Tetrahydrocurcumin Has Similar Anti-Amyloid Properties as Curcumin: In Vitro Comparative Structure-Activity Studies
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Molecular Docking and Molecular Dynamic Studies
2.3. Inhibition of Aβ-Aggregation by Cur/Turmeric Derivatives Using Dot-Blot Assays
2.4. Photo-Induced Cross-Linking of Unmodified Protein (PICUP)
2.5. Transmission Electron Microscopy (TEM)
2.6. Cell Culture
2.7. Treatment of Different Cur/Turmeric Derivatives
2.8. Cell Viability by MTT Assay
2.9. Western Blot
2.10. Animals
2.11. Tissue Processing and Aβ Plaque Labelling by Cur/Turmeric Derivatives
2.11.1. Aβ Labelling Using the Cryostat Section
2.11.2. Aβ Labelling Using the Paraffin Section
2.12. Statistical Analysis
3. Results
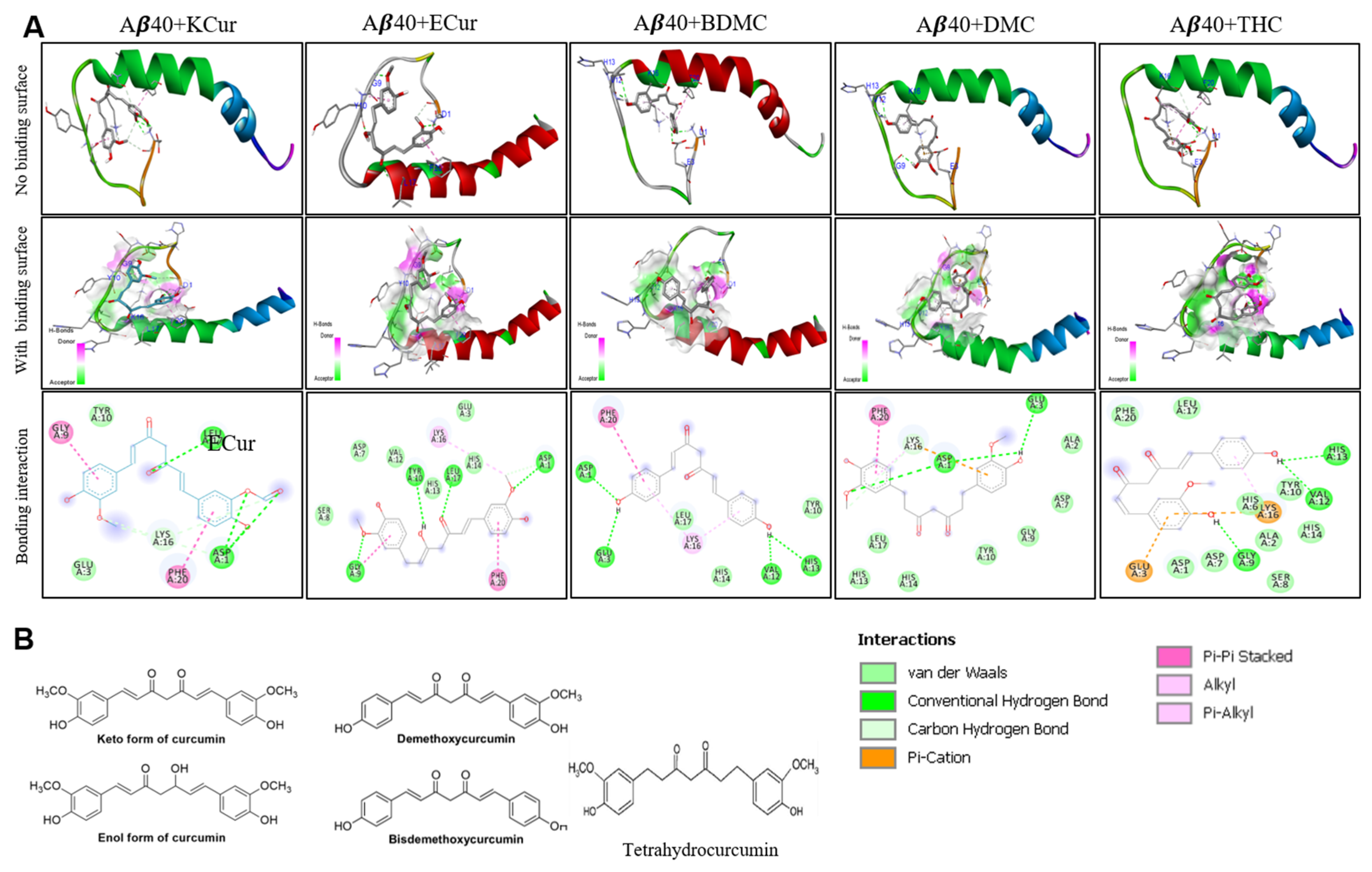
3.1. Curcumin Derivatives Interact with Aβ Similar to Curcumin
3.2. Binding Affinity of Different Cur Derivatives with Aβ40 and Aβ42
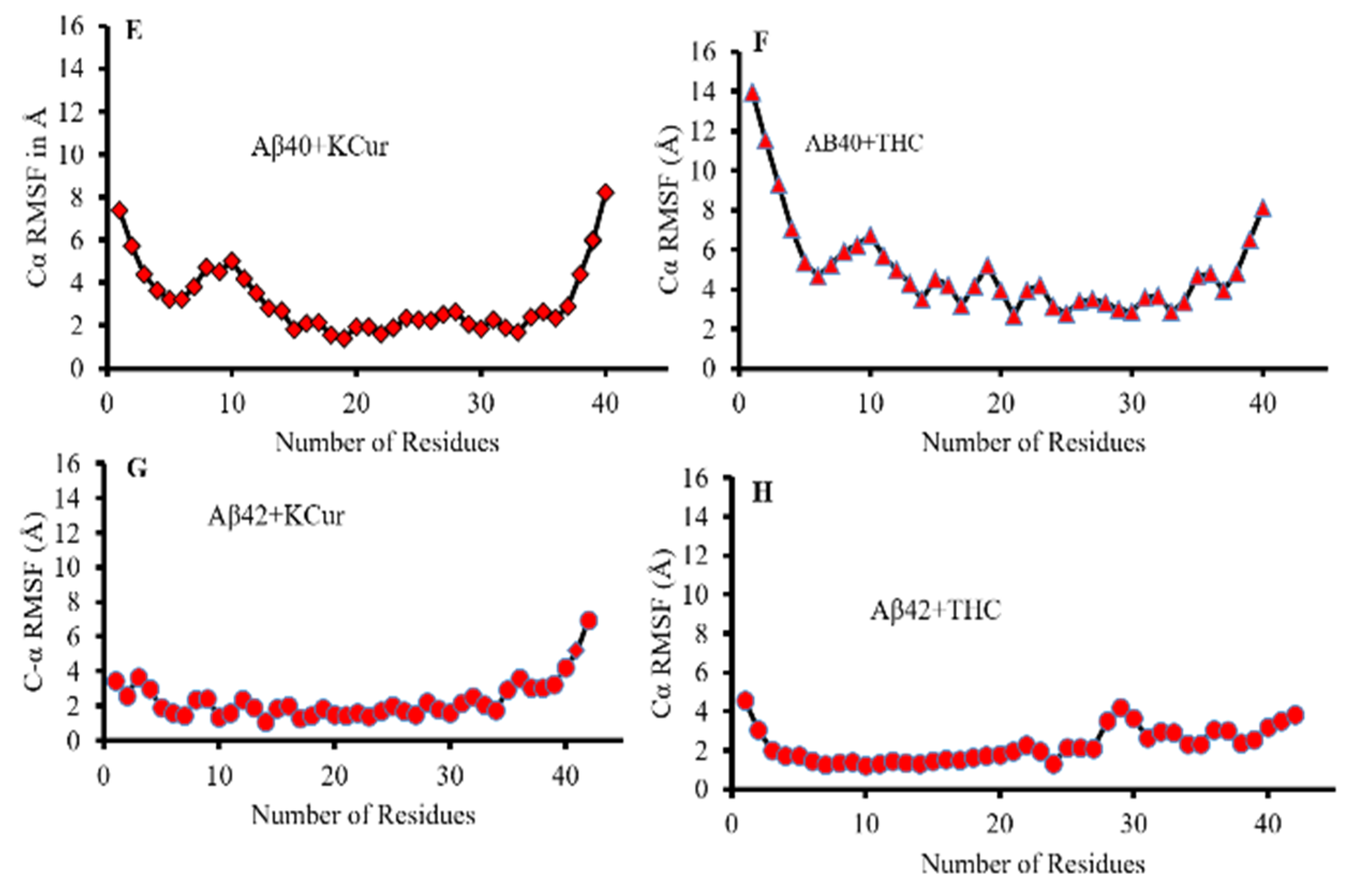
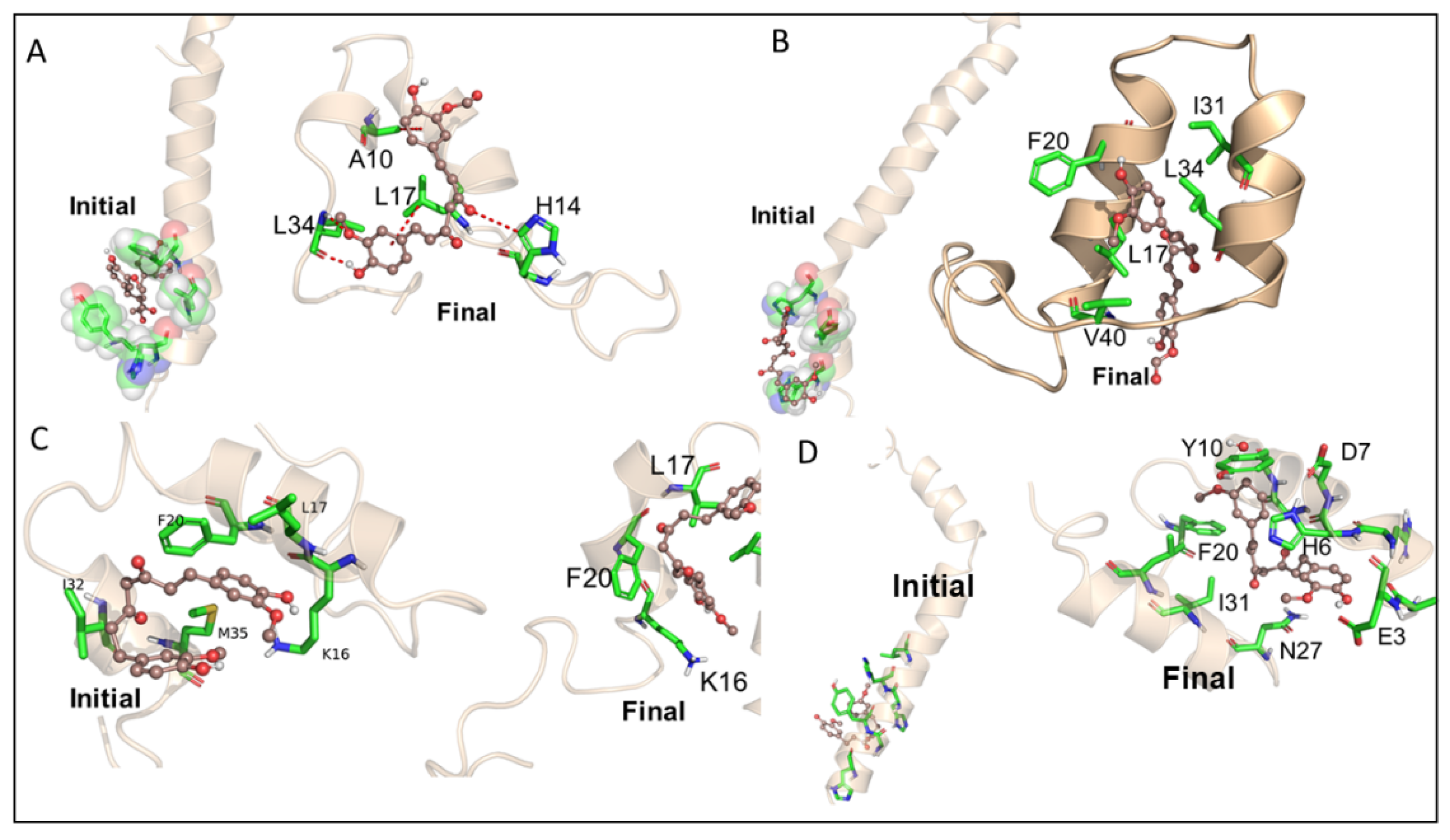
3.3. Molecular Dynamic Studies of Aβ40 and Aβ42 after Interaction with KCur and THC
3.4. Bonding Interactions of KCur and THC with Aβ
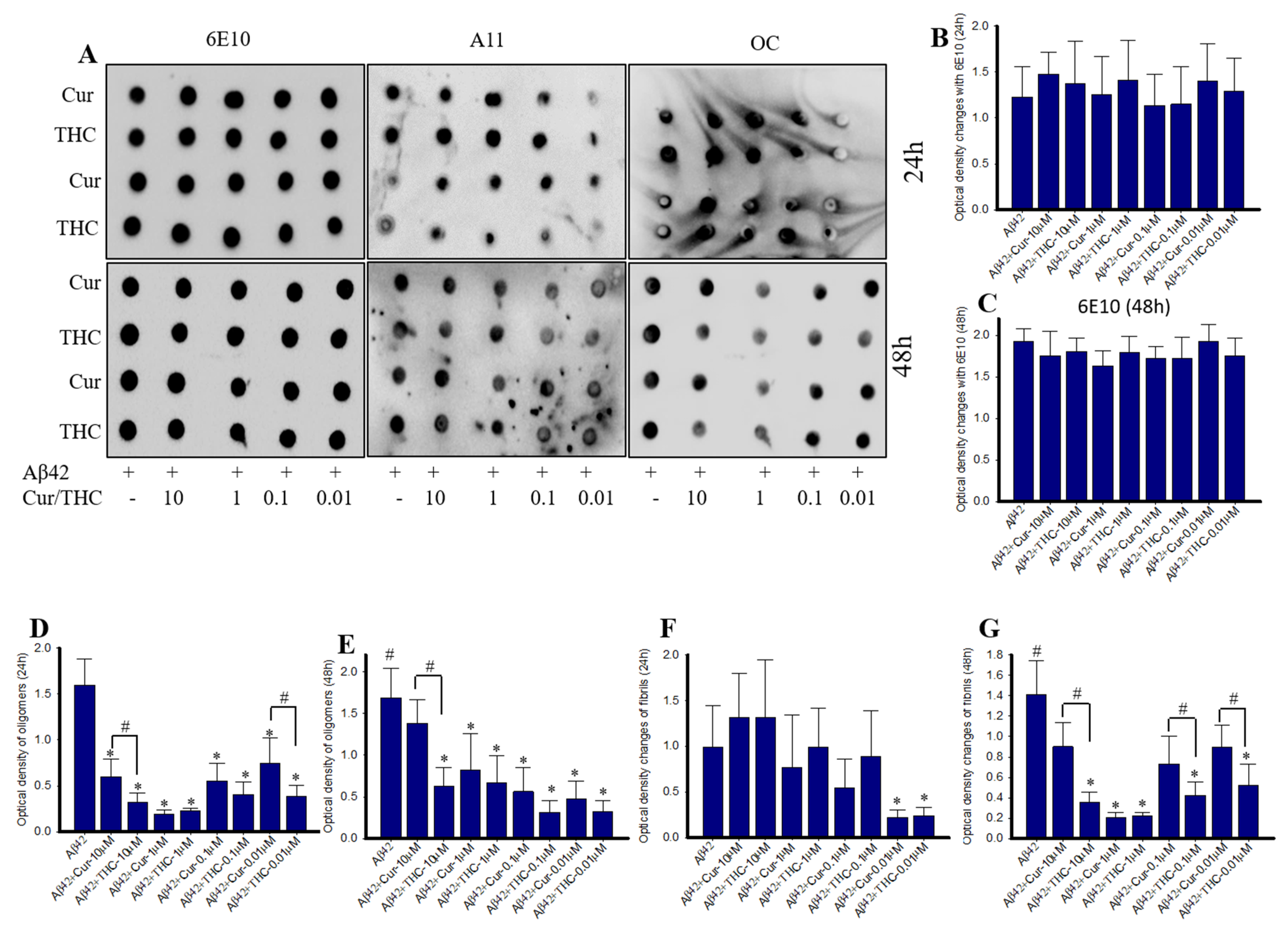
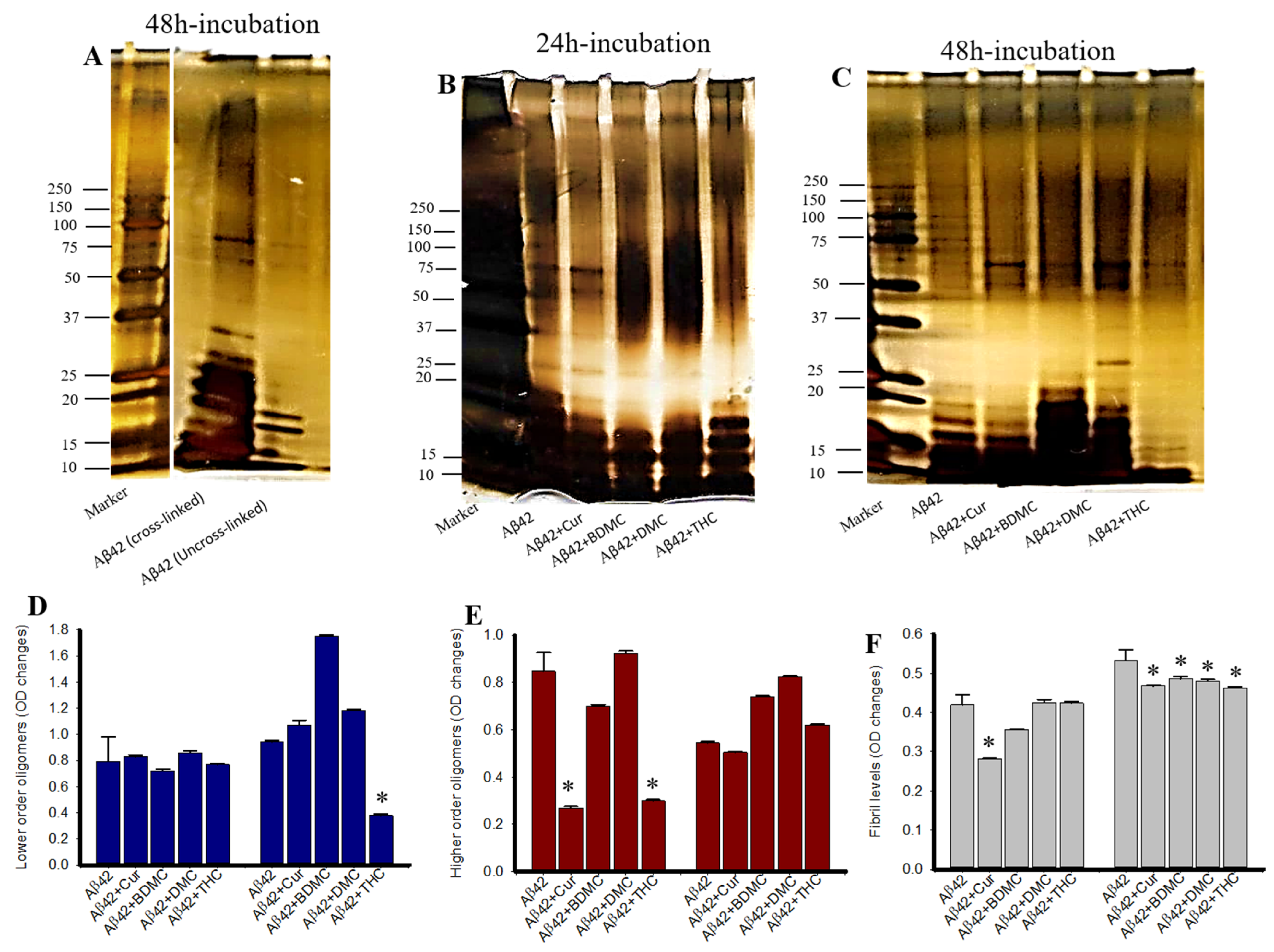
3.5. Cur Derivatives Inhibited Aβ42 Oligomer and Fibril Formation In Vitro
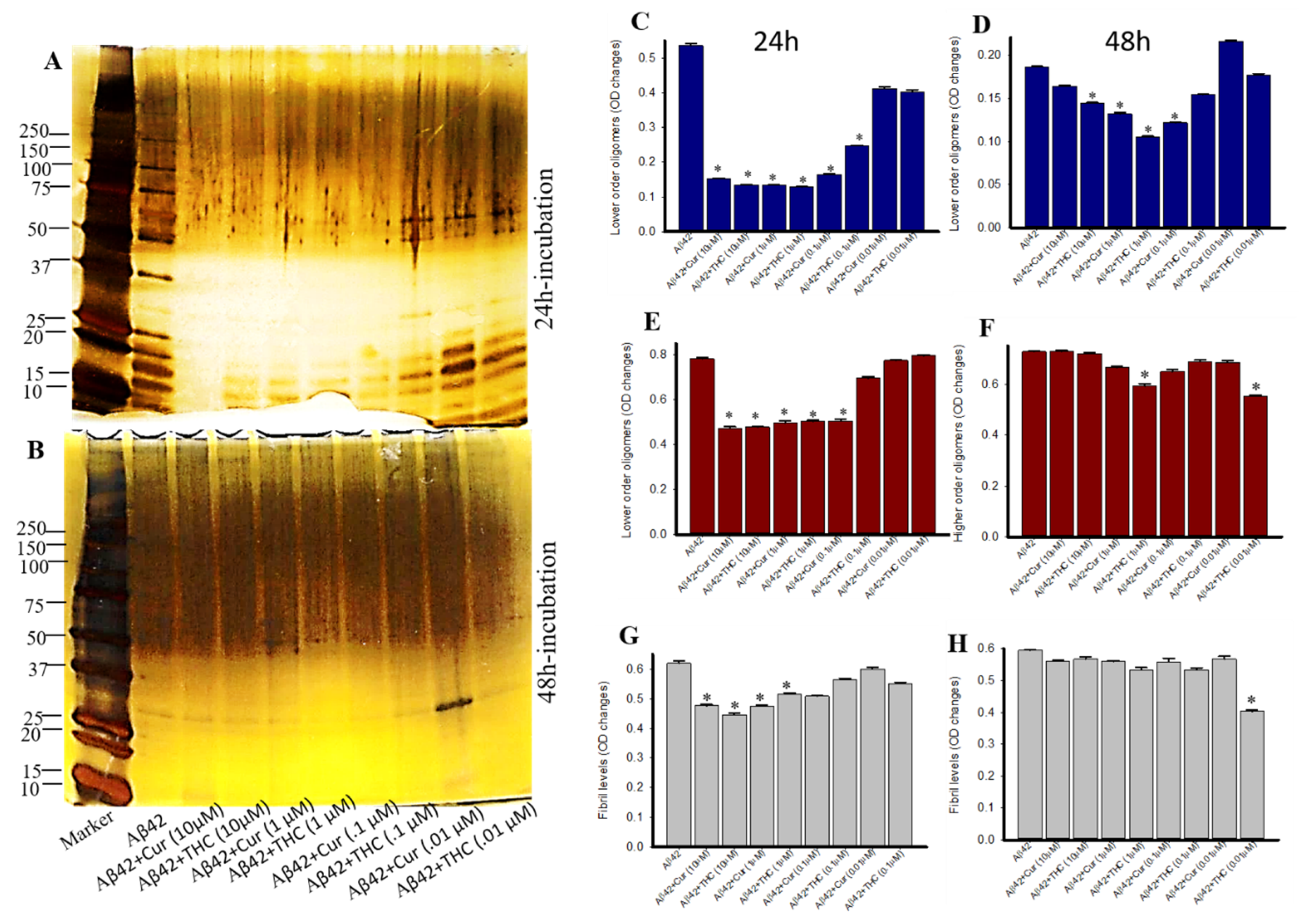
3.6. Lower Concentrations of Cur and THC Inhibited Aβ42 Oligomers and Fibril Formation Greater than Higher Concentrations
3.7. Photo-Induced Cross-Linking of Unmodified Protein (PICUP) of Aβ42 after Treatment with Cur/Turmeric Derivatives
3.8. Photo-Induced Cross-Linking of Unmodified Protein (PICUP) with Aβ42 after Treatment with Different Concentrations of Cur and THC
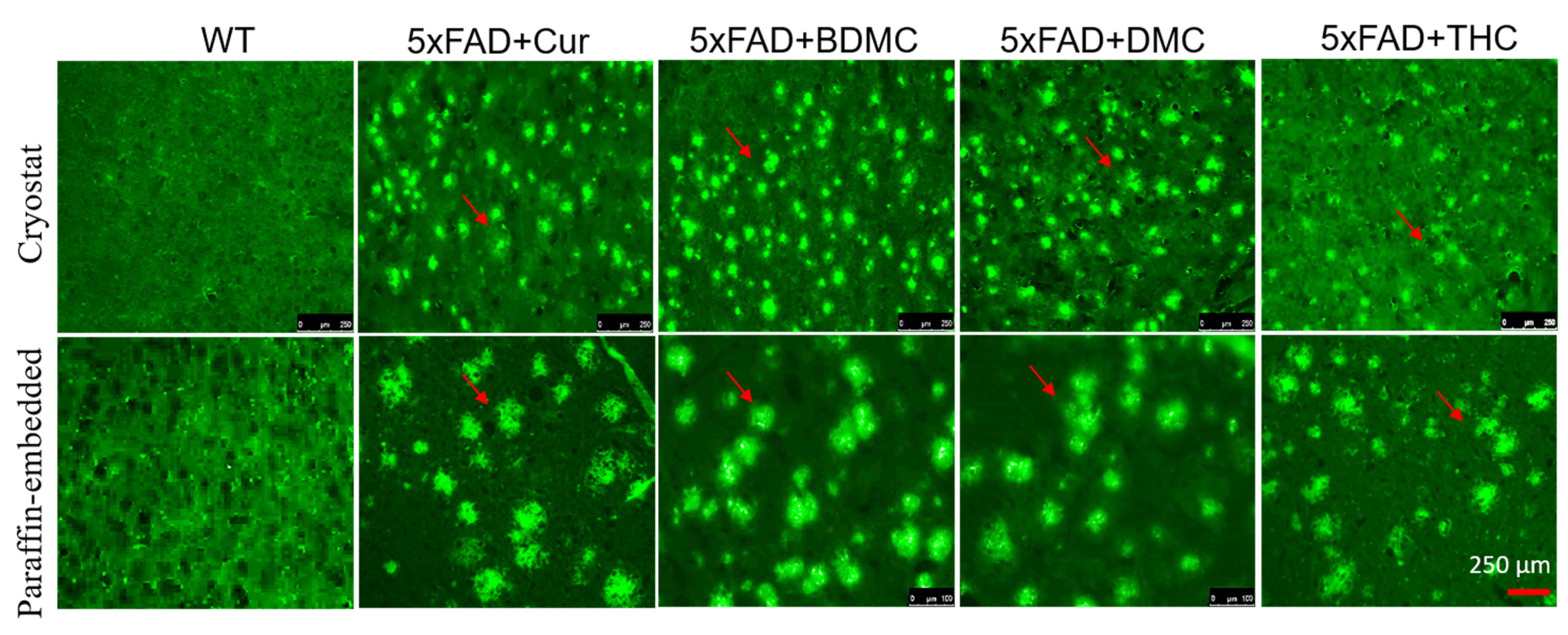
3.9. Curcumin/Turmeric Derivatives Are Equally Effective in Binding and Labeling Amyloid Plaques in 5× FAD Brain Tissue
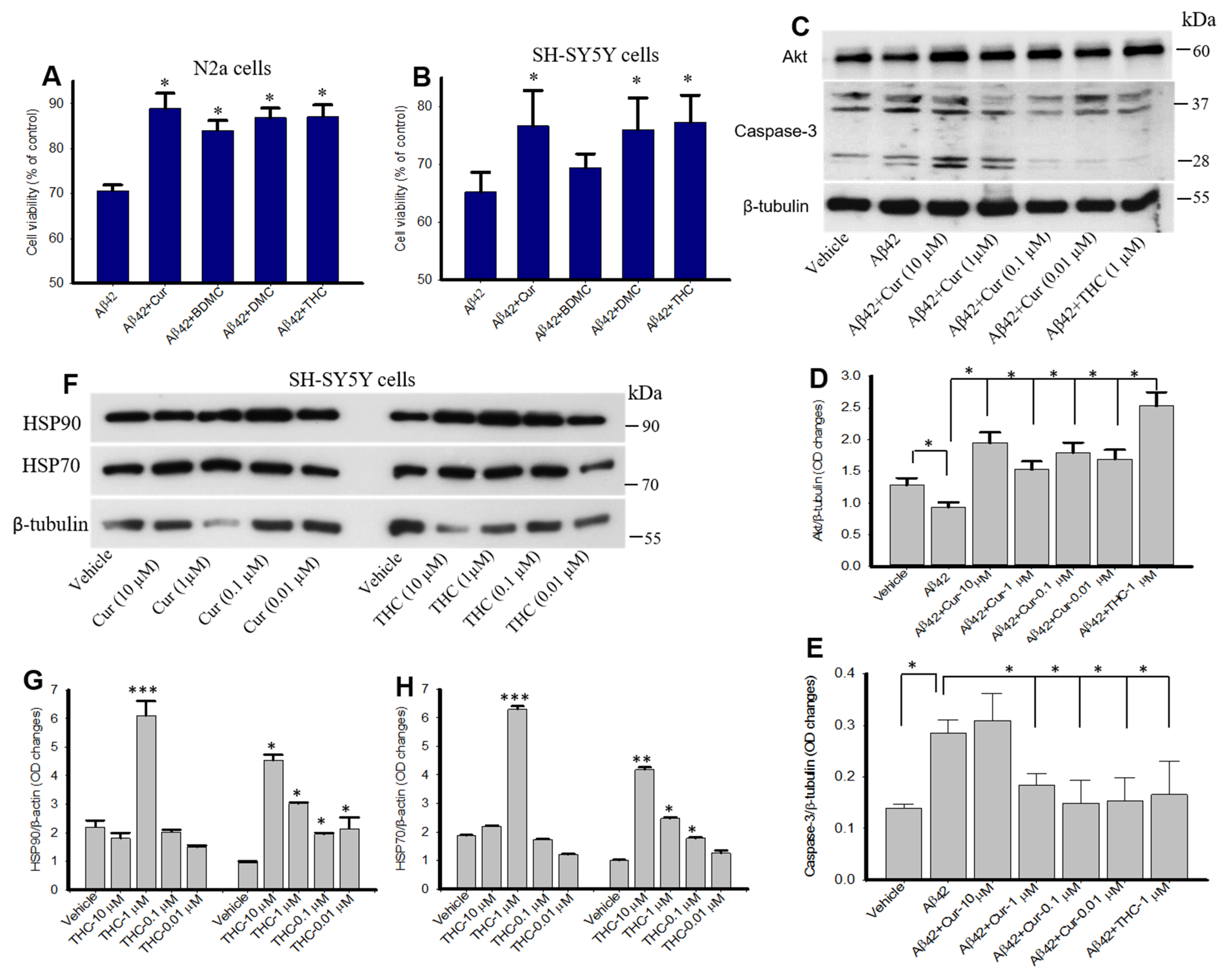
3.10. Curcumin/Turmeric Derivatives Protected Aβ42-Induced Neurotoxicity In Vitro
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Guo, T.; Zhang, D.; Zhang, Y.; Huang, T.Y.; Xu, H.; Zhao, Y. Molecular and cellular mechanisms underlying the pathogenesis of Alzheimer’s disease. Mol. Neurodegener. 2020, 15, 1–37. [Google Scholar] [CrossRef]
- DeTure, M.A.; Dickson, D.A. The neuropathological diagnosis of Alzheimer’s disease. Mol. Neurodegener. 2019, 2, 1–18. [Google Scholar] [CrossRef] [Green Version]
- Hashimoto, M.; Rockenstein, E.; Crews, L.; Masliah, E. Role of protein aggregation in mitochondrial dysfunction and neurodegeneration in Alzheimer’s and Parkinson’s diseases. NeuroMolecular Med. 2003, 4, 21–35. [Google Scholar] [CrossRef]
- Serrano-Pozo, A.; Frosch, M.P.; Masliah, E.; Hyman, B.T. Neuropathological alterations in Alzheimer disease. Cold Spring Harb. Perspect. Med. 2011, 1, a006189. [Google Scholar] [CrossRef]
- Selkoe, D.J. Cell biology of protein misfolding: The examples of Alzheimer’s and Parkinson’s diseases. Nat. Cell Biol. 2004, 6, 1054–1061. [Google Scholar] [CrossRef]
- Ferreira, S.T.; Lourenco, M.V.; Oliveira, M.M.; De Felice, F.G. Soluble amyloid-beta oligomers as synaptotoxins leading to cognitive impairment in Alzheimer’s disease. Front. Cell Neurosci. 2015, 9, 191. [Google Scholar] [CrossRef] [Green Version]
- Schenk, D.; Basi, G.S.; Pangalos, M.N. Treatment strategies targeting amyloid beta-protein. Cold Spring Harb. Perspect. Med. 2012, 2, a006387. [Google Scholar]
- Velander, P.; Wu, L.; Henderson, F.; Zhang, S.; Bevan, D.R.; Xu, B. Natural product-based amyloid inhibitors. Biochem. Pharmacol. 2017, 139, 40–55. [Google Scholar] [CrossRef] [Green Version]
- Yang, F.; Lim, G.P.; Begum, A.N.; Ubeda, O.J.; Simmons, M.R.; Ambegaokar, S.S.; Chen, P.P.; Kayed, R.; Glabe, C.G.; Frautschy, S.A.; et al. Curcumin inhibits formation of amyloid beta oligomers and fibrils, binds plaques, and reduces amyloid in vivo. J. Biol. Chem. 2005, 280, 5892–5901. [Google Scholar] [CrossRef] [Green Version]
- Ringman, J.M.; Frautschy, S.A.; Teng, E.; Begum, A.N.; Bardens, J.; Beigi, M.; Gylys, K.H.; Badmaev, V.; Heath, D.D.; Apostolova, L.G.; et al. Oral curcumin for Alzheimer’s disease: Tolerability and efficacy in a 24-week randomized, double blind, placebo-controlled study. Alzheimers Res. Ther. 2012, 4, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Begum, A.N.; Jones, M.R.; Lim, G.P.; Morihara, T.; Kim, P.; Heath, D.D.; Rock, C.L.; Pruitt, M.A.; Yang, F.; Hudspeth, B.; et al. Curcumin structure-function, bioavailability, and efficacy in models of neuroinflammation and Alzheimer’s disease. J. Pharmacol. Exp. Ther. 2008, 326, 196–208. [Google Scholar] [CrossRef] [Green Version]
- Prasad, S.; Aggarwal, B.B. Turmeric, the golden spice: From traditional medicine to modern medicine. In Herbal Medicine: Biomolecular and Clinical Aspects, 2nd ed.; Benzie, I.F.F., Wachtel-Galor, S., Eds.; CRC Press/Taylor & Francis: Boca Raton, FL, USA, 2011. [Google Scholar]
- Lee, W.-H.; Loo, J.C.Y.; Bebawy, M.; Luk, F.; Mason, R.S.; Rohanizadeh, R. Curcumin and its derivatives: Their application in neuropharmacology and neuroscience in the 21st century. Curr. Neuropharmacol. 2013, 11, 338–378. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aggarwal, B.B.; Deb, L.; Prasad, S. Curcumin differs from tetrahydrocurcumin for molecular targets, signaling pathways and cellular responses. Molecules 2014, 20, 185–205. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Garcia-Alloza, M.; Borrelli, L.A.; Rozkalne, A.; Hyman, B.T.; Bacskai, B.J. Curcumin labels amyloid pathologyin vivo, disrupts existing plaques, and partially restores distorted neurites in an Alzheimer mouse model. J. Neurochem. 2007, 102, 1095–1104. [Google Scholar] [CrossRef] [PubMed]
- Maiti, P.; Hall, T.C.; Paladugu, L.; Kolli, N.; Learman, C.; Rossignol, J.; Dunbar, G.L. A comparative study of dietary curcumin, nanocurcumin, and other classical amyloid-binding dyes for labeling and imaging of amyloid plaques in brain tissue of 5×-familial Alzheimer’s disease mice. Histochem. Cell Biol. 2016, 146, 609–625. [Google Scholar] [CrossRef]
- Jakubowski, J.M.; Orr, A.A.; Le, D.A.; Tamamis, P. Interactions between curcumin derivatives and amyloid-beta fibrils: Insights from molecular dynamics simulations. J. Chem. Inf. Model. 2020, 60, 289–305. [Google Scholar] [CrossRef]
- Ngo, S.T.; Li, M.S. Curcumin binds to Abeta1-40 peptides and fibrils stronger than ibuprofen and naproxen. J. Phys. Chem. B 2012, 116, 10165–10175. [Google Scholar] [CrossRef] [PubMed]
- Anand, P.; Thomas, S.G.; Kunnumakkara, A.B.; Sundaram, C.; Harikumar, K.B.; Sung, B.; Tharakan, S.T.; Misra, K.; Priyadarsini, I.K.; Rajasekharan, K.N.; et al. Biological activities of curcumin and its analogues (Congeners) made by man and Mother Nature. Biochem. Pharmacol. 2008, 76, 1590–1611. [Google Scholar] [CrossRef]
- Cole, G.M.; Teter, B.; Frautschy, S.A. Neuroprotective effects of curcumin. Adv. Exp. Med. Biol. 2007, 595, 197–212. [Google Scholar]
- Balachandran, V.S.; Divya, K.P.; Samateh, M.; Sagiri, S.S.; Satapathy, S.; Pradhan, P.; Raghavan, S.R.; Rakesh, L.; Sellers, M.S.; Karna, S.P.; et al. Freestanding organogels by molecular velcro of unsaturated amphiphiles. Soft Matter 2019, 15, 6263–6268. [Google Scholar] [CrossRef]
- Miao, Y.; Feher, V.A.; McCammon, J.A. Gaussian accelerated molecular dynamics: Unconstrained enhanced sampling and free energy calculation. J. Chem. Theory Comput. 2015, 11, 3584–3595. [Google Scholar] [CrossRef] [PubMed]
- Rakesh, L.; Howell, B.A.; Chai, M.; Mueller, A.; Kujawski, M.; Fan, D.; Ravi, S.; Slominski, C. Computer-aided applications of nanoscale smart materials for biomedical applications. Nanomedicine 2008, 3, 719–739. [Google Scholar] [CrossRef]
- Trivedi, M.K.; Panda, P.; Sethi, K.K.; Gangwar, M.; Mondal, S.C.; Jana, S. Solid and liquid state characterization of tetrahydrocurcumin using XRPD, FT-IR, DSC, TGA, LC-MS, GC-MS, and NMR and its biological activities. J. Pharma. Analysis 2020, 10, 334–345. [Google Scholar] [CrossRef] [PubMed]
- Humphrey, W.; Dalke, A.; Schulten, K. VMD: Visual molecular dynamics. J. Mol. Graph. 1996, 14, 33–38. [Google Scholar] [CrossRef]
- Maiti, P.; Paladugu, L.; Dunbar, G.L. Solid lipid curcumin particles provide greater anti-amyloid, anti-inflammatory and neuroprotective effects than curcumin in the 5 x FAD mouse model of Alzheimer’s disease. BMC Neurosci. 2018, 19, 7. [Google Scholar] [CrossRef] [Green Version]
- Bitan, G.; Kirkitadze, M.D.; Lomakin, A.; Vollers, S.S.; Benedek, G.B.; Teplow, D.B. Amyloid beta -protein (Abeta) assembly: Abeta 40 and Abeta 42 oligomerize through distinct pathways. Proc. Natl. Acad. Sci. USA 2003, 100, 330–335. [Google Scholar] [CrossRef] [Green Version]
- Bitan, G.; Teplow, D.B. Rapid photochemical cross-linking--a new tool for studies of metastable, amyloidogenic protein assemblies. Acc. Chem. Res. 2004, 37, 357–364. [Google Scholar] [CrossRef]
- Rahimi, F.; Maiti, P.; Bitan, G. Photo-induced cross-linking of unmodified proteins (PICUP) applied to amyloidogenic peptides. J. Vis. Exp. 2009, 12, 1071. [Google Scholar] [CrossRef]
- Maiti, P.; Lomakin, A.; Benedek, G.B.; Bitan, G. Despite its role in assembly, methionine 35 is not necessary for amyloid beta-protein toxicity. J. Neurochem. 2010, 113, 1252–1262. [Google Scholar]
- Maiti, P.; Piacentini, R.; Ripoli, C.; Grassi, C.; Bitan, G. Surprising toxicity and assembly behaviour of amyloid beta-protein oxidized to sulfone. Biochem. J. 2011, 433, 323–332. [Google Scholar] [CrossRef] [Green Version]
- Maiti, P.; Dunbar, G.L. Comparative neuroprotective effects of dietary curcumin and solid lipid curcumin particles in cultured mouse neuroblastoma cells after exposure to Abeta42. Int. J. Alzheimer’s Dis. 2017, 2017, 4164872. [Google Scholar]
- Maiti, P.; Bowers, Z.; Bourcier-Schultz, A.; Morse, J.; Dunbar, G.L. Preservation of dendritic spine morphology and postsynaptic signaling markers after treatment with solid lipid curcumin particles in the 5× FAD mouse model of Alzheimer’s amyloidosis. Alzheimers Res. Ther. 2021, 13, 1–22. [Google Scholar] [CrossRef]
- Manna, J.; Dunbar, G.; Maiti, P. Curcugreen treatment prevented splenomegaly and other peripheral organ abnormalities in 3 x Tg and 5 x FAD Mouse Models of Alzheimer’s Disease. Antioxidants 2021, 10, 899. [Google Scholar] [CrossRef] [PubMed]
- Maiti, P.; Plemmons, A.; Bowers, Z.; Weaver, C.; Dunbar, G. Labeling and imaging of amyloid plaques in brain tissue using the natural polyphenol curcumin. J. Vis. Exp. 2019, 153, e60377. [Google Scholar] [CrossRef] [PubMed]
- Mutsuga, M.; Chambers, J.K.; Uchida, K.; Tei, M.; Makibuchi, T.; Mizorogi, T.; Takashima, A.; Nakayama, H. Binding of curcumin to senile plaques and cerebral amyloid angiopathy in the aged brain of various animals and to neurofibrillary tangles in Alzheimer’s brain. J. Vet. Med. Sci. 2012, 74, 51–57. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- den Haan, J.; Morrema, T.H.J.; Rozemuller, A.J.; Bouwman, F.H.; Hoozemans, J.J.M. Different curcumin forms selectively bind fibrillar amyloid beta in postmortem Alzheimer’s disease brains: Implications for in-vivo diagnostics. Acta Neuropathol. Commun. 2018, 6, 1–12. [Google Scholar] [CrossRef]
- Sandur, S.K.; Pandey, M.K.; Sung, B.; Ahn, K.S.; Murakami, A.; Sethi, G.; Limtrakul, P.; Badmaev, V.; Aggarwal, B.B. Curcumin, demethoxycurcumin, bisdemethoxycurcumin, tetrahydrocurcumin and turmerones differentially regulate anti-inflammatory and anti-proliferative responses through a ROS-independent mechanism. Carcinogenesis 2007, 28, 1765–1773. [Google Scholar] [CrossRef]
- Priyadarsini, K.I. The chemistry of curcumin: From extraction to therapeutic agent. Molecules 2014, 19, 20091–20112. [Google Scholar] [CrossRef] [Green Version]
- Anand, P.; Kunnumakkara, A.B.; Newman, R.A. Bioavailability of curcumin: Problems and promises. Mol. Pharm. 2007, 4, 807–818. [Google Scholar] [CrossRef]
- Zhao, J.; Cao, Y.; Zhang, L. Exploring the computational methods for protein-ligand binding site prediction. Comput. Struct. Biotechnol. J. 2020, 18, 417–426. [Google Scholar] [CrossRef]
- Lill, M.A. Efficient incorporation of protein flexibility and dynamics into molecular docking simulations. Biochemistry 2011, 50, 6157–6169. [Google Scholar] [CrossRef] [Green Version]
- Du, X.; Li, Y.; Xia, Y.L.; Ai, S.M.; Liang, J.; Sang, P.; Ji, X.L.; Liu, S.Q. Insights into protein-ligand interactions: Mechanisms, models, and methods. Int J. Mol. Sci. 2016, 17, 144. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.M.M.; Kang, M.; Chang, C.E. Switches of hydrogen bonds during ligand-protein association processes determine binding kinetics. J. Mol. Recognit. 2014, 27, 537–548. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bonnet, P.; Bryce, R.A. Molecular dynamics and free energy analysis of neuraminidase-ligand interactions. Protein Sci. 2004, 13, 946–957. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Langkilde, A.; Kristensen, S.M.; Lo Leggio, L.; Molgaard, A.; Jensen, J.H.; Houk, A.R.; Navarro Poulsen, J.C.; Kauppinen, S.; Larsen, S. Short strong hydrogen bonds in proteins: A case study of rhamnogalacturonan acetylesterase. Acta Crystallogr. D Biol. Crystallogr. 2008, 64, 851–863. [Google Scholar] [CrossRef] [Green Version]
- Rao, P.P.; Mohamed, T.; Teckwani, K.; Tin, G. Curcumin binding to beta amyloid: A computational study. Chem. Biol. Drug Des. 2015, 86, 813–820. [Google Scholar] [CrossRef]
- Doytchinova, I.; Atanasova, M.; Salamanova, E.; Ivanov, S.; Dimitrov, I. Curcumin inhibits the primary nucleation of amyloid-beta peptide: A molecular dynamics study. Biomolecules 2020, 10, 1323. [Google Scholar] [CrossRef]
- Gupta, S.C.; Prasad, S.; Kim, J.H.; Patchva, S.; Webb, L.J.; Priyadarsini, I.K.; Aggarwal, B.B. Multitargeting by curcumin as revealed by molecular interaction studies. Nat. Prod. Rep. 2011, 28, 1937–1955. [Google Scholar] [CrossRef] [Green Version]
- Randino, R.; Grimaldi, M.; Persico, M.; De Santis, A.; Cini, E.; Cabri, W.; Riva, A.; D’Errico, G.; Fattorusso, C.; D’Ursi, A.M.; et al. Investigating the neuroprotective effects of turmeric extract: Structural interactions of beta-amyloid peptide with single curcuminoids. Sci. Rep. 2016, 6, 1–17. [Google Scholar] [CrossRef] [Green Version]
- Yanagisawa, D.; Kato, T.; Taguchi, H.; Shirai, N.; Hirao, K.; Sogabe, T.; Tomiyama, T.; Gamo, K.; Hirahara, Y.; Masaaki Kitada, M.; et al. Keto form of curcumin derivatives strongly binds to Aβ oligomers but not fibrils. Biomaterials 2021, 270, 120686. [Google Scholar] [CrossRef]
- Maiti, P.; Manna, J.; Veleri, S.; Frautschy, S. Molecular chaperone dysfunction in neurodegenerative diseases and effects of curcumin. Biomed. Res. Int. 2014, 2014, 495091. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maiti, P.; Dunbar, G.L. Use of curcumin, a natural polyphenol for targeting molecular pathways in treating age-related neurodegenerative diseases. Int. J. Mol. Sci. 2018, 19, 1637. [Google Scholar] [CrossRef] [PubMed] [Green Version]



| Components Error | Aβ40 + KCur | Aβ40 + THC | Aβ42 + KCur | Aβ42 + THC |
|---|---|---|---|---|
| ΔEVDW | −24.8503 ± 1.6431 | −31.9872 ± 1.9511 | −34.9977 ± 1.9897 | −31.5872 ± 1.8513 |
| ΔEEEL | −5.2240 ± 2.7017 | −8.7495 ± 3.5774 | 2.5387 ± 0.9428 | −10.7495 ± 3.9772 |
| ΔEGB | 13.4585 ± 2.6444 | 18.9348 ± 3.4770 | 11.8059± 1.2426 | 19.3348 ± 3.5790 |
| ΔESURF | −3.8720 ± 0.2205 | −4.6870 ± 0.3075 | −4.7245 ± 0.2368 | −4.3870 ± 0.3305 |
| ΔHTot | −20.4879 ± 1.4694 | −26.4890 ± 1.9629 | −30.4550 ± 1.6558 | −27.3890 ± 1.7620 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maiti, P.; Manna, J.; Thammathong, J.; Evans, B.; Dubey, K.D.; Banerjee, S.; Dunbar, G.L. Tetrahydrocurcumin Has Similar Anti-Amyloid Properties as Curcumin: In Vitro Comparative Structure-Activity Studies. Antioxidants 2021, 10, 1592. https://doi.org/10.3390/antiox10101592
Maiti P, Manna J, Thammathong J, Evans B, Dubey KD, Banerjee S, Dunbar GL. Tetrahydrocurcumin Has Similar Anti-Amyloid Properties as Curcumin: In Vitro Comparative Structure-Activity Studies. Antioxidants. 2021; 10(10):1592. https://doi.org/10.3390/antiox10101592
Chicago/Turabian StyleMaiti, Panchanan, Jayeeta Manna, Joshua Thammathong, Bobbi Evans, Kshatresh Dutta Dubey, Souvik Banerjee, and Gary L. Dunbar. 2021. "Tetrahydrocurcumin Has Similar Anti-Amyloid Properties as Curcumin: In Vitro Comparative Structure-Activity Studies" Antioxidants 10, no. 10: 1592. https://doi.org/10.3390/antiox10101592
APA StyleMaiti, P., Manna, J., Thammathong, J., Evans, B., Dubey, K. D., Banerjee, S., & Dunbar, G. L. (2021). Tetrahydrocurcumin Has Similar Anti-Amyloid Properties as Curcumin: In Vitro Comparative Structure-Activity Studies. Antioxidants, 10(10), 1592. https://doi.org/10.3390/antiox10101592







