||||||||||||||
| Propyl hexanoate | 1073 | 1172 | + | + | + | + | + | + | ||||||||||||||||||
| P-cymenene | 1085 | 1170 | + | + | + | + | + | + | + | + | + | + | + | |||||||||||||
| Nonan-2-one 3-nonenal | 1088 | 1200 | + | + | + | + | + | + | ||||||||||||||||||
| Linalool | 1091 | 1095 | + | + | + | |||||||||||||||||||||
| Terpinolene | 1102 | 1123 | + | + | + | + | + | + | ||||||||||||||||||
| Fenchol | 1118 | 1226 | + | + | + | |||||||||||||||||||||
| Limonene oxide | 1132 | 1193 | + | |||||||||||||||||||||||
| Maltol | 1134 | 1286 | + | + | + | + | ||||||||||||||||||||
| Citronellal | 1163 | 1240 | + | + | ||||||||||||||||||||||
| Camphor | 1181 | 1293 | + | + | ||||||||||||||||||||||
| Trans carveol | 1193 | 1367 | + | |||||||||||||||||||||||
| Terpinen-4-ol Alpha-terpineol | 1196 | 1271 | + | + | ||||||||||||||||||||||
| (E)-carveol | 1197 | 1372 | + | + | ||||||||||||||||||||||
| Propyl heptanoate | 1226 | 1252 | + | |||||||||||||||||||||||
| Geranial | 1258 | 1438 | + | + | ||||||||||||||||||||||
| P-menthadienhydro-peroxide | 1343 | 1514 | + | + | ||||||||||||||||||||||
| Gamma-nonalactone | 1347 | 1577 | + | + | + | + | ||||||||||||||||||||
| Eugenol | 1361 | 1526 | + | + | + | + | + | |||||||||||||||||||
| Trans-2-undecenal | 1375 | 1503 | + | |||||||||||||||||||||||
| Methyl eugenol | 1412 | 1618 | + | + | ||||||||||||||||||||||
| Vanilin | 1426 | 1697 | + | + | ||||||||||||||||||||||
| Methyl cinnamate | 1428 | 1524 | + | |||||||||||||||||||||||
| Isoeugenol | 1455 | 1658 | + | |||||||||||||||||||||||
| Delta-decalactone | 1467 | 1745 | + | |||||||||||||||||||||||
| Isoeugenol | 1479 | 1637 | + | + | + | + | ||||||||||||||||||||
| Beta-caryophyllene | 1501 | 1514 | + | + | ||||||||||||||||||||||
| Ethyl cinnamate | 1504 | 1612 | + | + | ||||||||||||||||||||||
| Methyl dodecanoate | 1524 | 1624 | + | |||||||||||||||||||||||
| Cumarin | 1527 | 1709 | + | + | + | + | ||||||||||||||||||||
| Isopropyl cinnamate | 1555 | 1654 | + | |||||||||||||||||||||||
| Cis-3-hexenyl benzoate | 1559 | 1697 | + |
| Herb Species | Total Aerobic Bacteria Count [log10 CFU/g] | |
|---|---|---|
| Non-Treated | Plasma Treated | |
| Echinacea purpurea | 8.53 ± 0.06 a | 6.13 ± 1.75 b |
| Salvia officinalis | 8.09 ± 0.02 a | 4.93 ± 0.02 b |
| Urtica dioica | 9.1 ± 0.61 | 9.19 ± 0.79 |
| Polygonum aviculare | 8.46 ± 0.06 a | 5.73 ± 0.01 b |
| Vaccinium myrtillus | 2.23 ± 1.97 | 1.0 ± 1.73 |
| Taraxacum officinale | 7.07 ± 0.72 a | 4.61 ± 0.13 b |
| Hypericum perforatum | 4,78 ± 0.42 a | 3.05 ± 0.02 b |
| Achillea millefolium | 8.81 ± 0.2 a | 5.59 ± 4.84 b |
| Sanguisorba officinalis | 8.63 ± 0.07 a | 5.8 ± 0.14 b |
| Leonurus cardiaca | 10.05 ± 0.1 a | 6.57 ± 0.11 b |
| Ballota nigra | 7.7 ± 0.26 | 1.33 ± 2.31 |
| Andrographis paniculata | 4.91 ± 0.18 a | 3.64 ± 0.08 b |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pogorzelska-Nowicka, E.; Hanula, M.M.; Brodowska-Trębacz, M.; Górska-Horczyczak, E.; Jankiewicz, U.; Mazur, T.; Marcinkowska-Lesiak, M.; Półtorak, A.; Wierzbicka, A. The Effect of Cold Plasma Pretreatment on Water-Suspended Herbs Measured in the Content of Bioactive Compounds, Antioxidant Activity, Volatile Compounds and Microbial Count of Final Extracts. Antioxidants 2021, 10, 1740. https://doi.org/10.3390/antiox10111740
Pogorzelska-Nowicka E, Hanula MM, Brodowska-Trębacz M, Górska-Horczyczak E, Jankiewicz U, Mazur T, Marcinkowska-Lesiak M, Półtorak A, Wierzbicka A. The Effect of Cold Plasma Pretreatment on Water-Suspended Herbs Measured in the Content of Bioactive Compounds, Antioxidant Activity, Volatile Compounds and Microbial Count of Final Extracts. Antioxidants. 2021; 10(11):1740. https://doi.org/10.3390/antiox10111740
Chicago/Turabian StylePogorzelska-Nowicka, Ewelina, Monika Maria Hanula, Marta Brodowska-Trębacz, Elżbieta Górska-Horczyczak, Urszula Jankiewicz, Tomasz Mazur, Monika Marcinkowska-Lesiak, Andrzej Półtorak, and Agnieszka Wierzbicka. 2021. "The Effect of Cold Plasma Pretreatment on Water-Suspended Herbs Measured in the Content of Bioactive Compounds, Antioxidant Activity, Volatile Compounds and Microbial Count of Final Extracts" Antioxidants 10, no. 11: 1740. https://doi.org/10.3390/antiox10111740
APA StylePogorzelska-Nowicka, E., Hanula, M. M., Brodowska-Trębacz, M., Górska-Horczyczak, E., Jankiewicz, U., Mazur, T., Marcinkowska-Lesiak, M., Półtorak, A., & Wierzbicka, A. (2021). The Effect of Cold Plasma Pretreatment on Water-Suspended Herbs Measured in the Content of Bioactive Compounds, Antioxidant Activity, Volatile Compounds and Microbial Count of Final Extracts. Antioxidants, 10(11), 1740. https://doi.org/10.3390/antiox10111740








