Genistein Modulated Lipid Metabolism, Hepatic PPARγ, and Adiponectin Expression in Bilateral Ovariectomized Rats with Nonalcoholic Steatohepatitis (NASH)
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Experimental Protocols
2.2. Liver Tissue Analyses
2.2.1. Hepatic Histopathology
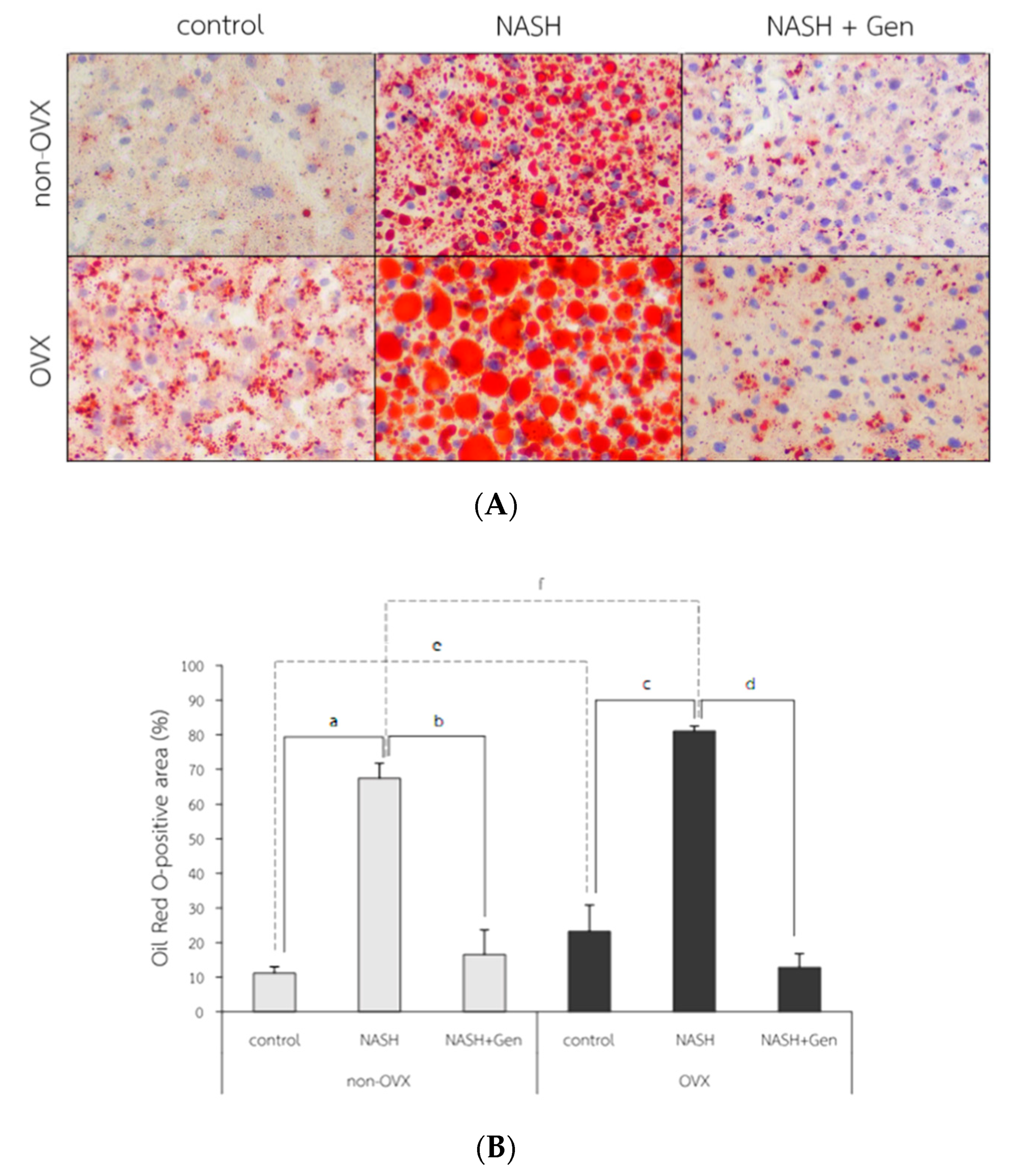
2.2.2. Hepatic Lipid Accumulation; Oil Red O (ORO) Stain, Free Fatty Acid (FFA) and Triglyceride (TG) Level
Oil Red O Stain
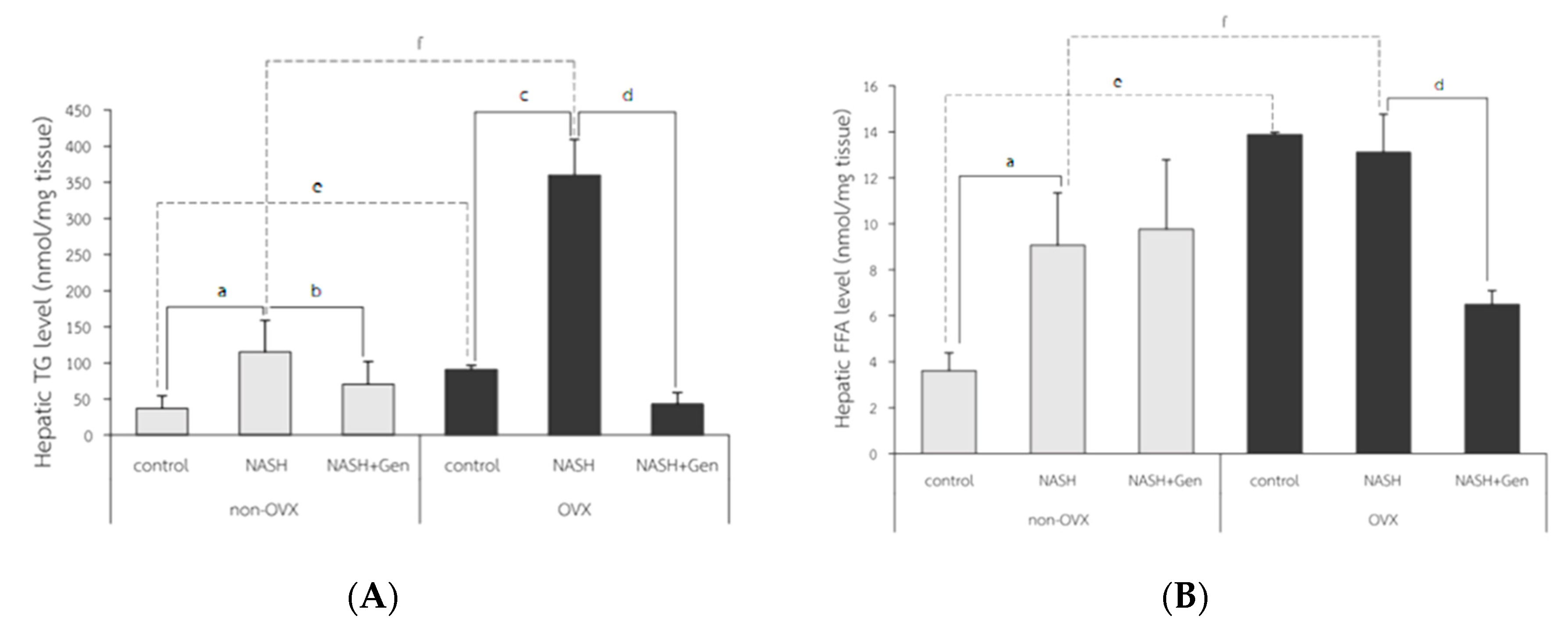
Colorimetry for Free Fatty Acid and Triglyceride Analysis
2.3. Hepatic Lipid Peroxidation by Thiobarbituric Acid Reactive (TBAR) Assay
2.4. Hepatocyte Apoptosis by TUNEL Assay
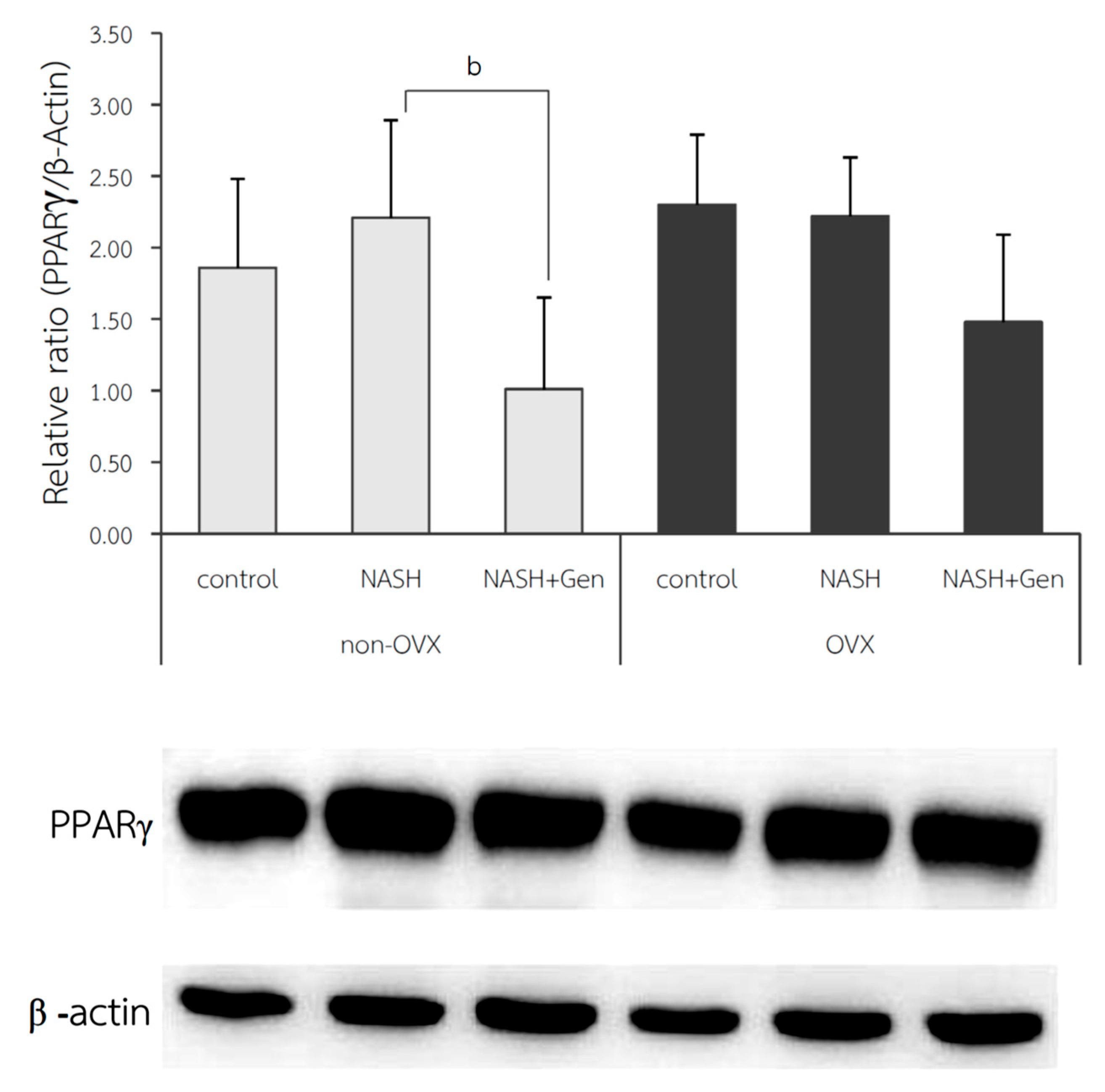
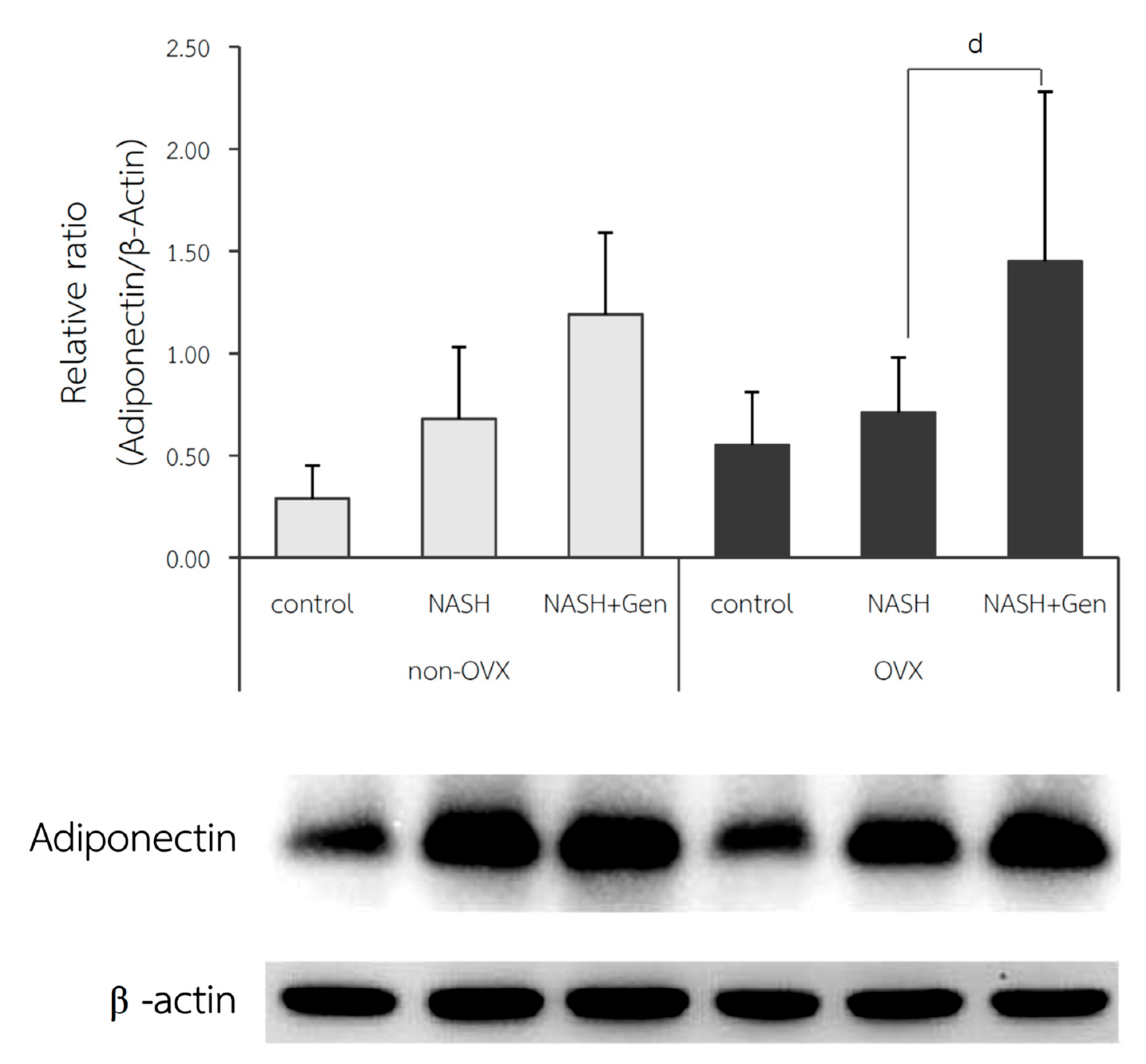
2.5. Hepatic Peroxisome Proliferator-Activated Receptor Gamma (PPARγ) and Adiponectin Protein Expression by Western Blot Analysis
2.6. Statistical Analysis
3. Results
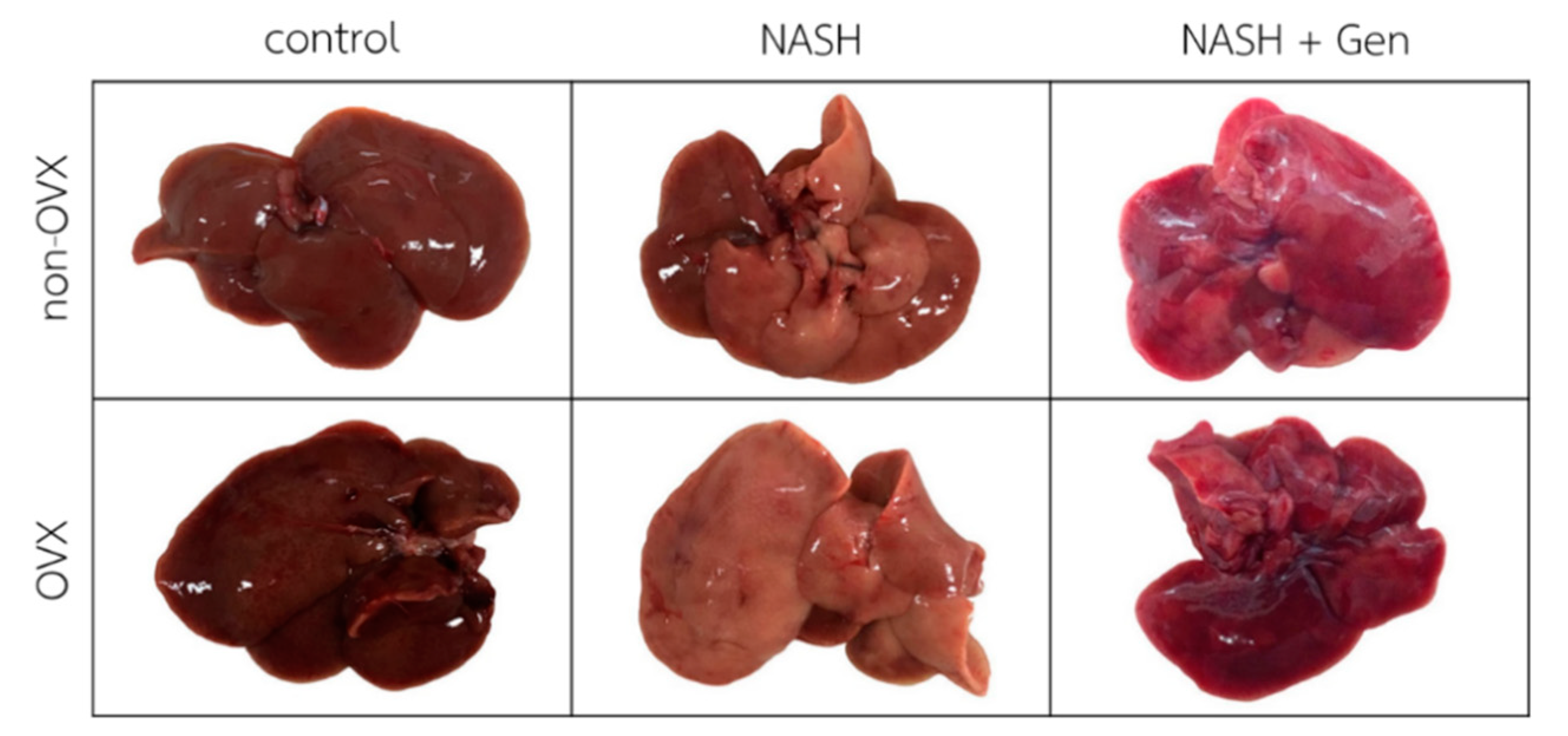
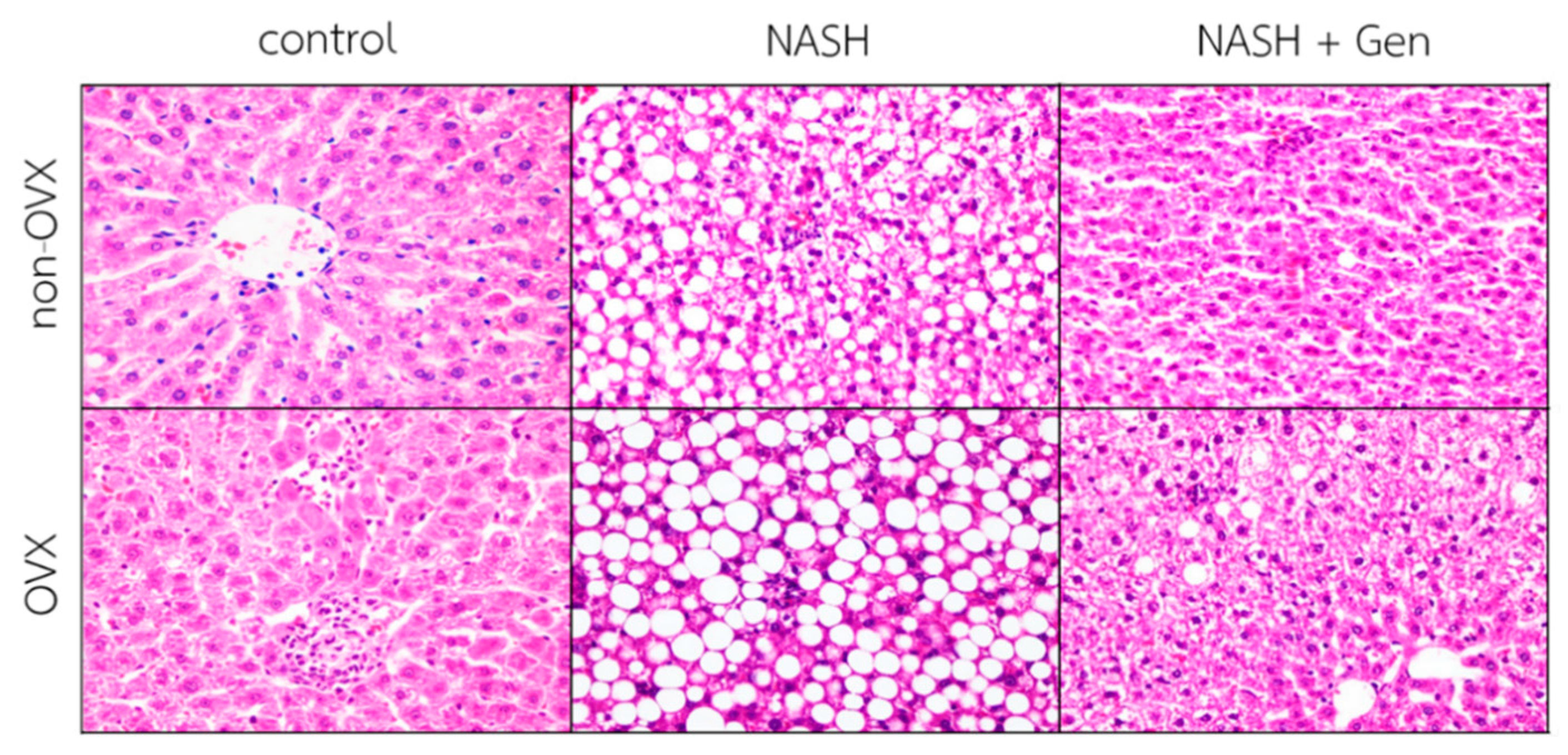
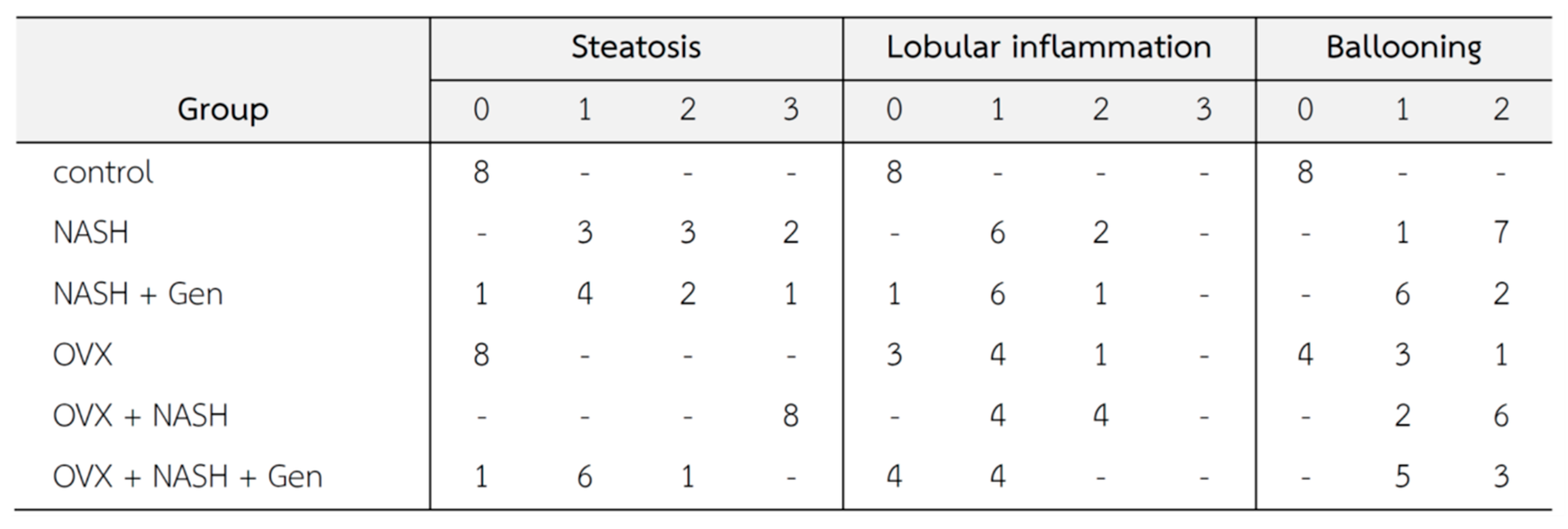
3.1. Gross Liver Appearance and Histopathology
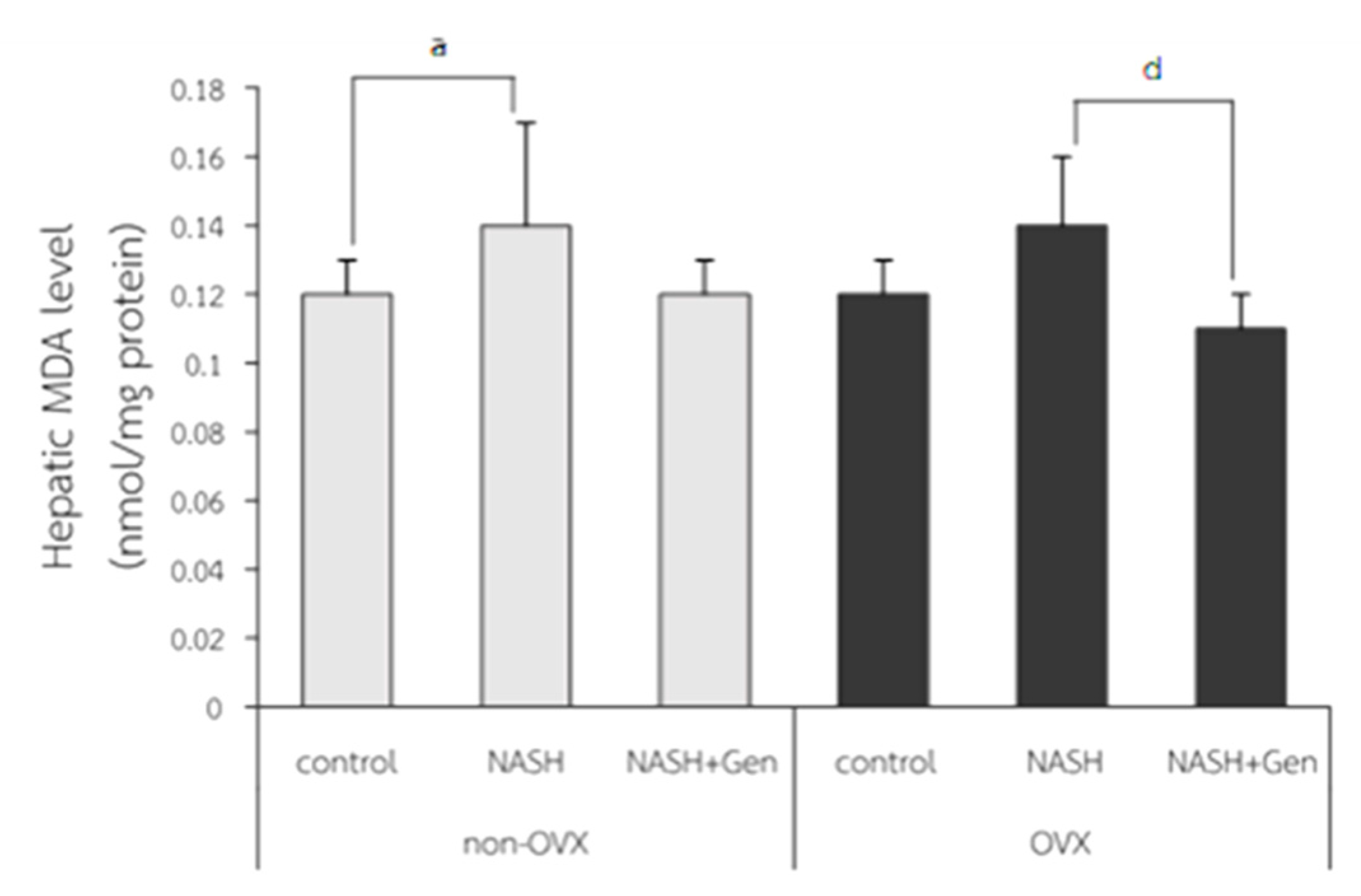
3.2. Hepatic Lipid Peroxidation Level
3.3. Hepatic Lipid Accumulation
3.4. Hepatocyte Apoptosis by TUNELs
3.5. Hepatic PPARγ Expression by Western Blot Analysis
3.6. Hepatic Adiponectin Expression by Western Blot Analysis
4. Discussion
4.1. High-Fat High-Fructose Diet and the Pathogenesis of NASH
4.2. Estrogen Deficiency Exacerbated the Pathogenesis of NASH
4.3. Genistein Ameliorated Liver Injuries in NASH through Its Anti-Lipogenic, Antioxidant, and Anti-Inflammatory Effects
4.4. Genistein Decreased the Severity of NASH by Down-Regulating Hepatic PPARγ Protein Expression
4.5. Genistein Improved NASH via Up-Regulated Adiponectin Protein Expression in Liver
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Marchesini, G.; Bugianesi, E.; Forlani, G.; Cerrelli, F.; Lenzi, M.; Manini, R.; Natale, S.; Vanni, E.; Villanova, N.; Melchionda, N.; et al. Nonalcoholic fatty liver, steatohepatitis, and the metabolic syndrome. Hepatology 2003, 37, 917–923. [Google Scholar] [CrossRef] [PubMed]
- Hsu, W.-F.; Sheen, L.-Y.; Lin, H.-J.; Chang, H.-H. A Review of Western and Traditional Chinese Medical Approaches to Managing Nonalcoholic Fatty Liver Disease. Evid. Based Complement. Altern. Med. 2016, 2016, 6491420. [Google Scholar] [CrossRef] [PubMed]
- Brunt, E.M. Nonalcoholic Steatohepatitis. Semin. Liver Dis. 2004, 24, 3–20. [Google Scholar] [CrossRef] [PubMed]
- Buzzetti, E.; Pinzani, M.; Tsochatzis, E. The multiple-hit pathogenesis of non-alcoholic fatty liver disease (NAFLD). Metabolism 2016, 65, 1038–1048. [Google Scholar] [CrossRef]
- Grodstein, F.; Manson, J.E.; Colditz, G.A.; Willett, W.C.; Speizer, F.E.; Stampfer, M.J. A prospective, observational study of postmenopausal hormone therapy and pri-mary prevention of cardiovascular disease. Ann. Intern. Med. 2000, 133, 933–941. [Google Scholar] [CrossRef]
- Cignarella, A.; Kratz, M.; Bolego, C. Emerging role of estrogen in the control of cardiometabolic disease. Trends Pharmacol. Sci. 2010, 31, 183–189. [Google Scholar] [CrossRef]
- Kamada, Y.; Kiso, S.; Yoshida, Y.; Chatani, N.; Kizu, T.; Hamano, M.; Tsubakio, M.; Takemura, T.; Ezaki, H.; Hayashi, N.; et al. Estrogen deficiency worsens steatohepatitis in mice fed high-fat and high-cholesterol diet. Am. J. Physiol. Liver Physiol. 2011, 301, G1031–G1043. [Google Scholar] [CrossRef]
- Yang, J.D.; Abdelmalek, M.F.; Pang, H.; Guy, C.D.; Smith, A.D.; Diehl, A.M.; Suzuki, A. Gender and menopause impact severity of fibrosis among patients with nonalcoholic steatohepatitis. Hepatology 2014, 59, 1406–1414. [Google Scholar] [CrossRef]
- Ji, G.; Yang, Q.; Hao, J.; Guo, L.; Chen, X.; Hu, J.; Leng, L.; Jiang, Z. Anti-inflammatory effect of genistein on non-alcoholic steatohepatitis rats induced by high fat diet and its potential mechanisms. Int. Immunopharmacol. 2011, 11, 762–768. [Google Scholar] [CrossRef]
- Choi, J.S.; Koh, I.-U.; Song, J. Genistein reduced insulin resistance index through modulating lipid metabolism in ovariectomized rats. Nutr. Res. 2012, 32, 844–855. [Google Scholar] [CrossRef]
- Susutlertpanya, W.; Werawatganon, D.; Siriviriyakul, P.; Klaikeaw, N. Genistein Attenuates Nonalcoholic Steatohepatitis and Increases Hepatic PPARgamma in a Rat Model. Evid. Based Complement. Alternat. Med. 2015, 2015, 509057. [Google Scholar]
- Jeon, S.; Park, Y.J.; Kwon, Y.H. Genistein alleviates the development of nonalcoholic steatohepatitis in ApoE(-/-) mice fed a high-fat diet. Mol. Nutr. Food Res. 2014, 58, 830–841. [Google Scholar] [CrossRef] [PubMed]
- Pickens, M.K.; Ogata, H.; Soon, R.K.; Grenert, J.P.; Maher, J.J. Dietary fructose exacerbates hepatocellular injury when incorporated into a methionine-choline-deficient diet. Liver Int. 2010, 30, 1229–1239. [Google Scholar] [CrossRef] [PubMed]
- Brunt, E.M.; Janney, C.G.; Di Bisceglie, A.M.; Neuschwander-Tetri, B.A.; Bacon, B.R. Nonalcoholic steatohepatitis: A proposal for grading and staging the histological lesions. Am. J. Gastroenterol. 1999, 94, 2467–2474. [Google Scholar] [CrossRef]
- Hasegawa, T.; Ito, Y.; Wijeweera, J.; Liu, J.; Malle, E.; Farhood, A.; McCuskey, R.S.; Jaeschke, H. Reduced inflammatory response and increased microcirculatory disturbances during hepatic ischemia-reperfusion injury in steatotic livers of ob/ob mice. Am. J. Physiol. Liver Physiol. 2007, 292, G1385–G1395. [Google Scholar] [CrossRef]
- Lim, J.S.; Mietus-Snyder, M.; Valente, A.; Schwarz, J.-M.; Lustig, R.H. The role of fructose in the pathogenesis of NAFLD and the metabolic syndrome. Nat. Rev. Gastroenterol. Hepatol. 2010, 7, 251–264. [Google Scholar] [CrossRef]
- Ishimoto, T.; Lanaspa, M.A.; Rivard, C.J.; Roncal-Jimenez, C.A.; Orlicky, D.J.; Cicerchi, C.; Mcmahan, R.H.; Abdelmalek, M.F.; Rosen, H.R.; Jackman, M.R.; et al. High-fat and high-sucrose (western) diet induces steatohepatitis that is dependent on fructokinase. Hepatology 2013, 58, 1632–1643. [Google Scholar] [CrossRef]
- Jegatheesan, P.; De Bandt, J. Fructose and NAFLD: The Multifaceted Aspects of Fructose Metabolism. Nutrients 2017, 9, 230. [Google Scholar] [CrossRef]
- Koruk, M.; Taysi, S.; Savas, M.C.; Yilmaz, O.; Akcay, F.; Karakok, M. Oxidative stress and enzymatic antioxidant status in patients with nonalcoholic steato-hepatitis. Ann. Clin. Lab. Sci. 2004, 34, 57–62. [Google Scholar]
- Li, Z.Z.; Berk, M.; McIntyre, T.M.; Feldstein, A.E. Hepatic lipid partitioning and liver damage in nonalcoholic fatty liver disease: Role of stearoyl-CoA desaturase. J. Biol. Chem. 2009, 284, 5637–5644. [Google Scholar] [CrossRef]
- Zou, C.; Ma, J.; Wang, X.; Guo, L.; Zhu, Z.; Stoops, J.; Eaker, E.A.; Johnson, C.J.; Strom, S.C.; Michalopoulos, G.K.; et al. Lack of Fas antagonism by Met in human fatty liver disease. Nat. Med. 2007, 13, 1078–1085. [Google Scholar] [CrossRef] [PubMed]
- Micheau, O.; Tschopp, J. Induction of TNF Receptor I-Mediated Apoptosis via Two Sequential Signaling Complexes. Cell 2003, 114, 181–190. [Google Scholar] [CrossRef]
- Minero, V.G.; Khadjavi, A.; Costelli, P.; Baccino, F.M.; Bonelli, G. JNK activation is required for TNFalpha-induced apoptosis in human hepatocarcinoma cells. Int. Immunopharmacol. 2013, 17, 92–98. [Google Scholar] [CrossRef] [PubMed]
- Yokoyama, Y.; Nimura, Y.; Nagino, M.; Bland, K.I.; Chaudry, I.H. Current understanding of gender dimorphism in hepatic patho-physiology. J. Surg. Res. 2005, 128, 147–156. [Google Scholar] [CrossRef]
- Yoneda, M.; Thomas, E.; Sumida, Y.; Eguchi, Y.; Schiff, E.R. The influence of menopause on the development of hepatic fibrosis in nonobese women with nonalcoholic fatty liver disease. Hepatology 2014, 60, 1792. [Google Scholar] [CrossRef]
- Zhang, Y.; Klein, K.; Sugathan, A.; Nassery, N.; Dombkowski, A.; Zanger, U.M.; Waxman, D.J. Transcriptional profiling of human liver identifies sex-biased genes associated with pol-ygenic dyslipidemia and coronary artery disease. PLoS ONE 2011, 6, e23506. [Google Scholar]
- Bryzgalova, G.; Lundholm, L.; Portwood, N.; Gustafsson, J.-Å.; Khan, A.; Efendic, S.; Dahlman-Wright, K. Mechanisms of antidiabetogenic and body weight-lowering effects of estrogen in high-fat diet-fed mice. Am. J. Physiol. Metab. 2008, 295, E904–E912. [Google Scholar] [CrossRef]
- Weigt, C.; Hertrampf, T.; Zoth, N.; Fritzemeier, K.H.; Diel, P. Impact of estradiol, ER subtype specific agonists and genistein on energy homeostasis in a rat model of nutrition induced obesity. Mol. Cell. Endocrinol. 2012, 351, 227–238. [Google Scholar] [CrossRef]
- Lundholm, L.; Zang, H.; Hirschberg, A.L.; Gustafsson, J.-Å.; Arner, P.; Dahlman-Wright, K. Key lipogenic gene expression can be decreased by estrogen in human adipose tissue. Fertil. Steril. 2008, 90, 44–48. [Google Scholar] [CrossRef]
- Tang, C.; Zhang, K.; Zhao, Q.; Zhang, J. Effects of Dietary Genistein on Plasma and Liver Lipids, Hepatic Gene Expression, and Plasma Metabolic Profiles of Hamsters with Diet-Induced Hyperlipidemia. J. Agric. Food Chem. 2015, 63, 7929–7936. [Google Scholar] [CrossRef]
- Weigt, C.; Hertrampf, T.; Kluxen, F.M.; Flenker, U.; Hülsemann, F.; Fritzemeier, K.H.; Diel, P. Molecular effects of ER alpha- and beta-selective agonists on regulation of energy homeostasis in obese female Wistar rats. Mol. Cell. Endocrinol. 2013, 377, 147–158. [Google Scholar] [CrossRef] [PubMed]
- Zhong, H.; Liu, H.; Jiang, Z. Genistein Ameliorates Fat Accumulation through AMPK Activation in Fatty Acid-Induced BRL Cells. J. Food Sci. 2017, 82, 2719–2725. [Google Scholar] [CrossRef] [PubMed]
- Yoo, N.Y.; Jeon, S.; Nam, Y.; Park, Y.J.; Won, S.B.; Kwon, Y.H. Dietary Supplementation of Genistein Alleviates Liver Inflammation and Fibrosis Mediated by a Methionine-Choline-Deficient Diet in db/db Mice. J. Agric. Food Chem. 2015, 63, 4305–4311. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.; Matsusue, K.; Kashireddy, P.; Cao, W.Q.; Yeldandi, V.; Yeldandi, A.V.; Rao, M.S.; Gonzalez, F.J.; Reddy, J.K. Adipocyte-specific gene expression and adipogenic steatosis in the mouse liver due to peroxisome proliferator-activated receptor gamma1 (PPARgamma1) overexpression. J. Biol. Chem. 2003, 278, 498–505. [Google Scholar] [CrossRef]
- Gavrilova, O.; Haluzik, M.; Matsusue, K.; Cutson, J.J.; Johnson, L.; Dietz, K.R.; Nicol, C.J.; Vinson, C.; Gonzalez, F.J.; Reitman, M.L. Liver peroxisome proliferator-activated receptor gamma contributes to hepatic steatosis, triglyceride clearance, and regulation of body fat mass. J. Biol. Chem. 2003, 278, 34268–34276. [Google Scholar] [CrossRef]
- García-Ruiz, I.; Rodríguez-Juan, C.; Díaz-Sanjuán, T.; Martínez, M.Á.; Muñoz-Yagüe, T.; Solís-Herruzo, J.A. Effects of rosiglitazone on the liver histology and mitochondrial function in ob/ob mice. Hepatology 2007, 46, 414–423. [Google Scholar] [CrossRef]
- Pettinelli, P.; Videla, L.A. Up-regulation of PPAR-gamma mRNA expression in the liver of obese patients: An additional rein-forcing lipogenic mechanism to SREBP-1c induction. J. Clin. Endocrinol. Metab. 2011, 96, 1424–1430. [Google Scholar] [CrossRef]
- Wu, C.W.; Chu, E.S.; Lam, C.N.; Cheng, A.S.L.; Lee, C.W.; Wong, V.W.S.; Sung, J.J.Y.; Yu, J. PPARgamma is essential for protection against nonalcoholic steatohepatitis. Gene Ther. 2010, 17, 790–798. [Google Scholar] [CrossRef]
- Grygiel-Gorniak, B. Peroxisome proliferator-activated receptors and their ligands: Nutritional and clinical implications—A re-view. Nutr. J. 2014, 13, 17. [Google Scholar] [CrossRef]
- Kim, M.-H.; Kang, K.-S.; Lee, Y.-S. The inhibitory effect of genistein on hepatic steatosis is linked to visceral adipocyte metabolism in mice with diet-induced non-alcoholic fatty liver disease. Br. J. Nutr. 2010, 104, 1333–1342. [Google Scholar] [CrossRef]
- Cui, S.; Wienhoefer, N.; Bilitewski, U. Genistein induces morphology change and G2/M cell cycle arrest by inducing p38 MAPK activation in macrophages. Int. Immunopharmacol. 2014, 18, 142–150. [Google Scholar] [CrossRef] [PubMed]
- Kansra, S.V.; Reddy, M.A.; Weng, Y.-I.; Shukla, S.D. Activation of mitogen activated protein kinase in human platelets by genistein. Pharmacol. Res. 1999, 39, 21–31. [Google Scholar] [CrossRef] [PubMed]
- Burns, K.A.; Heuvel, J.P.V. Modulation of PPAR activity via phosphorylation. Biochim. Biophys. Acta 2007, 1771, 952–960. [Google Scholar] [CrossRef] [PubMed]
- Tschritter, O.; Fritsche, A.; Thamer, C.; Haap, M.; Shirkavand, F.; Rahe, S.; Staiger, H.; Maerker, E.; Häring, H.; Stumvoll, M. Plasma adiponectin concentrations predict insulin sensitivity of both glucose and lipid metabolism. Diabetes 2003, 52, 239–243. [Google Scholar] [CrossRef] [PubMed]
- Lemoine, M.; Ratziu, V.; Kim, M.; Maachi, M.; Wendum, D.; Paye, F.; Bastard, J.P.; Poupon, R.; Housset, C.; Capeau, J.; et al. Serum adipokine levels predictive of liver injury in non-alcoholic fatty liver disease. Liver Int. 2009, 29, 1431–1438. [Google Scholar] [CrossRef] [PubMed]
- Musso, G.; Gambino, R.; Biroli, G.; Carello, M.; Faga, E.; Pacini, G.; De Michieli, F.; Cassader, M.; Durazzo, M.; Rizzetto, M.; et al. Hypoadiponectinemia Predicts the Severity of Hepatic Fibrosis and Pancreatic Beta-Cell Dysfunction in Nondiabetic Nonobese Patients with Nonalcoholic Steatohepatitis. Am. J. Gastroenterol. 2005, 100, 2438–2446. [Google Scholar] [CrossRef]
- Kaser, S.; Moschen, A.; Cayon, A.; Kaser, A.; Crespo, J.; Pons-Romero, F.; Ebenbichler, C.F.; Patsch, J.R.; Tilg, H. Adiponectin and its receptors in non-alcoholic steatohepatitis. Gut 2005, 54, 117–121. [Google Scholar] [CrossRef]
- Xu, A.; Wang, Y.; Keshaw, H.; Xu, L.Y.; Lam, K.S.; Cooper, G.J. The fat-derived hormone adiponectin alleviates alcoholic and nonalcoholic fatty liver diseases in mice. J. Clin. Investig. 2003, 112, 91–100. [Google Scholar] [CrossRef]
- Iwaki, M.; Matsuda, M.; Maeda, N.; Funahashi, T.; Matsuzawa, Y.; Makishima, M.; Shimomura, I. Induction of Adiponectin, a Fat-Derived Antidiabetic and Antiatherogenic Factor, by Nuclear Receptors. Diabetes 2003, 52, 1655–1663. [Google Scholar] [CrossRef]
- Yanagisawa, M.; Sugiya, M.; Iijima, H.; Nakagome, I.; Hirono, S.; Tsuda, T. Genistein and daidzein, typical soy isoflavones, inhibit TNF-alpha-mediated down-regulation of adiponectin expression via different mechanisms in 3T3-L1 adipocytes. Mol. Nutr. Food Res. 2012, 56, 1783–1793. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pummoung, S.; Werawatganon, D.; Chayanupatkul, M.; Klaikeaw, N.; Siriviriyakul, P. Genistein Modulated Lipid Metabolism, Hepatic PPARγ, and Adiponectin Expression in Bilateral Ovariectomized Rats with Nonalcoholic Steatohepatitis (NASH). Antioxidants 2021, 10, 24. https://doi.org/10.3390/antiox10010024
Pummoung S, Werawatganon D, Chayanupatkul M, Klaikeaw N, Siriviriyakul P. Genistein Modulated Lipid Metabolism, Hepatic PPARγ, and Adiponectin Expression in Bilateral Ovariectomized Rats with Nonalcoholic Steatohepatitis (NASH). Antioxidants. 2021; 10(1):24. https://doi.org/10.3390/antiox10010024
Chicago/Turabian StylePummoung, Sudaporn, Duangporn Werawatganon, Maneerat Chayanupatkul, Naruemon Klaikeaw, and Prasong Siriviriyakul. 2021. "Genistein Modulated Lipid Metabolism, Hepatic PPARγ, and Adiponectin Expression in Bilateral Ovariectomized Rats with Nonalcoholic Steatohepatitis (NASH)" Antioxidants 10, no. 1: 24. https://doi.org/10.3390/antiox10010024
APA StylePummoung, S., Werawatganon, D., Chayanupatkul, M., Klaikeaw, N., & Siriviriyakul, P. (2021). Genistein Modulated Lipid Metabolism, Hepatic PPARγ, and Adiponectin Expression in Bilateral Ovariectomized Rats with Nonalcoholic Steatohepatitis (NASH). Antioxidants, 10(1), 24. https://doi.org/10.3390/antiox10010024



