Protective Effects of Korean Herbal Remedy against Airway Inflammation in an Allergic Asthma by Suppressing Eosinophil Recruitment and Infiltration in Lung
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of Pyunkang-Tang (PGT) Mixture
2.2. Non-Targeted Metabolites Analysis of PGT by UPLC/MS
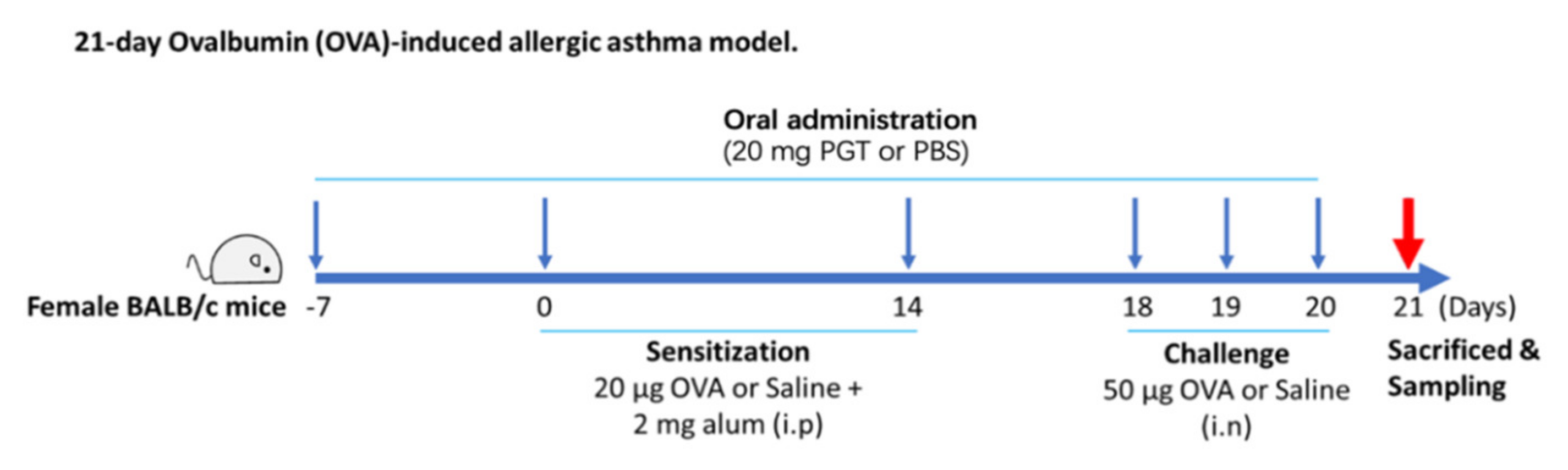
2.3. Ovalbumin-Induced Allergic Asthma in a Mouse Model and PGT Mixture Treatment
2.4. Isolation of Lung and Histological Analysis
2.5. RNA Preparation and Quantitative Reverse Transcriptase-Polymerase Chain Reaction (qRT-PCR)
2.6. Determination of Serum IgE
2.7. Flow Cytometry Analysis
2.8. In Vitro Experiment with Lung Epithelial Cells and Allergen
2.9. Statistical Analysis
3. Results
3.1. Non-Target Metabolites Profiling of PGT
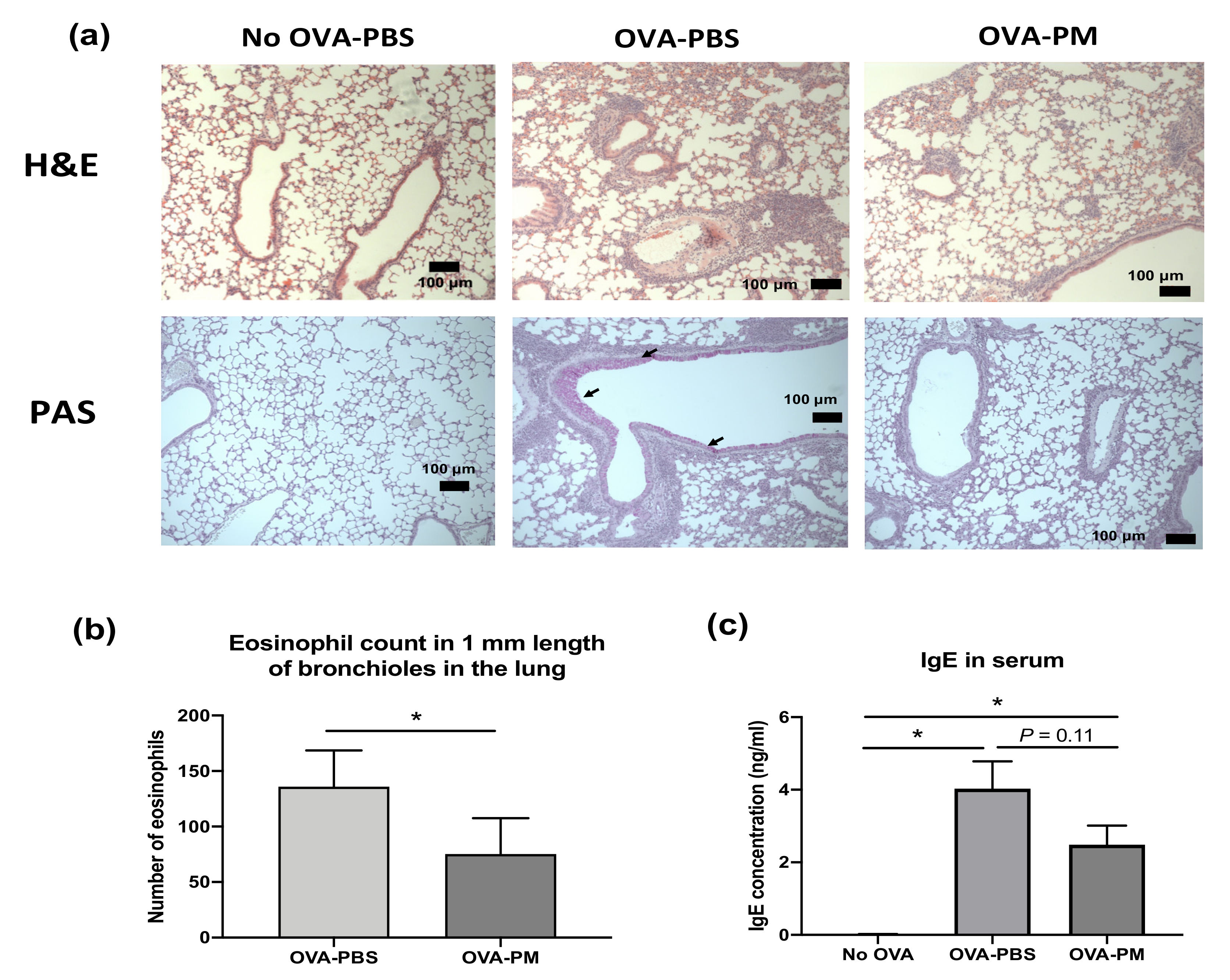
3.2. PGT Treatment Suppressed Airway Inflammation, Mucous Production and Goblet Cell Hyperplasia in OVA-Induced Allergic Asthma Model
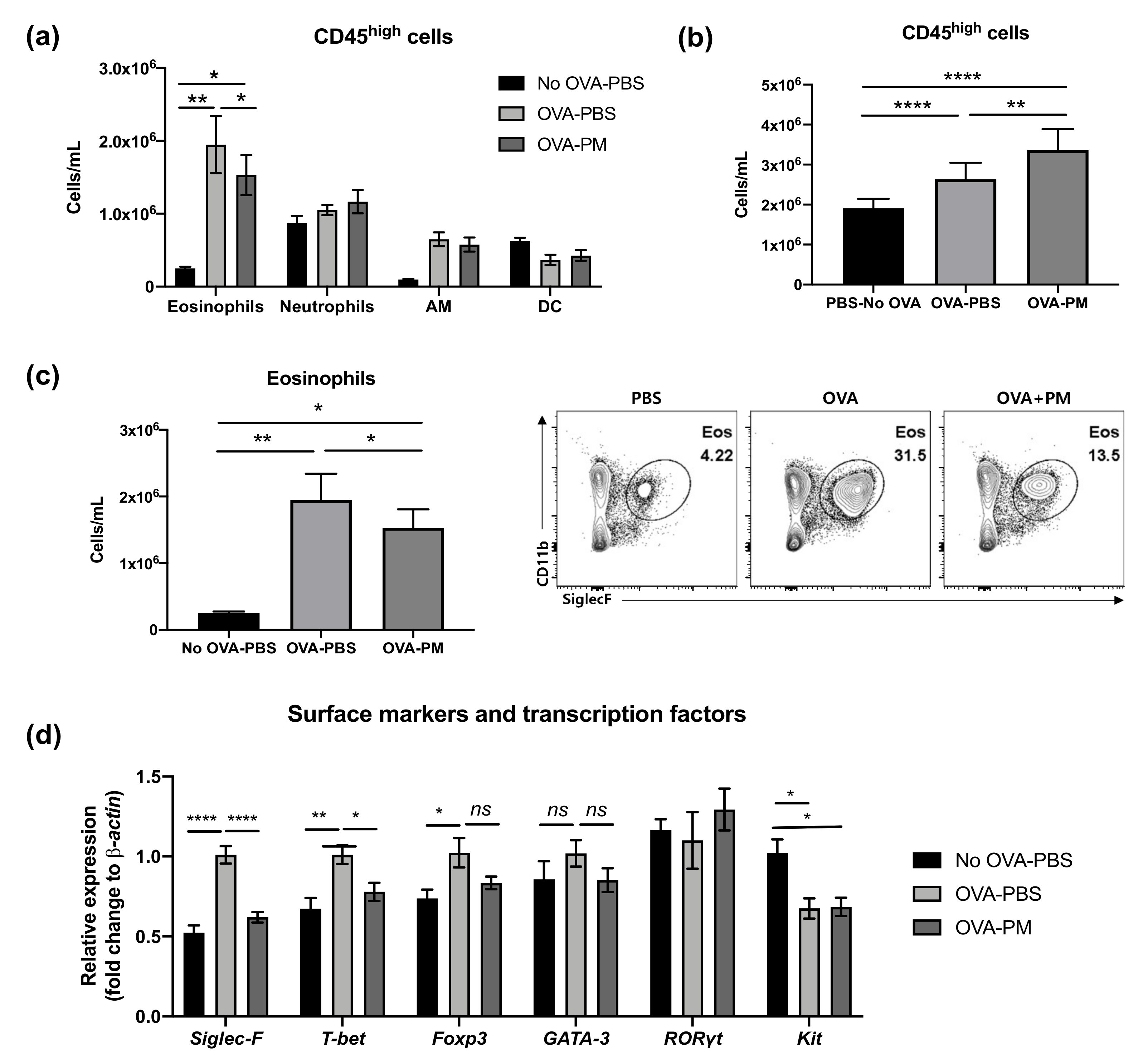
3.3. PGT Treatment Alleviated Eosinophilia Infiltration in Lung
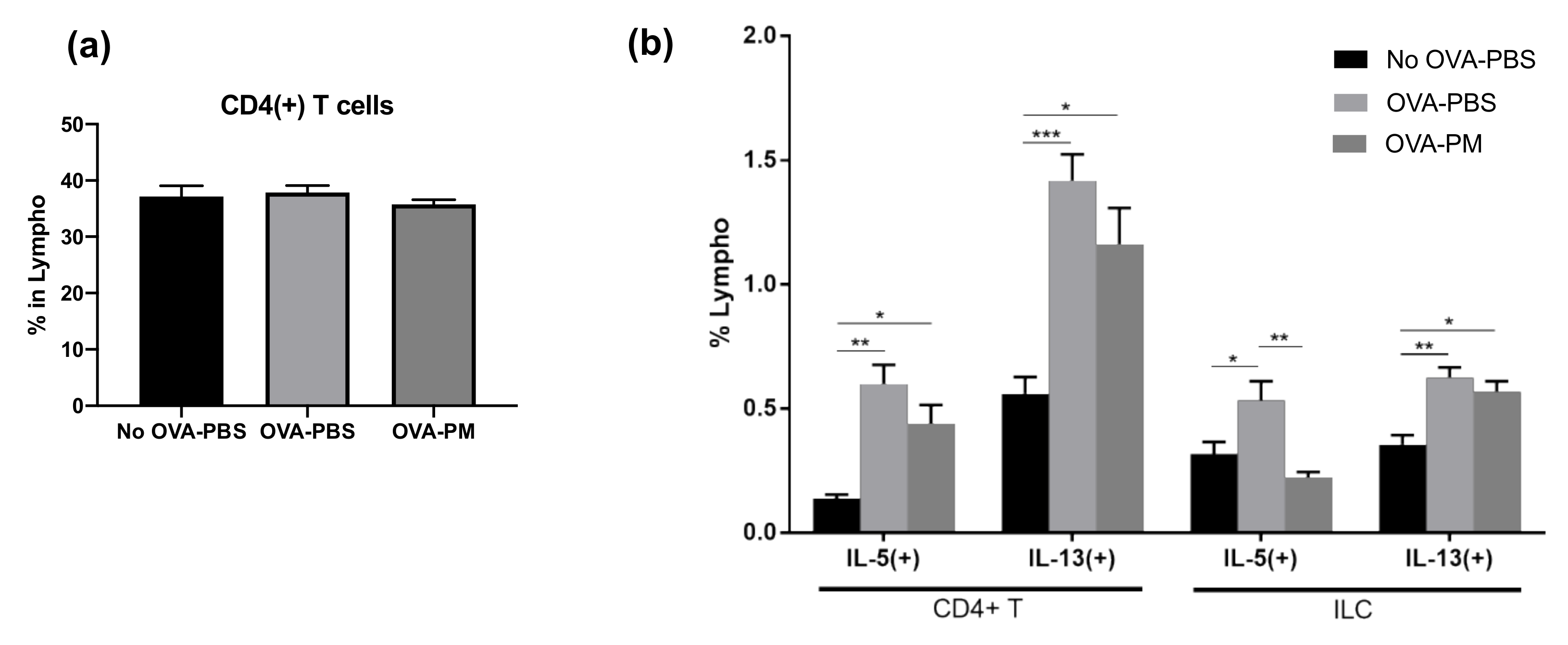
3.4. PGT Treatment Reduced Production of IL-5 and IL-13 in Lung
3.5. PGT Treatment Significantly Reduced IL-5-Producing Innate Lymphoid Cells
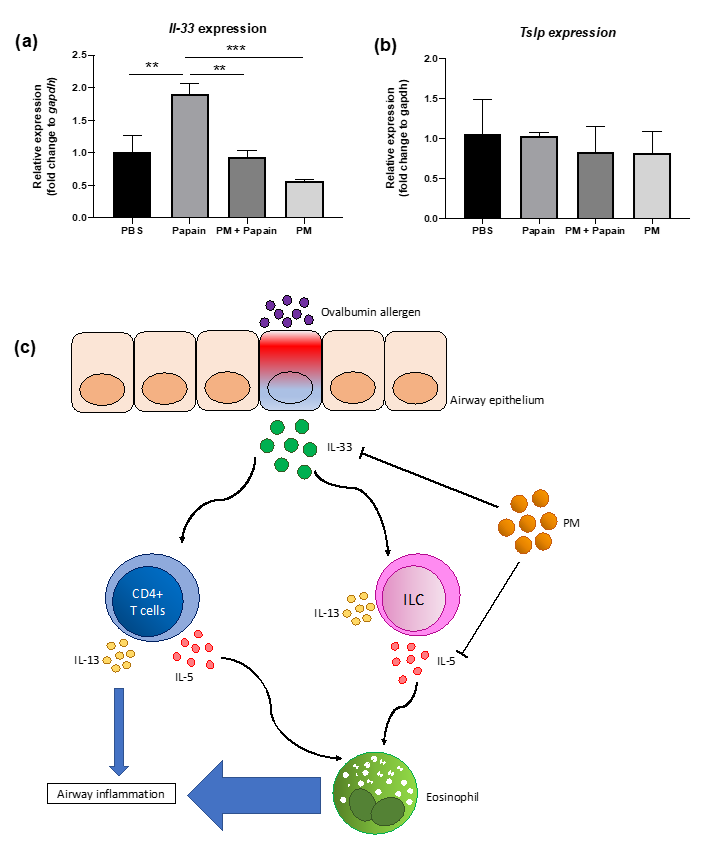
3.6. PGT Treatment Suppressed Production of IL-33, But not TSLP, in Murine Lung Epithelial Cells after Papain Stimulation
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Masoli, M.; Fabian, D.; Holt, S.; Beasley, R.; Global Initiative for Asthma (GINA) Program. The global burden of asthma: Executive summary of the GINA Dissemination Committee report. Allergy 2004, 59, 469–478. [Google Scholar] [CrossRef] [PubMed]
- Bahadori, K.; Doyle-Waters, M.M.; Marra, C.; Lynd, L.; Alasaly, K.; Swiston, J.; FitzGerald, J.M. Economic burden of asthma: A systematic review. BMC Pulm. Med. 2009, 9, 24. [Google Scholar] [CrossRef] [PubMed]
- Nunes, C.; Pereira, A.M.; Morais-Almeida, M. Asthma costs and social impact. Asthma Res. Pract. 2017, 3, 1. [Google Scholar] [CrossRef] [PubMed]
- Braman, S.S. The global burden of asthma. Chest 2006, 130, 4S–12S. [Google Scholar] [CrossRef]
- Asthma, G.I.f. Global strategy for asthma management and prevention. Updated. 2018. [Google Scholar]
- Bousquet, J.; Chanez, P.; Lacoste, J.Y.; Barnéon, G.; Ghavanian, N.; Enander, I.; Venge, P.; Ahlstedt, S.; Simony-Lafontaine, J.; Godard, P. Eosinophilic inflammation in asthma. N. Engl. J. Med. 1990, 323, 1033–1039. [Google Scholar] [CrossRef]
- Foster, P.S.; Hogan, S.P.; Ramsay, A.J.; Matthaei, K.I.; Young, I.G. Interleukin 5 deficiency abolishes eosinophilia, airways hyperreactivity, and lung damage in a mouse asthma model. J. Exp. Med. 1996, 183, 195–201. [Google Scholar] [CrossRef]
- Bosnjak, B.; Stelzmueller, B.; Erb, K.J.; Epstein, M.M. Treatment of allergic asthma: Modulation of Th2 cells and their responses. Respir. Res. 2011, 12, 114. [Google Scholar] [CrossRef]
- Barnes, P.J. Th2 cytokines and asthma: An introduction. Respir. Res. 2001, 2, 64–65. [Google Scholar] [CrossRef]
- Wenzel, S.E. Asthma phenotypes: The evolution from clinical to molecular approaches. Nat. Med. 2012, 18, 716. [Google Scholar] [CrossRef]
- Rosenberg, H.F.; Phipps, S.; Foster, P.S. Eosinophil trafficking in allergy and asthma. J. Allergy Clin. Immunol. 2007, 119, 1303–1310. [Google Scholar] [CrossRef]
- Walter, D.M.; McIntire, J.J.; Berry, G.; McKenzie, A.N.; Donaldson, D.D.; DeKruyff, R.H.; Umetsu, D.T. Critical role for IL-13 in the development of allergen-induced airway hyperreactivity. J. Immunol. 2001, 167, 4668–4675. [Google Scholar] [CrossRef] [PubMed]
- Webb, D.C.; McKenzie, A.N.; Koskinen, A.M.; Yang, M.; Mattes, J.; Foster, P.S. Integrated signals between IL-13, IL-4, and IL-5 regulate airways hyperreactivity. J. Immunol. 2000, 165, 108–113. [Google Scholar] [CrossRef] [PubMed]
- Olin, J.T.; Wechsler, M.E. Asthma: Pathogenesis and novel drugs for treatment. BMJ 2014, 349, g5517. [Google Scholar] [CrossRef] [PubMed]
- Kerrebijn, K.F.; van Essen-Zandvliet, E.; Neijens, H.J. Effect of long-term treatment with inhaled corticosteroids and beta-agonists on the bronchial responsiveness in children with asthma. J. Allergy Clin. Immunol. 1987, 79, 653–659. [Google Scholar] [CrossRef]
- Barnes, P.J.; Pedersen, S.; Busse, W.W. Efficacy and safety of inhaled corticosteroids: New developments. Am. J. Respir. Crit. Care Med. 1998, 157, S1–S53. [Google Scholar] [CrossRef]
- Guilbert, T.W.; Morgan, W.J.; Zeiger, R.S.; Mauger, D.T.; Boehmer, S.J.; Szefler, S.J.; Bacharier, L.B.; Lemanske, R.F., Jr.; Strunk, R.C.; Allen, D.B. Long-term inhaled corticosteroids in preschool children at high risk for asthma. N. Engl. J. Med. 2006, 354, 1985–1997. [Google Scholar] [CrossRef]
- Busse, W.; Corren, J.; Lanier, B.Q.; McAlary, M.; Fowler-Taylor, A.; Della Cioppa, G.; van As, A.; Gupta, N. Omalizumab, anti-IgE recombinant humanized monoclonal antibody, for the treatment of severe allergic asthma. J. Allergy Clin. Immunol. 2001, 108, 184–190. [Google Scholar] [CrossRef]
- Milgrom, H.; Fick, R.B., Jr.; Su, J.Q.; Reimann, J.D.; Bush, R.K.; Watrous, M.L.; Metzger, W.J. Treatment of allergic asthma with monoclonal anti-IgE antibody. N. Engl. J. Med. 1999, 341, 1966–1973. [Google Scholar] [CrossRef]
- Djukanovic, R.; Wilson, S.J.; Kraft, M.; Jarjour, N.N.; Steel, M.; Chung, K.F.; Bao, W.; Fowler-Taylor, A.; Matthews, J.; Busse, W.W. Effects of treatment with anti-immunoglobulin E antibody omalizumab on airway inflammation in allergic asthma. Am. J. Respir. Crit. Care Med. 2004, 170, 583–593. [Google Scholar] [CrossRef]
- Jutel, M.; Agache, I.; Bonini, S.; Burks, A.W.; Calderon, M.; Canonica, W.; Cox, L.; Demoly, P.; Frew, A.J.; O’hehir, R. International consensus on allergy immunotherapy. J. Allergy Clin. Immunol. 2015, 136, 556–568. [Google Scholar] [CrossRef]
- Cazzola, M.; Matera, M.G. Novel long-acting bronchodilators for COPD and asthma. Br. J. Pharmacol. 2008, 155, 291–299. [Google Scholar] [CrossRef] [PubMed]
- Cazzola, M.; Page, C.P.; Calzetta, L.; Matera, M.G. Pharmacology and therapeutics of bronchodilators. Pharmacol. Rev. 2012, 64, 450–504. [Google Scholar] [CrossRef] [PubMed]
- Martinez, F.D. New insights into the natural history of asthma: Primary prevention on the horizon. J. Allergy Clin. Immunol. 2011, 128, 939–945. [Google Scholar] [CrossRef] [PubMed]
- Warner, S.M.; Knight, D.A. Airway modeling and remodeling in the pathogenesis of asthma. Curr. Opin. Allergy Clin. Immunol. 2008, 8, 44–48. [Google Scholar] [CrossRef]
- Lipworth, B.J. Systemic adverse effects of inhaled corticosteroid therapy: A systematic review and meta-analysis. Arch. Intern. Med. 1999, 159, 941–955. [Google Scholar] [CrossRef]
- Kelly, H.W.; Sternberg, A.L.; Lescher, R.; Fuhlbrigge, A.L.; Williams, P.; Zeiger, R.S.; Raissy, H.H.; Van Natta, M.L.; Tonascia, J.; Strunk, R.C. Effect of inhaled glucocorticoids in childhood on adult height. N. Engl. J. Med. 2012, 367, 904–912. [Google Scholar] [CrossRef]
- Partridge, M.R.; van der Molen, T.; Myrseth, S.-E.; Busse, W.W. Attitudes and actions of asthma patients on regular maintenance therapy: The INSPIRE study. BMC Pulm. Med. 2006, 6, 13. [Google Scholar] [CrossRef]
- Rabe, K.; Vermeire, P.; Soriano, J.; Maier, W. Clinical management of asthma in 1999: The Asthma Insights and Reality in Europe (AIRE) study. Eur. Respir. J. 2000, 16, 802–807. [Google Scholar] [CrossRef]
- Lan, W.; Zhaojun, Z.; Zesheng, Z. Characterization of antioxidant activity of extracts from Flos Lonicerae. Drug Dev. Ind. Pharm. 2007, 33, 841–847. [Google Scholar] [CrossRef]
- Ku, S.-K.; Seo, B.-I.; Park, J.-H.; Park, G.-Y.; Seo, Y.-B.; Kim, J.-S.; Lee, H.-S.; Roh, S.-S. Effect of Lonicerae Flos extracts on reflux esophagitis with antioxidant activity. World J. Gastroenterol. WJG 2009, 15, 4799. [Google Scholar] [CrossRef]
- Lee, Y.-C.; Lee, J.-C.; Seo, Y.-B.; Kook, Y.-B. Liriopis tuber inhibit OVA-induced airway inflammation and bronchial hyperresponsiveness in murine model of asthma. J. Ethnopharmacol. 2005, 101, 144–152. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.-R.; Jung, C.-J.; Ku, S.-M.; Jung, D.-H.; Ku, S.-K.; Choi, J.-S. Antitussive, expectorant, and anti-inflammatory effects of Adenophorae Radix powder in ICR mice. J. Ethnopharmacol. 2019, 239, 111915. [Google Scholar] [CrossRef]
- Roh, S.-S.; Kim, S.-H.; Lee, Y.-C.; Seo, Y.-B. Effects of radix adenophorae and cyclosporine A on an OVA-induced murine model of asthma by suppressing to T cells activity, eosinophilia, and bronchial hyperresponsiveness. Mediat. Inflamm. 2008, 781425. [Google Scholar] [CrossRef]
- Huang, M.-H.; Wang, B.-S.; Chiu, C.-S.; Amagaya, S.; Hsieh, W.-T.; Huang, S.-S.; Shie, P.-H.; Huang, G.-J. Antioxidant, antinociceptive, and anti-inflammatory activities of Xanthii Fructus extract. J. Ethnopharmacol. 2011, 135, 545–552. [Google Scholar] [CrossRef] [PubMed]
- Won, A.-N.; Kim, S.A.; Ahn, J.Y.; Han, J.-H.; Kim, C.-H.; Lee, J.-H.; Kim, D.-I. HO-1 Induction by Selaginella tamariscina extract inhibits inflammatory response in lipopolysaccharide-stimulated RAW 264.7 Macrophages. Evid. Based Complement. Altern. Med. 2018, 7816923. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Jung, H.W.; Lee, J.Y.; Kim, J.S.; Kang, S.S.; Kim, Y.S.; Park, Y.-K. 2, 5-dihydroxyacetophenone isolated from Rehmanniae Radix Preparata inhibits inflammatory responses in lipopolysaccharide-stimulated RAW264. 7 macrophages. J. Med. Food 2012, 15, 505–510. [Google Scholar] [CrossRef] [PubMed]
- Lloyd, C.M.; Hessel, E.M. Functions of T cells in asthma: More than just T H 2 cells. Nat. Rev. Immunol. 2010, 10, 838. [Google Scholar] [CrossRef]
- Vock, C.; Hauber, H.-P.; Wegmann, M. The other T helper cells in asthma pathogenesis. J. Allergy 2010, 519298. [Google Scholar] [CrossRef]
- Lloyd, C.M.; Hawrylowicz, C.M. Regulatory T cells in asthma. Immunity 2009, 31, 438–449. [Google Scholar] [CrossRef]
- Kidd, P. Th1/Th2 balance: The hypothesis, its limitations, and implications for health and disease. Altern. Med. Rev. 2003, 8, 223–246. [Google Scholar]
- Halim, T.Y.; Steer, C.A.; Mathä, L.; Gold, M.J.; Martinez-Gonzalez, I.; McNagny, K.M.; McKenzie, A.N.; Takei, F. Group 2 innate lymphoid cells are critical for the initiation of adaptive T helper 2 cell-mediated allergic lung inflammation. Immunity 2014, 40, 425–435. [Google Scholar] [CrossRef] [PubMed]
- Cortez, V.S.; Robinette, M.L.; Colonna, M. Innate lymphoid cells: New insights into function and development. Curr. Opin. Immunol. 2015, 32, 71–77. [Google Scholar] [CrossRef] [PubMed]
- Camelo, A.; Rosignoli, G.; Ohne, Y.; Stewart, R.A.; Overed-Sayer, C.; Sleeman, M.A.; May, R.D. IL-33, IL-25, and TSLP induce a distinct phenotypic and activation profile in human type 2 innate lymphoid cells. Blood Adv. 2017, 1, 577–589. [Google Scholar] [CrossRef]
- Eberl, G.; Colonna, M.; Di Santo, J.P.; McKenzie, A.N. Innate lymphoid cells: A new paradigm in immunology. Science 2015, 348, aaa6566. [Google Scholar] [CrossRef] [PubMed]
- Kurowska-Stolarska, M.; Kewin, P.; Murphy, G.; Russo, R.C.; Stolarski, B.; Garcia, C.C.; Komai-Koma, M.; Pitman, N.; Li, Y.; McKenzie, A.N. IL-33 induces antigen-specific IL-5+ T cells and promotes allergic-induced airway inflammation independent of IL-4. J. Immunol. 2008, 181, 4780–4790. [Google Scholar] [CrossRef]
- Shaw, J.L.; Fakhri, S.; Citardi, M.J.; Porter, P.C.; Corry, D.B.; Kheradmand, F.; Liu, Y.-J.; Luong, A. IL-33–responsive innate lymphoid cells are an important source of IL-13 in chronic rhinosinusitis with nasal polyps. Am. J. Respir. Crit. Care Med. 2013, 188, 432–439. [Google Scholar] [CrossRef]

| Common Name | Genus | Family | Deposit No. | Dry Weight (g) |
|---|---|---|---|---|
| Lonicerae Flos (flowers) | Lonicerae japonica Thunberg | Caprofoliaceae | KHUOPS 2013-75 | 4 |
| Liriopis Tuber (underground stem/roots) | Liriope platyphylla Wang et Tang | Liliaceae | KHUOPS 2013-76 | 4 |
| Adenophorae Radix (roots) | Adenophora triphilla var. japonoica Hara | Campanulaceae | KHUOPS 2013-77 | 10 |
| Xanthii Fructus (fruits) | Xantium strumarinum Linne | Compositae | KHUOPS 2013-78 | 10 |
| Selaginellae Herba (leaves) | Selaginella tamariscina Spring | Selaginellaceae | KHUOPS 2013-79 | 10 |
| Rehmanniae Radix Preparata (roots) | Rehmannia glutinosa Liboschitz var. purpurea Making | Scrophulariaceae | KHUOPS 2013-80 | 2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yoon, S.; Song, S.; Shin, J.W.; Kang, S.; Kim, H.Y.; You, H.J. Protective Effects of Korean Herbal Remedy against Airway Inflammation in an Allergic Asthma by Suppressing Eosinophil Recruitment and Infiltration in Lung. Antioxidants 2021, 10, 6. https://doi.org/10.3390/antiox10010006
Yoon S, Song S, Shin JW, Kang S, Kim HY, You HJ. Protective Effects of Korean Herbal Remedy against Airway Inflammation in an Allergic Asthma by Suppressing Eosinophil Recruitment and Infiltration in Lung. Antioxidants. 2021; 10(1):6. https://doi.org/10.3390/antiox10010006
Chicago/Turabian StyleYoon, Soyon, Seokcheon Song, Jae Woo Shin, Sini Kang, Hye Young Kim, and Hyun Ju You. 2021. "Protective Effects of Korean Herbal Remedy against Airway Inflammation in an Allergic Asthma by Suppressing Eosinophil Recruitment and Infiltration in Lung" Antioxidants 10, no. 1: 6. https://doi.org/10.3390/antiox10010006
APA StyleYoon, S., Song, S., Shin, J. W., Kang, S., Kim, H. Y., & You, H. J. (2021). Protective Effects of Korean Herbal Remedy against Airway Inflammation in an Allergic Asthma by Suppressing Eosinophil Recruitment and Infiltration in Lung. Antioxidants, 10(1), 6. https://doi.org/10.3390/antiox10010006






