Antioxidants in Fish Sperm and the Potential Role of Melatonin
Abstract
1. Introduction
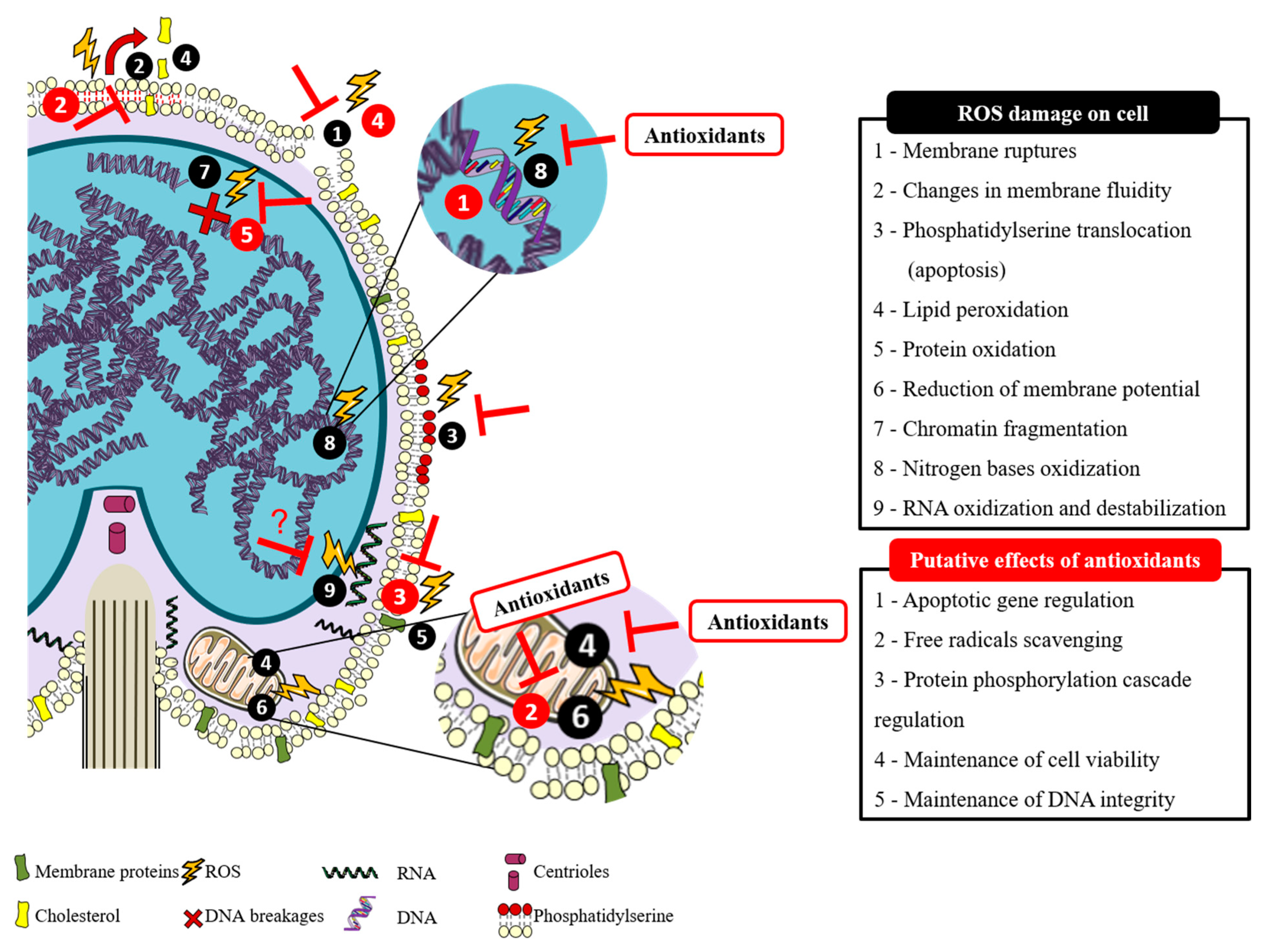
2. Spermatozoa Susceptibility to Reactive Oxygen Species and the Counteract Effect of Antioxidants
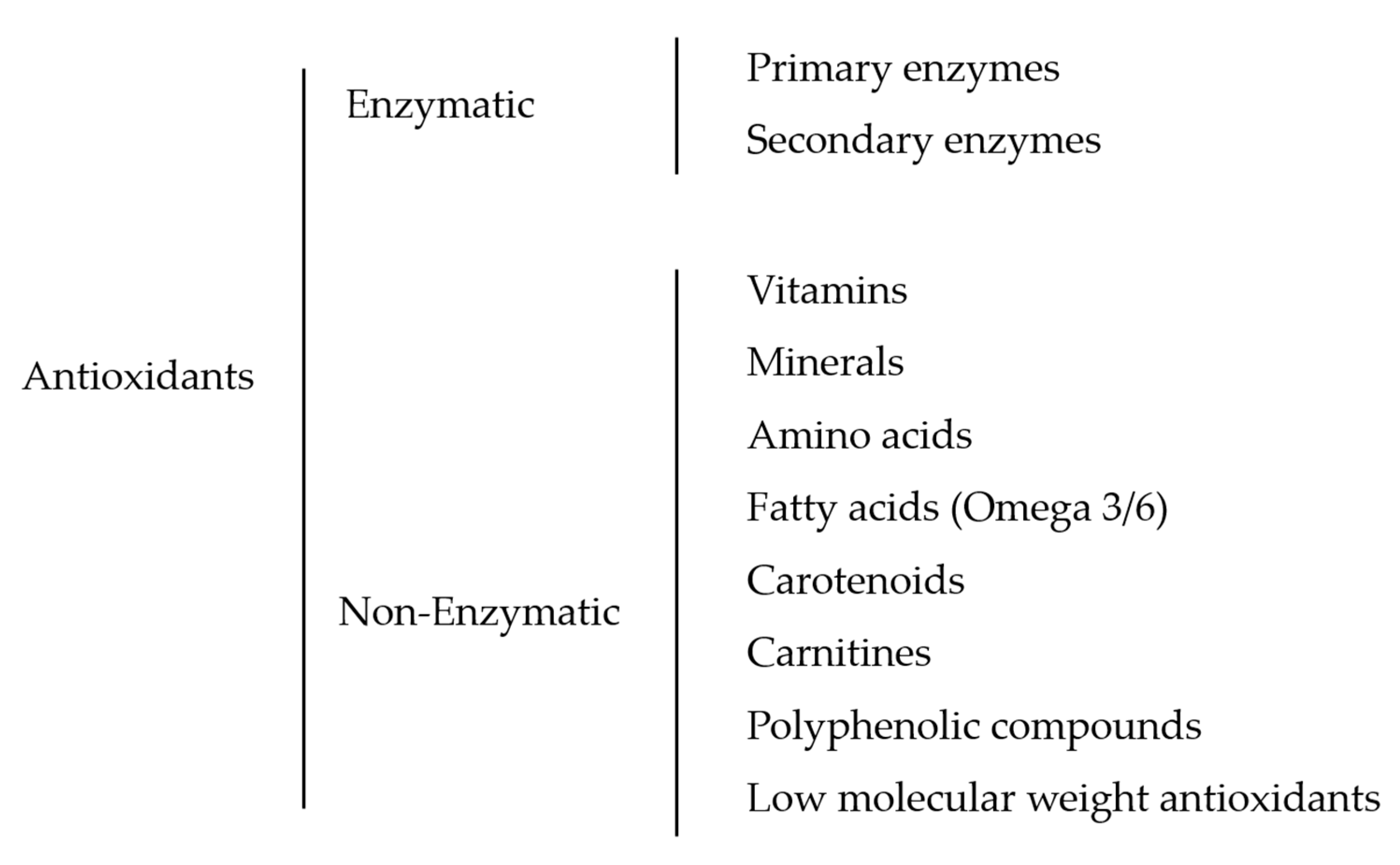
3. Fish Sperm Antioxidant System and Supplementation: Classification and Identification
3.1. Enzymatic Antioxidants
3.2. Non-Enzymatic Antioxidants
3.2.1. Vitamins
3.2.2. Minerals
3.2.3. Amino Acids
3.2.4. Omega-3 Fatty Acids
3.2.5. Carotenoids
3.2.6. Carnitines
3.2.7. Polyphenolic Antioxidants: Is There a Natural Solution?
| Phenolic Compound/Extract | Species | Target | Administration Mode | Main Effects | Ref | |
|---|---|---|---|---|---|---|
| Fish | MDPA | Beluga sturgeon | Sperm | CM | ↑ Fertilization rate | [121] |
| BHT | Common carp | Sperm | CM | ↑ Sperm motility, fertilization rate, eyed-egg rate | [122] | |
| Russian sturgeon | Sperm | CM | ↑ Sperm motility, ↓ LPO, | [124] | ||
| Coho salmon | Sperm | CM | ↑ Sperm motility, mitochondrial membrane potential, ↓ SOD• in spermatozoa | [123] | ||
| Curcumin + black pepper | African catfish | General health | Diet | ↑ Growth, ↓ hepatotoxic, nephrotoxic, and reprotoxic effects of cadmium | [135] | |
| Curcumin | Sand goby | General health | Diet | ↑ Digestive enzymes activity, growth | [140] | |
| Paprika | Senegalese sole | Sperm | Diet | ↑ Sperm concentration, ↓ skin injuries | [136] | |
| Ginger | Rainbow trout | General health | Diet | ↑ Growth, FCR and protein efficiency | [137] | |
| Asian seabass | General health | Diet | ↑ Survival, growth, FCR, immunological activity | [138] | ||
| Nile tilapia | General health | Diet | ↑ Growth, blood plasma total proteins ↓ mortality, blood glucose, triglycerides, and cholesterol | [141] | ||
| Blueberries | Artic char | Sperm | Diet | ↑ CAT-like activity, ↓ LPO | [6] | |
| Sesame seed | African catfish | Reproductive system | Diet | ↑ Sperm motility duration, hatchability, and egg survival rate | [142] | |
| Gracilaria sp. | European seabass | General health | Diet | ↑ TAC, down-regulated heat shock proteins ↓ LPO, delayed mortality | [143] | |
| Propolis | Common carp | Sperm | CM | ↑ Sperm integrity, motility, and hatchability | [144] | |
| Nile tilapia | Spleen | Diet | ↑ Survival, number of lymphocytes, GR | [145] | ||
| Rainbow trout | Brain | Diluted in water | ↑ CAT, ↓ MDA | [146] | ||
| Mammals | Rosemary | Boar | Sperm | CM | ↑ Sperm motility, fertilization capacity | [63] |
| Deer | Sperm | CM | ↑ Sperm motility, membrane integrity and live cells | [125] | ||
| White tea | Rat | Sperm | Extender | ↑ Sperm survival, ↓ LPO | [126] | |
| Green tea | Rabbit | General health | Oral | ↑ Reproductive performance, lipid metabolism, preserve hematological parameters, kidney, and liver functions | [128] | |
| Human | Sperm | Extender | ↑ Sperm motility, viability, phosphorylation of proteins, cell survival | [127] | ||
| Saffron | Buffalo | Sperm | CM | ↑ Sperm motility, viability, acrosome integrity, ↓ ROS, LPO | [130] | |
| Human | Sperm | Oral | ↓ Oxidative damage on sperm DNA | [129] | ||
| Curcumin | Human | Sperm | Oral | ↑ TAC, ↓ MDA, C-reactive protein, tumor necrosis factor | [131] | |
| Dog | Sperm | CM | ↑ Sperm DNA integrity, TAC, NOX-5 gene expression | [132] | ||
| Angora goat | Sperm | CM | ↑ Sperm motility, morphology, SOD activity | [133] | ||
| Rooster | Sperm | Diet | ↑ Sperm motility, viability, ↓ROS | [134] | ||
| Murtilla | Boar | Sperm | CM | ↑ Sperm motility, ↓ ROS, membrane damage | [147] | |
| Royal jelly | Buffalo | Sperm | Extender | ↑ Sperm viability, membrane and acrosome integrity, fertilization capacity | [148,149] | |
| Goat | Sperm | Extender | ↑ Sperm motility, membrane integrity ↓ acrosome damage | [150] | ||
| Ram | Sperm | Extender | ↑ Sperm motility, membrane integrity, cell viability | [151] | ||
| Rat | Sperm | Oral | ↑ Sperm motility, concentration, SOD, CAT and GSH activity ↓ Sperm abnormalities, MDA, apoptotic cells | [152] | ||
| Propolis | Rat | Sperm | Oral | ↑ Sperm motility, morphology, embryo development ↓ MDA | [153] | |
| Hazelnut | Rat | Sperm | Diet | ↑ Plasma testosterone, plasma oxidant-antioxidant balance | [154] |
3.2.8. Low Molecular Weight Antioxidants
3.3. Potential Role of Melatonin
3.3.1. Melatonin Production Sites on Fish
3.3.2. Melatonin-Mediated and Non-Mediated Mechanisms and Targets
3.3.3. Different Methods for Spermatozoa Protection
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Cabrita, E.; Martínez-Páramo, S.; Gavaia, P.J.; Riesco, M.F.; Valcarce, D.G.; Sarasquete, C.; Herráez, M.P.; Robles, V. Factors enhancing fish sperm quality and emerging tools for sperm analysis. Aquaculture 2014, 432, 389–401. [Google Scholar] [CrossRef]
- Aitken, R.J.; Drevet, J.R. The Importance of Oxidative Stress in Determining the Functionality of Mammalian Spermatozoa: A Two-Edged Sword. Antioxidants 2020, 9, 111. [Google Scholar] [CrossRef] [PubMed]
- Martin-Hidalgo, D.; Bragado, M.J.; Batista, A.R.; Oliveira, P.F.; Alves, M.G. Antioxidants and Male Fertility: From Molecular Studies to Clinical Evidence. Antioxidants 2019, 8, 89. [Google Scholar] [CrossRef] [PubMed]
- Sandoval-Vargas, L.; Mauricio, S.J.; González, J.R.; Figueroa, E.V.; Cabrita, E.; Valdebenito, I.I. Oxidative stress and use of antioxidants in fish semen cryopreservation. Rev. Aquac. 2020, 13, 365–387. [Google Scholar] [CrossRef]
- Cabrita, E.; Ma, S.; Diogo, P.; Martínez-Páramo, S.; Sarasquete, C.; Dinis, M.T. The influence of certain aminoacids and vitamins on post-thaw fish sperm motility, viability and DNA fragmentation. Anim. Reprod. Sci. 2011, 125, 189–195. [Google Scholar] [CrossRef]
- Mansour, N.; McNiven, M.A.; Richardson, G.F. The effect of dietary supplementation with blueberry, alpha-tocopherol or astaxanthin on oxidative stability of Arctic char (Salvelinus alpinus) semen. Theriogenology 2006, 66, 373–382. [Google Scholar] [CrossRef]
- Martínez-Páramo, S.; Dinis, M.; Soares, F.; Pacchiarini, T.; Sarasquete, C.; Cabrita, E. A nutritional approach to enhance the antioxidant system of fish teleosts. In Proceedings of the 4th International Workshop on the Biology of Fish Gametes, Albufeira, Portugal, 17–20 September 2013; pp. 120–121. [Google Scholar]
- Wischhusen, P.; Parailloux, M.; Geraert, P.-A.; Briens, M.; Bueno, M.; Mounicou, S.; Bouyssiere, B.; Antony Jesu Prabhu, P.; Kaushik, S.J.; Fauconneau, B.; et al. Effect of dietary selenium in rainbow trout (Oncorhynchus mykiss) broodstock on antioxidant status, its parental transfer and oxidative status in the progeny. Aquaculture 2019, 507, 126–138. [Google Scholar] [CrossRef]
- Martínez-Páramo, S.; Diogo, P.; Dinis, M.T.; Herraez, M.P.; Sarasquete, C.; Cabrita, E. Incorporation of ascorbic acid and alpha-tocopherol to the extender media to enhance antioxidant system of cryopreserved sea bass sperm. Theriogenology 2012, 77, 1129–1136. [Google Scholar] [CrossRef]
- Falcón, J.; Migaud, H.; Munoz-Cueto, J.A.; Carrillo, M. Current knowledge on the melatonin system in teleost fish. Gen. Comp. Endocrinol. 2010, 165, 469–482. [Google Scholar] [CrossRef]
- Maitra, S.K.; Hasan, K.N. The Role of Melatonin as a Hormone and an Antioxidant in the Control of Fish Reproduction. Front. Endocrinol. 2016, 7, 38. [Google Scholar] [CrossRef]
- Cosson, J.; Groison, A.L.; Suquet, M.; Fauvel, C.; Dreanno, C.; Billard, R. Studying sperm motility in marine fish: An overview on the state of the art. J. Appl. Ichthyol. 2008, 24, 460–486. [Google Scholar] [CrossRef]
- Mironczuk-Chodakowska, I.; Witkowska, A.M.; Zujko, M.E. Endogenous non-enzymatic antioxidants in the human body. Adv. Med. Sci. 2018, 63, 68–78. [Google Scholar] [CrossRef]
- Figueroa, E.; Farias, J.G.; Lee-Estevez, M.; Valdebenito, I.; Risopatrón, J.; Magnotti, C.; Romero, J.; Watanabe, I.; Oliveira, R.P.S. Sperm cryopreservation with supplementation of α-tocopherol and ascorbic acid in freezing media increase sperm function and fertility rate in Atlantic salmon (Salmo salar). Aquaculture 2018, 493, 1–8. [Google Scholar] [CrossRef]
- Len, J.S.; Koh, W.S.D.; Tan, S.X. The roles of reactive oxygen species and antioxidants in cryopreservation. Biosci. Rep. 2019, 39. [Google Scholar] [CrossRef]
- Beirão, J.; Soares, F.; Pousão-Ferreira, P.; Diogo, P.; Dias, J.; Dinis, M.T.; Herráez, M.P.; Cabrita, E. The effect of enriched diets on Solea senegalensis sperm quality. Aquaculture 2015, 435, 187–194. [Google Scholar] [CrossRef]
- Lahnsteiner, F.; Mansour, N.; Plaetzer, K. Antioxidant systems of brown trout (Salmo trutta f. fario) semen. Anim. Reprod. Sci. 2010, 119, 314–321. [Google Scholar] [CrossRef] [PubMed]
- Valcarce, D.G.; Robles, V. Evaluation of Intracellular Location of Reactive Oxygen Species in Solea Senegalensis Spermatozoa. J. Vis. Exp. 2018, e55323. [Google Scholar] [CrossRef]
- Kefer, J.C.; Agarwal, A.; Sabanegh, E. Role of antioxidants in the treatment of male infertility. Int. J. Urol. 2009, 16, 449–457. [Google Scholar] [CrossRef]
- Catoni, C.; Peters, A.; Martin Schaefer, H. Life history trade-offs are influenced by the diversity, availability and interactions of dietary antioxidants. Anim. Behav. 2008, 76, 1107–1119. [Google Scholar] [CrossRef]
- Flora, S.J.S. Structural, chemical and biological aspects of antioxidants for strategies against metal and metalloid exposure. Oxid. Med. Cell. Longev. 2009, 2, 191–206. [Google Scholar] [CrossRef]
- Bunaciu, A.A.; Aboul-Enein, H.Y.; Fleschin, S. FTIR Spectrophotometric Methods Used for Antioxidant Activity Assay in Medicinal Plants. Appl. Spectrosc. Rev. 2012, 47, 245–255. [Google Scholar] [CrossRef]
- Jacobsen, C. Oxidative Rancidity. In Encyclopedia of Food Chemistry; Elsevier Science & Technology: Gurgaon, Haryana, 2019; Volume 2, pp. 261–269. [Google Scholar]
- Hermund, D.B. Antioxidant properties of seaweed-derived substances. In Bioactive Seaweeds for Food Applications; Elsevier: Amsterdam, The Netherlands, 2018; pp. 201–221. [Google Scholar]
- Li, P.; Xi, M.D.; Du, H.; Qiao, X.M.; Liu, Z.G.; Wei, Q.W. Antioxidant supplementation, effect on post-thaw spermatozoan function in three sturgeon species. Reprod. Domest. Anim. 2018, 53, 287–295. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Wang, X.; Wang, W.; Zhang, X.; Xu, S.; Ma, D.; Xiao, Z.; Xiao, Y.; Li, J. Effect of the addition of six antioxidants on sperm motility, membrane integrity and mitochondrial function in red seabream (Pagrus major) sperm cryopreservation. Fish Physiol. Biochem. 2015, 41, 413–422. [Google Scholar] [CrossRef] [PubMed]
- Lahnsteiner, F.; Mansour, N. A comparative study on antioxidant systems in semen of species of the Percidae, Salmonidae, Cyprinidae, and Lotidae for improving semen storage techniques. Aquaculture 2010, 307, 130–140. [Google Scholar] [CrossRef]
- Hagedorn, M.; McCarthy, M.; Carter, V.L.; Meyers, S.A. Oxidative stress in zebrafish (Danio rerio) sperm. PLoS ONE 2012, 7. [Google Scholar] [CrossRef]
- Kutluyer, F.; Kayim, M.; Ogretmen, F.; Buyukleblebici, S.; Tuncer, P.B. Cryopreservation of rainbow trout Oncorhynchus mykiss spermatozoa: Effects of extender supplemented with different antioxidants on sperm motility, velocity and fertility. Cryobiology 2014, 69, 462–466. [Google Scholar] [CrossRef]
- Halliwell, B.; Gutteridge, J.M.C. Free Radicals in Biology and Medicine, 5th ed.; OUP: Oxford, UK, 2015. [Google Scholar]
- Izquierdo, M.; Fernandez-Palacios, H.; Tacon, A. Effect of broodstock nutrition on reproductive performance of fish. Aquaculture 2001, 197, 25–42. [Google Scholar] [CrossRef]
- Ahmadi, S.; Bashiri, R.; Ghadiri-Anari, A.; Nadjarzadeh, A. Antioxidant supplements and semen parameters: An evidence based review. Int. J. Reprod. Biomed. 2016, 14, 729–736. [Google Scholar] [CrossRef]
- Paul, T.; Young, M.J.; Hill, I.E.; Ingold, K. Strand cleavage of supercoiled DNA by water-soluble peroxyl radicals. The overlooked importance of peroxyl radical charge. Biochemistry 2000, 39, 4129–4135. [Google Scholar] [CrossRef]
- Lee, K.-J.; Dabrowski, K. Long-term effects and interactions of dietary vitamins C and E on growth and reproduction of yellow perch, Perca flavescens. Aquaculture 2004, 230, 377–389. [Google Scholar] [CrossRef]
- Mirzoyan, A.V.; Nebesikhina, N.A.; Voynova, N.V.; Chistyakov, V.A. Preliminary results on ascorbic acid and lysine suppression of clastogenic effect of deep-frozen sperm of the Russian sturgeon (Acipenser gueldenstaedti). Int. J. Refrig. 2006, 29, 374–378. [Google Scholar] [CrossRef]
- Prabhu, P.A.J.; Schrama, J.W.; Kaushik, S.J. Mineral requirements of fish: A systematic review. Rev. Aquac. 2016, 8, 172–219. [Google Scholar] [CrossRef]
- Salas-Huetos, A.; James, E.R.; Aston, K.I.; Jenkins, T.G.; Carrell, D.T. Diet and sperm quality: Nutrients, foods and dietary patterns. Reprod. Biol 2019, 19, 219–224. [Google Scholar] [CrossRef] [PubMed]
- Bell, J.; Pirie, B.; Adron, J.; Cowey, C. Some effects of selenium deficiency on glutathione peroxidase (EC 1.11. 1.9) activity and tissue pathology in rainbow trout (Salmo gairdneri). Br. J. Nutr. 1986, 55, 305–311. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Li, J.; Yan, L.; Cao, J.; Li, D.; Huang, G.Y.; Shi, W.J.; Dong, W.; Zha, J.; Ying, G.G.; et al. Subchronic effects of dietary selenium yeast and selenite on growth performance and the immune and antioxidant systems in Nile tilapia Oreochromis niloticus. Fish Shellfish Immunol. 2020, 97, 283–293. [Google Scholar] [CrossRef] [PubMed]
- Khan, K.U.; Zuberi, A.; Fernandes, J.B.K.; Ullah, I.; Sarwar, H. An overview of the ongoing insights in selenium research and its role in fish nutrition and fish health. Fish Physiol. Biochem. 2017, 43, 1689–1705. [Google Scholar] [CrossRef]
- Authman, M.M.; Zaki, M.S.; Khallaf, E.A.; Abbas, H.H. Use of fish as bio-indicator of the effects of heavy metals pollution. J. Aquac. Res. Dev. 2015, 6, 1–13. [Google Scholar] [CrossRef]
- Ibrahim, A.T.A.; Banaee, M.; Sureda, A. Selenium protection against mercury toxicity on the male reproductive system of Clarias gariepinus. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2019, 225, 108583. [Google Scholar] [CrossRef]
- Ashouri, S.; Keyvanshokooh, S.; Salati, A.P.; Johari, S.A.; Pasha-Zanoosi, H. Effects of different levels of dietary selenium nanoparticles on growth performance, muscle composition, blood biochemical profiles and antioxidant status of common carp (Cyprinus carpio). Aquaculture 2015, 446, 25–29. [Google Scholar] [CrossRef]
- Salgueiro, M.J.; Zubillaga, M.; Lysionek, A.; Sarabia, M.I.; Caro, R.; De Paoli, T.; Hager, A.; Weill, R.; Boccio, J. Zinc as an essential micronutrient: A review. Nutr. Res. 2000, 20, 737–755. [Google Scholar] [CrossRef]
- Swain, P.S.; Rao, S.B.N.; Rajendran, D.; Dominic, G.; Selvaraju, S. Nano zinc, an alternative to conventional zinc as animal feed supplement: A review. Anim. Nutr. 2016, 2, 134–141. [Google Scholar] [CrossRef] [PubMed]
- Salas-Huetos, A.; Rosique-Esteban, N.; Becerra-Tomas, N.; Vizmanos, B.; Bullo, M.; Salas-Salvado, J. The Effect of Nutrients and Dietary Supplements on Sperm Quality Parameters: A Systematic Review and Meta-Analysis of Randomized Clinical Trials. Adv. Nutr. 2018, 9, 833–848. [Google Scholar] [CrossRef] [PubMed]
- Lopes, F.C.; Junior, A.S.V.; Corcini, C.D.; Sanchez, J.A.A.; Pires, D.M.; Pereira, J.R.; Primel, E.G.; Fillmann, G.; Martins, C.M.G. Impacts of the biocide chlorothalonil on biomarkers of oxidative stress, genotoxicity, and sperm quality in guppy Poecilia vivipara. Ecotoxicol. Environ. Saf. 2020, 188, 109847. [Google Scholar] [CrossRef] [PubMed]
- Shahpar, Z.; Johari, S.A. Effects of Dietary Organic, Inorganic, and Nanoparticulate Zinc on Rainbow Trout, Oncorhynchus mykiss Larvae. Biol. Trace Elem. Res. 2019, 190, 535–540. [Google Scholar] [CrossRef] [PubMed]
- Tan, B.; Mai, K. Zinc methionine and zinc sulfate as sources of dietary zinc for juvenile abalone, Haliotis discus hannai Ino. Aquaculture 2001, 192, 67–84. [Google Scholar] [CrossRef]
- Kazemi, E.; Sourinejad, I.; Ghaedi, A.; Johari, S.A.; Ghasemi, Z. Effect of different dietary zinc sources (mineral, nanoparticulate, and organic) on quantitative and qualitative semen attributes of rainbow trout (Oncorhynchus mykiss). Aquaculture 2020, 515, 734529. [Google Scholar] [CrossRef]
- Kaliky, N.A.P.S.B.; Setiawati, M.; Carman, O.; Utomo, N.B.P. Effect of zinc (Zn) supplementation on quality and quantity of striped catfish Pangasianodon hypophthalmus sperm. J. Akua. Indonesia 2019, 18, 46–53. [Google Scholar] [CrossRef]
- Aripin, S.-A.; Jintasatap, O.; Yoonpundh, R. Effects of Exogenous Melatonin and Zinc Amino Acid on Male Clarias macrocephalus Broodstock. Asian J. Sci. Res. 2018, 11, 515–521. [Google Scholar] [CrossRef]
- Habte-Tsion, H.M. A review on fish immuno-nutritional response to indispensable amino acids in relation to TOR, NF-kappaB and Nrf2 signaling pathways: Trends and prospects. Comp. Biochem. Physiol. B, Biochem. Mol. Biol. 2020, 241, 110389. [Google Scholar] [CrossRef]
- Wu, G. Amino acids: Metabolism, functions, and nutrition. Amino Acids 2009, 37, 1–17. [Google Scholar] [CrossRef]
- Finn, R.N.; Fyhn, H.J. Requirement for amino acids in ontogeny of fish. Aquacult. Res. 2010, 41, 684–716. [Google Scholar] [CrossRef]
- Lahnsteiner, F. The role of free amino acids in semen of rainbow trout Oncorhynchus mykiss and carp Cyprinus carpio. J. Fish Biol. 2009, 75, 816–833. [Google Scholar] [CrossRef] [PubMed]
- Lahnsteiner, F. A comparative study on the composition and importance of free amino acids in semen of gilthead sea bream, Sparus aurata, and perch, Perca fluviatilis. Fish Physiol. Biochem. 2010, 36, 1297–1305. [Google Scholar] [CrossRef] [PubMed]
- Butts, I.A.E.; Hilmarsdóttir, G.S.; Zadmajid, V.; Gallego, V.; Støttrup, J.G.; Jacobsen, C.; Krüger-Johnsen, M.; Politis, S.N.; Asturiano, J.F.; Holst, L.K.; et al. Dietary amino acids impact sperm performance traits for a catadromous fish, Anguilla anguilla reared in captivity. Aquaculture 2020, 518. [Google Scholar] [CrossRef]
- Ogretmen, F.; Inanan, B.E.; Kutluyer, F.; Kayim, M. Effect of semen extender supplementation with cysteine on postthaw sperm quality, DNA damage, and fertilizing ability in the common carp (Cyprinus carpio). Theriogenology 2015, 83, 1548–1552. [Google Scholar] [CrossRef]
- Da Costa, B.B.; Marques, L.S.; Lassen, P.G.; Rodrigues, R.B.; Tais Da Rosa Silva, H.; Moreira, J.C.F.; Streit, D.P. Effects of cysteine supplementation on the quality of cryopreserved sperm of South American silver catfish. Aquacult. Res. 2019, 51, 455–464. [Google Scholar] [CrossRef]
- Kledmanee, K.; Taweedet, S.; Thaijongruk, P.; Chanapiwat, P.; Kaeoket, K. Effect of L-cysteine on chilled carp (Cyprinus carpio) semen qualities. Wetchasan Sattawaphaet 2013, 43, 91–97. [Google Scholar]
- Tuncer, P.B.; Bucak, M.N.; Buyukleblebici, S.; Sariozkan, S.; Yeni, D.; Eken, A.; Akalin, P.P.; Kinet, H.; Avdatek, F.; Fidan, A.F.; et al. The effect of cysteine and glutathione on sperm and oxidative stress parameters of post-thawed bull semen. Cryobiology 2010, 61, 303–307. [Google Scholar] [CrossRef]
- Malo, C.; Gil, L.; Gonzalez, N.; Martinez, F.; Cano, R.; de Blas, I.; Espinosa, E. Anti-oxidant supplementation improves boar sperm characteristics and fertility after cryopreservation: Comparison between cysteine and rosemary (Rosmarinus officinalis). Cryobiology 2010, 61, 142–147. [Google Scholar] [CrossRef]
- Sansone, R.A.; Sansone, L.A. Getting a knack for NAC: N-acetyl-cysteine. Innov. Clin. Neurosci. 2011, 8, 10. [Google Scholar]
- Stejskal, K.; Svobodova, Z.; Fabrik, I.; Adam, V.; Beklova, M.; Rodina, M.; Kizek, R. Content of cysteine, reduced and oxidized glutathione in spermatozoa of representatives of Acipenseriformes (Acipenser baerii and A. ruthenus) as well as teleosts (Perca fluviatilis and Sander lucioperca). J. Appl. Ichthyol. 2008, 24, 519–521. [Google Scholar] [CrossRef]
- Kutluyer, F.; Öğretmen, F.; İnanan, B.E. Effects of semen extender supplemented with L-methionine and packaging methods (straws and pellets) on post-thaw goldfish (Carassius auratus) sperm quality and DNA damage. CryoLetters 2015, 36, 336–343. [Google Scholar] [PubMed]
- Bouyeh, M. Effect of excess lysine and methionine on immune system and performance of broilers. Ann. Biol. Res. 2012, 3, 3218–3224. [Google Scholar]
- Lahnsteiner, F.; Mansour, N.; Kunz, F.A. The effect of antioxidants on the quality of cryopreserved semen in two salmonid fish, the brook trout (Salvelinus fontinalis) and the rainbow trout (Oncorhynchus mykiss). Theriogenology 2011, 76, 882–890. [Google Scholar] [CrossRef]
- Kutluyer, F.; Öğretmen, F.; Inanan, B.E. Cryopreservation of Goldfish (Carassius Auratus) Spermatozoa: Effects of Extender Supplemented with Taurine on Sperm Motility and Dna Damage. CryoLetters 2016, 37, 41–46. [Google Scholar]
- Li, P.; Mai, K.; Trushenski, J.; Wu, G. New developments in fish amino acid nutrition: Towards functional and environmentally oriented aquafeeds. Amino Acids 2008, 37, 43–53. [Google Scholar] [CrossRef]
- Schaffer, S.W.; Jong, C.J.; Ramila, K.; Azuma, J. Physiological roles of taurine in heart and muscle. J. Biomed. Sci. 2010, 17, S2. [Google Scholar] [CrossRef]
- Ekici, A.; Baran, A.; Yamaner, G.; Özdaş, Ö.B.; Sandal, A.İ.; Güven, E.; Baltacı, M.A. Effects of Different Doses of Taurine in the Glucose-Based Extender During Cryopreservation of Rainbow Trout (Oncorhynchus Mykiss) Semen. Biotechnol. Biotechnol. Equip. 2014, 26, 3113–3115. [Google Scholar] [CrossRef]
- Schaffer, S.W.; Azuma, J.; Mozaffari, M. Role of antioxidant activity of taurine in diabetes. Can. J. Physiol. Pharmacol. 2009, 87, 91–99. [Google Scholar] [CrossRef]
- Martínez-Páramo, S.; Diogo, P.; Dinis, M.T.; Soares, F.; Sarasquete, C.; Cabrita, E. Effect of two sulfur-containing amino acids, taurine and hypotaurine in European sea bass (Dicentrarchus labrax) sperm cryopreservation. Cryobiology 2013, 66, 333–338. [Google Scholar] [CrossRef]
- Higuchi, M.; Celino, F.T.; Shimizu-Yamaguchi, S.; Miura, C.; Miura, T. Taurine plays an important role in the protection of spermatogonia from oxidative stress. Amino Acids 2012, 43, 2359–2369. [Google Scholar] [CrossRef] [PubMed]
- Sarih, S.; Djellata, A.; Roo, J.; Hernández-Cruz, C.M.; Fontanillas, R.; Rosenlund, G.; Izquierdo, M.; Fernández-Palacios, H. Effects of increased protein, histidine and taurine dietary levels on egg quality of greater amberjack (Seriola dumerili, Risso, 1810). Aquaculture 2019, 499, 72–79. [Google Scholar] [CrossRef]
- Richard, D.M.; Dawes, M.A.; Mathias, C.W.; Acheson, A.; Hill-Kapturczak, N.; Dougherty, D.M. L-tryptophan: Basic metabolic functions, behavioral research and therapeutic indications. Int. J. Tryptophan Res. 2009, 2, IJTR.S2129. [Google Scholar] [CrossRef] [PubMed]
- Kutluyer, F. In vitro Effect of L-Tryptophan on the Quality and Fertilizing Capacity of Sperms of Endangered Species of Trouts. Pak. J. Zool. 2018, 50. [Google Scholar] [CrossRef]
- Kocabas, M.; Kutluyer, F.; Ertekin, O.; Aksu, O.; Bascinar, N. Improvement of sperm motility of Oncorhynchus mykiss and Salvelinus fontinalis by L-tryptophan. Syst. Biol. Reprod. Med. 2019, 65, 187–193. [Google Scholar] [CrossRef]
- Salamanca, N.; Morales, E.; Ruiz-Azcona, P.; Herrera, M. Endocrine and metabolic effects of Trp-enriched diets for attenuation of chronic stress in the Senegal soles (Solea senegalensis). Aquaculture 2020, 523. [Google Scholar] [CrossRef]
- Dabrowski, K.; Terjesen, B.F.; Zhang, Y.; Phang, J.M.; Lee, K.J. A concept of dietary dipeptides: A step to resolve the problem of amino acid availability in the early life of vertebrates. J. Exp. Biol. 2005, 208, 2885–2894. [Google Scholar] [CrossRef]
- Jobgen, W.S.; Fried, S.K.; Fu, W.J.; Meininger, C.J.; Wu, G. Regulatory role for the arginine–nitric oxide pathway in metabolism of energy substrates. J. Nutr. Biochem. 2006, 17, 571–588. [Google Scholar] [CrossRef]
- Estienne, M.J.; Harper, A.F.; Crawford, R.J. Dietary supplementation with a source of omega-3 fatty acids increases sperm number and the duration of ejaculation in boars. Theriogenology 2008, 70, 70–76. [Google Scholar] [CrossRef]
- Labbe, C.; Loir, M.; Kaushik, S.; Maisse, G. The influence of both rearing temperature and dietary lipid origin on fatty acid composition of spermatozoan polar lipids in rainbow trout (Oncorhynchus mykiss). Effect on sperm cryopreservation tolerance. In Proceedings of the Colloques de l’INRA, Tours, France, 13–14 November 2013. [Google Scholar]
- Asturiano, J.F. El Proceso Reproductivo de la Lubina Europea (Dicentrarchus labrax L.): Efectos de los Ácidos Grasos de la Dieta: Estudios In Vivo e In Vitro. Ph.D. Thesis, Facultat de Ciències Biològiques, Universidad de Valencia, Valencia, Spain, 1999. [Google Scholar]
- Koprucu, K.; Yonar, M.E.; Ozcan, S. Effect of dietary n-3 polyunsaturated fatty acids on antioxidant defense and sperm quality in rainbow trout (Oncorhynchus mykiss) under regular stripping conditions. Anim. Reprod. Sci. 2015, 163, 135–143. [Google Scholar] [CrossRef]
- Santos, M.C.; Milani, C.; Zucchini, P.; Quirino, C.R.; Romagnoli, S.; da Cunha, I.C.N. Residual effect after salmon oil supplementation on semen quality and serum levels of testosterone in dogs. Reprod. Domest. Anim. 2019, 54, 1393–1399. [Google Scholar] [CrossRef] [PubMed]
- Alavi, S.M.H.; Pšenička, M.; Policar, T.; Rodina, M.; Hamáčková, J.; Kozák, P.; Linhart, O. Sperm quality in male Barbus barbus L. fed different diets during the spawning season. Fish Physiol. Biochem. 2009, 35, 683–693. [Google Scholar] [CrossRef] [PubMed]
- Mortensen, A.; Skibsted, L.H.; Truscott, T.G. The interaction of dietary carotenoids with radical species. Arch. Biochem. Biophys. 2001, 385, 13–19. [Google Scholar] [CrossRef] [PubMed]
- Kohlmeier, L.; Kark, J.D.; Gomez-Gracia, E.; Martin, B.C.; Steck, S.E.; Kardinaal, A.F.M.; Ringstad, J.; Thamm, M.; Masaev, V.; Riemersma, R.; et al. Lycopene and Myocardial Infarction Risk in the EURAMIC Study. Am. J. Epidemiol. 1997, 146, 618–626. [Google Scholar] [CrossRef] [PubMed]
- Amarowicz, R. Lycopene as a natural antioxidant. Eur. J. Lipid Sci. Technol. 2011, 113, 675–677. [Google Scholar] [CrossRef]
- Subhash, K.; Bose, C.; Agrawal, B. Effect of short term supplementation of tomatoes on antioxidant enzymes and lipid peroxidation in type-II diabetes. Indian J. Clin. Biochem. 2007, 22, 95–98. [Google Scholar] [CrossRef]
- Abdel-Daim, M.M.; Eissa, I.A.M.; Abdeen, A.; Abdel-Latif, H.M.R.; Ismail, M.; Dawood, M.A.O.; Hassan, A.M. Lycopene and resveratrol ameliorate zinc oxide nanoparticles-induced oxidative stress in Nile tilapia, Oreochromis niloticus. Environ. Toxicol. Pharmacol. 2019, 69, 44–50. [Google Scholar] [CrossRef]
- Ibrahim, A.T.A. Protective role of lycopene and vitamin E against diazinon-induced biochemical changes in Oreochromis niloticus. Afr. J. Environ. Sci. Technol. 2015, 9, 557–565. [Google Scholar] [CrossRef]
- Hussein, M.M.A.; Elsadaawy, H.A.; El-Murr, A.; Ahmed, M.M.; Bedawy, A.M.; Tukur, H.A.; Swelum, A.A.; Saadeldin, I.M. Endosulfan toxicity in Nile tilapia (Oreochromis niloticus) and the use of lycopene as an ameliorative agent. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2019, 224, 108573. [Google Scholar] [CrossRef]
- Hamed, H.S.; Osman, A.G.M. Modulatory effect of lycopene against carbofuran toxicity in African catfish, Clarias gariepinus. Fish Physiol. Biochem. 2017, 43, 1721–1731. [Google Scholar] [CrossRef]
- Yonar, M.E. Protective effect of lycopene on oxidative stress and antioxidant status in Cyprinus carpio during cypermethrin exposure. Environ. Toxicol. 2013, 28, 609–616. [Google Scholar] [CrossRef] [PubMed]
- Yonar, M.E.; Sakin, F. Ameliorative effect of lycopene on antioxidant status in Cyprinus carpio during pyrethroid deltamethrin exposure. Pestic. Biochem. Physiol. 2011, 99, 226–231. [Google Scholar] [CrossRef]
- Ural, M.Ş. Chlorpyrifos-induced changes in oxidant/antioxidant status and haematological parameters of Cyprinus carpio carpio: Ameliorative effect of lycopene. Chemosphere 2013, 90, 2059–2064. [Google Scholar] [CrossRef]
- Yonar, M.E. The effect of lycopene on oxytetracycline-induced oxidative stress and immunosuppression in rainbow trout (Oncorhynchus mykiss, W.). Fish Shellfish Immunol. 2012, 32, 994–1001. [Google Scholar] [CrossRef] [PubMed]
- Aust, O.; Sies, H.; Stahl, W.; Polidori, M.C. Analysis of lipophilic antioxidants in human serum and tissues: Tocopherols and carotenoids. J. Chromatogr. 2001, 936, 83–93. [Google Scholar] [CrossRef]
- Jensen, C.; Birk, E.; Jokumsen, A.; Skibsted, L.H.; Bertelsen, G. Effect of dietary levels of fat, α-tocopherol and astaxanthin on colour and lipid oxidation during storage of frozen rainbow trout (Oncorhynchus mykiss) and during chill storage of smoked trout. Z. Lebensm. Unters. Forsch. A 1998, 207, 189–196. [Google Scholar] [CrossRef]
- Güroy, B.; Şahin, İ.; Mantoğlu, S.; Kayalı, S. Spirulina as a natural carotenoid source on growth, pigmentation and reproductive performance of yellow tail cichlid Pseudotropheus acei. Aquacult. Int. 2012, 20, 869–878. [Google Scholar] [CrossRef]
- Gulcin, I. Antioxidant and antiradical activities of L-carnitine. Life Sci. 2006, 78, 803–811. [Google Scholar] [CrossRef]
- Longobardi, V.; Salzano, A.; Campanile, G.; Marrone, R.; Palumbo, F.; Vitiello, M.; Zullo, G.; Gasparrini, B. Carnitine supplementation decreases capacitation-like changes of frozen-thawed buffalo spermatozoa. Theriogenology 2017, 88, 236–243. [Google Scholar] [CrossRef]
- Sariozkan, S.; Ozdamar, S.; Turk, G.; Canturk, F.; Yay, A. In vitro effects of l-carnitine and glutamine on motility, acrosomal abnormality, and plasma membrane integrity of rabbit sperm during liquid-storage. Cryobiology 2014, 68, 349–353. [Google Scholar] [CrossRef]
- Elokil, A.A.; Bhuiyan, A.A.; Liu, H.Z.; Hussein, M.N.; Ahmed, H.I.; Azmal, S.A.; Yang, L.; Li, S. The capability of L-carnitine-mediated antioxidant on cock during aging: Evidence for the improved semen quality and enhanced testicular expressions of GnRH1, GnRHR, and melatonin receptors MT 1/2. Poult. Sci. 2019, 98, 4172–4181. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Chen, Y.-K.; Ghonimy, A.; Yu, T.; Gao, Y.-S.; Wu, Z.-C.; Wang, Q.-J.; Zhang, D.-M. L-Carnitine supplementation improved population growth, photosynthetic pigment synthesis and antioxidant activity of marine Chlorella sp. Aquac. Rep. 2020, 17, 100394. [Google Scholar] [CrossRef]
- Morris, L.; Gibb, Z. Oral supplementation with L-carnitine improves stallion fertility. J. Equine Vet. Sci. 2016, S82. [Google Scholar] [CrossRef]
- Yeste, M.; Sancho, S.; Briz, M.; Pinart, E.; Bussalleu, E.; Bonet, S. A diet supplemented with L-carnitine improves the sperm quality of Pietrain but not of Duroc and Large White boars when photoperiod and temperature increase. Theriogenology 2010, 73, 577–586. [Google Scholar] [CrossRef]
- Al-Daraji, H.; Tahir, A. Effect of L-carnitine supplementation on drake semen quality. S. Afr. J. Anim. Sci. 2014, 44, 18–25. [Google Scholar] [CrossRef]
- Neuman, S.; Lin, T.; Heste, P. The effect of dietary carnitine on semen traits of white Leghorn roosters. Poult. Sci. 2002, 81, 495–503. [Google Scholar] [CrossRef]
- Sarica, S.; Corduk, M.; Suicmez, M.; Cedden, F.; Yildirim, M.; Kilinc, K. The effects of dietary L-carnitine supplementation on semen traits, reproductive parameters, and testicular histology of Japanese quail breeders. J. Appl. Poult. Res. 2007, 16, 178–186. [Google Scholar] [CrossRef]
- Abd-Elrazek, A.; Ahmed-Farid, O. Protective effect of L-carnitine and L-arginine against busulfan-induced oligospermia in adult rat. Andrologia 2018, 50, e12806. [Google Scholar] [CrossRef]
- Harmeyer, J. The physiological role of L-carnitine. Lohman Inf. 2002, 27, 15–21. [Google Scholar]
- Santulli, A.; Modica, A.; Curatolo, A.; d’Amelio, V. Carnitine administration to sea bass (Dicentrarchus labrax (L.)) during feeding on a fat diet: Modification of plasma lipid levels and lipoprotein pattern. Aquaculture 1988, 68, 345–351. [Google Scholar] [CrossRef]
- Chen, Y.; Sun, Z.; Liang, Z.; Xie, Y.; Tan, X.; Su, J.; Luo, Q.; Zhu, J.; Liu, Q.; Wang, A. Addition of l-carnitine to formulated feed improved growth performance, antioxidant status and lipid metabolism of juvenile largemouth bass, Micropterus salmoides. Aquaculture 2020, 518, 734434. [Google Scholar] [CrossRef]
- Sabzi, E.; Mohammadiazarm, H.; Salati, A.P. Effect of dietary l-carnitine and lipid levels on growth performance, blood biochemical parameters and antioxidant status in juvenile common carp (Cyprinus carpio). Aquaculture 2017, 480, 89–93. [Google Scholar] [CrossRef]
- Dhakal, S.; Kushairi, N.; Phan, C.W.; Adhikari, B.; Sabaratnam, V.; Macreadie, I. Dietary Polyphenols: A Multifactorial Strategy to Target Alzheimer’s Disease. Int. J. Mol. Sci. 2019, 20, 5090. [Google Scholar] [CrossRef] [PubMed]
- Sandoval-Acuna, C.; Ferreira, J.; Speisky, H. Polyphenols and mitochondria: An update on their increasingly emerging ROS-scavenging independent actions. Arch. Biochem. Biophys. 2014, 559, 75–90. [Google Scholar] [CrossRef] [PubMed]
- Osipova, V.P.; Kolyada, M.N.; Berberova, N.T.; Milaeva, E.R.; Ponomareva, E.N.; Belaya, M.M. Cryoprotective effect of phosphorous-containing phenolic anti-oxidant for the cryopreservation of beluga sperm. Cryobiology 2014, 69, 467–472. [Google Scholar] [CrossRef] [PubMed]
- Ogretmen, F.; Inanan, B.E. Effect of butylated hydroxytoluene (BHT) on the cryopreservation of common carp (Cyprinus carpio) spermatozoa. Anim. Reprod. Sci. 2014, 151, 269–274. [Google Scholar] [CrossRef]
- Merino, O.; Dumorne, K.; Leidy, S.V.; Figueroa, E.; Valdebenito, I.; Farias, J.G.; Risopatron, J. Short-term storage sperm of coho salmon (Oncorhynchus kisutch) at 4 degrees C: Effect of sperm: Extender dilution ratios and antioxidant butyl-hydroxytoluene (BHT) on sperm function. Cryobiology 2020, 95, 44–50. [Google Scholar] [CrossRef]
- Osipova, V.P.; Berberova, N.T.; Gazzaeva, R.A.; Kudryavtsev, K.V. Application of new phenolic antioxidants for cryopreservation of sturgeon sperm. Cryobiology 2016, 72, 112–118. [Google Scholar] [CrossRef]
- Zanganeh, Z.; Zhandi, M.; Zare-Shahneh, A.; Najafi, A.; Mahdi Nabi, M.; Mohammadi-Sangcheshmeh, A. Does rosemary aqueous extract improve buck semen cryopreservation? Small Rumin. Res. 2013, 114, 120–125. [Google Scholar] [CrossRef]
- Dias, T.R.; Alves, M.G.; Tomas, G.D.; Socorro, S.; Silva, B.M.; Oliveira, P.F. White tea as a promising antioxidant medium additive for sperm storage at room temperature: A comparative study with green tea. J. Agric. Food Chem. 2014, 62, 608–617. [Google Scholar] [CrossRef]
- De Amicis, F.; Santoro, M.; Guido, C.; Russo, A.; Aquila, S. Epigallocatechin gallate affects survival and metabolism of human sperm. Mol. Nutr. Food Res. 2012, 56, 1655–1664. [Google Scholar] [CrossRef] [PubMed]
- El-Ratel, I.T.; Abdel-Khal, A.E.; El-Harairy, M.A.; Fouda, S.F.; Y. El-Bnaw, L. Impact of Green Tea Extract on Reproductive Performance, Hematology, Lipid Metabolism and Histogenesis of Liver and Kidney of Rabbit Does. Asian J. Anim. Vet. Adv. 2017, 12, 51–60. [Google Scholar] [CrossRef]
- Maleki, B.H.; Tartibian, B.; Mooren, F.C.; Nezhad, F.Y.; Yaseri, M. Saffron supplementation ameliorates oxidative damage to sperm DNA following a 16-week low-to-intensive cycling training in male road cyclists. J. Funct. Foods 2016, 21, 153–166. [Google Scholar] [CrossRef]
- Sapanidou, V.; Taitzoglou, I.; Tsakmakidis, I.; Kourtzelis, I.; Fletouris, D.; Theodoridis, A.; Zervos, I.; Tsantarliotou, M. Antioxidant effect of crocin on bovine sperm quality and in vitro fertilization. Theriogenology 2015, 84, 1273–1282. [Google Scholar] [CrossRef]
- Alizadeh, F.; Javadi, M.; Karami, A.A.; Gholaminejad, F.; Kavianpour, M.; Haghighian, H.K. Curcumin nanomicelle improves semen parameters, oxidative stress, inflammatory biomarkers, and reproductive hormones in infertile men: A randomized clinical trial. Phytother. Res. 2018, 32, 514–521. [Google Scholar] [CrossRef]
- Aparnak, P.; Saberivand, A. Effects of curcumin on canine semen parameters and expression of NOX5 gene in cryopreserved spermatozoa. Vet. Res. Forum 2019, 10, 221–226. [Google Scholar] [CrossRef]
- Bucak, M.N.; Sarıözkan, S.; Tuncer, P.B.; Sakin, F.; Ateşşahin, A.; Kulaksız, R.; Çevik, M. The effect of antioxidants on post-thawed Angora goat (Capra hircus ancryrensis) sperm parameters, lipid peroxidation and antioxidant activities. Small Rumin. Res. 2010, 89, 24–30. [Google Scholar] [CrossRef]
- Yan, W.; Kanno, C.; Oshima, E.; Kuzuma, Y.; Kim, S.W.; Bai, H.; Takahashi, M.; Yanagawa, Y.; Nagano, M.; Wakamatsu, J.I.; et al. Enhancement of sperm motility and viability by turmeric by-product dietary supplementation in roosters. Anim. Reprod. Sci. 2017, 185, 195–204. [Google Scholar] [CrossRef]
- El-Houseiny, W.; Khalil, A.A.; Abd-Elhakim, Y.M.; Badr, H.A. The potential role of turmeric and black pepper powder diet supplements in reversing cadmium-induced growth retardation, ATP depletion, hepatorenal damage, and testicular toxicity in Clarias gariepinus. Aquaculture 2019, 510, 109–121. [Google Scholar] [CrossRef]
- Martínez-Páramo, S.; Arfuso, F.; Engrola, S.; Dias, J.; Faggio, C.; Aragão, C. Do Immunostimulants Affect Sperm Quality in Senegalense sole? In Proceedings of the Larvi’13—Fish and Shellfish Larviculture Symposium, Gent, Belgium, 2–5 September 2005; p. 265. [Google Scholar]
- Nya, E.J.; Austin, B. Use of dietary ginger, Zingiber officinale Roscoe, as an immunostimulant to control Aeromonas hydrophila infections in rainbow trout, Oncorhynchus mykiss (Walbaum). J. Fish Dis. 2009, 32, 971–977. [Google Scholar] [CrossRef]
- Talpur, A.D.; Ikhwanuddin, M.; Ambok Bolong, A.-M. Nutritional effects of ginger (Zingiber officinale Roscoe) on immune response of Asian sea bass, Lates calcarifer (Bloch) and disease resistance against Vibrio harveyi. Aquaculture 2013, 400–401, 46–52. [Google Scholar] [CrossRef]
- Shakya, S.R. Medicinal uses of ginger (Zingiber officinale Roscoe) improves growth and enhances immunity in aquaculture. Int. J. Chem. Stud. 2015, 3, 83–87. [Google Scholar]
- Rojtinnakorn, J.; Rittiplang, S.; Tongsiri, S.; Chaibu, P. Tumeric extract inducing growth biomarker in sand goby (Oxyeleotris marmoratus). In Proceedings of the 2nd International Conference on Chemical, Biological and Environment Sciences (ICCEBS’2012), Penang, Malaysia, 1–12 February 2012. [Google Scholar]
- Attalla, R. Growth response and physiological activities of the Nile tilapia (Oreochromis niloticus) fed basal diets supplemented with ginger (Zingiber officinale) as natural growth promoters. Egypt. J. Aquat. Biol. Fish. 2009, 13, 85–107. [Google Scholar] [CrossRef]
- Dada, A.A. Dietary sesame improves reproductive performance of male African catfish. In Proceedings of World Aquaculture; FAO: Rome, Italy, 2012; pp. 66–67. [Google Scholar]
- Peixoto, M.J.; Ferraz, R.; Magnoni, L.J.; Pereira, R.; Goncalves, J.F.; Calduch-Giner, J.; Perez-Sanchez, J.; Ozorio, R.O.A. Protective effects of seaweed supplemented diet on antioxidant and immune responses in European seabass (Dicentrarchus labrax) subjected to bacterial infection. Sci. Rep. 2019, 9, 16134. [Google Scholar] [CrossRef]
- Ogretmen, F.; Inanan, B.E.; Ozturk, M. Protective effects of propolis on cryopreservation of common carp (Cyprinus carpio) sperm. Cryobiology 2014, 68, 107–112. [Google Scholar] [CrossRef]
- Dotta, G.; de Andrade, J.I.A.; Garcia, P.; Alves Jesus, G.F.; Mourino, J.L.P.; Mattos, J.J.; Dias Bainy, A.C.; Martins, M.L. Antioxidant enzymes, hematology and histology of spleen in Nile tilapia fed supplemented diet with natural extracts challenged with Aeromonas hydrophila. Fish Shellfish Immunol. 2018, 79, 175–180. [Google Scholar] [CrossRef]
- Kakoolaki, S.; Talas, Z.S.; Cakir, O.; Ciftci, O.; Ozdemir, I. Role of propolis on oxidative stress in fish brain. Basic Clin. Neurosci. 2013, 4, 153–158. [Google Scholar]
- Jofre, I.; Cuevas, M.; de Castro, L.S.; de Agostini Losano, J.D.; Torres, M.A.; Alvear, M.; Scheuermann, E.; Andrade, A.F.C.; Nichi, M.; Assumpcao, M.E.O.; et al. Antioxidant Effect of a Polyphenol-Rich Murtilla (Ugni molinae Turcz.) Extract and Its Effect on the Regulation of Metabolism in Refrigerated Boar Sperm. Oxid. Med. Cell. Longev. 2019, 2019. [Google Scholar] [CrossRef]
- Shahzad, Q.; Mehmood, M.U.; Khan, H.; ul Husna, A.; Qadeer, S.; Azam, A.; Naseer, Z.; Ahmad, E.; Safdar, M.; Ahmad, M. Royal jelly supplementation in semen extender enhances post-thaw quality and fertility of Nili-Ravi buffalo bull sperm. Anim. Reprod. Sci. 2016, 167, 83–88. [Google Scholar] [CrossRef]
- Abd-Allah, S.M. Effect of royal jelly on the fertilizing ability of buffalo spermatozoa in vitro. J. Buffalo Sci. 2012, 1, 1–4. [Google Scholar] [CrossRef]
- Alcay, S.; Toker, M.B.; Onder, N.T.; Gokce, E. Royal jelly supplemented soybean lecithin-based extenders improve post-thaw quality and incubation resilience of goat spermatozoa. Cryobiology 2017, 74, 81–85. [Google Scholar] [CrossRef] [PubMed]
- Amini, S.; Masoumi, R.; Rostami, B.; Shahir, M.H.; Taghilou, P.; Arslan, H.O. Effects of supplementation of Tris-egg yolk extender with royal jelly on chilled and frozen-thawed ram semen characteristics. Cryobiology 2019, 88, 75–80. [Google Scholar] [CrossRef] [PubMed]
- Mahdivand, N.; Najafi, G.; Nejati, V.; Shalizar-Jalali, A.; Rahmani, F. Royal jelly protects male rats from heat stress-induced reproductive failure. Andrologia 2019, 51, e13213. [Google Scholar] [CrossRef] [PubMed]
- Seven, I.; Tatli Seven, P.; Gul Baykalir, B.; Parlak Ak, T.; Ozer Kaya, S.; Yaman, M. Bee glue (propolis) improves reproductive organs, sperm quality and histological changes and antioxidant parameters of testis tissues in rats exposed to excess copper. Andrologia 2020, 52, e13540. [Google Scholar] [CrossRef]
- Kara, H.; Orem, A.; Yulug, E.; Yucesan, F.B.; Kerimoglu, G.; Yaman, S.O.; Bodur, A.; Turedi, S.; Alasalvar, C. Hazelnut consumption improves testicular antioxidant function and semen quality in young and old male rats. Food Chem. 2019, 294, 1–8. [Google Scholar] [CrossRef]
- Abdelnour, S.A.; Abd El-Hack, M.E.; Alagawany, M.; Taha, A.E.; Elnesr, S.S.; Abd Elmonem, O.M.; Swelum, A.A. Useful impacts of royal jelly on reproductive sides, fertility rate and sperm traits of animals. J. Anim. Physiol. Anim. Nutr. 2020. [Google Scholar] [CrossRef]
- Kocot, J.; Kielczykowska, M.; Luchowska-Kocot, D.; Kurzepa, J.; Musik, I. Antioxidant Potential of Propolis, Bee Pollen, and Royal Jelly: Possible Medical Application. Oxid. Med. Cell. Longev. 2018, 2018, 7074209. [Google Scholar] [CrossRef]
- Chatterjee, A. Reduced glutathione: A radioprotector or a modulator of DNA-repair activity? Nutrients 2013, 5, 525–542. [Google Scholar] [CrossRef]
- Rahman, K. Studies on free radicals, antioxidants, and co-factors. Clin. Interv. Aging 2007, 2, 219. [Google Scholar]
- Muthmainnah, C.R.; Eriani, K.; Hasri, I.; Irham, M.; Batubara, A.S.; Muchlisin, Z.A. Effect of glutathione on sperm quality after short-term cryopreservation in seurukan fish Osteochilus vittatus (Cyprinidae). Theriogenology 2018, 122, 30–34. [Google Scholar] [CrossRef]
- Sarosiek, B.; Judycka, S.; Kowalski, R.K. Influence of antioxidants on spermatozoa in the short-term storage of Salmonidae milt. Pol. J. Nat. Sci. 2013, 28, 379–384. [Google Scholar]
- Sasaki, S.; Ohta, T.; Decker, E.A. Antioxidant activity of water-soluble fractions of salmon spermary tissue. J. Agric. Food Chem. 1996, 44, 1682–1686. [Google Scholar] [CrossRef]
- Townsend, D.M.; Tew, K.D.; Tapiero, H. The importance of glutathione in human disease. Biomed. Pharmacother. 2003, 57, 145–155. [Google Scholar] [CrossRef]
- Bentinger, M.; Tekle, M.; Dallner, G. Coenzyme Q—Biosynthesis and functions. Biochem. Biophys. Res. Commun. 2010, 396, 74–79. [Google Scholar] [CrossRef] [PubMed]
- Yousefian, I.; Zare-Shahneh, A.; Zhandi, M. The Effect of Coenzyme Q10 and α-Tocopherol in Skim Milk–Based Extender for Preservation of Caspian Stallion Semen in Cool Condition. J. Equine Vet. Sci. 2014, 34, 949–954. [Google Scholar] [CrossRef]
- Saeed, A.; El-Nagar, H.; Wafa, W.; Hussein, Y. Effect of coenzyme Q10 as an antioxidant added to semen extender during cryopreservation of buffalo and cattle semen. J. Anim. Poult. Prod. 2016, 7, 403–408. [Google Scholar] [CrossRef]
- Masoudi, R.; Sharafi, M.; Shahneh, A.Z. Effects of CoQ10 on the quality of ram sperm during cryopreservation in plant and animal based extenders. Anim. Reprod. Sci. 2019, 208. [Google Scholar] [CrossRef]
- Bobe, J.; Labbe, C. Egg and sperm quality in fish. Gen. Comp. Endocrinol. 2010, 165, 535–548. [Google Scholar] [CrossRef]
- Ciereszko, A.; Dabrowski, K.; Kucharczyk, D.; Dobosz, S.; Goryczko, K.; Glogowski, J. The presence of uric acid, an antioxidantive substance, in fish seminal plasma. Fish Physiol. Biochem. 1999, 21, 313–315. [Google Scholar] [CrossRef]
- Inanan, B.E.; Kanyilmaz, M. Effect of alpha-lipoic acid on oxidative stress, viability and motility in the common carp (Cyprinus carpio) spermatozoa after short-term storage and cryopreservation. Cryobiology 2020, 94, 73–79. [Google Scholar] [CrossRef]
- Reiter, R.J.; Rosales-Corral, S.; Tan, D.X.; Jou, M.J.; Galano, A.; Xu, B. Melatonin as a mitochondria-targeted antioxidant: One of evolution’s best ideas. Cell. Mol. Life Sci. 2017, 74, 3863–3881. [Google Scholar] [CrossRef] [PubMed]
- Manchester, L.C.; Coto-Montes, A.; Boga, J.A.; Andersen, L.P.; Zhou, Z.; Galano, A.; Vriend, J.; Tan, D.X.; Reiter, R.J. Melatonin: An ancient molecule that makes oxygen metabolically tolerable. J. Pineal Res. 2015, 59, 403–419. [Google Scholar] [CrossRef] [PubMed]
- Zhao, D.; Yu, Y.; Shen, Y.; Liu, Q.; Zhao, Z.; Sharma, R.; Reiter, R.J. Melatonin Synthesis and Function: Evolutionary History in Animals and Plants. Front. Endocrinol. 2019, 10, 249. [Google Scholar] [CrossRef] [PubMed]
- Acuna-Castroviejo, D.; Escames, G.; Rodriguez, M.I.; Lopez, L.C. Melatonin role in the mitochondrial function. Front. Biosci. 2007, 12, 947–963. [Google Scholar] [CrossRef] [PubMed]
- Reiter, R.J.; Mayo, J.C.; Tan, D.X.; Sainz, R.M.; Alatorre-Jimenez, M.; Qin, L. Melatonin as an antioxidant: Under promises but over delivers. J. Pineal Res. 2016, 61, 253–278. [Google Scholar] [CrossRef] [PubMed]
- Falcón, J. Cellular circadian clocks in the pineal. Prog. Neurobiol. 1999, 58, 121–162. [Google Scholar] [CrossRef]
- Iuvone, P.M.; Tosini, G.; Pozdeyev, N.; Haque, R.; Klein, D.C.; Chaurasia, S.S. Circadian clocks, clock networks, arylalkylamine N-acetyltransferase, and melatonin in the retina. Prog. Retin. Eye Res. 2005, 24, 433–456. [Google Scholar] [CrossRef]
- García-Allegue, R.; Madrid, J.; Sánchez-Vázquez, F. Melatonin rhythms in European sea bass plasma and eye: Influence of seasonal photoperiod and water temperature. J. Pineal Res. 2001, 31, 68–75. [Google Scholar] [CrossRef]
- Cahill, G.M. Circadian regulation of melatonin production in cultured zebrafish pineal and retina. Brain Res. 1996, 708, 177–181. [Google Scholar] [CrossRef]
- Iigo, M.; Furukawa, K.; Hattori, A.; Ohtani-Kaneko, R.; Hara, M.; Suzuki, T.; Tabata, M.; Aida, K. Ocular melatonin rhythms in the goldfish, Carassius auratus. J. Biol. Rhythms 1997, 12, 182–192. [Google Scholar] [CrossRef]
- Besseau, L.; Benyassi, A.; Møller, M.; Coon, S.L.; Weller, J.L.; Boeuf, G.; Klein, D.C.; Falcón, J. Melatonin pathway: Breaking the ‘high-at-night’rule in trout retina. Exp. Eye Res. 2006, 82, 620–627. [Google Scholar] [CrossRef]
- Zachmann, A.; Knijff, S.; Ali, M.; Anctil, M. Effects of photoperiod and different intensities of light exposure on melatonin levels in the blood, pineal organ, and retina of the brook trout (Salvelinus fontinalis Mitchill). Can. J. Zool. 1992, 70, 25–29. [Google Scholar] [CrossRef]
- Falcón, J.; Besseau, L.; Sauzet, S.; Boeuf, G. Melatonin effects on the hypothalamo-pituitary axis in fish. Trends Endocrinol. Metab. 2007, 18, 81–88. [Google Scholar] [CrossRef]
- Vera, L.; De Pedro, N.; Gomez-Milan, E.; Delgado, M.J.; Sanchez-Muros, M.J.; Madrid, J.A.; Sanchez-Vazquez, F.J. Feeding entrainment of locomotor activity rhythms, digestive enzymes and neuroendocrine factors in goldfish. Physiol. Behav. 2007, 90, 518–524. [Google Scholar] [CrossRef]
- Bubenik, G.A.; Pang, S.F. Melatonin levels in the gastrointestinal tissues of fish, amphibians, and a reptile. Gen. Comp. Endocrinol. 1997, 106, 415–419. [Google Scholar] [CrossRef]
- Fang, Y.; Zhao, C.; Xiang, H.; Zhao, X.; Zhong, R. Melatonin Inhibits Formation of Mitochondrial Permeability Transition Pores and Improves Oxidative Phosphorylation of Frozen-Thawed Ram Sperm. Front. Endocrinol. 2019, 10, 896. [Google Scholar] [CrossRef]
- Leon, J.; Acuna-Castroviejo, D.; Escames, G.; Tan, D.X.; Reiter, R.J. Melatonin mitigates mitochondrial malfunction. J. Pineal Res. 2005, 38, 1–9. [Google Scholar] [CrossRef]
- Sauzet, S.; Besseau, L.; Herrera Perez, P.; Coves, D.; Chatain, B.; Peyric, E.; Boeuf, G.; Munoz-Cueto, J.A.; Falcon, J. Cloning and retinal expression of melatonin receptors in the European sea bass, Dicentrarchus labrax. Gen. Comp. Endocrinol. 2008, 157, 186–195. [Google Scholar] [CrossRef]
- Tan, D.X.; Manchester, L.C.; Hardeland, R.; Lopez-Burillo, S.; Mayo, J.C.; Sainz, R.M.; Reiter, R.J. Melatonin: A hormone, a tissue factor, an autocoid, a paracoid, and an antioxidant vitamin. J. Pineal Res. 2003, 34, 75–78. [Google Scholar] [CrossRef]
- Mayo, J.C.; Aguado, A.; Cernuda-Cernuda, R.; Alvarez-Artime, A.; Cepas, V.; Quiros-Gonzalez, I.; Hevia, D.; Sainz, R.M. Melatonin Uptake by Cells: An Answer to Its Relationship with Glucose? Molecules 2018, 23, 1999. [Google Scholar] [CrossRef]
- Lombardo, F.; Gioacchini, G.; Fabbrocini, A.; Candelma, M.; D’Adamo, R.; Giorgini, E.; Carnevali, O. Melatonin-mediated effects on killifish reproductive axis. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2014, 172, 31–38. [Google Scholar] [CrossRef]
- Gaildrat, P.; Becq, F.; Falcón, J. First cloning and functional characterization of a melatonin receptor in fish brain: A novel one? J. Pineal Res. 2002, 32, 74–84. [Google Scholar] [CrossRef]
- Mechaly, A.S.; Viñas, J.; Piferrer, F. The kisspeptin system genes in teleost fish, their structure and regulation, with particular attention to the situation in Pleuronectiformes. Gen. Comp. Endocrinol. 2013, 188, 258–268. [Google Scholar] [CrossRef]
- Cebrian-Perez, J.A.; Casao, A.; Gonzalez-Arto, M.; dos Santos Hamilton, T.R.; Perez-Pe, R.; Muino-Blanco, T. Melatonin in sperm biology: Breaking paradigms. Reprod. Domest. Anim. 2014, 49 (Suppl. S4), 11–21. [Google Scholar] [CrossRef]
- Amano, M.; Iigo, M.; Ikuta, K.; Kitamura, S.; Okuzawa, K.; Yamada, H.; Yamamori, K. Disturbance of Plasma Melatonin Profile by High Dose Melatonin Administration Inhibits Testicular Maturation of Precocious Male Masu Salmon. Zool. Sci. 2004, 21, 79–85. [Google Scholar] [CrossRef]
- Bhattacharya, S.; Chattoraj, A.; Maitra, S.K. Melatonin in the regulation of annual testicular events in carp Catla catla: Evidence from the studies on the effects of exogenous melatonin, continuous light, and continuous darkness. Chronobiol. Int. 2007, 24, 629–650. [Google Scholar] [CrossRef]
- Ozgur, M.E.; Ulu, A.; Noma, S.A.A.; Ozcan, I.; Balcioglu, S.; Ates, B.; Koytepe, S. Melatonin protects sperm cells of Capoeta trutta from toxicity of titanium dioxide nanoparticles. Environ. Sci. Pollut. Res. 2020, 27, 17843–17853. [Google Scholar] [CrossRef]
- Gao, Y.; Yang, C.; Huang, C.; Zhang, G.; Ji, H.; Dong, W. The effects of melatonin supplement on paddlefish (Polyodon spathula) sperm quality and ATP content in sperm during in vitro storage. Aquaculture 2019, 503, 475–482. [Google Scholar] [CrossRef]
- Alvarado, M.V.; Carrillo, M.; Felip, A. Melatonin-induced changes in kiss/gnrh gene expression patterns in the brain of male sea bass during spermatogenesis. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2015, 185, 69–79. [Google Scholar] [CrossRef]
- Carnevali, O.; Gioacchini, G.; Maradonna, F.; Olivotto, I.; Migliarini, B. Melatonin induces follicle maturation in Danio rerio. PLoS ONE 2011, 6, e19978. [Google Scholar] [CrossRef]
- Mondal, P.; Hasan, K.N.; Pal, P.K.; Maitra, S.K. Influences of exogenous melatonin on the oocyte growth and oxidative status of ovary during different reproductive phases of an annual cycle in carp Catla catla. Theriogenology 2017, 87, 349–359. [Google Scholar] [CrossRef]
- Assis, I.D.L.; Palhares, P.C.; Machado, G.J.; Souza, J.G.S.; Souza França, T.; Felizardo, V.O.; Murgas, L.D.S. Effect of melatonin on cryopreserved sperm of Prochilodus lineatus (Characiformes). CryoLetters 2019, 40, 152–158. [Google Scholar]
- Succu, S.; Berlinguer, F.; Pasciu, V.; Satta, V.; Leoni, G.G.; Naitana, S. Melatonin protects ram spermatozoa from cryopreservation injuries in a dose-dependent manner. J. Pineal Res. 2011, 50, 310–318. [Google Scholar] [CrossRef]
- Aripin, S.-A.; Jintasataporn, O.; Yoonpundh, R. Effects of exogenous melatonin in Clarias macrocephalus male broodstock first puberty stage. J. Aquac. Res. Dev. 2015, 6, 1. [Google Scholar] [CrossRef]
- She, Q.; Han, Z.; Liang, S.; Xu, W.; Li, X.; Zhao, Y.; Wei, H.; Dong, J.; Li, Y. Impacts of circadian rhythm and melatonin on the specific activities of immune and antioxidant enzymes of the Chinese mitten crab (Eriocheir sinensis). Fish Shellfish Immunol. 2019, 89, 345–353. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Félix, F.; Oliveira, C.C.V.; Cabrita, E. Antioxidants in Fish Sperm and the Potential Role of Melatonin. Antioxidants 2021, 10, 36. https://doi.org/10.3390/antiox10010036
Félix F, Oliveira CCV, Cabrita E. Antioxidants in Fish Sperm and the Potential Role of Melatonin. Antioxidants. 2021; 10(1):36. https://doi.org/10.3390/antiox10010036
Chicago/Turabian StyleFélix, Francisca, Catarina C. V. Oliveira, and Elsa Cabrita. 2021. "Antioxidants in Fish Sperm and the Potential Role of Melatonin" Antioxidants 10, no. 1: 36. https://doi.org/10.3390/antiox10010036
APA StyleFélix, F., Oliveira, C. C. V., & Cabrita, E. (2021). Antioxidants in Fish Sperm and the Potential Role of Melatonin. Antioxidants, 10(1), 36. https://doi.org/10.3390/antiox10010036





