Oral Exposure to Tributyltin Induced Behavioral Abnormality and Oxidative Stress in the Eyes and Brains of Juvenile Japanese Medaka (Oryzias latipes)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Organisms
2.2. Chemicals and Preparation of Test Diets
2.3. Exposure Test
2.4. Behavioral Traits Measurement and Analysis
2.5. Biochemical Assays
2.6. Statistical Analysis
3. Results
3.1. Responses in the Behavioral Parameters Related to Locomotor Activity
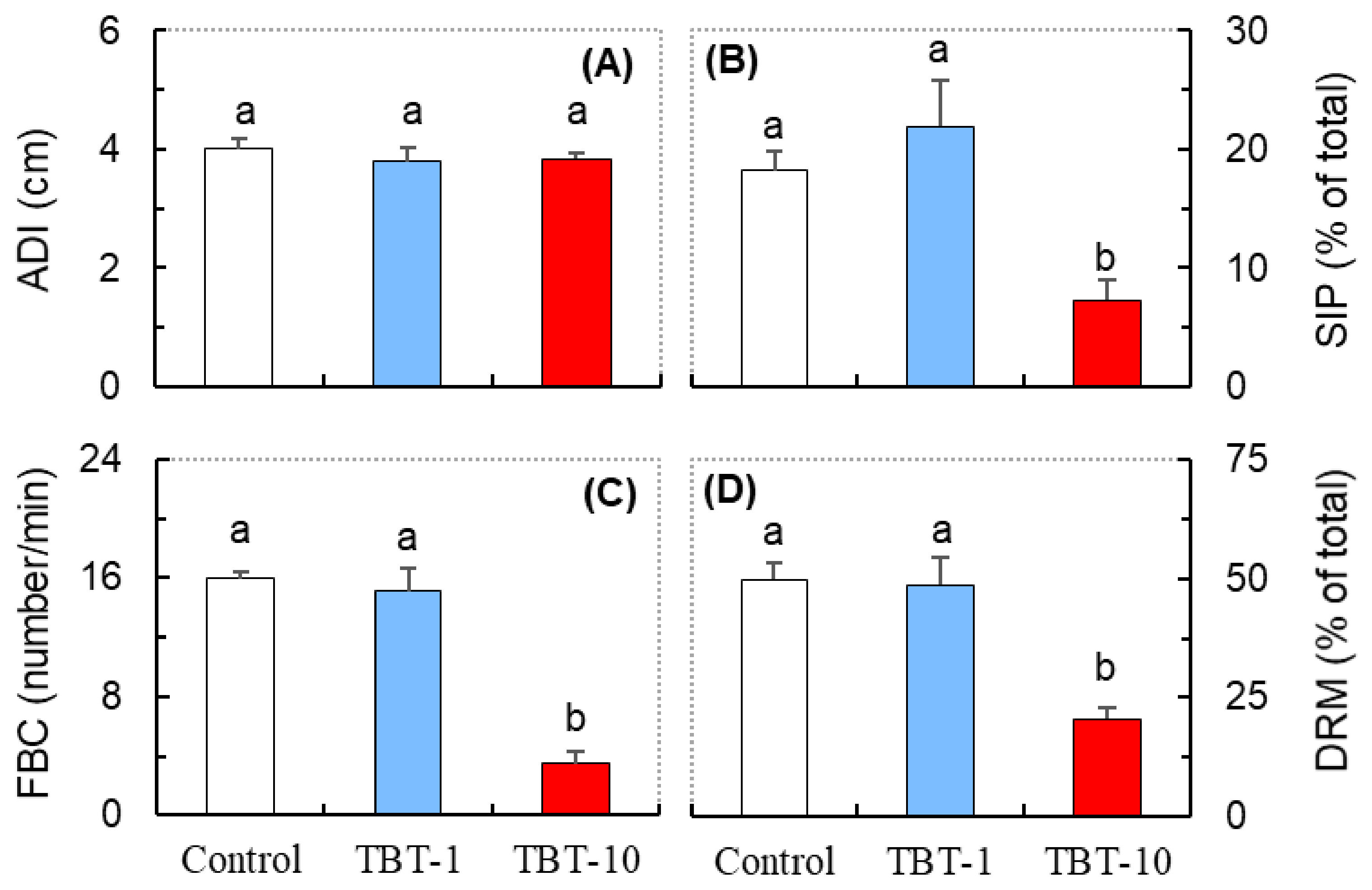
3.2. Responses in the Behavioral Parameters Related to Social Interaction
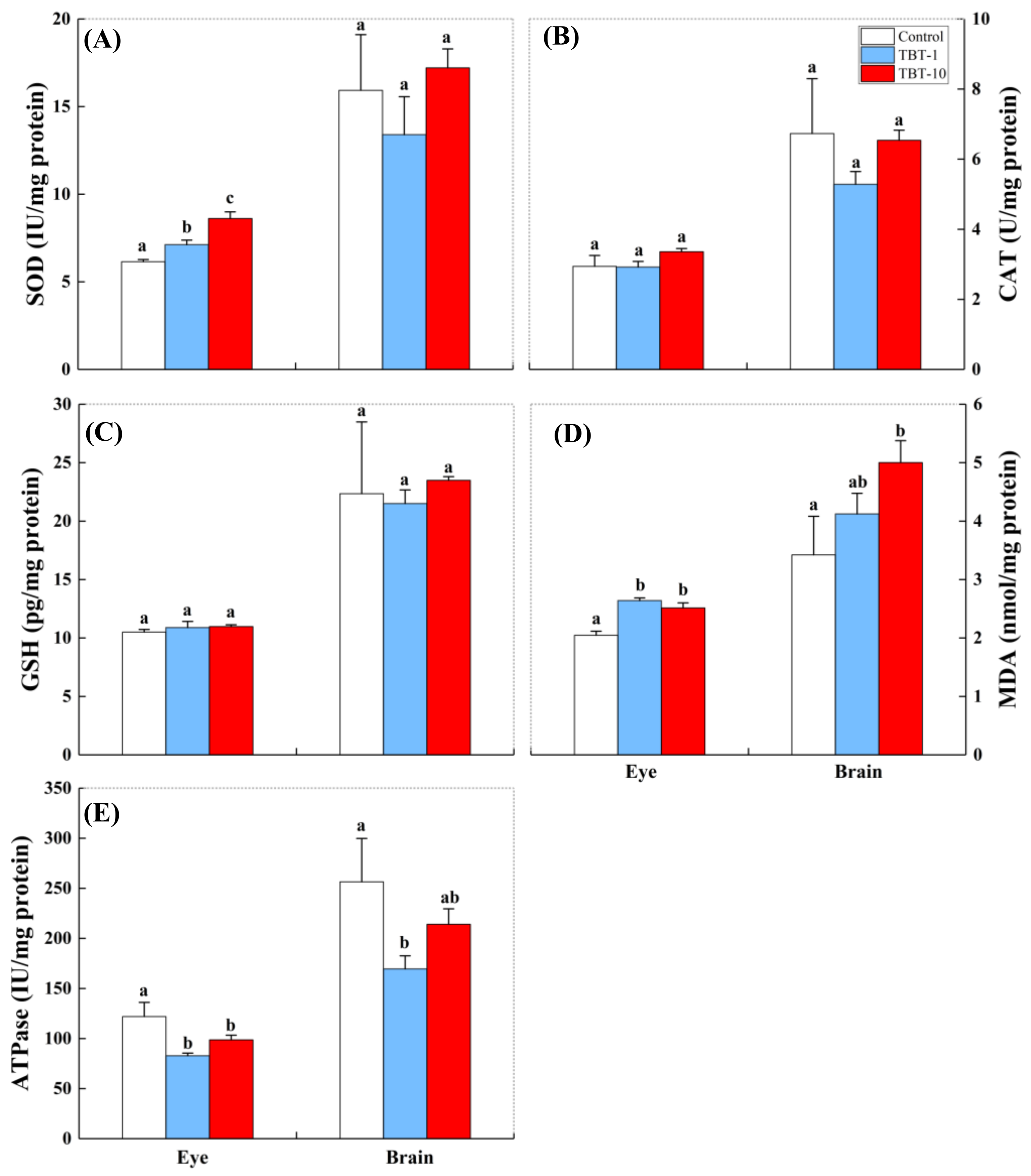
3.3. Responses in the Antioxidant Biomarkers and ATPase
3.4. Correlations Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fent, K. Ecotoxicology of Organotin Compounds. Crit. Rev. Toxicol. 1996, 26, 3–117. [Google Scholar] [CrossRef]
- Antizar-Ladislao, B. Environmental levels, toxicity and human exposure to tributyltin (TBT)-contaminated marine environment. A review. Environ. Int. 2008, 34, 292–308. [Google Scholar] [CrossRef]
- Broeg, K.; Theobald, N. Pollution with hazardous substances. In Handbook on Marine Environment Protection; Markus, S., Till, M., Eds.; Springer International Publishing AG: Cham, Switzerland, 2018; pp. 395–412. [Google Scholar]
- Champ, M.A.; Seligman, P.F. Organotin: Environmental Fate and Effects; Springer Science & Business Media: Berlin, Germany, 2012. [Google Scholar]
- Rantakokko, P.; Hallikainen, A.; Airaksinen, R.; Vuorinen, P.J.; Lappalainen, A.; Mannio, J.; Vartiainen, T. Concentrations of organotin compounds in various fish species in the Finnish lake waters and Finnish coast of the Baltic Sea. Sci. Total Environ. 2010, 408, 2474–2481. [Google Scholar] [CrossRef] [PubMed]
- Undap, S.L.; Matsunaga, S.; Honda, M.; Sekiguchi, T.; Suzuki, N.; Khalil, F.; Qiu, X.; Shimasaki, Y.; Ando, H.; Sato-Okoshi, W.; et al. Accumulation of organotins in wharf roach (Ligia exotica Roux) and its ability to serve as a biomonitoring species for coastal pollution. Ecotoxicol. Environ. Saf. 2013, 96, 75–79. [Google Scholar] [CrossRef] [PubMed]
- Ashraf, M.W.; Salam, A.; Mian, A. Levels of organotin compounds in selected fish species from the Arabian Gulf. Bull. Environ. Contam. Toxicol. 2017, 98, 811–816. [Google Scholar] [CrossRef] [PubMed]
- Shimasaki, Y.; Oshima, Y.; Inoue, S.; Inoue, Y.; Kang, I.J.; Nakayama, K.; Imoto, H.; Honjo, T. Effect of tributyltin on reproduction in Japanese whiting, Sillago japonica. Mar. Environ. Res. 2006, 62, S245–S248. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Zuo, Z.; Chen, Y.; Zhao, Y.; Hu, S.; Wang, C. Effect of tributyltin on the development of ovary in female cuvier (Sebastiscus marmoratus). Aquat. Toxicol. 2007, 83, 174–179. [Google Scholar] [CrossRef]
- Mochida, K.; Ito, K.; Kono, K.; Onduka, T.; Kakuno, A.; Fujii, K. Molecular and histological evaluation of tributyltin toxicity on spermatogenesis in a marine fish, the mummichog (Fundulus heteroclitus). Aquat. Toxicol. 2007, 83, 73–83. [Google Scholar] [CrossRef]
- Shimasaki, Y.; Kitano, T.; Oshima, Y.; Inoue, S.; Imada, N.; Honjo, T. Tributyltin causes masculinization in fish. Environ. Toxicol. Chem. 2003, 22, 141–144. [Google Scholar] [CrossRef]
- McAllister, B.G.; Kime, D.E. Early life exposure to environmental levels of the aromatase inhibitor tributyltin causes masculinisation and irreversible sperm damage in zebrafish (Danio rerio). Aquat. Toxicol. 2003, 65, 309–316. [Google Scholar] [CrossRef]
- Bryan, G.W.; Gibbs, P.E.; Hummerstone, L.G.; Burt, G.R. The decline of the gastropod Nucella Lapillus around South-West England: Evidence for the effect of tributyltin from antifouling paints. J. Mar. Biol. Assoc. UK 1986, 66, 611–640. [Google Scholar] [CrossRef]
- Hano, T.; Oshima, Y.; Kim, S.G.; Satone, H.; Oba, Y.; Kitano, T.; Inoue, S.; Shimasaki, Y.; Honjo, T. Tributyltin causes abnormal development in embryos of medaka, Oryzias latipes. Chemosphere 2007, 69, 927–933. [Google Scholar] [CrossRef]
- Inoue, S.; Oshima, Y.; Usuki, H.; Hamaguchi, M.; Hanamura, Y.; Kai, N.; Shimasaki, Y.; Honjo, T. Effects of tributyltin maternal and/or waterborne exposure on the embryonic development of the Manila clam, Ruditapes philippinarum. Chemosphere 2006, 63, 881–888. [Google Scholar] [CrossRef]
- Yu, A.; Wang, X.; Zuo, Z.; Cai, J.; Wang, C. Tributyltin exposure influences predatory behavior, neurotransmitter content and receptor expression in Sebastiscus marmoratus. Aquat. Toxicol. 2013, 128-129, 158–162. [Google Scholar] [CrossRef]
- Liu, Z.-H.; Li, Y.-W.; Hu, W.; Chen, Q.-L.; Shen, Y.-J. Mechanisms involved in tributyltin-enhanced aggressive behaviors and fear responses in male zebrafish. Aquat. Toxicol. 2020, 220, 105408. [Google Scholar] [CrossRef]
- Liang, X.; Souders, C.L.; Zhang, J.; Martyniuk, C.J. Tributyltin induces premature hatching and reduces locomotor activity in zebrafish (Danio rerio) embryos/larvae at environmentally relevant levels. Chemosphere 2017, 189, 498–506. [Google Scholar] [CrossRef]
- Qiu, X.; Takamura, T.; Enoki, S.; Kato-Unoki, Y.; Takai, Y.; Nagano, Y.; Kinoshita, M.; Kitano, T.; Shimasaki, Y.; Oshima, Y. Detoxification roles of tributyltin-binding protein type 2 in Japanese medaka (Oryzias latipes) exposed to tributyltin. Mar. Pollut. Bull. 2020, 159, 111445. [Google Scholar] [CrossRef] [PubMed]
- Lushchak, V.I. Contaminant-induced oxidative stress in fish: A mechanistic approach. Fish Physiol. Biochem. 2015, 42, 711–747. [Google Scholar] [CrossRef]
- Border, S.E.; DeOliveira, G.M.; Janeski, H.M.; Piefke, T.J.; Brown, T.J.; Dijkstra, P.D. Social rank, color morph, and social network metrics predict oxidative stress in a cichlid fish. Behav. Ecol. 2019, 30, 490–499. [Google Scholar] [CrossRef] [Green Version]
- Ferraz da Silva, I.; Freitas-Lima, L.C.; Graceli, J.B.; Rodrigues, L.C.d.M. Organotins in neuronal damage, brain function, and behavior: A short review. Front. Endocrinol. 2018, 8, 366. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mitra, S.; Gera, R.; Siddiqui, W.A.; Khandelwal, S. Tributyltin induces oxidative damage, inflammation and apoptosis via disturbance in blood–brain barrier and metal homeostasis in cerebral cortex of rat brain: An in vivo and in vitro study. Toxicology 2013, 310, 39–52. [Google Scholar] [CrossRef]
- Zhang, C.-n.; Zhang, J.-l.; Ren, H.-t.; Zhou, B.-h.; Wu, Q.-j.; Sun, P. Effect of tributyltin on antioxidant ability and immune responses of zebrafish (Danio rerio). Ecotoxicol. Environ. Saf. 2017, 138, 1–8. [Google Scholar] [CrossRef]
- Li, Z.-H.; Li, P.; Shi, Z.-C. Molecular responses in digestive tract of juvenile common carp after chronic exposure to sublethal tributyltin. Ecotoxicol. Environ. Saf. 2014, 109, 10–14. [Google Scholar] [CrossRef]
- Li, Z.-H.; Li, P. Evaluation of tributyltin toxicity in Chinese rare minnow larvae by abnormal behavior, energy metabolism and endoplasmic reticulum stress. Chem. Biol. Interact. 2015, 227, 32–36. [Google Scholar] [CrossRef]
- Zhang, J.; Sun, P.; Yang, F.; Kong, T.; Zhang, R. Tributyltin disrupts feeding and energy metabolism in the goldfish (Carassius auratus). Chemosphere 2016, 152, 221–228. [Google Scholar] [CrossRef]
- Chen, K.; Iwasaki, N.; Qiu, X.; Xu, H.; Takai, Y.; Tashiro, K.; Shimasaki, Y.; Oshima, Y. Obesogenic and developmental effects of TBT on the gene expression of juvenile Japanese medaka (Oryzias latipes). Aquat. Toxicol. 2021, 237, 105907. [Google Scholar] [CrossRef]
- Qiu, X.; Matsuyama, Y.; Furuse, M.; Shimasaki, Y.; Oshima, Y. Effects of Chattonella antiqua on the swimming behavior and brain monoamine metabolism of juvenile yellowtail (Seriola quinqueradiata). Mar. Pollut. Bull. 2020, 152, 110896. [Google Scholar] [CrossRef] [PubMed]
- Harino, H.; Fukushima, M.; Yamamoto, Y.; Kawai, S.; Miyazaki, N. Organotin Compounds in Water, Sediment, and Biological Samples from the Port of Osaka, Japan. Arch. Environ. Contam. Toxicol. 1998, 35, 558–564. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.C.; Hsieh, C.Y.; Tien, C.J. Factors influencing organotin distribution in different marine environmental compartments, and their potential health risk. Chemosphere 2006, 65, 547–559. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.; Qiu, X.; Chen, C.; Chen, K.; Li, M.; Xu, H.; Wu, X.; Shimasaki, Y.; Oshima, Y. Short-term and persistent impacts of sublethal exposure to diazepam on behavioral traits and brain GABA levels in juvenile zebrafish (Danio rerio). Sci. Total Environ. 2020, 740, 140392. [Google Scholar] [CrossRef]
- Qiu, X.; Saovany, S.; Takai, Y.; Akasaka, A.; Inoue, Y.; Yakata, N.; Liu, Y.; Waseda, M.; Shimasaki, Y.; Oshima, Y. Quantifying the vector effects of polyethylene microplastics on the accumulation of anthracene to Japanese medaka (Oryzias latipes). Aquat. Toxicol. 2020, 228, 105643. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.; Wu, M.; Chen, C.; Xu, H.; Wu, X.; Qiu, X. Impacts of chronic exposure to sublethal diazepam on behavioral traits of female and male zebrafish (Danio rerio). Ecotoxicol. Environ. Saf. 2021, 208, 111747. [Google Scholar] [CrossRef]
- Ohji, M.; Harino, H. Comparison of toxicities of metal pyrithiones including their degradation compounds and organotin antifouling biocides to the Japanese killifish Oryzias latipes. Arch. Environ. Contam. Toxicol. 2017, 73, 285–293. [Google Scholar] [CrossRef]
- Schmidt, K.; Steinberg, C.E.W.; Pflugmacher, S.; Staaks, G.B.O. Xenobiotic substances such as PCB mixtures (Aroclor 1254) and TBT can influence swimming behavior and biotransformation activity (GST) of carp (Cyprinus carpio). Environ. Toxicol. 2004, 19, 460–470. [Google Scholar] [CrossRef] [PubMed]
- Wong, B.; Candolin, U. Behavioral responses to changing environments. Behav. Ecol. 2015, 26, 665–673. [Google Scholar] [CrossRef] [Green Version]
- Brodin, T.; Piovano, S.; Fick, J.; Klaminder, J.; Heynen, M.; Jonsson, M. Ecological effects of pharmaceuticals in aquatic systems—Impacts through behavioural alterations. Philos. Trans. R. Soc. B Biol. Sci. 2014, 369, 20130580. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.; Zhang, C.; Sun, P.; Shao, X. Tributyltin affects shoaling and anxiety behavior in female rare minnow (Gobiocypris rarus). Aquat. Toxicol. 2016, 178, 80–87. [Google Scholar] [CrossRef]
- Xiao, W.-Y.; Li, Y.-W.; Chen, Q.-L.; Liu, Z.-H. Tributyltin impaired reproductive success in female zebrafish through disrupting oogenesis, reproductive behaviors and serotonin synthesis. Aquat. Toxicol. 2018, 200, 206–216. [Google Scholar] [CrossRef] [PubMed]
- Nakayama, K.; Oshima, Y.; Yamaguchi, T.; Tsuruda, Y.; Kang, I.J.; Kobayashi, M.; Imada, N.; Honjo, T. Fertilization success and sexual behavior in male medaka, Oryzias latipes, exposed to tributyltin. Chemosphere 2004, 55, 1331–1337. [Google Scholar] [CrossRef]
- Wester, P.W.; Canton, J.H.; Van Iersel, A.A.J.; Krajnc, E.I.; Vaessen, H.A.M.G. The toxicity of bis(tri-n-butyltin)oxide (TBTO) and di-n-butyltindichloride (DBTC) in the small fish species Oryzias latipes (medaka) and Poecilia reticulata (guppy). Aquat. Toxicol. 1990, 16, 53–72. [Google Scholar] [CrossRef]
- Kim, J.; Kim, C.Y.; Oh, H.; Ryu, B.; Kim, U.; Lee, J.M.; Jung, C.-R.; Park, J.-H. Trimethyltin chloride induces reactive oxygen species-mediated apoptosis in retinal cells during zebrafish eye development. Sci. Total Environ. 2019, 653, 36–44. [Google Scholar] [CrossRef]
- Fent, K.; Meier, W. Tributyltin-induced effects on early life stages of minnows Phoxinus phoxinus. Arch. Environ. Contam. Toxicol. 1992, 22, 428–438. [Google Scholar] [CrossRef]
- Bruno, D.W.; Ellis, A.E. Histopathological effects in Atlantic salmon, Salmo salar L. attributed to the use of tributyltin antifoulant. Aquaculture 1988, 72, 15–20. [Google Scholar] [CrossRef]
- Guo, S.; Qian, L.; Shi, H.; Barry, T.; Cao, Q.; Liu, J. Effects of tributyltin (TBT) on Xenopus tropicalis embryos at environmentally relevant concentrations. Chemosphere 2010, 79, 529–533. [Google Scholar] [CrossRef] [PubMed]
- Pereira, P.; Raimundo, J.; Canário, J.; Almeida, A.; Pacheco, M. Looking at the aquatic contamination through fish eyes – A faithful picture based on metals burden. Mar. Pollut. Bull. 2013, 77, 375–379. [Google Scholar] [CrossRef]
- Qiu, X.; Nomichi, S.; Chen, K.; Honda, M.; Kang, I.J.; Shimasaki, Y.; Oshima, Y. Short-term and persistent impacts on behaviors related to locomotion, anxiety, and startle responses of Japanese medaka (Oryzias latipes) induced by acute, sublethal exposure to chlorpyrifos. Aquat. Toxicol. 2017, 192, 148–154. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.-H.; Li, P.; Shi, Z.-C. Physiological and molecular responses in brain of juvenile common carp (Cyprinus carpio) following exposure to tributyltin. Environ. Toxicol. 2016, 31, 278–284. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Zuo, Z.; Chen, R.; Chen, Y.; Wang, C. Tributyltin exposure causes brain damage in Sebastiscus marmoratus. Chemosphere 2008, 73, 337–343. [Google Scholar] [CrossRef]
- Zhang, J.; Zuo, Z.; Sun, P.; Wang, H.; Yu, A.; Wang, C. Tributyltin exposure results in craniofacial cartilage defects in rockfish (Sebastiscus marmoratus) embryos. Mar. Environ. Res. 2012, 77, 6–11. [Google Scholar] [CrossRef]
- Pagliarani, A.; Bandiera, P.; Ventrella, V.; Trombetti, F.; Pirini, M.; Nesci, S.; Borgatti, A.R. Tributyltin (TBT) inhibition of oligomycin-sensitive Mg-ATPase activity in mussel mitochondria. Toxicol. In Vitro 2008, 22, 827–836. [Google Scholar] [CrossRef]
- Agrahari, S.; Gopal, K. Inhibition of Na+–K+-ATPase in different tissues of freshwater fish Channa punctatus (Bloch) exposed to monocrotophos. Pestic. Biochem. Physiol. 2008, 92, 57–60. [Google Scholar] [CrossRef]
- Wong-Riley, M.T. Energy metabolism of the visual system. Eye Brain 2010, 2, 99–116. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| Spearman’s Coefficients 1 | Eye | Brain | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| SOD | CAT | GSH | MDA | ATPase | SOD | CAT | GSH | MDA | ATPase | |
| Behavioral parameters related to locomotor activity | ||||||||||
| ASV | −0.643 * | −0.538 | −0.491 | −0.042 | −0.175 | −0.469 | −0.371 | −0.392 | −0.573 | −0.273 |
| DHM | −0.594 * | −0.559 | −0.456 | 0.105 | −0.287 | −0.573 | −0.483 | −0.441 | −0.587 * | −0.42 |
| DMM | −0.818 ** | −0.636 * | −0.47 | −0.301 | 0.028 | −0.329 | −0.056 | −0.371 | −0.622 * | 0.007 |
| DLM | 0.636 * | 0.594 * | 0.448 | −0.028 | 0.252 | 0.503 | 0.343 | 0.441 | 0.580 * | 0.301 |
| Behavioral parameters related to social interaction | ||||||||||
| ADI | −0.077 | 0.231 | −0.488 | −0.189 | 0.203 | 0.056 | 0.028 | 0.161 | 0.042 | 0.112 |
| SIP | −0.692 * | −0.671 * | 0.021 | −0.084 | −0.098 | −0.364 | −0.154 | −0.399 | −0.727 ** | −0.182 |
| FBC | −0.741 ** | −0.608 * | −0.137 | −0.210 | −0.021 | −0.406 | −0.287 | −0.315 | −0.769 ** | −0.196 |
| DRM | −0.622 * | −0.531 | −0.456 | 0.021 | −0.217 | −0.448 | −0.343 | −0.357 | −0.524 | −0.287 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shi, Y.; Chen, C.; Li, M.; Liu, L.; Dong, K.; Chen, K.; Qiu, X. Oral Exposure to Tributyltin Induced Behavioral Abnormality and Oxidative Stress in the Eyes and Brains of Juvenile Japanese Medaka (Oryzias latipes). Antioxidants 2021, 10, 1647. https://doi.org/10.3390/antiox10111647
Shi Y, Chen C, Li M, Liu L, Dong K, Chen K, Qiu X. Oral Exposure to Tributyltin Induced Behavioral Abnormality and Oxidative Stress in the Eyes and Brains of Juvenile Japanese Medaka (Oryzias latipes). Antioxidants. 2021; 10(11):1647. https://doi.org/10.3390/antiox10111647
Chicago/Turabian StyleShi, Yanhong, Chen Chen, Ming Li, Lei Liu, Kejun Dong, Kun Chen, and Xuchun Qiu. 2021. "Oral Exposure to Tributyltin Induced Behavioral Abnormality and Oxidative Stress in the Eyes and Brains of Juvenile Japanese Medaka (Oryzias latipes)" Antioxidants 10, no. 11: 1647. https://doi.org/10.3390/antiox10111647
APA StyleShi, Y., Chen, C., Li, M., Liu, L., Dong, K., Chen, K., & Qiu, X. (2021). Oral Exposure to Tributyltin Induced Behavioral Abnormality and Oxidative Stress in the Eyes and Brains of Juvenile Japanese Medaka (Oryzias latipes). Antioxidants, 10(11), 1647. https://doi.org/10.3390/antiox10111647





