Changes in Reactive Oxygen Species, Antioxidants and Carbohydrate Metabolism in Relation to Dormancy Transition and Bud Break in Apple (Malus × domestica Borkh) Cultivars
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials & Samples Preparation
2.2. Estimation of Chilling and Heat Accumulation during Dormancy
2.3. Carbohydrate Analysis
2.4. Hydrogen Peroxide and Superoxide Quantification
2.5. Extraction and Quantification of Glutathione
2.6. Enzymatic Assays
2.7. Statistical Analysis
3. Results
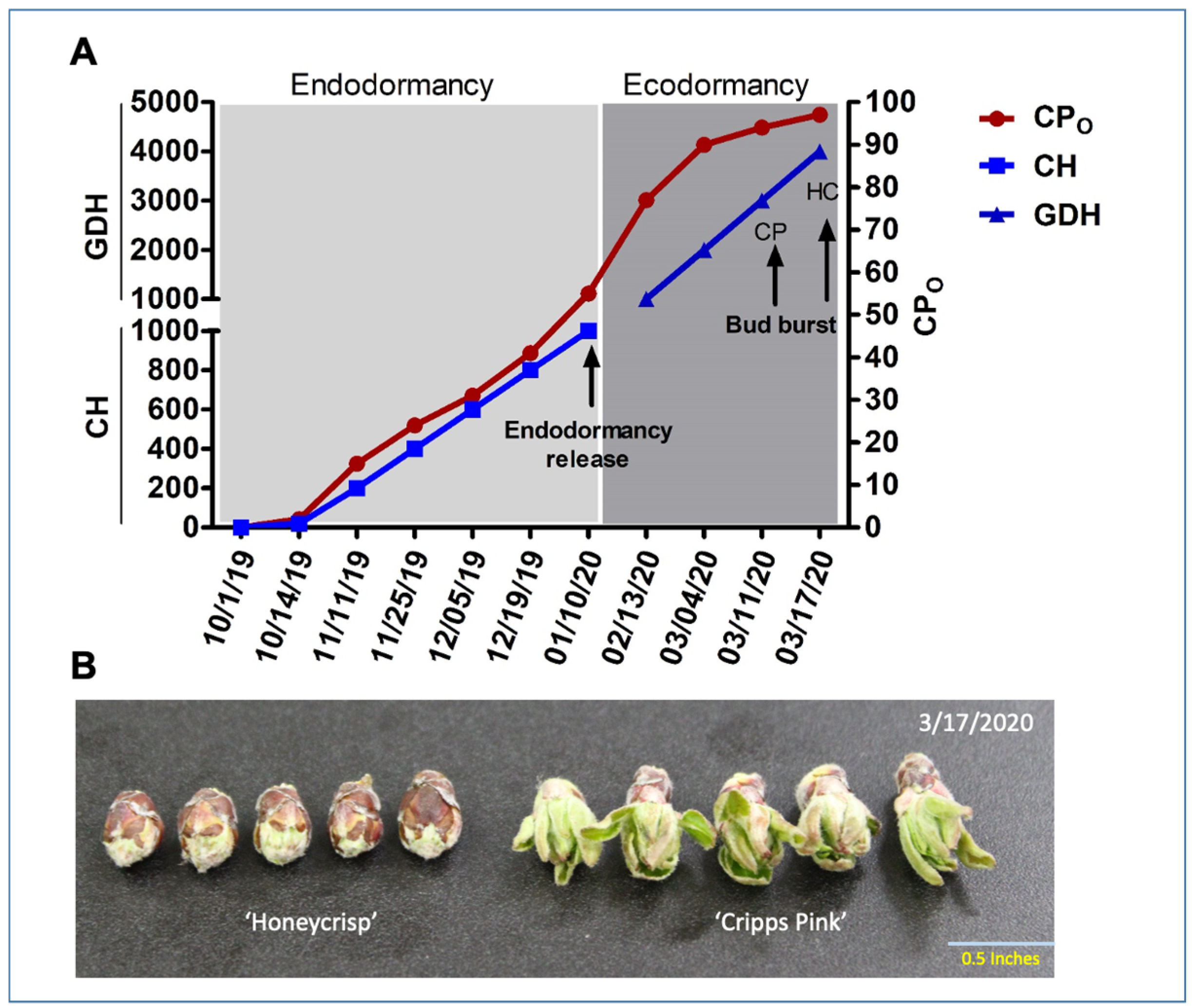
3.1. Phenological Variations between ‘Honeycrisp’ and ‘Cripps Pink’
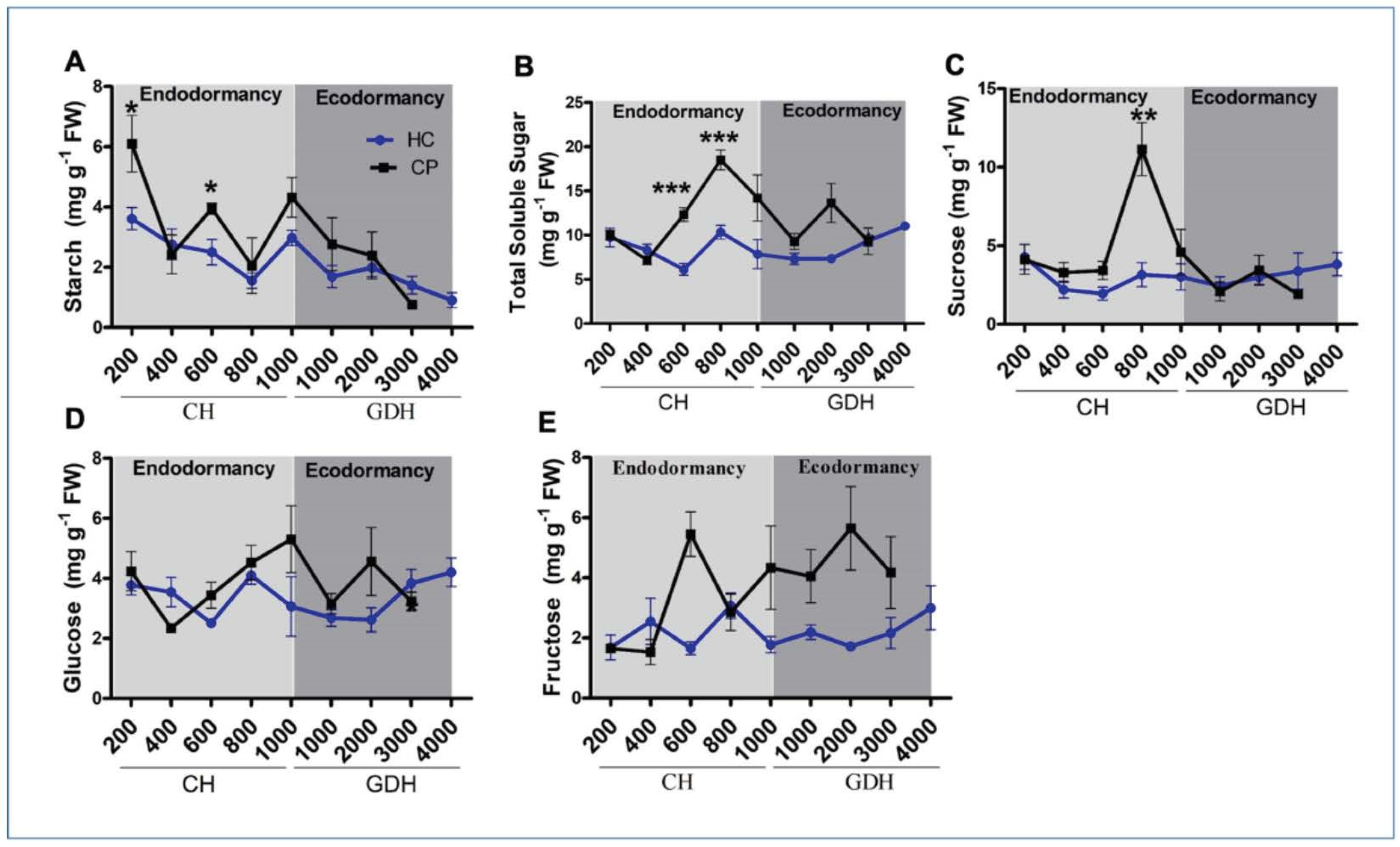
3.2. Changes in Carbohydrate Levels during Endodormancy and Ecodormancy
3.3. Accumulation Patterns of Hydrogen Peroxide, Superoxide Radicals and NADPH Oxidase during Dormancy
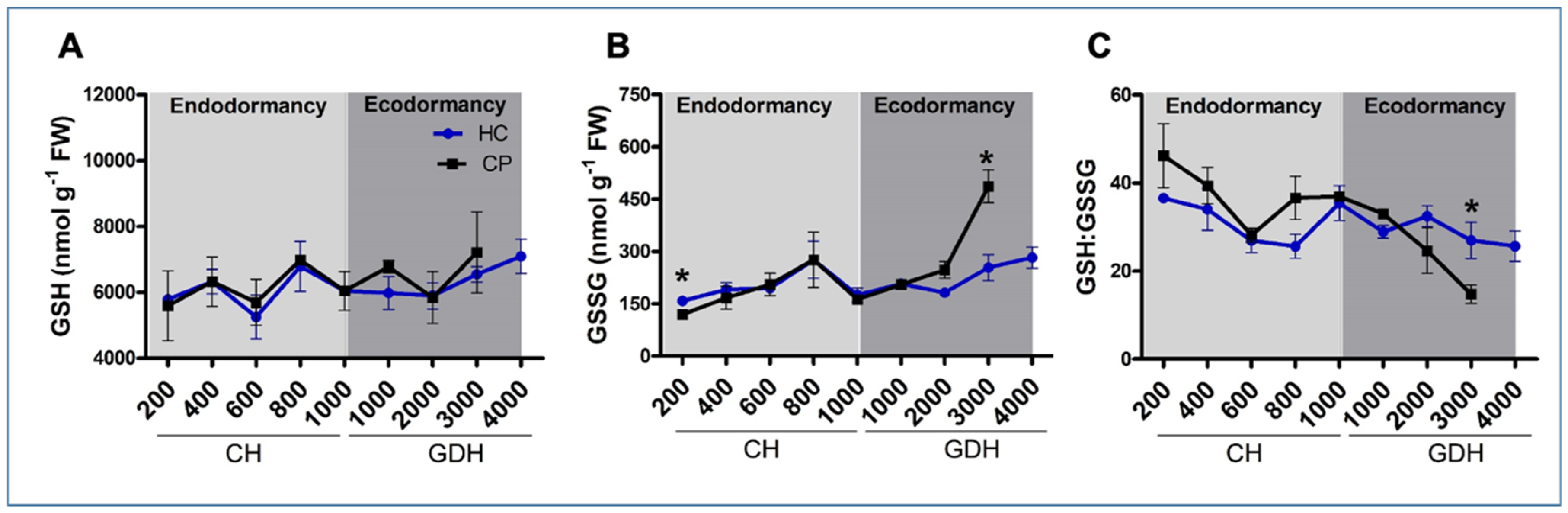
3.4. Redox Balance through Non-Enzymatic Antioxidants
3.5. Redox Balance through Enzymatic Antioxidants
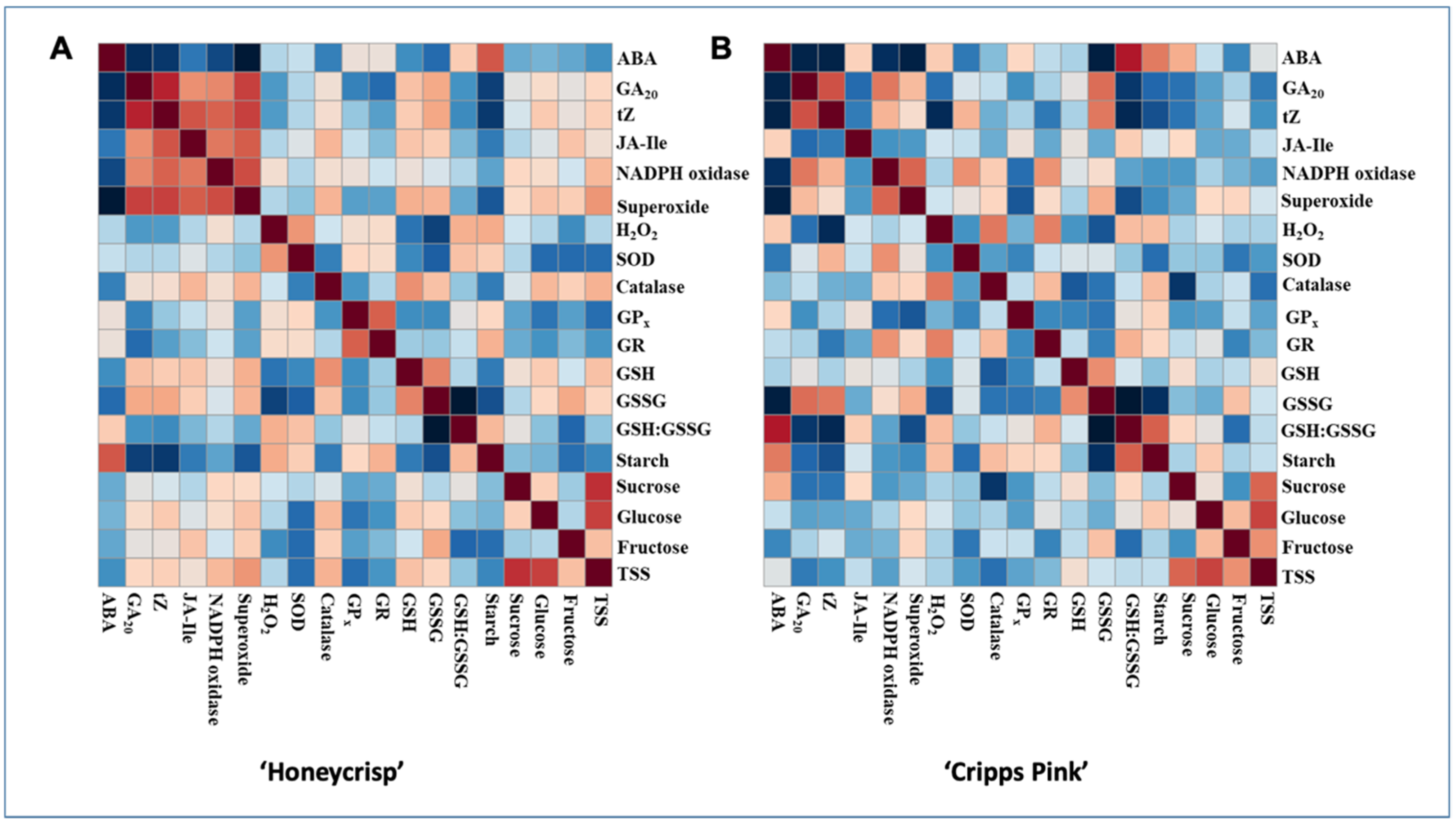
3.6. Persons Correlations between Hormones, Sugars and ROS
4. Discussion
4.1. Starch and Soluble Sugar Levels Differs between Apple Cultivars
4.2. Hydrogen Peroxide May Act as a Biological Marker for Dormancy Transition
4.3. Interplay of ROS, Carbohydrates, and Hormones
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Heide, O.M.; Prestrud, A.K. Low temperature, but not photoperiod, controls growth cessation and dormancy induction and release in apple and pear. Tree Physiol. 2005, 25, 109–114. [Google Scholar] [CrossRef]
- Lang, G. Dormancy: A new universal terminology. HortScience 1987, 22, 817–820. [Google Scholar]
- Drepper, B.; Gobin, A.; Remy, S.; van Orshoven, J. Comparing apple and pear phenology and model performance: What seven decades of observations reveal. Agronomy 2020, 10, 73. [Google Scholar] [CrossRef]
- Liu, J.; Islam, M.T.; Sapkota, S.; Ravindran, P.; Kumar, P.P.; Artlip, T.S.; Sherif, S.M. Ethylene-mediated modulation of bud phenology, cold hardiness, and hormone biosynthesis in peach (Prunus persica). Plants 2021, 10, 1266. [Google Scholar] [CrossRef] [PubMed]
- Persico, M.J.; Smith, D.E.; Centinari, M. Delaying Budbreak to Reduce Freeze Damage: Seasonal Vine Performance and Wine Composition in Two Vitis vinifera Cultivars. Am. J. Enol. Vitic. 2021. [Google Scholar] [CrossRef]
- Liu, J.; Sherif, S.M. Combating Spring Frost with Ethylene. Front. Plant Sci. 2019, 10, 1408. [Google Scholar] [CrossRef] [PubMed]
- Ionescu, I.A.; Møller, B.L.; Sánchez-Pérez, R. Chemical control of flowering time. J. Exp. Bot. 2017, 68, 369–382. [Google Scholar] [CrossRef]
- Takeuchi, T.; Matsushita, M.C.; Nishiyama, S.; Yamane, H.; Banno, K.; Tao, R. RNA-sequencing analysis identifies genes associated with chilling-mediated endodormancy release in apple. J. Am. Soc. Hortic. Sci. 2018, 143, 194–206. [Google Scholar] [CrossRef]
- Wang, S.Y.; Faust, M. Changes of fatty acids and sterols in apple buds during bud break induced by a plant bioregulator, thidiazuron. Physiol. Plant. 1988, 72, 115–120. [Google Scholar] [CrossRef]
- Yu, D.J.; Jun, S.H.; Park, J.; Kwon, J.H.; Lee, H.J. Transcriptome analysis of genes involved in cold hardiness of peach tree (Prunus persica) shoots during cold acclimation and deacclimation. Genes 2020, 11, 611. [Google Scholar] [CrossRef]
- Anderson, J.V.; Horvath, D.P.; Chao, W.S.; Foley, M.E. Bud Dormancy in Perennial Plants: A Mechanism for Survival. In Dormancy and Resistance in Harsh Environments; Lubzens, E., Cerda, J., Clark, M., Eds.; Springer: Berlin/Heidelberg, Germany, 2010; pp. 69–90. ISBN 978-3-642-12422-8. [Google Scholar]
- Sauter, J.J.; Wisniewski, M.; Witt, W. Interrelationships between ultrastructure, sugar levels, and frost hardiness of ray parenchyma cells during frost acclimation and deacclimation in poplar (Populus x canadensis Moench 〈robusta〉) wood. J. Plant Physiol. 1996, 149, 451–461. [Google Scholar] [CrossRef]
- Horikoshi, H.M.; Sekozawa, Y.; Kobayashi, M.; Saito, K.; Kusano, M.; Sugaya, S. Metabolomics analysis of ‘Housui’ Japanese pear flower buds during endodormancy reveals metabolic suppression by thermal fluctuation. Plant Physiol. Biochem. 2018, 126, 134–141. [Google Scholar] [CrossRef]
- Ito, A.; Sakamoto, D.; Moriguchi, T. Carbohydrate metabolism and its possible roles in endodormancy transition in Japanese pear. Sci. Hortic. 2012, 144, 187–194. [Google Scholar] [CrossRef]
- Rady, M.M.; Seif El-Yazal, M.A. Response of “ Anna” apple dormant buds and carbohydrate metabolism during floral bud break to onion extract. Sci. Hortic. 2013, 155, 78–84. [Google Scholar] [CrossRef]
- Sivaci, A. Seasonal changes of total carbohydrate contents in three varieties of apple (Malus sylvestris Miller) stem cuttings. Sci. Hortic. 2006, 109, 234–237. [Google Scholar] [CrossRef]
- Charrier, G.; Poirier, M.; Bonhomme, M.; Lacointe, A.; Améglio, T. Frost hardiness in walnut trees (Juglans regia L.): How to link physiology and modelling? Tree Physiol. 2013, 33, 1229–1241. [Google Scholar] [CrossRef] [PubMed]
- Gholizadeh, J.; Sadeghipour, H.R.; Abdolzadeh, A.; Hemmati, K.; Hassani, D.; Vahdati, K. Redox rather than carbohydrate metabolism differentiates endodormant lateral buds in walnut cultivars with contrasting chilling requirements. Sci. Hortic. 2017, 225, 29–37. [Google Scholar] [CrossRef]
- Hamman, R.A.; Dami, I.E.; Walsh, T.M.; Stushnoff, C. Seasonal carbohydrate changes and cold hardiness of chardonnay and riesling grapevines. Am. J. Enol. Vitic. 1996, 47, 31–36. [Google Scholar]
- Elle, D.; Sauter, J.J. Seasonal changes of activity of a starch granule bound endoamylase and of a starch phosphorylase in poplar wood (Populus x canadensis Moench 〈robusta〉) and their possible regulation by temperature and phytohormones. J. Plant Physiol. 2000, 156, 731–740. [Google Scholar] [CrossRef]
- El-Yazal, M.A.S.; Rady, M.M. Foliar-applied DormexTM or thiourea-enhanced proline and biogenic amine contents and hastened breaking bud dormancy in “Ain Shemer” apple trees. Trees-Struct. Funct. 2013, 27, 161–169. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhuo, X.; Zhao, K.; Zheng, T.; Han, Y.; Yuan, C.; Zhang, Q. Transcriptome Profiles Reveal the Crucial Roles of Hormone and Sugar in the Bud Dormancy of Prunus mume. Sci. Rep. 2018, 8, 5090. [Google Scholar] [CrossRef]
- Ben Mohamed, H.; Vadel, A.M.; Geuns, J.M.C.; Khemira, H. Biochemical changes in dormant grapevine shoot tissues in response to chilling: Possible role in dormancy release. Sci. Hortic. 2010, 124, 440–447. [Google Scholar] [CrossRef]
- Takemura, Y.; Kuroki, K.; Jiang, M.; Matsumoto, K.; Tamura, F. Identification of the expressed protein and the impact of change in ascorbate peroxidase activity related to endodormancy breaking in Pyrus pyrifolia. Plant Physiol. Biochem. 2015, 86, 121–129. [Google Scholar] [CrossRef]
- Hernández, J.A.; Díaz-Vivancos, P.; Acosta-Motos, J.R.; Alburquerque, N.; Martínez, D.; Carrera, E.; García-Bruntón, J.; Barba-Espín, G. Interplay among antioxidant system, hormone profile and carbohydrate metabolism during bud dormancy breaking in a high-chill peach variety. Antioxidants 2021, 10, 560. [Google Scholar] [CrossRef]
- Fernandez, E.; Cuneo, I.F.; Luedeling, E.; Alvarado, L.; Farias, D.; Saa, S. Starch and hexoses concentrations as physiological markers in dormancy progression of sweet cherry twigs. Trees-Struct. Funct. 2019, 33, 1187–1201. [Google Scholar] [CrossRef]
- Sachdev, S.; Ansari, S.A.; Ansari, M.I.; Fujita, M. Abiotic Stress and Reactive Oxygen Species: Generation. Antioxidants 2021, 10, 277. [Google Scholar] [CrossRef]
- Jajic, I.; Sarna, T.; Strzalka, K. Senescence, stress, and reactive oxygen species. Plants 2015, 4, 393–411. [Google Scholar] [CrossRef] [PubMed]
- Pérez, F.J.; Vergara, R.; Rubio, S. H2O2 is involved in the dormancy-breaking effect of hydrogen cyanamide in grapevine buds. Plant Growth Regul. 2008, 55, 149–155. [Google Scholar] [CrossRef]
- Kuroda, H.; Sugiura, T.; Ito, D. Changes in hydrogen peroxide content in flower buds of Japanese pear (Pyrus pyrifolia Nakai) in relation to breaking of endodormancy. J. Jpn. Soc. Hortic. Sci. 2002, 71, 610–616. [Google Scholar] [CrossRef]
- Khalil-Ur-Rehman, M.; Wang, W.; Dong, Y.; Faheem, M.; Xu, Y.; Gao, Z.; Shen, Z.G.; Tao, J. Comparative transcriptomic and proteomic analysis to deeply investigate the role of hydrogen cyanamide in grape bud dormancy. Int. J. Mol. Sci. 2019, 20, 3528. [Google Scholar] [CrossRef]
- Pérez, F.J.; Lira, W. Possible role of catalase in post-dormancy bud break in grapevines. J. Plant Physiol. 2005, 162, 301–308. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Zhao, S.; Tan, F.; Zhao, H.; Wang, D.D.; Si, H.; Chen, Q. Changes in ROS production and antioxidant capacity during tuber sprouting in potato. Food Chem. 2017, 237, 205–213. [Google Scholar] [CrossRef] [PubMed]
- Müller, K.; Carstens, A.C.; Linkies, A.; Torres, M.A.; Leubner-Metzger, G. The NADPH-oxidase AtrbohB plays a role in Arabidopsis seed after-ripening. New Phytol. 2009, 184, 885–897. [Google Scholar] [CrossRef]
- Ishibashi, Y.; Kasa, S.; Sakamoto, M.; Aoki, N.; Kai, K.; Yuasa, T.; Hanada, A.; Yamaguchi, S.; Iwaya-Inoue, M. A role for Reactive oxygen species produced by NADPH oxidases in the embryo and aleurone cells in barley seed germination. PLoS ONE 2015, 10, e0143173. [Google Scholar] [CrossRef]
- Nir, G.; Shulman, Y.; Fanberstein, L.; Lavee, S. Changes in the Activity of Catalase (EC 1.11.1.6) in Relation to the Dormancy of Grapevine (Vitis vinifera L.) Buds. Plant Physiol. 1986, 81, 1140–1142. [Google Scholar] [CrossRef]
- Porcher, A.; Guérin, V.; Leduc, N.; Lebrec, A.; Lothier, J.; Vian, A. Ascorbate–glutathione pathways mediated by cytokinin regulate H2O2 levels in light-controlled rose bud burst. Plant Physiol. 2021, 186, 910–928. [Google Scholar] [CrossRef] [PubMed]
- Porcher, A.; Guérin, V.; Montrichard, F.; Lebrec, A.; Lothier, J.; Vian, A. Ascorbate glutathione-dependent H2O2 scavenging is an important process in axillary bud outgrowth in rosebush. Ann. Bot. 2020, 126, 1049–1062. [Google Scholar] [CrossRef]
- Wang, S.Y.; Jiao, H.J.; Faust, M. Changes in the activities of catalase, peroxidase, and polyphenol oxidase in apple buds during bud break induced by thidiazuron. J. Plant Growth Regul. 1991, 10, 33–39. [Google Scholar] [CrossRef]
- Wang, S.Y.; Faust, M. Changes in the antioxidant system associated with budbreak in “Anna” apple (Malus domestica Borkh.) buds. J. Am. Soc. Hortic. Sci. 1994, 119, 735–741. [Google Scholar] [CrossRef]
- Ophir, R.; Pang, X.; Halaly, T.; Venkateswari, J.; Lavee, S.; Galbraith, D.; Or, E. Gene-expression profiling of grape bud response to two alternative dormancy-release stimuli expose possible links between impaired mitochondrial activity, hypoxia, ethylene-ABA interplay and cell enlargement. Plant Mol. Biol. 2009, 71, 403–423. [Google Scholar] [CrossRef]
- Walton, E.F.; Wu, R.M.; Richardson, A.C.; Davy, M.; Hellens, R.P.; Thodey, K.; Janssen, B.J.; Gleave, A.P.; Rae, G.M.; Wood, M.; et al. A rapid transcriptional activation is induced by the dormancy-breaking chemical hydrogen cyanamide in kiwifruit (Actinidia deliciosa) buds. J. Exp. Bot. 2009, 60, 3835–3848. [Google Scholar] [CrossRef]
- Considine, M.J.; Foyer, C.H. Redox regulation of plant development. Antioxid. Redox Signal. 2014, 21, 1305–1326. [Google Scholar] [CrossRef]
- Liu, Y.; Ye, N.; Liu, R.; Chen, M.; Zhang, J. H2O2 mediates the regulation of ABA catabolism and GA biosynthesis in Arabidopsis seed dormancy and germination. J. Exp. Bot. 2010, 61, 2979–2990. [Google Scholar] [CrossRef]
- Khalil-Ur-Rehman, M.; Sun, L.; Li, C.X.; Faheem, M.; Wang, W.; Tao, J.M. Comparative RNA-seq based transcriptomic analysis of bud dormancy in grape. BMC Plant Biol. 2017, 17, 18. [Google Scholar] [CrossRef]
- Liang, D.; Huang, X.; Shen, Y.; Shen, T.; Zhang, H.; Lin, L.; Wang, J.; Deng, Q.; Lyu, X.; Xia, H. Hydrogen cyanamide induces grape bud endodormancy release through carbohydrate metabolism and plant hormone signaling. BMC Genom. 2019, 20, 1034. [Google Scholar] [CrossRef] [PubMed]
- Ionescu, I.A.; López-Ortega, G.; Burow, M.; Bayo-Canha, A.; Junge, A.; Gericke, O.; Møller, B.L.; Sánchez-Pérez, R. Transcriptome and metabolite changes during hydrogen cyanamide-induced floral bud break in sweet cherry. Front. Plant Sci. 2017, 8, 1233. [Google Scholar] [CrossRef]
- Considine, M.J.; Foyer, C.H. Oxygen and reactive oxygen species-dependent regulation of plant growth and development. Plant Physiol. 2021, 186, 79–92. [Google Scholar] [CrossRef] [PubMed]
- Sapkota, S.; Liu, J.; Islam, M.T.; Ravindran, P.; Kumar, P.P.; Sherif, S.M. Contrasting bloom dates in two apple cultivars linked to differential levels of phytohormones and heat requirements during ecodormancy. Sci. Hortic. 2021, 288, 110413. [Google Scholar] [CrossRef]
- Xu, H.; Ediger, D. Rootstocks with Different Vigor Influenced Scion—Water Relations and Stress Responses in AmbrosiaTM Apple Trees (Malus Domestica var. Ambrosia). Plants 2021, 10, 614. [Google Scholar] [CrossRef]
- Lordan, J.; Fazio, G.; Francescatto, P.; Robinson, T. Effects of apple (Malus × domestica) rootstocks on scion performance and hormone concentration. Sci. Hortic. 2017, 225, 96–105. [Google Scholar] [CrossRef]
- Cesaraccio, C.; Spano, D.; Snyder, R.L.; Duce, P. Chilling and forcing model to predict bud-burst of crop and forest species. Agric. For. Meteorol. 2004, 126, 1–13. [Google Scholar] [CrossRef]
- Gibson, P.G.; Reighard, G.L. Chilling requirement and postrest heat accumulation in peach trees inoculated with peach latent mosaic viroid. J. Am. Soc. Hortic. Sci. 2002, 127, 333–336. [Google Scholar] [CrossRef][Green Version]
- Edwards, E.J.; Downie, A.F.; Clingeleffer, P.R. A Simple Microplate Assay to Quantify Non-Structural Carbohydrates of Grapevine Tissue. Am. J. Enol. Vitic. 2011, 62, 133–137. [Google Scholar] [CrossRef]
- Bradford, M.M. A Rapid and Sensitive Method for the Quantitation of Microgram Quantities of Protein Utilizing the Principle of Protein-Dye Binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Kaundal, A.; Rojas, C.M.; Mysore, K.S. Measurement of NADPH Oxidase Activity in Plants. Bio-Protocol 2012, 2, e278. [Google Scholar] [CrossRef]
- Welling, A.; Palva, E.T. Molecular control of cold acclimation in trees. Physiol. Plant. 2006, 127, 167–181. [Google Scholar] [CrossRef]
- Fadón, E.; Herrero, M.; Rodrigo, J. Dormant flower buds actively accumulate starch over winter in sweet cherry. Front. Plant Sci. 2018, 9, 171. [Google Scholar] [CrossRef]
- Park, J.Y.; Canam, T.; Kang, K.Y.; Unda, F.; Mansfield, S.D. Sucrose phosphate synthase expression influences poplar phenology. Tree Physiol. 2009, 29, 937–946. [Google Scholar] [CrossRef]
- Ben Mohamed, H.; Vadel, A.M.; Geuns, J.M.C.; Khemira, H. Carbohydrate changes during dormancy release in Superior Seedless grapevine cuttings following hydrogen cyanamide treatment. Sci. Hortic. 2012, 140, 19–25. [Google Scholar] [CrossRef]
- Charrier, G.; Lacointe, A.; Améglio, T. Dynamic modeling of carbon metabolism during the dormant period accurately predicts the changes in frost hardiness in walnut trees Juglans regia L. Front. Plant Sci. 2018, 871, 1746. [Google Scholar] [CrossRef]
- Rabot, A.; Henry, C.; Ben Baaziz, K.; Mortreau, E.; Azri, W.; Lothier, J.; Hamama, L.; Boummaza, R.; Leduc, N.; Pelleschi-Travier, S.; et al. Insight into the role of sugars in bud burst under light in the rose. Plant Cell Physiol. 2012, 53, 1068–1082. [Google Scholar] [CrossRef]
- Beauvieux, R.; Wenden, B.; Dirlewanger, E. Bud Dormancy in Perennial Fruit Tree Species: A Pivotal Role for Oxidative Cues. Front. Plant Sci. 2018, 9, 657. [Google Scholar] [CrossRef]
- Or, E.; Vilozny, I.; Eyal, Y.; Ogrodovitch, A. The transduction of the signal for grape bud dormancy breaking induced by hydrogen cyanamide may involve the SNF-like protein kinase GDBRPK. Plant Mol. Biol. 2000, 43, 483–494. [Google Scholar] [CrossRef]
- Rutschow, H.L.; Baskin, T.I.; Kramer, E.M. Regulation of solute flux through plasmodesmata in the root meristem. Plant Physiol. 2011, 155, 1817–1826. [Google Scholar] [CrossRef] [PubMed]
- Pérez, F.J.; Noriega, X.; Rubio, S. Hydrogen peroxide increases during endodormancy and decreases during budbreak in grapevine (Vitis vinifera l.) buds. Antioxidants 2021, 10, 873. [Google Scholar] [CrossRef] [PubMed]
- Scandroglio, F.; Tórtora, V.; Radi, R.; Castro, L. Metabolic control analysis of mitochondrial aconitase: Influence over respiration and mitochondrial superoxide and hydrogen peroxide production. Free Radic. Res. 2014, 48, 684–693. [Google Scholar] [CrossRef] [PubMed]
- Gilroy, S.; Suzuki, N.; Miller, G.; Choi, W.G.; Toyota, M.; Devireddy, A.R.; Mittler, R. A tidal wave of signals: Calcium and ROS at the forefront of rapid systemic signaling. Trends Plant Sci. 2014, 19, 623–630. [Google Scholar] [CrossRef]
- Kuroda, H.; Sagisaka, S.; Chiba, K. Seasonal Changes in Peroxide-scavenging Systems of Apple Trees in Relation to Cold Hardiness. J. Jpn. Soc. Hortic. Sci. 1990, 59, 399–408. [Google Scholar] [CrossRef][Green Version]
- Foyer, C.H.; Lopez-Delgado, H.; Dat, J.F.; Scott, I.M. Hydrogen peroxide- and glutathione-associated mechanisms of acclimatory stress tolerance and signalling. Physiol. Plant. 1997, 100, 241–254. [Google Scholar] [CrossRef]
- Abassi, N.A.; Kushad, M.M.; Endress, A.G. Active oxygen-scavenging enzymes activities in developing apple flowers and fruits. Sci. Hortic. 1998, 74, 183–194. [Google Scholar] [CrossRef]
- Rinne, P.; Tuominen, H.; Junttila, O. Seasonal changes in bud dormancy in relation to bud morphology, water and starch content, and abscisic acid concentration in adult trees of Betula pubescens. Tree Physiol. 1994, 14, 549–561. [Google Scholar] [CrossRef]
- Rubio, S.; Noriega, X.; Pérez, F.J. ABA promotes starch synthesis and storage metabolism in dormant grapevine buds. J. Plant Physiol. 2019, 234–235, 1–8. [Google Scholar] [CrossRef]
- Ishibashi, Y.; Aoki, N.; Kasa, S.; Sakamoto, M.; Kai, K.; Tomokiyo, R.; Watabe, G.; Yuasa, T.; Iwaya-Inoue, M. The interrelationship between abscisic acid and reactive oxygen species plays a key role in barley seed dormancy and germination. Front. Plant Sci. 2017, 8, 275. [Google Scholar] [CrossRef] [PubMed]
- Ye, N.; Zhu, G.; Liu, Y.; Li, Y.; Zhang, J. ABA controls H2O2 accumulation through the induction of OsCATB in rice leaves under water stress. Plant Cell Physiol. 2011, 52, 689–698. [Google Scholar] [CrossRef] [PubMed]
- Kwak, J.M.; Mori, I.C.; Pei, Z.M.; Leonhard, N.; Angel Torres, M.; Dangl, J.L.; Bloom, R.E.; Bodde, S.; Jones, J.D.G.; Schroeder, J.I. NADPH oxidase AtrbohD and AtrbohF genes function in ROS-dependent ABA signaling in arabidopsis. EMBO J. 2003, 22, 2623–2633. [Google Scholar] [CrossRef] [PubMed]
- Mittler, R.; Blumwald, E. The roles of ROS and ABA in systemic acquired acclimation. Plant Cell 2015, 27, 64–70. [Google Scholar] [CrossRef] [PubMed]
- Roman, H.; Girault, T.; Barbier, F.; Péron, T.; Brouard, N.; Pencík, A.; Novák, O.; Vian, A.; Sakr, S.; Lothier, J.; et al. Cytokinins are initial targets of light in the control of bud outgrowth. Plant Physiol. 2016, 172, 489–509. [Google Scholar] [CrossRef] [PubMed]
- Arnaud, D.; Lee, S.; Takebayashi, Y.; Choi, D.; Choi, J.; Sakakibara, H.; Hwang, I. Cytokinin-mediated regulation of reactive oxygen species homeostasis modulates stomatal immunity in arabidopsis. Plant Cell 2017, 29, 543–559. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Shen, W.; Chan, Z.; Wu, Y. Endogenous cytokinin overproduction modulates ROS homeostasis and decreases salt stress resistance in Arabidopsis thaliana. Front. Plant Sci. 2015, 6, 1004. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sapkota, S.; Liu, J.; Islam, M.T.; Sherif, S.M. Changes in Reactive Oxygen Species, Antioxidants and Carbohydrate Metabolism in Relation to Dormancy Transition and Bud Break in Apple (Malus × domestica Borkh) Cultivars. Antioxidants 2021, 10, 1549. https://doi.org/10.3390/antiox10101549
Sapkota S, Liu J, Islam MT, Sherif SM. Changes in Reactive Oxygen Species, Antioxidants and Carbohydrate Metabolism in Relation to Dormancy Transition and Bud Break in Apple (Malus × domestica Borkh) Cultivars. Antioxidants. 2021; 10(10):1549. https://doi.org/10.3390/antiox10101549
Chicago/Turabian StyleSapkota, Sangeeta, Jianyang Liu, Md Tabibul Islam, and Sherif M. Sherif. 2021. "Changes in Reactive Oxygen Species, Antioxidants and Carbohydrate Metabolism in Relation to Dormancy Transition and Bud Break in Apple (Malus × domestica Borkh) Cultivars" Antioxidants 10, no. 10: 1549. https://doi.org/10.3390/antiox10101549
APA StyleSapkota, S., Liu, J., Islam, M. T., & Sherif, S. M. (2021). Changes in Reactive Oxygen Species, Antioxidants and Carbohydrate Metabolism in Relation to Dormancy Transition and Bud Break in Apple (Malus × domestica Borkh) Cultivars. Antioxidants, 10(10), 1549. https://doi.org/10.3390/antiox10101549






